Effects of Faba Bean Hull Nanoparticles on Physical Properties, Protein and Lipid Oxidation, Colour Degradation, and Microbiological Stability of Burgers under Refrigerated Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. FBH-NPs Preparation
2.2. Preparation of FBH-NPs Methanolic Extract
2.3. FBH and FBH-NPs Methanolic Extract Analysis
2.4. Beef Burger Manufacture
2.5. Burger Physical Properties
2.5.1. pH Value and Water Holding Capacity (WHC)
2.5.2. Cooking Loss and Cooking Yield
2.6. Color Evaluation
2.6.1. Surface Colour Measurement
2.6.2. Oxymyoglobin and Metmyoglobin Determination
2.7. Lipid Oxidation
2.7.1. Peroxide Value Determination
2.7.2. Thiobarbituric Acid (TBARS) Determination
2.8. Total Protein Carbonyls Determination
2.9. Microbiological Analysis
3. Statistical Analysis
4. Results and Discussion
4.1. FBH-NPs Methanolic Extract Analysis
4.1.1. TP and TF Content
4.1.2. Antioxidant Activities
4.2. Effect of FBH-NPs on Burger Physical Properties
4.2.1. pH and WHC Values
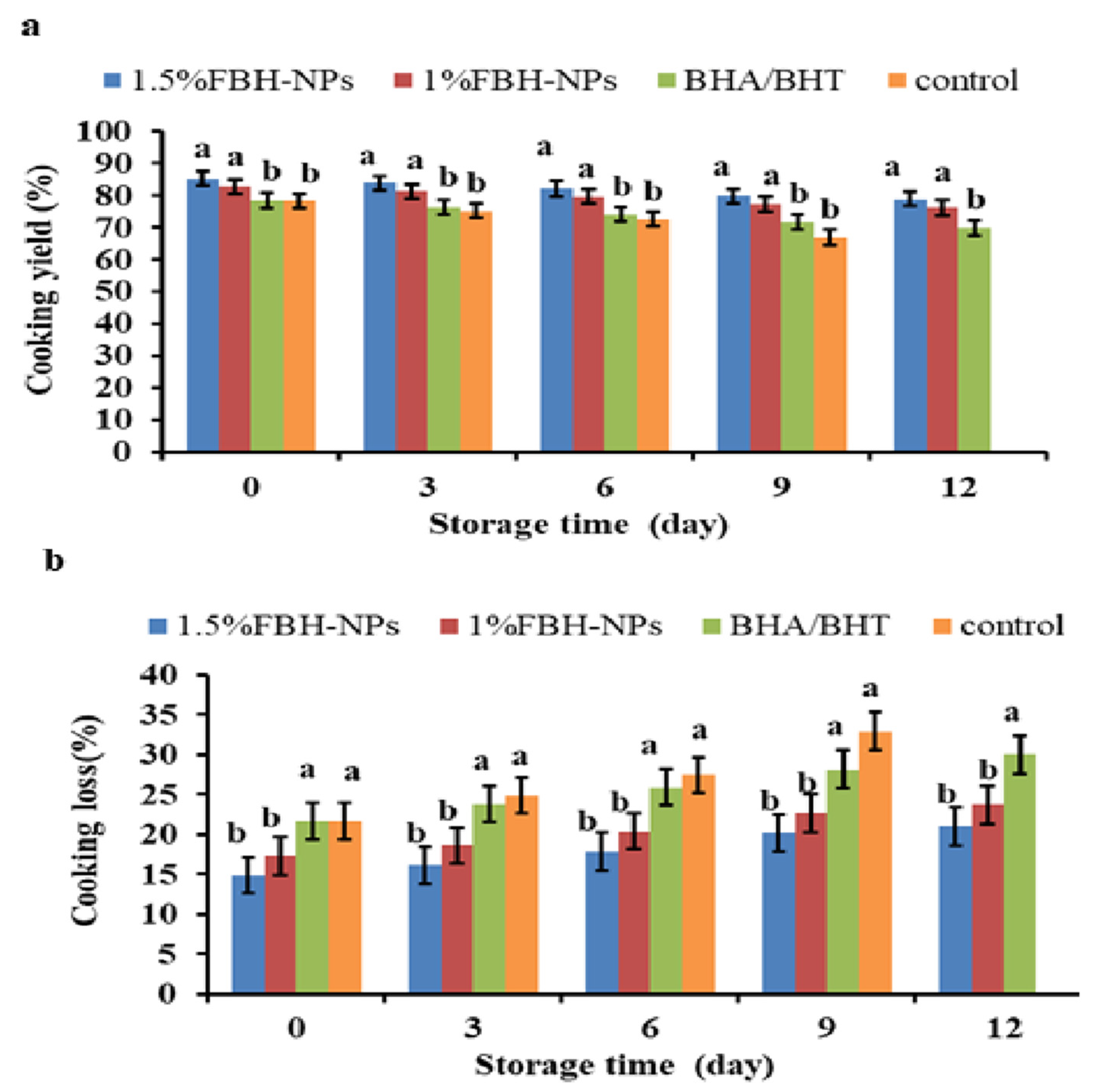
4.2.2. Cooking Yield and Cooking Loss
4.3. Effect of FBH-NPs on Burger Colour
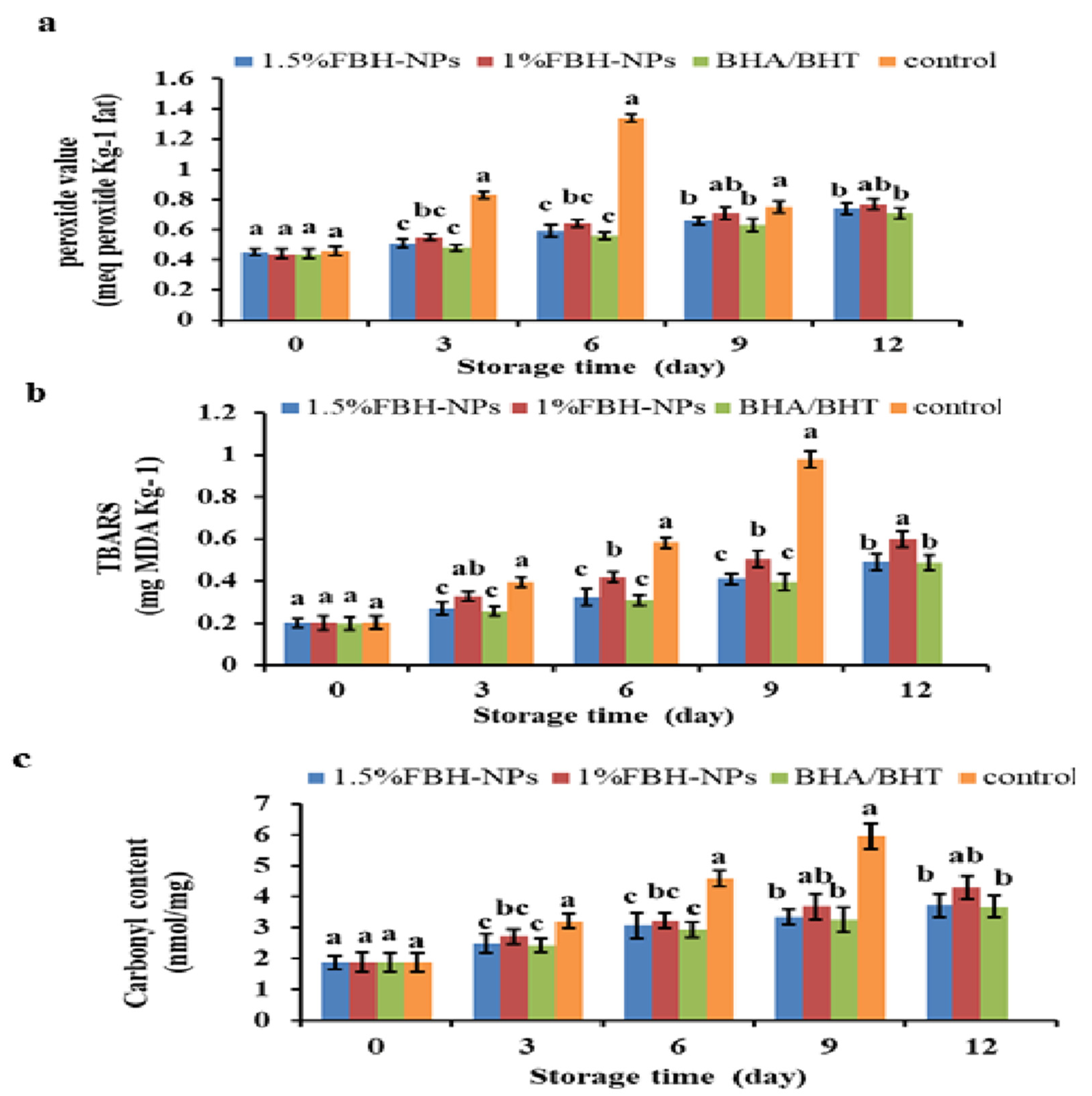
4.4. Effect of FBH-NPs on Burger Lipid Oxidation
4.5. Effect of FBH-NPs on Burger Protein Degradation
4.6. Microbiological Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Essa, R.; Elsebaie, E. Effect of using date pits powder as a fat replacer and anti-oxidative agent on beef burger quality. J. Food Dairy Sci. 2018, 9, 91–96. [Google Scholar] [CrossRef]
- Falowo, A.B.; Fayemi, P.O.; Muchenje, V. Natural antioxidants against lipid–protein oxidative deterioration in meat and meat products: A review. Food Res. Int. 2014, 64, 171–181. [Google Scholar] [CrossRef]
- Abdelhakam, O.S.; Elsebaie, E.M.; Ghazi, A.K.; Gouda, M.S. Quality characteristics of beef hamburger enriched with red grape pomace powder during freezing storage. Slov. Vet. Res. 2019, 56, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Crippa, A.; Larsson, S.C.; Discacciati, A.; Wolk, A.; Orsini, N. Red and processed meat consumption and risk of bladder cancer: A dose–response meta-analysis of epidemiological studies. Eur. J. Nutr. 2018, 57, 689–701. [Google Scholar] [CrossRef] [Green Version]
- Echegaray, N.; Gómez, B.; Barba, F.J.; Franco, D.; Estévez, M.; Carballo, J.; Marszałek, K.; Lorenzo, J.M. Chestnuts and by-products as source of natural antioxidants in meat and meat products: A review. Trends Food Sci. Technol. 2018, 82, 110–121. [Google Scholar] [CrossRef]
