The Impact of Mild Frost Occurring at Different Harvesting Times on the Volatile and Phenolic Composition of Virgin Olive Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Orchard Characteristics and Agricultural Aspects
2.3. Sample Selection and Processing
2.4. Olive Oil Extraction
2.5. Quality Parameters
2.6. Color
2.7. Determination of Phenolic Compounds
2.8. Total Phenolic Content
2.9. Hydrophilic Orac Assay (H-ORACFL)
2.10. Tocopherol Content
2.11. Volatile Compounds
2.12. Statistical Analysis
3. Results and Discussion
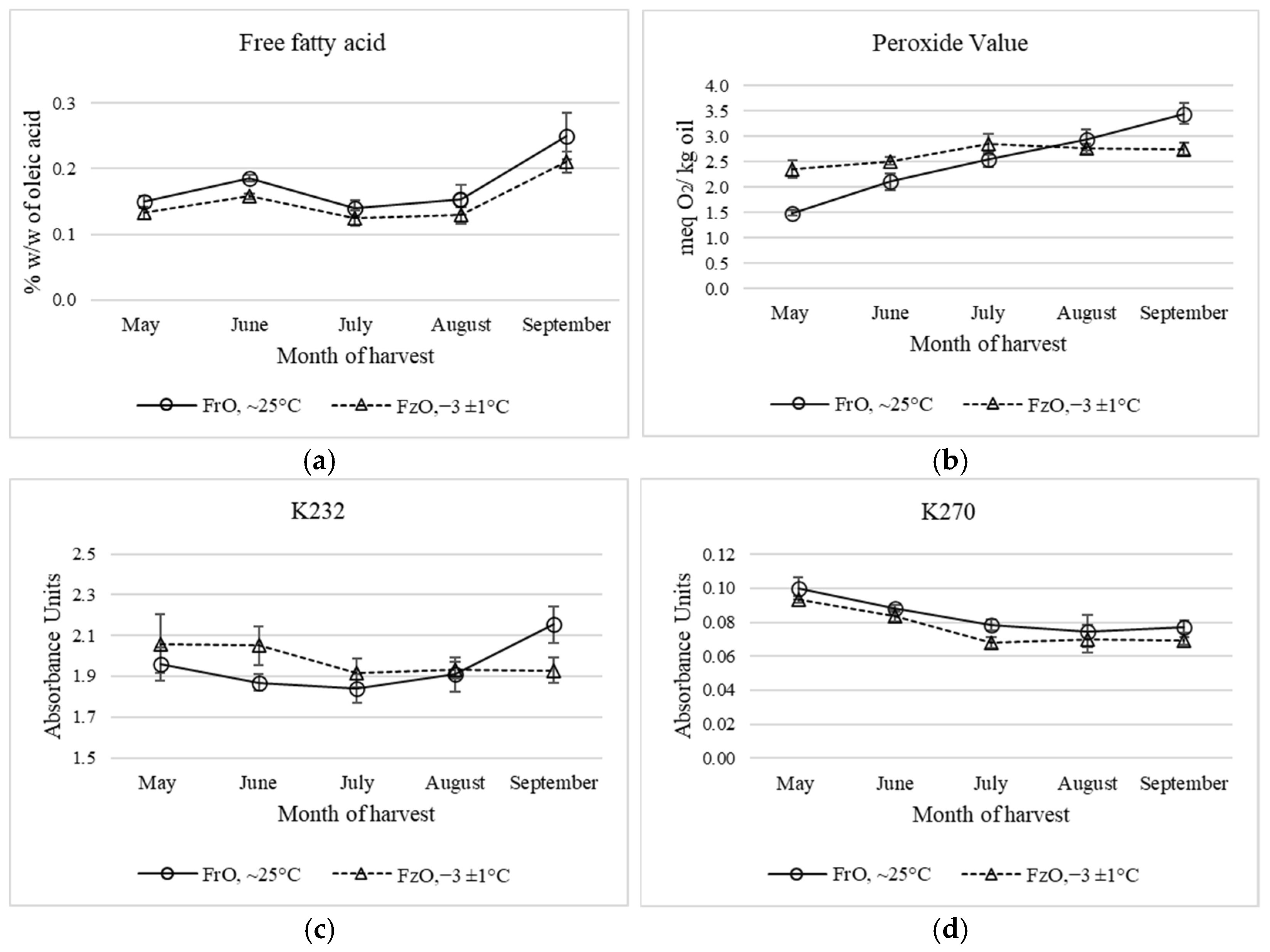
3.1. Quality Parameters
3.2. Evolution of Color Parameters
3.3. Behavior of Antioxidant Compounds
3.3.1. Phenolic Compounds
3.3.2. α-Tocopherol
3.4. Antioxidant Capacity
3.5. Volatile Compounds
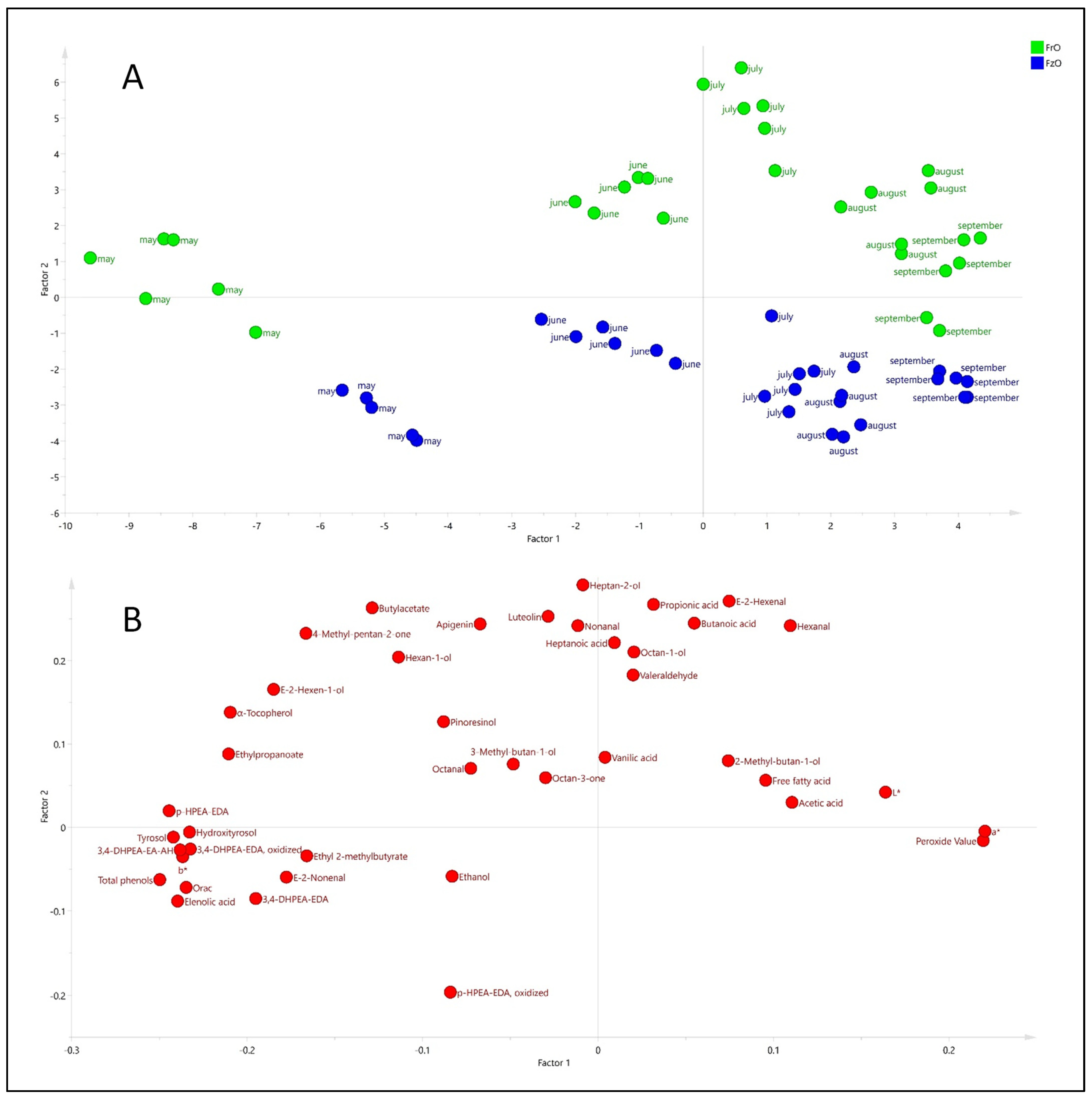
3.6. Multivariate Characterization of Samples Using PCA and PLS-DA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Del Río, C.; Caballero, J.M.; Fernández-García, M. Rendimiento Graso de La Aceituna (Banco de Germoplasma de Córdoba). In Variedades de Olivo en España; Rallo, L., Barranco, D., Caballero, J., Martín, A., del Río, C., Tous, J., Trujillo, I., Eds.; Junta de Andalucía, MAPA y Ediciones Mundi-Prensa: Madrid, Spain, 2005; pp. 347–356. [Google Scholar]
- Zohary, D. The Wild Genetic Resources of the Cultivated Olive. Acta Hortic. 1994, 356, 62–65. [Google Scholar] [CrossRef]
