Abstract
Oral submucous fibrosis (OSMF) is a chronic oral potentially malignant disorder (OPMD). It is described as a scarring disease of the oral mucosa associated with excess oxidants and insufficient antioxidants. While it is becoming increasingly accepted that oxidative stress results in excessive accumulation of collagen and progressive fibrosis of the submucosal tissues, there is limited data regarding the moderation of oxidative stress to initiate or prevent OSMF. To assess the scope for mechanism-based approaches to prevent or reverse OSMF, we systematically evaluated the existing literature and investigated the role of oxidative stress in the pathogenesis and chemoprevention of OSMF. A search for relevant articles on PubMed and Scopus was undertaken using pre-defined inclusion and exclusion criteria. A total of 78 articles were selected in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) guidelines. The articles eligible for assessment investigated both OSMF and/or oxidative stress biomarkers or specific antioxidants. Both in vitro and human studies consistently demonstrated variations in oxidative stress biomarker levels in OSMF and revealed an increase in oxidative stress, paralleling the development of the disease. Furthermore, the use of antioxidant supplements was overall associated with an improvement in clinical outcomes. Having identified the significance of oxidative stress in OSMF and the therapeutic potential of antioxidant supplements, this scoping review highlights the need for further well-designed studies in the development of mechanism-based interventions for managing OSMF.
1. Introduction
Oral submucous fibrosis (OSMF) is a chronic and subtle oral potentially malignant disorder (OPMD) affecting the oral cavity and oropharynx, in mainly the South-Asian populations [1]. Clinically, signs and symptoms of OSMF include but are not limited to, trismus, restricted tongue protrusion, burning mouth, marble-like appearance of the oral mucosa, xerostomia, recurrent ulceration, tongue papillae atrophy, and presence of palpable fibrous bands [2]. These oral complications are fundamentally due to a juxtaepithelial inflammatory reactions leading to fibroelastic changes of the lamina propria, and subsequently to the stiffness of the oral mucosa, which significantly affects patients’ quality of life by reducing their ability to eat and speak.
Although OSMF is multifactorial in origin, there is overwhelming evidence that long-term areca-nut chewing (alone or in a mixed package known also as betel quid (BQ)), is considered the main etiological factor. Constituents of BQ have in fact been shown to generate substantial amounts of reactive oxygen species (ROS) [3], which may create a biological imbalance between oxidants and antioxidants [4], playing a significant role in OSMF pathogenesis through an excessive accumulation of free radicals and production of lipid peroxides (LPO) [5,6]. BQ chewing is a time-honoured tradition for 10–20% of the world’s population [7]; therefore, attempting to eradicate a habit that has been passed down through countless generations may not be realistic in the short term. Rather than imposing a change in culture and way of life, a novel approach may be to elucidate ways to transpose oxidative stress pathways in favour of antioxidants, to prevent or reverse OSMF. While oxidative stress in OSMF unequivocally results in excessive collagen accumulation and marked fibrosis, a comprehensive review of the role of oxidative stress and antioxidant pathways in initiating and preventing OSMF is yet to be considered.
The aim of this study was to evaluate the existing literature systematically to assess the role of oxidative stress in OSMF. It was concluded that excess oxidants may drive the pathogenesis, while an excess of antioxidants may play a fundamental role in the chemoprevention of OSMF and the potential reversal of its debilitating effects.
2. Materials and Methods
2.1. Protocol and Search Strategy
This scoping review was done according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) guidelines. A search for relevant articles on Pubmed and Scopus published up to June 2021 was completed using the search string: ‘(“oral submucous fibrosis” OR betel OR “piper betel” OR “areca nut” OR gutka OR paan OR “pan masala” OR “slaked lime”) AND (“Reactive Oxygen Species” OR “oxidat*” OR “free radicals” OR “ROS” OR “Superoxide Dismutase” OR “Catalase” OR “Glutathione peroxidase” OR “antiox*” OR “Lipid Peroxides”)’.
2.2. Inclusion and Exclusion Criteria
Inclusion criteria included studies with OSMF human subjects, in vivo or in vitro models of OSMF, as well as studies assessing oxidative stress biomarkers or molecules or using antioxidants in models of fibrosis. Exclusion criteria included non-English language and non-peer-reviewed studies, systematic reviews, meta-analyses, and books/book chapters.
2.3. Study Selection and Data Extraction
The selection process involved three stages (Figure 1); the search string was imputed into databases, inclusion and exclusion criteria were applied, and duplicates were manually removed; then, papers obtained from stage 1 were screened based on titles and abstracts according to the inclusion and exclusion criteria under the guidance of the senior author (NC); full texts were analysed according to the inclusion and exclusion criteria. During all the stages, manuscripts that held any uncertainty regarding their relevance to aims of this scoping review were brought to the senior author (NC) and discussed collegially to come to a final decision.
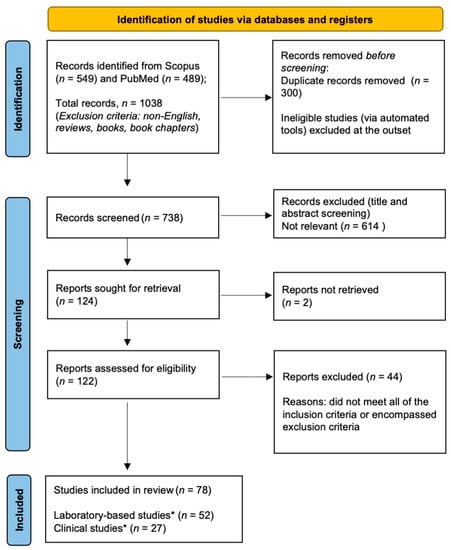
Figure 1.
Flow chart of the selection process according to PRISMA-ScR guidelines. Asterisk (*) denotes the inclusion of a dual (laboratory-based and clinical) study.
Data extracted from each article were the name of the first author, publication year, study type (in vitro, in vivo, human), PICO, oxidative stress biomarkers (if present), study design or model, assay methodology, details of treatment/intervention, if any.
3. Results
3.1. Overview of the Search Process
Details of the selection process are shown in Figure 1. A total of 549 and 489 (n = 1038) articles were retrieved from Scopus and PubMed, respectively. After removing 300 duplicates, the datasets were combined, giving a total of 738 manuscripts. A further 614 papers were excluded due to inconsistent titles and abstracts, leaving a total of 124 articles. After reading the full text, a further 45 manuscripts were excluded. The remaining 78 articles were deemed to be eligible for detailed assessment, of which 52 were laboratory-based studies (Table 1), 27 were clinical studies (Table 2), and 1 was a combination of the above-mentioned ones. There was often an overlap between clinical and laboratory-based studies, and these were included in the category that best represented the study. For example, the assessment of glutathione in a patient’s serum or saliva in the absence of clinical outcome measures was classified as a laboratory-based study.

