Structural Characterization of Two Short Unspecific Peroxygenases: Two Different Dimeric Arrangements
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protein Expression and Purification
2.2. Site-Directed Mutagenesis
2.3. Crystallization, Data Collection, and Processing
2.4. Structure Determination and Refinement
2.5. Size-Exclusion Chromatography and Analytical Ultracentrifugation
2.6. Enzyme Kinetics
2.7. Fatty Acid Enzymatic Oxygenation
3. Results
3.1. Production of Native UPOs and Site-Directed Variants
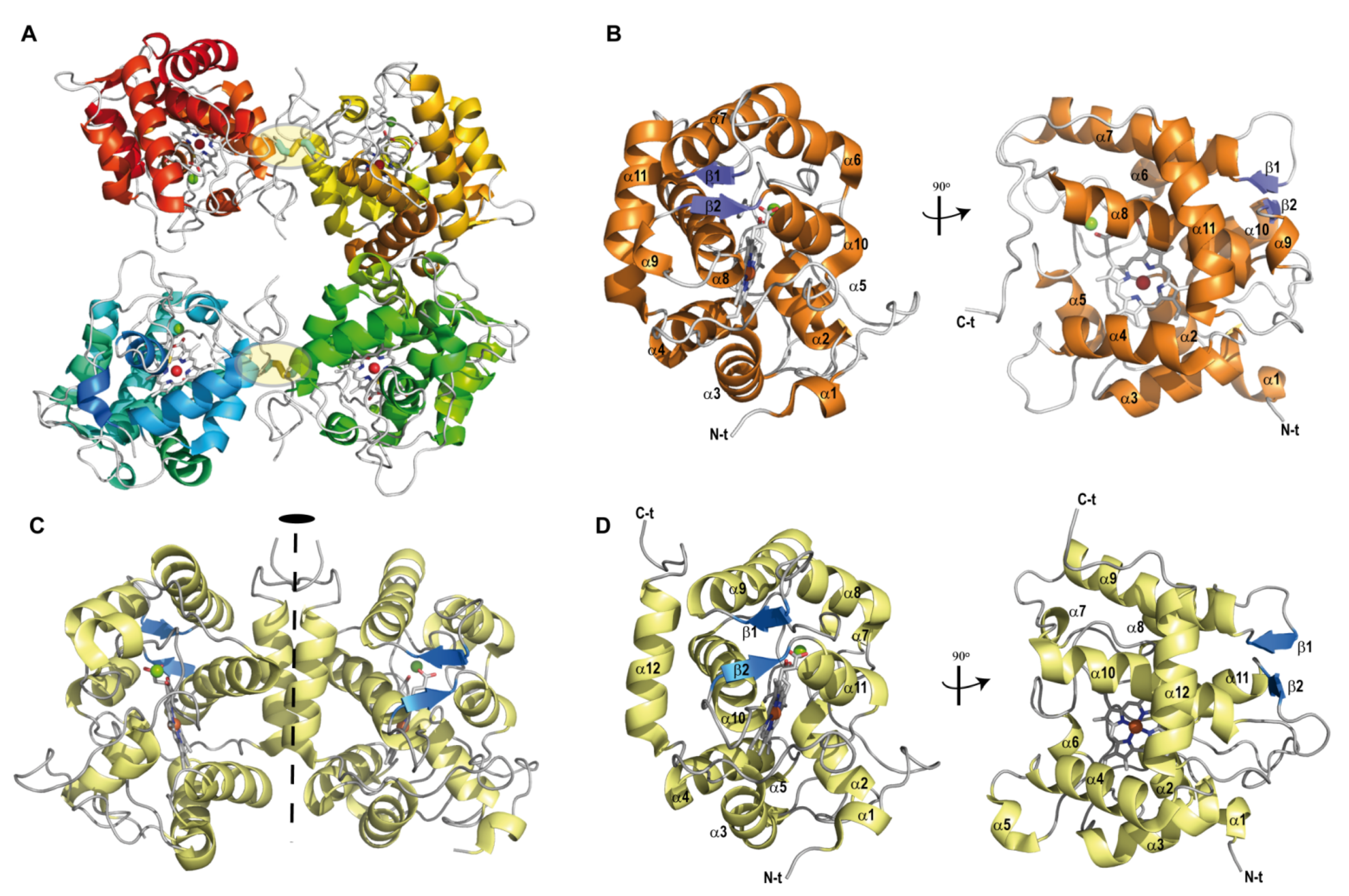
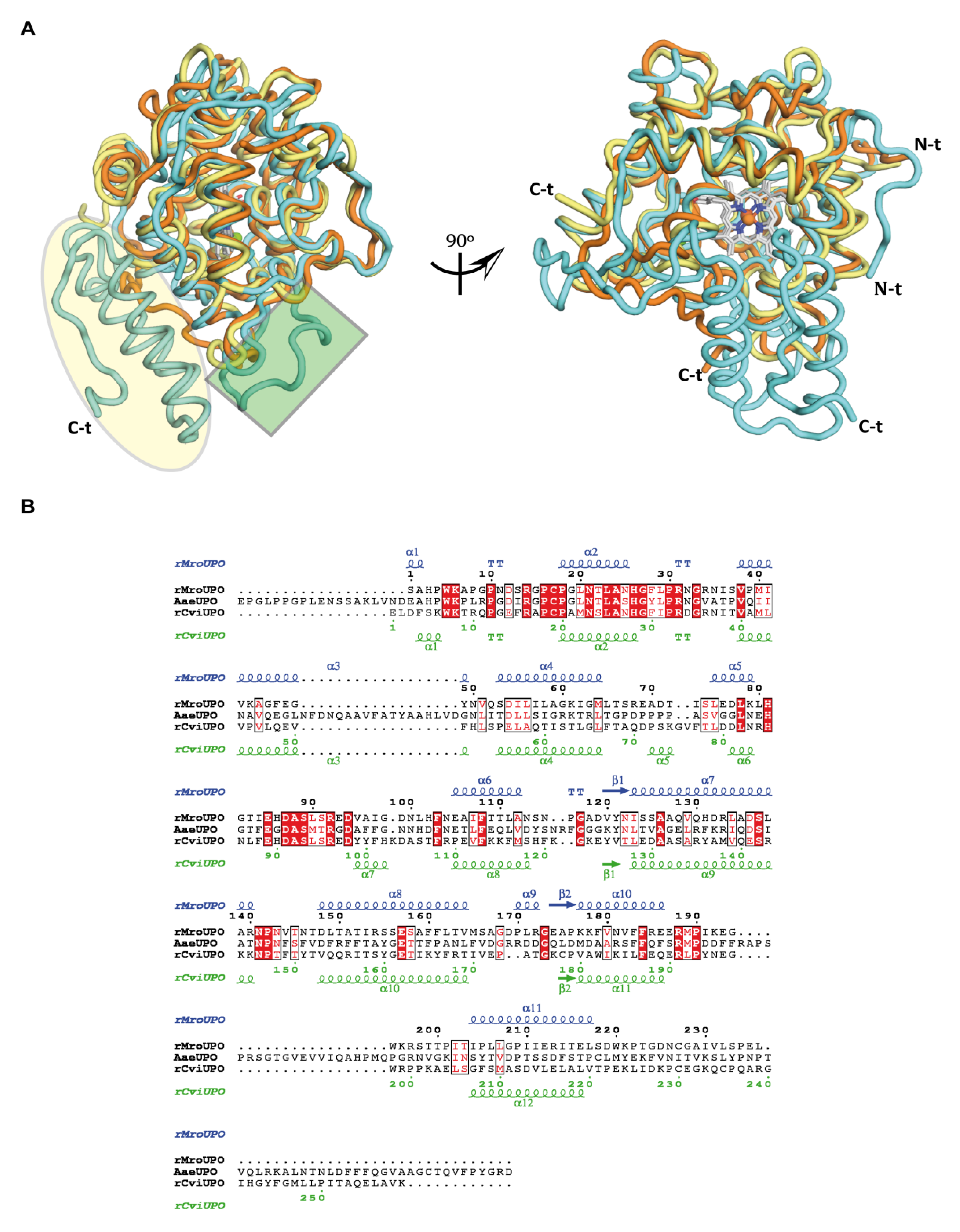
3.2. Overall Crystallographic Structures
3.3. Heme Pocket
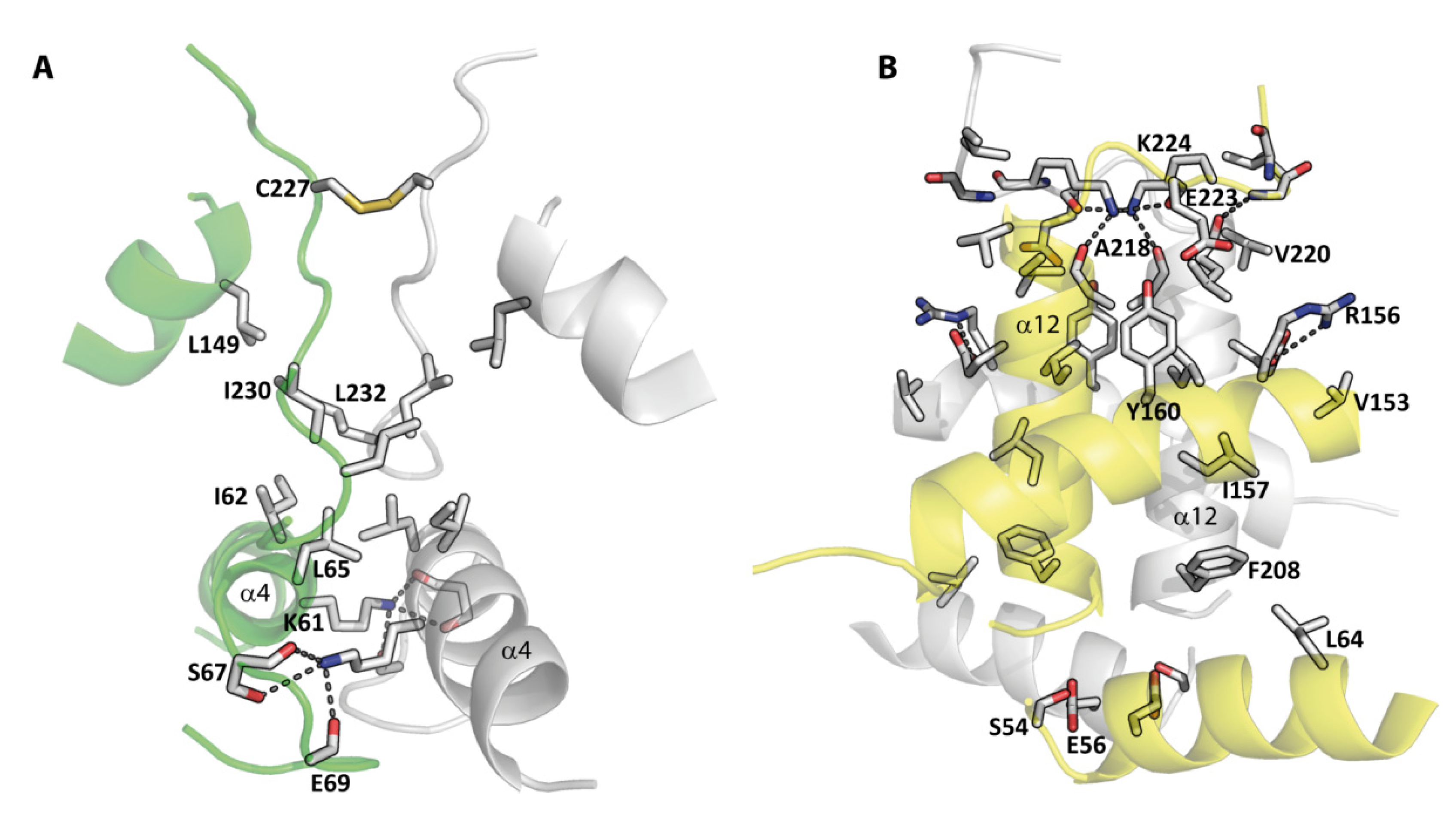
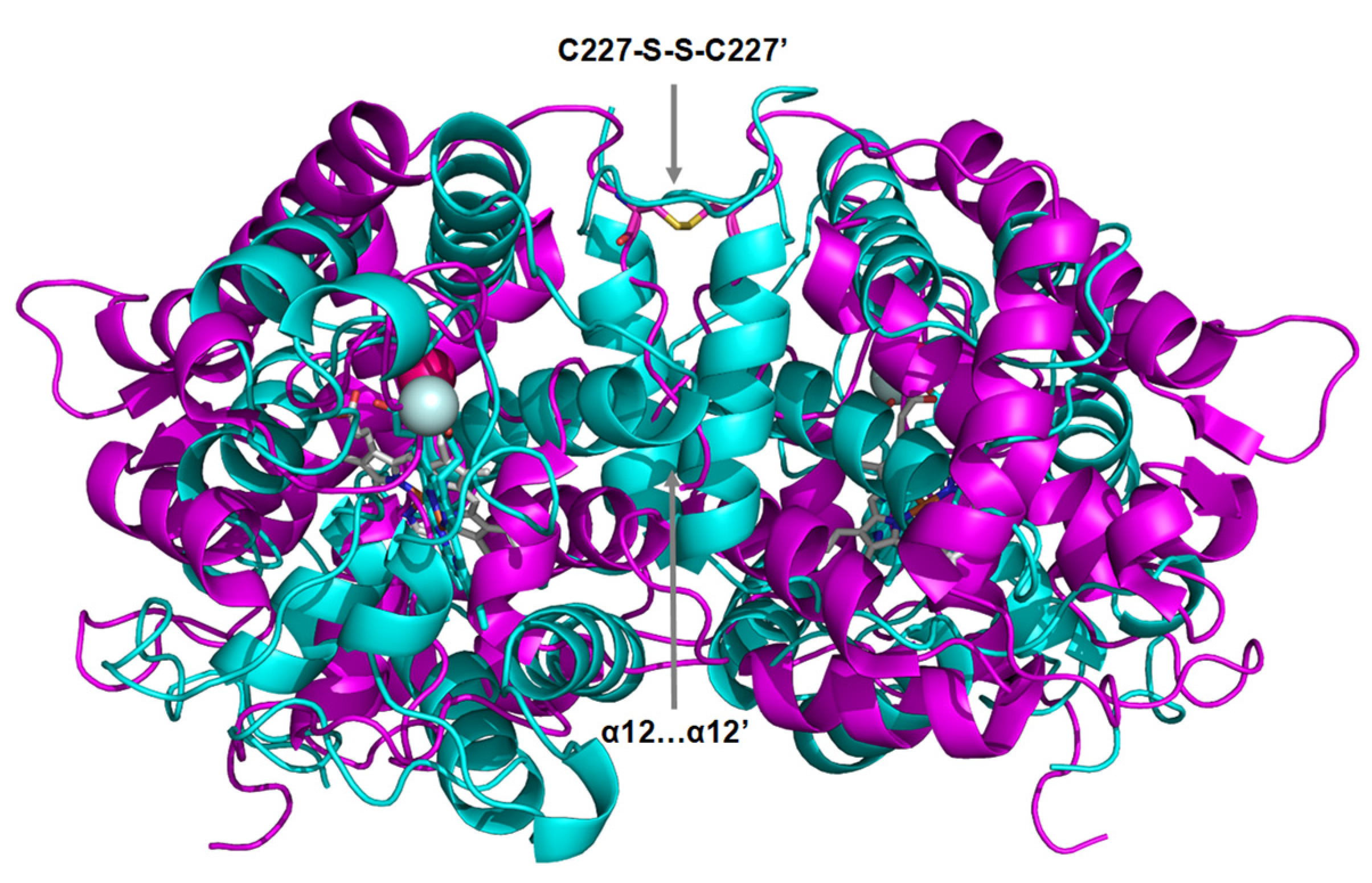
3.4. Dimeric Arrangements
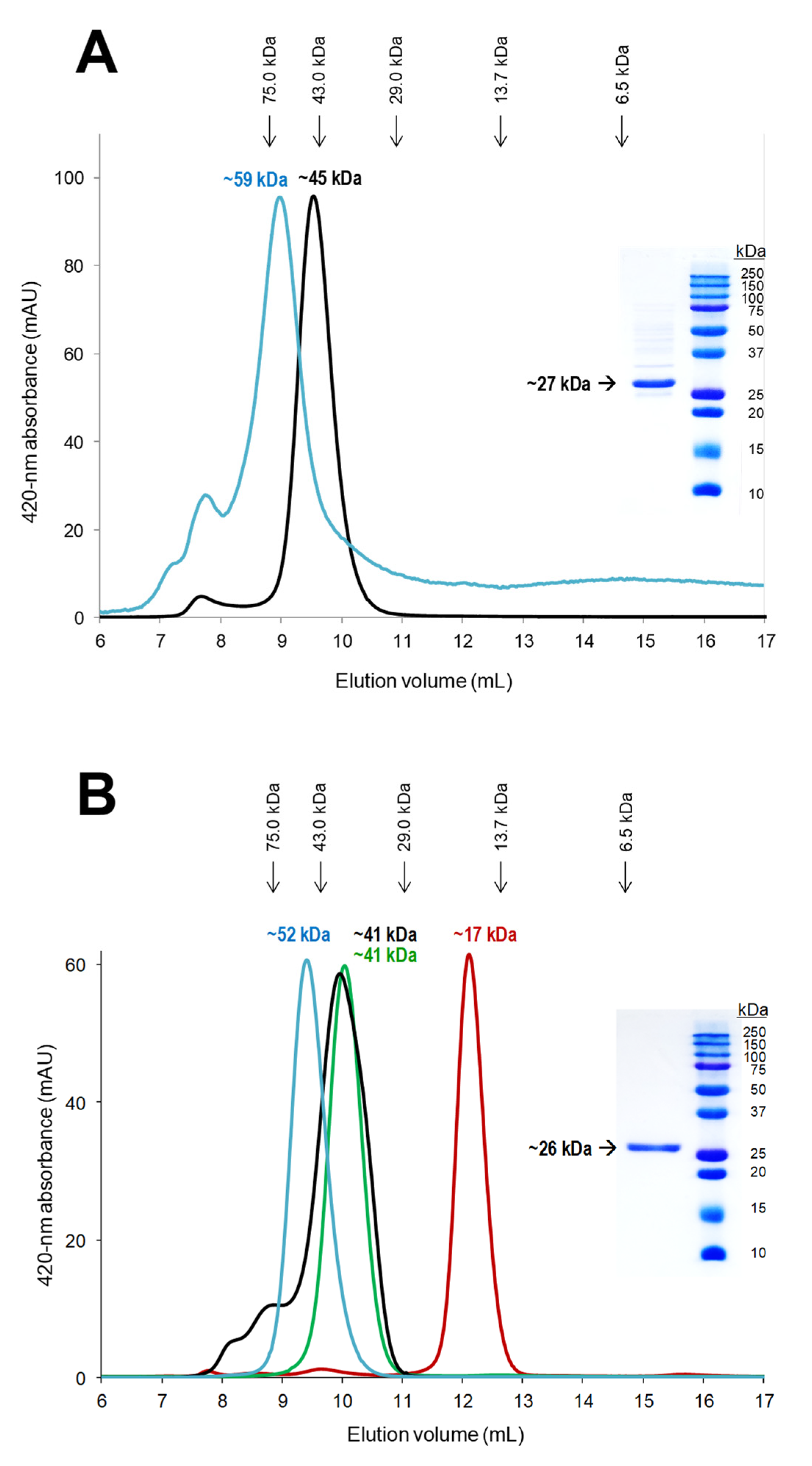
3.5. Biophysical Properties: Molecular-Mass and Oligomerization State
3.6. Comparison of Catalytic Properties
4. Discussion
4.1. Short and Long UPO Families
4.2. Two Different Dimeric Arrangements
4.3. Some (Bio)Technological Implications
4.4. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hofrichter, M.; Kellner, H.; Herzog, R.; Karich, A.; Liers, C.; Scheibner, K.; Wambui, V.; Ullrich, R. Fungal peroxygenases: A phylogenetically old superfamily of heme enzymes with promiscuity for oxygen transfer reactions. In Grand Challenges in Fungal Biotechnology; Nevalainen, H., Ed.; Springer: Cham, Switzerland, 2020; pp. 369–403. [Google Scholar]
- Ullrich, R.; Hofrichter, M. Enzymatic hydroxylation of aromatic compounds. Cell. Mol. Life Sci. 2007, 64, 271–293. [Google Scholar] [CrossRef]
- Aranda, C.; Carro, J.; González-Benjumea, A.; Babot, E.D.; Olmedo, A.; Linde, D.; Martínez, A.T.; Gutiérrez, A. Advances in enzymatic oxyfunctionalization of aliphatic compounds. Biotechnol. Adv. 2021, 51, 107703. