Modulating the Growth, Antioxidant Activity, and Immunoexpression of Proinflammatory Cytokines and Apoptotic Proteins in Broiler Chickens by Adding Dietary Spirulina platensis Phycocyanin
Abstract
:1. Introduction
2. Material and Methods
2.1. Isolation and Purification of Spirulina Platensis Phycocyanin
2.1.1. Micro-Organism Source
2.1.2. Culture Reparation
2.1.3. Phycocyanin Extraction and Purification
2.2. Birds
2.3. Experimental Design and Diets
2.4. Growth Performance
2.5. Sampling
2.6. Blood Biochemical Indices
2.7. Inflammatory and Antioxidant Indices
2.8. Histological and Immunohistochemical Examination
2.9. Statistical Analysis
3. Results
3.1. Phycocyanin Characterization
3.2. Growth Performance
3.3. Serum Biochemical Indices
3.4. Lipid Profile
3.5. Inflammatory and Antioxidant Indices
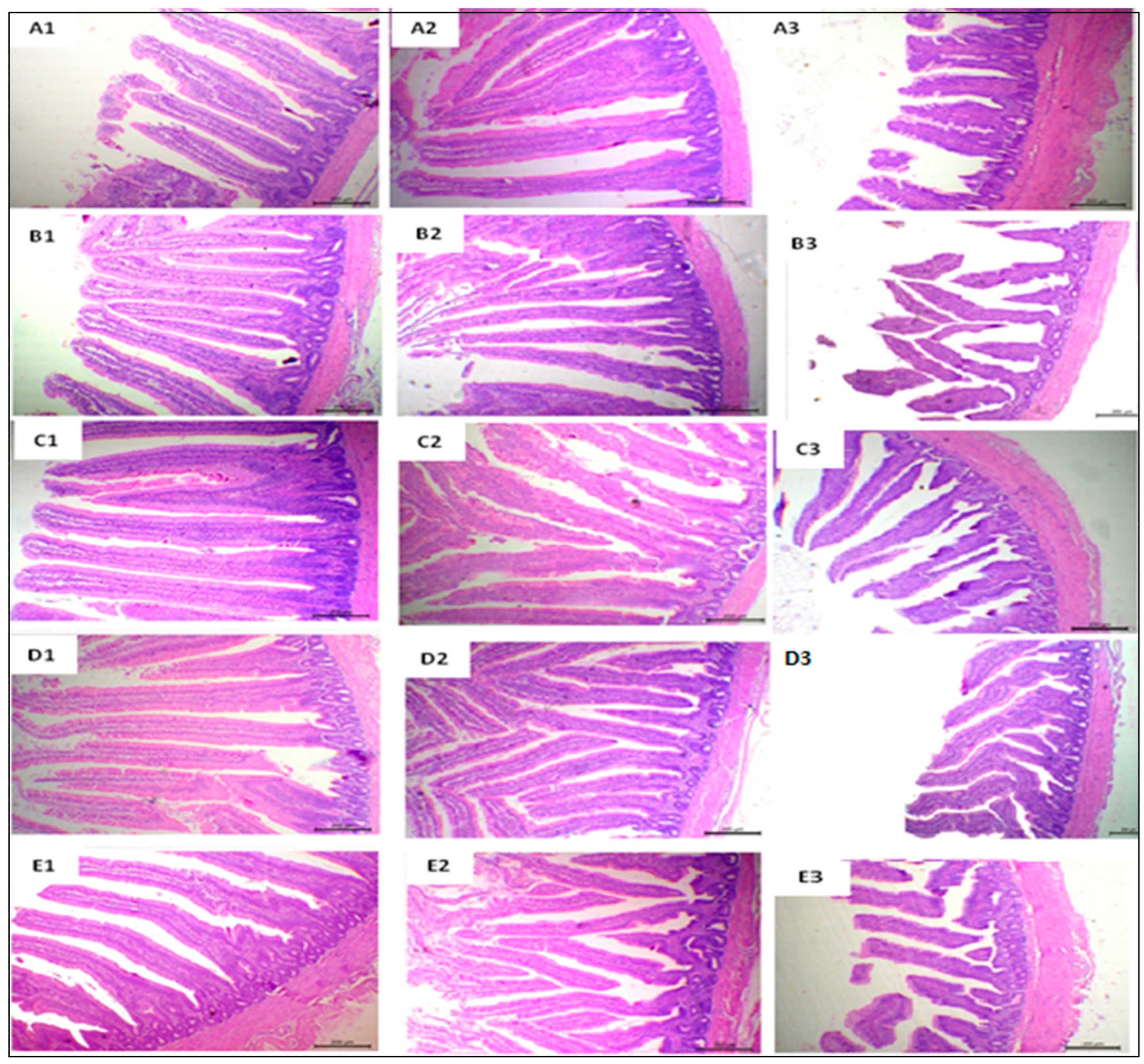
3.6. Histological Findings and Morphometric Measures
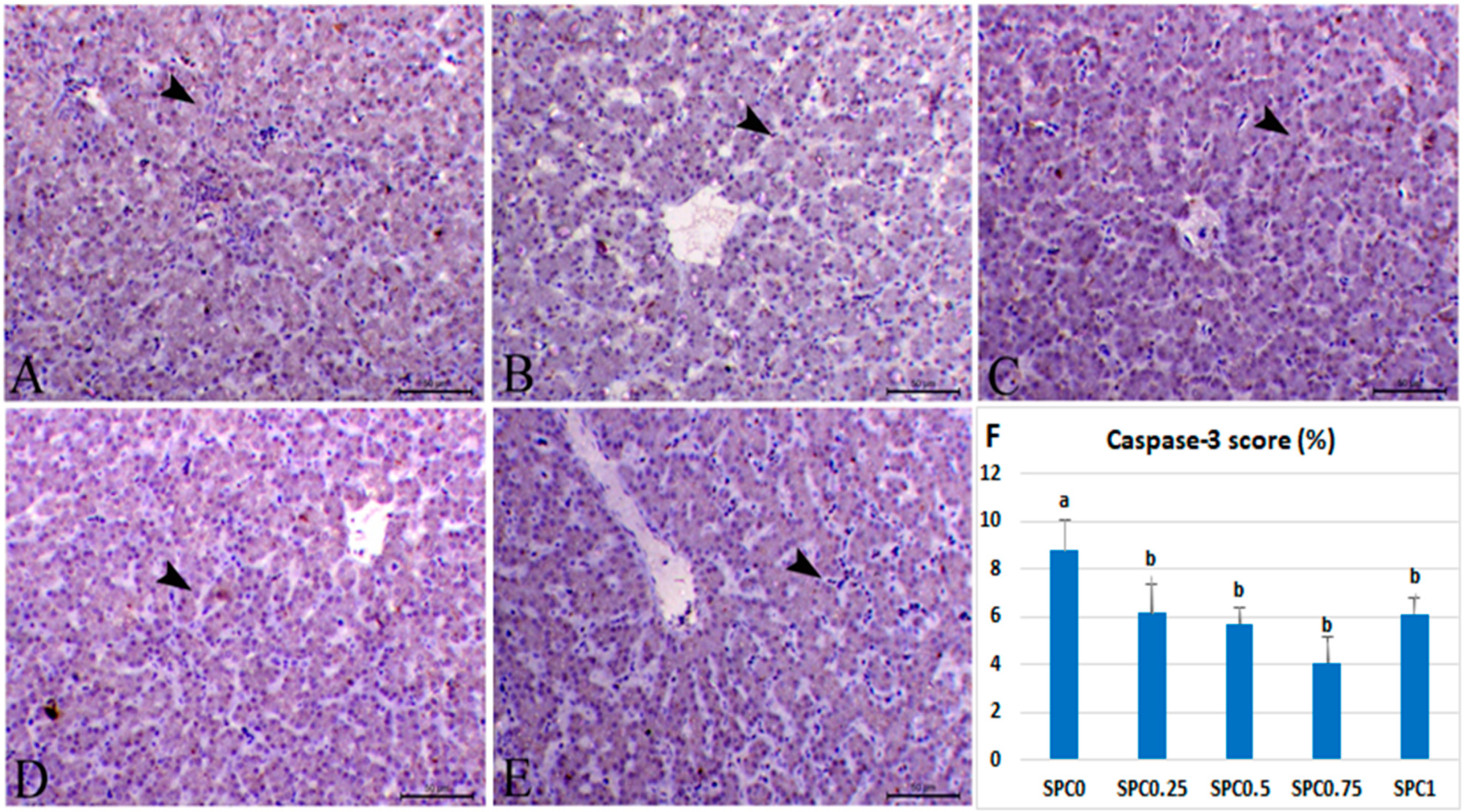
3.7. Immunohistochemical Analysis and Morphometric Measures
4. Discussion
4.1. Growth Performance
4.2. Blood Biochemical Parameters and Lipid Profile
4.3. Antioxidant Activity and Inflammatory Markers
4.4. Histological Findings
4.5. Immunohistochemical Examination
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sørum, H.; Sunde, M. Resistance to antibiotics in the normal flora of animals. Vet. Res. 2001, 32, 227–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, R.E.; Ahmed, S.A.; Amer, S.A.; Al-Gabri, N.A.; Ahmed, A.I.; Abdel-Warith, A.-W.A.; Younis, E.-S.M.; Metwally, A.E. Influence of vitamin C feed supplementation on the growth, antioxidant activity, immune status, tissue histomorphology, and disease resistance in Nile tilapia, Oreochromis niloticus. Aquac. Rep. 2020, 18, 100545. [Google Scholar] [CrossRef]
- Amer, S.A.; Mohamed, W.A.; Gharib, H.S.; Al-Gabri, N.A.; Gouda, A.; Elabbasy, M.T.; El-Rahman, A.; Ghada, I.; Omar, A.E. Changes in the growth, ileal digestibility, intestinal histology, behavior, fatty acid composition of the breast muscles, and blood biochemical parameters of broiler chickens by dietary inclusion of safflower oil and vitamin C. BMC Vet. Res. 2021, 17, 68. [Google Scholar] [CrossRef]
- Muthulakshmi, M.; Saranya, A.; Sudha, M.; Selvakumar, G. Extraction, partial purification, and antibacterial activity of phycocyanin from Spirulina isolated from fresh water body against various human pathogens. J. Algal Biomass Util. 2012, 3, 7–11. [Google Scholar]
- Mariey, Y.; Samak, H.; Ibrahem, M. Effect of using Spirulina platensis algae as a feed additive for poultry diets: 1-productive and reproductive performances of local laying hens. Poult. Sci. 2012, 32, 201–215. [Google Scholar]
- Xalxo, R.K.; Sao, S.; Sahu, P.K. Effect of antibacterial properties of cyanobacterial Spirulina platensis. Int. J. Pharm. Life Sci. 2013, 4, 7. [Google Scholar]
- Deng, R.; Chow, T.J. Hypolipidemic, antioxidant, and antiinflammatory activities of microalgae Spirulina. Cardiovasc. Ther. 2010, 28, e33–e45. [Google Scholar] [CrossRef] [Green Version]
- Hoseini, S.M.; Khosravi-Darani, K.; Mozafari, M.R. Nutritional and medical applications of spirulina microalgae. Mini Rev. Med. Chem. 2013, 13, 1231–1237. [Google Scholar] [CrossRef]
- Minkova, K.; Tchernov, A.; Tchorbadjieva, M.; Fournadjieva, S.; Antova, R.; Busheva, M.C. Purification of C-phycocyanin from Spirulina (Arthrospira) fusiformis. J. Biotechnol. 2003, 102, 55–59. [Google Scholar] [CrossRef]
- Safari, R.; Amiri, Z.R.; Kenari, R.E. Antioxidant and antibacterial activities of C-phycocyanin from common name Spirulina platensis. Iran. J. Fish. Sci. 2020, 19, 1911–1927. [Google Scholar]
