Plasma Free Thiol Levels during Early Sepsis Predict Future Renal Function Decline
Abstract
:1. Introduction
2. Methods
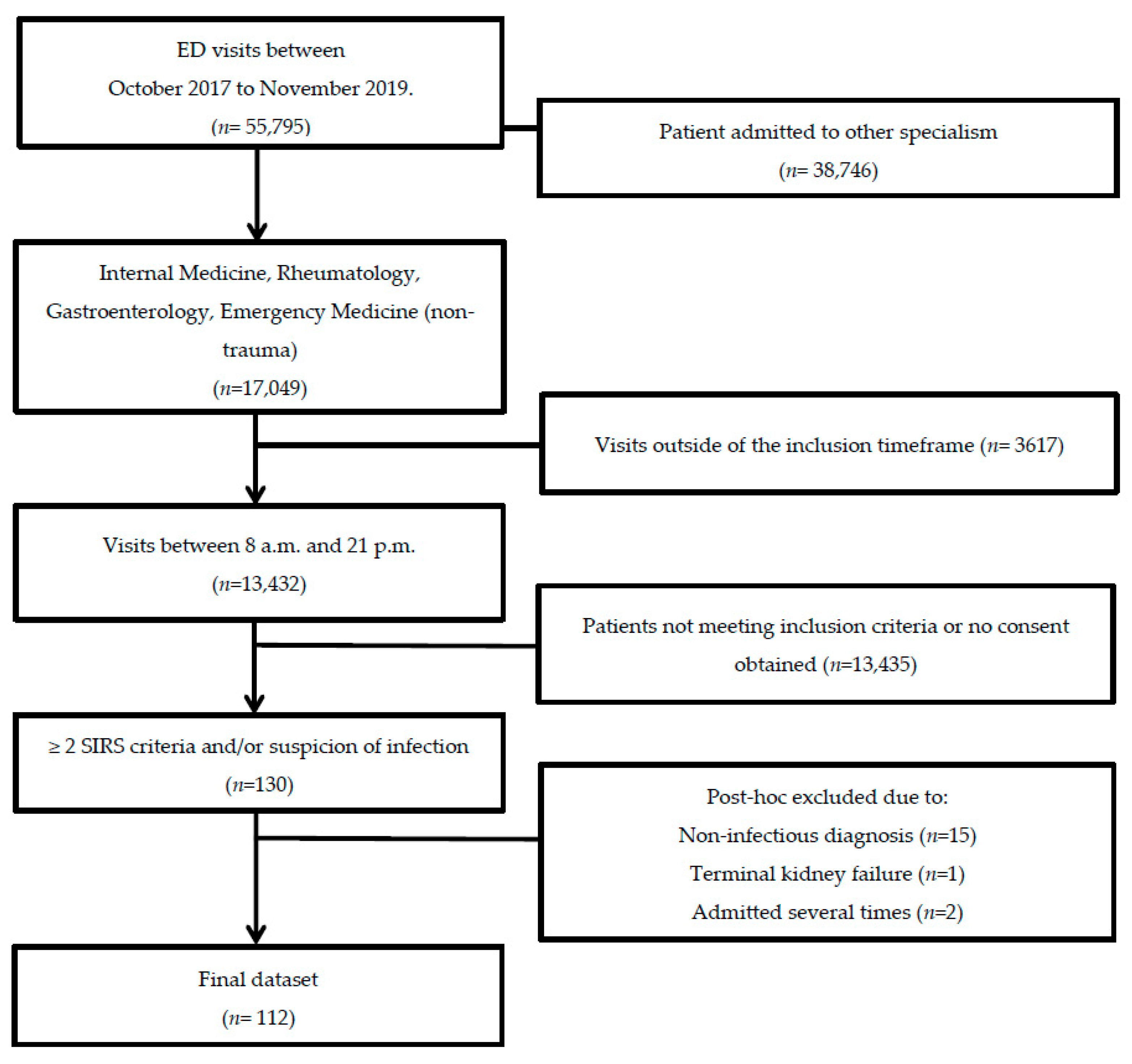
2.1. Study Population and Study Design
2.2. Data Collection and Laboratory Measurements
2.3. Post Hoc Sepsis Adjudication
2.4. Measurement of Plasma Free Thiol Levels
2.5. Ischemia-Modified Albumin Measurement
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
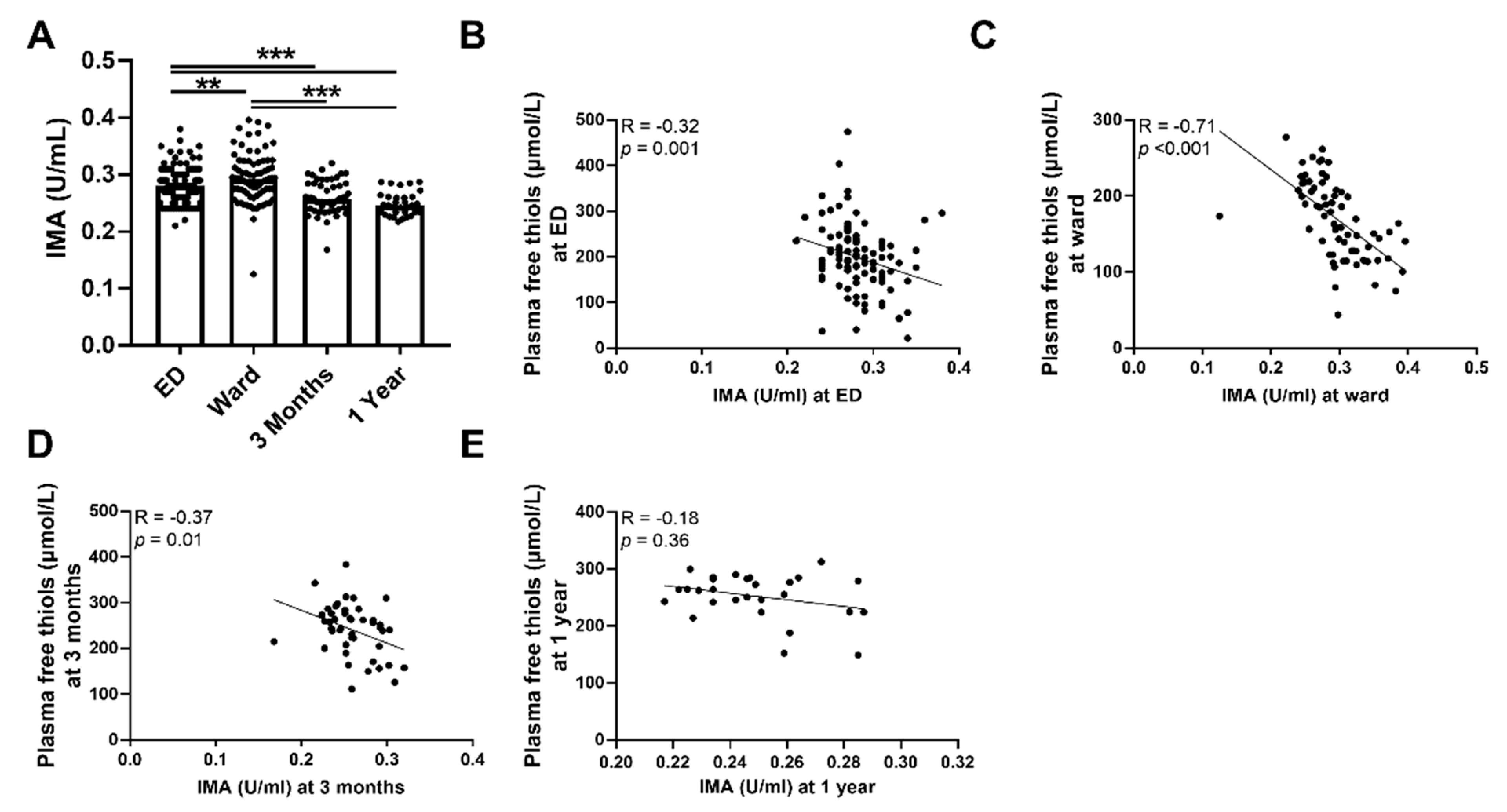
3.2. Plasma Free Thiol Levels Are Lower during Sepsis
3.3. Plasma Free Thiols Are Associated with Ischemia-Modified Albumin
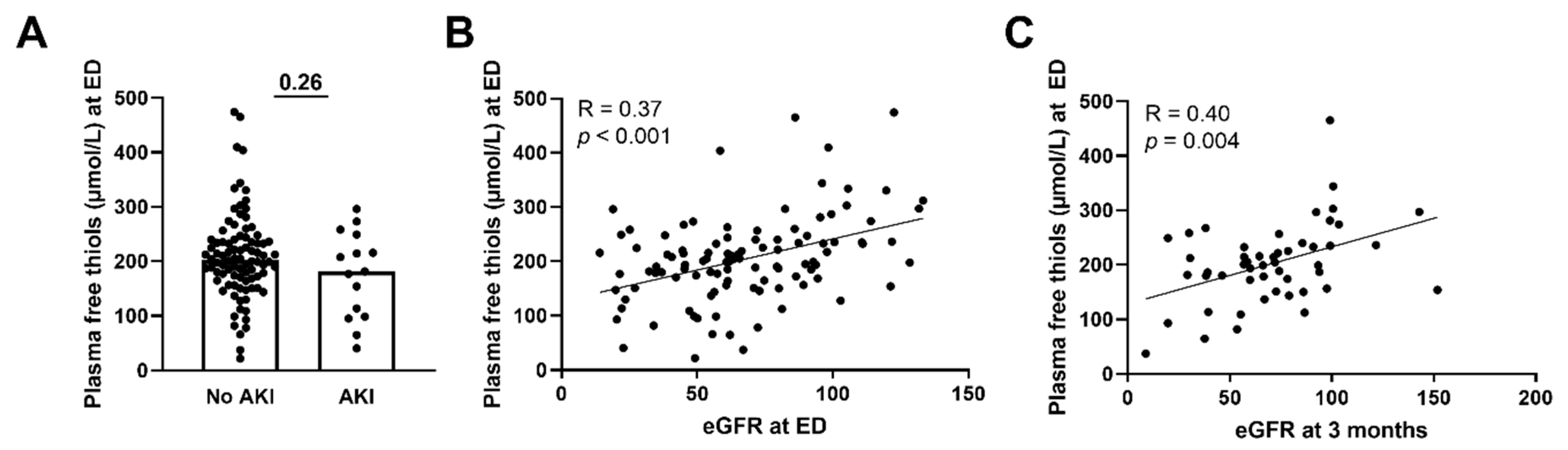
3.4. Lower Plasma Free Thiols Are Associated with Renal Function Decline
3.5. Plasma Free Thiols Are Associated with Long-Term Renal Function Decline
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Angus, D.C.; van der Poll, T. Severe sepsis and septic shock. N. Engl. J. Med. 2013, 369, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [Green Version]
- Cecconi, M.; Evans, L.; Levy, M.; Rhodes, A. Sepsis and septic shock. Lancet 2018, 392, 75–87. [Google Scholar] [CrossRef]
- De Blasi, R.A. Severe sepsis and septic shock. N. Engl. J. Med. 2013, 369, 2062–2063. [Google Scholar]
- Schrier, R.W.; Wang, W. Acute renal failure and sepsis. N. Engl. J. Med. 2004, 351, 159–169. [Google Scholar] [CrossRef]
- Bellomo, R.; Kellum, J.A.; Ronco, C.; Wald, R.; Martensson, J.; Maiden, M.; Bagshaw, S.M.; Glassford, N.J.; Lankadeva, Y.; Vaara, S.T.; et al. Acute kidney injury in sepsis. Intensive Care Med. 2017, 43, 816–828. [Google Scholar] [CrossRef] [Green Version]
- Chawla, L.S.; Eggers, P.W.; Star, R.A.; Kimmel, P.L. Acute kidney injury and chronic kidney disease as interconnected syndromes. N. Engl. J. Med. 2014, 371, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Noble, R.A.; Lucas, B.J.; Selby, N.M. Long-Term Outcomes in Patients with Acute Kidney Injury. Clin. J. Am. Soc. Nephrol. 2020, 15, 423–429. [Google Scholar] [CrossRef]
- Prowle, J.R. Sepsis-Associated AKI. Clin. J. Am. Soc. Nephrol. 2018, 13, 339–342. [Google Scholar] [CrossRef]
