High-Risk HPV with Multiple Infections Promotes CYP2E1, Lipoperoxidation and Pro-Inflammatory Cytokines in Semen of Asymptomatic Infertile Men
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Virus Detection and Genotyping
2.3. Oxidative Stress (OS) Biomarkers in the Seminal Plasma
2.3.1. Relative Gene Expression of CYP2E1 Enzyme and Antioxidant Enzymes Catalase (CAT) and Superoxide Dismutase (SOD) by qRT-PCR
2.3.2. Quantification of Total Antioxidant Capacity (TAC)
2.3.3. Assessment of Lipoperoxidation
2.3.4. 8-Hydroxydeoxyguanosine Assay
2.4. Cytokine Expression by Enzyme-Linked Immunosorbent Assays (ELISA)
2.5. Statistical Analysis
3. Results
3.1. Detected HPV Genotypes in the Population
3.2. Seminal Parameters in the Study Groups
3.3. Oxidative Damage in the Semen of Infertile Men
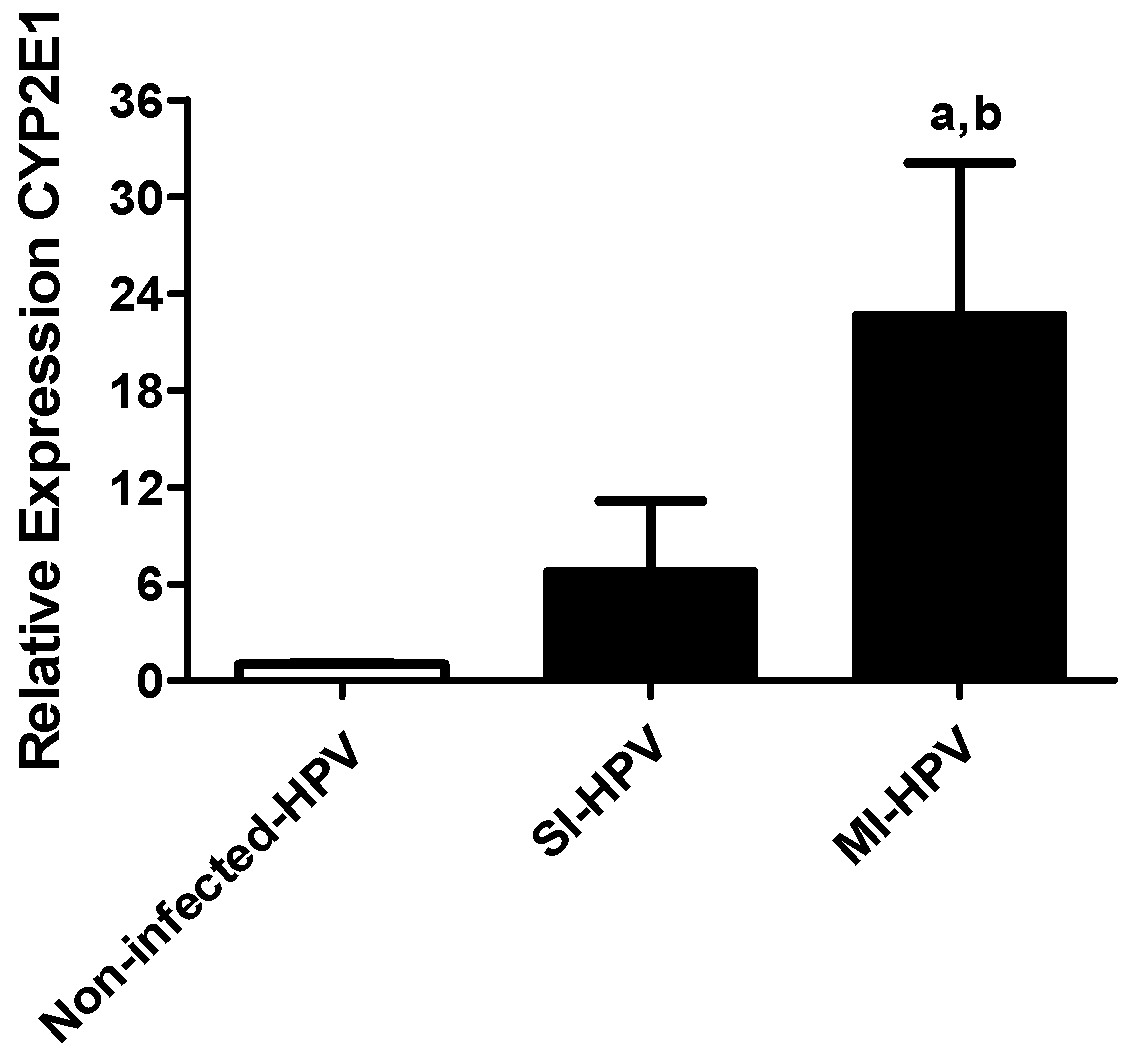
3.3.1. Relative Gene Expression of Cyp2E1
3.3.2. Lipoperoxidation and DNA Damage in the Semen
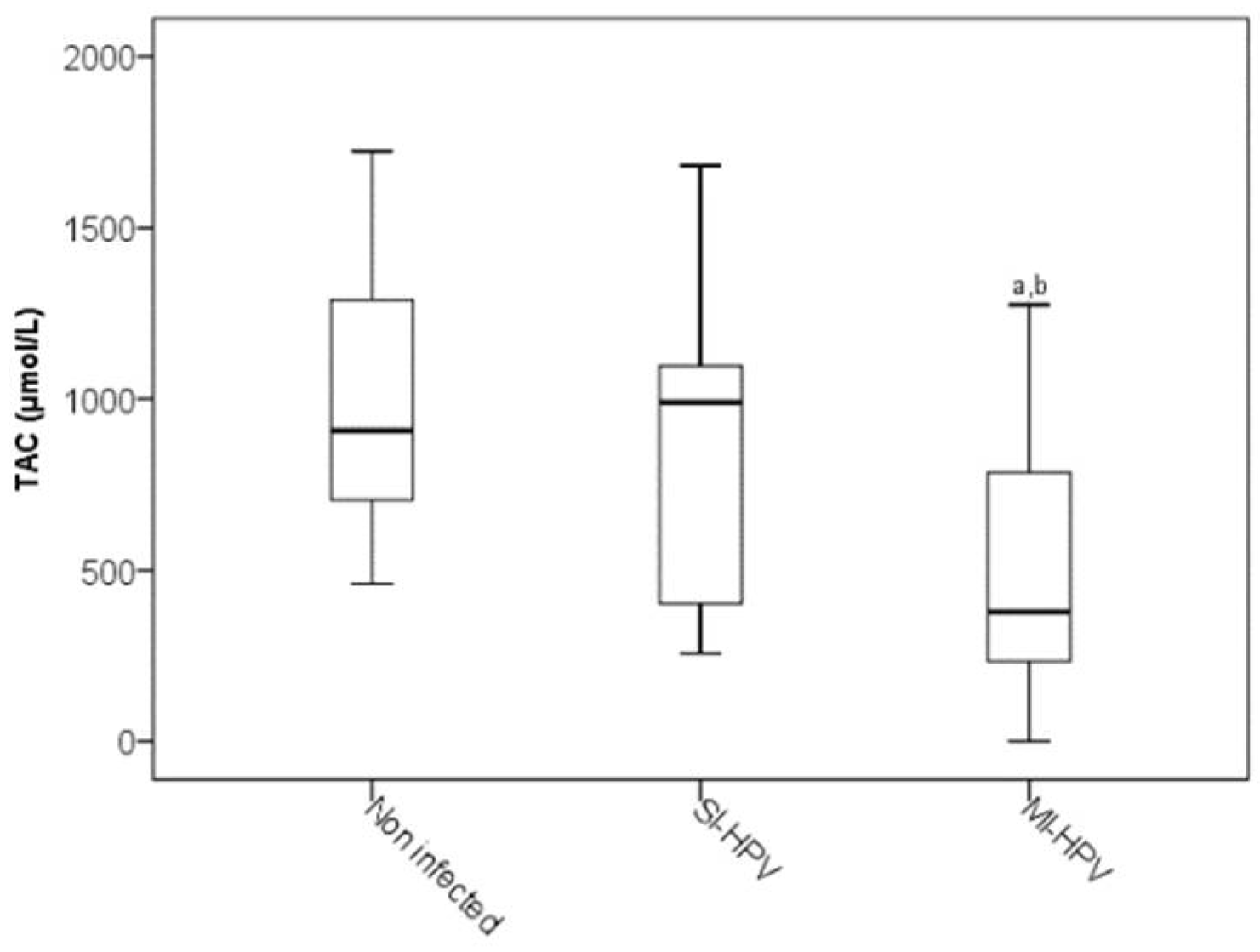
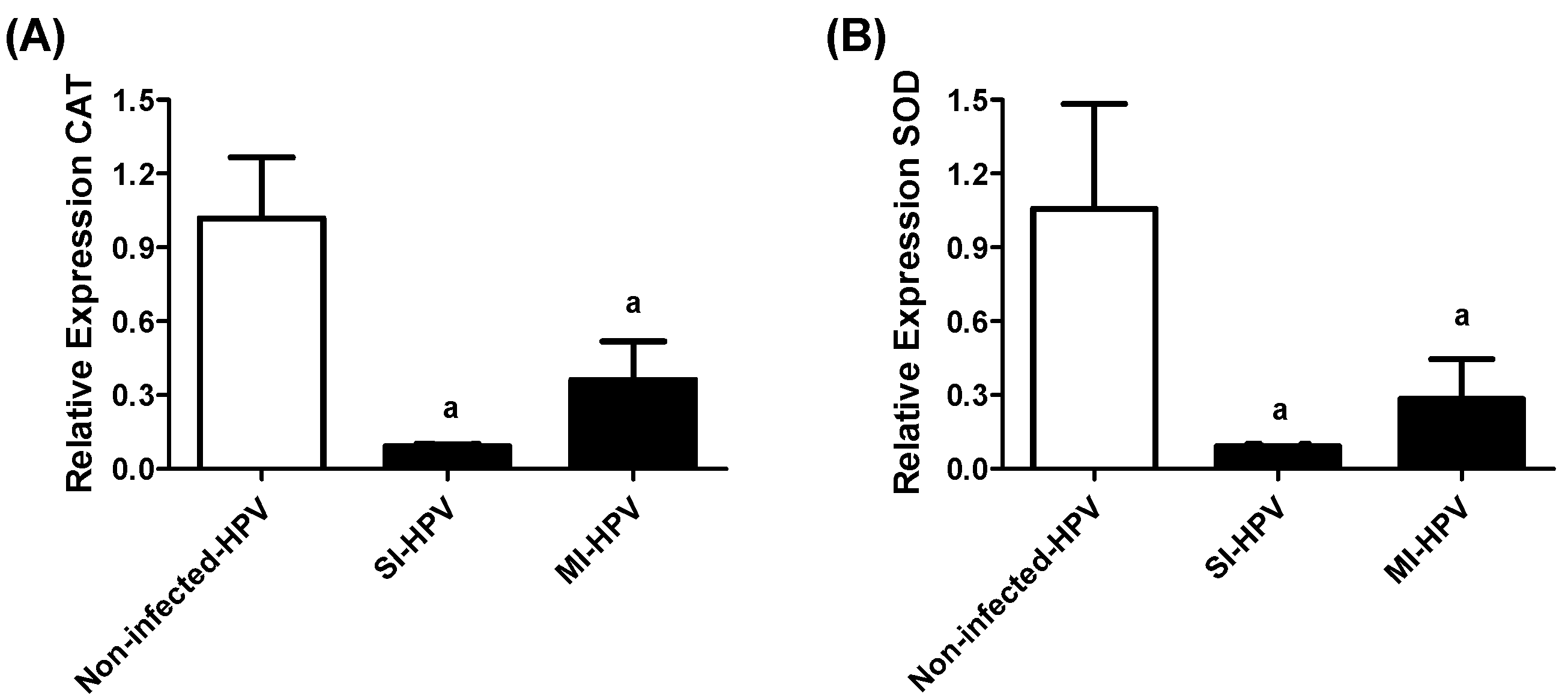
3.3.3. Antioxidants in the Semen
3.4. Correlation between OS Biomarkers and Sperm Morphology in the Infected Population
3.5. Seminal Pro-Inflammatory Cytokines
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foresta, C.; Noventa, M.; De Toni, L.; Gizzo, S.; Garolla, A. HPV-DNA sperm infection and infertility: From a systematic literature review to a possible clinical management proposal. Andrology 2015, 3, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Laprise, C.; Trottier, H.; Monnier, P.; Coutlée, F.M.M. Prevalence of human papillomaviruses in semen: A systematic review and meta-analysis. Hum. Reprod. 2014, 29, 640–651. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Z.; Feng, X.; Li, N.; Zhao, W.; Wei, L.; Chen, Y. Human papillomavirus in semen and the risk for male infertility: A systematic review and meta-analysis. BMC Infect. Dis. 2017, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jeršovienė, V.; Gudlevičienė, Z.; Rimienė, J.; Butkauskas, D. Human Papillomavirus and Infertility. Medicina 2019, 55, 377. [Google Scholar] [CrossRef]
- Pérez-Soto, E.; Fernández-Martínez, E.; Oros-Pantoja, R.; Medel-Flores, O.; Miranda-Covarrubias, J.C.; Sánchez-Monroy, V. Proinflammatory and Oxidative Stress States Induced by Human Papillomavirus and Chlamydia trachomatis Coinfection Affect Sperm Quality in Asymptomatic Infertile Men. Medicina 2021, 57, 862. [Google Scholar] [CrossRef]
