The Vascular Niche for Adult Cardiac Progenitor Cells
Abstract
1. Introduction
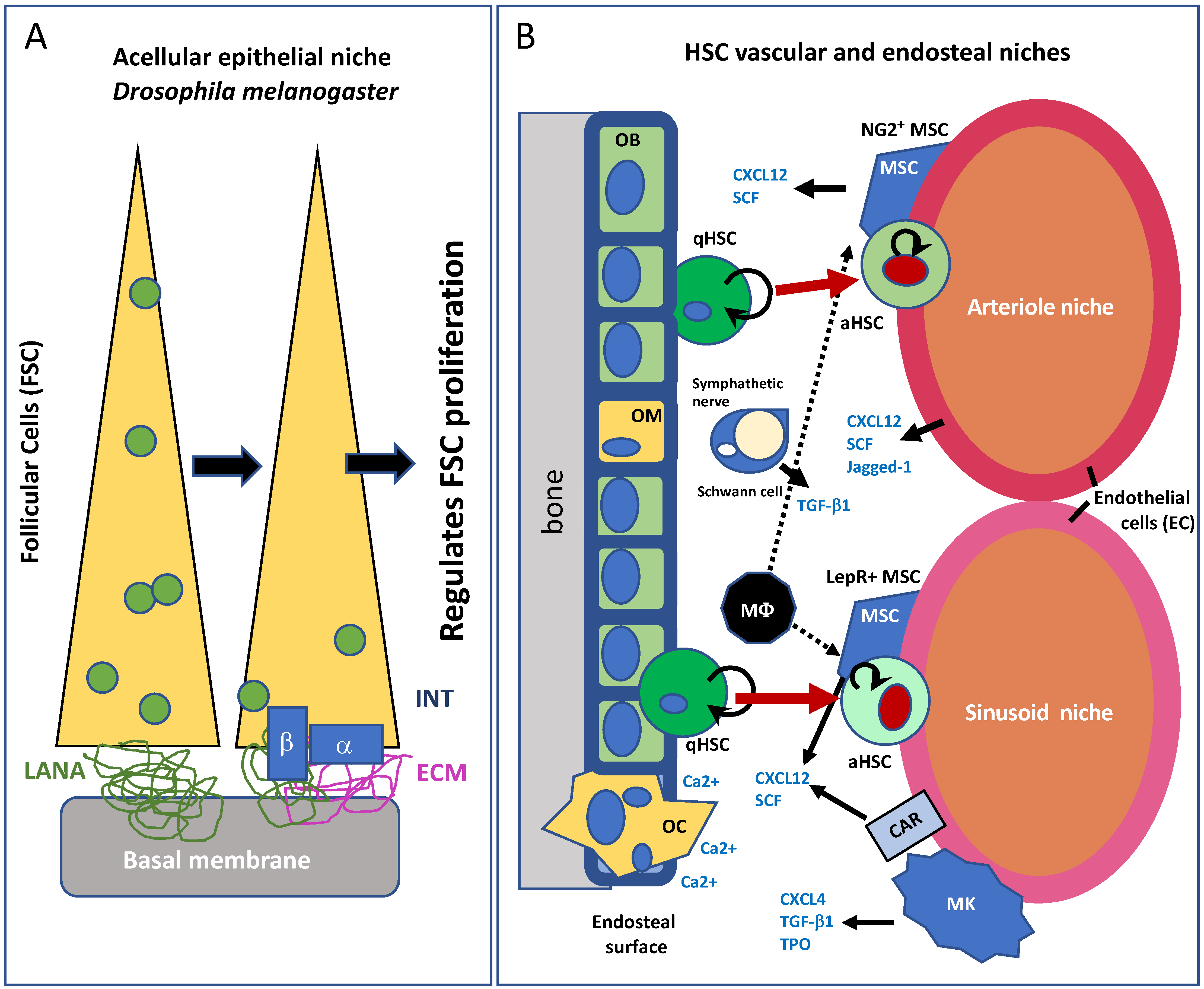
2. The Adult Progenitor Cell Niche
3. Translational Relevance of the Stem Cell Niche
4. Cardiac Cell Turnover: A Critical Overview
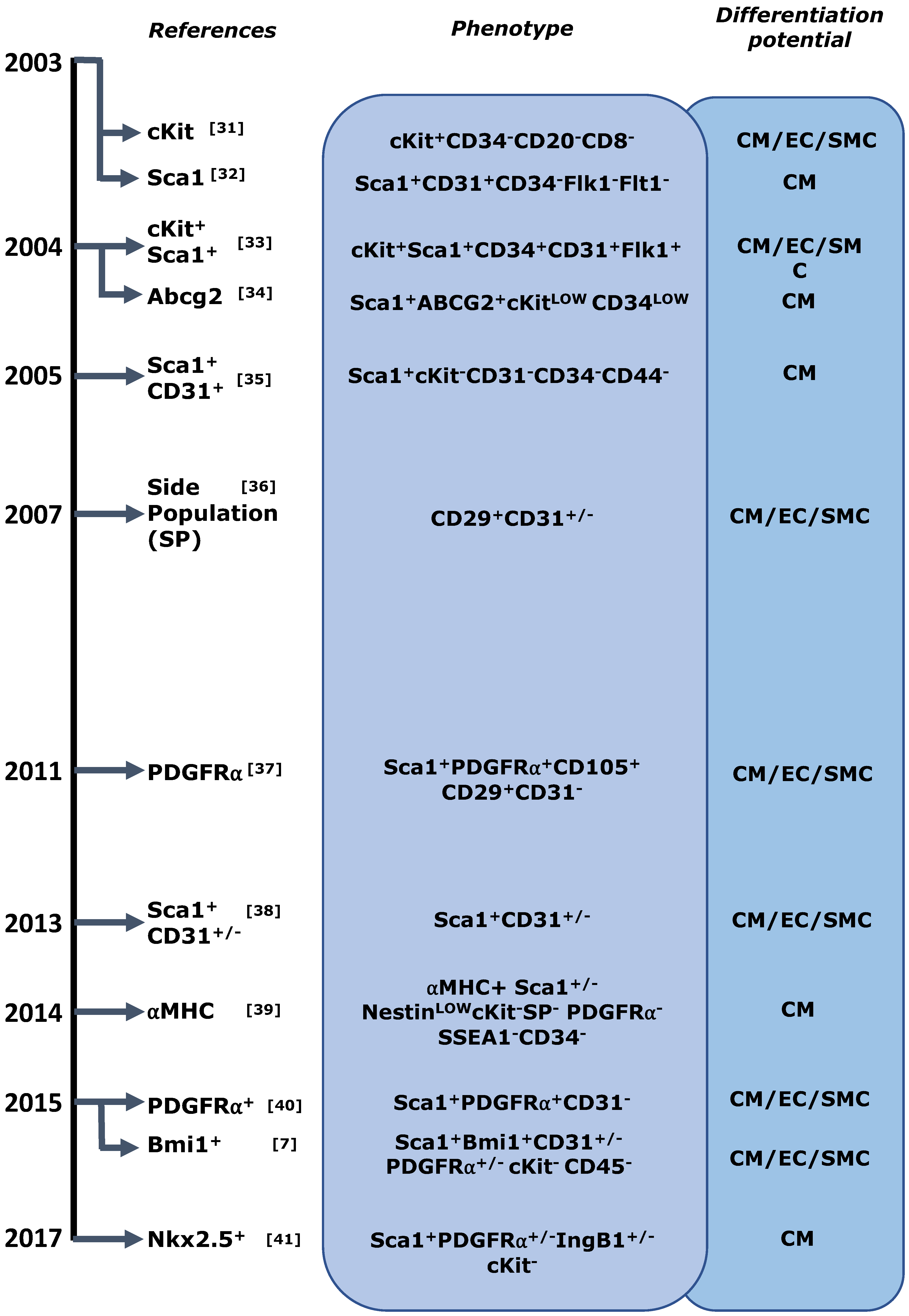
4.1. Cardiac Stem/Progenitor Cell Identification and Its Caveats
4.2. Progenitor Cell Interactions in the Adult Heart and Alternative Sources of Heart Turnover
5. The Cardiac Stem Cell Niche in the Adult Heart
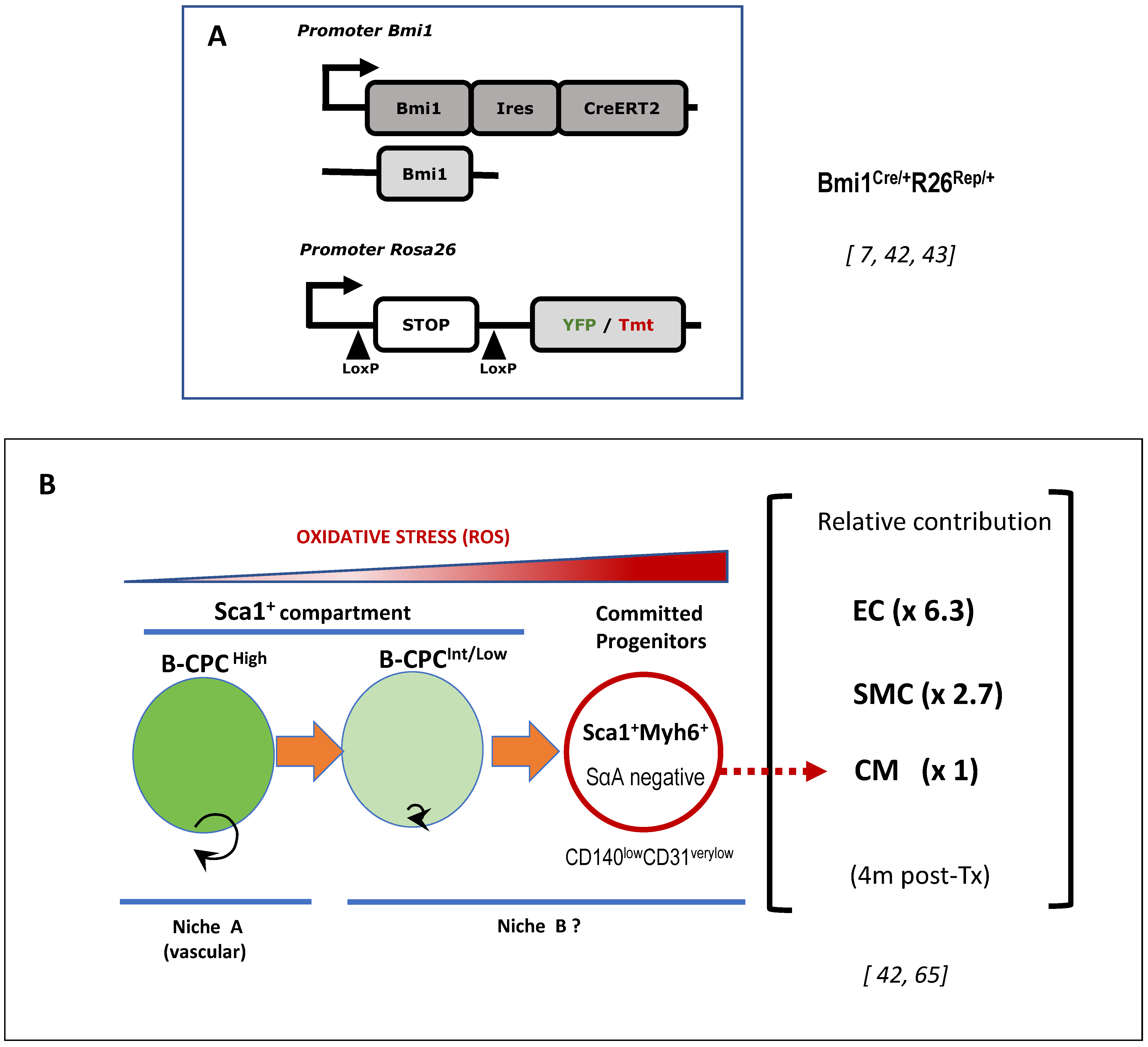
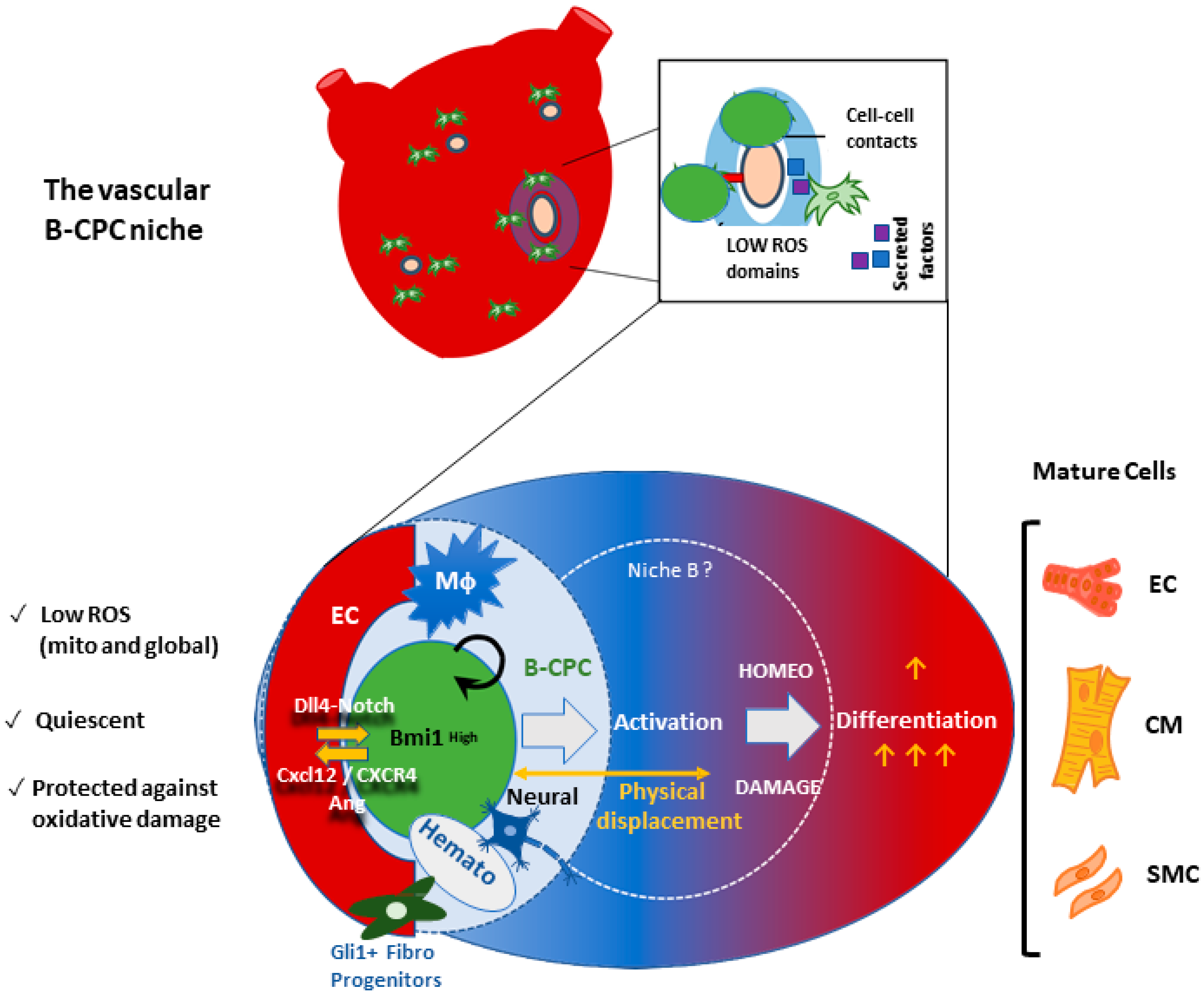
6. The Bmi1+ Cardiac Progenitor Cell Niche in the Adult Heart
6.1. Adult Cardiac Multipotent Progenitor Cells Are Characterized by the High Expression of Bmi1
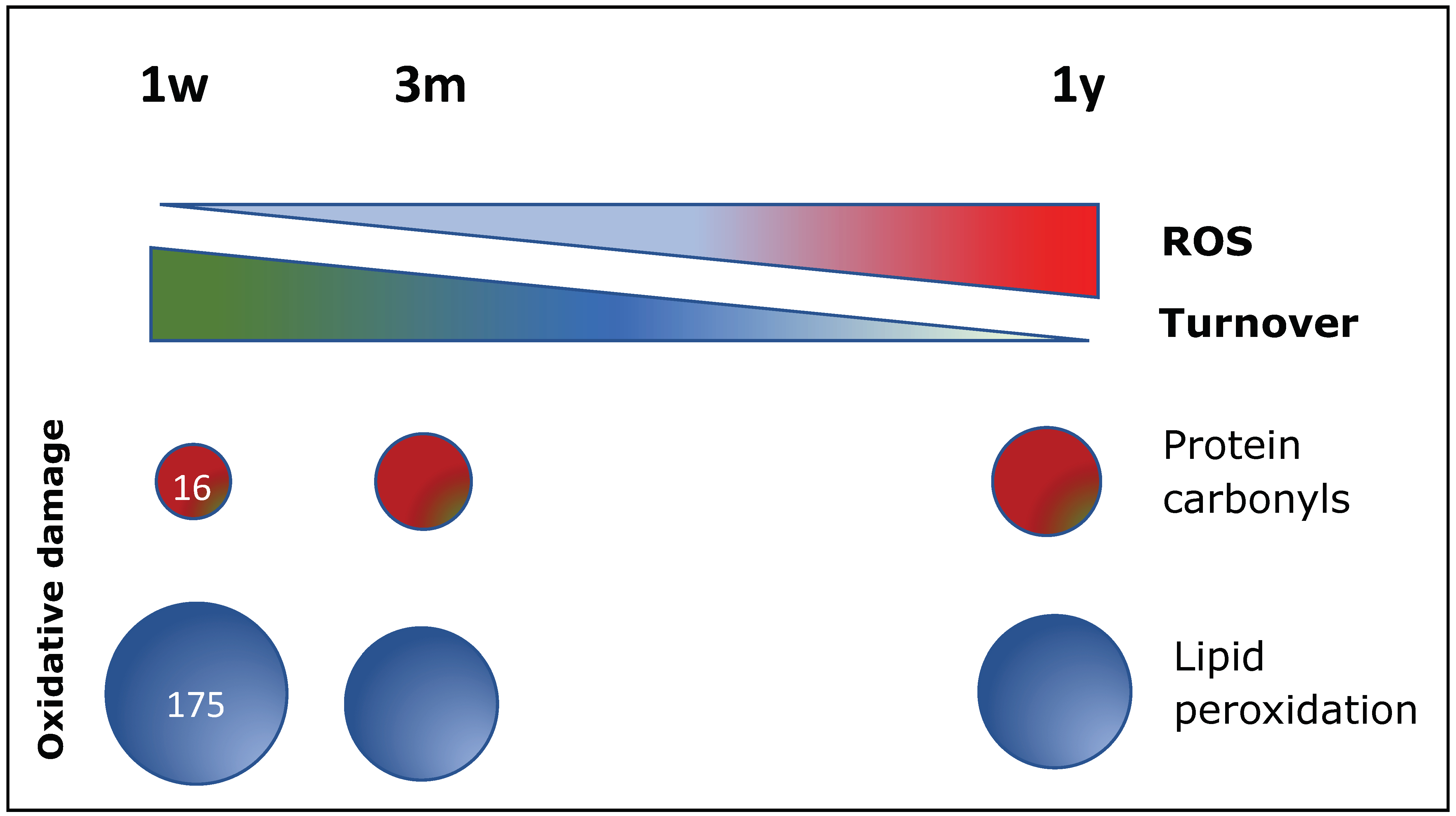
6.2. Oxidative Stress Plays a Central Role in B-CPC Regulation
6.3. The Vascular Niche for B-CPC in the Adult Mouse Heart
6.4. Preliminary Functional Definition of the B-CPC Vascular Niche Associated Processes
7. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ermolaeva, M.; Neri, F.; Ori, A.; Rudolph, K.L. Cellular and epigenetic drivers of stem cell ageing. Nat. Rev. Mol. Cell Biol. 2018, 19, 594–610. [Google Scholar] [CrossRef] [PubMed]
- Benitah, S.A.; Welz, P.S. Circadian Regulation of Adult Stem Cell Homeostasis and Aging. Cell Stem Cell 2020, 26, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Voog, J.; Jones, D.L. Stem cells and the niche: A dynamic duo. Cell Stem Cell 2010, 6, 103–115. [Google Scholar] [CrossRef]
- Hurwitz, S.N.; Jung, S.K.; Kurre, P. Hematopoietic stem and progenitor cell signaling in the niche. Leukemia 2020, 34, 3136–3148. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Negredo, P.; Yeo, R.W.; Brunet, A. Aging and Rejuvenation of Neural Stem Cells and Their Niches. Cell Stem Cell 2020, 27, 202–223. [Google Scholar] [CrossRef]
- Kann, A.P.; Hung, M.; Kraus, R.S. Cell-cell contact and signaling in the muscle stem cell niche. Curr. Opin. Cell Biol. 2021, 73, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Valiente-Alandi, I.; Albo-Castellanos, C.; Herrero, D.; Arza, E.; Garcia-Gomez, M.; Segovia, J.C.; Capecchi, M.; Bernad, A. Cardiac Bmi1(+) cells contribute to myocardial renewal in the murine adult heart. Stem Cell Res. Ther. 2015, 6, 205. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, D.; Annovazzi, L.; Casalone, C.; Corona, C.; Mellai, M. Glioblastoma: Microenvironment and Niche Concept. Cancers 2018, 11, 5. [Google Scholar] [CrossRef]
- Zhang, B.; Nguyen, L.X.T.; Li, L.; Zhao, D.; Kumar, B.; Wu, H.; Lin, A.; Pellicano, F.; Hopcroft, L.; Su, Y.L. Bone marrow niche trafficking of miR-126 controls the self-renewal of leukemia stem cells in chronic myelogenous leukemia. Nat. Med. 2018, 24, 450–462. [Google Scholar] [CrossRef]
- Duarte, D.; Hawkins, E.D.; Akinduro, O.; Ang, H.; De Filippo, K.; Kong, I.Y.; Haltalli, M.; Ruivo, N.; Straszkowski, L.; Vervoort, S.J. Inhibition of Endosteal Vascular Niche Remodeling Rescues Hematopoietic Stem Cell Loss in AML. Cell Stem Cell 2018, 22, 64–77.e6. [Google Scholar] [CrossRef]
- Schofield, R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells 1978, 4, 7–25. [Google Scholar] [PubMed]
- Bozdağ, S.C.; Kurt Yüksel, M.; Demirer, T. Adult Stem Cells and Medicine. Adv. Exp. Med. Biol. 2018, 1079, 17–36. [Google Scholar] [PubMed]
- Rojas–Rios, P.; Gonzalez–Reyes, A. The Plasticity of Stem Cell Niches: A General Property Behind Tissue Homeostasis and Repair. Stem Cells 2014, 32, 852–859. [Google Scholar] [CrossRef]