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lan, D.; Durrani, R.; Hollmann, F. Peroxygenases en route to becoming dream catalysts. What are the opportunities and challenges? Curr. Opin. Chem. Biol. 2017, 37, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.T.; Ruiz-Dueñas, F.J.; Camarero, S.; Serrano, A.; Linde, D.; Lund, H.; Vind, J.; Tovborg, M.; Herold-Majumdar, O.M.; Hofrichter, M.; et al. Oxidoreductases on their way to industrial biotransformations. Biotechnol. Adv. 2017, 35, 815–831. [Google Scholar] [CrossRef] [Green Version]
- Grogan, G. Hemoprotein catalyzed oxygenations: P450s, UPOs, and progress toward scalable reactions. JACS Au 2021, 1, 1312–1329. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Nogal, A.; Sánchez-Moreno, I.; Méndez-Sánchez, D.; Gómez de Santos, P.; Hollmann, F.; Alcalde, M. Surfing the wave of oxyfunctionalization chemistry by engineering fungal unspecific peroxygenases. Curr. Opin. Struct. Biol. 2022, 73, 102342. [Google Scholar] [CrossRef]
- Ortiz de Montellano, P.R. Cytochrome P450: Structure, Mechanism, and Biochemistry; Springer: New York, NY, USA, 2015; p. 912. [Google Scholar]
- Hofrichter, M.; Kellner, H.; Pecyna, M.J.; Ullrich, R. Fungal unspecific peroxygenases: Heme-thiolate proteins that combine peroxidase and cytochrome P450 properties. Adv. Exp. Med. Biol. 2015, 851, 341–368. [Google Scholar]
- Poulos, T.L.; Finzel, B.C.; Howard, A.J. High-resolution crystal structure of cytochrome P450cam. J. Mol. Biol. 1987, 195, 687–700. [Google Scholar] [CrossRef]
- Burley, S.K.; Bhikadiya, C.; Bi, C.; Bittrich, S.; Chen, L.; Crichlow, G.V.; Duarte, J.M.; Dutta, S.; Fayazi, M.; Feng, Z.; et al. RCSB Protein Data Bank: Celebrating 50 years of the PDB with new tools for understanding and visualizing biological macromolecules in 3D. Protein Sci. 2022, 31, 187–208. [Google Scholar] [CrossRef]
- Hofrichter, M.; Kellner, H.; Herzog, R.; Karich, A.; Kiebist, J.; Scheibner, K.; Ullrich, R. Peroxide-mediated oxygenation of organic compounds by fungal peroxygenases. Antioxidants 2022, 11, 163. [Google Scholar] [CrossRef]