- Sivasankari, S.; Ravindran, D.; Ravindran, D. Comparison of different extraction methods for phycocyanin extraction and yield from Spirulina platensis. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 904–909. [Google Scholar]
- Kuddus, M.; Singh, P.; Thomas, G.; Ali, A. Production of cphycocyanin and its potential applications. In Biotechnology of Bioactive Compounds: Sources and Applications; Wiley: Hoboken, NJ, USA, 2015; pp. 283–299. [Google Scholar]
- Romay, C.; Gonzalez, R.; Ledon, N.; Remirez, D.; Rimbau, V. C-phycocyanin: A biliprotein with antioxidant, anti-inflammatory and neuroprotective effects. Curr. Protein Pept. Sci. 2003, 4, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Amer, S.A.; Al-Khalaifah, H.S.; Gouda, A.; Osman, A.; Goda, N.I.; Mohammed, H.A.; Darwish, M.I.; Hassan, A.M.; Mohamed, S.K.A. Potential Effects of Anthocyanin-Rich Roselle (Hibiscus sabdariffa L.) Extract on the Growth, Intestinal Histomorphology, Blood Biochemical Parameters, and the Immune Status of Broiler Chickens. Antioxidants 2022, 11, 544. [Google Scholar] [CrossRef] [PubMed]
- Doan, H.V.; Hoseinifar, S.H.; Hung, T.Q.; Lumsangkul, C.; Jaturasitha, S.; El-Haroun, E.; Paolucci, M. Dietary inclusion of chestnut (Castanea sativa) polyphenols to Nile tilapia reared in biofloc technology: Impacts on growth, immunity, and disease resistance against Streptococcus agalactiae. Fish Shellfish. Immunol. 2020, 105, 319–326. [Google Scholar] [CrossRef] [PubMed]
- El-Araby, D.A.; Amer, S.A.; Attia, G.A.; Osman, A.; Fahmy, E.M.; Altohamy, D.E.; Alkafafy, M.; Elakkad, H.A.; Tolba, S.A. Dietary Spirulina platensis phycocyanin improves growth, tissue histoarchitecture, and immune responses, with modulating immunoexpression of CD3 and CD20 in Nile tilapia, Oreochromis niloticus. Aquaculture 2022, 546, 737413. [Google Scholar] [CrossRef]
- Demirel, Z.; Yilmaz-Koz, F.F.; Karabay-Yavasoglu, U.N.; Ozdemir, G.; Sukatar, A. Antimicrobial and antioxidant activity of brown algae from the Aegean Sea. J. Serb. Chem. Soc. 2009, 74, 619–628. [Google Scholar] [CrossRef]
- Sarada, R.; Pillai, M.G.; Ravishankar, G. Phycocyanin from Spirulina sp.: Influence of processing of biomass on phycocyanin yield, analysis of efficacy of extraction methods and stability studies on phycocyanin. Process Biochem. 1999, 34, 795–801. [Google Scholar] [CrossRef]
- Salama, A.; Ghany, A.A.; Osman, A.; Sitohy, M. Maximising phycocyanin extraction from a newly identified Egyptian cyanobacteria strain: Anabaena oryzae SOS13. Int. Food Res. J. 2015, 22, 517–525. [Google Scholar]
- Patel, A.; Mishra, S.; Pawar, R.; Ghosh, P. Purification and characterization of C-Phycocyanin from cyanobacterial species of marine and freshwater habitat. Protein Expr. Purif. 2005, 40, 248–255. [Google Scholar] [CrossRef]
- Aviagen, R. Ross Broiler Management Manual, 2009; ROSS: Richmond, VA, USA, 2014; Volume 9, pp. 350–364. [Google Scholar]
- American Veterinary Medical Association. AVMA Guidelines for the Euthanasia of Animals: 2013 Edition; American Veterinary Medical Association: Schaumburg, IL, USA, 2013. [Google Scholar]
- Grant, G.H.; Silverman, L.M.; Christenson, R.H. Amino acids and proteins. In Fundamentals of Clinical Chemistry; Tietz, N.Z., Ed.; WB Saunders: Philadelphia, PA, USA, 1987; pp. 291–345. [Google Scholar]
- Doumas, B.; Baysa, D.; Carter, R.; Peters, T.; Schaffer, R. Determination of serum total protein. Clin Chem 1981, 27, 1642. [Google Scholar] [CrossRef] [PubMed]
- Doumas, B.; Biggs, H. Determination of serum albumin in standard method of clinical chemistry. Stand. Methods Clin. Chem. 1972, 7, 175–188. [Google Scholar]