Table 1.
Overview of laboratory-based studies that were eligible for analysis.

Table 2.
Overview of clinical intervention studies that were eligible for analysis.
3.2. Laboratory-Based Studies
Of the 51 laboratory-based studies and 1 dual study [8] included in this scoping review, 14 (26.9%) investigated the pathogenesis of OSMF, 13 (25%) demonstrated the significance of oxidative stress in the pathogenesis of OSMF, 7 (13.5%) investigated other biomarkers present in OSMF, 9 (17.3%) investigated antioxidant enzyme status in OSMF patients versus healthy controls, and ultimately 9 (17.3%) examined potential therapeutics for OSMF.
3.2.1. Pathogenesis of OSMF (Role of Betel Nut/Arecoline, Copper, and Eugenol)
Areca nut extracts (ANE) have been shown to have cytotoxic effects on hamster cheek pouch [3], normal mucosal cells [9,10], and gingival keratinocytes [11,12]. Consumption of BQ has been suggested to be associated with OSMF in oral epithelial cells [13,14], normal buccal mucosa fibroblasts (BMFs) [15], keratinocytes [16], gingival fibroblasts [17], HaCaT and HPL1D epithelial cell lines [18], and human umbilical vein endothelial cells [19]. Furthermore, copper has been shown to enhance the cytotoxicity of arecoline on epithelial cells [20], while eugenol is involved in the pathogenesis of OSMF in oral mucosal fibroblasts [21].
3.2.2. Oxidative Stress Biomarkers in the Pathogenesis of OSMF
Arecoline induces ROS generation and cell cycle arrest in human keratinocytes [22]. Compared to healthy subjects, OSMF patients had elevated levels of serum malondialdehyde (MDA) [8,23,24,25,26], copper [27,28], LPO, ceruloplasmin [28,29], nitric oxide [30], calcium, magnesium, potassium, iron, conjugated dienes, and hydroxyl radicals [28]; as well as elevated levels of salivary 8-Hydroxy-2′-deoxyguanosine (8-OHdG) [31], 8-isoprostane [32,33], lactoperoxidase, and total protein [34]. Conversely, OSMF patients had relatively decreased levels of hydrogen peroxide (H2O2) and sodium, compared to healthy controls [28].
On the other hand, there are conflicting results from the nine studies (17.3%) comparing antioxidant enzyme status in OSMF patients versus their healthy counterparts. Of these, three found decreased levels [28,35,36], while six found increased levels [8,23,34,37,38,39] of antioxidant enzymes. Banerjee and colleagues (2020) [40] evaluated mitochondrial antioxidant levels and found higher GSH and peroxiredoxins 3 levels, as well as lower glutaredoxin 2 and catalase levels. Five articles (55.5%) found more significant fluctuations with higher OSMF clinical grading and staging. Specifically, Sadaksharm (2018) [30] found lower levels of superoxide dismutase in OSMF samples, and Gupta and colleagues (2004) [8] found decreased serum β-carotene and vitamin E antioxidant levels. Lee and co-workers (2016) [41] found higher levels of transglutaminase-2 (TGM-2) expression.
3.2.3. Other Molecular Markers in OSMF
In OSMF serum samples, there were increased platelets, eosinophils, and erythrocyte sedimentation rates, and decreased haemoglobin, iron, ceruloplasmin, copper, and zinc levels, which were well correlated with disease progression [42]. There were also decreased levels of ascorbic acid and increased levels of fatty acids (FAA) [43], serum citrate, oxaloacetate, 8-OHdG [44], and 8-iso-prostaglandin F2 alpha [43]. Other markers in OSMF include tissue inhibitors of metalloproteinases (TIMP-1 and TIMP-2) [45], cyclophilin A [46], and serum uric acid level [47].
3.2.4. Potential Therapeutic Agents for OSMF
Chang et al. (2012) [48] found that N-Acetyl-L-Cysteine (NAC), apoptosis signal-regulating kinase 1 inhibitor thioredoxin, and c-Jun NH2-terminal kinase inhibitor SP600125 significantly reduced thrombin-induced connective tissue growth factor (CCN2) synthesis in human Bcl2-modified factors, while epigallocatechin-3-gallate (EGCG) completely inhibited thrombin-induced CCN2 synthesis. EGCG was later proposed as a potential therapeutic agent for OSMF [49,50,51,52]. In addition, EGCG, glutathione, and NAC were shown to be effective in inhibiting IL-6-induced epithelial-mesenchymal transition by ANE [53]. Other proposed antioxidants for managing OSMF include lycopene [54], curcumin [55], and angiotensin 1–7 [56].
3.3. Clinical Studies
Chemopreventive Effects of Nutrient Antioxidants as a Potential OSMF Treatment
A total of 26 clinical studies and one dual study [8] supported the chemoprotective role of antioxidant supplementation. Clinical improvements in at least one OSMF symptom (mouth opening, mucosal burning sensation, tongue protrusion, cheek flexibility, difficulty in swallowing and speech, pain associated with the lesion, oral health-related quality of life) were observed after administration of lycopene [57,58,59,60,61,62,63,64,65], alpha-lipoic acid [66], allicin [67], rebamipide [68], pentoxifylline [69,70], oxitard [59,71,72], aloe vera [71,73,74,75,76,77], curcumin [77,78,79,80,81], and spirulina [75,82] (Table 2).
4. Discussion
The aim of this review was to evaluate the existing literature and assess the role of oxidative stress in the pathogenesis and chemoprevention of OSMF. Overall, both laboratory-based and clinical studies consistently demonstrated variations in oxidative stress biomarker levels in OSMF, highlighting their important roles in OSMF pathogenesis and their potential as diagnostic, prognosis, or therapeutic biomarkers. Administration of nutrient antioxidants is a potentially efficacious treatment for OSMF by having chemopreventive effects with clinical improvement.
4.1. Oxidative Stress Biomarkers Is OSMF
Serum samples from OSMF patients revealed increased MDA [24], ceruloplasmin [29], LPOs [8], nitric oxide [30], and decreased levels of beta-carotene, vitamin E [8], and SOD levels [30]. Human studies largely demonstrated elevated levels of oxidative stress biomarkers serum MDA [8,23,25,26,83], salivary 8-hydroxy-2′-deoxyguanosine [31], salivary 8-isoprostane [33], salivary lactoperoxidase and total salivary protein [34], and serum copper [27,28], calcium, magnesium, potassium, iron, LPOs, conjugated dienes, and hydroxyl radicals [28]. MDA was found by two articles to be more significant with higher clinical OSMF staging and grading [8,83], while 8-OHdG (salivary oxidative stress biomarker) levels were almost double in OSMF patients [31]. Interestingly, studies investigating the level of antioxidant enzymes in OSMF have produced conflicting results. Lower antioxidant enzyme activity in OSMF patients found in some studies [28,35,36] may be due to depletion of antioxidant defence systems occurring as the consequence of overwhelming free radicals by the elevated levels of oxidative stress. Chitra et al. [28] also demonstrated decreased levels of H2O2 and sodium in OSMF.