- Destalminil-Letourneau, M.; Morin-Poulard, I.; Tian, Y.; Vanzo, N.; Crozatier, M. The vascular niche controls Drosophila hematopoiesis via fibroblast growth factor signaling. Elife 2021, 10, e64672. [Google Scholar] [CrossRef]
- O’Reilly, A.M.; Lee, H.-H.; Simon, M.A. Integrins control the positioning and proliferation of follicle stem cells in the Drosophila ovary. J. Cell Biol. 2008, 182, 801–815. [Google Scholar] [CrossRef] [PubMed]
- Wolock, S.L.; Krishnan, I.; Tenen, D.E.; Matkins, V.; Camacho, V.; Patel, S.; Agarwal, P.; Bhatia, R.; Tenen, D.G.; Klein, A.M.; et al. Mapping Distinct Bone Marrow Niche Populations and Their Differentiation Paths. Cell Rep. 2019, 28, 302–311.e5. [Google Scholar] [CrossRef]
- Wei, Q.; Frenette, P.S. Niches for Hematopoietic Stem Cells and Their Progeny. Immunity 2018, 48, 632–648. [Google Scholar] [CrossRef]
- Andreotti, J.P.; Silva, W.N.; Costa, A.C.; Picoli, C.C.; Bitencourt, F.C.O.; Coimbra-Campos, L.M.C.; Resende, R.R.; Magno, L.A.V.; Romano-Silva, M.A.; Mintz, A.; et al. A neural stem cell niche heterogeneity. Semin. Cell Dev. Biol. 2019, 95, 42–53. [Google Scholar] [CrossRef]
- Leatherman, J. Stem cells supporting other stem cells. Front. Genet. 2013, 4, 257. [Google Scholar] [CrossRef]
- Nishina, H.; Katou-Ichikawa, C.; Kuramochi, M.; Izawa, T.; Kuwamura, M.; Yamate, J. The localization and distribution of cells labeled by a somatic stem cell-recognizing antibody (A3) in rat colon development; possible presence of a new cell type forming the intestinal stem cell niche. J. Toxicol. Pathol. 2019, 32, 37–48. [Google Scholar] [CrossRef]
- Puig, T.; Kádár, E.; Limón, A.; Cancelas, J.A.; Eixarch, H.; Luquín, L.; García, M.; Barquinero, J. Myeloablation enhances engraftment of transduced murine hematopoietic cells, but does not influence long-term expression of the transgene. Gene Ther. 2002, 9, 1472–1479. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Río, P.; Navarro, S.; Wang, W.; Sánchez-Domínguez, R.; Pujol, R.M.; Segovia, J.C.; Bogliolo, M.; Merino, E.; Wu, N.; Salgado, R. Successful engraftment of gene-corrected hematopoietic stem cells in non-conditioned patients with Fanconi anemia. Nat. Med. 2019, 25, 1396–1401. [Google Scholar] [CrossRef] [PubMed]
- Tucci, F.; Scaramuzza, S.; Aiuti, A.; Mortellaro, A. Update on Clinical Ex Vivo Hematopoietic Stem Cell Gene Therapy for Inherited Monogenic Diseases. Mol. Ther. 2021, 29, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Eschenhagen, T.; Bolli, R.; Braun, T.; Field, L.J.; Fleischmann, B.K.; Frisén, J.; Giacca, M.; Hare, J.M.; Steven Houser, S. Cardiomyocyte Regeneration: A Consensus Statement. Circulation 2017, 136, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, M.; Liu, F.; Zhang, Y.H.; Li, R.B.; Zhai, M.; Liu, F.; Zhou, L.Y.; Liu, C.Y.; Yan, K.W.; Dong, Y.H.; et al. Long Noncoding RNA CPR (Cardiomyocyte Proliferation Regulator) Regulates Cardiomyocyte Proliferation and Cardiac Repair. Circulation 2019, 139, 2668–2684. [Google Scholar] [CrossRef]
- Mollova, M.; Bersell, K.; Walsh, S.; Savla, J.; Das, L.T.; Park, S.Y.; Silberstein, L.E.; Dos Remedios, C.G.; Graham, D.; Colan, S.; et al. Cardiomyocyte proliferation contributes to heart growth in young humans. Proc. Natl. Acad. Sci. USA 2013, 110, 1446–1451. [Google Scholar] [CrossRef]
- Gabisonia, K.; Prosdocimo, G.; Aquaro, G.D.; Carlucc, L.; Zentilin, L.; Secco, I.; Ali, H.; Braga, L.; Gorgodze, N.; Bernini, F.; et al. MicroRNA therapy stimulates uncontrolled cardiac repair after myocardial infarction in pigs. Nature 2019, 569, 418–422. [Google Scholar] [CrossRef]
- Tang, J.; Wang, H.; Huang, X.; Li, F.; Zhu, H.; Li, Y.; Lingjuan He, L.; Zhang, H.; Pu, W.; Liu, K.; et al. Arterial Sca1+ Vascular Stem Cells Generate De Novo Smooth Muscle for Artery Repair and Regeneration. Cell Stem Cell 2020, 26, 81–96.e4. [Google Scholar] [CrossRef]
