Abstract
Alzheimer’s disease (AD) is a progressive degenerative disorder and a leading cause of dementia in the elderly. The etiology of AD is multifactorial, including an increased oxidative state, deposition of amyloid plaques, and neurofibrillary tangles of the tau protein. The formation of amyloid plaques is considered one of the first signs of the illness, but only in the central nervous system (CNS). Interestingly, results indicate that AD is not just localized in the brain but is also found in organs distant from the brain, such as the cardiovascular system, gut microbiome, liver, testes, and kidney. These observations make AD a complex systemic disorder. Still, no effective medications have been found, but regular physical activity has been considered to have a positive impact on this challenging disease. While several articles have been published on the benefits of physical activity on AD development in the CNS, its peripheral effects have not been discussed in detail. The provocative question arising is the following: is it possible that the beneficial effects of regular exercise on AD are due to the systemic impact of training, rather than just the effects of exercise on the brain? If so, does this mean that the level of fitness of these peripheral organs can directly or indirectly influence the incidence or progress of AD? Therefore, the present paper aims to summarize the systemic effects of both regular exercise and AD and point out how common exercise-induced adaptation via peripheral organs can decrease the incidence of AD or attenuate the progress of AD.
1. Introduction
Alzheimer’s disease (AD) is known as a progressive degenerative disorder of the central nervous system (CNS), characterized by neuronal dysfunctions and changes in brain structure and function [1,2,3]. AD is the most common irreversible cause of dementia in elderly patients, estimated to comprise 60–80% of cases [1,4]. Pathophysiology of AD includes the oxidative state, the aggregation of pathological amyloid-β (Aβ), and abnormal accumulation of hyperphosphorylated neurofibrillary tangles of the tau protein, followed by inflammation in the central cortex and limbic system of the brain, which results in neuronal atrophy and a loss of synapses [5,6]. While amyloidogenesis is a well-controlled process in healthy tissues, pathologic amyloid plaques can accumulate in various tissues in AD. The formation of amyloid plaques is considered the first sign of the illness, not only in the CNS but also in peripheral organs, resulting in a systemic disorder [7,8,9]. Available evidence supports the belief that the decreased clearance of Aβ is one of the key processes in AD’s pathomechanism [10]. The most common early clinical symptom of amyloid plaque accumulation is difficulty remembering recent events [11]. Furthermore, longitudinal studies have shown that depressive symptoms that occur more than ten years before the onset of AD are associated with mild cognitive impairment [12] and memory complaints [13]. Physical disability continues to decline in cognitive functions and behavioral and social skills. AD reduces functioning mobility, lower quality of life conditions, and higher dependence on other people [11]. Due to its increased prevalence and incurability, AD is one of the major priorities of the healthcare system and one of the most significant social and economic challenges in modern society (World Health Organization (WHO) and Alzheimer’s Disease International (ADI), 2012). In addition, several other common disorders often appear with AD, such as diabetes [14], vascular abnormalities [15], and inflammation [16].
The dependence of AD on environmental and lifestyle factors is noted by the close relationship between AD and DNA methylation (DNAm)-based biomarkers, such as PhenoAge. DNAm PhenoAge is a powerful epigenetic biomarker that predicts various aging outcomes, including all-cause mortality, cancer, health, physical activity, and AD. DNAm PhenoAge is based on complex clinical measures of phenotypic age that record differences in lifespan and healthspan. The association between pathologically diagnosed AD and DNAm PhenoAge suggests that those diagnosed with AD present with more than the one-year-older dorsolateral prefrontal cortex than same-aged individuals who were not diagnosed with the disease. Interestingly, age-adjusted DNAm is positively associated with neuropathological hallmarks of AD, such as amyloid load, neuritic plaques, and neurofibrillary tangles [17].