Copper (in high levels in AN) initiates fibrinogenesis through the upregulation of lysyl oxidase, thereby inhibiting collagen degradation. High serum copper levels generate high levels of free radicals by metal-catalysed Haber–Weiss reaction, being one of the drivers of carcinogenesis in areca nut users. As a consequence, this compromises blood supply resulting in decreased flow of nutrients and ultimately will impact antioxidant levels. In line with this, Khan et al. (2015) [20] revealed that treating keratinocytes with arecoline and copper resulted in enhanced cytotoxicity, which becomes comparable to IC50 of ANE.
Glutathione S-transferase (GST), a family of Phase II detoxification enzymes that function to protect cellular macromolecules from attack by reactive electrophiles, are long-time known to protect cells from oxidative stress [84]. However, Bathi, Rao, and Mutalik (2009) [35] and Madhulatha et al. (2018) [36] did not observe strong associations between GST gene polymorphisms (GSTM1 and GSTT1) and OSMF. Compared to healthy controls, OSMF patients had considerably elevated levels of glutathione, ceruloplasmin, and malondialdehyde, but reduced levels of beta-carotene, vitamin E, and glutathione peroxidase (GPx). The three major families of SOD, with the availability of lowering the toxic effects of superoxide (O2−) and H2O2 by converting them into water, are copper/zinc, iron/manganese, and nickel type [85], and were found in decreased levels in most studies [24,30,39], but increased in others [28]. Lower antioxidant enzyme activity in OSMF patients may be due to depletion of antioxidant defence systems occurring as a consequence of overwhelming free radicals by the elevated levels of oxidative stress. In particular, increased MDA levels in serum may serve as a valuable surrogate marker in the early diagnosis, treatment, and prognosis of OSMF. Indeed, Gupta et al. (2004) [8] demonstrated that beta-carotene and vitamin E levels in plasma, increased after 6 weeks of their oral administration to OSMF patients, along with decreased MDA levels associated with clinical improvement.
In summary, there is sound evidence that oxidative stress biomarkers are altered in OSMF tissues as well as in patients’ blood.
4.2. Chemopreventive Effects of Nutrient Antioxidants as an Efficacious Treatment for OSMF
Multiple compounds with antioxidant and anti-cariogenic properties were investigated to assess their effectiveness in managing or preventing OSMF. The mechanism of action of antioxidants may involve immune system stimulation and breakage of the free radical chain reactions [86].
Six articles investigated the efficacy of aloe vera in managing OSMF with substantial antioxidant vitamins and enzymes, topical aloe vera was effective in improving at least one of the following parameters: mouth burning and opening [71,72,73,74,76,77], tongue protrusion [71,73,76], cheek flexibility [73,76], and speech and swallowing [71].
Lycopene is a carotene, carotenoid pigment, and phytochemical with potent antioxidant and anti-carcinogenic properties. Documented as a non-invasive treatment option, it yields significant improvements in OSMF signs and symptoms [54]. Its administration improved mouth opening [57,58,60,61,62,63,64,65], tongue protrusion [58,61], cheek flexibility [61], and burning sensation [58,59,60,61,64,65]. When compared to betamethasone injections, Goel and Ahmed (2015) [57] found that lycopene was more effective in improving mouth opening in subjects with an initial mouth opening distance of less than 19 mm, but less effective with distances between 20–44 mm.
Curcumin, the main natural polyphenol found in Curcuma species [87], has been shown to target multiple signalling molecules. The current literature suggests that the use of curcumin (either as a topical gel or oral tablets) in OSMF patients improved burning sensation [77,79,80], mouth opening [77,79,80,81], and tongue protrusion [79]. Curcumin was found to be more effective than intralesional steroid injections in improving tongue protrusion and mouth burning [80]. Meanwhile, arecoline can stimulate CCN2, enhancing OSMF’s pro-fibrotic activity. Curcumin can block the arecoline-induced CCN2 expression, thus making it a potentially useful agent in controlling OSMF [16,55]. Furthermore, curcumin influences levels of oxidative stress markers and antioxidants: it decreased MDA and 8-oxo-2′-deoxyguanosine levels [78] while increasing salivary and serum vitamin C/E levels. Vitamin C plays a protective role in carcinogenesis as an antioxidant, reducing vitamin E degradation and enhancing detoxification via cytochrome P450 [88].
Epigallocatechin gallate (EGCG) is a plant-based potent antioxidant protecting against cellular damage caused by free radicals [53], with potential uses in OSMF. Hsieh and colleagues (2018) noted that EGCG dose-dependently inhibited arecoline-induced transforming growth factor 1 (TGFb1) activation in BMFs. BMFs exposed to arecoline resulted in the generation of mitochondrial ROS, which activated latent TGFb1, and in turn, stimulated CCN2 and early growth response-1 (Egr-1) synthesis. TGFb1 may play a pivotal role in the pathogenesis of OSMF; thus, EGCG can be a useful agent in the chemoprevention and treatment of OSMF. EGCG blocks TGFb1-induced CCN2 synthesis by suppressing c-JunNH2-terminal kinase (JNK), p38 mitogen-activated protein kinase, and activin receptor-like kinase 5 (ALK5) [49]. Hsieh and co-workers (2017) concluded that ALK5, Smad3, extracellular signal-regulated kinase, and JNK are involved in the TGFb1-induced Egr-1 protein production in BMFs. Egr-1 mediates COL1A1 and COL1A2 mRNA expression and acid-soluble collagen production in BMFs. EGCG can block TGFb1-induced collagen production by attenuating Egr-1 expression, which is a key mediator in the TGFb1-induced pathogenesis of OSMF. Hsieh et al., 2015, noted that arecoline induces an overexpression of Egr-1, which enhances the profibrotic activity seen in OSMF. EGCG was shown to completely block arecoline-induced Egr-1 expression in human buccal fibroblasts.