- Monroe, T.O.; Hill, M.C.; Morikawa, Y.; Leach, J.P.; Heallen, T.; Cao, S.; Krijger, P.H.L.; de Laat, W.; Wehrens, X.H.T.; Rodney, G.G.; et al. YAP Partially Reprograms Chromatin Accessibility to Directly Induce Adult Cardiogenesis In Vivo. Dev. Cell 2019, 48, 765–779.e7. [Google Scholar] [CrossRef]
- Senyo, S.E.; Steinhauser, M.L.; Pizziment, C.L.; Yang, V.K.; Cai, L.; Wang, M.; Wu, T.D.; Guerquin-Kern, J.L.; Lechene, C.P.; Lee, R.T. Mammalian heart renewal by pre-existing cardiomyocytes. Nature 2013, 493, 433–436. [Google Scholar] [CrossRef]
- Beltrami, A.P.; Barlucchi, L.; Torella, D.; Baker, M.; Limana, F.; Chimenti, S.; Kasahara, H.; Rota, M.; Musso, E.; Urbanek, K. Adult Cardiac Stem Cells Are Multipotent and Support Myocardial Regeneration. Cell 2003, 114, 763–776. [Google Scholar] [CrossRef]
- Oh, H.; Bradfute, S.B.; Gallardo, T.D.; Nakamura, T.; Gaussin, V.; Mishina, Y.; Pocius, J.; Michael, L.H.; Behringer, R.R.; Garry, D.J.; et al. Cardiac progenitor cells from adult myocardium: Homing, differentiation, and fusion after infarction. Proc. Natl. Acad. Sci. USA 2003, 100, 12313–12318. [Google Scholar] [CrossRef] [PubMed]
- Messina, E.; De Angelis, L.; Frati, G.; Morrone, S.; Chimenti, S.; Fiordaliso, F.; Salio, M.; Battagli, M.; Latronico, M.V.; Coletta, M.; et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ. Res. 2004, 95, 911–921. [Google Scholar] [CrossRef]
- Maher, T.J.; Ren, Y.; Li, Q.; Barueinm, E.; Garry, M.G.; Sorrentino, P.; Martin, C.M. ATP-binding cassette transporter Abcg2 lineage contributes to the cardiac vasculature after oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, 1610–1618. [Google Scholar] [CrossRef] [PubMed]
- Pfister, O.; Mouquet, F.; Jain, M.; Summer, R.; Helmes, M.; Fine, A.; Colucci, W.S.; Liao, R. CD31- but not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ. Res. 2005, 97, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Oyama, T.; Nagai, T.; Wada, H.; Naito, A.T.; Matsuura, K.; Iwanaga, K.; Takahashi, T.; Goto, M.; Mikami, Y.; Yasuda, N.; et al. Cardiac side population cells have a potential to migrate and differentiate into cardiomyocytes in vitro and in vivo. J. Cell Biol. 2007, 176, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.J.H.; Chandrakanthan, V.; Xaymardan, M.; Asli, N.S.; Li, J.; Ishtiaq Ahmed, I.; Heffernan, C.; Menon, M.K.; Scarlett, C.J.; Rashidianfar, A.; et al. Adult Cardiac-Resident MSC-like Stem Cells with a Proepicardial Origin. Cell Stem Cell 2013, 9, 527–540. [Google Scholar] [CrossRef]
- Uchida, S.; De Gaspari, P.; Kostin, S.; Jenniches, K.; Kilic, A.; Izumiya, Y.; Shiojima, I.; Grosse-Kreymborg, K.; Renz, H.; Walsh, K.; et al. Sca1-derived cells are a source of myocardial renewal in the murine adult heart. Stem Cell Rep. 2013, 1, 397–410. [Google Scholar] [CrossRef]
- Malliaras, K.; Ibrahim, A.; Tseliou, E.; Liu, W.; Sun, B.; Middleton, R.C.; Seinfeld, J.; Wang, L.; Sharifi, B.G.; Eduardo Marbán, E. Stimulation of endogenous cardioblasts by exogenous cell therapy after myocardial infarction. EMBO Mol. Med. 2014, 6, 760–777. [Google Scholar] [CrossRef]
- Noseda, M.; Harada, M.; McSweeney, S.; Leja, T.; Belian, E.; Stuckey, D.J.; Abreu Paiva, M.S.; Habib, J.; Macaulay, I.; de Smith, A.J.; et al. PDGFRalpha demarcates the cardiogenic clonogenic Sca1+ stem/progenitor cell in adult murine myocardium. Nat. Commun. 2015, 6, 6930. [Google Scholar] [CrossRef]