Many different signaling pathway molecules have been investigated at the cellular level as possible targets in AD treatment. One of these promising agents could be pituitary-adenylate-cyclase-activating polypeptide (PACAP), a member of the vasoactive intestinal polypeptide (VIP)—a secretin-growth-hormone-releasing hormone (GHRH)—glucagon superfamily [18]. PACAP, due to its oxidative-stress-reducing effects [19], has a protective impact against aging [20,21], amyloidosis [21], and neurodegenerative diseases. For example, in AD, correlating with accumulating amounts of Aβ, decreased PACAP signaling activity has been observed [21]. The preventive influence of PACAP on AD has been reported to protect against Aβ toxicity and attenuate AD’s severity in animal models [18,22]. PACAP neuropeptide has three G-protein-coupled seven-transmembrane receptors: pituitary-adenylate-cyclase-activating polypeptide type 1 receptor (PAC1R), binds PACAP with the highest affinity, and vasoactive intestinal peptide receptors (VPAC1R and VPAC2R) bind PACAP with lower binding affinity. All three receptors have been detected in CNS and in many peripheral tissues. PACAP can activate intracellular messengers such as adenylate cyclase, cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA), the more active, phosphorylated form of PKA (P-PKA), and protein phosphatase 2A (PP2A) [23,24]. These signaling pathways were reported in CNS and suggested to lead to deterioration of neural function, leading to AD’s well-known symptoms in CNS. However, recent observations indicate that this AD-associated cellular signaling pathway is also present in peripheral organs.
2. AD and Exercise
Great efforts have been made to find reliable prevention methods and develop drugs or disease-modifying therapies to treat AD, yet without success [25,26,27,28]. These findings emphasize the importance of prevention. It is known that genes can predispose to AD [29,30], but it is also known that lifestyle habits are associated with the incidence of AD [31,32]. It has been reported that physical inactivity increases the incidence of AD [28]. Therefore, it is not surprising that physical activity can reduce the occurrence of AD [32]. Regular exercise has been shown to induce adaptation in the whole body, including the neuronal, cardiovascular, skeletal, immune, digestive, and reproductive systems [33,34]. Furthermore, training in AD (TAD) is known to have a positive impact on alteration of neurotrophin synthesis, attenuation of oxidative stress, inflammation, induction of amyloid-β (Aβ) degrading enzymes, an increase in vascularization and blood flow, and energy metabolism of the brain [32]. It is also known that exercise can reduce the concentration of Aβ in plasma [35] and can be protective against Aβ neurotoxicity with the disease [11].
3. Exercise and Peripheral Organs with AD
Physical exercise is recommended as a nonpharmacological method for preventing cognitive decline [36]. Training has antioxidant [37] and anti-inflammatory effects that contribute to neuroprotection and cognitive improvements seen in animal models and AD patients [32,38]. AD is also regarded as a metabolic disorder and most of the attention has been paid to brain metabolism [39]. Interestingly, results indicate that AD is localized in the brain and organs distant from the brain. The cardiovascular system, gut microbiome, liver, testes, and kidney are also affected [40,41,42]. AD may also affect metabolism, such as overproduction and accumulation of pathological amyloid plaques in peripheral organs [32,43,44,45]. These observations make AD a complex systemic disorder [16,46]. In this paper, therefore, we have examined metabolic changes in the cardiovascular system, gut microbiome, liver, testes, and kidney of the amyloid precursor protein/presenilin 1 (APP/PS1) mice [47,48,49,50]. Metabolomic results reveal that the liver was one of the earliest affected organs in APP/PS1 mice during amyloid pathology progression, followed by the kidney and heart [51]. Interestingly, the CNS-related symptoms of AD, such as loss of memory, are readily recognizable, while impaired functions in the liver or kidney are less prevalent.
Several articles have been published on the benefits of physical activity in developing AD in CNS [11], but the peripheral effects have not been discussed in detail. Regular training is known to reduce Aβ biomarker concentrations in the blood and cerebrospinal fluid [52], but only a few data are available to elucidate the peripheral mechanisms. It is interesting to examine the effect of mobility because there may be potential positive systemic effects and consequences on different tissue in AD [32].