- Exline, M.C.; Crouser, E.D. Mitochondrial mechanisms of sepsis-induced organ failure. Front. Biosci. 2008, 13, 5030–5041. [Google Scholar] [PubMed]
- Crouser, E.D. Mitochondrial dysfunction in septic shock and multiple organ dysfunction syndrome. Mitochondrion 2004, 4, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Turell, L.; Radi, R.; Alvarez, B. The thiol pool in human plasma: The central contribution of albumin to redox processes. Free. Radic. Biol. Med. 2013, 65, 244–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortese-Krott, M.M.; Koning, A.; Kuhnle, G.G.C.; Nagy, P.; Bianco, C.L.; Pasch, A.; Wink, D.A.; Fukuto, J.M.; Jackson, A.A.; van Goor, H.; et al. The Reactive Species Interactome: Evolutionary Emergence, Biological Significance, and Opportunities for Redox Metabolomics and Personalized Medicine. Antioxid. Redox Signal. 2017, 27, 684–712. [Google Scholar] [CrossRef] [Green Version]
- Banne, A.F.; Amiri, A.; Pero, R.W. Reduced level of serum thiols in patients with a diagnosis of active disease. J. Anti Aging Med. 2003, 6, 327–334. [Google Scholar] [CrossRef]
- Sutton, T.R.; Minnion, M.; Barbarino, F.; Koster, G.; Fernandez, B.O.; Cumpstey, A.F.; Wischmann, P.; Madhani, M.; Frenneaux, M.P.; Postle, A.D.; et al. A robust and versatile mass spectrometry platform for comprehensive assessment of the thiol redox metabolome. Redox Biol. 2018, 16, 359–380. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Abdulle, A.E.; Bourgonje, M.F.; Binnenmars, S.H.; Gordijn, S.J.; Bulthuis, M.L.C.; van Gemert, S.B.; Kieneker, L.M.; Gansevoort, R.T.; Bakker, S.J.L. Serum free sulfhydryl status associates with new-onset chronic kidney disease in the general population. Redox Biol. 2021, 48, 102211. [Google Scholar] [CrossRef]
- Koning, A.M.; Meijers, W.C.; Pasch, A.; Leuvenink, H.G.D.; Frenay, A.S.; Dekker, M.M.; Feelisch, M.; de Boer, R.A.; van Goor, H. Serum free thiols in chronic heart failure. Pharmacol. Res. 2016, 111, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Abdulle, A.E.; Bourgonje, A.R.; Kieneker, L.M.; Koning, A.M.; la Bastide-van Gemert, S.; Bulthuis, M.L.C.; Dijkstra, G.; Nico Faber, K.; Dullaart, R.P.F.; Bakker, S.J.L.; et al. Serum free thiols predict cardiovascular events and all-cause mortality in the general population: A prospective cohort study. BMC Med. 2020, 18, 130. