Enhancement of Bioactive Constituents in Fresh Cauliflower By-Products in Challenging Climate Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Chemicals and Reagents
2.3. Growth Parameters Analysis
2.4. Total Soluble Sugars Content Analysis
2.5. Determination of Free radical Scavenging Ability by the Use of ABTS•+ Radical Cation Assay and Assessment of the Total Phenolic Content (TPC)
2.6. Determination and Quantification of Polyamines by UPLC-UV
2.7. Statistical Analysis
3. Results and Discussion
3.1. Analysis of Height and Biomass of Cauliflower Leaves
3.2. Total Soluble Sugar Content
3.3. Antioxidant Activity
3.4. Content of Polyamines
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aggarwal, P.; Vyas, S.; Thornton, P.; Campbell, B.; Kropff, M. Importance of considering technology growth in impact assessments of climate change on agriculture. Glob. Food Secur. 2019, 23, 41–48. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Akram, N.A.; Ashraf, M. Osmoprotection in plants under abiotic stresses: New insights into a classical phenomenon. Planta 2020, 251, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhry, S.; Sidhu, G.P.S. Climate change regulated abiotic stress mechanisms in plants: A comprehensive review. Plant Cell Rep. 2021, 41, 1–31. [Google Scholar] [CrossRef]
- Santos, A.R.; Kano, C.; Maia Chaves, F.C.; Borborema da Cunha, A.L.; Felipe de Oliveira Gentil, D.; Batisrta da Costa Junior, A. Inflorescence production of broccoli cultivars in the hot and humid climate of central amazonia. Rev. Caatinga 2020, 33, 54–61. [Google Scholar] [CrossRef]
- Ahmar, S.; Liaqat, N.; Hussain, M.; Salim, M.A.; Shabbir, M.A.; Ali, M.Y.; Noushahi, H.A.; Bilal, M.; Atta, B.; Rizwan, M. Effect of abiotic stresses on Brassica Species and role of transgenic breeding for adaptation. Asian J. Crop Sci. 2019, 3, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Piñero, M.C.; Pérez-Jiménez, M.; López-Marín, J.; Del Amor, F.M. Amelioration of boron toxicity in sweet pepper as affected by calcium management under an elevated CO2 concentration. Environ. Sci. Pollut. Res. 2017, 24, 10893–10899. [Google Scholar] [CrossRef]