- Piontek, K.; Ullrich, R.; Liers, C.; Diederichs, K.; Plattner, D.A.; Hofrichter, M. Crystallization of a 45 kDa peroxygenase/peroxidase from the mushroom Agrocybe aegerita and structure determination by SAD utilizing only the haem iron. Acta Crystallogr. F 2010, 66, 693–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piontek, K.; Strittmatter, E.; Ullrich, R.; Gröbe, G.; Pecyna, M.J.; Kluge, M.; Scheibner, K.; Hofrichter, M.; Plattner, D.A. Structural basis of substrate conversion in a new aromatic peroxygenase: Cytochrome P450 functionality with benefits. J. Biol. Chem. 2013, 288, 34767–34776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramírez-Escudero, M.; Molina-Espeja, P.; Gómez de Santos, P.; Hofrichter, M.; Sanz-Aparicio, J.; Alcalde, M. Structural insights into the substrate promiscuity of a laboratory evolved peroxygenase. ACS Chem. Biol. 2018, 13, 3259–3268. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, R.; Nuske, J.; Scheibner, K.; Spantzel, J.; Hofrichter, M. Novel haloperoxidase from the agaric basidiomycete Agrocybe aegerita oxidizes aryl alcohols and aldehydes. Appl. Environ. Microbiol. 2004, 70, 4575–4581. [Google Scholar] [CrossRef] [Green Version]
- Rotilio, L.; Swoboda, A.; Ebner, K.; Rinnofner, C.; Glieder, A.; Kroutil, W.; Mattevi, A. Structural and biochemical studies enlighten the unspecific peroxygenase from Hypoxylon sp. EC38 as an efficient oxidative biocatalyst. ACS Catal. 2021, 11, 11511–11525. [Google Scholar] [CrossRef]
- Sundaramoorthy, M.; Terner, J.; Poulos, T.L. The crystal structure of chloroperoxidase: A heme peroxidase-cytochrome P450 functional hybrid. Structure 1995, 3, 1367–1377. [Google Scholar] [CrossRef] [Green Version]
- Sundaramoorthy, M.; Terner, J.; Poulos, T.L. Stereochemistry of the chloroperoxidase active site: Crystallographic and molecular-modeling studies. Chem. Biol. 1998, 5, 461–473. [Google Scholar] [CrossRef] [Green Version]
- Anh, D.H.; Ullrich, R.; Benndorf, D.; Svatos, A.; Muck, A.; Hofrichter, M. The coprophilous mushroom Coprinus radians secretes a haloperoxidase that catalyzes aromatic peroxygenation. Appl. Environ. Microbiol. 2007, 73, 5477–5485. [Google Scholar] [CrossRef] [Green Version]