- McGowan, M.W.; Artiss, J.D.; Strandbergh, D.R.; Zak, B. A peroxidase-coupled method for the colorimetric determination of serum triglycerides. Clin. Chem. 1983, 29, 538–542. [Google Scholar] [CrossRef]
- Allain, C.C.; Poon, L.S.; Chan, C.S.; Richmond, W.; Fu, P.C. Enzymatic determination of total serum cholesterol. Clin. Chem. 1974, 20, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Vassault, A.; Grafmeyer, D.; Naudin, C.; Dumont, G.; Bailly, M.; Henny, J.; Gerhardt, M.; Georges, P. Protocole de validation de techniques. Ann. Biol. Clin. 1986, 44, 45. [Google Scholar]
- Mcdonald, R.E.; Hultin, H.O. Some characteristics of the enzymic lipid peroxidation system in the microsomal fraction of flounder skeletal muscle. J. Food Sci. 1987, 52, 15–21. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.J. 241 Total antioxidant status in plasma and body fluids. Methods Enzymol. 1994, 234, 279–293. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Nishikimi, M.; Rao, N.A.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Suvarna, S.; Layton, C.; Bancroft, J. The hematoxylins and eosin. In Bancroft’s Theory and Practice of Histological Techniques, 7th ed.; Churchill Livingstone: London, UK, 2013; pp. 172–186. [Google Scholar]
- Saber, S.; Khalil, R.M.; Abdo, W.S.; Nassif, D.; El-Ahwany, E. Olmesartan ameliorates chemically-induced ulcerative colitis in rats via modulating NFκB and Nrf-2/HO-1 signaling crosstalk. Toxicol. Appl. Pharmacol. 2019, 364, 120–132. [Google Scholar] [CrossRef]
- Rizzardi, A.E.; Johnson, A.T.; Vogel, R.I.; Pambuccian, S.E.; Henriksen, J.; Skubitz, A.P.; Metzger, G.J.; Schmechel, S.C. Quantitative comparison of immunohistochemical staining measured by digital image analysis versus pathologist visual scoring. Diagn. Pathol. 2012, 7, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Lee, S.; Kim, I. Effect of dietary Spirulina (Arthrospira) platensis on the growth performance, antioxidant enzyme activity, nutrient digestibility, cecal microflora, excreta noxious gas emission, and breast meat quality of broiler chickens. Poult. Sci. 2018, 97, 2451–2459. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.; Smith, D.; Moritz, J. Effects of algae incorporation into broiler starter diet formulations on nutrient digestibility and 3 to 21 d bird performance. J. Appl. Poult. Res. 2015, 24, 206–214. [Google Scholar] [CrossRef]
- Tavernari, F.D.C.; Roza, L.; Surek, D.; Sordi, C.; Silva, M.; Albino, L.; Migliorini, M.; Paiano, D.; Boiago, M. Apparent metabolisable energy and amino acid digestibility of microalgae Spirulina platensis as an ingredient in broiler chicken diets. Br. Poult. Sci. 2018, 59, 562–567. [Google Scholar] [CrossRef]
- Abdelnour, S.A.; Swelum, A.A.; Salama, A.; Al-Ghadi, M.Q.; Qattan, S.Y.; El-Hack, M.E.A.; Khafaga, A.F.; Alhimaidi, A.R.; Almutairi, B.O.; Ammari, A.A. The beneficial impacts of dietary phycocyanin supplementation on growing rabbits under high ambient temperature. Ital. J. Anim. Sci. 2020, 19, 1046–1056. [Google Scholar] [CrossRef]
- Alwaleed, E.A.; El-Sheekh, M.; Abdel-Daim, M.M.; Saber, H. Effects of Spirulina platensis and Amphora coffeaeformis as dietary supplements on blood biochemical parameters, intestinal microbial population, and productive performance in broiler chickens. Environ. Sci. Pollut. Res. 2021, 28, 1801–1811. [Google Scholar] [CrossRef]
- Xie, Y.; Li, W.; Zhu, L.; Zhai, S.; Qin, S.; Du, Z. Effects of phycocyanin in modulating the intestinal microbiota of mice. MicrobiologyOpen 2019, 8, e00825. [Google Scholar] [CrossRef] [Green Version]