- de Almeida, P.L.; de Lima, S.N.; Costa, L.L.; de Oliveira, C.C.; Damasceno, K.A.; dos Santos, B.A.; Campagnol, P.C.B. Effect of jabuticaba peel extract on lipid oxidation, microbial stability and sensory properties of Bologna-type sausages during refrigerated storage. Meat Sci. 2015, 110, 9–14. [Google Scholar] [CrossRef]
- Kumar, Y.; Yadav, D.N.; Ahmad, T.; Narsaiah, K. Recent trends in the use of natural antioxidants for meat and meat products. Compr. Rev. Food Sci. Food Saf. 2015, 14, 796–812. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, R.; Trindade, M.; Tonin, F.; Pugine, S.; Lima, C.; Lorenzo, J.; De Melo, M. Evaluation of oxidative stability of lamb burger with Origanum vulgare extract. Food Chem. 2017, 233, 101–109. [Google Scholar] [CrossRef]
- Kryževičūtė, N.; Jaime, I.; Diez, A.M.; Rovira, J.; Venskutonis, P.R. Effect of raspberry pomace extracts isolated by high pressure extraction on the quality and shelf-life of beef burgers. Int. J. Food Sci. Technol. 2017, 52, 1852–1861. [Google Scholar] [CrossRef]
- Aly, A.A. Utilization of Pomegranate Peels to Increase the Shelf Life of Chicken Burger during Cold Storage. Egypt. J. Food Sci. 2019, 47, 1–10. [Google Scholar] [CrossRef]
- Morsy, M.K.; Mekawi, E.; Elsabagh, R. Impact of pomegranate peel nanoparticles on quality attributes of meatballs during refrigerated storage. LWT 2018, 89, 489–495. [Google Scholar] [CrossRef]
- Smaoui, S.; Hlima, H.B.; Mtibaa, A.C.; Fourati, M.; Sellem, I.; Elhadef, K.; Ennouri, K.; Mellouli, L. Pomegranate peel as phenolic compounds source: Advanced analytical strategies and practical use in meat products. Meat Sci. 2019, 158, 107914. [Google Scholar] [CrossRef]
- Barakat, O. Utilization of Faba Bean (Vicia faba) Hulls in Producing Some Biological Compounds. Master’s Thesis, Faculty of Agriculture, Kafrelsheikh University, Kafr El Sheikh, Egypt, 2018. [Google Scholar]
- Oomah, B.D.; Caspar, F.; Malcolmson, L.J.; Bellido, A.-S. Phenolics and antioxidant activity of lentil and pea hulls. Food Res. Int. 2011, 44, 436–441. [Google Scholar] [CrossRef]
- Gnanasambandam, A.; Paull, J.; Torres, A.; Kaur, S.; Leonforte, T.; Li, H.; Zong, X.; Yang, T.; Materne, M. Impact of molecular technologies on faba bean (Vicia faba L.) breeding strategies. Agronomy 2012, 2, 132–166. [Google Scholar] [CrossRef] [Green Version]
- Barakat, O.; Elsebaie, E.; Ammar, A.; Elnemr, K. Utilization of Faba Bean Hulls (Seeds Coat) as a Source to Produce Antioxidants. J. Food Dairy Sci. 2017, 8, 275–278. [Google Scholar] [CrossRef] [Green Version]