Lastly, other compounds such as alpha-lipoic acid (ALA) are worth noting due to their effects in alleviating burning sensation and improving mouth opening. Along with conventional intralesional steroid injections, ALA was indeed able to reverse higher clinical stages [66]. Jiang et al. [67] demonstrated that when compared to triamcinolone acetonide, allicin, a defence molecule derived from garlic exhibited greater and more stable augmentation in mouth opening, alleviation of mucosal burning sensation, and improvement in ‘Oral Health Related Quality of Life’ (OHRQoL) score. Rebamipide, an amino acid derivative of 2-quinolinone used for gastrointestinal mucosal protection was found effective in managing burning sensation [68]. Oral pentoxifylline, as an adjunct to surgical reconstruction, improves mouth opening, burning sensation, and relapse [70]. Oxitard capsules resulted in significant clinical improvements in mouth-opening, tongue protrusion, swallowing, speech pain associated with the lesion, and burning sensation when compared with placebo [72]. Turmeric/black pepper and Nigella sativa improved mouth opening, burning sensation, and SOD levels [89]. Lastly, spirulina, which has multiple antioxidant effects, was shown to improve mouth opening and burning sensation [82].
Eight papers compared the chemopreventive effects of nutrient antioxidants against each other. When compared to standard antioxidant capsules, aloe vera produced greater improvements in burning sensation and mouth opening [74], while pentoxifylline showed further reduction in dysphagia and burning sensation [69]. Aloe vera was less effective in improving mouth opening when compared to spirulina [75], and also less effective in improving swallowing, speech, and pain associated with the lesion when compared with oxitard capsules [71]. However, when compared to curcumin, aloe vera brought larger improvements in burning sensation, but the two were equally effective in increasing mouth opening [77]. Three studies compared lycopene to other antioxidants, yielding conflicting results. Saran et al. [58] found lycopene to be more effective than curcumin in improving mouth opening and burning sensation, while Piyush et al. [61] found no differences between the two. Patil et al. [59] found that lycopene was less effective than oxitard in improving mouth opening and tongue protrusion. Compared to Nigella sativa, turmeric/black pepper showed greater improvements in mouth opening, burning sensation, and SOD levels [89]).
In summary, results of clinical studies show that there is clear scope for investigating the preventive and therapeutic effects of antioxidants in betel nut chewers and patients with OSMF through well-designed, large, controlled studies.
4.3. Strengths and Limitations
By providing a broad overview of relevant studies, this scoping review has established the current understanding of the topic of interest while identifying gaps in the literature. However, we must acknowledge the possibility that we may have missed some relevant studies including non-English articles and articles with no full text. The balance of breadth and depth of the data reported in this article was also challenging due to the volume of articles identified and time constraints.
This present review was not aimed at providing a comprehensive pathogenic model of OSMF. While oxidative stress is indeed associated with OSMF, tissue inflammation is crucial for the induction of tissue fibrosis. The involvement of prostanoids and other inflammatory mediators [90,91] as well as immune cells (recently reviewed in [92]) is central to OSMF pathogenesis but was not in the scope of our review.
4.4. Future Directions
The current literature is in favour of antioxidants being used for OSMF prevention and management because it is relatively non-toxic and can be easily supplemented in the diet. Our findings have important public health implications for the management and reversal of OSMF’s debilitating effects. Additionally, our results can be used as a helpful precursor for future systematic reviews to deepen knowledge of pathogenesis and chemoprevention of OSMF. This review may also serve as a framework for future studies to help inform the development of mechanism-based interventions and a clear guidelines for managing patients with OSMF using antioxidants.
5. Conclusions
Understanding the molecular profile of distinct BQ components and how these mediate the pathogenesis of OSMF is a key challenge if we are to develop mechanism-based preventive or therapeutic strategies for this potentially malignant disease [93,94]. This scoping review highlighted the role of oxidative stress in OSMF’s pathogenesis, as shown by altered levels of various oxidative stress biomarkers, including MDA and 8-OHdG. As such, there is the potential for these oxidative stress biomarkers to be used in OSMF diagnosis, prognosis, and potential therapeutic targets. Similarly, antioxidant enzymes, (e.g., serum SOD and ceruloplasmin) may also be used in OSMF diagnosis and prognosis as their levels were also changed in OSMF patients. Furthermore, this scoping review identified various nutrient antioxidants, (e.g., aloe vera, lycopene, curcumin, and EGCG) effective in improving the signs and symptoms of OSMF such as mouth opening, burning sensation, tongue protrusion, and cheek flexibility.
Author Contributions
Conceptualization: L.S. and N.C.; methodology: N.C.; investigation, data curation and formal analysis: A.R., E.N., K.N. and S.L.; writing—original draft preparation: A.R., E.N., K.N., S.L. and N.C.; writing—review and editing: L.S., P.Z., P.J.F., G.A. and U.R.; supervision: N.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors would like to acknowledge the support of the Melbourne Dental School, The University of Melbourne.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pindborg, J.J.; Murti, P.R.; Bhonsle, R.B.; Gupta, P.C.; Daftary, D.K.; Mehta, F.S. Oral submucous fibrosis as a precancerous condition. Scand. J. Dent. Res. 1984, 92, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.H.; Wang, T.H.; Shieh, T.M.; Tseng, Y.H. Oral Submucous Fibrosis: A Review on Etiopathogenesis, Diagnosis, and Therapy. Int. J. Mol. Sci. 2019, 20, 2940. [Google Scholar] [CrossRef] [PubMed]
- Nair, U.J.; Obe, G.; Friesen, M.; Goldberg, M.T.; Bartsch, H. Role of lime in the generation of reactive oxygen species from betel-quid ingredients. Environ. Health Perspect. 1992, 98, 203–205. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Frei, B.; Stocker, R.; Ames, B.N. Antioxidant defenses and lipid peroxidation in human blood plasma. Proc. Natl. Acad. Sci. USA 1988, 85, 9748–9752. [Google Scholar] [CrossRef]
- Sardaro, N.; Della Vella, F.; Incalza, M.A.; DI Stasio, D.; Lucchese, A.; Contaldo, M.; Laudadio, C.; Petruzzi, M. Oxidative Stress and Oral Mucosal Diseases: An Overview. In Vivo 2019, 33, 289–296. [Google Scholar] [CrossRef]
- World Health Organization. Review of Areca (Betel) Nut and Tobacco Use in the Pacific: A Technical Report; WHO Regional Office for the Western Pacific: Manila, Philippines, 2012.