- Serpooshan, V.; Liu, Y.H.; Buikema, J.W.; Galdos, F.X.; Chirikian, O.; Paige, S.; Venkatraman, S.; Kumar, A.; Rawnsley, D.R.; Huang, X.; et al. Nkx2.5+ Cardiomyoblasts Contribute to Cardiomyogenesis in the Neonatal Heart. Sci. Rep. 2017, 7, 12590. [Google Scholar] [CrossRef] [PubMed]
- Herrero, D.; Tomé, M.; Cañón, S.; Cruz, F.M.; Carmona, R.M.; Fuster, E.; Roche, E.; Bernad, A. Redox-dependent BMI1 activity drives in vivo adult cardiac progenitor cell differentiation. Cell Death Differ. 2018, 25, 809–822. [Google Scholar] [CrossRef] [PubMed]
- Herrero, D.; Cañón, S.; Pelacho, B.; Salvador-Bernáldez, M.; Aguilar, S.; Pogontke, C.; Carmona, R.C.; Salvador, J.M.; Perez-Pomares, J.M.; Klein, O.D.; et al. Bmi1-Progenitor Cell Ablation Impairs the Angiogenic Response to Myocardial Infarction. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2160–2173. [Google Scholar] [CrossRef] [PubMed]
- Hesse, M.; Fleischmann, B.K.; Kotlikoff, M.I. The role of c-kit expressing cells in heart repair at the neonatal and adult stage. Stem Cells 2014, 32, 1701–1712. [Google Scholar] [CrossRef]
- Chien, K.R.; Frisén, J.; Fritsche-Danielson, R.; Melton, D.A.; Murry, C.E.; Weissman, I.W. Regenerating the field of cardiovascular cell therapy. Nat. Biotechnol. 2019, 37, 232–237. [Google Scholar] [CrossRef]
- Higuera, M.; Bernad, A. Review of: Setd4 controlled quiescent c-Kit+ cells contribute to cardiac neovascularization of capillaries beyond activation. QEIOS 2021, 11, 11603. [Google Scholar] [CrossRef]
- Vicinanza, C.; Aquila, I.; Cianflone, E.; Scalise, M.; Marino, F.; Mancuso, T.; Fumagalli, F.; Giovannone, E.D.; Cristiano, F.; Iaccino, E.; et al. Kitcre knock-in mice fail to fate-map cardiac stem cells. Nature 2018, 555, E1–E5. [Google Scholar] [CrossRef]
- Vicinanza, C.; Aquila, I.; Scalise, M.; Cristiano, F.; Marino, F.; Cianflone, E.; Mancuso, T.; Marotta, P.; Sacco, W.; Lewis, F.C.; et al. Adult cardiac stem cells are multipotent and robustly myogenic: c-kit expression is necessary but not sufficient for their identification. Cell Death Differ. 2017, 24, 2101–2116. [Google Scholar] [CrossRef]
- Aquila, I.; Marino, F.; Cianflone, E.; Marotta, P.; Torella, M.; Mollace, V.; Indolfi, C.; Nadal-Ginard, B.; Torella, D. The use and abuse of Cre/Lox recombination to identify adult cardiomyocyte renewal rate and origin. Pharmacol. Res. 2018, 127, 116–128. [Google Scholar] [CrossRef]
- Vagnozzi, R.J.; Maillet, M.; Sargent, M.A.; Khalil, H.; Johansen, A.K.Z.; Schwanekamp, J.A.; York, A.J.; Huang, V.; Nahrendorf, M.; Sadayappan, S.; et al. An acute immune response underlies the benefit of cardiac stem cell therapy. Naturec 2020, 577, 405–409. [Google Scholar] [CrossRef]
- Hosoda, T. C-kit-positive cardiac stem cells and myocardial regeneration. Am. J. Cardiovasc. Dis. 2012, 2, 58–67. [Google Scholar] [PubMed]
- Israeli-Rosenberg, S.; Manso, A.M.; Okada, H.; Ross, R.S. Integrins and integrin-associated proteins in the cardiac myocyte. Circ. Res. 2014, 114, 572–586. [Google Scholar] [CrossRef] [PubMed]
- Goumans, M.J.; de Boer, T.P.; Smits, A.M.; van Laake, L.W.; van Vliet, P.; Metz, C.H.; Korfage, T.H.; Kats, K.P.; Hochstenbach, R.; Pasterkamp, G.; et al. TGF-beta1 induces efficient differentiation of human cardiomyocyte progenitor cells into functional cardiomyocytes in vitro. Stem Cell Res. 2007, 1, 138–149. [Google Scholar] [CrossRef]
- Besser, R.R.; Ishahak, M.; Mayo, V.; Carbonero, D.; Claure, I.; Agarwal, A. Engineered Microenvironments for Maturation of Stem Cell Derived Cardiac Myocytes. Theranostics 2018, 8, 124–140. [Google Scholar] [CrossRef] [PubMed]
- Renko, O.; Tolonen, A.-M.; Rysä, J.; Magga, J.; Mustonen, E.; Ruskoaho, H.; Serpi, R. SDF1 gradient associates with the distribution of c-Kit+ cardiac cells in the heart. Sci. Rep. 2018, 8, 1160. [Google Scholar] [CrossRef] [PubMed]