- Gimenes, F.; Medina, F.S.; De Abreu, A.L.P.; Irie, M.M.T.; Esquicąti, I.B.; Malagutti, N.; Vasconcellos, V.R.B.; Discacciati, M.G.; Bonini, M.G.; Maria-Engler, S.S. Sensitive simultaneous detection of seven sexually transmitted agents in semen by multiplex-PCR and of HPV by single PCR. PLoS ONE 2014, 9, e98862. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P.; Roychoudhury, S.; Chakravarthi, S.; Wang, C.W.; Slama, P. Antioxidant Paradox in Male Infertility: ‘A Blind Eye’ on Inflammation. Antioxidants 2022, 11, 167. [Google Scholar] [CrossRef]
- Agarwal, A.; Rana, M.; Qiu, E.; AlBunni, H.; Bui, A.D.; Henkel, R. Role of oxidative stress, infection and inflammation in male infertility. Andrologia 2018, 50, e13126. [Google Scholar] [CrossRef]
- Farin, F.M.; Bigler, L.G.; Oda, D.; Mcdougall, J.K.; Omiecinski, C.J. Expression of cytochrome P450 and microsomal epoxide hydrolase in cervical and oral epithelial cells immortalized by human papillomavirus type 16 E6/E7 genes. Carcinogenesis 1995, 16, 1391–1401. [Google Scholar] [CrossRef]
- Shayakhmetova, G.M.; Bondarenko, L.B.; Matvienko, A.V.; Kovalenko, V.M. Correlation between spermatogenesis disorders and rat testes CYP2E1 mRNA contents under experimental alcoholism or type I diabetes. Adv. Med. Sci. 2014, 59, 183–189. [Google Scholar] [CrossRef]
- Duale, N.; Steffensen, I.L.; Andersen, J.; Brevik, A.; Brunborg, G.; Lindeman, B. Impaired sperm chromatin integrity in obese mice. Andrology 2014, 2, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P.; Cheng, Q. Orphans in the Human Cytochrome P450 Superfamily: Approaches to Discovering Functions and and relevance in pharmacology. Pharmacol. Rev. 2011, 63, 684–699. [Google Scholar] [CrossRef]
- Trafalis, D.T.; Panteli, E.S.; Grivas, A.; Tsigris, C.; Karamanakos, P.N. CYP2E1 and risk of chemically mediated cancers. Expert. Opin. Drug Metab. Toxicol. 2010, 6, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.; Costa, C.; Bassaizteguy, V.; Santos, M.; Cardozo, R.; Montes, J.; Settineri, R.; Nicolson, G.L. Incubation of human sperm with micelles made from glycerophospholipid mixtures increases sperm motility and resistance to oxidative stress. PLoS ONE 2018, 13, e0197897. [Google Scholar] [CrossRef] [PubMed]
- Collodel, G.; Castellini, C.; Lee, J.C.Y.; Signorini, C. Relevance of Fatty Acids to Sperm Maturation and Quality. Oxid. Med. Cell Longev. 2020, 2020, 1–14. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Who Laboratory Manual for the Examination and Processing of Human Semen. V.; WHO Press: Geneva, Switzerland, 2010. [Google Scholar]