- Osman, A.; Abd-Elaziz, S.; Salama, A.; Eita, A.A.; Sitohy, M. Health protective actions of phycocyanin obtained from an Egyptian isolate of Spirulina platensis on albino rats. EurAsian J. BioSciences 2019, 13, 105–112. [Google Scholar]
- Wu, Q.; Liu, L.; Miron, A.; Klímová, B.; Wan, D.; Kuča, K. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: An overview. Arch. Toxicol. 2016, 90, 1817–1840. [Google Scholar] [CrossRef]
- Aladaileh, S.H.; Khafaga, A.F.; El-Hack, M.E.A.; Al-Gabri, N.A.; Abukhalil, M.H.; Alfwuaires, M.A.; Bin-Jumah, M.; Alkahtani, S.; Abdel-Daim, M.M.; Aleya, L. Spirulina platensis ameliorates the sub chronic toxicities of lead in rabbits via anti-oxidative, anti-inflammatory, and immune stimulatory properties. Sci. Total Environ. 2020, 701, 134879. [Google Scholar] [CrossRef]
- Farag, M.R.; Alagawany, M.; El-Hack, M.; Dhama, K. Nutritional and healthical aspects of Spirulina (Arthrospira) for poultry, animals and human. Int. J. Pharmacol. 2016, 12, 36–51. [Google Scholar] [CrossRef] [Green Version]
- Mirzaie, S.; Zirak-Khattab, F.; Hosseini, S.A.; Donyaei-Darian, H. Effects of dietary Spirulina on antioxidant status, lipid profile, immune response and performance characteristics of broiler chickens reared under high ambient temperature. Asian-Australas. J. Anim. Sci. 2018, 31, 556. [Google Scholar] [CrossRef]
- Qureshi, M.; Garlich, J.; Kidd, M. Dietary Spirulina platensis enhances humoral and cell-mediated immune functions in chickens. Immunopharmacol. Immunotoxicol. 1996, 18, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Kharde, S.; Shirbhate, R.; Bahiram, K.; Nipane, S. Effect of Spirulina supplementation on growth performance of broilers. Indian J. Vet. Res. 2012, 21, 66–69. [Google Scholar]
- Shanmugapriya, B.; Babu, S.S. Supplementary effect of Spirulina platensis on performance, hematology and carcass yield of broiler chicken. Indian Streams Res. J. 2014, 4, 1–7. [Google Scholar]
- Zhang, L.; Jin, P.; Qin, S.; Liu, J.; Yang, Z.; Zhao, H.; Sheng, Q. Effects of dietary supplementation with S. platensis and probiotics on the growth performance, immune response and the fecal Lactobacillus spp. and E. coli contents of weaned piglets. Livest. Sci. 2019, 225, 32–38. [Google Scholar] [CrossRef]
- Bonos, E.; Kasapidou, E.; Kargopoulos, A.; Karampampas, A.; Nikolakakis, I.; Christaki, E.; Florou-Paneri, P. Spirulina as a functional ingredient in broiler chicken diets. South Afr. J. Anim. Sci. 2016, 46, 94–102. [Google Scholar] [CrossRef] [Green Version]
- Gongnet, G.; Niess, E.; Rodehutscord, M.; Pfeffer, E. Algae-meal (Spirulina platensis) from lake Chad replacing soybean-meal in broiler diets. Archiv fur Geflugelkunde 2001, 65, 265–268. [Google Scholar]
- Toyomizu, M.; Sato, K.; Taroda, H.; Kato, T.; Akiba, Y. Effects of dietary Spirulina on meat colour in muscle of broiler chickens. Br. Poult. Sci. 2001, 42, 197–202. [Google Scholar] [CrossRef]
- Zahir, U.; AnwarulHaque, B.; Maksuda, B.; Mahfuj, U.M. Effect of dietary supplement of algae (Spirulina platensis) as an alternative to antibiotics on growth performance and health status of broiler chickens. J. Poult. Sci. 2019, 18, 576–584. [Google Scholar]
- Ruth, M.R.; Field, C.J. The immune modifying effects of amino acids on gut-associated lymphoid tissue. J. Anim. Sci. Biotechnol. 2013, 4, 27. [Google Scholar] [CrossRef] [Green Version]