- Zinta, G.; AbdElgawad, H.; Peshev, D.; Weedon, J.T.; Van den Ende, W.; Nijs, I.; Janssens, I.A.; Beemster, G.T.; Asard, H. Dynamics of metabolic responses to periods of combined heat and drought in Arabidopsis thaliana under ambient and elevated atmospheric CO2. J. Exp. Bot. 2018, 69, 2159–2170. [Google Scholar] [CrossRef]
- Kapusta-Duch, J.; Szeląg-Sikora, A.; Sikora, J.; Niemiec, M.; Gródek-Szostak, Z.; Kuboń, M.; Leszczyńska, T.; Borczak, B. Health-Promoting Properties of Fresh and Processed Purple Cauliflower. Sustainability 2019, 11, 4008. [Google Scholar] [CrossRef] [Green Version]
- Collado-González, J.; Piñero, M.C.; Otálora, G.; López-Marín, J.; del Amor, F.M. Exogenous spermidine modifies nutritional and bioactive constituents of cauliflower (Brassica oleracea var. botrytis L.) florets under heat stress. Sci. Hortic. 2021, 277, 109818. [Google Scholar] [CrossRef]
- Drabińska, N.; Jeż, M.; Nogueira, M. Variation in the Accumulation of Phytochemicals and Their Bioactive Properties among the Aerial Parts of Cauliflower. Antioxidants 2021, 10, 1597. [Google Scholar] [CrossRef]
- Muñoz-Esparza, N.C.; Latorre-Moratalla, M.L.; Comas-Basté, O.; Toro-Funes, N.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. Polyamines in Food. Front. Nutr. 2019, 6, 108. [Google Scholar] [CrossRef] [PubMed]
- Ebert, A.W. Potential of underutilized traditional vegetables and legume crops to contribute to food and nutritional security, income and more sustainable production systems. Sustainability 2014, 6, 319–335. [Google Scholar] [CrossRef] [Green Version]
- Nuñez-Gómez, V.; Baenas, N.; Navarro-González, I.; García-Alonso, J.; Moreno, D.A.; González-Barrio, R.; Periago-Castón, M. Seasonal variation of health-promoting bioactives in broccoli and methyl-jasmonate pre-harvest treatments to enhance their contents. Foods 2020, 9, 1371. [Google Scholar] [CrossRef] [PubMed]
