The Therapeutic and Prophylactic Potential of Quercetin against COVID-19: An Outlook on the Clinical Studies, Inventive Compositions, and Patent Literature
Abstract
:1. Introduction
2. Searching for Non-Patent Literature
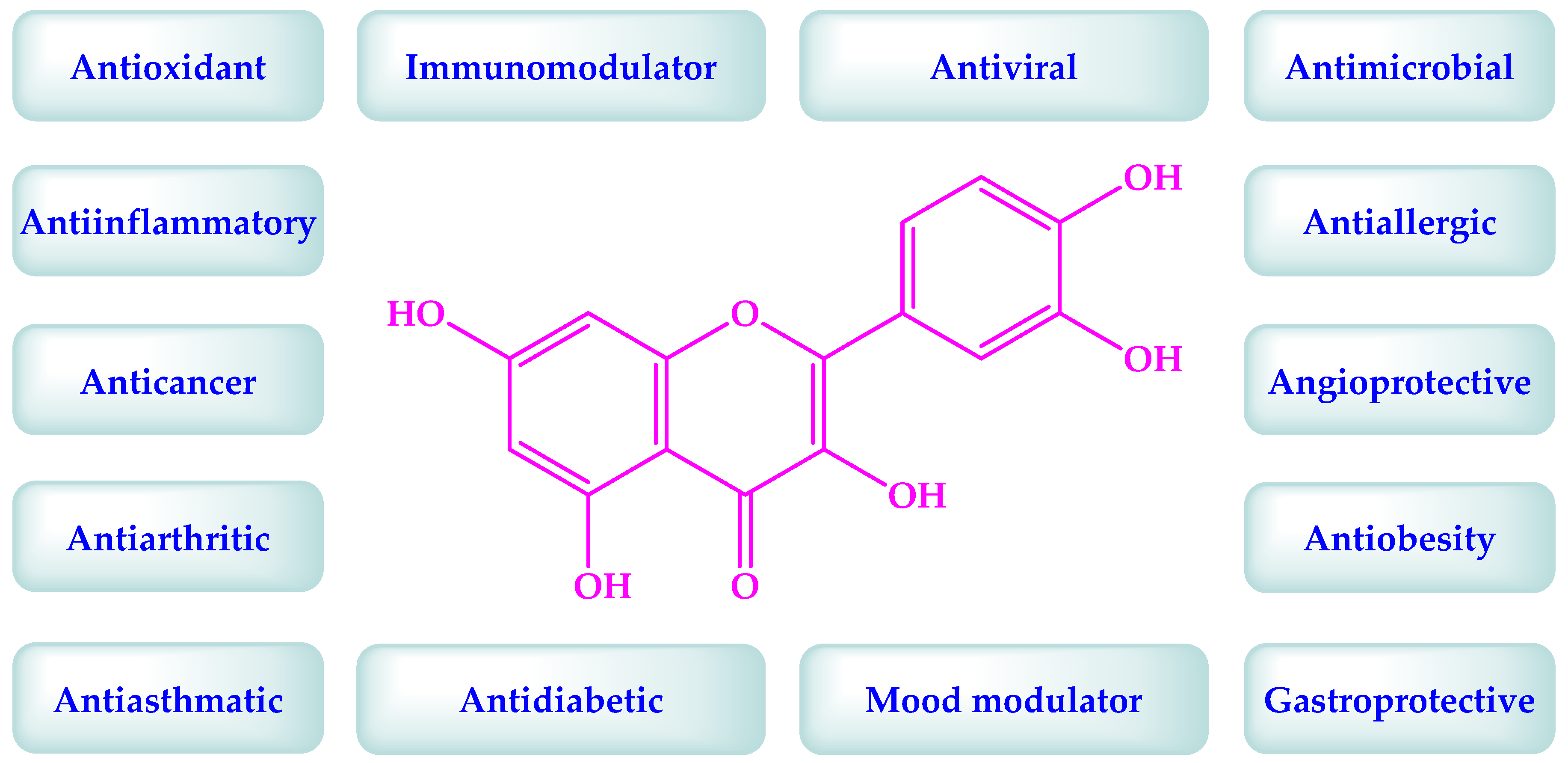
3. Quercetin
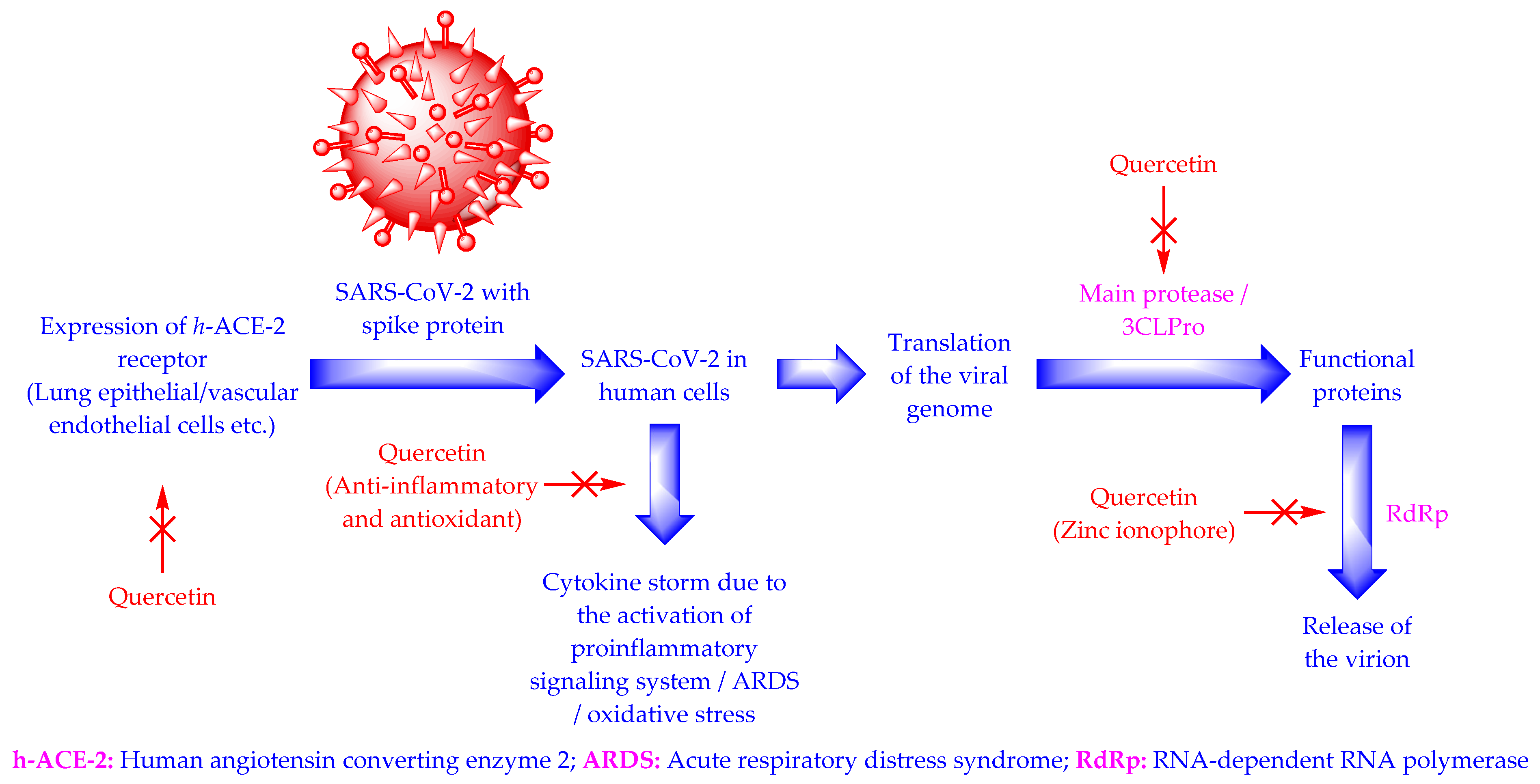
4. Mechanism of Action of Quercetin against SARS-CoV-2
5. Clinical Studies of Quercetin against COVID-19
6. Patent Searching
7. Conclusions
8. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pollard, C.A.; Morran, M.P.; Nestor-Kalinoski, A.L. The COVID-19 pandemic: A global health crisis. Physiol. Genom. 2020, 52, 549–557. [Google Scholar] [CrossRef]
- Coronavirus Disease (COVID-19) Pandemic. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 4 March 2022).
- Lane, A.; Hunter, K.; Lee, E.L.; Hyman, D.; Bross, P.; Alabd, A.; Betchen, M.; Terrigno, V.; Talwar, S.; Ricketti, D.; et al. Clinical characteristics and symptom duration among outpatients with COVID-19. Am. J. Infect. Control 2021, 50, 383–389. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Y.; Zhang, F.; Zhang, L.; Li, L. COVID-19 in Elderly Adults: Clinical Features, Molecular Mechanisms, and Proposed Strategies. Aging Dis. 2020, 11, 1481–1495. [Google Scholar] [CrossRef]
- Imran, M.; Alshrari, A.S.; Asdaq, S.M.B.; Abida. Trends in the development of remdesivir based inventions against COVID-19 and other disorders: A patent review. J. Infect. Public Health 2021, 14, 1075–1086. [Google Scholar] [CrossRef]
- Imran, M.; Kumar, A.M.; Asdaq, S.M.B.; Khan, S.A.; Alaqel, S.I.; Alshammari, M.K.; Alshehri, M.M.; Alshrari, A.S.; Mateq, A.A.; Al-Shammeri, A.M.; et al. Discovery, Development, and Patent Trends on Molnupiravir: A Prospective Oral Treatment for COVID-19. Molecules 2021, 26, 5795. [Google Scholar] [CrossRef]
- Hashemian, S.M.; Farhadi, T.; Velayati, A.A. A review on favipiravir: The properties, function, and usefulness to treat COVID-19. Expert Rev. Anti. Infect. Ther. 2021, 19, 1029–1037. [Google Scholar] [CrossRef]
- Asdaq, S.M.B.; Rabbani, S.I.; Alkahtani, M.; Aldohyan, M.M.; Alabdulsalam, A.M.; Alshammari, M.S.; Alajlan, S.A.; Binrokan, A.; Mohzari, Y.; Alrashed, A.; et al. A Patent Review on the Therapeutic Application of Monoclonal Antibodies in COVID-19. Int. J. Mol. Sci. 2021, 22, 11953. [Google Scholar] [CrossRef]
- Alshrari, A.S.; Hudu, S.A.; Imran, M.; Asdaq, S.M.B.; Ali, A.M.; Rabbani, S.I. Innovations and development of COVID-19 vaccines: A patent review. J. Infect. Public Health 2022, 15, 123–131. [Google Scholar] [CrossRef]
- Vishwakarma, S.; Panigrahi, C.; Barua, S.; Sahoo, M.; Mandliya, S. Food nutrients as inherent sources of immunomodulation during COVID-19 pandemic. Lebensm. Wiss. Technol. 2022, 158, 113154. [Google Scholar] [CrossRef]