- Sinha, R.A.; Singh, B.K.; Yen, P.M. Direct effects of thyroid hormones on hepatic lipid metabolism. Nat. Rev. Endocrinol. 2018, 14, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, I.J.; Huang, L.-S.; Huggins, L.A.; Yu, S.; Nagareddy, P.R.; Scanlan, T.S.; Ehrenkranz, J.R. Thyroid hormone reduces cholesterol via a non-LDL receptor-mediated pathway. Endocrinology 2012, 153, 5143–5149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Viscarra, J.; Kim, S.-J.; Sul, H.S. Transcriptional regulation of hepatic lipogenesis. Nat. Rev. Mol. Cell Biol. 2015, 16, 678–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Moneim, A.-M.E.; Shehata, A.M.; Mohamed, N.G.; Elbaz, A.M.; Ibrahim, N.S. Synergistic effect of Spirulina platensis and selenium nanoparticles on growth performance, serum metabolites, immune responses, and antioxidant capacity of heat-stressed broiler chickens. Biol. Trace Elem. Res. 2022, 200, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, E.S.; Alsanie, W.F.; Gaber, A.; Kamel, N.N.; Alaqil, A.A.; Abbas, A.O. Blue-green algae (Spirulina platensis) alleviates the negative impact of heat stress on broiler production performance and redox status. Animals 2021, 11, 1243. [Google Scholar] [CrossRef]
- Hwang, J.H.; Chen, J.C.; Yang, S.Y.; Wang, M.F.; Liu, T.C.; Chan, Y.C. Expression of COX-2 and NMDA receptor genes at the cochlea and midbrain in salicylate-induced tinnitus. Laryngoscope 2011, 121, 361–364. [Google Scholar] [CrossRef]
- Bhat, V.B.; Madyastha, K. C-phycocyanin: A potent peroxyl radical scavenger in vivo and in vitro. Biochem. Biophys. Res. Commun. 2000, 275, 20–25. [Google Scholar] [CrossRef]
- Estrada, J.P.; Bescós, P.B.; Fresno, A.V.D. Antioxidant activity of different fractions of Spirulina platensis protean extract. Il Farmaco 2001, 56, 497–500. [Google Scholar] [CrossRef]
- Khan, Z.; Bhadouria, P.; Bisen, P. Nutritional and therapeutic potential of Spirulina. Curr. Pharm. Biotechnol. 2005, 6, 373–379. [Google Scholar] [CrossRef] [Green Version]
- Bermejo, P.; Piñero, E.; Villar, Á.M. Iron-chelating ability and antioxidant properties of phycocyanin isolated from a protean extract of Spirulina platensis. Food Chem. 2008, 110, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Biologic basis for interleukin-1 in disease. Blood 1996, 87, 2095–2147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romay, C.; Ledón, N.; González, R. Further studies on anti-inflammatory activity of phycocyanin in some animal models of inflammation. Inflamm. Res. 1998, 47, 334–338. [Google Scholar] [CrossRef]
- Amer, S.A.; Beheiry, R.R.; Fattah, D.M.A.; Roushdy, E.M.; Hassan, F.A.; Ismail, T.A.; Zaitoun, N.; Abo-Elmaaty, A.; Metwally, A.E. Effects of different feeding regimens with protease supplementation on growth, amino acid digestibility, economic efficiency, blood biochemical parameters, and intestinal histology in broiler chickens. BMC Vet. Res. 2021, 17, 283. [Google Scholar] [CrossRef] [PubMed]
- Amer, S.A.; Naser, M.A.; Abdel-Wareth, A.A.; Saleh, A.A.; Elsayed, S.A.; Metwally, A.E. Effect of dietary supplementation of alpha-galactosidase on the growth performance, ileal digestibility, intestinal morphology, and biochemical parameters in broiler chickens. BMC Vet. Res. 2020, 16, 144. [Google Scholar] [CrossRef]
- Amer, S.A.; Tolba, S.A.; AlSadek, D.M.; Fattah, D.M.A.; Hassan, A.M.; Metwally, A.E. Effect of supplemental glycerol monolaurate and oregano essential oil blend on the growth performance, intestinal morphology, and amino acid digestibility of broiler chickens. BMC Vet. Res. 2021, 17, 312. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Mobashar, M.; Mahsood, F.K.; Javaid, S.; Abdel-Wareth, A.; Ammanullah, H.; Mahmood, A. Spirulina inclusion levels in a broiler ration: Evaluation of growth performance, gut integrity, and immunity. Trop. Anim. Health Prod. 2020, 52, 3233–3240. [Google Scholar] [CrossRef]