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Gabriels, R.Y.; de Borst, M.H.; Bulthuis, M.L.C.; Faber, K.N.; van Goor, H.; Dijkstra, G. Serum Free Thiols Are Superior to Fecal Calprotectin in Reflecting Endoscopic Disease Activity in Inflammatory Bowel Disease. Antioxidants 2019, 8, 351. [Google Scholar] [CrossRef] [Green Version]
- Boekhoud, L.; Koeze, J.; van der Slikke, E.C.; Bourgonje, A.R.; Moser, J.; Zijlstra, J.G.; Muller Kobold, A.C.; Bulthuis, M.L.C.; van Meurs, M.; van Goor, H.; et al. Acute Kidney Injury is Associated with Lowered Plasma-Free Thiol Levels. Antioxidants 2020, 9, 1135. [Google Scholar] [CrossRef] [PubMed]
- Bone, R.C.; Balk, R.A.; Cerra, F.B.; Dellinger, R.P.; Fein, A.M.; Knaus, W.A.; Schein, R.M.H.; Sibbald, W.J. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992, 101, 1644–1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kellum, J.A.; Aspelin, P.L.N.; Barsoum, R.S.; Burdmann, E.A.; Goldstein, S.L.; Herzog, C.A.; Joannidis, M.; Kribben, A.; Levey, A.S.; MacLeod, M.A.; et al. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar]
- Billings, F.T., IV; Shaw, A.D. Clinical trial endpoints in acute kidney injury. Nephron. Clin. Pract. 2014, 127, 89–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semler, M.W.; Rice, T.W.; Shaw, A.D.; Siew, E.D.; Self, W.H.; Kumar, A.B.; Byrne, D.W.; Ehrenfeld, J.M.; Wanderer, J.P. Identification of Major Adverse Kidney Events within the Electronic Health Record. J. Med. Syst. 2016, 40, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Hu, M.L.; Louie, S.; Cross, C.E.; Motchnik, P.; Halliwell, B. Antioxidant protection against hypochlorous acid in human plasma. J. Lab. Clin. Med. 1993, 121, 257–262. [Google Scholar]