- Manos, M.M.; Ting, Y.; Wright, D.K.; Lewis, A.J.; Broker, T.R.; Wolinsky, S.M. Use of polymerase chain reaction amplification for the detection of genital human papillomaviruses. Cancer Cells 1989, 7, 209–214. [Google Scholar]
- Georgescu, S.R.; Mitran, C.I.; Mitran, M.I.; Caruntu, C.; Sarbu, M.I.; Matei, C.; Nicolae, I.; Tocut, S.M.; Popa, M.I.; Tampa, M. New insights in the pathogenesis of HPV infection and the associated carcinogenic processes: The role of chronic inflammation and oxidative stress. J. Immunol. Res. 2018, 2018, 1–5. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Benzie, I.F. An automated, specific, spectrophotometric method for measuring ascorbic acid in plasma (EFTSA). Clin. Biochem. 1996, 29, 111–116. [Google Scholar] [CrossRef]
- Layali, I.; Tahmasbpour, E.; Joulaei, M.; Jorsaraei, S.G.; Farzanegi, P. Total antioxidant capacity and lipid peroxidation in semen of patient with hyperviscosity. Cell J. 2015, 16, 554–559. [Google Scholar]
- Hosen, M.B.; Islam, M.R.; Begum, F.; Kabir, Y.; Howlader, M.Z.H. Oxidative stress induced sperm DNA damage, a possible reason for male infertility. Iran J. Reprod. Med. 2015, 13, 525–532. [Google Scholar] [PubMed]
- Damke, E.; Kurscheidt, F.A.; Balani, V.A.; Takeda, K.I.; Irie, M.M.T.; Gimenes, F.; Consolaro, M.E.L. Male Partners of Infertile Couples with Seminal Infections of Human Papillomavirus Have Impaired Fertility Parameters. Biomed. Res. Int. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jia, C.W.; Ma, Y.M.; Zhou, L.Y.; Wang, S.Y. Correlation between HPV sperm infection and male infertility. Asian J. Androl. 2013, 15, 529–532. [Google Scholar] [CrossRef]
- Cortés-Gutiérrez, E.I.; Dávila-Rodrígu, I.; Fernández, J.L.; de la O-Pérez, L.O.; Garza-Flores, M.E.; Eguren-Garza, R.; Gosálvez, J. The presence of human papillomavirus in semen does not affect the integrity of sperm DNA. Andrologia 2017, 2017, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Calogero, A.E.; Duca, Y.; Condorelli, R.A.; La Vignera, S. Male accessory gland inflammation, infertility, and sexual dysfunctions: A practical approach to diagnosis and therapy. Andrology 2017, 5, 1064–1072. [Google Scholar] [CrossRef] [PubMed]