The provocative question that arises is the following: is it possible that the beneficial effects of regular exercise on AD are due to the systemic effects of training, rather than only the impact of exercise on the brain? If so, does this mean that the level of fitness of peripheral organs can directly or indirectly influence the incidence or progress of AD? Therefore, the present paper aims to summarize the systemic effects of both regular exercise and AD and point out how common exercise-induced adaptation via peripheral organs can decrease the incidence of AD or attenuate the progress of AD.
4. Exercise and the Cardiovascular System with AD
With the progress of the availability of genetic screening, accumulating evidence suggests a possible link between AD-associated allele variants of Apo E4, presenilin 1–2, and heart failure [53,54,55]. However, a recent Mendelian randomization study did not find causal relationships between AD and heart failure in a European population [56]. One of the reasons behind heart failure and cardiac events related to mortalities is due to greater left ventricular (lv) diameter and lv mass [57]. The results of a longitudinal study on middle-aged American aboriginals revealed a 25 gm increase in lv mass in mid-life, which was independent of physical activity and was associated with lower hippocampal volume and higher white matter grade in older life [58]. Moreover, lv hypertrophy was independently related to cortical thickness, pointing out the relationship between lv hypertrophy with adverse changes in brain structure [59].
Metabolic syndrome does not just increase the risk of cardiovascular diseases, including heart failure, but also increases the incidence of AD [60]. The organism’s metabolism level and the organ itself seems to play an important role in the aging process. AD is characterized by metabolic impairment in CNS [32] and the cardiovascular system [61], and has been proposed to be one of the reasons for AD-induced cardiovascular disorders [62]. It has been demonstrated in an animal model of AD that transgenic mice expressing five mutations in human AβPP and PS1 (5XFAD) showed decreased cardiac contractility and mitochondrial functions compared with wild mice. Increased activation of AMP-activated protein kinase (AMPK) levels in the lv and inflammatory markers were also observed in 5XFAD mice [63].
Exercise, especially endurance exercise, has a powerful effect on the cardiovascular system [64]. Molecular mechanisms associated with the cardiovascular system and pathologies of AD have many common pathways. These could be linked to the increased neuronal and myocardial deaths observed in the mixed etiology. Indeed, decreased blood flow and oxygen supply have been reported prior to Aβ deposition in the brain of humans and mouse models and have been suggested to play a role in AD pathology [65,66]. Endothelial nitric oxide (NO) was first recognized as a major vasodilator involved in cardiovascular homeostasis, regulation of blood pressure, and local blood flow [67]. Exercise also stimulates NO release from endothelial cells [68]. This finding may suggest an association between endothelial NO, training, cerebrovascular, and cognition functions in AD. Previous research has recognized NO’s role in causing excessive amyloidogenic processing of APP and elevated local concentrations of Aβ, thus influencing the functional state of microglia and cognitive functions [65]. NO is produced by constitutively active endothelial nitric oxide synthase (eNOS). In addition, increased expressions of APP and β-site APP cleavage enzyme 1 (BACE1) and increased production of Aβ peptides in the cerebral tissue of eNOS-deficient mice were connected to AD [69].
5. Exercise and Gut Microbiome in AD
Gastrointestinal comorbidities are prevalent in AD [70], but relatively few studies emphasize the connection between exercise and gut microbiota in AD. This complex mass of microbiota [71,72] contributes to several functions through neuronal, immune, endocrine, and metabolic pathways [73,74]. The genomes of all microorganisms can be helpful, but they are also potentially harmful to the human body. Unfortunately, this large number of microorganisms can influence the development of various diseases outside the digestive system. It has been proven that specific microbiome alterations are associated with cognitive impairment and deficiencies in synaptogenesis [75]. Furthermore, accumulated evidence suggests that the microbiota–gut–brain axis affects neurodegenerative disorders, including dementia and AD [76,77,78]. The microbiome affects both disease progression and cognitive abilities in AD [79,80,81]. Vice versa, AD causes changes in the microbiome, mainly due to the deposition of Aβ plaque, which is associated with the inflammatory response [82]. Short-chain fatty acids (SCFAs) may be involved in these changes: for example, butyrate plays an essential role in suppressing inflammation, increases intestinal mucin production, and affects the expression of tight-junction proteins, which may cause enhanced intestinal permeability [83,84,85].