- Collado-González, J.; Piñero, M.C.; Otálora, G.; López-Marín, J.; del Amor, F.M. Effects of Different Nitrogen Forms and Exogenous Application of Putrescine on Heat Stress of Cauliflower: Photosynthetic Gas Exchange, Mineral Concentration and Lipid Peroxidation. Plants 2021, 10, 152. [Google Scholar] [CrossRef] [PubMed]
- Khedkar, M.A.; Nimbalkar, P.R.; Chavan, P.V.; Chendake, Y.J.; Bankar, S.B. Cauliflower waste utilization for sustainable biobutanol production: Revelation of drying kinetics and bioprocess development. Bioproc. Biosyst. Eng. 2017, 40, 1493–1506. [Google Scholar] [CrossRef]
- Del Amor, F.M.; Cuadra-Crespo, P.; Walker, D.J.; Cámara, J.M.; Madrid, R. Effect of foliar application of antitranspirant on photosynthesis and water relations of pepper plants under different levels of CO2 and water stress. J. Plant Physiol. 2010, 167, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- Saffeullah, P.; Siddiqi, T.O.; Umar, S. Analysis of genetic, developmental and spatio-temporal patterns of nitrate accumulation in cauliflower and cabbage genotypes. Plant Physiol. Rep. 2021, 26, 671–686. [Google Scholar] [CrossRef]
- Balibrea, M.E.; Cuartero, J.; Bolarín, M.C.; Pérez-Alfocea, F. Sucrolytic activities during fruit development of Lycopersicon genotypes differing in tolerance to salinity. Physiol. Plant. 2003, 118, 38–46. [Google Scholar] [CrossRef]
- Cano-Lamadrid, M.; Hernández, F.; Corell, M.; Burló, F.; Legua, P.; Moriana, A.; Carbonell-Barrachina, Á.A. Antioxidant capacity, fatty acids profile, and descriptive sensory analysis of table olives as affected by deficit irrigation. J. Sci. Food Agric. 2017, 97, 444–451. [Google Scholar] [CrossRef] [Green Version]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.-P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef]
- Rodriguez, S.; López, B.; Chaves, A.R. Effect of different treatments on the evolution of polyamines during refrigerated storage of eggplants. J. Agric. Food Chem. 2001, 49, 4700–4705. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of climate change on crops adaptation and strategies to tackle its outcome: A review. Plants 2019, 8, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, J.M. The Influences of Elevated Carbon Dioxide on Trichome Densities and Trichome Molecular Pathways. Doctoral Dissertation, University of Kansas, Lawrence, KS, USA, 2020. [Google Scholar]