- El-Saber, M.M.M. Biochemical studies on faba bean under rainfed at Maryout conditions. Master’s Thesis, Biochemistry Department, Faculty of Agriculture, Zagazig University, Zagazig, Egypt, 2010. [Google Scholar]
- Güzel, D.; Sayar, S. Effect of cooking methods on selected physicochemical and nutritional properties of barlotto bean, chickpea, faba bean, and white kidney bean. J. Food Sci. Technol. 2012, 49, 89–95. [Google Scholar] [CrossRef] [Green Version]
- Kanatt, S.R.; Arjun, K.; Sharma, A. Antioxidant and antimicrobial activity of legume hulls. Food Res. Int. 2011, 44, 3182–3187. [Google Scholar] [CrossRef]
- Rodrigues, S.M.; Demokritou, P.; Dokoozlian, N.; Hendren, C.O.; Karn, B.; Mauter, M.S.; Sadik, O.A.; Safarpour, M.; Unrine, J.M.; Viers, J. Nanotechnology for sustainable food production: Promising opportunities and scientific challenges. Environ. Sci. Nano 2017, 4, 767–781. [Google Scholar] [CrossRef]
- Morsy, M.K.; Khalaf, H.H.; Sharoba, A.M.; El-Tanahi, H.H.; Cutter, C.N. Incorporation of essential oils and nanoparticles in pullulan films to control foodborne pathogens on meat and poultry products. J. Food Sci. 2014, 79, M675–M684. [Google Scholar] [CrossRef]
- Khataee, A.; Fathinia, S.; Fathinia, M. Production of pyrite nanoparticles using high energy planetary ball milling for sonocatalytic degradation of sulfasalazine. Ultrason. Sonochem. 2017, 34, 904–915. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z.; Rahmat, A. Antioxidant activities, total phenolics and flavonoids content in two varieties of Malaysia young ginger (Zingiber officinale Roscoe). Molecules 2010, 15, 4324–4333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 3. [Google Scholar]
- Turkmen, N.; Sari, F.; Velioglu, Y.S. The effect of cooking methods on total phenolics and antioxidant activity of selected green vegetables. Food Chem. 2005, 93, 713–718. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Boudjou, S.; Oomah, B.D.; Zaidi, F.; Hosseinian, F. Phenolics content and antioxidant and anti-inflammatory activities of legume fractions. Food Chem. 2013, 138, 1543–1550. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Gnoyke, S.; Popken, A.M.; Böhm, V. Antioxidant capacity and related parameters of different fruit formulations. LWT-Food Sci. Technol. 2010, 43, 992–999. [Google Scholar] [CrossRef]
- Yousefi, N.; Zeynali, F.; Alizadeh, M. Optimization of low-fat meat hamburger formulation containing quince seed gum using response surface methodology. J. Food Sci. Technol. 2018, 55, 598–604. [Google Scholar] [CrossRef]