- Giustarini, D.; Dalle-Donne, I.; Milzani, A.; Rossi, R. Low molecular mass thiols, disulfides and protein mixed disulfides in rat tissues: Influence of sample manipulation, oxidative stress and ageing. Mech. Ageing Dev. 2011, 132, 141–148. [Google Scholar] [CrossRef]
- Bar-Or, D.; Lau, E.; Winkler, J.V. A novel assay for cobalt-albumin binding and its potential as a marker for myocardial ischemia-a preliminary report. J. Emerg. Med. 2000, 19, 311–315. [Google Scholar] [CrossRef]
- Lee, E.; Eom, J.E.; Jeon, K.H.; Kim, T.H.; Kim, E.; Jhon, G.J.; Kwon, Y. Evaluation of albumin structural modifications through cobalt-albumin binding (CAB) assay. J. Pharm. Biomed. Anal. 2014, 91, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Gaze, D.C.; Crompton, L.; Collinson, P. Ischemia-modified albumin concentrations should be interpreted with caution in patients with low serum albumin concentrations. Med. Princ. Pract. 2006, 15, 322–324. [Google Scholar] [CrossRef] [PubMed]
- Victor, V.M.; Espulgues, J.V.; Hernandez-Mijares, A.; Rocha, M. Oxidative stress and mitochondrial dysfunction in sepsis: A potential therapy with mitochondria-targeted antioxidants. Infect. Disord. Drug Targets 2009, 9, 376–389. [Google Scholar] [CrossRef] [PubMed]
- Cowley, H.C.; Bacon, P.J.; Goode, H.F.; Webster, N.R.; Jones, J.G.; Menon, D.K. Plasma antioxidant potential in severe sepsis: A comparison of survivors and nonsurvivors. Crit. Care Med. 1996, 24, 1179–1183. [Google Scholar] [CrossRef] [PubMed]
- Deneke, S.M. Thiol-based antioxidants. Curr. Top Cell Regul. 2000, 36, 151–180. [Google Scholar]
- Hofer, S.; Rosenhagen, C.; Nakamura, H.; Yodoi, J.; Bopp, C.; Zimmermann, J.B.; Goebel, M.; Schemmer, P.; Hoffmann, K.; Schulze-Osthoff, K.; et al. Thioredoxin in human and experimental sepsis. Crit. Care Med. 2009, 37, 2155–2159. [Google Scholar] [CrossRef] [Green Version]
- Villa, P.; Saccani, A.; Sica, A.; Ghezzi, P. Glutathione protects mice from lethal sepsis by limiting inflammation and potentiating host defense. J. Infect. Dis. 2002, 185, 1115–1120. [Google Scholar] [CrossRef]