- Anh, D.H. Novel Extracellular Haloperoxidase-Peroxygenases from the Coprophilous Fungi Coprinus radians and Coprinus verticillatus: Production, Purification and Biochemical Characterization. Ph.D. Thesis, International Graduate School of Zittau, Zittau, Germany, 2008. [Google Scholar]
- Gröbe, G.; Ullrich, M.; Pecyna, M.; Kapturska, D.; Friedrich, S.; Hofrichter, M.; Scheibner, K. High-yield production of aromatic peroxygenase by the agaric fungus Marasmius rotula. AMB Express 2011, 1, 31–42. [Google Scholar] [CrossRef] [Green Version]
- Ullrich, R.; Poraj-Kobielska, M.; Scholze, S.; Halbout, C.; Sandvoss, M.; Pecyna, M.J.; Scheibner, K.; Hofrichter, M. Side chain removal from corticosteroids by unspecific peroxygenase. J. Inorg. Biochem. 2018, 183, 84–93. [Google Scholar] [CrossRef]
- Kimani, V.W. New Secretory Peroxidases and Peroxygenases from Saprotrophic Fungi of Kenyan Forests. Ph.D. Thesis, TU Dresden, Zittau, Germany, 2019. [Google Scholar]
- Kiebist, J.; Schmidtke, K.U.; Zimmermann, J.; Kellner, H.; Jehmlich, N.; Ullrich, R.; Zänder, D.; Hofrichter, M.; Scheibner, K. A peroxygenase from Chaetomium globosum catalyzes the selective oxygenation of testosterone. ChemBioChem 2017, 18, 563–569. [Google Scholar] [CrossRef] [Green Version]
- Molina-Espeja, P.; Garcia-Ruiz, E.; Gonzalez-Perez, D.; Ullrich, R.; Hofrichter, M.; Alcalde, M. Directed evolution of unspecific peroxygenase from Agrocybe aegerita. Appl. Environ. Microbiol. 2014, 80, 3496–3507. [Google Scholar] [CrossRef] [Green Version]
- Molina-Espeja, P.; Ma, S.; Maté, D.M.; Ludwig, R.; Alcalde, M. Tandem-yeast expression system for engineering and producing unspecific peroxygenase. Enzyme Microb. Technol. 2015, 73–74, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Püllmann, P.; Knorrscheidt, A.; Münch, J.; Palme, P.R.; Hoehenwarter, W.; Marillonnet, S.; Alcalde, M.; Westermann, B.; Weissenborn, M.J. A modular two yeast species secretion system for the production and preparative application of unspecific peroxygenases. Commun. Biol. 2021, 4, 562. [Google Scholar] [CrossRef]