- Essa, R.Y.; Elsebaie, E.M. New fat replacement agent comprised of gelatin and soluble dietary fibers derived from date seed powder in beef burger preparation. LWT 2022, 156, 113051. [Google Scholar] [CrossRef]
- Carlez, A.; Veciana-Nogues, T.; Cheftel, J.-C. Changes in colour and myoglobin of minced beef meat due to high pressure processing. LWT-Food Sci. Technol. 1995, 28, 528–538. [Google Scholar] [CrossRef]
- Richards, M.P.; Hultin, H.O. Contributions of blood and blood components to lipid oxidation in fish muscle. J. Agric. Food Chem. 2002, 50, 555–564. [Google Scholar] [CrossRef]
- Yoon, D.-k.; Kim, J.-H.; Cho, W.-Y.; Ji, D.-S.; Lee, H.-J.; Kim, J.-H.; Lee, C.-H. Effect of allium hookeri root on physicochemical, lipid, and protein oxidation of longissimus dorsi muscle meatball. Korean J. Food Sci. Anim. Resour. 2018, 38, 1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, E.E.; Younis, E.R.; Mohamed, E.A. Impact of atmospheric cold plasma (ACP) on maintaining bolti fish (Tilapia nilotica) freshness and quality criteria during cold storing. J. Food Process. Preserv. 2021, 45, e15442. [Google Scholar] [CrossRef]
- Yousef, A.E.; Carlstrom, C. Food Microbiology: A Laboratory Manual; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Hashemi, Z.; Ebrahimzadeh, M. Evaluation of three methods for the extraction of antioxidants from vicia faba L. bean and hulls. Lat. Am. Appl. Res. 2014, 44, 203–208. [Google Scholar] [CrossRef]
- Troszynska, A.; Ciska, E. Phenolic compounds of seed coats of white and coloured varieties of pea (Pisum sativum L.) and their total antioxidant activity. Czech J. Food Sci. 2002, 20, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Dawi, F.; El-Beltagi, H.S.; Abdel-Mobdy, Y.E.; Salah, S.M.; Ghaly, I.S.; Abdel-Rahim, E.A.; Mohamed, H.I.; Soliman, A.M. Synergistic impact of the pomegranate peels and its nanoparticles against the infection of tobacco mosaic virus (TMV). Fresenius Environ. Bull. 2021, 30, 731–746. [Google Scholar]
- Carpes, S.T.; Begnini, R.; Alencar, S.M.d.; Masson, M.L. Study of preparations of bee pollen extracts, antioxidant and antibacterial activity. Ciência Agrotecnologia 2007, 31, 1818–1825. [Google Scholar] [CrossRef] [Green Version]
- de Florio Almeida, J.; dos Reis, A.S.; Heldt, L.F.S.; Pereira, D.; Bianchin, M.; de Moura, C.; Plata-Oviedo, M.V.; Haminiuk, C.W.I.; Ribeiro, I.S.; da Luz, C.F.P. Lyophilized bee pollen extract: A natural antioxidant source to prevent lipid oxidation in refrigerated sausages. LWT-Food Sci. Technol. 2017, 76, 299–305. [Google Scholar] [CrossRef]