AD treated with probiotics resulted in changes in the microbiome environment and increased brain function [86]. Physical activity is also a well-known cause of alterations in the microbiome’s composition, even in AD [82,87,88,89,90,91]. A group of AD transgenic mice APP/SP1 which received exercise and probiotic treatment significantly outperformed all other groups in terms of cognitive function, such as the spatial memory, assessed by the Morris Maze Test [82]. Training with probiotic supplementation also altered the level of different bacterial genera in the gut microbiome in AD transgenic mice. Changes were observed mainly in the genera of Bacteroides, Clostridia, Eubacteria, Lactobacillus, Prevotella, and Roseburia (Table 1) [82]. Some of these microorganisms are involved in butyrogenesis, such as Prevotella, Bacteroides, and Lactobacillus [92]. Their abundance increased in trained groups. Furthermore, the level of Lactobacillus johnsonii was positively correlated with Aβ content and covered area in the hippocampus. Elevated levels of L. reuteri were also observed due to training [82]. L. reuteri is known as a vitamin B12 producer, which is obligatory for brain health and low levels of vitamin B12, and is found to be associated with an increased risk of AD [93,94,95]. These observations suggest that regular exercise and nutritional interventions, such as probiotic supplementation, are being proposed to reduce the incidence and decrease the progression of AD [70,96,97].

Table 1.
Investigation of the systemic effects of exercise on peripheral organs in AD.
6. Exercise and Liver with AD
Increasing evidence suggests an important role of liver function in the pathophysiology of AD. Proteins, such as amyloid-β and hyperphosphorylated tau, are vital contributors to the onset or progression of AD. The liver is theoretically involved in the peripheral clearance of circulating Aβ in the blood [98].
In addition to the beneficial systemic effects, exercise positively impacts liver function in AD. For example, abnormal levels of liver enzymes associated with the diagnosis of Alzheimer’s and correlated with poor memory and thinking scores have been observed [99]. Furthermore, microbial changes caused by exercise and probiotic treatment alter liver metabolism, mitochondrial content, and antioxidant capacity in APP/PS1 transgenic mice. Training and probiotic supplementation did not significantly raise mitochondrial counts in the AD animal’s liver, based on cytochrome c oxidase subunit 4 (COX4) and peroxisome-proliferator-activated receptor–gamma coactivator 1 alpha (PGC-1α) protein levels. However, on the other hand, the mitochondrial antioxidant capacity changed positively due to regular exercise. Antioxidant signaling proteins, such as nuclear factor erythroid 2-related factor 2 (NRF-2) and superoxide dismutase 2 (SOD2), were also investigated. NRF-2, the major regulator of antioxidant protection, showed an increase in the group of trained and probiotic supplemented animals compared with the AD group. Furthermore, decreased superoxide SOD2 levels were observed in AD, while training prevented this alteration [47].
7. Exercise and Gonads with AD
Testes are also peripheral organs affected by AD. In AD organs, decreased numbers of spermatogonia, spermatocytes, and interstitial Leydig cells have been observed. Immunoreactivity of collagen type IV in seminiferous tubules’ basement membrane (bm) was hardly detectable, and decreased bm thickness in AD testes, resulting in changes in blood–testes barrier function.
Studies show that testicular degradation can be compensated for by regular physical activity in a mouse model of AD [100]. In TAD animals, the number of convoluted seminiferous tubules’ cells was partially recovered, and the number of Leydig cells was elevated after physical activity. As a result of training, the thickness of the basement membrane became almost as thick as in WT mice. Expression of the type IV collagen molecule was also elevated, maintaining the integrity of the basal membrane, and thus compensating for the adverse effects of AD [48].