- Kumar, A.; Rai, A.; Khan, M.S.; Kumar, A.; Haque, Z.U.; Fazil, M.; Rabbani, G. Role of herbal medicines in the management of patients with COVID-19: A systematic review and meta-analysis of randomized controlled trials. J. Tradit. Complement. Med. 2022, 12, 100–113. [Google Scholar] [CrossRef]
- Derosa, G.; Maffioli, P.; D’Angelo, A.; Di Pierro, F. A role for quercetin in coronavirus disease 2019 (COVID-19). Phytother. Res. 2021, 35, 1230–1236. [Google Scholar] [CrossRef]
- Bernini, R.; Velotti, F. Natural Polyphenols as Immunomodulators to Rescue Immune Response Homeostasis: Quercetin as a Research Model against Severe COVID-19. Molecules 2021, 26, 5803. [Google Scholar] [CrossRef]
- Manjunath, S.H.; Thimmulappa, R.K. Antiviral, immunomodulatory, and anticoagulant effects of quercetin and its derivatives: Potential role in prevention and management of COVID-19. J. Pharm. Anal. 2021, 12, 29–34. [Google Scholar] [CrossRef]
- Diniz, L.R.L.; Souza, M.T.S.; Duarte, A.B.S.; Sousa, D.P. Mechanistic Aspects and Therapeutic Potential of Quercetin against COVID-19-Associated Acute Kidney Injury. Molecules 2020, 25, 5772. [Google Scholar] [CrossRef]
- DI Pierro, F.; Khan, A.; Bertuccioli, A.; Maffioli, P.; Derosa, G.; Khan, S.; Khan, B.A.; Nigar, R.; Ujjan, I.; Devrajani, B.R. Quercetin Phytosome® as a potential candidate for managing COVID-19. Minerva Gastroenterol. Torino 2021, 67, 190–195. [Google Scholar] [CrossRef]
- Colunga Biancatelli, R.M.L.; Berrill, M.; Catravas, J.D.; Marik, P.E. Quercetin and Vitamin C: An Experimental, Synergistic Therapy for the Prevention and Treatment of SARS-CoV-2 Related Disease (COVID-19). Front. Immunol. 2020, 11, 1451. [Google Scholar] [CrossRef]
- D’Andrea, G. Quercetin: A flavonol with multifaceted therapeutic applications? Fitoterapia 2015, 106, 256–271. [Google Scholar] [CrossRef]
- Dabbagh-Bazarbachi, H.; Clergeaud, G.; Quesada, I.M.; Ortiz, M.; O’Sullivan, C.K.; Fernández-Larrea, J.B. Zinc ionophore activity of quercetin and epigallocatechin-gallate: From Hepa 1-6 cells to a liposome model. J. Agric. Food. Chem. 2014, 62, 8085–8093. [Google Scholar] [CrossRef]
- Ansari, M.A.; Abdul, H.M.; Joshi, G.; Opii, W.O.; Butterfield, D.A. Protective effect of quercetin in primary neurons against Abeta(1–42): Relevance to Alzheimer’s disease. J. Nutr. Biochem. 2009, 20, 269–275. [Google Scholar] [CrossRef] [Green Version]
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar]
- Yang, D.; Wang, T.; Long, M.; Li, P. Quercetin: Its Main Pharmacological Activity and Potential Application in Clinical Medicine. Oxid. Med. Cell Longev. 2020, 2020, 8825387. [Google Scholar] [CrossRef]
- Grass Notices. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices&id=341 (accessed on 4 March 2022).