- Farsanipour, A.; Khodanazary, A.; Hosseini, S.M. Effect of chitosan-whey protein isolated coatings incorporated with tarragon Artemisia dracunculus essential oil on the quality of Scomberoides commersonnianus fillets at refrigerated condition. Int. J. Biol. Macromol. 2020, 155, 766–771. [Google Scholar] [CrossRef]
- Zhang, M.; Mittal, G.; Barbut, S. Effects of test conditions on the water holding capacity of meat by a centrifugal method. LWT-Food Sci. Technol. 1995, 28, 50–55. [Google Scholar] [CrossRef]
- Kaya, E.; Tuncel, N.B.; Yılmaz Tuncel, N. The effect of ultrasound on some properties of pulse hulls. J. Food Sci. Technol. 2017, 54, 2779–2788. [Google Scholar] [CrossRef]
- Soltanizadeh, N.; Mirmoghtadaie, L.; Nejati, F.; Najafabadi, L.I.; Heshmati, M.K.; Jafari, M. Solid-state protein–carbohydrate interactions and their application in the food industry. Compr. Rev. Food Sci. Food Saf. 2014, 13, 860–870. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Barber, X.; Pérez-Álvarez, J.A.; Fernández-López, J. Assessment of chemical, physico-chemical, techno-functional and antioxidant properties of fig (Ficus carica L.) powder co-products. Ind. Crops Prod. 2015, 69, 472–479. [Google Scholar] [CrossRef]
- Embaby, H.; Mokhtar, S.; Mostafa, A.; Gaballah, A. Effect of Lentil (Lens culinaris) Coat Powder Addition on Lipid Oxidation and Quality Characteristics of Beef Burgers Stored at 4 °C. Suez Canal Univ. J. Food Sci. 2016, 3, 35–44. [Google Scholar]
- Warner, K.; Inglett, G. Flavor and texture characteristics of foods containing Z-trim corn and oat fibers as fat and flour replacers. Cereal Foods World 1997, 42, 821–825. [Google Scholar]
- Suman, S.; Sharma, B. Effect of grind size and fat levels on the physico-chemical and sensory characteristics of low-fat ground buffalo meat patties. Meat Sci. 2003, 65, 973–976. [Google Scholar] [CrossRef]
- Rodríguez-Carpena, J.G.; Morcuende, D.; Estévez, M. Avocado by-products as inhibitors of color deterioration and lipid and protein oxidation in raw porcine patties subjected to chilled storage. Meat Sci. 2011, 89, 166–173. [Google Scholar] [CrossRef]
- Faustman, C.; Sun, Q.; Mancini, R.; Suman, S.P. Myoglobin and lipid oxidation interactions: Mechanistic bases and control. Meat Sci. 2010, 86, 86–94. [Google Scholar] [CrossRef]
- Haak, L.; Raes, K.; De Smet, S. Effect of plant phenolics, tocopherol and ascorbic acid on oxidative stability of pork patties. J. Sci. Food Agric. 2009, 89, 1360–1365. [Google Scholar] [CrossRef]