- Shanmugapriya, B.; Babu, S.; Hariharan, T.; Sivaneswaran, S.; Anusha, M.; Raja, P.U. Synergistic effect of Spirulina platensis on performance and gut microbial load of broiler chicks. Ind. Asian J. Multidiscip. Res. 2015, 1, 149–155. [Google Scholar]
- Saini, M.K.; Vaish, V.; Sanyal, S.N. Role of cytokines and Jak3/Stat3 signaling in the 1, 2-Dimethylhydrazine Dihydrochloride-induced rat model of colon carcinogenesis. Eur. J. Cancer Prev. 2013, 22, 215–228. [Google Scholar] [CrossRef]
- Reddy, C.M.; Bhat, V.B.; Kiranmai, G.; Reddy, M.N.; Reddanna, P.; Madyastha, K. Selective inhibition of cyclooxygenase-2 by C-phycocyanin, a biliprotein from Spirulina platensis. Biochem. Biophys. Res. Commun. 2000, 277, 599–603. [Google Scholar] [CrossRef] [Green Version]
- Ou, Y.; Zheng, S.; Lin, L.; Jiang, Q.; Yang, X. Protective effect of C-phycocyanin against carbon tetrachloride-induced hepatocyte damage in vitro and in vivo. Chem. Biol. Interact. 2010, 185, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Martinez, S.E.; Chen, Y.; Ho, E.A.; Martinez, S.A.; Davies, N.M. Pharmacological effects of a C-phycocyanin-based multicomponent nutraceutical in an in-vitro canine chondrocyte model of osteoarthritis. Can. J. Vet. Res. 2015, 79, 241–249. [Google Scholar] [PubMed]
- Shih, C.-M.; Cheng, S.-N.; Wong, C.-S.; Kuo, Y.-L.; Chou, T.-C. Antiinflammatory and antihyperalgesic activity of C-phycocyanin. Anesth. Analg. 2009, 108, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
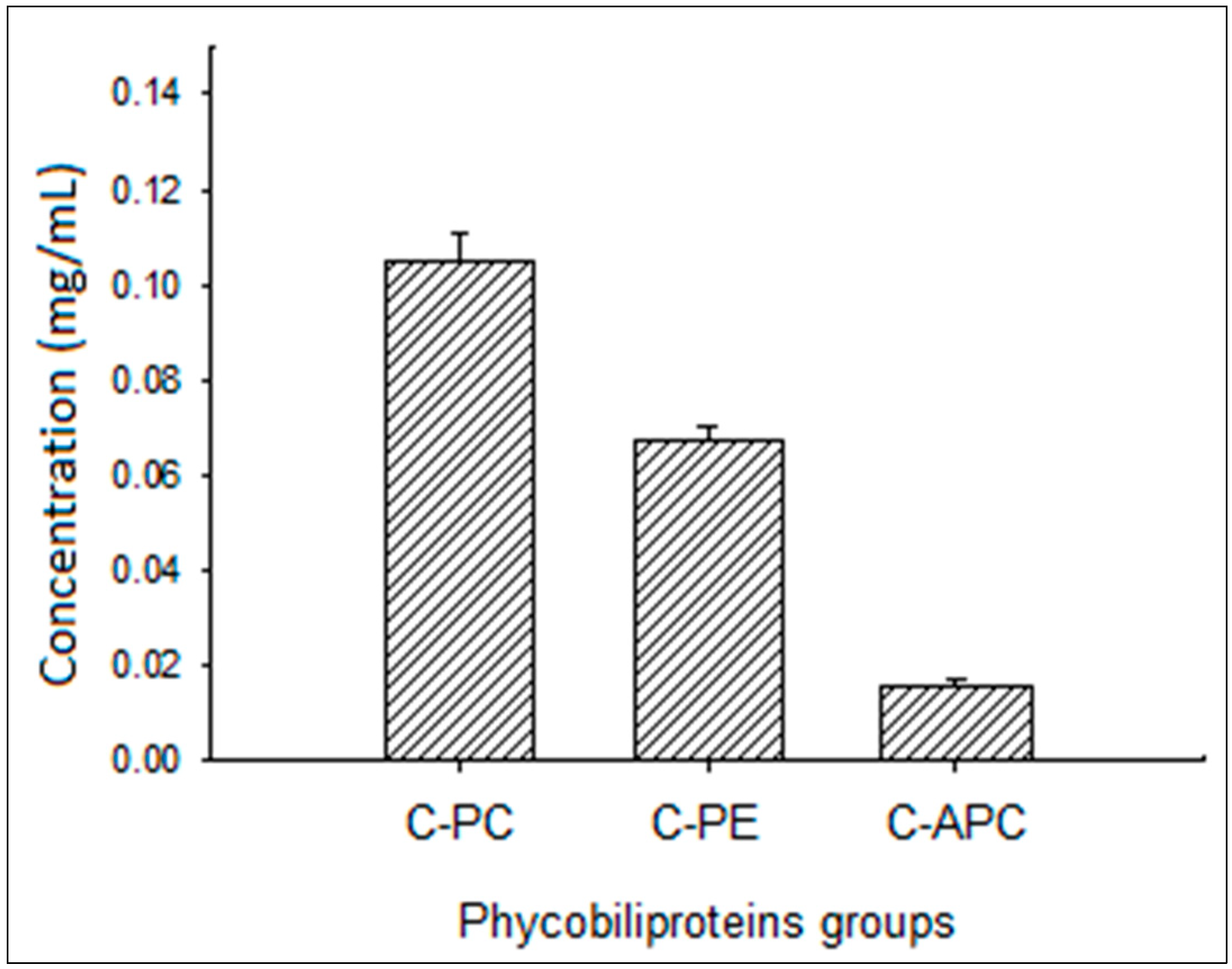

| Ingredients | Starter Period (4–10 Day) | Grower Period (11–23 Day) | Finisher Period (24–35 Day) |
|---|---|---|---|
| Yellow corn | 56 | 59.3 | 62 |
| Soybean meal, 48% | 33.33 | 28.1 | 23.825 |
| Corn gluten, 60% | 3.925 | 5.275 | 6.07 |
| Soybean oil | 2.2 | 3 | 4 |
| Calcium carbonate | 1.2 | 1.2 | 1.1 |
| Calcium dibasic phosphate | 1.5 | 1.4 | 1.3 |
| Common salt | 0.15 | 0.15 | 0.15 |
| Premix * | 0.3 | 0.3 | 0.3 |
| DL-methionine, 98% | 0.4 | 0.3 | 0.33 |
| Lysine HCl, 78% | 0.47 | 0.45 | 0.40 |
| Choline | 0.07 | 0.07 | 0.07 |
| Threonine | 0.1 | 0.1 | 0.1 |
| Phytase | 0.005 | 0.005 | 0.005 |
| Na2CO3 | 0.25 | 0.25 | 0.25 |
| Antimycotoxin | 0.1 | 0.1 | 0.1 |
| Chemical composition | |||
| ME (Kcal/kg) | 3003.9 | 3100.4 | 3200.8 |
| Crude protein % | 23.01 | 21.52 | 20.15 |
| Calcium % | 0.941 | 0.904 | 0.833 |
| Available phosphorus % | 0.481 | 0.449 | 0.418 |
| Lysine % | 1.465 | 1.315 | 1.163 |
| Methionine % | 0.721 | 0.610 | 0.626 |
| Threonine % | 0.823 | 0.765 | 0.713 |
| Trait Studied | SPC0 | SPC0.25 | SPC0.5 | SPC0.75 | SPC1 | Regression # | |
|---|---|---|---|---|---|---|---|
| Linear | Quadratic | ||||||
| Initial BW (g) | 88.96 ± 0.95 | 89.17 ± 1.30 | 90.00 ± 0.63 | 89.59 ± 0.96 | 89.59 ± 0.36 | 0.333 | 0.41 |
| Starter Period (4–10 Days) | |||||||
| BW (g) | 320.60 ± 4.93 bc | 315.61 ± 7.64 c | 333.25 ± 8.39 ab | 331.23 ± 9.78 ab | 337.81 ± 7.59 a | <0.01 | 0.84 |
| BWG (g) | 231.64 ± 5.78 bc | 226.44 ± 6.41 c | 243.26 ± 8.94 ab | 241.65 ± 9.01 ab | 248.23 ± 7.66 a | <0.01 | 0.76 |
| FI (g) | 240.84 ± 6.26 | 236.88 ± 16.16 | 241.25 ± 9.44 | 247.29 ± 12.35 | 238.13 ± 15.27 | 0.83 | 0.75 |
| FCR | 1.04 ± 0.01 | 1.05 ± 0.07 | 0.99 ± 0.02 | 1.02 ± 0.06 | 0.96 ± 0.07 | 0.09 | 0.62 |
| Grower Period (11–23 Days) | |||||||
| BW (g) | 1140.97 ± 0.87 bc | 1100.90 ± 15.89 c | 1211.72 ± 25.68 ab | 1243.33 ± 67.66 a | 1205.17 ± 47.86 ab | <0.01 | 0.40 |
| BWG (g) | 820.37 ± 5.46 bc | 785.30 ± 23.30 c | 878.47 ± 28.36 ab | 912.10 ± 60.87 a | 867.36 ± 40.88 ab | 0.01 | 0.34 |