- Gupta, S.; Reddy, M.V.; Harinath, B.C. Role of oxidative stress and antioxidants in aetiopathogenesis and management of oral submucous fibrosis. Indian J. Clin. Biochem. 2004, 19, 138–141. [Google Scholar] [CrossRef]
- Jeng, J.H.; Kuo, M.L.; Hahn, L.J.; Kuo, M.Y. Genotoxic and non-genotoxic effects of betel quid ingredients on oral mucosal fibroblasts in vitro. J. Dent. Res. 1994, 73, 1043–1049. [Google Scholar] [CrossRef]
- Singh, V.; Mohammad, S.; Pant, A.P.; Saimbi, C.S.; Srivastava, R. Therapeutic interventions in oral submucous fibrosis: An experimental and clinical study. J. Oral Maxillofac. Surg. 2015, 14, 278–290. [Google Scholar] [CrossRef]
- Chang, M.C.; Ho, Y.S.; Lee, J.J.; Kok, S.H.; Hahn, L.J.; Jeng, J.H. Prevention of the areca nut extract-induced unscheduled DNA synthesis of gingival keratinocytes by vitamin C and thiol compounds. Oral Oncol. 2002, 38, 258–265. [Google Scholar] [CrossRef]
- Chang, M.C.; Chan, C.P.; Chen, Y.J.; Hsien, H.C.; Chang, Y.C.; Yeung, S.Y.; Jeng, P.Y.; Cheng, R.H.; Hahn, L.J.; Jeng, J.H. Areca nut components stimulate ADAM17, IL-1α, PGE2 and 8-isoprostane production in oral keratinocyte: Role of reactive oxygen species, EGF and JAK signaling. Oncotarget 2016, 7, 16879–16894. [Google Scholar] [CrossRef]
- Chang, M.C.; Ho, Y.S.; Lee, P.H.; Chan, C.P.; Lee, J.J.; Hahn, L.J.; Wang, Y.J.; Jeng, J.H. Areca nut extract and arecoline induced the cell cycle arrest but not apoptosis of cultured oral KB epithelial cells: Association of glutathione, reactive oxygen species and mitochondrial membrane potential. Carcinogenesis 2001, 22, 1527–1535. [Google Scholar] [CrossRef]
- Jeng, J.H.; Wang, Y.J.; Chang, W.H.; Wu, H.L.; Li, C.H.; Uang, B.J.; Kang, J.J.; Lee, J.J.; Hahn, L.J.; Lin, B.R.; et al. Reactive oxygen species are crucial for hydroxychavicol toxicity toward KB epithelial cells. Cell. Mol. Life Sci. 2004, 61, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.H.; Yang, S.F.; Lee, S.S.; Chang, Y.C. Augmented heme oxygenase-1 expression in areca quid chewing-associated oral submucous fibrosis. Oral Dis. 2009, 15, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C.; Chen, Y.J.; Chang, H.H.; Chan, C.P.; Yeh, C.Y.; Wang, Y.L.; Cheng, R.H.; Hahn, L.J.; Jeng, J.H. Areca nut components affect COX-2, cyclin B1/cdc25C and keratin expression, PGE2 production in keratinocyte is related to reactive oxygen species, CYP1A1, Src, EGFR and Ras signaling. PLoS ONE 2014, 9, e101959. [Google Scholar] [CrossRef] [PubMed]
- Illeperuma, R.P.; Kim, D.K.; Park, Y.J.; Son, H.K.; Kim, J.Y.; Kim, J.; Lee, D.Y.; Kim, K.Y.; Jung, D.W.; Tilakaratne, W.M.; et al. Areca nut exposure increases secretion of tumor-promoting cytokines in gingival fibroblasts that trigger DNA damage in oral keratinocytes. Int. J. Cancer 2015, 137, 2545–2557. [Google Scholar] [CrossRef]
- Pant, I.; Rao, S.G.; Kondaiah, P. Role of areca nut induced JNK/ATF2/Jun axis in the activation of TGF-β pathway in precancerous Oral Submucous Fibrosis. Sci. Rep. 2016, 6, 34314. [Google Scholar] [CrossRef]
- Li, J.; Yao, M.; Zhu, X.; Li, Q.; He, J.; Chen, L.; Wang, W.; Zhu, C.; Shen, T.; Cao, R.; et al. YAP-induced endothelial-mesenchymal transition in oral submucous fibrosis. J. Dent. Res. 2019, 98, 920–929. [Google Scholar] [CrossRef]
- Khan, I.; Pant, I.; Narra, S.; Radhesh, R.; Ranganathan, K.; Rao, S.G.; Kondaiah, P. Epithelial atrophy in oral submucous fibrosis is mediated by copper (II) and arecoline of areca nut. J. Cell. Mol. Med. 2015, 19, 2397–2412. [Google Scholar] [CrossRef]
- Jeng, J.H.; Hahn, L.J.; Lu, E.J.; Wang, Y.J.; Kuo, M.Y. Eugenol triggers different pathobiological effects on human oral mucosal fibroblasts 1. J. Dent. Res. 1994, 73, 1050–1055. [Google Scholar] [CrossRef]
- Thangjam, G.S.; Kondaiah, P. Regulation of oxidative-stress responsive genes by arecoline in human keratinocytes. J. Periodontal Res. 2009, 44, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Tejasvi, M.A.; Bangi, B.B.; Geetha, P.; Avinash, C.A.; Chittaranjan, B.; Bhayya, H.; Donempudi, P. Estimation of serum superoxide dismutase and serum malondialdehyde in oral submucous fibrosis: A clinical and biochemical study. J. Cancer Res. Ther. 2014, 1, 722. [Google Scholar]
- Shakunthala, G.K.; Annigeri, R.G.; Arunkumar, S. Role of oxidative stress in the pathogenesis of oral submucous fibrosis: A preliminary prospective study. Contemp. Clin. Dent. 2015, 6 (Suppl. S1), S172. [Google Scholar] [CrossRef] [PubMed]
- Paulose, S.; Rangdhol, V.; Ramesh, R.; Jeelani, S.A.; Brooklyin, S. Estimation of serum malondialdehyde and assessment of DNA damage using comet assay in patients with oral submucous fibrosis. J. Investig. Clin. Dent. 2016, 7, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Kapgate, T.D.; Bhowate, R.R.; Dangore, S.; Meshram, M.; Lohe, V.K. Effect of Turmeric on Serum Malondialdehyde in Oral Submucous Fibrosis. Int. J. Cur. Res. Rev. 2020, 12, 129. [Google Scholar] [CrossRef]
- Khanna, S.; Udas, A.C.; Kumar, G.K.; Suvarna, S.; Karjodkar, F.R. Trace elements (copper, zinc, selenium and molybdenum) as markers in oral sub mucous fibrosis and oral squamous cell carcinoma. J. Trace Elem. Med. Biol. 2013, 27, 307–311. [Google Scholar] [CrossRef]
- Chitra, S.; Balasubramaniam, M.; Hazra, J. Effect of α-tocopherol on salivary reactive oxygen species and trace elements in oral submucous fibrosis. Ann. Clin. Biochem. 2012, 49, 262–265. [Google Scholar] [CrossRef]