- Lajous, M.; Mueller, N.; Cruz-Valdéz, A.; Aguilar, L.V.; Franceschi, S.; Hernández-Ávila, M.; Lazcano-Ponce, E. Determinants of prevalence, acquisition, and persistence of human papillomavirus in healthy Mexican military men. Cancer Epidemiol. Biomarkers Prev. 2005, 14, 1710–1716. [Google Scholar] [CrossRef][Green Version]
- Reed-Maldonado, A.B.; Madden, K.C. Infertility and the Military Male. In Seminars in Reproductive Medicine; Thieme Medical Publishers: New York, NY, USA, 2019; Volume 37, pp. 5–11. [Google Scholar]
- Bernton, E.; Hoover, D.; Galloway, R.; Popp, K. Adaptation to Chronic Stress in Military Trainees. Ann. N. Y. Acad. Sci. 2006, 774, 217–231. [Google Scholar] [CrossRef]
- Xiong, Y.Q.; Chen, Y.X.; Cheng, M.J.; He, W.Q.; Chen, Q. The risk of human papillomavirus infection for male fertility abnormality: A meta-analysis. Asian J. Androl. 2018, 20, 493–497. [Google Scholar]
- Tavakolian, S.; Goudarzi, H.; Nazarian, H.; Raee, P.; Niakan, S.; Faghihloo, E. The evaluation of Human papilloma virus and human herpes viruses (EBV, CMV, VZV HSV-1 and HSV-2) in semen samples. Andrologia 2021, 53, 1–8. [Google Scholar] [CrossRef]
- Yuki, K.; Kazuyoshi, S.; Tomomi, N.; Hiroki, N.; Masashi, I.; Kazufumi, N.; Kawaguchi, S.; Izumi, K.; Kadono, Y.; Mizokami, A. Human papillomavirus detected in sperm of Japanese infertile males affects reproductive parameters. Int. J. Infect. Dis. 2021, 112, 294–299. [Google Scholar]
- Lehti, M.S.; Sironen, A. Formation and function of sperm tail structures in association with sperm motility defects. Biol. Reprod. 2017, 97, 522–536. [Google Scholar] [CrossRef]
- Ozer, O.F.; Akbulut, H.; Guler, E.M.; Caglar, H.G.; Gevher, F.; Koktasoglu, F.; Selek, S. Oxidative stress and phenotype frequencies of paraoxonase-1 in teratozoospermia. Andrologia 2019, 51, 1–8. [Google Scholar] [CrossRef]
- Kurkowska, W.; Bogacz, A.; Janiszewska, M.; Gabryś, E.; Tiszler, M.; Bellanti, F.; Kasperczyk, S.; Machón-Grecka, A.; Dobrakowski, M.; Kasperczyk, A. Oxidative Stress is Associated with Reduced Sperm Motility in Normal Semen. Am. J. Mens Health 2020, 14, 1–8. [Google Scholar] [CrossRef]
- Tuominen, H.; Rautava, J.; Kero, K.; Syrjänen, S.; Collado, M.C.; Rautava, S. HPV infection and bacterial microbiota in the semen from healthy men. BMC Infect. Dis. 2021, 373, 1–9. [Google Scholar] [CrossRef]
- Zheng, J.J.; Miao, J.R.; Wu, Q.; Yu, C.X.; Mu, L.; Song, J.H. Correlation between HPV-negative cervical lesions and cervical microenvironment. Taiwan J. Obstet. Gynecol. 2020, 59, 855–861. [Google Scholar] [CrossRef]
