Peter Eckl: Research on the Pro-/Antioxidant Balance
Funding
Conflicts of Interest
References
- Esterbauer, H.; Eckl, P.; Ortner, A. Possible mutagens derived from lipids and lipid precursors. Mutat. Res. 1990, 238, 223–233. [Google Scholar] [CrossRef]
- Eckl, P.M.; Ortner, A.; Esterbauer, H. Genotoxic properties of 4-hydroxyalkenals and analogous aldehydes. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1993, 290, 183–192. [Google Scholar] [CrossRef]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef]
- Brambilla, G.; Sciabà, L.; Faggin, P.; Maura, A.; Marinari, U.M.; Ferro, M.; Esterbauer, H. Cytotoxicity, DNA fragmentation and sister-chromatid exchange in Chinese hamster ovary cells exposed to the lipid peroxidation product 4-hydroxynonenal and homologous aldehydes. Mutat. Res. 1986, 171, 169–176. [Google Scholar] [CrossRef]
- Poli, G.; Schaur, R.J.; Siems, W.G.; Leonarduzzi, G. 4-hydroxynonenal: A membrane lipid oxidation product of medicinal interest. Med. Res. Rev. 2008, 28, 569–631. [Google Scholar] [CrossRef]
- Schaur, R.; Siems, W.; Bresgen, N.; Eckl, P. 4-Hydroxy-nonenal—A Bioactive Lipid Peroxidation Product. Biomolecules 2015, 5, 2247. [Google Scholar] [CrossRef] [Green Version]
- Karlhuber, G.M.; Bauer, H.C.; Eckl, P.M. Cytotoxic and genotoxic effects of 4-hydroxynonenal in cerebral endothelial cells. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1997, 381, 209–216. [Google Scholar] [CrossRef]
- Bresgen, N.; Karlhuber, G.; Krizbai, I.; Bauer, H.; Bauer, H.C.; Eckl, P.M. Oxidative stress in cultured cerebral endothelial cells induces chromosomal aberrations, micronuclei, and apoptosis. J. Neurosci. Res. 2003, 72, 327–333. [Google Scholar] [CrossRef]
- Bresgen, N.; Jaksch, H.; Bauer, H.; Eckl, P.; Krizbai, I.; Tempfer, H. Astrocytes are more resistant than cerebral endothelial cells toward geno- and cytotoxicity mediated by short-term oxidative stress. J. Neurosci. Res. 2006, 84, 1821–1828. [Google Scholar] [CrossRef]
- Omenn, G.S.; Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Glass, A.; Keogh, J.P.; Meyskens, F.L., Jr.; Valanis, B.; Williams, J.H., Jr.; et al. Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. J. Natl. Cancer Inst. 1996, 88, 1550–1559. [Google Scholar] [CrossRef]