- Newton, C.; Lorre, C.; Sauvage, C.; Ivorra, S.; Terral, J.-F. On the Origins and Spread of Olea Europaea L. (Olive) Domestication: Evidence for Shape Variation of Olive Stones at Ugarit, Late Bronze Age, Syria—A Window on the Mediterranean Basin and on the Westward Diffusion of Olive Varieties. Veg. Hist. Archaeobot. 2014, 23, 567–575. [Google Scholar] [CrossRef]
- Tena, N.; Aparicio, R.; Baeten, V.; García-González, D.L.; Fernández-Pierna, J.A. Assessment of Vibrational Spectroscopy Performance in Geographical Identification of Virgin Olive Oils: A World Level Study. Eur. J. Lipid Sci. Technol. 2019, 121, 1900035. [Google Scholar] [CrossRef]
- Lavee, S.; Wodner, M. Factors Affecting the Nature of Oil Accumulation in Fruit of Olive ( Olea Europaea L.) Cultivars. J. Hortic. Sci. 1991, 66, 583–591. [Google Scholar] [CrossRef]
- Rallo, L.; Díez, C.M.; Morales-Sillero, A.; Miho, H.; Priego-Capote, F.; Rallo, P. Quality of Olives: A Focus on Agricultural Preharvest Factors. Sci. Hortic. (Amst.) 2018, 233, 491–509. [Google Scholar] [CrossRef]
- Fitó, M.; De La Torre, R.; Covas, M.I. Olive Oil and Oxidative Stress. Mol. Nutr. Food Res. 2007, 51, 1215–1224. [Google Scholar] [CrossRef]
- International Olive Council (IOC). COI/T.15/NC No 3/Rev. 16; Trade Standard Applying to Olive Oil and Olive Pomace Oil. International Olive Council: Madrid, Spain, 2021.