- Borgogna, J.C.; Santori, E.K.; Nelson, T.M.; Rath, J.M.; Glover, E.D.; Ravel, J. The vaginal metabolome and microbiota of cervical HPV-positive and HPV-negative women: A cross-sectional analysis. BJOG 2019, 127, 1–11. [Google Scholar]
- Pellavio, G.; Todaro, F.; Alberizzi, P.; Scotti, C.; Gastaldi, G.; Lolicato, M.; Omes, C.; Caliogna, L.; Nappi, R.E.; Laforenza, U. HPV Infection A ff ects Human Sperm Functionality by Inhibition of Aquaporin-8. Cells 2020, 17, 1–15. [Google Scholar]
- Barati, E.; Nikzad, H.; Karimian, M. Oxidative stress and male infertility: Current knowledge of pathophysiology and role of antioxidant therapy in disease management. Cell Mol. Life Sci. 2020, 77, 93–113. [Google Scholar] [CrossRef] [PubMed]
- Gholinezhad, M.; Aliarab, A.; Abbaszadeh-Goudarzi, G.; Yousefnia-Pasha, Y.; Samadaian, N.; Rasolpour-Roshan, K.; Aghagolzadeh-Haji, H.; Mohammadoo-Khorasani, M. Nitric oxide, 8-hydroxydeoxyguanosine, and total antioxidant capacity in human seminal plasma of infertile men and their relationship with sperm parameters. Clin. Exp. Reprod. Med. 2020, 47, 54–60. [Google Scholar] [CrossRef]
- Signorini, C.; Moretti, E.; Noto, D.; Micheli, L.; Ponchia, R.; Collodel, G. Fatty Acid Oxidation and Pro-Resolving Lipid Mediators Are Related to Male Infertility. Antioxidants 2022, 11, 107. [Google Scholar] [CrossRef] [PubMed]
- Loveland, K.L.; Klein, B.; Pueschl, D.; Indumathy, S.; Bergmann, M.; Loveland, B.E.; Hedger, M.P.; Schuppe, H.C. Cytokines in male fertility and reproductive pathologies: Immunoregulation and beyond. Front. Endocrinol. 2017, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Fraczek, M.; Kurpisz, M. Cytokines in the male reproductive tract and their role in infertility disorders. J. Reprod. Immunol. 2015, 108, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Fraczek, M.; Czernikiewicz, A.; Kurpisz, M. Cytokines and Oxidative stress in the germ line. In Studies on Men′s Health and Fertility; Oxidative Stress in Applied Basic Research and Clinical Practice; Agarwal, A., Aitken, R., Alvarez, J., Eds.; Human Press: Poznan, Poland, 2012; pp. 179–205. [Google Scholar]
- Maksymyuk, H.; Vorobets, Z.; Maksymyuk, V. The Level of Proinflammatory and Antiinflammatory Cytokines in Sperm Plasma of Fertile and Infertile Men. Health Probl. Civiliz. 2015, 9, 21–25. [Google Scholar] [CrossRef]
- Depuydt, C.E.; Beert, J.; Bosmans, E.; Salembier, G. Human Papillomavirus (HPV) virion induced cancer and subfertility, two sides of the same coin. Facts Views Vis. Obgyn. 2016, 8, 211–222. [Google Scholar]