- Knorrscheidt, A.; Soler, J.; Hünecke, N.; Püllmann, P.; Garcia-Borràs, M.; Weissenborn, M.J. Accessing chemo- and regioselective benzylic and aromatic oxidations by protein engineering of an unspecific peroxygenase. ACS Catal. 2021, 11, 7327–7338. [Google Scholar] [CrossRef] [PubMed]
- Babot, E.D.; del Río, J.C.; Kalum, L.; Martínez, A.T.; Gutiérrez, A. Oxyfunctionalization of aliphatic compounds by a recombinant peroxygenase from Coprinopsis cinerea. Biotechnol. Bioeng. 2013, 110, 2332. [Google Scholar] [CrossRef] [Green Version]
- Bormann, S.; Kellner, H.; Hermes, J.; Herzog, R.; Ullrich, R.; Liers, C.; Ulber, R.; Hofrichter, M.; Holtmann, D. Broadening the biocatalytic toolbox—Screening and expression of new unspecific peroxygenases. Antioxidants 2022, 11, 223. [Google Scholar] [CrossRef]
- Fernández-Fueyo, E.; Aranda, C.; Gutiérrez, A.; Martínez, A.T. Method of Heterologous Expression of Active Fungal Unspecific Peroxygenase in Bacterial Host Cells for Fatty-Acid Epoxidation and Other Oxygenation Reactions. European Patent EP18382514.0, 10 July 2018. [Google Scholar]
- Linde, D.; Olmedo, A.; González-Benjumea, A.; Renau, C.; Estévez, M.; Carro, J.; Fernández-Fueyo, E.; Gutiérrez, A.; Martínez, A.T. Two new unspecific peroxygenases from heterologous expression of fungal genes in Escherichia coli. Appl. Environ. Microbiol. 2020, 86, e02899-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carro, J.; González-Benjumea, A.; Fernández-Fueyo, E.; Aranda, C.; Guallar, V.; Gutiérrez, A.; Martínez, A.T. Modulating fatty acid epoxidation vs. hydroxylation in a fungal peroxygenase. ACS Catal. 2019, 9, 6234–6242. [Google Scholar] [CrossRef] [Green Version]
- Municoy, M.; González-Benjumea, A.; Carro, J.; Aranda, C.; Linde, D.; Renau-Mínguez, C.; Ullrich, R.; Hofrichter, M.; Guallar, V.; Gutiérrez, A.; et al. Fatty-acid oxygenation by fungal peroxygenases: From computational simulations to preparative regio- and stereo-selective epoxidation. ACS Catal. 2020, 10, 13584–13595. [Google Scholar] [CrossRef]