- García-González, D.L.; Romero, N.; Aparicio, R. Comparative Study of Virgin Olive Oil Quality from Single Varieties Cultivated in Chile and Spain. J. Agric. Food Chem. 2010, 58, 12899–12905. [Google Scholar] [CrossRef]
- Aparicio, R.; Conte, L.S.; Fiebig, H.J. Olive Oil Authentication. In Handbook of Olive Oil: Analysis and Properties; Aparicio, R., Harwood, J., Eds.; Springer: Boston, MA, USA, 2013; pp. 589–653. [Google Scholar]
- Ferreiro, L.; Aparicio, R. Influence of Altitude on Chemical Composition of Andalusian Virgin Olive Oil. Mathematical Classification Equations. Grasas Y Aceites 1992, 43, 149–156. [Google Scholar] [CrossRef]
- Aparicio, R.; Ferreiro, L.; Alonso, V. Effect of Climate on the Chemical Composition of Virgin Olive Oil. Anal. Chim. Acta 1994, 292, 235–241. [Google Scholar] [CrossRef]
- Angerosa, F.; Bréas, O.; Contento, S.; Guillou, C.; Reniero, F.; Sada, E. Application of Stable Isotope Ratio Analysis to the Characterization of the Geographical Origin of Olive Oils. J. Agric. Food Chem. 1999, 47, 1013–1017. [Google Scholar] [CrossRef]
- Romero, N.; Saavedra, J.; Tapia, F.; Sepúlveda, B.; Aparicio, R. Influence of Agroclimatic Parameters on Phenolic and Volatile Compounds of Chilean Virgin Olive Oils and Characterization Based on Geographical Origin, Cultivar and Ripening Stage. J. Sci. Food Agric. 2016, 96, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Bontempo, L.; Paolini, M.; Franceschi, P.; Ziller, L.; García-González, D.L.; Camin, F. Characterisation and Attempted Differentiation of European and Extra-European Olive Oils Using Stable Isotope Ratio Analysis. Food Chem. 2019, 276, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Quintanilla-Casas, B.; Torres-Cobos, B.; Guardiola, F.; Servili, M.; Alonso-Salces, R.M.; Valli, E.; Bendini, A.; Toschi, T.G.; Vichi, S.; Tres, A. Geographical Authentication of Virgin Olive Oil by GC—MS Sesquiterpene Hydrocarbon Fingerprint: Verifying EU and Single Country Label-Declaration. Food Chem. 2022, 378, 132104. [Google Scholar] [CrossRef] [PubMed]
- International Olive Council (IOC). World Olive Oil and Table Olive Figures. Production; International Olive Council: Madrid, Spain, 2021. [Google Scholar]
- Bernardini, E.; Visioli, F. High Quality, Good Health: The Case for Olive Oil. Eur. J. Lipid Sci. Technol. 2017, 119, 1500505. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, D.; Farooqi, T.J.A.; Ma, L.; Deng, Y.; Jia, Z. The Olive (Olea Europaea L.) Industry in China: Its Status, Opportunities and Challenges. Agrofor. Syst 2019, 93, 395–417. [Google Scholar] [CrossRef]
- Fuchs, Y.; Zauberman, G.; Yanko, U. Freeze Injuries in Avocado Fruit. HortScience 1975, 10, 64–65. [Google Scholar]
- Sanzani, S.M.; Schena, L.; Nigro, F.; Sergeeva, V.; Ippolito, A.; Salerno, M.G. Abiotic Diseases in Olives. J. Plant Pathol. 2012, 94, 469–491. [Google Scholar]
- Cansev, A.; Gulen, H.; Eris, A. Cold-Hardiness of Olive (Olea Europaea L.) Cultivars in Cold-Acclimated and Non-Acclimated Stages: Seasonal Alteration of Antioxidative Enzymes and Dehydrin-like Proteins. J. Agric. Sci. 2009, 147, 51–61. [Google Scholar] [CrossRef]
- Lodolini, E.M.; Alfei, B.; Santinelli, A.; Cioccolanti, T.; Polverigiani, S.; Neri, D. Frost Tolerance of 24 Olive Cultivars and Subsequent Vegetative Re-Sprouting as Indication of Recovery Ability. Sci. Hortic. (Amst.) 2016, 211, 152–157. [Google Scholar] [CrossRef]
- Kozlowski, T.; Pallardy, S. Growth Control in Woody Plants; Academic Press: San Diego, CA, USA, 1997. [Google Scholar]
- Morelló, J.R.; Motilva, M.J.; Ramo, T.; Romero, M.P. Effect of Freeze Injuries in Olive Fruit on Virgin Olive Oil Composition. Food Chem. 2003, 81, 547–553. [Google Scholar] [CrossRef]
- Romero, I.; Aparicio-Ruiz, R.; Oliver-Pozo, C.; Aparicio, R.; García-González, D.L. Characterization of Virgin Olive Oils with Two Kinds of ‘Frostbitten Olives’ Sensory Defect. J. Agric. Food Chem. 2016, 64, 5590–5597. [Google Scholar] [CrossRef] [PubMed]
- International Olive Council (IOC). COI/T.20/Doc. No 15/Rev. 10; Sensory Analysis of Olive Oil. Method for the Organoleptic Assessment of Virgin Olive Oil. International Olive Council: Madrid, Spain, 2018.