- Politch, J.A.; Tucker, L.; Bowman, F.P.; Anderson, D.J. Concentrations and significance of cytokines and other immunologic factors in semen of healthy fertile men. Hum. Reprod. 2007, 22, 2928–2935. [Google Scholar] [CrossRef]
- Seshadri, S.; Bates, M.; Vince, G.; Jones, D.I.L. The role of cytokine expression in different subgroups of subfertile men. Am. J. Reprod. Immunol. 2009, 62, 275–282. [Google Scholar] [CrossRef]
- Syriou, V.; Papanikolaou, D.; Kozyraki, A.; Goulis, D.G. Cytokines and male infertility. Eur. Cytokine Netw. 2018, 29, 73–82. [Google Scholar] [CrossRef]
- La Vignera, S.; Vicari, E.; Condorelli, R.A.; Franchina, C.; Scalia, G. Prevalence of human papilloma virus infection in patients with male accessory. Reprod. Biomed. Online 2015, 30, 385–391. [Google Scholar] [CrossRef][Green Version]
- Perletti, G.; Monti, E.; Magri, V.; Cai, T.; Cleves, A.; Trinchieri, A.; Montanari, E. The association between prostatitis and prostate cancer. Systematic review and meta-analysis. Arch. Ital. Urol. Androl. 2017, 89, 259–265. [Google Scholar] [CrossRef]
- Wang, R.Y.; Chen, X.W.; Zhang, W.W.; Jiang, F.; Liu, M.Q.; Shen, X.B. CYP2E1 changes the biological function of gastric cancer cells via the PI3K/Akt/mTOR signaling pathway. Mol. Med. Rep. 2020, 21, 842–850. [Google Scholar] [CrossRef]
- Medel-Flores, O.; Valenzuela-Rodríguez, V.A.; Ocadiz-Delgado, R.; Castro-Muñoz, L.J.; Hernández-Leyva, S.; Lara-Hernández, G.; Silva-Escobedo, J.G.; Gariglio-Vidal, P.; Sánchez-Monroy, V. Association between HPV infection and prostate cancer in a Mexican population. Genet. Mol. Biol. 2018, 41, 781–789. [Google Scholar] [CrossRef] [PubMed]
| Group | HPV Genotypes | n | Percentage (%) |
|---|---|---|---|
| Single infection (SI-HPV) | HPV52 | 15 | 18.5 |
| HPV33 | 9 | 11.1 | |
| HPV31 | 2 | 2.5 | |
| HPV6 | 2 | 2.5 | |
| HPV16 | 1 | 1.2 | |
| HPV11 | 1 | 1.2 | |
| 30 | 37.00% | ||
| Multiple infection (MI-HPV, all with HR-HPV) | HPV 33, 52 | 9 | 11.1 |
| HPV 18, 31 | 5 | 6.2 | |
| HPV 16, 31, 33, 52 | 3 | 3.7 | |
| HPV 31, 52 | 2 | 2.5 | |
| HPV 16, 18 | 1 | 1.2 | |
| HPV 16, 31 | 1 | 1.2 | |
| HPV 18, 33 | 1 | 1.2 | |
| HPV 18, 52 | 1 | 1.2 | |
| HPV 18, 58 | 1 | 1.2 | |
| HPV 33, 51 | 1 | 1.2 | |
| HPV 16, 18, 33 | 1 | 1.2 | |
| HPV 16, 33, 52 | 1 | 1.2 | |
| HPV 16, 31, 52 | 1 | 1.2 | |
| HPV 33, 52, 58 | 1 | 1.2 | |