- González-Benjumea, A.; Carro, J.; Renau, C.; Linde, D.; Fernández-Fueyo, E.; Gutiérrez, A.; Martínez, A.T. Fatty acid epoxidation by Collariella virescens peroxygenase and heme-channel variants. Catal. Sci. Technol. 2020, 10, 717–725. [Google Scholar] [CrossRef] [Green Version]
- González-Benjumea, A.; Linde, D.; Carro, J.; Ullrich, R.; Hofrichter, M.; Martínez, A.T.; Gutiérrez, A. Regioselective and stereoselective epoxidation of n-3 and n-6 fatty acids by fungal peroxygenases. Antioxidants 2021, 10, 1888. [Google Scholar] [CrossRef] [PubMed]
- Lund, H.; Kalum, L.; Hofrichter, M.; Peter, S. Epoxidation Using Peroxygenase. Patent (USA) US 9908860 B2, 6 March 2018. [Google Scholar]
- Puigbò, P.; Guzmán, E.; Romeu, A.; Garcia-Vallvé, S. OPTIMIZER: A web server for optimizing the codon usage of DNA sequences. Nucleic Acids Res. 2007, 35, W126–W131. [Google Scholar] [CrossRef] [Green Version]
- Studier, F.W. Protein production by auto-induction in high-density shaking cultures. Protein Express Purif. 2005, 41, 207–234. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Kabsch, W. XDS. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 125–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, P.R.; Murshudov, G.N. How good are my data and what is the resolution? Acta Crystallogr. Sect. D 2013, 69, 1204–1214. [Google Scholar] [CrossRef]
- McCoy, A.J.; Grosse-Kunstleve, R.W.; Adams, P.D.; Winn, M.D.; Storoni, L.C.; Read, R.J. Phaser crystallographic software. J. Appl. Cryst. 2007, 40, 658–674. [Google Scholar] [CrossRef] [Green Version]
- Murshudov, G.N.; Skubak, P.; Lebedev, A.A.; Pannu, N.S.; Steiner, R.A.; Nicholls, R.A.; Winn, M.D.; Long, F.; Vagin, A.A. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr. Sect. D 2011, 67, 355–367. [Google Scholar] [CrossRef] [Green Version]
- Emsley, P.; Lohkamp, B.; Scott, W.G.; Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 486–501. [Google Scholar] [CrossRef] [Green Version]
- Schrödinger. The PyMOL Molecular Graphics System, Version 2.0; Schrödinger, LLC: New York, NY, USA, 2017; Available online: https://pymol.org (accessed on 1 March 2022).