- Yu, J.; Ahmedna, M.; Goktepe, I. Potential of peanut skin phenolic extract as antioxidative and antibacterial agent in cooked and raw ground beef. Int. J. Food Sci. Technol. 2010, 45, 1337–1344. [Google Scholar] [CrossRef]
- Suman, S.P.; Faustman, C.; Stamer, S.L.; Liebler, D.C. Redox instability induced by 4-hydroxy-2-nonenal in porcine and bovine myoglobins at pH 5.6 and 4 °C. J. Agric. Food Chem. 2006, 54, 3402–3408. [Google Scholar] [CrossRef]
- Ladikos, D.; Lougovois, V. Lipid oxidation in muscle foods: A review. Food Chem. 1990, 35, 295–314. [Google Scholar] [CrossRef]
- Trujillo-Santiago, E.; Villalobos-Delgado, L.H.; Guzmán-Pantoja, L.; López, M.; Zafra-Ciprián, D.; Nevárez-Moorillón, G.; Santiago-Castro, J. The effects of Hierba Santa (Piper auritum Kunth) on the inhibition of lipid oxidation in beef burgers. LWT 2021, 146, 111428. [Google Scholar] [CrossRef]
- El-Sattar, A. Effect of natural antioxidant extracted from hulls of Vicia faba on cake shelf life. J. Food Dairy Sci. 2015, 6, 523–529. [Google Scholar] [CrossRef]
- Sohaib, M.; Anjum, F.M.; Arshad, M.S.; Imran, M.; Imran, A.; Hussain, S. Oxidative stability and lipid oxidation flavoring volatiles in antioxidants treated chicken meat patties during storage. Lipids Health Dis. 2017, 16, 27. [Google Scholar] [CrossRef] [Green Version]
- Jia, R.; Ge, S.; Ren, S.; Luo, Y.; Xiu, L.; Liu, H.; Cai, D. Antibacterial mechanism of adzuki bean seed coat polyphenols and their potential application in preservation of fresh raw beef. Int. J. Food Sci. Technol. 2021, 56, 5025–5039. [Google Scholar] [CrossRef]

| Samples | Total Phenols (mg GAE/g dw) | Total Flavonoids (QE/g Extract) |
|---|---|---|
| FBH | 82.31 ± 1.04 A | 8.42 ± 0.47 B |
| FBH-NPs | 103.14 ± 0.98 B | 10.23 ± 0.54 A |
| Samples | DPPH (µg/mL) IC50 | β-Carotene/Linoleic Acid (%) | ABTS (µmol/g de Trolox) | FRAP (µmol Fe+2/g) |
|---|---|---|---|---|
| FBH | 123.65 ± 0.42 A | 84.19 ± 0.80 B | 202.28 ± 1.25 D | 345.80 ± 0.71 D |
| FBH-NPs | 112.51 ± 0.48 B | 91.37 ± 0.84 A | 226.66 ± 1.31 C | 387.09 ± 0.68 C |
| BHA/BHT | 93.46 ± 0.55 C | 65.87 ± 0.96 D | 4274.91 ± 66.28 B | 2513.95 ± 101.30 A |
| α-tocopherol | 62.08 ± 0.19 D | 71.24 ± 0.73 C | 4773.82 ± 91.15 A | 1325.76 c ± 24.08 B |
| Treatments | 0 day | 3rd Day | 6th Day | 9th Day | 12th Day |
|---|---|---|---|---|---|
| a* | |||||
| Control | 15.23 ± 0.76 A, a | 12.60 ± 0.52 B, b | 10.47 ± 0.85 C, c | 8.36 ± 0.83 C, d | Not determined |
| BHA/BHT | 15.62 ± 0.91 A, a | 15.41 ± 0.66 A, a | 15.37 ± 0.92 A, a | 15.28 ± 0.90 A, a | 15.23 ± 0.96 A, a |
| 1% FBH-NPs | 13.84 ± 0.84 B, a | 12.70 ± 0.78 B, b | 11.41 ± 0.73 B, c | 10.43 ± 1.08 B, d | 9.52 ± 0.99 B, e |