The PACAP, as mentioned above, is expressed in not only the CNS but also peripheral organs, with the highest level in testes [101]. PACAP regulation seems essential for maintaining the typical structure of testes and spermatogenesis and male sex hormone production [102]. It is proven that a lack of PACAP protein or a mistake in PACAP downstream signaling causes morphological and functional changes in testis [21]. The messenger RNA (mRNA) expression and the protein level of the PAC1R were decreased in AD animals compared with WT [48]. Interestingly, this reduction in PAC1R has also been demonstrated in the CNS of AD models [18]. In contrast, the mRNA and protein expression of VPAC1R and VPAC2R did not show a significant difference in AD. In the TAD experimental group, an increase in PAC1R and VPAC1R was noted, whereas a decrease in VPAC2 receptor protein expression was observed. The downstream PACAP signaling pathway elements, such as the cAMP, PKA, P-PKA, and PP2A protein expressions, were also significantly decreased in the samples of AD mice. In TAD testes, expression of the PKA protein was elevated almost to WT levels, and P-PKA and PPA2 were augmented considerably by physical activity [48].
There are few data available about ovary in AD. The most common metabolic disorder in premenopausal women is polycystic ovary syndrome (PCOS) [103]. Patients with PCOS have increased LH-FSH levels, decreased vitamin D and insulin resistance, and obesity [104]. These are important factors also in AD and may increase the risk of the disease [105]. Moderate exercise (guidelines for PCOS suggest at least 150 min of physical activity per week) is helpful in PCOS, so it can contribute to minimize the chance of developing AD [106]. Furthermore, women with premature menopause have an increased risk of AD [107]. Menopausal hormone therapy supplemented with regular training may give the chance to reduce the risk of AD in later life [108,109].
8. Exercise and Kidney in AD
Pathological Aβ accumulation has also been observed in kidneys of AD animals leading to possible fibrosis, causing filtration disorders and renal insufficiency [44]. In AD mice, homogenous eosinophilic deposits in the tubular systems and strong Aβ positivity were visible in the kidneys [49].
Physical exercise has been shown to positively affect the morphology and function of kidneys in the AD mouse model. Training can reduce kidney fibrosis, which induces Aβ clearance and may help inhibit the disease’s progression [110]. First of all, exercise reduced Aβ accumulation and diminished eosinophilic deposits [49]. After physical activity in AD mice, the amount of interstitial collagen type I was reduced compared with the untrained group. Furthermore, both normalized mRNA and protein expression of collagen type I were measured in TAD animals [50]. Additionally, as a result of exercise, the immunopositivity and (mRNA and protein) the expression of collagen type IV were normalized/elevated in trained animals [49]. The normalized basement membrane formation can play a role in Aβ elimination via kidneys and help inhibit the disease’s progression [49,110]. Accordingly, collagen type IV has been observed to inhibit Aβ plaque formation [111].
Pathological fibrosis in the kidneys of AD animals raises the role of transforming the growth factor β (TGFβ) pathway [112,113]. TGFβ is an essential factor in the pathogenesis of AD in the brain and a master regulator of renal inflammation and fibrosis, consequently responsible for appropriate filtration [114,115]. TGFβ causes increased collagen expression and accumulation [116]. Physical activity, through TGFβ signalization, may prevent renal fibrosis and support Aβ clearance in the periphery. Activation of canonical and non-canonical TGFβ pathways was observed in AD, which was normalized in TAD mice. Although TGFβ1′s mRNA and protein expression did not significantly differ between WT and AD kidneys, increased expressions were found in TAD samples. As for the receptors, the expression of TGFβRI protein was reduced in AD mice and normalized after physical activity, while the expression of mRNA and protein of TGFβRII changed to the contrary [50].