- Romero, I.; García-González, D.L.; Aparicio-Ruiz, R.; Morales, M.T. Study of Volatile Compounds of Virgin Olive Oils with ‘Frostbitten Olives’ Sensory Defect. J. Agric. Food Chem. 2017, 65, 4314–4320. [Google Scholar] [CrossRef] [PubMed]
- Bartolozzi, F.; Cerquaglia, F.; Coppari, L.; Fontanazza, G. Frost Tolerance Induced by Cold Acclimation in Olive (Olea Europaea L.). Acta Hortic. 2002, 586, 473–477. [Google Scholar] [CrossRef]
- Wang, J.W.; Ma, L.Y.; Gómez-del-Campo, M.; Zhang, D.S.; Deng, Y.; Jia, Z.K. Youth Tree Behavior of Olive (Olea Europaea L.) Cultivars in Wudu, China: Cold and Drought Resistance, Growth, Fruit Production, and Oil Quality. Sci. Hortic. (Amst.) 2018, 236, 106–122. [Google Scholar] [CrossRef]
- Centeno, A.; Hueso, A.; Gómez-del-Campo, M. Long-Term Evaluation of Growth and Production of Olive Cultivars in Super High-Density Orchard under Cold-Weather Conditions. Sci. Hortic. (Amst.) 2019, 257, 108657. [Google Scholar] [CrossRef]
- Herrera-Cáceres, C.; Pérez-Galarce, F.; Álvarez-Miranda, E.; Candia-Véjar, A. Optimization of the Harvest Planning in the Olive Oil Production: A Case Study in Chile. Comput. Electron. Agric. 2017, 141, 147–159. [Google Scholar] [CrossRef]
- Caipo, L.; Sandoval, A.; Sepúlveda, B.; Fuentes, E.; Valenzuela, R.; Metherel, A.H.; Romero, N. Effect of Storage Conditions on the Quality of Arbequina Extra Virgin Olive Oil and the Impact on the Composition of Flavor-Related Compounds (Phenols and Volatiles). Foods 2021, 10, 2161. [Google Scholar] [CrossRef]
- Hermoso, M.; Uceda, M.; Frias, L.; Beltrán, G. Maduración. In El Cultivo Del Olivo; Barranco, D., Fernández-Escobar, R., Rallo, L., Eds.; Mundi Prensa: Madrid, Spain, 2000; pp. 153–166. [Google Scholar]
- AOCS Press. American Oil Chemists’s Society. In Official Methods and Recommended Practices of the AOCS; AOCS Press: Champaign, IL, USA, 1993. [Google Scholar]
- Mundi Prensa. Asociación Española de Normalizacion; Colorimetry. Part 4: CIE 1976 L*a*b* Colour Space (UNE-EN ISO/CIE 11664-4:2019); Mundi Prensa: Madrid, Spain, 2020; pp. 6–9. [Google Scholar]
- International Olive Council (IOC). COI/T.20/Doc No 29; Determination of Biophenols in Olive Oils by HPLC. International Olive Council: Madrid, Spain, 2009.
- Mateos, R.; Espartero, J.L.; Trujillo, M.; Ríos, J.J.; León-Camacho, M.; Alcudia, F.; Cert, A. Determination of Phenols, Flavones, and Lignans in Virgin Olive Oils by Solid-Phase Extraction and High-Performance Liquid Chromatography with Diode Array Ultraviolet Detection. J. Agric. Food Chem. 2001, 49, 2185–2192. [Google Scholar] [CrossRef]
- Fuentes, E.; Paucar, F.; Tapia, F.; Ortiz, J.; Jimenez, P.; Romero, N. Effect of the Composition of Extra Virgin Olive Oils on the Differentiation and Antioxidant Capacities of Twelve Monovarietals. Food Chem. 2018, 243, 285–294. [Google Scholar] [CrossRef]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for Hydrophilic and Lipophilic Antioxidant Capacity (Oxygen Radical Absorbance Capacity (ORAC FL )) of Plasma and Other Biological and Food Samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-Regression: A Basic Tool of Chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Cen, H.; He, Y.; Huang, M. Combination and Comparison of Multivariate Analysis for the Identification of Orange Varieties Using Visible and near Infrared Reflectance Spectroscopy. Eur. Food Res. Technol. 2007, 225, 699–705. [Google Scholar] [CrossRef]