- Guex, N.; Peitsch, M.C. SWISS-MODEL and the Swiss-Pdb Viewer: An environment for comparative protein modeling. Electrophoresis 1997, 18, 2714–2723. [Google Scholar] [CrossRef] [PubMed]
- Krissinel, E. Crystal contacts as nature’s docking solutions. J. Comp. Chem. 2010, 31, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Chen, V.B.; Arendall, W.B.; Headd, J.J.; Keedy, D.A.; Immormino, R.M.; Kapral, G.J.; Murray, L.W.; Richardson, J.S.; Richardson, D.C. MolProbity: All-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 12–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Židek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Mirdita, M.; Ovchinnikov, S.; Steinegger, M. ColabFold—Making protein folding accessible to all. bioRxiv 2022. [Google Scholar] [CrossRef]
- Schuck, P.; Rossmanith, P. Determination of the sedimentation coefficient distribution by least-squares boundary modeling. Biopolymers 2000, 54, 328–341. [Google Scholar] [CrossRef]
- Maqueda, F. Oxyfunctionalization of Organic Compounds by an Enzyme with Peroxygenase Activity. Master’s Thesis, UCM, Madrid, Spain, 2018. [Google Scholar]
- Hofrichter, M.; Ullrich, R. Oxidations catalyzed by fungal peroxygenases. Curr. Opin. Chem. Biol. 2014, 19, 116–125. [Google Scholar] [CrossRef]
- Lucas, F.; Babot, E.D.; del Río, J.C.; Kalum, L.; Ullrich, R.; Hofrichter, M.; Guallar, V.; Martínez, A.T.; Gutiérrez, A. Molecular determinants for selective C25-hydroxylation of vitamins D2 and D3 by fungal peroxygenases. Catal. Sci. Technol. 2016, 6, 288–295. [Google Scholar] [CrossRef] [Green Version]
- Olmedo, A.; del Río, J.C.; Kiebist, J.; Scheibner, K.; Martínez, A.T.; Gutiérrez, A. Fatty acid chain shortening by a fungal peroxygenase. Chem. Eur. J. 2017, 23, 16985–16989. [Google Scholar] [CrossRef] [Green Version]
- Aranda, C.; Ullrich, R.; Kiebist, J.; Scheibner, K.; del Río, J.C.; Hofrichter, M.; Martínez, A.T.; Gutiérrez, A. Selective synthesis of the resveratrol analogue 4,4’-dihydroxy-trans-stilbene and stilbenoids modification by fungal peroxygenases. Catal. Sci. Technol. 2018, 8, 2394–2401. [Google Scholar] [CrossRef] [Green Version]
- Aranda, C.; Municoy, M.; Guallar, V.; Kiebist, J.; Scheibner, K.; Ullrich, R.; del Río, J.C.; Hofrichter, M.; Martínez, A.T.; Gutiérrez, A. Selective synthesis of 4-hydroxyisophorone and 4-ketoisophorone by fungal peroxygenases. Catal. Sci. Technol. 2019, 9, 1398–1405. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-López, J.N.; Lowe, D.J.; Hernández-Ruíz, J.; Hiner, A.N.P.; García-Canovas, F.; Thorneley, R.N.F. Mechanism of reaction of hydrogen peroxide with horseradish peroxidase: Identification of intermediates in the catalytic cycle. J. Am. Chem. Soc. 2001, 123, 11838–11847. [Google Scholar] [CrossRef] [PubMed]
- Howlett, G.J.; Minton, A.P.; Rivas, G. Analytical ultracentrifugation for the study of protein association and assembly. Curr. Opin. Chem. Biol. 2006, 10, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, A.; Textor, L.C.; Santos, J.C.; Cuadrado, N.H.; Kostetsky, E.Y.; Roig, M.G.; Bavro, V.N.; Muniz, J.R.; Shnyrov, V.L.; Polikarpov, I. Crystal structure analysis of peroxidase from the palm tree Chamaerops excelsa. Biochimie 2015, 111, 58–69. [Google Scholar] [CrossRef]
- Biermann, U.; Friedt, W.; Lang, S.; Lühs, W.; Machmüller, G.; Metzger, U.O.; Klaas, M.R.; Schäfer, H.J.; Schneider, M.P. New syntheses with oils and fats as renewable raw materials for the chemical industry. In Biorefineries-Industrial Processes and Products; Kamm, B., Gruber, P.R., Kamm, M., Eds.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2006; pp. 253–289. [Google Scholar]
- Corma, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Aranda, C.; Olmedo, A.; Kiebist, J.; Scheibner, K.; del Río, J.C.; Martínez, A.T.; Gutiérrez, A. Selective epoxidation of fatty acids and fatty acid methyl esters by fungal peroxygenases. ChemCatChem 2018, 10, 3964–3968. [Google Scholar] [CrossRef] [Green Version]