| 1.5% FBH-NPs | 13.69 ± 0.85 B, a | 12.85 ± 0.84 B, b | 11.77 ± 0.91 B, c | 10.74 ± 1.10 B, d | 9.95 ± 0.73 B, e |
| b* | |||||
| Control | 14.10 ± 0.69 A, a | 12.71 ± 0.60 C, b | 11.59 ± 1.08 C, c | 9.22 ± 0.67 C, d | Not determined |
| BHA/BHT | 14.47 ± 1.01 A, a | 14.10 ± 0.73 A, a | 13.86 ± 0.55 A, a | 13.55 ± 0.74 A, a | 13.30 ± 0.69 A, a |
| 1% FBH-NPs | 13.85 ± 0.58 B, a | 13.25 ± 0.92 B, a | 12.87 ± 0.87 B, b | 12.48 ± 0.98 B, b | 11.88 ± 1.00 B, c |
| 1.5% FBH-NPs | 13.98 ± 0.77 B, a | 13.36 ± 1.10 B, a | 12.93 ± 0.64 B, b | 12.57 ± 0.59 B, b | 11.93 ± 0.76 B, c |
| L* | |||||
| Control | 43.28 ± 0.94 A, a | 42.74 ± 0.64 A, b | 42.17 ± 0.69 A, c | 41.10 ± 0.55 A, d | Not determined |
| BHA/BHT | 42.36 ± 0.75 B, a | 41.95 ± 1.18 A, B, a, b | 41.60 ± 0.84 B, b, c | 41.08 ± 0.80 A, c, d | 40.87 ± 0.67 A, d |
| 1% FBH-NPs | 42.68 ± 0.89 B, a | 42.14 ± 0.56 A, a | 41.88 ± 0.95 B, a | 41.35 ± 1.07 A, a | 41.07 ± 0.99 A, a |
| 1.5% FBH-NPs | 41.85 ± 1.03 C, a | 41.62 ± 0.75 B, a | 41.28 ± 0.72 B, a | 40.93 ± 0.61 A, B, a | 40.62 ± 0.58 A, a |
| Treatments | 0 Day | 3rd Day | 6th Day | 9th Day | 12th Day |
|---|---|---|---|---|---|
| Oxymyoglobin (%) | |||||
| Control | 63.25 ± 0.42 B, a | 45.49 ± 0.72 D, b | 37.92 ± 0.66 D, c | 22.71 ± 0.84 D, d | Not determined |
| BHA/BHT | 64.78 ± 0.33 A, a | 56.80 ± 0.64 A, b | 53.49 ± 0.59 A, c | 51.88 ± 0.70 A, d | 50.32 ± 0.61 A, e |
| 1% FBH-NPs | 58.23 ± 0.39 C, a | 50.54 ± 0.85 C, b | 46.63 ± 0.70 C, c | 37.64 ± 0.75 C, d | 31.07 ± 0.82 B, e |
| 1.5% FBH-NPs | 56.14 ± 0.50 D, a | 51.97 ± 0.74 B, b | 48.02 ± 0.81 B, c | 41.87 ± 0.69 B, d | 38.17 ± 0.79 A, e |
| Metmyoglobin (%) | |||||
| Control | 14.78 ± 0.48 C, d | 27.37 ± 0.60 A, c | 31.58 ± 0.56 A, b | 52.87 ± 0.63 A, a | Not determined |
| BHA/BHT | 12.40 ± 0.44 D, e | 18.20 ± 0.73 D, d | 21.67 ± 0.49 D, c | 23.79 ± 0.54 D, b | 24.96 ± 0.74 B, a |
| 1% FBH-NPs | 15.73 ± 0.72 B, e | 23.74 ± 0.59 B, d | 25.97 ± 0.66 B, c | 32.84 ± 0.70 B, b | 36.01 ± 0.41 A, a |
| 1.5% FBH-NPs | 18.76 ± 0.81 A, e | 22.63 ± 0.62 C, d | 24.09 ± 0.57 C, c | 29.74 ± 0.62 C, b | 31.10 ± 0.55 B, a |
| Treatments | 0 Day | 3rd Day | 6th Day | 9th Day | 12th Day |
|---|---|---|---|---|---|
| Total plate count (Log CFU/g) | |||||
| Control | 3.51 ± 0.21 A, d | 4.92 ± 0.31 A, c | 6.80 ± 0.35 A, b | 7.43 ± 0.41 A, a | Not determined |
| BHA/BHT | 3.50 ± 0.33 A, c | 4.02 ± 0.28 C, b | 4.98 ± 0.27 C, a | 5.18 ± 0.38 B, a | 5.42 ± 0.33 A, a |
| 1% FBH-NPs | 3.51 ± 0.27 A, d | 4.35 ± 0.26 B, c | 5.29 ± 0.24 B, b | 5.42 ± 0.32 B, a, b | 5.86 ± 0.41 A, a |