- Blumberg, J.; Block, G. The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study in Finland. Nutr. Rev. 1994, 52, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Sommerburg, O.; Langhans, C.D.; Arnhold, J.; Leichsenring, M.; Salerno, C.; Crifò, C.; Hoffmann, G.F.; Debatin, K.M.; Siems, W.G. Beta-carotene cleavage products after oxidation mediated by hypochlorous acid—A model for neutrophil-derived degradation. Free Radic. Biol. Med. 2003, 35, 1480–1490. [Google Scholar] [CrossRef] [PubMed]
- Siems, W.; Sommerburg, O.; Schild, L.; Augustin, W.; Langhans, C.D.; Wiswedel, I. Beta-carotene cleavage products induce oxidative stress in vitro by impairing mitochondrial respiration. FASEB J. 2002, 16, 1289–1291. [Google Scholar] [CrossRef] [PubMed]
- Alija, A.J.; Bresgen, N.; Sommerburg, O.; Langhans, C.D.; Siems, W.; Eckl, P.M. Beta-carotene breakdown products enhance genotoxic effects of oxidative stress in primary rat hepatocytes. Carcinogenesis 2006, 27, 1128–1133. [Google Scholar] [CrossRef] [Green Version]
- Alija, A.J.; Bresgen, N.; Sommerburg, O.; Siems, W.; Eckl, P.M. Cytotoxic and genotoxic effects of beta-carotene breakdown products on primary rat hepatocytes. Carcinogenesis 2004, 25, 827–831. [Google Scholar] [CrossRef]
- Alija, A.J.; Bresgen, N.; Sommerburg, O.; Langhans, C.D.; Siems, W.; Eckl, P.M. Cyto- and genotoxic potential of beta-carotene and cleavage products under oxidative stress. Biofactors 2005, 24, 159–163. [Google Scholar] [CrossRef]
- Haider, C.; Ferk, F.; Bojaxhi, E.; Martano, G.; Stutz, H.; Bresgen, N.; Knasmüller, S.; Alija, A.; Eckl, P.M. Effects of β-Carotene and Its Cleavage Products in Primary Pneumocyte Type II Cells. Antioxidants 2017, 6, 37. [Google Scholar] [CrossRef] [Green Version]
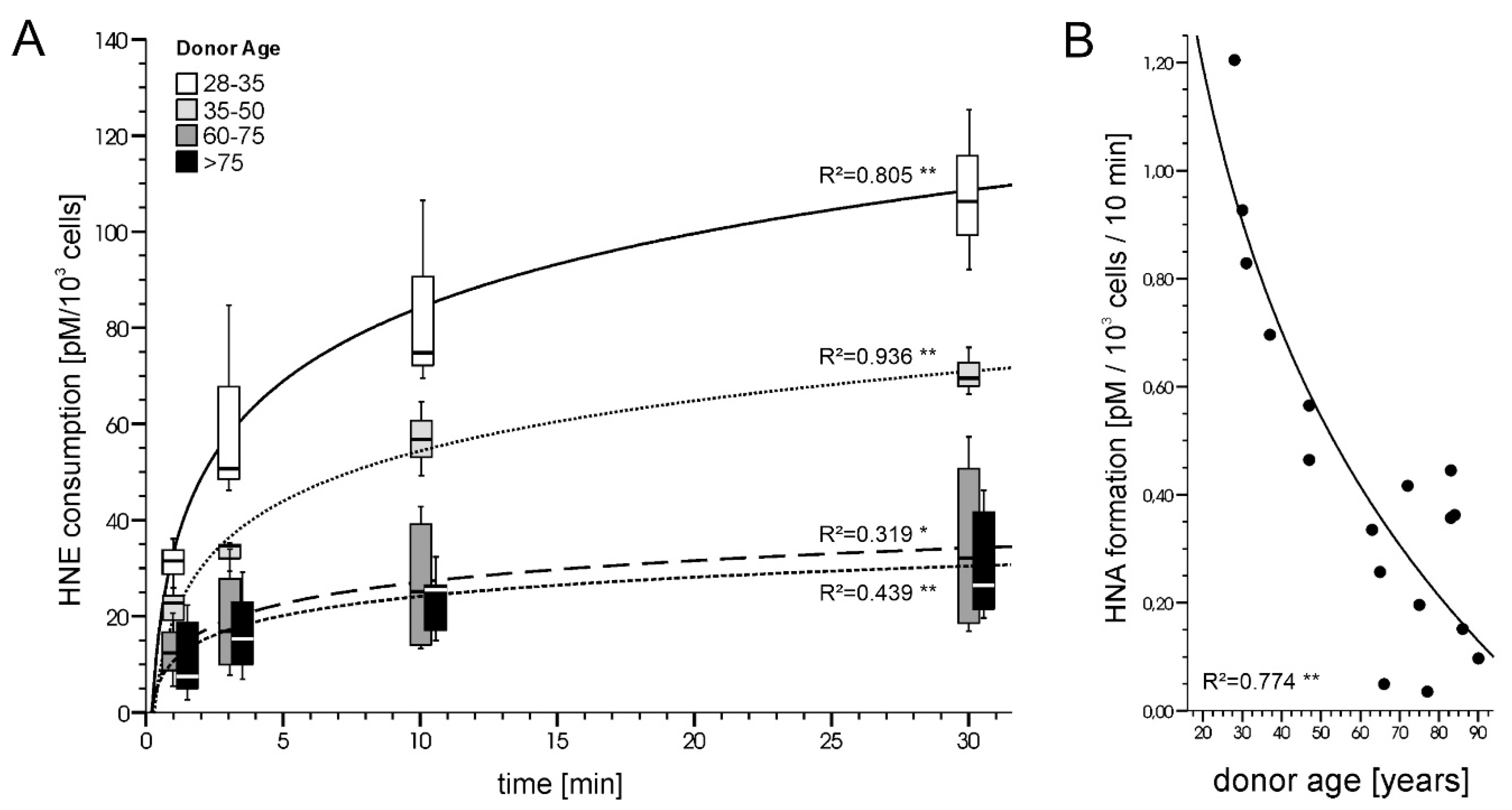
- Petkovic, I.; Bresgen, N.; Gilardoni, E.; Regazzoni, L.; Uchida, K.; Aldini, G.; Siems, W.; Eckl, P. In Vitro Aging of Human Skin Fibroblasts: Age-Dependent Changes in 4-Hydroxynonenal Metabolism. Antioxidants 2020, 9, 150. [Google Scholar] [CrossRef] [Green Version]
- Siems, W.; Voss, P.; Bresgen, N.; Eckl, P.; Grune, T. unpublished work.