- Ortolani, O.; Conti, A.; De Gaudio, A.R.; Moraldi, E.; Cantini, Q.; Novelli, G. The effect of glutathione and N-acetylcysteine on lipoperoxidative damage in patients with early septic shock. Am. J. Respir. Crit. Care Med. 2000, 161, 1907–1911. [Google Scholar] [CrossRef]
- Nielsen, M.B.; Jespersen, B.; Birn, H.; Krogstrup, N.V.; Bourgonje, A.R.; Leuvenink, H.G.D.; van Goor, H.; Norregaard, R. Elevated plasma free thiols are associated with early and one-year graft function in renal transplant recipients. PLoS ONE 2021, 16, e0255930. [Google Scholar] [CrossRef]
- Damba, T.; Bourgonje, A.R.; Abdulle, A.E.; Pasch, A.; Sydor, S.; van den Berg, E.H.; Gansevoort, R.T.; Bakker, S.J.L.; Blokzjil, H.; Dullaart, R.P.F.; et al. Oxidative stress is associated with suspected non-alcoholic fatty liver disease and all-cause mortality in the general population. Liver Int. 2020, 40, 2148–2159. [Google Scholar] [CrossRef]
- Wiedermann, C.J. Hypoalbuminemia as Surrogate and Culprit of Infections. Int. J. Mol. Sci. 2021, 22, 4496. [Google Scholar] [CrossRef]
- Caironi, P.; Tognoni, G.; Masson, S.; Fumagalli, R.; Pesenti, A.; Romero, M.; Fanizza, C.; Caspani, L.; Faenza, S.; Grasselli, G.; et al. Albumin replacement in patients with severe sepsis or septic shock. N. Engl. J. Med. 2014, 370, 1412–1421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delaney, A.P.; Dan, A.; McCaffrey, J.; Finfer, S. The role of albumin as a resuscitation fluid for patients with sepsis: A systematic review and meta-analysis. Crit. Care Med. 2011, 39, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martinez, R.; Noiret, L.; Sen, S.; Mookerjee, R.; Jalan, R. Albumin infusion improves renal blood flow autoregulation in patients with acute decompensation of cirrhosis and acute kidney injury. Liver Int. 2015, 35, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Makris, D.; Mertens, P.R.; Dounousi, E.; Giamouzis, G.; Nseir, S. Editorial: Oxidative Stress in the Critically Ill Patients: Pathophysiology and Potential Interventions. Oxidative Med. Cell Longev. 2018, 2018, 2353128. [Google Scholar] [CrossRef] [Green Version]