- Bhat, I.U.H.; Bhat, R. Quercetin: A Bioactive Compound Imparting Cardiovascular and Neuroprotective Benefits: Scope for Exploring Fresh Produce, Their Wastes, and By-Products. Biology 2021, 10, 586. [Google Scholar] [CrossRef]
- Costela-Ruiz, V.J.; Illescas-Montes, R.; Puerta-Puerta, J.M.; Ruiz, C.; Melguizo-Rodríguez, L. SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020, 54, 62–75. [Google Scholar] [CrossRef]
- Markoulaki, D.; Iordanou, S.; Koukios, D.; Christoldoulou, I.; Papadopoulos, P.; Timiliotou-Matsentidou, C. Severe Multisystem Inflammatory Syndrome Associated with SARS-CoV-2 in a 31-Year-Old Male Patient: The First Clinical Case Report from the Republic of Cyprus. Cureus 2022, 14, e22640. [Google Scholar] [CrossRef]
- Rondanelli, M.; Perna, S.; Gasparri, C.; Petrangolini, G.; Allegrini, P.; Cavioni, A.; Faliva, M.A.; Mansueto, F.; Patelli, Z.; Peroni, G.; et al. Promising Effects of 3-Month Period of Quercetin Phytosome® Supplementation in the Prevention of Symptomatic COVID-19 Disease in Healthcare Workers: A Pilot Study. Life 2022, 12, 66. [Google Scholar] [CrossRef]
- Saeedi-Boroujeni, A.; Mahmoudian-Sani, M.R. Anti-inflammatory potential of Quercetin in COVID-19 treatment. J. Inflamm. Lond. 2021, 18, 3. [Google Scholar] [CrossRef]
- Aucoin, M.; Cooley, K.; Saunders, P.R.; Cardozo, V.; Remy, D.; Cramer, H.; Neyre Abad, C.; Hannan, N. The effect of quercetin on the prevention or treatment of COVID-19 and other respiratory tract infections in humans: A rapid review. Adv. Integr. Med. 2020, 7, 247–251. [Google Scholar] [CrossRef]
- Clinical Trial Database. Available online: https://www.clinicaltrials.gov/ (accessed on 8 March 2022).
- Shohan, M.; Nashibi, R.; Mahmoudian-Sani, M.R.; Abolnezhadian, F.; Ghafourian, M.; Alavi, S.M.; Sharhani, A.; Khodadadi, A. The therapeutic efficacy of quercetin in combination with antiviral drugs in hospitalized COVID-19 patients: A randomized controlled trial. Eur. J. Pharmacol. 2022, 914, 174615. [Google Scholar] [CrossRef]
- Margolin, L.; Luchins, J.; Margolin, D.; Margolin, M.; Lefkowitz, S. 20-Week Study of Clinical Outcomes of Over-the-Counter COVID-19 Prophylaxis and Treatment. J. Evid. Based Integr Med. 2021, 26, 2515690X211026193. [Google Scholar] [CrossRef]
- Di Pierro, F.; Iqtadar, S.; Khan, A.; Ullah Mumtaz, S.; Masud Chaudhry, M.; Bertuccioli, A.; Derosa, G.; Maffioli, P.; Togni, S.; Riva, A.; et al. Potential Clinical Benefits of Quercetin in the Early Stage of COVID-19: Results of a Second, Pilot, Randomized, Controlled and Open-Label Clinical Trial. Int. J. Gen. Med. 2021, 14, 2807–2816. [Google Scholar] [CrossRef]
- Di Pierro, F.; Derosa, G.; Maffioli, P.; Bertuccioli, A.; Togni, S.; Riva, A.; Allegrini, P.; Khan, A.; Khan, S.; Khan, B.A.; et al. Possible Therapeutic Effects of Adjuvant Quercetin Supplementation Against Early-Stage COVID-19 Infection: A Prospective, Randomized, Controlled, and Open-Label Study. Int. J. Gen. Med. 2021, 14, 2359–2366. [Google Scholar] [CrossRef]
- Önal, H.; Arslan, B.; Üçüncü Ergun, N.; Topuz, Ş.; Yilmaz Semerci, S.; Kurnaz, M.E.; Molu, Y.M.; Bozkurt, M.A.; Süner, N.; Kocataş, A. Treatment of COVID-19 patients with quercetin: A prospective, single center, randomized, controlled trial. Turk. J. Biol. 2021, 45, 518–529. [Google Scholar] [CrossRef]
- Xia, L.; Shi, Y.; Su, J.; Friedemann, T.; Tao, Z.; Lu, Y.; Ling, Y.; Lv, Y.; Zhao, R.; Geng, Z.; et al. Shufeng Jiedu, a promising herbal therapy for moderate COVID-19: Antiviral and anti-inflammatory properties, pathways of bioactive compounds, and a clinical real-world pragmatic study. Phytomedicine 2021, 85, 153390. [Google Scholar] [CrossRef]
- Feng, Y.; Zhu, B.; Liu, Y.; Liu, Y.; Zhou, G.; Yang, L.; Liu, L.; Ren, J.; Hou, Y.; Yu, H.; et al. Yindan Jiedu granules exhibit anti-inflammatory effect in patients with novel Coronavirus disease (COVID-19) by suppressing the NF-κB signaling pathway. Phytomedicine 2022, 95, 153784. [Google Scholar] [CrossRef]
- Imran, M.; Khan, S.A.; Abida’ Alshrari, A.S.; Eltahir Mudawi, M.M.; Alshammari, M.K.; Harshan, A.A.; Alshammari, N.A. Small molecules as kinetoplastid specific proteasome inhibitors for Leishmaniasis: A patent review from 1998 to 2021. Expert Opin. Ther. Pat. 2022, 27, 887–906. [Google Scholar] [CrossRef]
- Imran, M.; Khan, S.A.; Alshammari, M.K.; Alreshidi, M.A.; Alreshidi, A.A.; Alghonaim, R.S.; Alanazi, F.A.; Alshehri, S.; Ghoneim, M.M.; Shakeel, F. Discovery, Development, Inventions, and Patent Trends on Mobocertinib Succinate: The First-in-Class Oral Treatment for NSCLC with EGFR Exon 20 Insertions. Biomedicines 2021, 9, 1938. [Google Scholar] [CrossRef]
- Imran, M.; Khan, S.A.; Alshammari, M.K.; Alqahtani, A.M.; Alanazi, T.A.; Kamal, M.; Jawaid, T.; Ghoneim, M.M.; Alshehri, S.; Shakeel, F. Discovery, Development, Inventions and Patent Review of Fexinidazole: The First All-Oral Therapy for Human African Trypanosomiasis. Pharmaceuticals 2022, 15, 128. [Google Scholar] [CrossRef]
- Imran, M.; Alsharari, A.S.; Thabet, H.K.; Abida; Bakht, A.M. Synthetic molecules as DprE1 inhibitors: A patent review. Expert Opin. Ther. Pat. 2021, 31, 759–772. [Google Scholar] [CrossRef]