- Mohammadi, H.; Ghorbanpour, M.; Brestic, M. Exogenous putrescine changes redox regulations and essential oil constituents in field-grown Thymus vulgaris L. under well-watered and drought stress conditions. Ind. Crops Prod. 2018, 122, 119–132. [Google Scholar] [CrossRef]
- Chen, D.; Shao, Q.; Yin, L.; Younis, A.; Zheng, B. Polyamine function in plants: Metabolism, regulation on development, and roles in abiotic stress responses. Front. Plant Sci. 2019, 9, 1945. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, S.R.; Kwak, J.-H. Chemical Composition and Antioxidant Activity in Different Tissues of Brassica Vegetables. Molecules 2015, 20, 1228–1243. [Google Scholar] [CrossRef] [Green Version]
- Guarise, M.; Borgonovo, G.; Bassoli, A.; Ferrante, A. Evaluation of two wild populations of Hedge Mustard (Sisymbrium officinale (L.) Scop.) as a potential leafy vegetable. Horticulturae 2019, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Piñero, M.C.; Otálora, G.; Collado, J.; López-Marín, J.; Del Amor, F.M. Foliar application of putrescine before a short-term heat stress improves the quality of melon fruits (Cucumis melo L.). J. Sci. Food Agric. 2021, 101, 1428–1435. [Google Scholar] [CrossRef]
- Gamage, D.; Thompson, M.; Sutherland, M.; Hirotsu, N.; Makino, A.; Seneweera, S. New insights into the cellular mechanisms of plant growth at elevated atmospheric carbon dioxide concentrations. Plant Cell Environ. 2018, 41, 1233–1246. [Google Scholar] [CrossRef]
- Agüera, E.; De la Haba, P. Leaf senescence in response to elevated atmospheric CO2 concentration and low nitrogen supply. Biol. Plant. 2018, 62, 401–408. [Google Scholar] [CrossRef]