- Bonifazi, M.; Meessen, J.; Perez, A.; Vasques, F.; Busana, M.; Vassalli, F.; Novelli, D.; Bernasconi, R.; Signori, C.; Masson, S.; et al. Albumin Oxidation Status in Sepsis Patients Treated with Albumin or Crystalloids. Front. Physiol. 2021, 12, 682877. [Google Scholar] [CrossRef] [PubMed]
- Bourgonje, A.R.; von Martels, J.Z.H.; Bulthuis, M.L.C.; van Londen, M.; Faber, K.N.; Dijkstra, G.; van Goor, H. Crohn’s Disease in Clinical Remission Is Marked by Systemic Oxidative Stress. Front. Physiol. 2019, 10, 499. [Google Scholar] [CrossRef]
- van Dijk, P.R.; Abdulle, A.E.; Bulthuis, M.L.C.; Perton, F.G.; Connelly, M.A.; van Goor, H.; Dullaart, R.P.F. The Systemic Redox Status Is Maintained in Non-Smoking Type 2 Diabetic Subjects without Cardiovascular Disease: Association with Elevated Triglycerides and Large VLDL. J. Clin. Med. 2019, 9, 49. [Google Scholar] [CrossRef] [Green Version]
- van Eijk, L.E.; Tami, A.; Hillebrands, J.L.; den Dunnen, W.F.A.; de Borst, M.H.; van der Voort, P.H.J.; Bulthuis, M.L.C.; Veloo, A.C.M.; old, K.I.; Gonzalez, M.F.V.; et al. Mild Coronavirus Disease 2019 (COVID-19) Is Marked by Systemic Oxidative Stress: A Pilot Study. Antioxidants 2021, 10, 2022. [Google Scholar] [CrossRef]
- Schillern, E.E.M.; Pasch, A.; Feelisch, M.; Waanders, F.; Hendriks, S.H.; Mencke, R.; Harms, G.; Groenier, K.H.; Bilo, H.J.; Hillebrands, J.-L.; et al. Serum free thiols in type 2 diabetes mellitus: A prospective study. J. Clin. Transl. Endocrinol. 2019, 16, 100182. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Feelisch, M.; Faber, K.N.; Pasch, A.; Dijkstra, G.; van Goor, H. Oxidative Stress and Redox-Modulating Therapeutics in Inflammatory Bowel Disease. Trends Mol. Med. 2020, 26, 1034–1046. [Google Scholar] [CrossRef]

| All Patients n = 112 | |
|---|---|
| Age (years) | 66 (17) |
| Gender, males | 67 (60%) |
| Diabetes mellitus Malignancy Cardiovascular disease Chronic Kidney Failure | 28 (25%) 48 (43%) 42 (38%) 12 (11%) |
| Thrombocytes (×109 L) | 209 (129) |
| CRP (mg/L) | 81 (112) |
| Leukocytes (×109 L) | 9.7 (8.6) |
| Creatinine (µmol/L) | 97 (49) |
| Albumin (g/L) | 39 (8) |
| eGFR mL/min/1.73 m2 | 63 (41) |