- Imran, M.; Asdaq, S.M.B.; Khan, S.A.; Unnikrishnan Meenakshi, D.; Alamri, A.S.; Alsanie, W.F.; Alhomrani, M.; Mohzari, Y.; Alrashed, A.; AlMotairi, M.; et al. Innovations and Patent Trends in the Development of USFDA Approved Protein Kinase Inhibitors in the Last Two Decades. Pharmaceuticals 2021, 14, 710. [Google Scholar] [CrossRef]
- Niedzwiecki, A.; Rath, M.W.; Ivanov, V.O.; Goc, A. Pharmaceutical Micronutrient Composition, and Its Use to Simultaneously Inhibit Multiple Cellular Mechanisms of Infectivity Caused by Coronavirus, Its Variants and Mutants. U.S. Patent Application No. US2021315857A1, 14 October 2021. [Google Scholar]
- Khalil, I.T.I.; Abd El-Latif, S.A.M. A New and Safe Pharmaceutical Preparation for Prophylaxis and Treatment of Respiratory Viral Infections, Especially Corona Viruses. PCT Patent Application Publication No. WO2021259441A1, 30 December 2021. [Google Scholar]
- Hofleitner, P. Composition for Treating Viral Infections. PCT Patent Application Publication No. WO2021257252A1, 23 December 2021. [Google Scholar]
- Hahn, N. Nutraceutical Composition. PCT Patent Application Publication No. WO2021255464A1, 23 December 2021. [Google Scholar]
- Salunke, P.P.; Salunke, V.P.; Patil, P.E. A Composition for Management of COVID-19 and Associated Disorders. PCT Patent Application Publication No. WO2021240481A2, 2 December 2021. [Google Scholar]
- Spadavecchia, J.; Giousuè Balzanelli, M.; Derrien, M. Composition for the Prevention or Treatment of COVID-19. PCT Patent Application Publication No. WO2021205083A2, 14 October 2021. [Google Scholar]
- Suzman, P.; Fisherman, J.; Lunsmann, W. Methods and Compositions for Treating Viral Respiratory Infections. PCT Patent Application Publication No. WO2021168173A1, 26 August 2021. [Google Scholar]
- Voelkel, N.F.; Magolske, C. Method and Composition for Treating Coronavirus, Influenza, and Acute Respiratory Distress Syndrome. U.S. Patent Application No. US2022040227A1, 10 February 2022. [Google Scholar]
- Schack, D.M.; Campbell, A.W. Anthocyanin and Quercetin Based Formulations for Improved Respiratory Health. U.S. Patent Application No. US2021393579A1, 23 December 2021. [Google Scholar]
- Margolin, L. Compositions and Methods for Dietary Enhancement of Immune System Function. U.S. Patent Application No. US2021386779A1, 16 December 2021. [Google Scholar]
- Meydani, S.N.; Mozaffarian, D. Nutritional Supplement to Combat COVID-19 and Aid Vaccination. U.S. Patent Application No. US2021361700A1, 25 November 2021. [Google Scholar]
- Hazan, S. Methods of Preventing and Treating COVID-19 Infection. U.S. Patent Application No. US2021290718A1, 23 September 2021. [Google Scholar]
- Li, Y.; Miao, L.; Zhao, W.; Xu, Z.; Pan, X.; Zhou, W.; Sun, M.; Zuo, Y. Small- Molecule Inhibitor for Blocking Combination of COVID-19 Spinous Protein and Human Angiotensin Converting Enzyme 2 and Application Thereof. Chinese Patent Application Publication No. CN112457281A, 9 March 2021. [Google Scholar]
- Su, W.; Yang, Z.; Zhong, N.; Ma, Q.; Li, R.; Li, P.; Peng, W.; Wu, H.; Shi, R.; Wang, Y. Herba Hyperici Japonici Extract and Application Thereof in Preparation of Novel Coronavirus Resistant Drug. Chinese Patent Application Publication No. CN112263598A, 26 January 2021. [Google Scholar]
- Feng, Y.; Xu, C.; Gao, J.; Ge, Q.; Lu, Y.; Wu, Z.; Zhang, Y. Application of Quercetin to Preparation of Anti-Novel Coronavirus Drugs. Chinese Patent Application Publication No. CN112022845A, 4 December 2020. [Google Scholar]
- Nezami, M. Compositions and Methods for Preventing and/or Treating Viral Infection. PCT Patent Application Publication No. WO2021262749A1, 30 December 2021. [Google Scholar]
- Newman, R.A.; Addington, O.C. Method and Compositions for Treating Coronavirus Infection. U.S. Patent 1,072,9735 B1, 4 August 2020. [Google Scholar]
- Barnhill, S. Antiviral Compositions and Methods for Their Use. PCT Patent Application Publication No. WO2022024097A1, 3 February 2022. [Google Scholar]
- St. John, A. Targeting Immune Pathologies Induced by Highly Pathogenic Coronaviruses. PCT Patent Application Publication No. WO2022019828A1, 27 January 2022. [Google Scholar]
- Yimam, M.; Jiao, P.; Horm, T.; Brownell, L.; Hong, M.; O’Neal, A.; Jia, Q. Aloe Based Compositions Comprising Polysaccharides and Polyphenols for Regulation of Homeostasis of Immunity. U.S. Patent Application No. US2022016196A1, 20 January 2022. [Google Scholar]
- Kelesidis, T.; Arumugaswami, V.; Garcia, Jr. Compositions and Methods for Inhibiting and Treating Coronavirus Infections. PCT Patent Application Publication No. WO2022015570A1, 20 January 2022. [Google Scholar]
- Ghaderi, L. Compositions and Methods for Inducing Biological Mimicry in a Mammal for the Prevention and/or Treatment of COVID-19 and Other Diseases. U.S. Patent Application No. US20220001014A1, 6 January 2022. [Google Scholar]
- Robitaille, M.; Laurin, P.; Gagnon, L.; Cesari, F. Pharmacotherapeutic Doses of Hesperidin or Related Bioflavonoids to Adress Infectious and/or Inflammatory Diseases. PCT Patent Application Publication No. WO2021248254A2, 16 December 2021. [Google Scholar]
- Hickok, S.S. Improved Immunomodulator Compositions and Viral Pathogen Treatments. PCT Patent Application Publication No. WO2021245365A1, 9 December 2021. [Google Scholar]
- Obiso, R.; Newman, R.; Addington, O. Extract Containing Oleandrin and Method of Production Thereof. PCT Patent Application Publication No. WO2021242590A1, 2 December 2021. [Google Scholar]