| FI (g) | 1070.09 ± 16.56 ab | 1170.36 ± 52.13 a | 1042.92 ± 26.25 b | 1091.25 ± 63.36 ab | 1077.08 ± 75.44 ab | 0.51 | 0.65 |
| FCR | 1.30 ± 0.02 b | 1.49 ± 0.05 a | 1.19 ± 0.07 c | 1.20 ± 0.06 c | 1.24 ± 0.05 bc | <0.01 | 0.86 |
| Finisher Period (24–35 Days) | |||||||
| BW (g) | 2126.22 ± 32.50 b | 2363.89 ± 38.08 a | 2487.50 ± 78.70 a | 2499.17 ± 80.55 a | 2455.83 ± 82.17 a | <0.01 | <0.01 |
| BWG (g) | 985.25 ± 31.82 b | 1262.99 ± 127.54 a | 1275.78 ± 53.23 a | 1255.83 ± 80.86 a | 1250.67 ± 54.17 a | <0.01 | <0.01 |
| FI (g) | 1709.82 ± 105.53 | 1710.12 ± 127.23 | 1736.51 ± 233.40 | 1752.08 ± 165.63 | 1748.61 ± 185.14 | 0.71 | 0.96 |
| FCR | 1.73 ± 0.08 | 1.37 ± 0.23 | 1.36 ± 0.13 | 1.40 ± 0.21 | 1.40 ± 0.14 | 0.06 | 0.05 |
| Overall Performance (1–35 Days) | |||||||
| Final BW, g | 2126.22 ± 32.50 b | 2363.89 ± 138.08 a | 2487.50 ± 78.70 a | 2499.17 ± 80.55 a | 2455.83 ± 82.17 a | <0.01 | 0.01 |
| Total BWG, g | 2037.27 ± 32.11 b | 2274.72 ± 138.99 a | 2397.50 ± 78.26 a | 2409.58 ± 80.04 a | 2366.25 ± 81.89 a | <0.01 | 0.01 |
| Total FI, g | 3020.75 ± 85.47 | 3117.35 ± 142.64 | 3020.68 ± 216.28 | 3090.63 ± 238.36 | 3063.82 ± 257.01 | 0.87 | 0.86 |
| FCR | 1.48 ± 0.03 a | 1.38 ± 0.14 ab | 1.26 ± 0.05 b | 1.28 ± 0.11 b | 1.30 ± 0.08 b | 0.02 | 0.09 |
| SPC0 | SPC0.25 | SPC0.5 | SPC0.75 | SPC1 | Regression # | ||
|---|---|---|---|---|---|---|---|
| Linear | Quadratic | ||||||
| Glucose (mg/dL) | 335.00 ± 2.65 | 337.33 ± 7.57 | 339.33 ± 6.43 | 338.67 ± 6.43 | 340.33 ± 6.11 | 0.30 | 0.77 |
| Total proteins (g/dL) | 3.21 ± 0.25 b | 4.32 ± 0.89 a | 4.69 ± 0.36 a | 4.88 ± 0.53 a | 5.05 ± 0.27 a | <0.01 | 0.10 |
| Albumin (g/dL) | 1.21 ± 0.03 c | 1.42 ± 0.13 bc | 1.54 ± 0.17 b | 1.87 ± 0.31 a | 2.02 ± 0.04 a | <0.01 | 0.79 |
| Total globulins (g/dL) | 2.00 ± 0.24 b | 2.90 ± 0.80 a | 3.15 ± 0.28 a | 3.02 ± 0.25 a | 3.03 ± 0.24 a | 0.02 | 0.05 |
| GH (ng/mL) | 2.90 ± 0.30 c | 4.20 ± 0.30 b | 4.90 ± 0.44 ab | 5.30 ± 0.46 a | 5.60 ± 0.72 a | <0.01 | 0.05 |
| Leptin (ng/mL) | 2.18 ± 0.06 | 2.19 ± 0.02 | 1.65 ± 0.51 | 2.02 ± 0.32 | 2.06 ± 0.52 | 0.54 | 0.24 |
| T3 (ng/mL) | 3.44 ± 0.21 c | 4.42 ± 0.10 b | 4.29 ± 0.11 b | 4.41 ± 0.10 b | 4.85 ± 0.22 a | <0.01 | 0.03 |
| T4 (ng/mL) | 18.55 ± 1.47 c | 23.06 ± 0.57 b | 24.62 ± 0.88 ab | 25.12 ± 1.02 a | 24.41 ± 0.25 ab | <0.01 | <0.01 |
| SPC0 | SPC0.25 | SPC0.5 | SPC0.75 | SPC1 | Regression # | ||
|---|---|---|---|---|---|---|---|
| Linear | Quadratic | ||||||
| TC (mmol/L) | 3.54 ± 0.06 a | 3.35 ± 0.01 c | 3.46 ± 0.05 ab | 3.48 ± 0.03 a | 3.39 ± 0.02 bc | 0.04 | 0.23 |
| HDL-C (mmol/L) | 1.98 ± 0.03 | 2.07 ± 0.12 | 2.07 ± 0.13 | 2.08 ± 0.08 | 2.13 ± 0.09 | 0.11 | 0.79 |
| LDL-C (mmol/L) | 1.32 ± 0.03 a | 1.05 ± 0.13 b | 1.17 ± 0.15 ab | 1.16 ± 0.12 ab | 1.03 ± 0.09 b | 0.04 | 0.50 |
| VLDL-C (mmol/L) | 0.23 ± 0.02 | 0.23 ± 0.01 | 0.23 ± 0.02 | 0.24 ± 0.01 | 0.23 ± 0.02 | 0.91 | 0.93 |
| TG (mmol/L) | 1.27 ± 0.04 a | 1.17 ± 0.04 b | 1.19 ± 0.04 ab | 1.15 ± 0.03 b | 1.17 ± 0.07 b | 0.02 | 0.09 |
| SPC0 | SPC0.25 | SPC0.5 | SPC0.75 | SPC1 | Regression # | ||
|---|---|---|---|---|---|---|---|
| Linear | Quadratic | ||||||
| TAC (U/mL) | 9.69 ± 0.63 c | 11.59 ± 1.51 b | 13.11 ± 0.81 ab | 13.12 ± 0.46 ab | 13.69 ± 0.41 a | <0.01 | 0.04 |
| CAT (U/mL) | 2.57 ± 0.45 b | 5.03 ± 1.92 a | 6.50 ± 0.57 a | 6.43 ± 0.55 a | 6.57 ± 0.60 a | <0.01 | 0.02 |
| SOD (U/mL) | 135.96 ± 3.34 b | 152.21 ± 8.28 a | 156.32 ± 1.97 a | 162.44 ± 3.39 a | 153.49 ± 9.03 a | 0.01 | 0.02 |
| MDA nmol/mL | 4.59 ± 0.39 a | 4.60 ± 1.18 a | 3.63 ± 0.75 b | 3.17 ± 0.90 b | 3.43 ± 0.71 b | 0.03 | 0.59 |
| Serum IL1β (μg/mL) | 155 ± 11.79 | 157 ± 12.12 | 142 ± 6.08 | 157 ± 15.13 | 165 ± 7.94 | 0.35 | 0.11 |
