Extraction of Antioxidant Compounds from Onion Bulb (Allium cepa L.) Using Individual and Simultaneous Microwave-Assisted Extraction Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Standards
2.2. Biological Material Preparation
2.3. Microwave-Assisted Extraction Procedure
2.4. Total-Phenolic Compounds
2.5. Antioxidant Activity
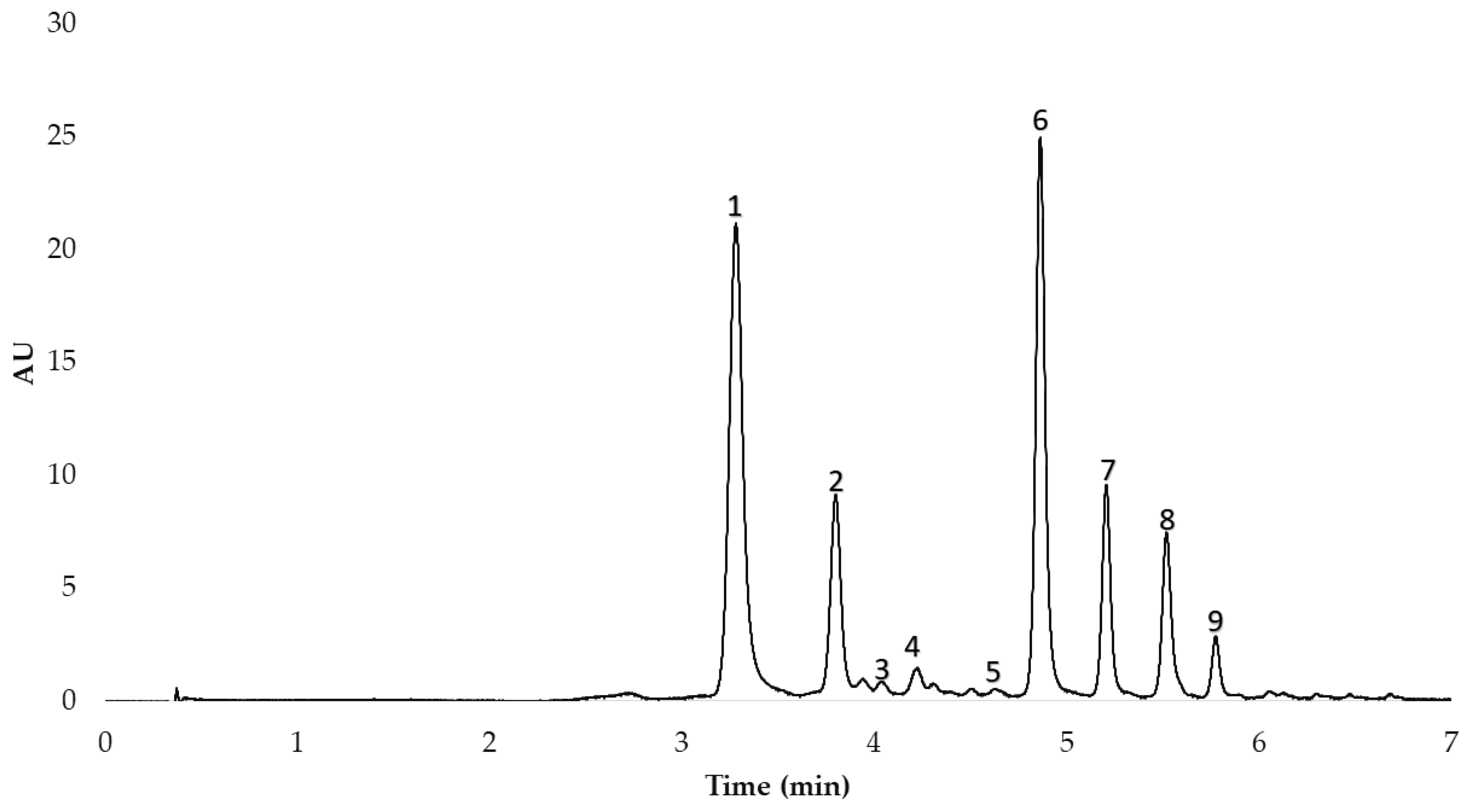
2.6. UHPLC-MS-QToF Conditions
2.7. HPLC Conditions
2.8. Optimization Procedure and Data Analysis
3. Results and Discussion
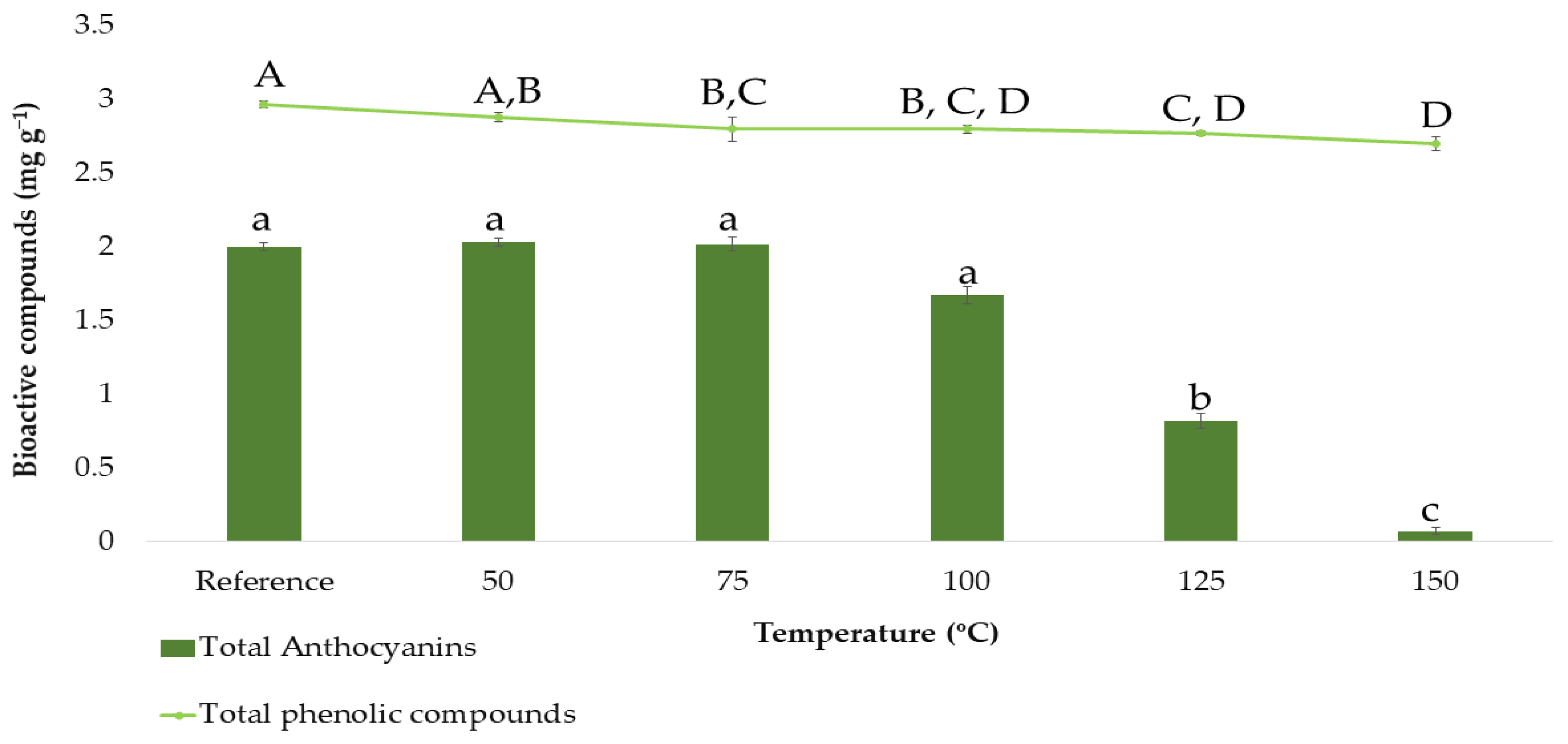
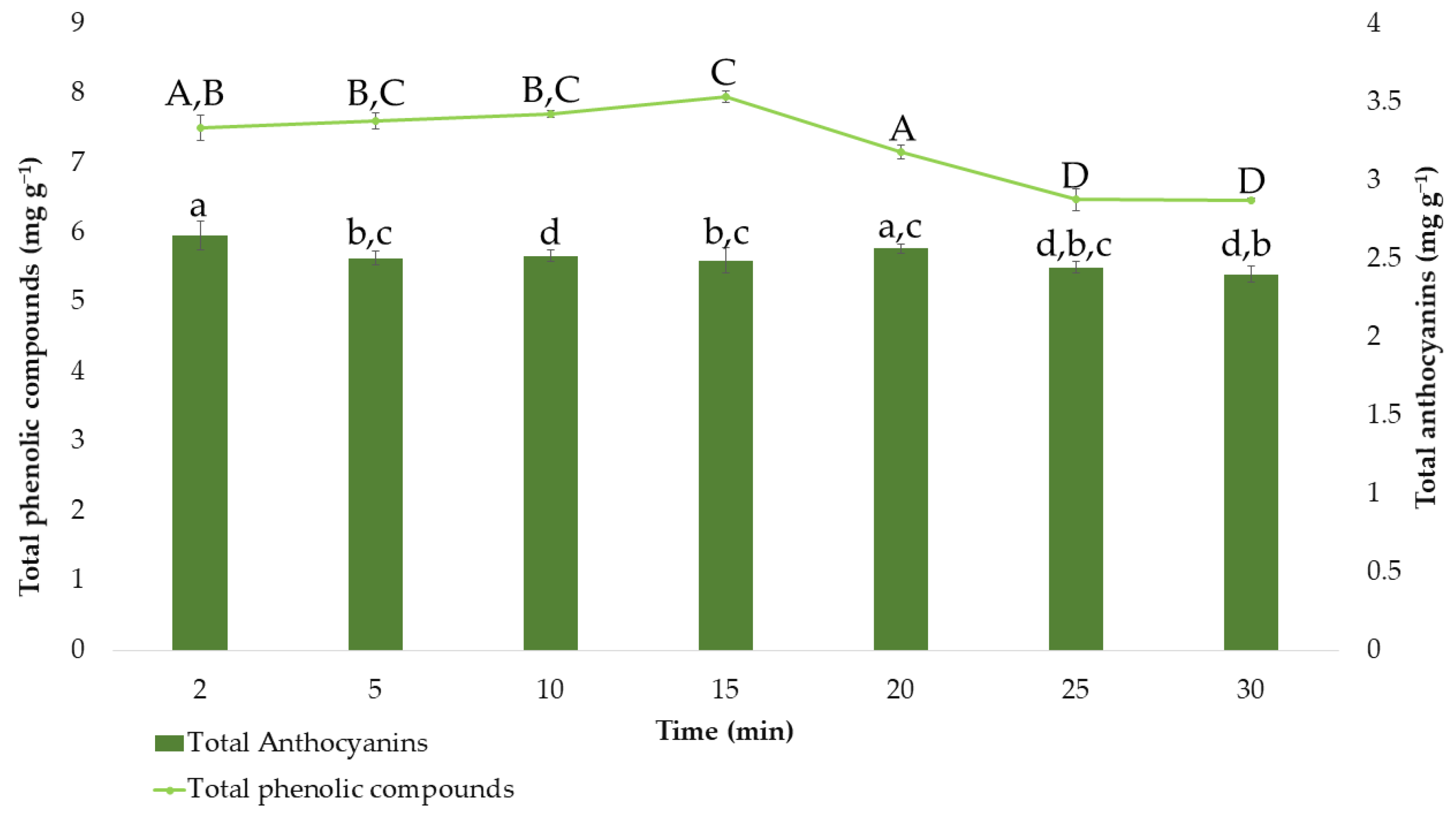
3.1. Temperature Stability Study
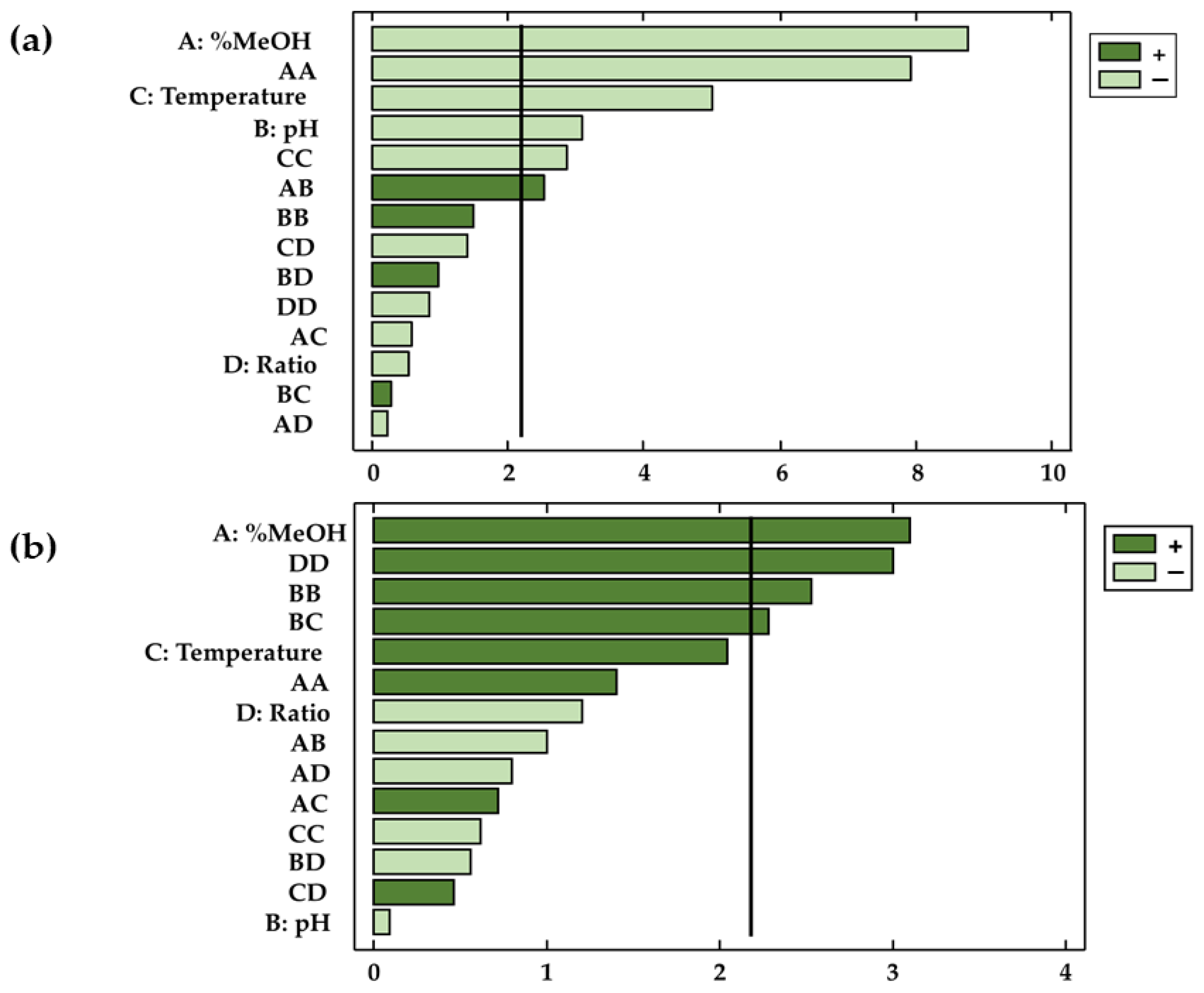
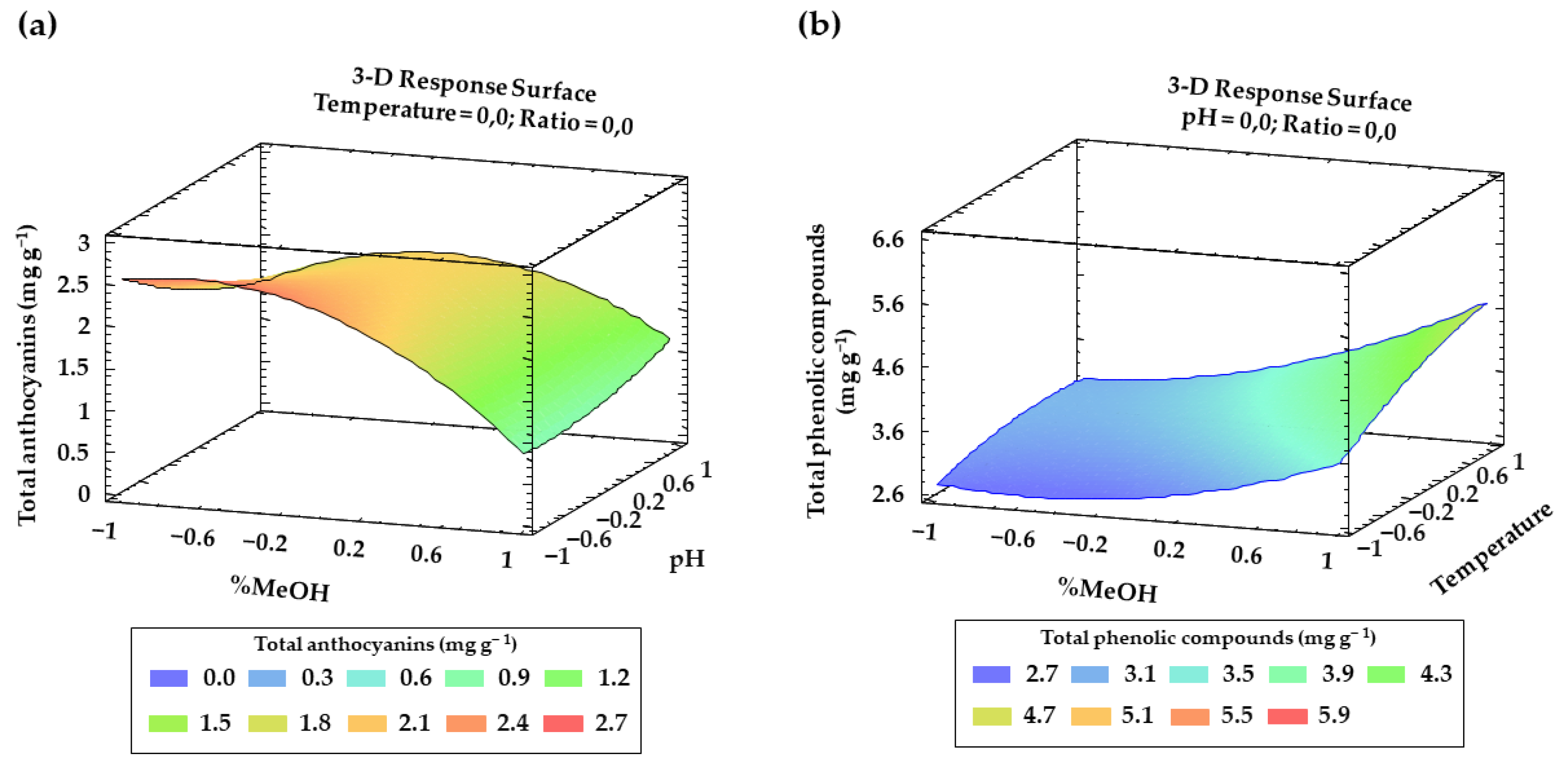
3.2. Box-Behken Designs and RSM
0.05·X1X3 − 0.02·X1X4 + 0.12·X2 2 + 0.03·X2X3 + 0.09·X2X4 − 0.21·X32 − 0.12·X3X4 − 0.06·X42,
0.22·X1X3 − 0.24·X1X4 + 0.70 X22 + 0.70·X2X3 − 0.17·X2X4 − 0.16·X32 + 0.14·X3X4 + 0.77·X42,
3.3. Optimal Conditions
3.4. Extraction Time and Precision
3.5. Multiresponse-Optimization
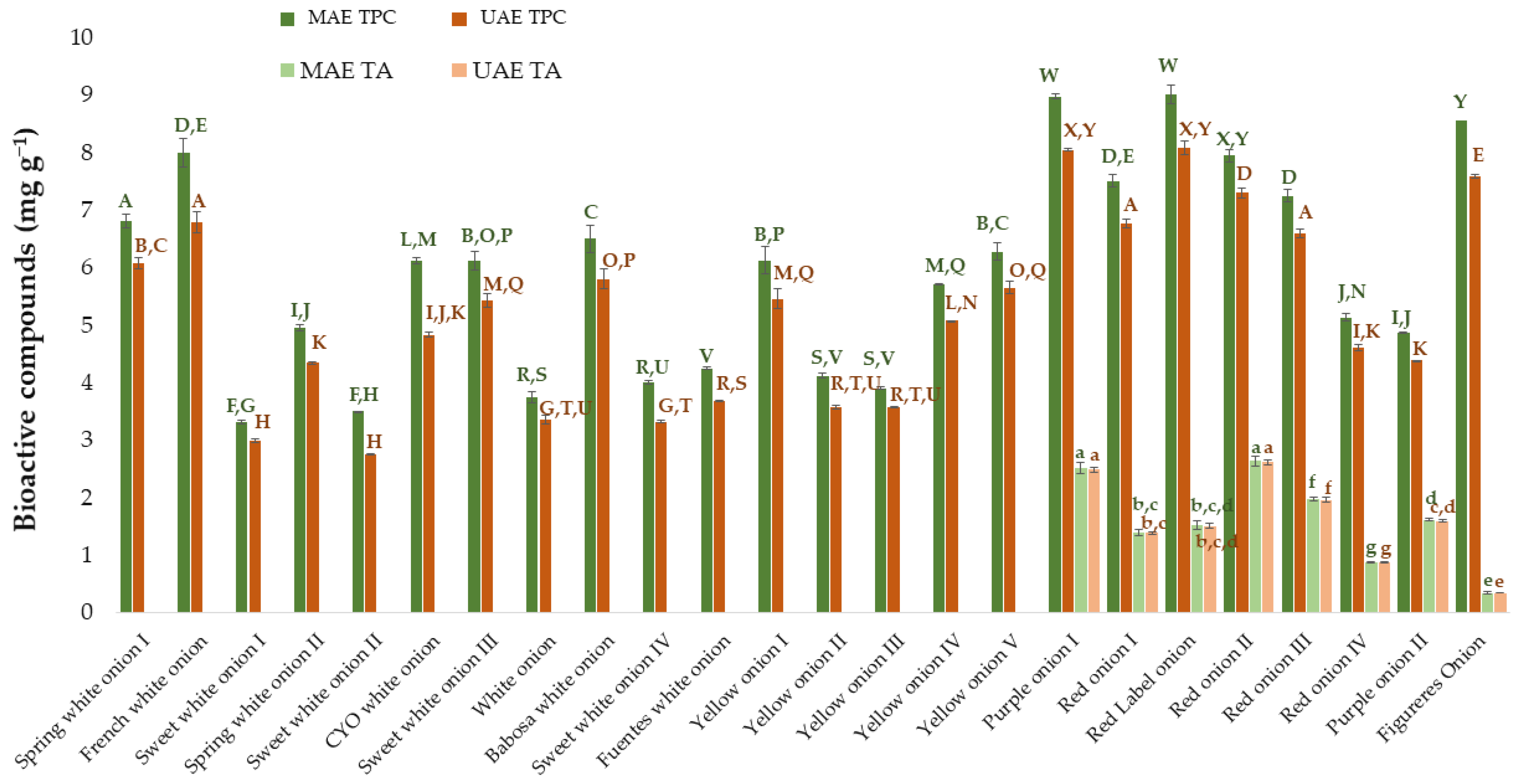
3.6. Microwave-Assisted Extraction vs. Other Assisted Extraction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations and Nomenclatures
| ANOVA | Analysis of Variance |
| BBD | Box-Behnken Design |
| CV | Coefficient of variation |
| DOE | Design of Experiments |
| HPLC | High-Performance Liquid Chromatography |
| MAE | Microwave-Assisted Extraction |
| n | Number of replicates |
| RSM | Response Surface Methodology |
| SD | Standard Deviation |
| TA | Total Anthocyanins |
| TE | Trolox Equivalents |
| TPC | Total Phenolic Compounds |
| UAE | Ultrasound-Assisted Extraction |
| UHPLC | Ultra-High-Performance Liquid Chromatography |
| 3D | Three-dimensional |
| X1 | Percentage of methanol of the solvent |
| X2 | pH of the solvent |
| X3 | Extraction temperature |
| X4 | Ratio between sample weight and volume of solvent |
| k | Represents the number of factors |
| C0 | Represents the number of central points |
| Y | Represents the responses |
| β0 | Represents the model constant |
| βi | Represents the coefficient for each main effect |
| βij | Represents the coefficient corresponding to the interactions between factor i and factor j |
| βii | Represents the coefficient of the quadratic factors that represent the curvature of the surface |
| X | Represents each one of the factors studied |
| r | Represents the residual value (random error) |
References
- Aguiar, J.; Goncalves, J.L.; Alves, V.L.; Camara, J.S. Chemical fingerprint of free polyphenols and antioxidant activity in dietary fruits and vegetables using a non-targeted approach based on quechers ultrasound-assisted extraction combined with UHPLC-PDA. Antioxidants 2020, 9, 305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michiu, D.; Socaciu, M.-I.; Fogarasi, M.; Jimborean, A.M.; Ranga, F.; Muresan, V.; Semeniuc, C.A. Implementation of an Analytical Method for Spectrophotometric Evaluation of Total Phenolic Content in Essential Oils. Molecules 2022, 27, 1345. [Google Scholar] [CrossRef] [PubMed]