- Winter, E.M.; van Oorschotm, A.A.; Hogers, B.; van der Graaf, L.M.; Doevendans, P.A.; Poelmann, R.E.; Atsma, D.E.; Gittenberger-de Groot, A.C.; Goumans, M.J. A new direction for cardiac regeneration therapy: Application of synergistically acting epicardium-derived cells and cardiomyocyte progenitor cells. Circ. Heart Fail. 2009, 2, 643–653. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, M.R.; Xu, Z.D.; Hu, Z.; Chen, C.; Chi, Y.L.; Kong, Z.D.; Li, Z.F.; Li, X.T.; Guo, S.L.; et al. Cardiomyocyte differentiation induced in cardiac progenitor cells by cardiac fibroblast-conditioned medium. Exp. Biol. Med. 2014, 239, 628–637. [Google Scholar] [CrossRef]
- Sánchez, P.L.; Fernández-Santos, M.E.; Costanza, S.; Climent, A.M.; Moscoso, I.; Gonzalez-Nicolas, M.A.; Sanz-Ruiz, R.; Rodríguez, H.; Kren, S.M.; Garrido, G. Acellular human heart matrix: A critical step toward whole heart grafts. Biomaterials 2015, 61, 279–289. [Google Scholar] [CrossRef]
- French, K.M.; Maxwell, J.T.; Bhutani, S.; Ghosh-Choudhary, S.; Fierro, M.J.; Johnson, T.D.; Christman, K.L.; Taylor, W.R.; Davis, M.E. Fibronectin and Cyclic Strain Improve Cardiac Progenitor Cell Regenerative Potential In Vitro. Stem Cells Int. 2016, 2016, 8364382. [Google Scholar] [CrossRef]
- Gude, N.; Joyo, E.; Toko, H.; Quijada, P.; Villanueva, M.; Hariharan, N.; Sacchi, V.; Truffa, S.; Joyo, A.; Voelkers, M.; et al. Notch activation enhances lineage commitment and protective signaling in cardiac progenitor cells. Basic Res. Cardiol. 2015, 110, 29. [Google Scholar] [CrossRef]
- Urbich, C.; Aicher, A.; Heeschen, C.; Dernbach, E.; Hofmann, W.K.; Zeiher, A.M.; Dimmeler, S. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J. Mol. Cell Cardiol. 2005, 39, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Moscoso, I.; Tejados, N.; Barreiro, O.; Sepúlveda, P.; Izarra, A.; Calvo, E.; Dorronsoro, A.; Salcedo, J.M.; Sádaba, R.; Díez-Juan, A.; et al. Podocalyxin-like protein 1 is a relevant marker for human c-kit(pos) cardiac stem cells. J. Tissue Eng. Regen. Med. 2016, 10, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Van Oorschot, A.A.M.; Smits, A.M.; Pardal, E.; Doevendans, P.A.; Goumans, M.-J. Low oxygen tension positively influences cardiomyocyte progenitor cell function. J. Cell Mol. Med. 2011, 15, 2723–2734. [Google Scholar] [CrossRef] [PubMed]
- Kirton, J.P.; Xu, Q. Endothelial precursors in vascular repair. Microvasc. Res. 2010, 79, 193–199. [Google Scholar] [CrossRef]
- Herrero, D.; Cañón, S.; Albericio, G.; Carmona, R.-M.; Aguilar, S.; Mañes, S.; Bernad, A. Age-related oxidative stress confines damage-responsive Bmi1 + cells to perivascular regions in the murine adult heart. Redox Biol. 2019, 22, 101156. [Google Scholar] [CrossRef]
- Tallquist, M.D.; Molkentin, J.D. Redefining the identity of cardiac fibroblasts. Nat. Rev. Cardiol. 2017, 14, 484–491. [Google Scholar] [CrossRef]
- Fioret, B.A.; Heimfeld, J.D.; Paik, D.T.; Hatzopoulos, A.K. Endothelial cells contribute to generation of adult ventricular myocytes during cardiac homeostasis. Cell Rep. 2014, 8, 229–241. [Google Scholar] [CrossRef]
- Smart, N.; Riley, P.R. The epicardium as a candidate for heart regeneration. Future Cardiol. 2012, 8, 53–69. [Google Scholar] [CrossRef]
- Kimura, W.; Sadek, H.A. The cardiac hypoxic niche: Emerging role of hypoxic microenvironment in cardiac progenitors. Cardiovasc. Diagn. Ther. 2012, 2, 278–289. [Google Scholar]
- Li, Q.; Dasari, C.; Li, D.; Arshia, A.; Ume, A.M.; Abouzid, M.R.A.; Guo, Y.; Bolli, R. Effects of Heme Oxygenase-1 on c-Kit-Positive Cardiac Cells. Int. J. Mol. Sci. 2021, 22, 13448. [Google Scholar] [CrossRef]