| SPC0 | SPC0.25 | SPC0.5 | SPC0.75 | SPC1 | Regression # | ||
|---|---|---|---|---|---|---|---|
| Linear | Quadratic | ||||||
| Duodenum | |||||||
| VH µm | 626.55 ± 36.97 d | 706.20 ± 31.64 c | 921.23 ± 40.00 b | 1042.44 ± 20.14 a | 922.34 ± 29.14 b | <0.01 | <0.01 |
| VW µm | 130.36 ± 1.64 | 82.46 ± 35.09 | 115.57 ± 41.43 | 118.39 ± 9.57 | 65.51 ± 15.68 | 0.07 | 0.64 |
| CD µm | 115.26 ± 13.38 d | 119.01 ± 19.78 cd | 153.78 ± 24.18 b | 232.42 ± 13.51 a | 148.40 ± 7.78 b | <0.01 | <0.01 |
| VH:CD | 4.66 ± 0.44 c | 5.85 ± 0.32 ab | 5.81 ± 0.39 ab | 5.49 ± 0.18 b | 6.29 ± 0.21 a | <0.01 | 0.02 |
| GCC | 100.70 ± 11.84 c | 125.90 ± 11.64 b | 139.23 ± 6.60 ab | 155.59 ± 5.16 a | 131.39 ± 10.24 b | <0.01 | <0.01 |
| Jejunum | |||||||
| VH µm | 854.95 ± 77.77 d | 995.13 ± 66.11 c | 1213.0 ± 38.47 b | 1373.18 ± 104.11 a | 1038.93 ± 56.37 c | <0.01 | <0.01 |
| VW µm | 83.30 ± 14.85 | 98.86 ± 8.94 | 95.14 ± 5.05 | 93.77 ± 8.11 | 90.82 ± 17.21 | 0.36 | 0.17 |
| CD µm | 163.78 ± 6.42 d | 210.36 ± 24.55 c | 254.38 ± 16.90 b | 321.66 ± 17.43 a | 195.31 ± 13.12 c | <0.01 | <0.01 |
| VH:CD | 1.28 ± 0.09 c | 5.18 ± 0.93 a | 4.78 ± 0.19 ab | 4.27 ± 0.11 b | 5.42 ± 0.18 a | <0.01 | <0.01 |
| GCC | 170.45 ± 17.64 c | 210.36 ± 24.55 b | 242.98 ± 22.74 b | 298.98 ± 13.12 a | 210.45 ± 11.48 b | <0.01 | <0.01 |
| Ileum | |||||||
| VH µm | 254.76 ± 44.46 c | 382.31 ± 14.48 b | 392.03 ± 10.37 b | 506.08 ± 41.73 a | 349.00 ± 28.15 b | <0.01 | <0.01 |
| VW µm | 72.72 ± 7.33 c | 107.17 ± 5.56 ab | 74.97 ± 11.78 c | 91.41 ± 9.98 bc | 124.69 ± 16.28 a | <0.01 | 0.07 |
| CD µm | 86.15 ± 8.35 c | 92.34 ± 4.22 bc | 100.49 ± 2.51 ab | 110.11 ± 10.54 a | 87.73 ± 2.56 c | 0.09 | <0.01 |
| VH:CD | 626.55 ± 36.97 d | 3.93 ± 0.22 b | 3.90 ± 0.18 b | 4.60 ± 0.17 a | 3.98 ± 0.24 b | <0.01 | <0.01 |
| GCC | 130.36 ± 1.64 | 71.56 ± 6.23 b | 80.55 ± 2.57 ab | 89.01 ± 4.02 a | 74.01 ± 5.14 b | <0.01 | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omar, A.E.; Al-Khalaifah, H.S.; Osman, A.; Gouda, A.; Shalaby, S.I.; Roushdy, E.M.; Abdo, S.A.; Ali, S.A.; Hassan, A.M.; Amer, S.A. Modulating the Growth, Antioxidant Activity, and Immunoexpression of Proinflammatory Cytokines and Apoptotic Proteins in Broiler Chickens by Adding Dietary Spirulina platensis Phycocyanin. Antioxidants 2022, 11, 991. https://doi.org/10.3390/antiox11050991
Omar AE, Al-Khalaifah HS, Osman A, Gouda A, Shalaby SI, Roushdy EM, Abdo SA, Ali SA, Hassan AM, Amer SA. Modulating the Growth, Antioxidant Activity, and Immunoexpression of Proinflammatory Cytokines and Apoptotic Proteins in Broiler Chickens by Adding Dietary Spirulina platensis Phycocyanin. Antioxidants. 2022; 11(5):991. https://doi.org/10.3390/antiox11050991
Chicago/Turabian StyleOmar, Anaam E., Hanan S. Al-Khalaifah, Ali Osman, Ahmed Gouda, Shimaa I. Shalaby, Elshimaa M. Roushdy, Samar A. Abdo, Sozan A. Ali, Aziza M. Hassan, and Shimaa A. Amer. 2022. "Modulating the Growth, Antioxidant Activity, and Immunoexpression of Proinflammatory Cytokines and Apoptotic Proteins in Broiler Chickens by Adding Dietary Spirulina platensis Phycocyanin" Antioxidants 11, no. 5: 991. https://doi.org/10.3390/antiox11050991
APA StyleOmar, A. E., Al-Khalaifah, H. S., Osman, A., Gouda, A., Shalaby, S. I., Roushdy, E. M., Abdo, S. A., Ali, S. A., Hassan, A. M., & Amer, S. A. (2022). Modulating the Growth, Antioxidant Activity, and Immunoexpression of Proinflammatory Cytokines and Apoptotic Proteins in Broiler Chickens by Adding Dietary Spirulina platensis Phycocyanin. Antioxidants, 11(5), 991. https://doi.org/10.3390/antiox11050991