- Routray, W.; Orsat, V. Microwave-Assisted Extraction of Flavonoids: A Review. Food Bioproc. Tech. 2012, 5, 409–424. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Acylated anthocyanins from edible sources and their applications in food systems. Biochem. Eng. J. 2003, 14, 217–225. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; Pacheco-Hernández, M.D.L.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Özcan, M.M.; Doğu, S.; Uslu, N. Effect of species on total phenol, antioxidant activity and phenolic compounds of different wild onion bulbs. Food Meas. Charact. 2018, 12, 902–905. [Google Scholar] [CrossRef]
- Hossain, M.B.; Lebelle, J.; Birsan, R.; Kai, D.K. Enrichment and assessment of the contributions of the major polyphenols to the total antioxidant activity of onion extracts: A fractionation by flash chromatography approach. Antioxidants 2018, 7, 175. [Google Scholar] [CrossRef] [Green Version]
- Saptarini, N.M.; Herawati, I.E. Extraction methods and varieties affect total anthocyanins content in acidified extract of papery skin of onion (Allium cepa L.). Drug Invent. 2018, 10, 471–474. [Google Scholar]
- Barros, L.; Carvalho, J.; Morais, J.S.; Ferreira, I.C.F.R. Strawberry-tree, blackthorn and rose fruits: Detailed characterisation in nutrients and phytochemicals with antioxidant. Food Chem. 2010, 120, 247–254. [Google Scholar] [CrossRef]
- Gironés-Vilaplana, A.; Calín-Sanchez, A.; Moreno, D.A.; Carbonell-Barrachina, A.A.; García-Viguera, C. Novel maqui liquor using traditional pacharán processing. Food Chem. 2015, 173, 1228–1235. [Google Scholar] [CrossRef]
- Wu, X.; Prior, R.L. Identification and characterization of anthocyanins by high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry in common foods in the United States: Vegetables, nuts, and grains. J. Agric. Food Chem. 2005, 53, 3101–3113. [Google Scholar] [CrossRef] [PubMed]
- Oancea, S.; Drǎghici, O. pH and thermal stability of anthocyanin-based optimised extracts of romanian red onion cultivars. Czech J. Food Sci. 2013, 31, 283–291. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Gregorio, M.R.R.; García-Falcón, M.S.S.; Simal-Gándara, J. Flavonoids changes in fresh-cut onions during storage in different packaging systems. Food Chem. 2011, 124, 652–658. [Google Scholar] [CrossRef]
- Zhang, C.; Li, X.; Zhan, Z.; Cao, L.; Zeng, A.; Chang, G.; Liang, Y. Transcriptome Sequencing and Metabolism Analysis Reveals the role of Cyanidin Metabolism in Dark-red Onion (Allium cepa L.). Bulbs. Sci. Rep. 2018, 8, 14109. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, I.; Carbone, V.; Spagnuolo, C.; Minasi, P.; Russo, G.L. Identification and quantification of flavonoids from two southern italian cultivars of Allium cepa L., Tropea (Red Onion) and Montoro (Copper Onion), and their capacity to protect human erythrocytes from oxidative stress. J. Agric. Food Chem. 2015, 63, 5229–5238. [Google Scholar] [CrossRef]