| 29 | 35.80% | ||
| Multiple infection (MI-HPV, all with low and high HPV) | HPV 18, 6 | 6 | 7.4 |
| HPV 6, 52 | 3 | 3.7 | |
| HPV 11, 16 | 2 | 2.5 | |
| HPV 6, 18, 52 | 2 | 2.5 | |
| HPV 6, 11, 52 | 2 | 2.5 | |
| HPV 6, 11, 16 | 2 | 2.5 | |
| HPV 11, 16, 58 | 1 | 1.2 | |
| HPV 11, 16, 52 | 1 | 1.2 | |
| HPV 11, 33, 52 | 1 | 1.2 | |
| HPV 6, 11, 16, 18, 58 | 1 | 1.2 | |
| 27.20% |
| Test | Non-Infected HPV 1 (n = 20) | SI-HPV 2 (n = 30) | MI-HPV 3 (n = 56) | p |
|---|---|---|---|---|
| Volume (mL) | 2.65 ± 1.46 | 3.37 ± 1.59 | 3.11 ± 1.21 | 0.142 |
| pH | 8 [7.50, 8] | 8 [7, 8] | 8 [7, 8] | 0.135 |
| Total sperm number (×106/ejaculate) | 56 [38.0, 133.5] | 93 [38.7, 95] | 94 [51, 162.5] | 0.332 |
| Sperm concentration (×106/mL) | 28.75 [14.65, 66.00] | 28.5 [8.25, 76] | 27.5 [18, 57] | 0.819 |
| Normal morphology (%) | 5 [3, 7] | 2 [1, 3] a | 2 [1, 4] a | 0.004 |
| Abnormal sperm morphology (%) | 95 [97, 99] | 98 [97, 99] a | 98 [97, 99] a | 0.004 |
| Head defects (%) | 43.93 ± 11.20 | 42.82 ± 11.03 | 41.71 ± 12.30 | 0.737 |
| Midpiece defects (%) | 22.80 ± 13.04 | 24.82 ± 9.09 | 24.31 ± 7.90 | 0.723 |
| Tail defects (%) | 28.20 ± 16.70 | 28.21 ± 13.86 | 28.80 ± 13.42 | 0.988 |
| Total progressive motility (% A+B) | 43.64 ± 17.56 | 44.65 ± 23.05 | 45.05 ± 19.35 | 0.974 |
| Fast progressive motility (% A) | 2 [1, 41] | 2 [1, 5] | 2 [0, 3] | 0.113 |
| Low progressive motility (% B) | 6 [0, 48] | 37 [20, 52.50] | 45.50 [30, 58.75] a | 0.018 |
| Leukocytes (106) | 0.491 [0.14, 1.42] | 0.70 [0.20, 1.37] | 0.80 [0.50, 1.17] | 0.152 |
| Parameter | Non-Infected HPV (n = 20) | SI-HR-HPV (n = 30) | MI-HR-HPV (n = 51) | p | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median | Percentile 25th and 75th | IQR | Median | Percentile 25th and 75th | IQR | Median | Percentile 25th and 75th | IQR | ||
| LPO (nmoles MDA/mg protein) | 3.35 | 2.51, 4.80 | 2.29 | 7.43 a | 5.55, 8.61 | 3.22 | 12.23 a,b | 6.89, 12.23 | 5.48 | 0.000 |
| 8OH-dG (ng/mL) | 8.2 | 1.85, 8.54 | 6.68 | 8.34 | 8.03, 8.80 | 0.78 | 8.20 | 7.70, 8.48 | 0.79 | 0.208 |
| Correlation | Correlation, p-Value | |
|---|---|---|
| CYP2E1 | SI-HR-HPV, MI-HR-HPV | 0.841 **, 0.000 |
| CYP2E1 | LPO | 0.323 **, 0.005 |
| Defects in the tail | 8OH-dG | 0.246 **, 0.039 |
| CYP2E1 | TAC | −0.411 **, 0.000 |
| 8OH-dG | % Normal morphology | −0.259 *, 0.030 |
| TAC | Defects in the intermediate piece | −0.283 *, 0.020 |
| Defects in the tail | Defects in the head | −0.512 **, 0.000 |