- Dite, G.S.; Murphy, N.M.; Allman, R. Methods of Assessing Risk of Developing a Severe Response to Coronavirus Infection. PCT Patent Application Publication No. WO2021237292A1, 2 December 2021. [Google Scholar]
- Adnot, S.; Trottein, F.; Bernard, D. Method to Treat a Pathogen Lung Infection. PCT Patent Application Publication No. WO2021233948A1, 25 November 2021. [Google Scholar]
- Zhang, H. Compositions and Methods for Preventing and/or Treating Microbial Infections. PCT Patent Application Publication No. WO2021237215A1, 25 November 2021. [Google Scholar]
- Weiguang, W.; Vinodh, K. Topical Disulfiram for Treating Viral Infections. PCT Patent Application Publication No. WO2021234362A1, 25 November 2021. [Google Scholar]
- Ichim, T.; Lin, F.; Pingle, S.; Ashili, S. Augmentation of Cell Therapy Efficacy by Inhibition of Complement Activation Pathways. U.S. Patent Application No. US20210353685A1, 18 November 2021. [Google Scholar]
- O’heeron, P.; Ichim, T. Reduction of Cytokine Storm and Pathological Inflammation Including Caused by Coronavirus Using Sphagnum and Extracts Thereof. PCT Patent Application Publication No. WO2021226627A1, 11 November 2021. [Google Scholar]
- Fliri, A. Drug Combinations for Inhibiting Infectivity of Influenza and Corona Viruses. PCT Patent Application Publication No. WO2021222240A1, 4 November 2021. [Google Scholar]
- Lecanu, L. Pannexin-1 Inhibitors for the Treatment of SARS-COV-2 Infected COVID-19 Patients with or without an Associated Acute Respiratory Syndrome. PCT Patent Application Publication No. WO2021220137A2, 4 November 2021. [Google Scholar]
- Finzi, E. Zinc for Treating COVID-19. PCT Patent Application Publication Number WO2021216562A1, 28 October 2021. [Google Scholar]
- Hoag, G.G.; Salerno, J. Method for Treating Viral and Bacterial Infection Through Inhalation Therapy. PCT Patent Application Publication No. WO2021216749A1, 28 October 2021. [Google Scholar]
- Parkin, J.; Wells, J.M.; Dransfield, M. Methods Involving Neutrophil Elastase Inhibitor Alvelestat for Treating Coronavirus Infection. PCT Patent Application Publication No. WO2021209740A1, 21 October 2021. [Google Scholar]
- Vecht-Lifshitz, S.E. Pharmaceutical Compositions for Treating Corona Virus Disease. PCT Patent Application Publication Number WO2021205437A1, 14 October 2021. [Google Scholar]
- Mcmahon, C.D.; Watson, D. Viral Treatments Involving Manuka Honey and Components Thereof. PCT Patent Application Publication No. WO2021206566A1, 14 October 2021. [Google Scholar]
- Newman, R.A.; Addington, O.C.; Obiso, R.J. Method and Compositions for Treating Coronavirus Infection. PCT Patent Application Publication No. WO2021202103A2, 7 October 2021. [Google Scholar]
- Newman, R.A.; Addington, O.C.; Obiso, R. Method and Compositions for Treating Coronavirus Infection. PCT Patent Application Publication No. WO2021201903A1, 7 October 2021. [Google Scholar]
- Ichim, T.; O’heeron, P. Fibroblast Mediated Expansion and Augmentation of Immune Regulatory Cells for Treatment of Acute Respiratory Distress Syndrome (ARDS). PCT Patent Application Publication No. WO2021202032A1, 7 October 2021. [Google Scholar]
- Budak, G.G.; Budak, M. The Natural Antiviral and Anti-Inflammatory Compound Consist of Bioflavonoids Which Extracted from Papaver Rhoeas Red Petals. PCT Patent Application Publication No. WO2021194436A1, 30 September 2021. [Google Scholar]
- Davidoff, A. Methods of Treating Viral Infections and Health Consequences. PCT Patent Application Publication No. WO2021186250A2, 23 September 2021. [Google Scholar]
- Popov, T.; Josling, P.D. Compositions and Applications Thereof. PCT Patent Application Publication No. WO2021160982A1, 19 August 2021. [Google Scholar]
- Xiao, Z.; Xiao, S.; Peng, G.; He, Z. Formulations Comprising Botanical Extracts. Australian Patent Application Publication No. AU2021106876A4, 25 November 2021. [Google Scholar]
- Jain, S. Method and System for Detecting and Treating Exposure to an Infectious Pathogen. PCT Patent Application Publication No. WO2021211620A1, 21 October 2021. [Google Scholar]
- Winchester, H. Methods of Diagnosing Risk of Serious Symptoms from COVID-19 Infection. U.S. Patent Application No. US2021293827A1, 23 September 2021. [Google Scholar]
- Kang, S.; Kwon, J.; Jung, Y.; Choi, S.; Lee, Y. SARS-CoV-2 Composition for the Prevention or Treatment of SARS-CoV-2 Infection Comprising the Extract of Agrimonia pilosa as an Active Ingredient. Korean Patent Application Publication No. KR20210141341A, 23 November 2021. [Google Scholar]
- Chung, J.Y.; Lee, P.G.; Kim, Y.P.; Lee, J.H.; Lee, J.J. Coronavirus Therapeutic Agent Comprising Zanthoxylum piperitum Leaf Extract as Active Ingredient. PCT Patent Application Publication No. WO2021230667A1, 18 November 2021. [Google Scholar]
- Ye, D.; Lu, Y.; Zhou, J.; Lin, Y.; Li, H.; Deng, C. Medicinal and Edible Traditional Chinese Medicine Preparation and Application Thereof. Chinese Patent Application Publication No. CN111773282A, 16 October 2020. [Google Scholar]
- Rincome, U.; Rincome, S. Fermented—Concentrated Extraction Method of Houttuynia cordata for Medical Using. PCT Patent Application Publication No. WO2021236023A1, 25 November 2021. [Google Scholar]