- Stoica, F.; Aprodu, L.; Enachi, E.; Stănciuc, N.; Condurache, N.N.; Duță, D.E.; Bahrim, G.E.; Râpeanu, G. Bioactive’s characterization, biological activities, and in silico studies of red onion (Allium cepa L.) skin extracts. Plants 2021, 10, 2330. [Google Scholar] [CrossRef]
- Moreno-Rojas, J.M.; Moreno-Ortega, A.; Ordóñez, J.J.; Moreno-Rojas, R.; Pérez-Aparicio, J.; Pereira-Caro, G. Development and validation of UHPLC-HRMS methodology for the determination of flavonoids, amino acids and organosulfur compounds in black onion, a novel derived product from fresh shallot onions (Allium cepa var. Aggregatum). LWT 2018, 97, 376–383. [Google Scholar] [CrossRef]
- Terahara, N.; Yamaguchi, M.-A.; Honda, T. Malonylated Anthocyanins from Bulbs of Red Onion, Allium cepa L. Biosci. Biotechnol. Biochem. 2009, 58, 1324–1325. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Pérez-Gregorio, M.R.; García-Falcón, M.S.; Simal-Gándara, J.; Almeida, D.P.F. Effect of meteorological conditions on antioxidant flavonoids in Portuguese cultivars of white and red onions. Food Chem. 2011, 124, 303–308. [Google Scholar] [CrossRef]
- Pérez-Gregorio, M.R.; González-Barreiro, C.; Rial-Otero, R.; Simal-Gándara, J. Comparison of sanitizing technologies on the quality appearance and antioxidant levels in onion slices. Food Control 2011, 22, 2052–2058. [Google Scholar] [CrossRef]
- Li, H.; Deng, Z.; Zhu, D.; Hu, C.; Liu, R.; Young, J.C.; Tsao, R. Highly pigmented vegetables: Anthocyanin compositions and their role in antioxidant activities. Food Res. Int. 2012, 46, 250–259. [Google Scholar] [CrossRef]
- Pérez-Gregorio, R.M.; García-Falcón, M.S.; Simal-Gándara, J.; Rodrigues, A.S.; Almeida, D.P.F. Identification and quantification of flavonoids in traditional cultivars of red and white onions at harvest. J. Food Compos. Anal. 2010, 23, 592–598. [Google Scholar] [CrossRef]
- Rosa, R.; Ferrari, E.; Veronesi, P. From Field to Shelf: How Microwave-Assisted Extraction Techniques Foster an Integrated Green Approach. In Emerging Microwave Technologies in Industrial, Agricultural, Medical and Food Processing; Kok, Y.Y., Ed.; IntechOpen: London, UK, 2018; p. 179. [Google Scholar]
- Chaves, J.O.; de Souza, M.C.; da Silva, C.L.; Lachos-Perez, D.; Torres-Mayanga, P.C.; Machado, A.P.D.F.; Forster-Carneiro, T.; Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Barbero, G.F.; et al. Extraction of Flavonoids From Natural Sources Using Modern Techniques. Front. Chem. 2020, 8, 507887. [Google Scholar] [CrossRef] [PubMed]
- González-de-Peredo, A.V.; Vázquez-Espinosa, M.; Espada-Bellido, E.; Ferreiro-González, M.; Carrera, C.; Barbero, G.F.; Palma, M. Development of Optimized Ultrasound-Assisted Extraction Methods for the Recovery of Total Phenolic Compounds and Anthocyanins from Onion Bulbs. Antioxidants 2021, 10, 1755. [Google Scholar] [CrossRef]
- Rehman, M.U.; AbdullahKhan, F.; Niaz, K. Introduction to natural products analysis. In Recent Advances in Natural Products Analysis, 1st ed.; Silva, A.S., Nabavi, S.F., Saeedi, M., Nabavi, S.M., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 3–15. [Google Scholar]