- Negre-Salvayre, A.; Auge, N.; Ayala, V.; Basaga, H.; Boada, J.; Brenke, R.; Chapple, S.; Cohen, G.; Feher, J.; Grune, T.; et al. Pathological aspects of lipid peroxidation. Free Radic. Res. 2010, 44, 1125–1171. [Google Scholar] [CrossRef]
- Xi, Y.; Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef]
- Chevolleau, S.; Noguer-Meireles, M.H.; Mervant, L.; Martin, J.F.; Jouanin, I.; Pierre, F.; Naud, N.; Guéraud, F.; Debrauwer, L. Towards Aldehydomics: Untargeted Trapping and Analysis of Reactive Diet-Related Carbonyl Compounds Formed in the Intestinal Lumen. Antioxidants 2021, 10, 1261. [Google Scholar] [CrossRef] [PubMed]
- Eckl, P.M.; Strom, S.C.; Michalopoulos, G.; Jirtle, R.L. Induction of sister chromatid exchanges in cultured adult rat hepatocytes by directly and indirectly acting mutagens/carcinogens. Carcinogenesis 1987, 8, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Eckl, P.M.; Alati, T.; Jirtle, R.L. The effects of a purified diet on sister chromatid exchange frequencies and mitotic activity in adult rat hepatocytes. Carcinogenesis 1991, 12, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Eckl, P.M.; Raffelsberger, I. The primary rat hepatocyte micronucleus assay: General features. Mutat. Res. 1997, 392, 117–124. [Google Scholar] [CrossRef]
- Sommerburg, O.; Hämmerling, S.; Schneider, S.P.; Okun, J.; Langhans, C.D.; Leutz-Schmidt, P.; Wielpütz, M.O.; Siems, W.; Gräber, S.Y.; Mall, M.A.; et al. CFTR Modulator Therapy with Lumacaftor/Ivacaftor Alters Plasma Concentrations of Lipid-Soluble Vitamins A and E in Patients with Cystic Fibrosis. Antioxidants 2021, 10, 483. [Google Scholar] [CrossRef]
- Altomare, A.; Baron, G.; Brioschi, M.; Longoni, M.; Butti, R.; Valvassori, E.; Tremoli, E.; Carini, M.; Agostoni, P.; Vistoli, G.; et al. N-Acetyl-Cysteine Regenerates Albumin Cys34 by a Thiol-Disulfide Breaking Mechanism: An Explanation of Its Extracellular Antioxidant Activity. Antioxidants 2020, 9, 367. [Google Scholar] [CrossRef]
- Mónico, A.; Guzmán-Caldentey, J.; Pajares, M.A.; Martín-Santamaría, S.; Pérez-Sala, D. Molecular Insight into the Regulation of Vimentin by Cysteine Modifications and Zinc Binding. Antioxidants 2021, 10, 1039. [Google Scholar] [CrossRef]
- Benedetti, A.; Comporti, M.; Esterbauer, H. Identification of 4-hydroxynonenal as a cytotoxic product originating from the peroxidation of liver microsomal lipids. Biochim. Biophys. Acta 1980, 620, 281–296. [Google Scholar] [CrossRef]
- Schauenstein, E.; Wünschmann, B.; Esterbauer, H. On the extensively selective in-vivo destruction of subcutaneously implanted Ehrlich ascites tumor cells using 4-hydroxy-enales, especially 4-hydroxy-pentenal. Z Krebsforsch 1968, 71, 21–29. [Google Scholar] [CrossRef]
- György, P.; Tomarelli, R.; Ostergard, R.P.; Brown, J.B. Unsaturated fatty acids in the dietary destruction of N,N-dimethylaminoazobenzene (butter yellow) and in the production of anemia in rats. J. Exp. Med. 1942, 76, 413–420. [Google Scholar] [CrossRef]
- Paniker, N.V.; Srivastava, S.K.; Beutler, E. Glutathione metabolism of the red cells. Effect of glutathione reductase deficiency on the stimulation of hexose monophosphate shunt under oxidative stress. Biochim. Biophys. Acta 1970, 215, 456–460. [Google Scholar] [CrossRef]
- Sies, H.; Chance, B. The steady state level of catalase compound I in isolated hemoglobin-free perfused rat liver. FEBS Lett. 1970, 11, 172–176. [Google Scholar] [CrossRef] [Green Version]