- Prileschajew, N. Oxydation ungesättigter Verbindungen mittels organischer Superoxyde. Ber. Dtsch. Chem. Ges. 1909, 42, 4811–4815. [Google Scholar] [CrossRef] [Green Version]
- Björkling, F.; Frykman, H.; Godtfredsen, S.E.; Kirk, O. Lipase catalyzed synthesis of peroxycarboxylic acids and lipase mediated oxidations. Tetrahedron 1992, 48, 4587–4592. [Google Scholar] [CrossRef]


| rMroUPO | rCviUPO | |
|---|---|---|
| Wavelength (Å) | 0.9792 | 0.9792 |
| Space group | C2221 | P212121 |
| Unit cell dimensions: | ||
| a | 100.3 Å | 78.5 Å |
| b | 107.4 Å | 140.1 Å |
| c | 186.2 Å | 42.2 Å |
| Resolution (Å) | 73.3–1.45 | 70.1–1.95 |
| Rmeas a | 0.094 (0.81) | 0.191 (0.99) |
| Rpim | 0.037 (0.33) | 0.057 (0.40) |
| I/σI | 12.0 (2.3) | 10.1 (2.4) |
| Completeness (%) | 99.4 (99.5) | 100 (100) |
| Redundancy | 6.1 (5.8) | 10.6 (6.2) |
| CC1/2 b (%) | 99.7 (66.2) | 99.4 (83.5) |
| rMroUPO | rCviUPO | |
|---|---|---|
| Refinement: | ||
| Resolution (Å) | 73.3–1.45 | 70.1–1.95 |
| No. unique reflections | 167,101 (12,200) | 33,334 (2439) |
| Rwork/Rfree | 0.158/0.185 | 0.191/0.243 |
| No. atoms: | ||
| Protein | 7276 | 3664 |
| Ligand | 172 | 86 |
| Ion | 4 | 2 |
| Water | 1066 | 268 |
| B-factors (Å2): | ||
| Protein | 15.7 | 26.0 |
| Ligand | 12.6 | 20.3 |
| Ion | 8.38 | 14.97 |
| Water | 28.54 | 34.1 |
| R.m.s deviations: | ||
| Bond lengths (Å) | 0.013 | 0.010 |
| Bond angles (°) | 1.85 | 1.53 |
| Ramachandran: | ||
| Favored (%) | 96.41 | 94.21 |
| Allowed (%) | 3.17 | 5.57 |
| Outliers (%) | 0.42 | 0.22 |
| PDB codes: | 7ZBP | 7ZCL |
| MroUPO | rMroUPO | C227A | rCviUPO | C235A | K228stop | ||
|---|---|---|---|---|---|---|---|
| Veratryl alcohol | Km | 279 | 54.2 ± 16.7 | - | 2940 ± 160 | 7240 ± 820 | 55,300 ± 10,000 |
| kcat | 49 | 2.49 ± 0.16 | 0 | 2.24 ± 0.03 | 11.2 ± 0.4 | 56.4 ± 6.6 | |
| kcat/Km | 176 | 46 ± 12 | - | 0.75 ± 0.03 | 1.5 ± 0.2 | 1.0 ± 0.2 | |
| ABTS | Km | 71 | 246 ± 3 | - | 239 ± 8 1 | 87 ± 7 | 110 ± 15 |
| kcat | 25 | 15.0 ± 3.3 | 0 | 157.2 ± 2.8 | 257.0 ± 4.9 | 209 ± 7 | |
| kcat/Km | 350 | 62 ± 14 | - | 656 ± 26 | 2970 ± 190 | 1900 ± 210 | |
| DMP | Km | 133 | 206 ± 38 | - | 4930 ± 470 | 4500 ± 424 | 3720 ± 250 |
| kcat | 70 | 66.2 ± 3.1 | 0 | 325 ± 11 | 812 ± 26 | 728 ± 16 | |
| kcat/Km | 530 | 320 ± 50 | - | 66 ± 5 | 180 ± 12 | 195 ± 10 | |
| H2O2 | Km | 3140 | 1880 ± 130 | - | 2250 ± 750 2 | 420 ± 6 3 | 680 ± 120 4 |
| kcat/Km | 24.2 | 25.8 ± 0.4 | - | 1120 ± 475 | 5170 ± 130 | 3930 ± 170 |
| Conversion | Products | (%) | Epoxidation | ||||
|---|---|---|---|---|---|---|---|
| (%) | 12-Epoxy | 9-Epoxy | Diepoxy | Hydroxy | OH-Epoxy | Yield (%) | |
| rCviUPO | 97 | 56 | 10 | - | 8 | 26 | 45 |
| K228stop | 98 | 14 | 16 | 51 | 13 | 6 | 68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linde, D.; Santillana, E.; Fernández-Fueyo, E.; González-Benjumea, A.; Carro, J.; Gutiérrez, A.; Martínez, A.T.; Romero, A. Structural Characterization of Two Short Unspecific Peroxygenases: Two Different Dimeric Arrangements. Antioxidants 2022, 11, 891. https://doi.org/10.3390/antiox11050891
Linde D, Santillana E, Fernández-Fueyo E, González-Benjumea A, Carro J, Gutiérrez A, Martínez AT, Romero A. Structural Characterization of Two Short Unspecific Peroxygenases: Two Different Dimeric Arrangements. Antioxidants. 2022; 11(5):891. https://doi.org/10.3390/antiox11050891
Chicago/Turabian StyleLinde, Dolores, Elena Santillana, Elena Fernández-Fueyo, Alejandro González-Benjumea, Juan Carro, Ana Gutiérrez, Angel T. Martínez, and Antonio Romero. 2022. "Structural Characterization of Two Short Unspecific Peroxygenases: Two Different Dimeric Arrangements" Antioxidants 11, no. 5: 891. https://doi.org/10.3390/antiox11050891
APA StyleLinde, D., Santillana, E., Fernández-Fueyo, E., González-Benjumea, A., Carro, J., Gutiérrez, A., Martínez, A. T., & Romero, A. (2022). Structural Characterization of Two Short Unspecific Peroxygenases: Two Different Dimeric Arrangements. Antioxidants, 11(5), 891. https://doi.org/10.3390/antiox11050891