- Zhou, H.-Y.; Liu, C.-Z. Microwave-assisted extraction of solanesol from tobacco leaves. J. Chromatogr. A 2006, 1129, 135–139. [Google Scholar] [CrossRef]
- Lopez-Avila, V.; Luque de Castro, M.D. Microwave-Assisted Extraction. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1389–1398. [Google Scholar]
- Stoica, F.; Râpeanu, G.; Nistor, O.V.; Enachi, E.; Stănciuc, N.; Mureșan, C.; Bahrim, G.E. Recovery of bioactive compounds from red onion skins using conventional solvent extraction and microwave assisted extraction. Ann. Univ. Dunarea Jos Galati Fascicle VI-Food Technol. 2020, 44, 104–126. [Google Scholar] [CrossRef]
- Razali, M.A.A.; Sanusi, N.; Ismail, H.; Othman, N.; Ariffin, A. Application of response surface methodology (RSM) for optimization of cassava starch grafted polyDADMAC synthesis for cationic properties. Starch/Staerke 2012, 64, 935–943. [Google Scholar] [CrossRef]
- Aydar, A.Y. Utilization of Response Surface Methodology in Optimization of Extraction of Plant Materials. In Statistical Approaches with Emphasis on Design of Experiments Applied to Chemical Processes; Silva, V., Ed.; InTech: London, UK, 2018; pp. 157–169. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Miliauskas, G.; Venskutonis, P.R.; Van Beek, T.A. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004, 85, 231–237. [Google Scholar] [CrossRef]
- Škrovánková, S.; Mišurcová, L.; Machů, L. Antioxidant Activity and Protecting Health Effects of Common Medicinal Plants. Adv. Food Nutr. Res. 2012, 67, 75–139. [Google Scholar] [CrossRef]
- Dini, I.; Tenore, G.C.; Dini, A. S-alkenyl cysteine sulfoxide and its antioxidant properties from Allium cepa var. tropeana (red onion) seeds. J. Nat. Prod. 2008, 71, 2036–2037. [Google Scholar] [CrossRef]
- Majid, I.; Nanda, V. Instrumental texture and flavonoid profile of paste developed from sprouted onion varieties of Indian origin. Int. J. Food Prop. 2017, 20, 2511–2526. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, S.L.C.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandao, G.C.; da Silva, E.G.P.; Portugal, L.A.; dos Reis, P.S.; Souza, A.S.; et al. Box-Behnken design: An alternative for the optimization of analytical methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef]
- Maran, J.; Manikandan, S.; Thirugnanasambandham, K.; Vigna Nivetha, C.; Dinesh, R. Box-Behnken design based statistical modeling for ultrasound-assisted extraction of corn silk polysaccharide. Carbohydr. Polym. 2013, 92, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M.; Goulas, V.; Tsakona, S.; Manganaris, G.A.; Gekas, V. A Knowledge Base for The Recovery of Natural Phenols with Different Solvents. Int. J. Food Prop. 2012, 16, 382–396. [Google Scholar] [CrossRef] [Green Version]
- Jiménez, N.; Bohuon, P.; Dornier, M.; Bonazzi, C.; Pérez, A.M.; Vaillant, F. Effect of water activity on anthocyanin degradation and browning kinetics at high temperatures (100–140 °C). Food Res. Int. 2012, 47, 106–115. [Google Scholar] [CrossRef]