- Niedzwiecki, A.; Rath, M.W.; Ivanov, V.; Goc, A. Micronutrient Combination to Inhibit Coronavirus Cell Infection. U.S. Patent Application No. US20220047545A1, 17 February 2022. [Google Scholar]
- Ichim, T.; Lin, F.; Pingle, S.; Ashili, S. Treatment of Acute Respiratory Distress Syndrome by T Regulatory Cells. U.S. Patent Application No. US20210308186A1, 7 October 2021. [Google Scholar]
- Stafford, V.R. Method to Mitigate Morbidity and Mortality in Virally Induced forms of ACE2 Receptor Pathology Progressing to SARS or ARDS. U.S. Patent Application No. US2021315910A1, 14 October 2021. [Google Scholar]




| Intervention | Primary Purpose (Phase; Status; Results) | NCT Number (Completion Date) | Sponsor (Location of the Clinical Trial) |
|---|---|---|---|
| Standard care + Quercetin phytosome (400 mg/day for 30 days) | Treatment (3; completed; published) | NCT04578158 (15 April 2021) | Liaquat University of Medical & Health Sciences (Pakistan) |
| Quercetin (500 mg/day) for non-COVID-19 individual group for 3 months | Prevention (not mentioned; completed; published) | NCT04377789 (31 August 2020) | Kanuni Sultan Suleyman Training and Research Hospital (Turkey) |
| Standard care + 600 mg/day of quercetin for the first week and 400 mg/day of quercetin for the second week | Treatment (not mentioned; completed; published) | NCT04861298 (29 August 2021) | King Edward Medical University Teaching Hospital (Pakistan) |
| Dietary supplement (zinc and vitamin C) + quercetin (500 mg/day) to COVID-19 patients for 4 weeks | Treatment (4; recruiting; not posted) | NCT04468139 (30 July 2020) | Ministry of Health, First Health Cluster (Saudi Arabia) |
| Standard care + a combination of quercetin and curcumin (No dose mentioned) | Treatment (not mentioned; completed; not posted) | NCT05130671 (31 December 2021) | King Edward Medical University (Pakistan) |
| Standard care + quercetin (268 mg/day) + curcumin (168 mg/day) + vitamin D3 (360 IU) for 14 days | Treatment (not mentioned; recruiting; not posted) | NCT05008003 (31 March 2022) | Liaquat University of Medical & Health Sciences (Pakistan) |
| Quercetin (500 mg, 2 times/day) for 3 months | Prevention (not mentioned; completed; published) | NCT05037240 (25 May 2021) | Personal Services Company of Pavia (Italy) |
| Quercetin tablet 3 times/day (dose not mentioned) for 10 days before the meal | Treatment (1; completed; not posted) | NCT04851821 (30 June 2021) | Sahloul University Hospital (Tunisia) |
| Masitinib (3 mg/kg/day for 4 days, then 4.5 mg/kg/day) + isoquercetin (prodrug of quercetin, 1 g/day orally) for 15 days | Treatment (2; recruiting; not posted) | NCT04622865 (June 2022) | AB Science (France) |
| Two quercetin tablets (dose not mentioned) per day before meal for 10–30 days | Treatment (1; recruiting; not posted) | NCT04853199 (30 August 2021) | Sahloul University Hospital (Tunisia) |
| Standard care (zinc and vitamin C) + 2 capsules of the extract of Psidii guava (a source of quercetin) 3 times a day for 1 week | Treatment (3; completed; not posted) | NCT04810728 (30 January 2021) | Baiturrahmah University (Indonesia) |
| Hydroxychloroquine (0–400 mg) + azithromycin (0–500 mg + dietary supplements, including 0–600 mg quercetin (exact doses not mentioned) | Prevention (1; withdrawn; not posted) | NCT04590274 (December 2021) | International Brain Research Foundation (Not mentioned) |
| Isoquercetin (prodrug of quercetin) 1 g 2 times/day on day 1, followed by 500 mg 2 times/day for 27 days | Treatment (2; not yet recruiting; not posted) | NCT04536090 (June 2023) | Montreal Clinical Research Institute (Not mentioned) |
| Ivermectin (0.4 mg/kg) to treat outpatients, including supplemental treatment comprising quercetin (dose not mentioned) | Observational (not mentioned; not yet recruiting; not posted) | NCT05045937 (20 September 2023) | Patrick Robinson (United States) |
| NASAFYTOL (capsules encompassing quercetin, turmeric extract, and vitamin D3) for 14 days | Treatment (not mentioned; completed; not posted) | NCT04844658 (29 October 2021) | Tilman and Artialis (Belgium) |
| Patent/Application Number (Applicant; Publication Date; Priority Country) | Summary of the Claimed Invention to Prevent/Treat COVID-19 | Examples of Studies on Quercetin or Its Compositions |
|---|---|---|
| US2021315857A1 (Rath Matthias W.; 14 October 2021; United States) | A composition comprising many ingredients, including quercetin [43] | In vitro |
| WO2021259441A1 (Khalil et al.; 30 December 2021; Egypt) | A composition containing quercetin and tamarixin [44] | Clinical |
| WO2021257252A1 (Hofleitner Peter; 23 December 2021; United States) | A composition encompassing luteolin, quercetin, kaempferol, vitamin C [45] | Clinical |
| WO2021255464A1 (Hahn Norman; 23 December 2021; United Kingdom) | A nutraceutical composition comprising many ingredients, including quercetin [46] | Clinical |
| WO2021240481A2 (Vedicinals India Private Limited; 2 December 2021; India) | A composition comprising many active ingredients, including quercetin [47] | In vitro and clinical |
| WO2021205083A2 (Spadavecchia et al.; 14 October 2021; France) | A composition of 2,6-di-tert-butyl-4-methyl-phenol that may optionally contain quercetin [48] | In vitro |
| WO2021168173A1 (Synkine Therapeutics; 26 August 2021; United States) | A composition of quercetin and iota-carrageenan [49] | In vitro |