- Navajas-Porras, B.; Pérez-Burillo, S.; Morales-Pérez, J.; Rufián-Henares, J.A.; Pastoriza, S. Relationship of Quality Parameters, Antioxidant Capacity and Total Phenolic Content of EVOO with Ripening State and Olive Variety. Food Chem. 2020, 325, 126926. [Google Scholar] [CrossRef] [PubMed]
- Trypidis, D.; García-González, D.L.; Lobo-Prieto, A.; Nenadis, N.; Tsimidou, M.Z.; Tena, N. Real Time Monitoring of the Combined Effect of Chlorophyll Content and Light Filtering Packaging on Virgin Olive Oil Photo-Stability Using Mesh Cell-FTIR Spectroscopy. Food Chem. 2019, 295, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Konuskan, D.B.; Mungan, B. Effects of Variety, Maturation and Growing Region on Chemical Properties, Fatty Acid and Sterol Compositions of Virgin Olive Oils. J. Am. Oil Chem. Soc. 2016, 93, 1499–1508. [Google Scholar] [CrossRef]
- Allouche, Y.; Jiménez, A.; Gaforio, J.J.; Uceda, M.; Beltrán, G. How Heating Affects Extra Virgin Olive Oil Quality Indexes and Chemical Composition. J. Agric. Food Chem. 2007, 55, 9646–9654. [Google Scholar] [CrossRef] [PubMed]
- Gandul-Rojas, B.; Gallardo-Guerrero, L.; Roca, M.; Aparicio-Ruiz, R. Chromatographic Methodologies: Compounds for Olive Oil Color Issues. In Handbook of Olive Oil: Analysis and Properties; Aparicio, R., Harwood, J., Eds.; Springer: Boston, MA, USA, 2013; pp. 220–254. [Google Scholar]
- Gandul-Rojas, B.; Cepero, M.R.L.; Mínguez-Mosquera, M.I. Use of Chlorophyll and Carotenoid Pigment Composition to Determine Authenticity of Virgin Olive Oil. JAOCS J. Am. Oil Chem. Soc. 2000, 77, 853–858. [Google Scholar] [CrossRef]
- Lobo-Prieto, A.; Tena, N.; Aparicio-Ruiz, R.; García-González, D.L.; Sikorska, E. Monitoring Virgin Olive Oil Shelf-Life by Fluorescence Spectroscopy and Sensory Characteristics: A Multidimensional Study Carried out under Simulated Market Conditions. Foods 2020, 9, 1846. [Google Scholar] [CrossRef]
- Romero-Segura, C.; García-Rodríguez, R.; Sánchez-Ortiz, A.; Sanz, C.; Pérez, A.G. The Role of Olive β-Glucosidase in Shaping the Phenolic Profile of Virgin Olive Oil. Food Res. Int. 2012, 45, 191–196. [Google Scholar] [CrossRef]
- Conde, C.; Delrot, S.; Gerós, H. Physiological, Biochemical and Molecular Changes Occurring during Olive Development and Ripening. J. Plant Physiol. 2008, 165, 1545–1562. [Google Scholar] [CrossRef]
- García-Vico, L.; García-Rodríguez, R.; Sanz, C.; Pérez, A.G. Biochemical Aspects of Olive Freezing-Damage: Impact on the Phenolic and Volatile Profiles of Virgin Olive Oil. LWT—Food Sci. Technol. 2017, 86, 240–246. [Google Scholar] [CrossRef] [Green Version]
- García-Rodríguez, R.; Romero-Segura, C.; Sanz, C.; Sánchez-Ortiz, A.; Pérez, A.G. Role of Polyphenol Oxidase and Peroxidase in Shaping the Phenolic Profile of Virgin Olive Oil. Food Res. Int. 2011, 44, 629–635. [Google Scholar] [CrossRef]
- Pourcel, L.; Routaboul, J.M.; Cheynier, V.; Lepiniec, L.; Debeaujon, I. Flavonoid Oxidation in Plants: From Biochemical Properties to Physiological Functions. Trends Plant Sci. 2007, 12, 29–36. [Google Scholar] [CrossRef]
- Guillaume, C.; Ravetti, L.; Gwyn, S. Characterisation of Phenolic Compounds in Oils Produced from Frosted Olives. JAOCS J. Am. Oil Chem. Soc. 2010, 87, 247–254. [Google Scholar] [CrossRef]
- Peres, F.; Martins, L.L.; Mourato, M.; Vitorino, C.; Ferreira-Dias, S. Bioactive Compounds of Portuguese Virgin Olive Oils Discriminate Cultivar and Ripening Stage. JAOCS J. Am. Oil Chem. Soc. 2016, 93, 1137–1147. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms and Factors for Edible Oil Oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Krieger-Liszkay, A.; Trebst, A. Tocopherol Is the Scavenger of Singlet Oxygen Produced by the Triplet States of Chlorophyll in the PSII Reaction Centre. J. Exp. Bot. 2006, 57, 1677–1684. [Google Scholar] [CrossRef]