| 1.5% FBH-NPs | 3.50 ± 0.29 A, c | 4.17 ± 0.30 C, b | 5.11 ± 0.36 C, a | 5.26 ± 0.29 B, a | 5.50 ± 0.37 A, a |
| Psychrotrophic count (Log CFU/g) | |||||
| Control | Not detected | 1.67 ± 0.26 A, c | 2.43 ± 0.42 A, b | 3.17 ± 0.36 A, a | Not determined |
| BHA/BHT | Not detected | 0.87 ± 0.15 C, c | 1.04 ± 0.33 C, b | 1.56 ± 0.35 C, a | 1.88 ± 0.30 B, a |
| 1% FBH-NPs | Not detected | 1.12 ± 0.30 B, b | 1.44 ± 0.34 B, b | 1.80 ± 0.31 B, a | 2.25 ± 0.38 A, a |
| 1.5% FBH-NPs | Not detected | 0.94 ± 0.28 C, b | 1.13 ± 0.29 C, b | 1.69 ± 0.39 A, B, a | 1.99 ± 0.44 A, a |
| Lipolytic count (Log CFU/g) | |||||
| Control | Not detected | 1.52 ± 0.33 A, c | 1.96 ± 0.29 A, b | 2.37 ± 0.47 A, a | Not determined |
| BHA/BHT | Not detected | 0.77 ± 0.36 C, c | 0.95 ± 0.32 C, b | 1.12 ± 0.22 C, a, b | 1.36 ± 0.27 B, a |
| 1% FBH-NPs | Not detected | 0.96 ± 0.28 B, d | 1.23 ± 0.15 B, c | 1.61 ± 0.35 B, b | 2.07 ± 0.36 A, a |
| 1.5% FBH-NPs | Not detected | 0.84 ± 0.20 C, c | 1.04 ± 0.26 C, b | 1.25 ± 0.28 C, a, b | 1.40 ± 0.19 B, a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsebaie, E.M.; Elmahdy, A.; El-Gezawy, E.S.; Badr, M.R.; Asker, G.A.; El-Gawish, A.M.; Essa, R.Y. Effects of Faba Bean Hull Nanoparticles on Physical Properties, Protein and Lipid Oxidation, Colour Degradation, and Microbiological Stability of Burgers under Refrigerated Storage. Antioxidants 2022, 11, 938. https://doi.org/10.3390/antiox11050938
Elsebaie EM, Elmahdy A, El-Gezawy ES, Badr MR, Asker GA, El-Gawish AM, Essa RY. Effects of Faba Bean Hull Nanoparticles on Physical Properties, Protein and Lipid Oxidation, Colour Degradation, and Microbiological Stability of Burgers under Refrigerated Storage. Antioxidants. 2022; 11(5):938. https://doi.org/10.3390/antiox11050938
Chicago/Turabian StyleElsebaie, Essam Mohamed, Ahmed Elmahdy, Eman S. El-Gezawy, Mohamed Reda Badr, Galila Ali Asker, Asmaa M. El-Gawish, and Rowida Younis Essa. 2022. "Effects of Faba Bean Hull Nanoparticles on Physical Properties, Protein and Lipid Oxidation, Colour Degradation, and Microbiological Stability of Burgers under Refrigerated Storage" Antioxidants 11, no. 5: 938. https://doi.org/10.3390/antiox11050938
APA StyleElsebaie, E. M., Elmahdy, A., El-Gezawy, E. S., Badr, M. R., Asker, G. A., El-Gawish, A. M., & Essa, R. Y. (2022). Effects of Faba Bean Hull Nanoparticles on Physical Properties, Protein and Lipid Oxidation, Colour Degradation, and Microbiological Stability of Burgers under Refrigerated Storage. Antioxidants, 11(5), 938. https://doi.org/10.3390/antiox11050938