- Sies, H. Findings in redox biology: From H(2)O(2) to oxidative stress. J. Biol. Chem. 2020, 295, 13458–13473. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Biochemistry of oxidative stress. Angew. Chem. Int. Ed. Engl. 1986, 25, 1058–1071. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress. Introductory remarks. In Oxidative Stress; Sies, H., Ed.; Academic Press: London, UK, 1985; pp. 1–8. [Google Scholar]
- Horvathova, E.; Eckl, P.M.; Bresgen, N.; Slamenova, D. Evaluation of genotoxic and cytotoxic effects of H2O2 and DMNQ on freshly isolated rat hepatocytes; protective effects of carboxymethyl chitin-glucan. Neuroendocrinol. Lett. 2008, 29, 644–648. [Google Scholar]
- Khader, M.; Bresgen, N.; Eckl, P.M. Antimutagenic effects of ethanolic extracts from selected Palestinian medicinal plants. J. Ethnopharmacol. 2010, 127, 319–324. [Google Scholar] [CrossRef]
- Chiou, B.; Neal, E.H.; Bowman, A.B.; Lippmann, E.S.; Simpson, I.A.; Connor, J.R. Endothelial cells are critical regulators of iron transport in a model of the human blood–brain barrier. J. Cereb. Blood Flow Metab. 2019, 39, 2117–2131. [Google Scholar] [CrossRef]
- Khader, M.; Bresgen, N.; Eckl, P.M. In vitro toxicological properties of thymoquinone. Food Chem. Toxicol. 2009, 47, 129–133. [Google Scholar] [CrossRef]
- Bresgen, N.; Rolinek, R.; Hochleitner, E.; Lottspeich, F.; Eckl, P.M. Induction of apoptosis by a hepatocyte conditioned medium. J. Cell. Physiol. 2004, 198, 452–460. [Google Scholar] [CrossRef]
- Bresgen, N.; Jaksch, H.; Lacher, H.; Ohlenschläger, I.; Uchida, K.; Eckl, P.M. Iron mediated oxidative stress plays an essential role in ferritin induced cell death. Free Radic. Biol. Med. 2010, 48, 1347–1357. [Google Scholar] [CrossRef]
- Krenn, M.A.; Schürz, M.; Teufl, B.; Uchida, K.; Eckl, P.M.; Bresgen, N. Ferritin stimulated lipid peroxidation, lysosomal leak and macroautophagy promote lysosomal “metastability” in primary hepatocytes determining in-vitro cell survival. Free Radic. Biol. Med. 2014, 80, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Korovila, I.; Höhn, A.; Jung, T.; Grune, T.; Ott, C. Reduced Liver Autophagy in High-Fat Diet Induced Liver Steatosis in New Zealand Obese Mice. Antioxidants 2021, 10, 501. [Google Scholar] [CrossRef] [PubMed]

| Year of First Entry | Entries in Year 1987 1 | Entries in Year 2021 | Total Entries 2 | |
|---|---|---|---|---|
| Antioxidants | 1942 * | 4181 | 41,666 | 669,369 |
| Oxidative stress | 1960 ** | 95 | 28,257 | 280,672 |
| Lipid peroxidation | 1958 | 578 | 3889 | 79,687 |
| 4-Hydroxynonenal 3 | 1980 4 | 19 | 281 | 5664 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bresgen, N.; Siems, W. Peter Eckl: Research on the Pro-/Antioxidant Balance. Antioxidants 2022, 11, 1079. https://doi.org/10.3390/antiox11061079
Bresgen N, Siems W. Peter Eckl: Research on the Pro-/Antioxidant Balance. Antioxidants. 2022; 11(6):1079. https://doi.org/10.3390/antiox11061079
Chicago/Turabian StyleBresgen, Nikolaus, and Werner Siems. 2022. "Peter Eckl: Research on the Pro-/Antioxidant Balance" Antioxidants 11, no. 6: 1079. https://doi.org/10.3390/antiox11061079
APA StyleBresgen, N., & Siems, W. (2022). Peter Eckl: Research on the Pro-/Antioxidant Balance. Antioxidants, 11(6), 1079. https://doi.org/10.3390/antiox11061079