- Ballus, C.A.; Meinhart, A.D.; de Souza Campos, F.A.; Teixeira, H. Total phenolics of virgin olive oils highly correlate with the hydrogen atom transfer mechanism of antioxidant capacity. JAOCS J. Am. Oil Chem. Soc. 2015, 92, 843–851. [Google Scholar] [CrossRef]
- Wang, Z.M.; Lu, Z.M.; Shi, J.S.; Xu, Z.H. Exploring Flavour-Producing Core Microbiota in Multispecies Solid-State Fermentation of Traditional Chinese Vinegar. Sci. Rep. 2016, 6, 26818. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, L.; Johansson, E.; Kettaneh-Wold, N.; Trygg, J.; Wikstrom, C.; Wold, S. Multi—And Megavariate Data Analysis, 3rd ed.; Umetrics Academy: Malmö, Sweeden, 2013. [Google Scholar]




| Months of Harvest | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Phenolic Compound (mg/kg) | May | June | July | August | September | |||||
| FrO | FzO | FrO | FzO | FrO | FzO | FrO | FzO | FrO | FzO | |
| Elenolic acid | 231 ± 6Aa | 226 ± 14Aa | 93 ± 9Ab | 116 ± 6Bb | 74 ± 3Ac | 113 ± 6Bb | 66 ± 3Ad | 73 ± 3Bc | 64 ± 8Ad | 68 ± 5Ac |
| Hydroxityrosol | 3.8 ± 0.6Aa | 1.2 ± 0.2aB | 0.89 ± 0.08Ab | 1.6 ± 0.2Bb | 0.23 ± 0.01Ac | 0.28 ± 0.04Bc | 0.20 ± 0.02Ac | 0.43 ± 0.04Bd | 0.21 ± 0.04Ac | 0.08 ± 0.02Be |
| Tyrosol | 2.3 ± 0.2Aa | 1.3 ± 0.1Ba | 0.99 ± 0.151Ab | 0.8 ± 0.1Bb | 0.31 ± 0.03Acd | 0.33 ± 0.06Ac | 0.22 ± 0.04Ac | 0.34 ± 0.05Bc | 0.42 ± 0.05Ad | 0.27 ± 0.02Bd |
| Vanilic acid | 0.07 ± 0.01Aa | 0.09 ± 0.0Ba | 0.06 ± 0.01Ab | 0.06 ± 0.01Ab | 0.09 ± 0.01Ac | 0.07 ± 0.01Bc | 0.09 ± 0.01Aac | 0.07 ± 0.01Bc | 0.12 ± 0.03Ad | 0.05 ± 0.01Bd |
| p-Coumaric acid | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 3,4-DHPEA-EDA | 68 ± 5Aa | 85 ± 8Ba | 65 ± 9Aa | 89 ± 4Ba | 31 ± 2Ab | 49 ± 5Bb | 9 ± 1Ac | 50 ± 3Bb | 23 ± 2Ad | 0.46 ± 0.01Bc |
| 3,4-DHPEA-EDA, oxidized | 3.0 ± 0.2Aa | 3.0 ± 0.2Aa | 1.0 ± 0.1Ab | 2.7 ± 0.2Bb | 1.1 ± 0.1Ac | 0.1 ± 0.0Bc | 0.1 ± 0.0Ad | 0.4 ± 0.0Bd | 0.1 ± 0.0d | ND |
| 3,4-DHPEA-EDA-DOA | 0.07 ± 0.00a | ND | 0.09 ± 0.02Ab | 0.05 ± 0.00Ba | 0.05 ± 0.01Ac | 0.08 ± 0.01Ba | 0.06 ± 0.01Aac | 0.06 ± 0.01Aa | 0.10 ± 0.02Ad | 0.03 ± 0.00Ba |
| p-HPEA-EDA | 50 ± 2Aa | 43 ± 2Ba | 39 ± 1Ab | 29 ± 2Bb | 21 ± 1Ac | 20 ± 1Bc | 21 ± 2Ac | 23 ± 2Ad | 12 ± 2Ad | 11 ± 1Ae |
| p-HPEA-EDA, oxidized | 0.33 ± 0.02Aa | 1.37 ± 0.27Ba | 0.30 ± 0.04Ab | 0.88 ± 0.09Bb | 0.06 ± 0.01Ac | 0.19 ± 0.01Bc | 0.13 ± 0.01Ad | 1.29 ± 0.12Ba | 0.16 ± 0.02e | ND |
| Pinoresinol1 | 9 ± 1Aa | 8.8 ± 0.4Aa | 12 ± 1Ab | 11 ± 0Bb | 12 ± 1Ab | 8.5 ± 0.3Ba | 8 ± 1Ac | 9 ± 1Ba | 6.9 ± 0.3Ac | 7 ± 1Ac |
| p-HPEA-EDA-DLA | 1.8 ± 0.1Aa | 2.6 ± 2.0Aa | 1.6 ± 0.1Ab | 1.7 ± 0.1Ab | 1.6 ± 0.1Ab | 0.6 ± 0.1Bc | 0.8 ± 0.1Ac | 1.1 ± 0.1Bc | 0.9 ± 0.1Ac | 0.8 ± 0.1Bc |
| 3,4-DHPEA-EA-AH | 30 ± 2Aa | 36 ± 2Ba | 13 ± 3Ab | 14 ± 1Ab | 10 ± 1Ac | 2 ± 0Bc | 6 ± 1Ad | 7 ± 1Bd | 5 ± 0Ad | 0.3 ± 0.0Be |
| Luteolin | 8.0 ± 0.6Aa | 6.6 ± 1.5Ba | 8.9 ± 0.8Aa | 8.5 ± 0.3Ab | 14 ± 2Ab | 5.4 ± 0.2Bc | 8.8 ± 2.2Aa | 8 ± 1Bb | 8 ± 1Aa | 3.4 ± 0.3Bd |
| Apigenin | 3.1 ± 0.3Aa | 2.5 ± 0.4Ba | 3.1 ± 0.3Aac | 2.8 ± 0.2Ab | 4.5 ± 0.7Ab | 2.4 ± 0.2Ba | 2.7 ± 0.5Acd | 2.4 ± 0.5Aa | 2.6 ± 0.3Ad | 1.6 ± 0.3Bc |
| Methyl luteolin | 1.6 ± 0.1Aa | 1.7 ±0.7Aab | 2.0 ± 0.3Ab | 1.8 ± 0.2Aa | 2.0 ± 0.2Ab | 1.8 ± 0.2Aa | 1.3 ± 0.3Ac | 1.3 ± 0.2Abc | 1.4 ± 0.4Aac | 1.0 ± 0.3Bc |
| Total phenols | 413 ± 7Aa | 419 ± 10Aa | 241 ± 6Ab | 279 ± 10Bb | 171 ± 5Ac | 204 ± 9Bc | 124 ± 3Ad | 177 ± 8Bd | 124 ± 8Ad | 93 ± 5Be |