- González-de-Peredo, A.V.; Vázquez-Espinosa, M.; Espada-Bellido, E.; Jiménez-Cantizano, A.; Ferreiro-González, M.; Amores-Arrocha, A.; Palma, M.; Barroso, C.G.; Barbero, G.F. Development of New Analytical Microwave-Assisted Extraction Methods for Bioactive Compounds from Myrtle (Myrtus communis L.). Molecules 2018, 23, 2992. [Google Scholar] [CrossRef] [Green Version]
- Vázquez-Espinosa, M.; Espada-Bellido, E.; González-de-Peredo, A.V.; Ferreiro-González, M.; Carrera, C.; Palma, M.; Barroso, C.G.; Barbero, G.F. Optimization of Microwave-Assisted Extraction for the Recovery of Bioactive Compounds from the Chilean Superfruit (Aristotelia chilensis (Mol.) Stuntz). Agronomy 2018, 8, 240. [Google Scholar] [CrossRef] [Green Version]
- Xiang, Z.; Wu, X. Ultrasonic-Microwave Assisted Extraction of Total Flavonoids from Scutellaria baicalensis Using Response Surface Methodology. Pharm. Chem. J. 2017, 51, 318–324. [Google Scholar] [CrossRef]
- Machado, A.P.D.F.; Pasquel-Reátegui, J.L.; Barbero, G.F.; Martínez, J. Pressurized liquid extraction of bioactive compounds from blackberry (Rubus fruticosus L.) residues: A comparison with conventional methods. Food Res. Int. 2015, 77, 675–683. [Google Scholar] [CrossRef]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Wei, G.; Zhou, H.; Gu, C.; Vimolmangkang, S.; Liao, L.; Han, Y. Unraveling the Mechanism Underlying the Glycosylation and Methylation of Anthocyanins in Peach. Plant Physiol. 2014, 166, 1044–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno, J.; Peinado, R. Chapter 5-Polyphenols. Enol. Chem. 2012, 1, 53–76. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [Green Version]
- Amić, D.; Davidović-Amić, D.; Trinajstić, N. Application of topological indices to chromatographic data: Calculation of the retention indices of anthocyanins. J. Chromatogr. A 1993, 653, 115–121. [Google Scholar] [CrossRef]
- Setford, P.C.; Jeffery, D.W.; Grbin, P.R.; Muhlack, R. Mass Transfer of Anthocyanins during Extraction from Pre-Fermentative Grape Solids under Simulated Fermentation Conditions: Effect of Convective Conditions. Molecules 2019, 24, 73. [Google Scholar] [CrossRef] [Green Version]
- Ambigaipalan, P.; de Camargo, A.C.; Shahidi, F. Identification of phenolic antioxidants and bioactives of pomegranate seeds following juice extraction using HPLC-DAD-ESI-MSn. Food Chem. 2017, 221, 1883–1894. [Google Scholar] [CrossRef]
- Sang, J.; Sang, J.; Ma, Q.; Hou, X.-F.; Li, C.-Q. Extraction optimization and identification of anthocyanins from Nitraria tangutorun Bobr. seed meal and establishment of a green analytical method of anthocyanins. Food Chem. 2017, 218, 386–395. [Google Scholar] [CrossRef]
- Santos, D.T.; Veggi, P.C.; Meireles, M.A.A. Optimization and economic evaluation of pressurized liquid extraction of phenolic compounds from jabuticaba skins. J. Food Eng. 2012, 108, 444–452. [Google Scholar] [CrossRef] [Green Version]
- Türker, N.; Erdogdu, F. Effects of pH and temperature of extraction medium on effective diffusion coefficient of anthocynanin pigments of black carrot (Daucus carota var. L.). J. Food Eng. 2006, 76, 579–583. [Google Scholar] [CrossRef]