| US2022040227A1 (ReversPAH; 10 February 2022; United States) | A composition comprising a copper chelator, a 5-lipoxygenase inhibitor, and quercetin [50] | Not present |
| US2021393579A1 (Zestt Wellness Limited; 23 December 2021; United States) | A composition comprising anthocyanins and quercetin [51] | Prophetic clinical study |
| US2021386779A1 (Margolin Leon; 16 December 2021; United States) | A composition containing a zinc ionophore (quercetin) and a bio-assimilable form of zinc (Zn+2) [52] | Clinical |
| US2021361700A1 (Tufts College; 25 November 2021; United States) | A composition encompassing zinc, quercetin, vitamin E, and epigallocatechin gallate [53] | Prophetic clinical study |
| US2021290718A1 (Hazan Sabine; 23 September 2021; United States) | A composition comprising quercetin, vitamin C, vitamin D, zinc, and artemisia [54] | Clinical |
| CN112457281A (Dalian University of Technology; 9 March 2021; China) | Quercetin disulfonated derivatives [55] | In vitro |
| CN112263598A (Sun Yat-Sen University; 26 January 2021; China) | Aqueous ethanol extract of Tianjihuang containing quercetin [56] | In vitro |
| CN112022845A (Zhejiang Provincial Center for Disease Control and Prevention; 4 December 2020; China) | Use of quercetin (dose 12.5 to 100 μg/mL) to treat COVID-19 [57] | In vitro |
| WO2021262749A1 (Research Cancer Institute of America; 30 December 2021; United States) | A composition consisting of quercetin and hydrogen peroxide [58] | Clinical |
| US10729735B1 (Phoenix Biotechnology; 4 August 2020; United States) | A composition comprising oleandrin that may optionally contain quercetin [59] | Not present |
| WO2022024097A1 (AI Pharmaceuticals; 3 February 2022; United States) | A composition comprising edible mushrooms that may optionally contain quercetin [60] | Not present |
| WO2022019828A1 (National University of Singapore; 27 January 2022; Singapore) | A composition comprising mast cell stabilizers, including quercetin [61] | Not present |
| US2022016196A1 (Unigen; 20 January 2022; United States) | A composition containing aloe extract, poria extract, and rosemary extract [62] | Not present |
| WO2022015570A1 (University of California; 20 January 2022; United States) | A composition comprising one or more mitochondrial antioxidants, including quercetin [63] | Not present |
| US20220001014A1 (Ghaderi Lida; 6 January 2022; United States) | A composition comprising an inflammation/protease/cytokine inhibitors, and angiotensin receptor 1 inhibitor (quercetin) [64] | Not present |
| WO2021248254A2 (Ingenew Pharma; 16 December 2021; United States) | A composition including a flavonoid (hesperidin, hesperetin, naringin, naringenin, diosmin, quercetin, and myricetin) [65] | Not present |
| WO2021245365A1 (Remedy Research Limited; 9 December 2021; United Kingdom) | An aqueous composition comprising zinc ions or zinc ionophores (quercetin), sulfur ions, and ammonium ions [66] | Not present |
| WO2021242590A1 (Phoenix Biotechnology; 2 December 2021; United States) | A composition encompassing oleandrin and another compound (quercetin) [67] | Not present |
| WO2021237292A1 (Genetic Technologies Limited; 2 December 2021; Australia) | A method of treating COVID-19 using drugs (favipiravir, remdesivir, quercetin, etc.) [68] | Not present |
| WO2021233948A1 (INSERM; 25 November 2021; Europe) | A composition of senotherapeutic compounds (navitoclax, dasatinib, quercetin, etc.) [69] | Not present |
| WO2021237215A1 (University of Louisville Research Foundation; 25 November 2021; United States) | A composition comprising plant-derived (curcumin, resveratrol, quercetin, etc.) exosome-like particles [70] | Not present |
| WO2021234362A1 (University of Wolverhampton; 25 November 2021; United Kingdom) | A non-orally administered composition encompassing disulfiram that may optionally contain quercetin [71] | Not present |
| US20210353685A1 (Brain Cancer Research Institute; 18 November 2021; United States) | A method to increase the efficacy of the cell therapy using antioxidants (ascorbic acid, tocopherol, rutin, quercetin, etc.) [72] | Not present |
| WO2021226627A1 (Figene; 11 November 2021; United States) | A composition of sphagnum extract that may optionally contain quercetin [73] | Not present |
| WO2021222240A1 (Systamedic; 4 November 2021; United States) | A composition containing a polymerase inhibitor (rucaparib, fisetin, quercetin, etc.) and a viral replication inhibitor (remdesivir, molnupiravir, favipiravir, etc.) [74] | Not present |
| WO2021220137A2 (Sapir Pharmaceuticals; 4 November 2021; United States) | A composition of pannexin-1 inhibitor (probenecid) that may optionally contain quercetin [75] | Not present |
| WO2021216562A1 (Finzi Eric; 28 October 2021; United States) | A composition of zinc that may optionally include a zinc ionophore (quercetin) [76] | Not present |
| WO2021216749A1 (Hoag George Edward and Salerno John; 28 October 2021; United States) | A composition comprising TRPA1 antagonists (1,8-cineole), a plant extract of antibacterial/antiviral compounds (b-caryophyllene, etc.), and a plant extract of antioxidants (quercetin, etc.) [77] | Not present |