- Korski, K.I.; Kubli, D.A.; Wang, B.J.; Khalafalla, F.G.; Monsanto, M.M.; Firouzi, F.; Echeagaray, O.H.; Kim, T.; Adamson, R.M.; Dembitsky, W.P.; et al. Hypoxia Prevents Mitochondrial Dysfunction and Senescence in Human c-Kit(+) Cardiac Progenitor Cells. Stem Cells 2019, 37, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-Y.; Qin, L.; Tellides, G.; Simons, M. Fibroblast growth factor receptor 1 is a key inhibitor of TGFβ signaling in the endothelium. Sci. Signal. 2014, 7, ra90. [Google Scholar] [CrossRef] [PubMed]
- Valiente-Alandi, V.; Albo-Castellanos, C.; Herrero, D.; Sanchez, I.; Bernad, A. Bmi1 (+) cardiac progenitor cells contribute to myocardial repair following acute injury. Stem Cell Res. Ther. 2016, 7, 100. [Google Scholar] [CrossRef] [PubMed]
- Sá da Bandeira, S.; Casamitjana, J.; Crisan, M. Pericytes, integral components of adult hematopoietic stem cell niches. Pharmacol Ther. 2017, 171, 104–113. [Google Scholar] [CrossRef]
- Rosa, I.; Marini, M.; Manetti, M. Telocytes: An Emerging Component of Stem Cell Niche Microenvironment. J. Histochem. Cytochem. 2021, 69, 795–818. [Google Scholar] [CrossRef]
- Spencer, J.A.; Ferraro, F.; Roussakis, E.; Klein, A.; Wu, J.; Runnels, J.M.; Zaher, W.; Mortensen, L.J.; Alt, C.; Turcotte, R. Direct measurement of local oxygen concentration in the bone marrow of live animal. Nature 2014, 508, 269–273. [Google Scholar] [CrossRef]
- Chen, J.; Kang, J.G.; Keyvanfar, K.; Young, N.S.; Hwang, P.M. Long-term adaptation to hypoxia preserves hematopoietic stem cell function. Exp. Hematol. 2016, 44, 866–873.e4. [Google Scholar] [CrossRef]
- Yang, D.; Liu, H.-Q.; Yang, Z.; Fan, D.; Tang, Q.-Z. BMI1 in the heart: Novel functions beyond tumorigenesis. EBioMedicine 2021, 63, 103193. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, L.; Vaseghi, H.R.; Liu, Z.; Lu, R.; Alimohamad, S.; Yin, C.; Fu, J.D.; Wang, G.G.; Liu, J.; et al. Bmi1 Is a Key Epigenetic Barrier to Direct Cardiac Reprogramming. Cell Stem Cell 2016, 18, 382–395. [Google Scholar] [CrossRef]
- Sangiorgi, E.; Capecchi, M.R. Bmi1 lineage tracing identifies a self-renewing pancreatic acinar cell subpopulation capable of maintaining pancreatic organ homeostasis. Proc. Natl. Acad. Sci. USA 2009, 106, 7101–7106. [Google Scholar] [CrossRef]
- Hosen, N.; Yamane, T.; Muijtjens, M.; Pham, K.; Clarke, M.F.; Weissman, I.L. Bmi-1-Green Fluorescent Protein-Knock-In Mice Reveal the Dynamic Regulation of Bmi-1 Expression in Normal and Leukemic Hematopoietic Cells. Stem Cells 2007, 25, 1635–1644. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhao, M.; Xie, Y.; Zhu, T.; Liang, W.; Sun, B.; Liu, W.; Wu, L.; Lu, G.; Li, T.S.; et al. Bmi-1 high-expressing cells enrich cardiac stem/progenitor cells and respond to heart injury. J. Cell Mol. Med. 2019, 23, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Inomata, K.; Aoto, T.; Binh, N.T.; Okamoto, N.; Tanimura, S.; Wakayama, T.; Iseki, S.; Hara, E.; Masunaga, T.; Shimizu, H.; et al. Genotoxic stress abrogates renewal of melanocyte stem cells by triggering their differentiation. Cell 2009, 137, 1088–1099. [Google Scholar] [CrossRef] [PubMed]
- Nombela-Arrieta, C.; Pivarnik, G.; Winkel, B.; Canty, K.J.; Harley, B.; Mahoney, J.E.; Park, S.-Y.; Lu, J.; Protopopov, J.; Silberstein, L.E. Quantitative imaging of haematopoietic stem and progenitor cell localization and hypoxic status in the bone marrow microenvironment. Nat. Cell Biol. 2013, 15, 533–543. [Google Scholar] [CrossRef]