- Peron, D.V.; Fraga, S.; Antelo, F. Thermal degradation kinetics of anthocyanins extracted from juçara (Euterpe edulis Martius) and ‘Italia’ grapes (Vitis vinifera L.), and the effect of heating on the antioxidant capacity. Food Chem. 2017, 232, 836–840. [Google Scholar] [CrossRef] [PubMed]
- Frond, A.D.; Iuhas, C.I.; Stirnu, I.; Leopold, L.; Soacaci, S.; Andreea, S.; Ayvaz, H.; Mihai, S.; Diaconeasa, Z.; Carmen, S. Phytochemical characterization of five edible purple-reddish vegetables: Anthocyanins, flavonoids, and phenolic acid derivatives. Molecules 2019, 24, 1536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khoo, H.E.; Chew, L.Y.; Ismail, A. Azlan, A. Anthocyanins in purple colored fruits. In Polyphenols: Chemistry, Dietary Sources and Health Benefits; Sun, J., Prasad, K.N., Ismail, A., Eds.; Nova Science Publisher: New York, NY, USA, 2012; pp. 133–152. [Google Scholar]
- Carrera, C.; Ruiz-Rodríguez, A.; Barroso, C.G. Ultrasound assisted extraction of phenolic compounds from grapes. Anal. Chim. Acta 2012, 732, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.T.V.; Tarone, A.G.; Cazarin, C.B.B.; Barbero, G.F.; Martínez, J. Pressurized liquid extraction of bioactive compounds from grape marc. J. Food Eng. 2018, 240, 105–113. [Google Scholar] [CrossRef]
- Ju, Z.Y.; Howard, L.R. Effects of solvent and temperature on pressurized liquid extraction of anthocyanins and total phenolics from dried red grape skin. J. Agric. Food Chem. 2003, 51, 5207–5213. [Google Scholar] [CrossRef] [PubMed]
- Simpson, K.L. Chemical changes in natural food pigments. In Chemical Changes in Food During Processing. Ift Basic Symposium Series; Richardson, T., Finley, J.W., Eds.; Springer: Dordrecht, The Netherlands, 1985; pp. 409–411. [Google Scholar] [CrossRef]
- Espada-Bellido, E.; Ferreiro-González, M.; Carrera, C.; Palma, M.; Barroso, C.G.; Barbero, G.F. Optimization of the ultrasound-assisted extraction of anthocyanins and total phenolic compounds in mulberry (Morus nigra) pulp. Food Chem. 2017, 219, 23–32. [Google Scholar] [CrossRef]
- Association of Official Agricultural Chemists. Peer Verified Methods Advisory Committee. In AOAC Peer Verified Methods Program; AOAC International: Gaithersburg, MD, USA, 1998; pp. 1–35. [Google Scholar]
- Aksezer, C.S. On the sensitivity of desirability functions for multiresponse optimization. J. Ind. Manag. Optim. 2008, 4, 685–696. [Google Scholar] [CrossRef]
- Jeya, K.S.; Sathiyasree, B.; Beniz, T.E.; Ramarajan, C.; Gurushankar, K. Optimization of extraction parameters and stabilization of anthocyanin from onion peel. Crit. Rev. Food Sci. Nutr. 2022, 62, 2560–2567. [Google Scholar] [CrossRef]
- Hendrawan, Y.; Maharani, D.M.; Hawa, L.C. Effect of ultrasonic assisted extraction on Dayak onion powder extraction (Eleutherine palmifolia). In IOP Conference Series: Earth and Environmental Science; IOP Publishing Ltd.: Bristol, UK, 2020; Volume 475, p. 012015. [Google Scholar]
- Ko, E.Y.; Nile, S.H.; Jung, Y.S.; Keum, Y.S. Antioxidant and antiplatelet potential of different methanol fractions and flavonols extracted from onion (Allium cepa L.). 3 Biotech 2018, 8, 155. [Google Scholar] [CrossRef]