| non-oxidized phenols | 410 ± 7 | 415 ± 10 | 240 ± 6 | 276 ± 10 | 170 ± 5 | 204 ± 9 | 124 ± 3 | 175 ± 8 | 124 ± 8 | 93 ± 5 |
| Oxidized phenols | 3.40 ± 0.16 | 4.41 ± 0.34 | 1.25 ± 0.06 | 3.60 ± 0.22 | 1.15 ± 6 | 0.23 ± 0.01 | 0.24 ± 0.02 | 1.74 ± 0.16 | 0.26 ± 0.02 | ND |
| α-Tocopherol (mg/kg) | 166 ± 8Aa | 123 ± 3Ba | 131 ± 4Ab | 115 ± 6Bb | 125 ± 7Ab | 114 ± 2Bb | 117 ± 5Ac | 97 ± 6Bc | 114 ± 1Ac | 99 ± 5Bc |
| ORAC (µmol TE/g) | 5.2 ± 0.6Aa | 4.8 ± 0.2Ba | 2.9 ± 0.2Ab | 3.6 ± 0.7Bb | 2.4 ± 0.1Ac | 2.9 ± 0.2Bc | 2.9 ± 0.1Ad | 3.0 ± 0.1Ac | 2.3 ± 0.1Ac | 2.1 ± 0.3Bd |
| Months of Harvest | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| May | June | July | August | September | ||||||||
| Volatile Compounds (mg/kg) | FrO | FzO | FrO | FzO | FrO | FzO | FrO | FzO | FrO | FzO | Sensory Atributes | OT in Oil (mg/kg) |
| Ethanol | 6.61 ± 4.64Aa | 1.47 ± 0.45Ba | 0.51 ± 0.03Ab | 0.81 ± 0.06Ba | 1.04 ± 0.50Ab | 3.04 ± 1.64Bbc | 2.46 ± 0.41Ab | 3.77 ± 0.58Bc | 1.87 ± 0.30Ab | 2.78 ± 0.75Bb | Apple, sweet | 30 |
| Ethylpropanoate | 0.14 ± 0.02Aa | 0.08 ± 0.01Ba | 0.09 ± 0.00Ab | 0.08 ± 0.00Ba | 0.08 ± 0.01Ac | 0.08 ± 0.01Aa | 0.08 ± 0.01Ac | 0.07 ± 0.01Ab | 0.07 ± 0.00Ac | 0.06 ± 0.00Bb | Strawberry, apple, fruity | 0.10 |
| Pentanal | 0.09 ± 0.02Aa | 0.08 ± 0.01Aa | 0.25 ± 0.04Ab | 0.17 ± 0.08Bb | 0.15 ± 0.02Ac | 0.08 ± 0.01Bac | 0.12 ± 0.02Ad | 0.06 ± 0.03Ba | 0.16 ± 0.02Ac | 0.13 ± 0.07Bbc | Woody, bitter, oily | 0.24 |
| 4-Methyl-pentan-2-one | 0.09 ± 0.01Aa | 0.07 ± 0.01Ba | 0.10 ± 0.00Ab | 0.06 ± 0.00Ba | 0.09 ± 0.01Ac | 0.04 ± 0.01Bb | 0.06 ± 0.00Ad | 0.03 ± 0.01Bb | 0.05 ± 0.00Ae | 0.04 ± 0.00Bb | Strawberry, fruity, sweet, ethereal | 0.30 |
| Ethyl 2-methylbutyrate | 0.03 ± 0.01Aa | 0.02 ± 0.00Ba | 0.02 ± 0.01Ab | 0.02 ± 0.00Aa | 0.01 ± 0.00Ab | 0.02 ± 0.01Ba | 0.02 ± 0.01Ab | 0.02 ± 0.00Aa | 0.02 ± 0.00Ab | 0.02 ± 0.00Aa | Fruity | 0.72 |
| Butylacetate | 0.07 ± 0.01Aa | 0.04 ± 0.01Ba | 0.06 ± 0.00Aa | 0.05 ± 0.00Ab | 0.07 ± 0.01Aa | 0.04 ± 0.01Bad | 0.05 ± 0.00Ab | 0.03 ± 0.00Bc | 0.04 ± 0.00Ac | 0.03 ± 0.00Bcd | Green, fruity, pungent, sweet | 0.10 |
| Hexanal | 4.28 ± 0.38Aa | 4.49 ± 0.24Aa | 6.98 ± 0.16Ab | 4.71 ± 0.12Bab | 6.19 ± 0.31Ac | 4.38 ± 0.50Ba | 6.35 ± 0.57Ac | 4.66 ± 0.41Bab | 7.21 ± 0.89Ab | 5.02 ± 0.34Bb | Green apple, grass | 0.08 |
| 2-Methyl-butan-1-ol | 0.07 ± 0.03Aab | 0.03 ± 0.00Ba | 0.05 ± 0.01Aa | 0.05 ± 0.01Ab | 0.05 ± 0.00Aa | 0.06 ± 0.01Ab | 0.07 ± 0.02Aab | 0.05 ± 0.00Bb | 0.08 ± 0.02Ab | 0.07 ± 0.01Ac | Winey, spicy | 0.48 |
| 3-Methyl-butan-1-ol | 0.18 ± 0.12Aa | 0.04 ± 0.01Ba | 0.04 ± 0.01Ab | 0.04 ± 0.01Aa | 0.07 ± 0.01Abc | 0.07 ± 0.02Ab | 0.11 ± 0.10Abd | 0.06 ± 0.01Ab | 0.12 ± 0.09Aad | 0.07 ± 0.01Ab | Woody, sweet | 0.10 |
| (E)-2-Hexenal | 6.43 ± 0.98Aa | 6.42 ± 0.47Aa | 11.07 ± 0.40Ab | 7.31 ± 0.28Bb | 9.56 ± 0.88Ac | 6.32 ± 1.02Ba | 9.58 ± 0.79Ac | 6.58 ± 0.69Bab | 9.38 ± 0.94Ac | 7.07 ± 0.62Bab | Bitter almonds, green- fruity | 0.42 |
| Octan-3-one | 0.20 ± 0.01Aa | 0.19 ± 0.00Ba | 0.19 ± 0.00Ab | 0.19 ± 0.00Aa | 0.19 ± 0.00Ab | 0.19 ± 0.00Aa | 0.19 ± 0.00Ab | 0.19 ± 0.00Aa | 0.20 ± 0.00Aa | 0.19 ± 0.00Aa | Pungent, resinous | 0.75 |
| Octanal | 0.06 ± 0.01Aa | 0.09±0.02Ba | 0.06 ± 0.00Aa | 0.05 ± 0.01Ab | 0.06 ± 0.00Aa | 0.04 ± 0.01Bb | 0.07 ± 0.02Aab | 0.05 ± 0.01Bb | 0.06 ± 0.01Aa | 0.05 ± 0.01Ab | Fatty, Sharp | 0.32 |
| (E)-2-heptenal | 0.59 ± 0.29Aa | 0.53 ± 0.02Aa | 0.66 ± 0.16Aa | 0.65 ± 0.13Aab | 0.91 ± 0.15Ab | 0.63 ± 0.12Bab | 0.52 ± 0.11Aa | 0.55 ± 0.23Aab | 0.51 ± 0.17Aa | 0.74 ± 0.03Bb | Sweet, grassy, woody | 5.00 × 10−3 |