- Shen, Q.; Goderie, S.K.; Jin, L.; Karanth, N.; Sun, Y.; Abramova, N.; Vincent, P.; Pumiglia, K.; Temple, S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science 2004, 304, 1338–1340. [Google Scholar] [CrossRef] [PubMed]
- Kimura, W.; Xiao, F.; Canseco, D.C.; Muralidhar, S.; Thet, S.; Zhang, H.M.; Abderrahman, Y.; Chen, R.; Garcia, J.A.; Shelton, J.M.; et al. Hypoxia fate mapping identifies cycling cardiomyocytes in the adult heart. Nature 2015, 523, 226–230. [Google Scholar] [CrossRef]
- Kocabas, F.; Mahmoud, A.I.; Sosic, D.; Porrello, E.R.; Chen, R.; Garcia, J.A.; DeBerardinis, R.J.; Sadek, H.A. The hypoxic epicardial and subepicardial microenvironment. J. Cardiovasc. Transl. Res. 2012, 5, 654–665. [Google Scholar] [CrossRef]
- Nóbrega-Pereira, S.; Fernandez-Marcos, P.J.; Brioche, T.; Gomez-Cabrera, M.C.; Salvador-Pascual, A.; Flores, J.M.; Viña, J.; Serrano, M. G6PD protects from oxidative damage and improves healthspan in mice. Nat. Commun. 2016, 7, 10894. [Google Scholar] [CrossRef]
- Carlsson, L.M.; Jonsson, J.; Edlund, T.; Marklund, S.L. Mice lacking extracellular superoxide dismutase are more sensitive to hyperoxia. Proc. Natl. Acad. Sci. USA 1995, 92, 6264–6268. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Cao, Y.; Fang, Y.; Cai, J.; Li, X.; Xu, F.; Yuan, N.; Zhang, S.; Wang, J. ROS functions as an upstream trigger for autophagy to drive hematopoietic stem cell differentiation. Hematology 2016, 21, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Puente, B.N.; Kimura, W.; Muralidhar, S.A.; Moon, J.; Amatruda, J.F.; Phelps, K.L.; Grinsfelder, D.; Rothermel, B.A.; Chen, R.; Garcia, J.A.; et al. The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle. Cell 2014, 157, 565–579. [Google Scholar] [CrossRef] [PubMed]
- Banerjee Mustafi, S.; Aznar, N.; Dwivedi, S.K.; Chakraborty, P.K.; Basak, R.; Mukherjee, P.; Ghosh, P.; Bhattacharya, R. Mitochondrial BMI1 maintains bioenergetic homeostasis in cells. FASEB J. 2016, 30, 4042–4055. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, L.; Ni, W.; Zhang, Y.; Sun, S.; Miao, D.; Chai, R.; Li, H. Bmi1 regulates auditory hair cell survival by maintaining redox balance. Cell Death Dis. 2015, 6, e1605. [Google Scholar] [CrossRef]
- Landais, S.; D’Alterio, C.; Jones, D.L. Persistent replicative stress alters polycomb phenotypes and tissue homeostasis in Drosophila melanogaster. Cell Rep. 2014, 7, 859–870. [Google Scholar] [CrossRef]
- Kramann, R.; Schneider, R.K.; Di Rocco, D.P.; Machado, F.; Fleig, S.; Bondzie, P.A.; Henderson, J.M.; Ebert, B.L.; Humphreys, B.D. Perivascular Gli1+ progenitors are key contributors to injury- induced organ fibrosis. Cell Stem Cell 2015, 16, 51–66. [Google Scholar] [CrossRef]
- Kunisaki, Y.; Bruns, I.; Scheiermann, C.; Ahmed, J.; Pinho, S.; Zhang, D.; Mizoguchi, T.; Wei, Q.; Lucas, D.; Ito, K.; et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 2013, 502, 637–643. [Google Scholar] [CrossRef]
- Ottone, C.; Krusche, B.; Whitby, A.; Clements, M.; Quadrato, G.; Pitulescu, M.E.; Adams, R.A.; Parrinello, S. Direct cell-cell contact with the vascular niche maintains quiescent neural stem cells. Nat. Cell Biol. 2014, 16, 1045–1056. [Google Scholar] [CrossRef]
- Jang, K.; Kim, K.; Gilbert, C.A.; Simpkins, F.; Ince, T.A.; Slingerland, J.M. VEGFA activates an epigenetic pathway upregulating ovarian cancer-initiating cells. EMBO Mol. Med. 2017, 9, 304–318. [Google Scholar] [CrossRef]
- Wang, H.U.; Chen, Z.F.; Anderson, D.J. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell 1998, 93, 741–753. [Google Scholar] [CrossRef]