- Shah, P.H.; Venkatesh, R.; More, C.B. Determination of role of ceruloplasmin in oral potentially malignant disorders and oral malignancy—A cross-sectional study. Oral Dis. 2017, 23, 1066–1071. [Google Scholar] [CrossRef]
- Sadaksharam, J. Significance of serum nitric oxide and superoxide dismutase in oral submucous fibrosis and squamous cell carcinoma: A comparative study. Contemp. Clin. Dent. 2018, 9, 283. [Google Scholar] [CrossRef]
- Nandakumaar, A.; Nataraj, P.; James, A.; Krishnan, R.; Mahesh, K.M. Estimation of Salivary 8-Hydroxydeoxyguanosine (8-OHdG) as a Potential Biomarker in Assessing Progression towards Malignancy: A Case-Control Study. Asian Pac. J. Cancer Prev. 2020, 21, 2325–2329. [Google Scholar] [CrossRef]
- Senghore, T.; Li, Y.F.; Sung, F.C.; Tsai, M.H.; Hua, C.H.; Liu, C.S.; Huang, R.J.; Yeh, C.C. Biomarkers of Oxidative Stress Associated with the Risk of Potentially Malignant Oral Disorders. Anticancer Res. 2018, 38, 5211–5216. [Google Scholar] [CrossRef] [PubMed]
- Meera, S.; Sarangarajan, R.; Rajkumar, K. 8-Isoprostane: A salivary oxidative stress biomarker for oral submucous fibrosis and oral squamous cell carcinoma. J. Oral Maxillofac. Pathol. 2020, 24, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Divyambika, C.V.; Sathasivasubramanian, S.; Vani, G.; Vanishree, A.J.; Malathi, N. Correlation of Clinical and Histopathological Grades in Oral Submucous Fibrosis Patients with Oxidative Stress Markers in Saliva. Indian J. Clin. Biochem. 2018, 33, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Bathi, R.J.; Rao, R.; Mutalik, S. GST null genotype and antioxidants: Risk indicators for oral pre-cancer and cancer. Indian J. Dent. Res. 2009, 20, 298–303. [Google Scholar]
- Madhulatha, G.; Das, S.; Venkateswarlu, N.; Pujar, A.; Jyothy, A.; Munshi, A. GSTM1 and GSTT1 null polymorphism and antioxidant levels in oral submucous fibrosis, leukoplakia and oral cancer patients among a South Indian Population. J. Oral Maxillofac. Surg. Med. Pathol. 2018, 30, 169–174. [Google Scholar] [CrossRef]
- Aggarwal, A.; Shetti, A.; Keluskar, V.; Bagewadi, A. Estimation of serum beta carotene levels in patients with oral submucous fibrosis in India. J. Oral Sci. 2011, 53, 427–431. [Google Scholar] [CrossRef][Green Version]
- Rathod, Y.G.; Kulkarni, S.P.; Khairnar, M.R.; Joshi, P.N.; Patle, B.K.; Pagare, J.S. Estimation of serum beta-carotene level in patients suffering from oral submucous fibrosis. J. Exp. Ther. 2018, 12, 267–271. [Google Scholar]
- Gurudath, S.; Ganapathy, K.; Sujatha, D.; Pai, A.; Ballal, S.; Ml, A. Estimation of superoxide dismutase and glutathione peroxidase in oral submucous fibrosis, oral leukoplakia and oral cancer—A comparative study. Asian Pac. J. Cancer Prev. 2012, 13, 4409–4412. [Google Scholar] [CrossRef]
- Banerjee, S.; Mukherjee, S.; Mitra, S.; Singhal, P. Comparative Evaluation of Mitochondrial Antioxidants in Oral Potentially Malignant Disorders. Kurume Med. J. 2020, 66, 15–27. [Google Scholar] [CrossRef]
- Lee, S.S.; Chen, Y.J.; Tsai, C.H.; Huang, F.M.; Chang, Y.C. Elevated transglutaminase-2 expression mediates fibrosis in areca quid chewing-associated oral submucocal fibrosis via reactive oxygen species generation. Clin. Oral Investig. 2016, 20, 1029–1034. [Google Scholar] [CrossRef]
- Anuradha, C.D.; Shyamala Devi, C.S. Studies on the hematological profile and trace elements in oral submucous fibrosis. J. Clin. Biochem. Nutr. 1995, 19, 9–17. [Google Scholar] [CrossRef]
- Rai, V.; Bose, S.; Saha, S.; Chakraborty, S. Evaluation of oxidative stress and the microenvironment in oral submucous fibrosis. Heliyon 2019, 5, e01502. [Google Scholar] [CrossRef] [PubMed]
- Kulasekaran, C.; Devi, M.; Dhivya, K.; Vijayalakshmi, D.; Sathishkumar, M.; Madhanmohan, A. Immunohistochemical detection of 8-hydroxydeoxyguanosine: A biomarker of oxidative DNA damage in oral submucous fibrosis. J. Oral Maxillofac. Pathol. 2020, 24, 536–541. [Google Scholar] [PubMed]
- Pitiyage, G.N.; Lim, K.P.; Gemenitzidis, E.; Teh, M.T.; Waseem, A.; Prime, S.S.; Tilakaratne, W.M.; Fortune, F.; Parkinson, E.K. Increased secretion of tissue inhibitors of metalloproteinases 1 and 2 (TIMPs -1 and -2) in fibroblasts are early indicators of oral sub-mucous fibrosis and ageing. J. Oral Pathol. Med. 2012, 41, 454–462. [Google Scholar] [CrossRef]
- Hou, X.; Liu, R.; Huang, C.; Jiang, L.; Zhou, Y.; Chen, Q. Cyclophilin A was revealed as a candidate marker for human oral submucous fibrosis by proteomic analysis. Cancer Biomark. 2017, 20, 345–356. [Google Scholar] [CrossRef]
- Yadav, K.D.; Patil, B.A.; Raheel, S.A.; Abuderman, A.; Patil, S.; Gaballah, K.; Kujan, O. Serum uric acid levels in patients with oral cancer, leukoplakia and submucous fibrosis: A cross-sectional study. Transl. Cancer Res. 2020, 9, 3084–3091. [Google Scholar] [CrossRef]
- Chang, J.Z.; Yang, W.H.; Deng, Y.T.; Chen, H.M.; Kuo, M.Y. Thrombin-stimulated connective tissue growth factor (CTGF/CCN2) production in human buccal mucosal fibroblasts: Inhibition by epigallocatechin-3-gallate. Head Neck 2012, 34, 1089–1094. [Google Scholar] [CrossRef]
- Chang, J.Z.; Yang, W.H.; Deng, Y.T.; Chen, H.M.; Kuo, M.Y. EGCG blocks TGFβ1-induced CCN2 by suppressing JNK and p38 in buccal fibroblasts. Clin. Oral Investig. 2013, 17, 455–461. [Google Scholar] [CrossRef]
- Hsieh, Y.P.; Chen, H.M.; Chang, J.Z.; Chiang, C.P.; Deng, Y.T.; Kuo, M.Y. Arecoline stimulated early growth response-1 production in human buccal fibroblasts: Suppression by epigallocatechin-3-gallate. Head Neck 2015, 37, 493–497. [Google Scholar] [CrossRef]