- Vicente, R.; Pérez, P.; Martínez-Carrasco, R.; Feil, R.; Lunn, J.E.; Watanabe, M.; Arrivault, S.; Stitt, M.; Hoefgen, R.; Morcuende, R. Metabolic and transcriptional analysis of durum wheat responses to elevated CO2 at low and high nitrate supply. Plant Cell Physiol. 2016, 57, 2133–2146. [Google Scholar] [CrossRef] [Green Version]
- Mageney, V.; Neugart, S.; Albach, D.C. A guide to the variability of flavonoids in Brassica oleracea. Molecules 2017, 22, 252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cömert, E.D.; Mogol, B.A.; Gökmen, V. Relationship between color and antioxidant capacity of fruits and vegetables. Curr. Res. Nutr. Food Sci. 2020, 2, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Soengas, P.; Rodríguez, V.M.; Velasco, P.; Cartea, M.E. Effect of temperature stress on antioxidant defenses in Brassica oleracea. ACS Omega 2018, 3, 5237–5243. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, S.; Mitra, S. Elevated atmospheric CO2 and the future of crop plants. Plant Breed. 2021, 140, 1–11. [Google Scholar] [CrossRef]
- Pintó-Marijuan, M.; Joffre, R.; Casals, I.; De Agazio, M.; Zacchini, M.; García-Plazaola, J.I.; Esteban, R.; Aranda, X.; Guàrdia, M.; Fleck, I. Antioxidant and photoprotective responses to elevated CO2 and heat stress during holm oak regeneration by resprouting, evaluated with NIRS (near-infrared reflectance spectroscopy). Plant Biol. 2013, 15, 5–17. [Google Scholar] [CrossRef]
- Zinta, G.; AbdElgawad, H.; Domagalska, M.A.; Vergauwen, L.; Knapen, D.; Nijs, I.; Janssens, I.A.; Beemster, G.T.; Asard, H. Physiological, biochemical, and genome-wide transcriptional analysis reveals that elevated CO2 mitigates the impact of combined heat wave and drought stress in Arabidopsis thaliana at multiple organizational levels. Glob. Chang. Biol. 2014, 20, 3670–3685. [Google Scholar] [CrossRef]
- Collado-González, J.; Piñero, M.C.; Otalora, G.; Lopez-Marín, J.; Del Amor, F.M. Unraveling the nutritional and bioactive constituents in baby-leaf lettuce for challenging climate conditions. Food Chem. 2022, 384, 132506. [Google Scholar] [CrossRef]