| SIRS > 2 (sepsis-2) | 112 (100%) |
| SOFA score ≥ 2 (sepsis-3) | 66 (59%) |
| AKI AKI 1 AKI 2 AKI 3 | 15 (13%) 13 (12%) 1 (1%) 1 (1%) |
| ICU admission | 8 (7%) |
| FACTORS | MAKE 90 | MAKE 365 | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| OR (95%CI) | p Value | OR (95% CI) | p Value | OR (95%CI) | p Value | OR (95% CI) | p Value | |
| Plasma free thiols at ED (per SD) | 0.43 (0.22–0.82) | 0.011 | 0.58 (0.34–0.96) | 0.035 | 0.52 (0.29–0.93) | 0.028 | ||
| SOFA Score (per point) | 1.47 (1.13–1.91) | 0.004 | 1.41 (1.07–1.85) | 0.014 | 1.28 (1.03–1.61) | 0.028 | ||
| eGFR at ED (per SD) | 0.52 (0.96–0.99) | 0.028 | 0.66 (0.97–1.00) | 0.071 | ||||
| Age per 10 years | 1.23 (9.86–1.78) | 0.28 | 1.40 (1.00–1.96) | 0.047 | ||||
| Malignancy | 2.14 (0.75–6.1) | 0.16 | 4.0 (1.61–9.96) | 0.003 | 4.40 (1.66–11.58) | 0.003 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van der Slikke, E.C.; Boekhoud, L.; Bourgonje, A.R.; Olgers, T.J.; ter Maaten, J.C.; Henning, R.H.; van Goor, H.; Bouma, H.R. Plasma Free Thiol Levels during Early Sepsis Predict Future Renal Function Decline. Antioxidants 2022, 11, 800. https://doi.org/10.3390/antiox11050800
van der Slikke EC, Boekhoud L, Bourgonje AR, Olgers TJ, ter Maaten JC, Henning RH, van Goor H, Bouma HR. Plasma Free Thiol Levels during Early Sepsis Predict Future Renal Function Decline. Antioxidants. 2022; 11(5):800. https://doi.org/10.3390/antiox11050800
Chicago/Turabian Stylevan der Slikke, Elisabeth C., Lisanne Boekhoud, Arno R. Bourgonje, Tycho J. Olgers, Jan C. ter Maaten, Robert H. Henning, Harry van Goor, and Hjalmar R. Bouma. 2022. "Plasma Free Thiol Levels during Early Sepsis Predict Future Renal Function Decline" Antioxidants 11, no. 5: 800. https://doi.org/10.3390/antiox11050800
APA Stylevan der Slikke, E. C., Boekhoud, L., Bourgonje, A. R., Olgers, T. J., ter Maaten, J. C., Henning, R. H., van Goor, H., & Bouma, H. R. (2022). Plasma Free Thiol Levels during Early Sepsis Predict Future Renal Function Decline. Antioxidants, 11(5), 800. https://doi.org/10.3390/antiox11050800