- Hsieh, Y.P.; Chen, H.M.; Lin, H.Y.; Yang, H.; Chang, J.Z. Epigallocatechin-3-gallate inhibits transforming-growth-factor-β1-induced collagen synthesis by suppressing early growth response-1 in human buccal mucosal fibroblasts. J. Formos. Med. 2017, 116, 107–113. [Google Scholar] [CrossRef]
- Hsieh, Y.P.; Wu, K.J.; Chen, H.M.; Deng, Y.T. Arecoline activates latent transforming growth factor β1 via mitochondrial reactive oxygen species in buccal fibroblasts: Suppression by epigallocatechin-3-gallate. J. Formos. Med. 2018, 117, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Illeperuma, R.P.; Kim, J. The Protective Effect of Antioxidants in Areca Nut Extract-Induced Oral Carcinogenesis. Asian Pac. J. Cancer Prev. 2020, 21, 2447–2452. [Google Scholar] [CrossRef]
- Francis, T.; Priya, V.V.; Gayathri, R.A. comparative study on the efficacy of lycopene and quercetin in oral submucous fibrosis cell lines. Drug Invent. Today 2019, 12, 1349–1351. [Google Scholar]
- Deng, Y.T.; Chen, H.M.; Cheng, S.J.; Chiang, C.P.; Kuo, M.Y. Arecoline-stimulated connective tissue growth factor production in human buccal mucosal fibroblasts: Modulation by curcumin. Oral Oncol. 2009, 45, e99–e105. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Huang, Y.; Wang, D.; Li, Y.; Wang, G.; Jin, S.; Zhu, X.; Wu, B.; Du, X.; Li, X. Angiotensin (1-7) inhibits arecoline-induced migration and collagen synthesis in human oral myofibroblasts via inhibiting NLRP3 inflammasome activation. J. Cell. Physiol. 2019, 234, 4668–4680. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Ahmed, J.A. comparative study on efficacy of different treatment modalities of oral submucous fibrosis evaluated by clinical staging in population of Southern Rajasthan. J. Cancer Res. Ther. 2015, 11, 113–118. [Google Scholar] [CrossRef]
- Saran, G.; Umapathy, D.; Misra, N.; Channaiah, S.G.; Singh, P.; Srivastava, S.; Shivakumar, S.A. Comparative study to evaluate the efficacy of lycopene and curcumin in oral submucous fibrosis patients: A randomized clinical trial. Indian J. Dent. Res. 2018, 29, 303–312. [Google Scholar] [CrossRef]
- Patil, S.R.; Yadav, S.; Al-Zoubi, I.A.; Maragathavalli, G.; Sghaireen, M.G.; Gudipaneni, R.K.; Alam, M.K. Comparative study of the efficacy of newer antioxitands lycopene and oxitard in the treatment of oral submucous fibrosis. Pesqui. Bras. Odontopediatria Clín. Integr. 2018, 16, 4059. [Google Scholar] [CrossRef]
- Johny, J.; Bhagvandas, S.C.; Mohan, S.P.; Punathil, S.; Moyin, S.; Bhaskaran, M.K. Comparison of Efficacy of Lycopene and Lycopene-Hyaluronidase Combination in the Treatment of Oral Submucous Fibrosis. J. Pharm. Bioallied Sci. 2019, 11, S260–S264. [Google Scholar] [CrossRef]
- Piyush, P.; Mahajan, A.; Singh, K.; Ghosh, S.; Gupta, S. Comparison of therapeutic response of lycopene and curcumin in oral submucous fibrosis: A randomized controlled trial. Oral Dis. 2019, 25, 73–79. [Google Scholar] [CrossRef]
- Kumar, A.; Bagewadi, A.; Keluskar, V.; Singh, M. Efficacy of lycopene in the management of oral submucous fibrosis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 103, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Karemore, T.V.; Motwani, M. Evaluation of the effect of newer antioxidant lycopene in the treatment of oral submucous fibrosis. Indian J. Dent. Res. Off. Publ. Indian Soc. Dent. Res. 2012, 23, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Arakeri, G.; Patil, S.; Maddur, N.; Rao Us, V.; Subash, A.; Patil, S.; Gao, S.; Brennan, P.A. Long-term effectiveness of lycopene in the management of oral submucous fibrosis (OSMF): A 3-years follow-up study. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2020, 49, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Gowda, B.B.K.; Yathish, T.R.; Sinhasan, S.; Naik, H.; Somayaji, P.; Anand, D. The response of oral submucous fibrosis to lycopene—A carotenoid antioxidant. A clinicopathological study. J. Clin. Diagn. Res. 2011, 5, 882–888. [Google Scholar]
- Rao, P.K. Efficacy of alpha lipoic acid in adjunct with intralesional steroids and hyaluronidase in the management of oral submucous fibrosis. J. Cancer Res. Ther. 2010, 6, 508–510. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, Y.; Li, F.; Zhu, Y.; Chen, Y.; Yang, S.; Sun, G. Allicin as a possible adjunctive therapeutic drug for stage II oral submucous fibrosis: A preliminary clinical trial in a Chinese cohort. Int. J. Oral Maxillofac. Surg. 2015, 44, 1540–1546. [Google Scholar] [CrossRef]
- Baptist, J.; Shakya, S.; Ongole, R. Rebamipide to Manage Stomatopyrosis in Oral Submucous Fibrosis. J. Contemp. Dent. Pract. 2016, 17, 1009–1012. [Google Scholar] [CrossRef]
- Kalkur, C.; Sattur, A.P.; Guttal, K.S.; Lakshman, A.R. “Introducing Modified Dakkak and Bennett Grading System for Indian Food in Oral Submucous Fibrosis”: A Dharwad Study. J. Diet. Suppl. 2019, 16, 207–214. [Google Scholar] [CrossRef]
- Kholakiya, Y.; Jose, A.; Rawat, A.; Nagori, S.A.; Jacob, S.; Roychoudhury, A. Surgical management of oral submucous fibrosis with “Seagull-nasolabial flap” combined with short-term oral pentoxifylline for preventing relapse. J. Stomatol. Oral Maxillofac. Surg. 2020, 121, 512–516. [Google Scholar] [CrossRef]
- Patil, S.; Halgatti, V.; Maheshwari, S.; Santosh, B.S. Comparative study of the efficacy of herbal antioxdants oxitard and aloe vera in the treatment of oral submucous fibrosis. J. Clin. Exp. Dent. 2014, 6, e265–e270. [Google Scholar] [CrossRef]