| Run | Factors | Responses | ||||||
|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | YTPC (mg g−1) | YTA (mg g−1) | |||
| Experimental | Predicted | Experimental | Predicted | |||||
| 1 | 0 | −1 | 0 | 0 | 3.4833 | 4.0118 | 2.0412 | 2.3208 |
| 2 | 1 | −1 | 0 | 0 | 5.7664 | 5.5038 | 0.8868 | 0.8339 |
| 3 | 0 | 1 | 0 | 0 | 3.1456 | 3.9761 | 2.0438 | 1.9783 |
| 4 | 1 | 1 | 0 | 0 | 4.7861 | 4.5235 | 1.2305 | 1.1776 |
| 5 | 0 | 0 | −1 | −1 | 3.6146 | 3.8842 | 2.0176 | 1.9154 |
| 6 | 0 | 0 | 1 | −1 | 3.7680 | 4.3269 | 1.6192 | 1.6535 |
| 7 | 0 | 0 | −1 | 1 | 3.6630 | 3.1813 | 2.1397 | 2.1060 |
| 8 | 0 | 0 | 1 | 1 | 4.3753 | 4.1829 | 1.2535 | 1.3563 |
| 9 | 0 | 0 | 0 | 0 | 3.2191 | 3.2825 | 1.8125 | 2.0290 |
| 10 | −1 | 0 | 0 | −1 | 4.1747 | 3.7766 | 1.8150 | 1.8508 |
| 11 | 1 | 0 | 0 | −1 | 5.3465 | 5.5262 | 0.8095 | 0.8702 |
| 12 | −1 | 0 | 0 | 1 | 3.7587 | 3.8429 | 1.7955 | 1.8381 |
| 13 | 1 | 0 | 0 | 1 | 3.9511 | 4.6131 | 0.7089 | 0.7764 |
| 14 | 0 | −1 | −1 | 0 | 3.5470 | 4.1913 | 2.4131 | 2.3890 |
| 15 | 0 | 1 | −1 | 0 | 2.8873 | 2.7622 | 1.7712 | 1.9972 |
| 16 | 0 | −1 | 1 | 0 | 3.4709 | 3.5201 | 2.0102 | 1.8339 |
| 17 | 0 | 1 | 1 | 0 | 5.5978 | 4.8777 | 1.4669 | 1.5407 |
| 18 | 0 | 0 | 0 | 0 | 3.4444 | 3.2825 | 2.3189 | 2.0290 |
| 19 | 0 | −1 | 0 | −1 | 5.4355 | 4.8213 | 2.3473 | 2.3718 |
| 20 | 0 | 1 | 0 | −1 | 5.1206 | 5.1248 | 1.9102 | 1.8571 |
| 21 | 0 | −1 | 0 | 1 | 5.0823 | 4.7371 | 2.1971 | 2.1463 |
| 22 | 0 | 1 | 0 | 1 | 4.0890 | 4.3621 | 2.1043 | 1.9760 |
| 23 | −1 | 0 | −1 | 0 | 2.7386 | 2.7427 | 1.9456 | 1.8986 |
| 24 | 1 | 0 | −1 | 0 | 3.8788 | 3.5676 | 0.9988 | 0.9798 |
| 25 | −1 | 0 | 1 | 0 | 2.7200 | 3.0298 | 1.5266 | 1.4952 |
| 26 | 1 | 0 | 1 | 0 | 4.7302 | 4.7248 | 0.3750 | 0.3717 |
| 27 | 0 | 0 | 0 | 0 | 3.8636 | 3.2825 | 2.0626 | 2.0290 |
| Source | Coefficient | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|---|
| Model | 2.03 | 7.44 | 14 | 0.53 | 17.29 | <0.0001 |
| X1: %MeOH | −0.51 | 2.37 | 1 | 2.37 | 77.05 | <0.0001 |
| X2: pH | −0.17 | 0.29 | 1 | 0.29 | 9.54 | 0.01 |
| X3: Temperature | −0.25 | 0.77 | 1 | 0.77 | 24.96 | 0.00 |
| X4: Ratio | −0.03 | 0.01 | 1 | 0.01 | 0.28 | 0.61 |
| X12 | −0.63 | 1.94 | 1 | 1.94 | 62.97 | <0.0001 |
| X1X2 | 0.34 | 0.19 | 1 | 0.19 | 6.38 | 0.03 |
| X1X3 | −0.05 | 0.01 | 1 | 0.01 | 0.34 | 0.57 |
| X1X4 | −0.02 | 0.00 | 1 | 0.02 | 0.05 | 0.82 |
| X22 | 0.12 | 0.07 | 1 | 0.07 | 2.23 | 0.16 |
| X2X3 | 0.03 | 0.00 | 1 | 0.00 | 0.08 | 0.78 |
| X2X4 | 0.09 | 0.03 | 1 | 0.03 | 0.96 | 0.35 |
| X32 | −0.21 | 0.25 | 1 | 0.25 | 8.15 | 0.02 |
| X3X4 | −0.12 | 0.06 | 1 | 0.06 | 1.93 | 0.19 |
| X42 | −0.06 | 0.02 | 1 | 0.02 | 0.71 | 0.42 |
| Residual | 0.37 | 12 | 0.03 | |||
| Lack of fit | 0.24 | 10 | 0.02 | 0.38 | ||
| Pure Error | 0.13 | 2 | 0.06 | |||
| Cor Total | 7.81 | 26 |
| Source | Coefficient | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|---|
| Model | 3.28 | 16.00 | 14 | 1.14 | 3.06 | 0.03 |