- Yang, D.; Jin, C.; Ma, H.; Huang, M.; Shi, G.-P.; Wang, J.; Xiang, M. EphrinB2/EphB4 pathway in postnatal angiogenesis: A potential therapeutic target for ischemic cardiovascular disease. Angiogenesis 2016, 19, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.J.; Spradling, A.C. Stem Cells and Niches: Mechanisms That Promote Stem Cell Maintenance throughout Life. Cell 2008, 132, 598–611. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Asakura, Y.; Murakonda, B.S.R.; Pengo, T.; Latroche, C.; Chazaud, B.; McLoon, L.K.; Asakura, A. Muscle Satellite Cell Cross-Talk with a Vascular Niche Maintains Quiescence via VEGF and Notch Signaling. Cell Stem Cell 2018, 23, 530–543.e9. [Google Scholar] [CrossRef] [PubMed]
- Giordani, L.; He, G.J.; Negroni, E.; Sakai, H.; Law, J.Y.L.; Siu, M.M.; Wan, R.; Corneau, A.; Tajbakhsh, S.; Cheung, T.H.; et al. High-Dimensional Single-Cell Cartography Reveals Novel Skeletal Muscle-Resident Cell Populations. Mol. Cell 2019, 74, 609–621.e6. [Google Scholar] [CrossRef]
- Barruet, E.; Steven MGarcia, S.M.; Striedinger, K.; Wu, J.; Lee, S.; Byrnes, L.; Wong, A.; Xuefeng, S.; Tamaki, S.A.S.; Pomerantz, J.H. Functionally heterogeneous human satellite cells identified by single cell RNA sequencing. Elife 2020, 9, e51576. [Google Scholar] [CrossRef]
- Bergmann, O.; Zdunek, S.; Felker, A.; Salehpour, M.; Alkass, K.; Bernard, S.; Sjostrom, S.L.; Szewczykowska, M.; Jackowska, T.; Dos Remedios, C.; et al. Dynamics of Cell Generation and Turnover in the Human Heart. Cell 2015, 161, 1566–1575. [Google Scholar] [CrossRef]
- Ma, Y.; Mouton, A.J.; Lindsey, M.L. Cardiac macrophage biology in the steady-state heart, the aging heart, and following myocardial infarction. Transl. Res. 2018, 191, 15–28. [Google Scholar] [CrossRef]
- Nicolás-Ávila, J.A.; Lechuga-Vieco, A.V.; Esteban-Martínez, L.; Sánchez-Díaz, M.; Díaz-García, E.; Santiago, D.J.; Rubio-Ponce, A.; Li, J.L.; Balachander, A.; Quintana, J.A.; et al. Network of Macrophages Supports Mitochondrial Homeostasis in the Heart. Cell 2020, 183, 94–109.e23. [Google Scholar] [CrossRef]
- Ratnayake, R.; Nguyen, P.D.; Rossello, F.J.; Wimmer, V.C.; Tan, J.L.; Galvis, L.A.; Julier, Z.; Wood, A.J.; Boudier TIsiaku, A.I. Macrophages provide a transient muscle stem cell niche via NAMPT secretion. Nature 2021, 591, 281–287. [Google Scholar] [CrossRef]
- Li, J.; Li, S.H.; Dong, J.; Alibhai, F.J.; Zhang, C.; Shao, Z.B.; Song, H.F.; He, S.; Yin, W.J.; Wu, J.; et al. Long-term repopulation of aged bone marrow stem cells using young Sca-1 cells promotes aged heart rejuvenation. Aging Cell 2019, 18, e13026. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrero, D.; Albericio, G.; Higuera, M.; Herranz-López, M.; García-Brenes, M.A.; Cordero, A.; Roche, E.; Sepúlveda, P.; Mora, C.; Bernad, A. The Vascular Niche for Adult Cardiac Progenitor Cells. Antioxidants 2022, 11, 882. https://doi.org/10.3390/antiox11050882
Herrero D, Albericio G, Higuera M, Herranz-López M, García-Brenes MA, Cordero A, Roche E, Sepúlveda P, Mora C, Bernad A. The Vascular Niche for Adult Cardiac Progenitor Cells. Antioxidants. 2022; 11(5):882. https://doi.org/10.3390/antiox11050882
Chicago/Turabian StyleHerrero, Diego, Guillermo Albericio, Marina Higuera, María Herranz-López, Miguel A. García-Brenes, Alejandra Cordero, Enrique Roche, Pilar Sepúlveda, Carmen Mora, and Antonio Bernad. 2022. "The Vascular Niche for Adult Cardiac Progenitor Cells" Antioxidants 11, no. 5: 882. https://doi.org/10.3390/antiox11050882
APA StyleHerrero, D., Albericio, G., Higuera, M., Herranz-López, M., García-Brenes, M. A., Cordero, A., Roche, E., Sepúlveda, P., Mora, C., & Bernad, A. (2022). The Vascular Niche for Adult Cardiac Progenitor Cells. Antioxidants, 11(5), 882. https://doi.org/10.3390/antiox11050882