| Defects in the tail | Defects in the intermediate piece | −0.461 **, 0.000 |
| Cytokine | Non-Infected HPV (n = 20) | SI-HPV (n = 30) | MI-HPV (n = 51) | p | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median | Percentile 25th and 75th | IQR | Median | Percentile 25th and 75th | IQR | Median | Percentile 25th and 75th | IQR | ||
| IFN-γ (pg/mL) | 0.000 | 0.000, 0.000 | 0.000 | 398.80 a | 206.50, 909 | 704.38 | 826.50 a | 154.00, 724.00 | 571.25 | 0.000 |
| IL-1β (pg/mL) | 0.000 | 0.000, 1.33 | 1.33 | 193.98 a | 11.33, 1303.00 | 1289.00 | 157.16 a | 0.000, 328 | 328 | 0.000 |
| IL-4 (pg/mL) | 6.25 | 0.000, 26.47 | 26.47 | 35.00 a | 22.50, 69.00 | 48.06 | 41.25 a | 17.50, 69.00 | 52.13 | 0.000 |
| IL-6 (pg/mL) | 0.000 | 0.000, 12.80 | 12.80 | 98.80 a | 0.000, 351.20 | 351.20 | 203.33 a | 36.80, 203.33 | 166.53 | 0.001 |
| IL-8 (pg/mL) | 395.45 | 0.000, 395.90 | 19.3 | 565.35 | 272.83, 917.15 | 644.3 | 550.00 a | 291.63, 1065.64 | 775.4 | 0.086 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Soto, E.; Medel-Flores, M.O.; Fernández-Martínez, E.; Oros-Pantoja, R.; Miranda-Covarrubias, J.C.; Sánchez-Monroy, V. High-Risk HPV with Multiple Infections Promotes CYP2E1, Lipoperoxidation and Pro-Inflammatory Cytokines in Semen of Asymptomatic Infertile Men. Antioxidants 2022, 11, 1051. https://doi.org/10.3390/antiox11061051
Pérez-Soto E, Medel-Flores MO, Fernández-Martínez E, Oros-Pantoja R, Miranda-Covarrubias JC, Sánchez-Monroy V. High-Risk HPV with Multiple Infections Promotes CYP2E1, Lipoperoxidation and Pro-Inflammatory Cytokines in Semen of Asymptomatic Infertile Men. Antioxidants. 2022; 11(6):1051. https://doi.org/10.3390/antiox11061051
Chicago/Turabian StylePérez-Soto, Elvia, María Olivia Medel-Flores, Eduardo Fernández-Martínez, Rigoberto Oros-Pantoja, José Cruz Miranda-Covarrubias, and Virginia Sánchez-Monroy. 2022. "High-Risk HPV with Multiple Infections Promotes CYP2E1, Lipoperoxidation and Pro-Inflammatory Cytokines in Semen of Asymptomatic Infertile Men" Antioxidants 11, no. 6: 1051. https://doi.org/10.3390/antiox11061051
APA StylePérez-Soto, E., Medel-Flores, M. O., Fernández-Martínez, E., Oros-Pantoja, R., Miranda-Covarrubias, J. C., & Sánchez-Monroy, V. (2022). High-Risk HPV with Multiple Infections Promotes CYP2E1, Lipoperoxidation and Pro-Inflammatory Cytokines in Semen of Asymptomatic Infertile Men. Antioxidants, 11(6), 1051. https://doi.org/10.3390/antiox11061051