| X1: %MeOH | 0.63 | 3.61 | 1 | 3.61 | 9.66 | 0.01 |
| X2: pH | −0.02 | 0.00 | 1 | 0.00 | 0.01 | 0.93 |
| X3: Temperature | 0.36 | 1.56 | 1 | 1.56 | 4.19 | 0.06 |
| X4: Ratio | −0.21 | 0.54 | 1 | 0.54 | 1.44 | 0.25 |
| X12 | 0.40 | 0.73 | 1 | 0.73 | 1.96 | 0.19 |
| X1X2 | −0.47 | 0.37 | 1 | 0.37 | 0.99 | 0.34 |
| X1X3 | 0.22 | 0.19 | 1 | 0.19 | 0.51 | 0.49 |
| X1X4 | −0.24 | 0.24 | 1 | 0.24 | 0.64 | 0.44 |
| X22 | 0.70 | 2.39 | 1 | 2.39 | 6.39 | 0.03 |
| X2X3 | 0.70 | 1.94 | 1 | 1.94 | 5.20 | 0.04 |
| X2X4 | −0.17 | 0.12 | 1 | 0.12 | 0.31 | 0.59 |
| X32 | −0.16 | 0.14 | 1 | 0.14 | 0.37 | 0.55 |
| X3X4 | 0.14 | 0.08 | 1 | 0.08 | 0.21 | 0.66 |
| X42 | 0.77 | 3.37 | 1 | 3.37 | 9.01 | 0.01 |
| Residual | 4.48 | 12 | 0.37 | |||
| Lack of fit | 4.27 | 10 | 0.43 | 3.99 | 0.22 | |
| Pure Error | 0.21 | 2 | 0.11 | |||
| Cor Total | 20.48 | 26 |
| mg of Trolox Equivalents g−1 of Dry Onion 1 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Onion 1 2 | Onion 2 | Onion 3 | Onion 4 | Onion 5 | Onion 6 | Onion 7 | Onion 8 | Onion 9 | Onion 10 | Onion 11 | Onion 12 |
| 5.89 ± 0.23 a,b | 5.34 ± 0.27 a,c | 4.26 ± 0.10 d | 4.10 ± 1.34 d | 4.87 ± 0.28 c | 8.95 ± 0.22 e | 6.21 ± 0.49 b,f | 8.12 ± 0.18 g | 8.25 ± 0.14 g | 3.12 ± 0.66 h | 4.95 ± 0.59 c | 6.72 ± 0.11 f,i |
| Onion 13 | Onion 14 | Onion 15 | Onion 16 | Onion 17 | Onion 18 | Onion 19 | Onion 20 | Onion 21 | Onion 22 | Onion 23 | Onion 24 |
| 7.89 ± 0.12 g | 7.12 ± 0.18 i | 4.95 ± 0.29 c | 3.89 ± 0.56 d | 7.89 ± 0.29 g | 6.12 ± 0.02 b | 7.21 ± 0.21 i | 6.75 ± 0.19 f,i | 9.12 ± 0.21 e | 9.21 ± 0.12 e | 5.45 ± 0.93 c | 2.86 ± 0.11 h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-de-Peredo, A.V.; Vázquez-Espinosa, M.; Espada-Bellido, E.; Ferreiro-González, M.; Carrera, C.; Barbero, G.F.; Palma, M. Extraction of Antioxidant Compounds from Onion Bulb (Allium cepa L.) Using Individual and Simultaneous Microwave-Assisted Extraction Methods. Antioxidants 2022, 11, 846. https://doi.org/10.3390/antiox11050846
González-de-Peredo AV, Vázquez-Espinosa M, Espada-Bellido E, Ferreiro-González M, Carrera C, Barbero GF, Palma M. Extraction of Antioxidant Compounds from Onion Bulb (Allium cepa L.) Using Individual and Simultaneous Microwave-Assisted Extraction Methods. Antioxidants. 2022; 11(5):846. https://doi.org/10.3390/antiox11050846
Chicago/Turabian StyleGonzález-de-Peredo, Ana V., Mercedes Vázquez-Espinosa, Estrella Espada-Bellido, Marta Ferreiro-González, Ceferino Carrera, Gerardo F. Barbero, and Miguel Palma. 2022. "Extraction of Antioxidant Compounds from Onion Bulb (Allium cepa L.) Using Individual and Simultaneous Microwave-Assisted Extraction Methods" Antioxidants 11, no. 5: 846. https://doi.org/10.3390/antiox11050846
APA StyleGonzález-de-Peredo, A. V., Vázquez-Espinosa, M., Espada-Bellido, E., Ferreiro-González, M., Carrera, C., Barbero, G. F., & Palma, M. (2022). Extraction of Antioxidant Compounds from Onion Bulb (Allium cepa L.) Using Individual and Simultaneous Microwave-Assisted Extraction Methods. Antioxidants, 11(5), 846. https://doi.org/10.3390/antiox11050846