- Mostafa, H.A.M.; Hassanein, R.A.; Khalil, S.I.; El-Khawas, S.A.; El-Bassiouny, H.M.S.; El-Monem, A.A.A. Effect of arginine or putrescine on growth, yield and yield components of late sowing wheat. Res. J. Appl. Sci. 2010, 6, 177–183. [Google Scholar]
- Toscano, S.; Trivellini, A.; Cocetta, G.; Bulgari, R.; Francini, A.; Romano, D.; Ferrante, A. Effect of Preharvest Abiotic Stresses on the Accumulation of Bioactive Compounds in Horticultural Produce. Front. Plant Sci. 2019, 1212. [Google Scholar] [CrossRef] [Green Version]
- Martínez, S.; Armesto, J.; Gómez-Limia, L.; Carballo, J. Impact of processing and storage on the nutritional and sensory properties and bioactive components of Brassica spp. A review. Food Chem. 2020, 313, 126065. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, Q.; Shi, Y.; Li, X.; Hou, L.; Xing, G.; Ahammed, G.J. Elevated CO2 improves antioxidant capacity, ion homeostasis, and polyamine metabolism in tomato seedlings under Ca (NO3)2-induced salt stress. Sci. Hortic. 2020, 273, 109644. [Google Scholar] [CrossRef]
- Pál, M.; Szalai, G.; Janda, T. Speculation: Polyamines are important in abiotic stress signaling. Plant Sci. 2015, 237, 16–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luna-Esquivel, E.N.; Ojeda-Barrios, D.L.; Guerrero-Prieto, V.M.; Ruiz-Anchondo, T.; Martínez-Téllez, J.J. Poliaminas como indicadores de estrés en plantas. Rev. Chapingo Ser. Hortic. 2014, 20, 283–295. [Google Scholar] [CrossRef]
- Park, K.Y.; Seo, S.Y.; Kim, Y.J. Increasing polyamine contents enhances the stress tolerance via reinforcement of antioxidative properties. Front. Plant Sci. 2019, 10, 1331. [Google Scholar]

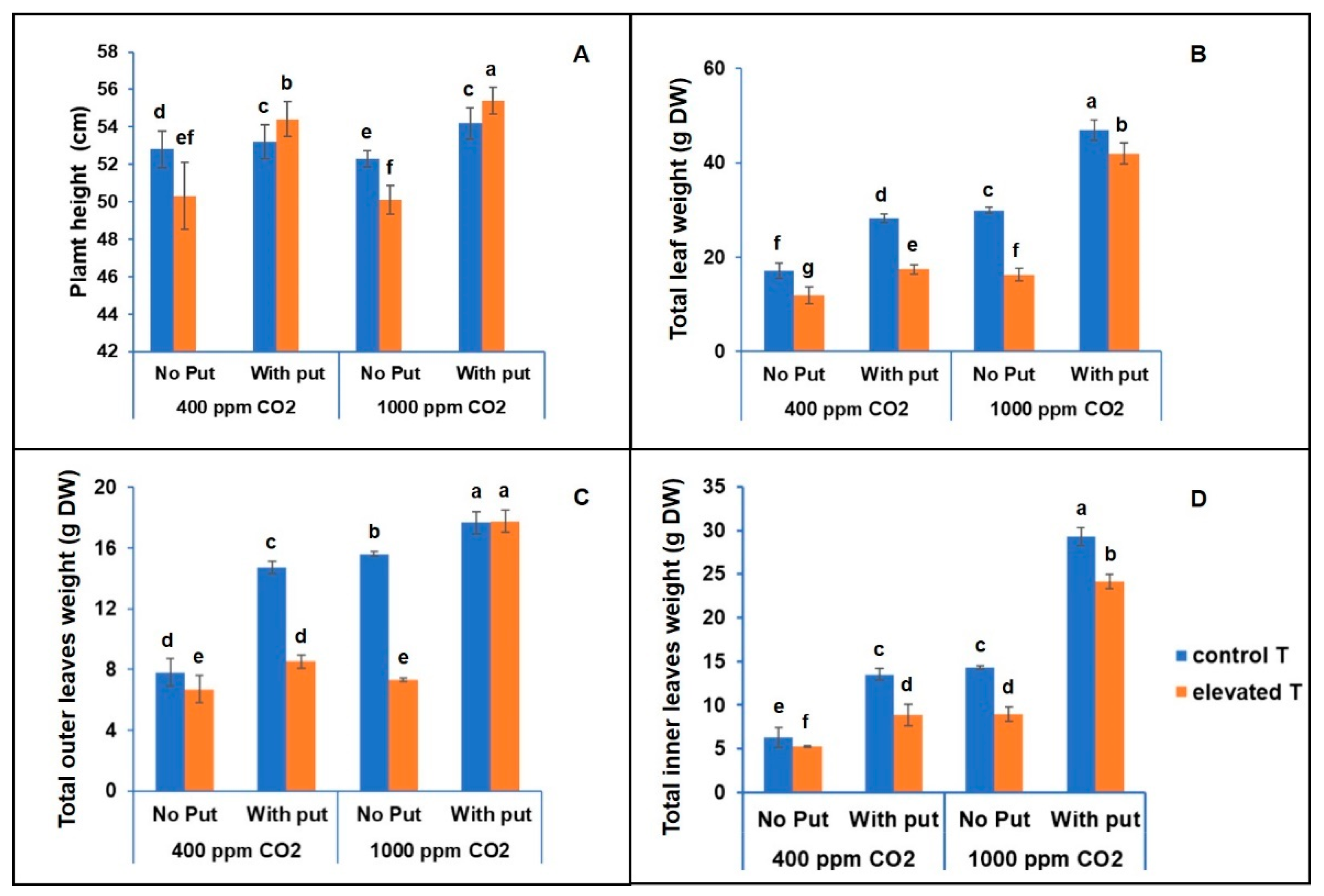
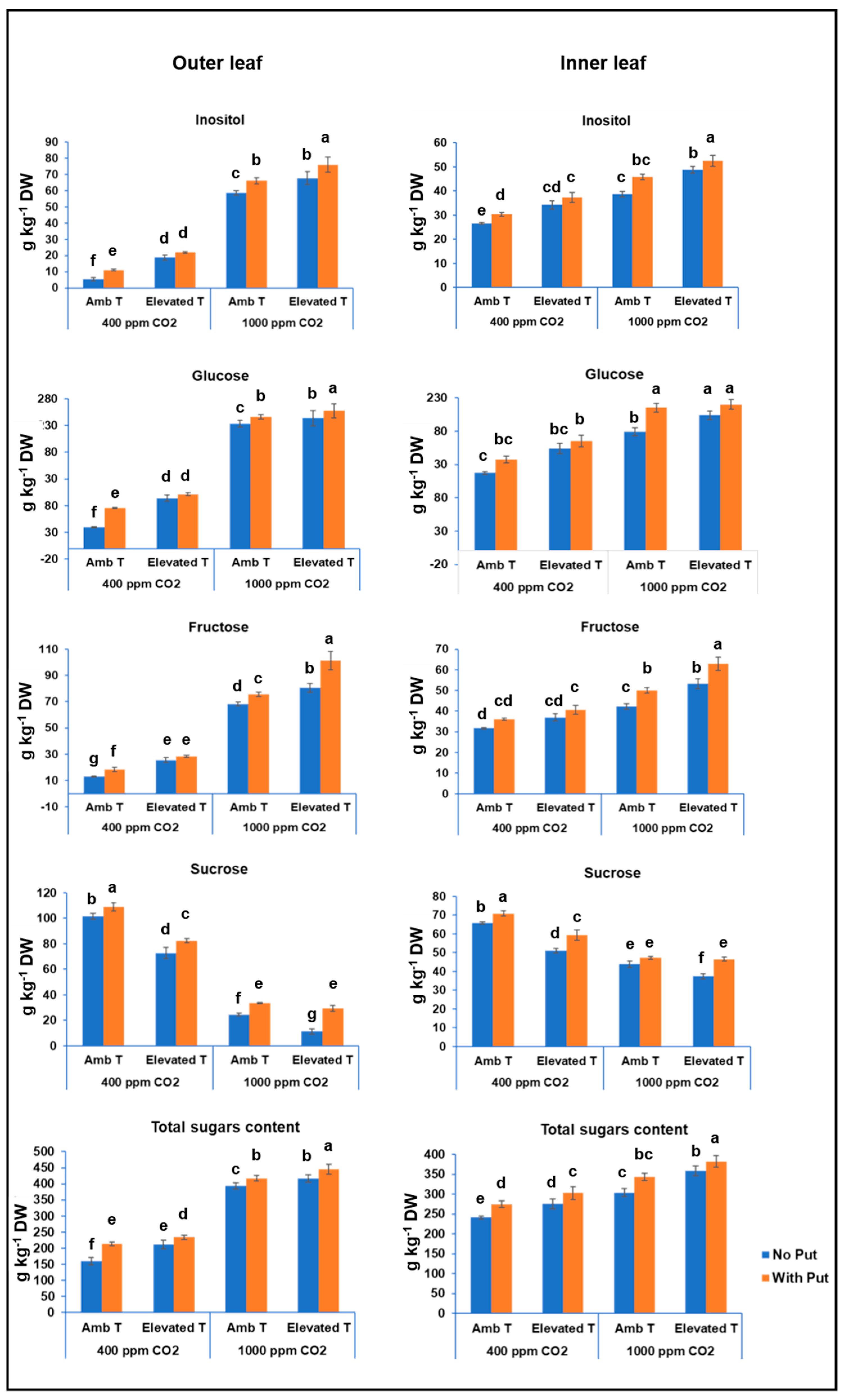

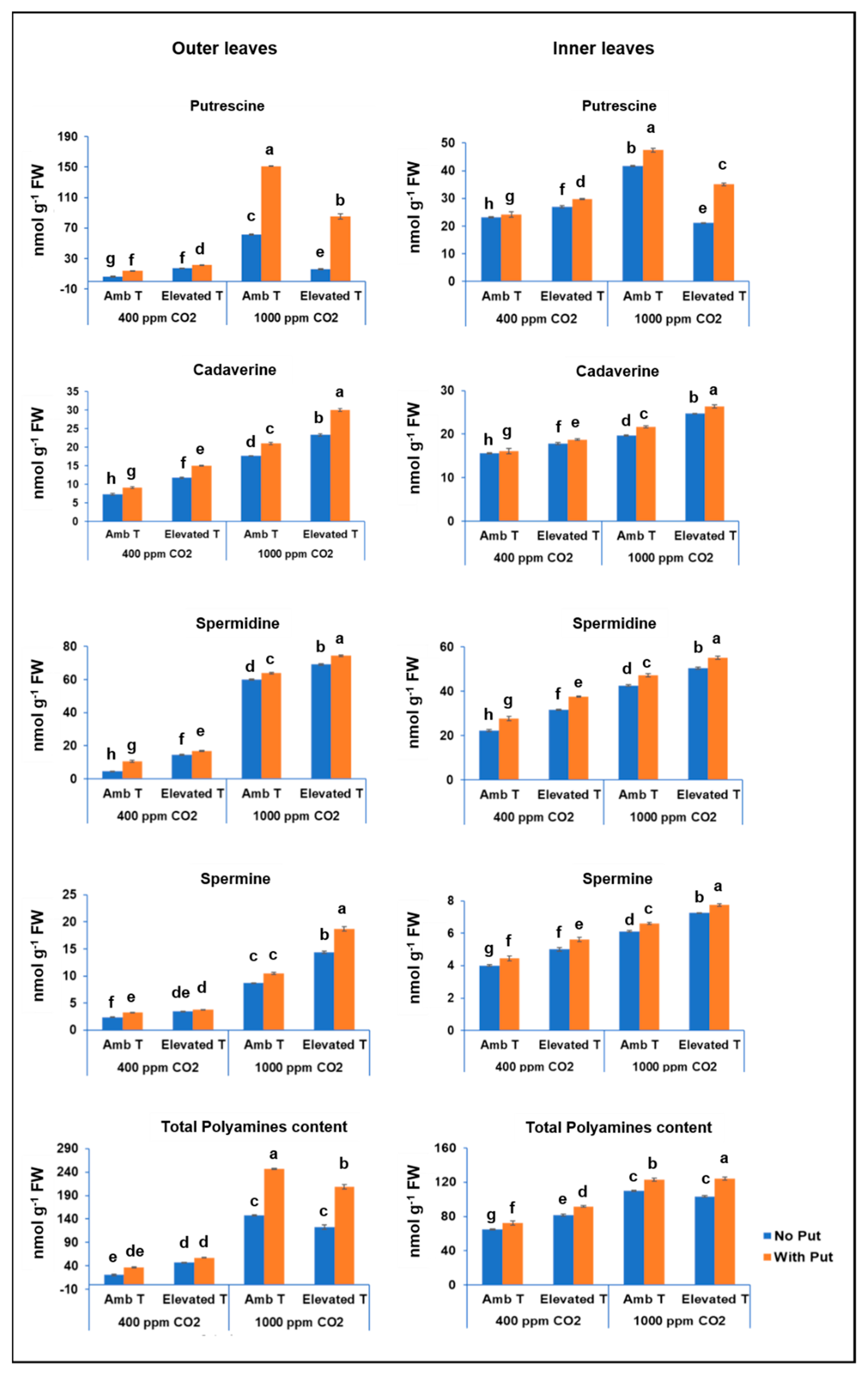
| Attribute | T | Put | CO2 | AL | T * Put | T * CO2 | T * AL | Put * CO2 | Put * AL | CO2 * AL | T * Put * CO2 | T * Put * AL | T * CO2 * AL | Put * CO2 * AL | T * Put * CO2 * AL |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inositol | *** | *** | *** | *** | ns | ns | ** | ** | ns | *** | ns | ns | ns | ns | ns |