| WO2021209740A1 (Mereo Biopharma 4 Limited and UAB Research Foundation; 21 October 2021; United Kingdom) | A composition of alvelestat that may optionally include quercetin [78] | Not present |
| WO2021205437A1 (Vecht-Lifshitz Susan Eve; 14 October 2021; Israel) | A composition comprising a methyltransferase inhibitor, a viral enzyme inhibitor, and a SAHH (s-adenosylhomocysteine hydrolase) inhibitor that may optionally contain quercetin [79] | Not present |
| WO2021206566A1 (Manukamed Limited Partnership; 14 October 2021; New Zealand) | A composition of manuka honey that may optionally contain quercetin [80] | Not present |
| WO2021202103A2 (Phoenix Biotechnology; 7 October 2021; United States) | A composition comprising oleandrin, and digoxin that may optionally include quercetin [81] | Not present |
| WO2021201903A1 (Phoenix Biotechnology; 7 October 2021; United States) | A composition comprising oleandrin, and digoxin that may optionally include quercetin [82] | Not present |
| WO2021202032A1 (Figene; 7 October 2021; United States) | A composition containing fibroblasts and/or fibroblast-derived exosomes, and immune regulatory cells that may optionally include NF-kappa B inhibitor (quercetin) [83] | Not present |
| WO2021194436A1 (Nanobiomed Saglik Ve Yasam Bilimleri; 30 September 2021; Turkey) | A composition of quercetin, which is isolated from Papaver rhoeas red petals [84] | Not present |
| WO2021186250A2 (Davidoff Allen; 23 September 2021; United States) | A composition comprising a uric acid lowering agent (uricase) that may optionally contain antioxidants (quercetin, etc.) [85] | Not present |
| WO2021160982A1 (Nasaleze Patents; 19 August 2021; United Kingdom) | A composition including HPMC particles, and a signaling agent (menthol, strawberry, etc.) that may optionally contain a biologically active agent (quercetin) [86] | Not present |
| AU2021106876A4 (Apex Biotech Research; 25 November 2021; Australia) | A composition of andrographolide, ursolic acid, and piceid that may optionally contain an antiviral compound (quercetin) [87] | Not present |
| WO2021211620A1 (Sun Genomics; 21 October 2021; United States) | A composition comprising a probiotic, prebiotic, and/or metabolite of the gut microbiome that may optionally contain an antiviral (quercetin) [88] | Not present |
| US2021293827A1 (Winchester Henry; 23 September 2021; United States) | A composition of an anti-inflammatory drug, an anticoagulant, a thrombolytic, an immunosuppressive agent, a metal chelator, an iron supplement, or a nutraceutical (quercetin) [89] | Not present |
| KR20210141341A (APRG Company; 23 November 2021; South Korea) | A composition comprising ursolic acid or quercetin isolated from Yongacho [90] | Not present |
| WO2021230667A1 (Mecox Curemed and Choongang Ocean; 18 November 2021; South Korea) | A composition comprising Zanthoxylum piperitum leaf extract, or a compound isolated from it (quercetin of its analog) [91] | Not present |
| CN111773282A (Golden Health Foshan Technology Company; 16 October 2020; China) | A Chinese composition containing many natural ingredients, including quercetin [92] | Not present |
| WO2021236023A1 (Rincome Udom; 25 November 2021; Thailand) | A fermented Plu kow leaf powder containing quercetin [93] | Not present |
| US20220047545A1 (Rath Matthias; 17 February 2022; United States) | A composition containing many natural compounds including quercetin [94] | Present, but no comment on quercetin |
| US20210308186A1 (Brain Cancer Research Institute; 7 October 2021; United States) | A composition of NF-kappa B activity blockers (quercetin, etc.) [95] | Not present |
| US2021315910A1 (Stafford Vivi Robyn; 14 October 2021; United States) | A composition of doxycycline that may optionally contain quercetin [96] | Clinical |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imran, M.; Thabet, H.K.; Alaqel, S.I.; Alzahrani, A.R.; Abida, A.; Alshammari, M.K.; Kamal, M.; Diwan, A.; Asdaq, S.M.B.; Alshehri, S. The Therapeutic and Prophylactic Potential of Quercetin against COVID-19: An Outlook on the Clinical Studies, Inventive Compositions, and Patent Literature. Antioxidants 2022, 11, 876. https://doi.org/10.3390/antiox11050876
Imran M, Thabet HK, Alaqel SI, Alzahrani AR, Abida A, Alshammari MK, Kamal M, Diwan A, Asdaq SMB, Alshehri S. The Therapeutic and Prophylactic Potential of Quercetin against COVID-19: An Outlook on the Clinical Studies, Inventive Compositions, and Patent Literature. Antioxidants. 2022; 11(5):876. https://doi.org/10.3390/antiox11050876
Chicago/Turabian StyleImran, Mohd, Hamdy Khamees Thabet, Saleh I. Alaqel, Abdullah R. Alzahrani, Abida Abida, Mohammed Kanan Alshammari, Mehnaz Kamal, Anupama Diwan, Syed Mohammed Basheeruddin Asdaq, and Sultan Alshehri. 2022. "The Therapeutic and Prophylactic Potential of Quercetin against COVID-19: An Outlook on the Clinical Studies, Inventive Compositions, and Patent Literature" Antioxidants 11, no. 5: 876. https://doi.org/10.3390/antiox11050876
APA StyleImran, M., Thabet, H. K., Alaqel, S. I., Alzahrani, A. R., Abida, A., Alshammari, M. K., Kamal, M., Diwan, A., Asdaq, S. M. B., & Alshehri, S. (2022). The Therapeutic and Prophylactic Potential of Quercetin against COVID-19: An Outlook on the Clinical Studies, Inventive Compositions, and Patent Literature. Antioxidants, 11(5), 876. https://doi.org/10.3390/antiox11050876