| Heptan-2-ol | 2.28 ± 0.35Aa | 0.96 ± 0.52Bac | 3.90 ± 0.62Ab | 3.38 ± 0.12Ab | 4.49 ± 0.69Ab | 0,62 ± 0,77Ba | 3.79 ± 0.85Ab | 0,59 ± 0,81Ba | 2.05 ± 0.62Aa | 1.62 ± 0.81Ac | Earthy, sweety | 0.01 |
| Hexan-1-ol | 5.29 ± 0.91Aa | 3.94 ± 0.15Ba | 5.07 ± 0.12Aa | 3.81 ± 0.06Bb | 4.72 ± 0.71Aab | 4.24 ± 0.23Ac | 4.06 ± 0.47Ab | 3.79 ± 0.04Ad | 4.37 ± 0.43Ab | 4.00 ± 0.28Abd | Fruity, soft, aromatic | 0.40 |
| Nonanal | 0.92 ± 0.14Aa | 1.09 ± 0.06Ba | 0.63 ± 0.02Ab | 0.61 ± 0.06Abc | 0.63 ± 0.04Ab | 0.56 ± 0.02Bb | 0,66 ± 0.03Ab | 0.65 ± 0.01Adc | 0.69 ± 0.04Ab | 0.71 ± 0.05Ad | Fatty, waxy, pungent | 0.15 |
| (E)-2-nonenal | 1.08 ± 0.23Aa | 1.21 ± 0.34Aa | 0.79 ± 0.09Aa | 0.72 ± 0.05Ab | 1.05 ± 0.21Aa | 0.78 ± 0.14Ab | 1.92 ± 0.83Ab | 0.74 ± 0.04Bb | 1.10 ± 0.25Aa | 1.07 ± 0.07Ab | Fatty, rancid, paper-like, penetrating, waxy, beany | 0.90 |
| (E)-2-Hexen-1-ol | 4.93 ± 0.19Aa | 4.30 ± 0.05Ba | 4.65 ± 0.16Ab | 4.34 ± 0.00Bb | 4.44 ± 0.07Ac | 4.24 ± 0.03Bb | 4.33 ± 0.05Ac | 4.19 ± 0.03Bb | 4.30 ± 0.04Ac | 4.27 ± 0.03Aa | Green grass, leaves, fruity, astringent, bitter | 5.00 |
| Acetic acid | 1.73 ± 0.10Aa | 1.78 ± 0.04Aa | 1.72 ± 0.03Aa | 1.67 ± 0.03Aa | 1.72 ± 0.03Aa | 2.02 ± 0.38Bbc | 2.68 ± 0.93Ab | 2.10 ± 0.15Ac | 2.72 ± 0.70Ab | 1.85 ± 0.08Bab | Sour, vinegary | 0.50 |
| Propionic acid | 0.30 ± 0.02Aa | 0.29 ± 0.01Aac | 0.34 ± 0.02Ab | 0.30 ± 0.00Bab | 0.34 ± 0.01Ab | 0.30 ± 0.01Bb | 0.34 ± 0.03Ab | 0.30 ± 0.01Bb | 0.30 ± 0.03Aa | 0.28 ± 0.01Ac | Pungent, sour, mould | 0.72 |
| Octan-1-ol | 0.41 ± 0.00Aa | 0.41 ± 0.00Aab | 0.41 ± 0.01Aa | 0.41 ± 0.00Aab | 0.44 ± 0.00Ab | 0.41 ± 0.00Bb | 0.43 ± 0.03Ab | 0.40 ± 0.00Ba | 0.41 ± 0.00Aa | 0.41 ± 0.01Aab | Green, fusty, musty, sweet, waxy | 0.48 |
| Butanoic acid | 0.31 ± 0.03Aa | 0.31 ± 0.01Aac | 0.32 ± 0.01Aa | 0.29 ± 0.00Bb | 0.46 ± 0.03Ab | 0.32 ± 0.01Bc | 0.44 ± 0.04Ab | 0.30 ± 0.00Bab | 0.31 ± 0.01Aa | 0.31 ± 0.02Aabc | Rancid, cheese, sweat | 0.65 |
| Heptanoic acid | 2.31 ± 0.09Aab | 2.27 ± 0.01Aa | 2.26 ± 0.01Aa | 2.22 ± 0.01Bb | 2.36 ± 0.08Abc | 2.25 ± 0.01Bac | 2.40 ± 0.02Ac | 2.24 ± 0.03Bbc | 2.28 ± 0.05Aa | 2.25 ± 0.02Aac | Rancid, fatty | 0.1 |
| Total Volatiles | 38.4 ± 2.2Aa | 30.1 ± 1.7Ba | 40.2 ± 2.8Aa | 31.9 ± 2.0Bab | 39.1 ± 2.5Aa | 30.7 ± 1.8Ba | 40.7 ± 2.5Aa | 31.4 ± 1.9Bab | 38.3 ± 2.5Aa | 33.0 ± 1.9Bb | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pino, C.; Sepúlveda, B.; Tapia, F.; Saavedra, J.; García-González, D.L.; Romero, N. The Impact of Mild Frost Occurring at Different Harvesting Times on the Volatile and Phenolic Composition of Virgin Olive Oil. Antioxidants 2022, 11, 852. https://doi.org/10.3390/antiox11050852
Pino C, Sepúlveda B, Tapia F, Saavedra J, García-González DL, Romero N. The Impact of Mild Frost Occurring at Different Harvesting Times on the Volatile and Phenolic Composition of Virgin Olive Oil. Antioxidants. 2022; 11(5):852. https://doi.org/10.3390/antiox11050852
Chicago/Turabian StylePino, Catalina, Betsabet Sepúlveda, Francisco Tapia, Jorge Saavedra, Diego L. García-González, and Nalda Romero. 2022. "The Impact of Mild Frost Occurring at Different Harvesting Times on the Volatile and Phenolic Composition of Virgin Olive Oil" Antioxidants 11, no. 5: 852. https://doi.org/10.3390/antiox11050852
APA StylePino, C., Sepúlveda, B., Tapia, F., Saavedra, J., García-González, D. L., & Romero, N. (2022). The Impact of Mild Frost Occurring at Different Harvesting Times on the Volatile and Phenolic Composition of Virgin Olive Oil. Antioxidants, 11(5), 852. https://doi.org/10.3390/antiox11050852