- Patil, S.; Al-Zarea, B.K.; Maheshwari, S.; Sahu, R. Comparative evaluation of natural antioxidants spirulina and aloe vera for the treatment of oral submucous fibrosis. J. Oral Biol. Craniofacial Res. 2015, 5, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Hebbale, M.; Mhapuskar, A.; Ul Nisa, S.; Thopte, S.; Singh, S. Effectiveness of Aloe Vera and Antioxidant along with Physiotherapy in the Management of Oral Submucous Fibrosis. J. Contemp. Dent. Pract. 2016, 17, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Sudarshan, R.; Annigeri, R.G.; Sree Vijayabala, G. Aloe vera in the treatment for oral submucous fibrosis—A preliminary study. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2012, 41, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Santosh, B.S.; Maheshwari, S.; Deoghare, A.; Chhugani, S.; Rajesh, P.R. Efficacy of oxitard capsules in the treatment of oral submucous fibrosis. J. Cancer Res. Ther. 2015, 11, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Anuradha, A.; Patil, B.; Asha, V.R. Evaluation of efficacy of Aloe vera in the treatment of oral submucous fibrosis—A clinical study. J. Oral Pathol. Med. 2017, 46, 50–55. [Google Scholar] [CrossRef]
- Nerkar Rajbhoj, A.; Kulkarni, T.M.; Shete, A.; Shete, M.; Gore, R.; Sapkal, R.A. Comparative Study to Evaluate Efficacy of Curcumin and Aloe Vera Gel along with Oral Physiotherapy in the Management of Oral Submucous Fibrosis: A Randomized Clinical Trial. Asian Pac. J. Cancer Prev. 2021, 22, 107–112. [Google Scholar] [CrossRef]
- Rai, B.; Kaur, J.; Jacobs, R.; Singh, J. Possible action mechanism for curcumin in pre-cancerous lesions based on serum and salivary markers of oxidative stress. J. Oral Sci. 2010, 52, 251–256. [Google Scholar] [CrossRef]
- Rai, A.; Kaur, M.; Gombra, V.; Hasan, S.; Kumar, N. Comparative evaluation of curcumin and antioxidants in the management of oral submucous fibrosis. J. Investig. Clin. Dent. 2019, 10, e12464. [Google Scholar] [CrossRef]
- Yadav, M.; Aravinda, K.; Saxena, V.S.; Srinivas, K.; Ratnakar, P.; Gupta, J.; Sachdev, A.S.; Shivhare, P. Comparison of curcumin with intralesional steroid injections in Oral Submucous Fibrosis—A randomized, open-label interventional study. J. Oral Biol. Craniofacial Res. 2014, 4, 169–173. [Google Scholar] [CrossRef]
- Kapoor, S.; Arora, P. Effect of curcumin in management of potentially Malignant disorders-A comparative study. Onkol. Radioter. 2019, 13, 1–4. [Google Scholar]
- Shetty, P.; Shenai, P.; Chatra, L.; Rao, P.K. Efficacy of spirulina as an antioxidant adjuvant to corticosteroid injection in management of oral submucous fibrosis. Indian J. Dent. Res. 2013, 24, 347. [Google Scholar] [CrossRef] [PubMed]
- Bale, R.; Kattappagari, K.K.; Vidya, D.; Vuddandi, S.; Gummalla, C.; Baddam, V.R. Oral submucous fibrosis: A quantitative assessment of serum malondialdehyde, superoxide dismutase and correlation with clinical staging. J. Oral Maxillofac. Pathol. 2017, 21, 41. [Google Scholar] [CrossRef] [PubMed]
- Parl, F.F. Glutathione S-transferase genotypes and cancer risk. Cancer Lett. 2005, 221, 123–129. [Google Scholar] [CrossRef]
- Fukai, T.; Ushio-Fukai, M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid. Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef] [PubMed]
- Boone, C.W.; Kelloff, G.J.; Freedman, L.S. Intraepithelial and postinvasive neoplasia as a stochastic continuum of clonal evolution, and its relationship to mechanisms of chemopreventive drug action. J. Cell. Biochem. 1993, 53, 14–25. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A review of its effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Guruprasad, R.; Nair, P.P.; Singh, M.; Singh, M.; Singh, M.P.; Jain, A. Serum vitamin C and iron levels in oral submucous fibrosis. Indian J. Dent. 2014, 5, 21–25. [Google Scholar] [CrossRef]
- Pipalia, P.R.; Annigeri, R.G.; Mehta, R. Clinicobiochemical evaluation of turmeric with black pepper and Nigella sativa in management of oral submucous fibrosis—a double-blind, randomized preliminary study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, 705–712. [Google Scholar] [CrossRef]
- Jeng, J.H.; Ho, Y.S.; Chan, C.P.; Wang, Y.J.; Hahn, L.J.; Lei, D.; Hsu, C.C.; Chang, M.C. Areca nut extract up-regulates prostaglandin production, cyclooxygenase-2 mRNA and protein expression of human oral keratinocytes. Carcinogenesis 2000, 21, 1365–1370. [Google Scholar] [CrossRef]
- Patel, P.N.; Thennavan, A.; Sen, S.; Chandrashekar, C.; Radhakrishnan, R. Translational approach utilizing COX-2, p53, and MDM2 expressions in malignant transformation of oral submucous fibrosis. J. Oral Sci. 2015, 57, 169–176. [Google Scholar] [CrossRef]
- Wang, L.; Tang, Z. Immunopathogenesis of oral submucous fibrosis by chewing the areca nut. J. Leukoc. Biol. 2022, 111, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Chua, N.Q.E.; Dang, S.; Davis, A.; Chong, K.W.; Prime, S.S.; Cirillo, N. Molecular Mechanisms of Malignant Transformation of Oral Submucous Fibrosis by Different Betel Quid Constituents-Does Fibroblast Senescence Play a Role? Int. J. Mol. Sci. 2022, 23, 1637. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.H.; Wang, Y.P.; Chang, J.Y.; Pan, Y.H.; Chang, M.C.; Jeng, J.H. Genetic Susceptibility and Protein Expression of Extracellular Matrix Turnover-Related Genes in Oral Submucous Fibrosis. Int. J. Mol. Sci. 2020, 21, 8104. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).