| Glucose | *** | *** | *** | *** | *** | *** | ns | ns | ns | *** | ns | ns | ns | * | * |
| Fructose | *** | *** | *** | *** | * | *** | *** | *** | * | ** | ** | ns | ns | * | ** |
| Sucrose | *** | *** | *** | *** | *** | *** | *** | * | *** | *** | * | ns | *** | * | ns |
| Total Sugars | *** | *** | *** | *** | ns | ns | ns | ** | ns | *** | ns | ns | ns | ns | ** |
| AA | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| TPC | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | ns | ns | ** | ns | *** |
| Putrescine | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Cadaverine | *** | *** | *** | *** | ns | ns | ns | * | ns | *** | * | ns | ns | ns | ** |
| Spermidine | *** | *** | *** | *** | * | ns | ns | ** | ns | *** | ** | *** | *** | ** | *** |
| Spermine | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Total PAs | *** | *** | *** | *** | *** | ns | *** | *** | *** | *** | ns | *** | *** | ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Collado-González, J.; Piñero, M.C.; Otalora, G.; López-Marín, J.; Del Amor, F.M. Enhancement of Bioactive Constituents in Fresh Cauliflower By-Products in Challenging Climate Conditions. Antioxidants 2022, 11, 958. https://doi.org/10.3390/antiox11050958
Collado-González J, Piñero MC, Otalora G, López-Marín J, Del Amor FM. Enhancement of Bioactive Constituents in Fresh Cauliflower By-Products in Challenging Climate Conditions. Antioxidants. 2022; 11(5):958. https://doi.org/10.3390/antiox11050958
Chicago/Turabian StyleCollado-González, Jacinta, María Carmen Piñero, Ginés Otalora, Josefa López-Marín, and Francisco M. Del Amor. 2022. "Enhancement of Bioactive Constituents in Fresh Cauliflower By-Products in Challenging Climate Conditions" Antioxidants 11, no. 5: 958. https://doi.org/10.3390/antiox11050958
APA StyleCollado-González, J., Piñero, M. C., Otalora, G., López-Marín, J., & Del Amor, F. M. (2022). Enhancement of Bioactive Constituents in Fresh Cauliflower By-Products in Challenging Climate Conditions. Antioxidants, 11(5), 958. https://doi.org/10.3390/antiox11050958