TGFβ signaling interacts with the group of mitogen-activated protein kinases (MAPKs), such as extracellular signal-regulated kinase 1/2 (ERK1/2), p38 mitogen-activated protein kinase (p38), and Jun N-terminal kinase (JNK) [117]. Members of the MAPK family strongly support distinct functions in the pathogenesis of renal fibrosis. For example, ERK contributes to renal fibrotic transformation, whereas inhibition of ERK activity reduces interstitial fibrosis in AD [118]. The MAPK family shows alteration in the kidney of AD after long-term training. After physical activity, standard ERK and a more active form of the kinase, phospho-ERK expression, and normalized renal function were detected in TAD samples [50]. The protein expression of ERK1/2 kinase was reduced in the kidneys of AD mice, with normalization after physical exercise. The amount of the phosphorylated ERK was significantly elevated in AD samples and showed a significant decrease in TAD mice. The other member of the MAPK family is the p38 protein. Its expression was increased in AD mice, inducing fibrosis, while the phosphorylated form of p38 changed to the contrary. Exercise normalized p38 levels suggest a balancing function of training in p38-mediated fibrosis formation [50,119]. Therefore, inhibition of p38 may be a good target in fibrosis treatment [120]. Furthermore, JNK kinase also affects glomerular filtration [121], and the inhibition of the kinase may suppress interstitial fibrosis [122]. The protein expression of the two isoforms of JNK revealed a significant reduction in AD mice but showed elevation in TAD animals [50].
Matrix metalloproteinase 9 (MMP9) is also involved in fibrotic processes by degrading extracellular matrix elements such as type I, IV, and type V of collagen and other extracellular matrix proteins [123,124]. MMP9 is regulated by TGF signaling in the kidney, and its activation is p38-dependent [123,125]. Surprisingly, a significant increase in MMP9 protein expression was observed in the kidneys of AD mice, but MMP9 expression was dramatically increased after exercise, congruent with the marked decrease in collagen type I in the tubular system [50].
The TGF signaling pathway’s activation can also play an important role in AD pathogenesis through cell cycle/cellular proliferation and apoptosis [126]. Cell proliferation markers, such as cyclin-dependent kinase inhibitor 1 (CDKN1/p21) and proliferating cell nuclear antigen (PCNA) expressions, were measured in AD: CDKN1/p21 activation and substantial PCNA reductions in the tubular system of AD mice were normalized by physical activity [49]. The CDKN1/p21 elevation suggests a cell cycle arrest in AD, and CDKN1/p21 interaction with PCNA can block cells in the S-phase of the cell cycle [127]. Caspase activation can mediate via Aβ accumulation: the more active, cleaved form of caspase 3 appeared in AD samples, indicating increased apoptosis and the loss of tubular cell function, while physical activity reduced its expression [49,128].
The abovementioned neuroprotective PACAP also has a nephroprotective role in various renal pathologies [129]. PACAP is produced in kidneys, where the most dramatic amyloid deposition was found in PACAP knockout mice. This signal molecule may be one of the most promising targets for the renal elimination of Aβ [21]. Examining molecular processes in AD mice, the protein expressions of PAC1R, VPAC1R, and VPAC2R were demonstrated in WT kidneys. In contrast, the expressions of all PACAP receptors were almost undetectable or significantly decreased in AD samples. Interestingly, the level of all PACAP receptors increased in TAD mice: most dominantly, expressions of PAC1R and VPAC1R were elevated [49]. PACAP equally binds both PAC1R and VPAC1R in kidney diseases [130]. PACAP signaling pathway can be regulated through PKA [101]. In AD animals, reduced PKA protein expression was observed, almost normalized after physical activity [49]. The activation/phosphorylation of PKA increases the level of transcription factor cAMP-response-element-binding protein (CREB) [131], the protein level of which was also decreased (or its activated form was undetectable) in AD mice and augmented after exercise [49]. These data suggest PACAP and PKA signaling may be involved in “physical-activity-mediated defense mechanisms” in AD. Furthermore, PACAP also plays an essential role in bone morphogenetic protein (BMP) signaling [132], as does a member of the TGFβ superfamily [133]. Initially, BMP was identified as “only” an osteogenic factor, but nowadays, it is known that BMPs perform several other functions [133,134,135,136]. PACAP and BMP signaling is involved in preventing diseases through physical activity. For example, modified BMP/Smad signaling has been reported in AD mice [49,130]. BMPs and Smad transcription factors regulate the expression of genes associated with fibrosis and basement membrane components, such as collagen [137,138]. Both BMP receptor type 1 (BMPR1) mRNA and protein levels and the expression of bone morphogenetic protein 4 (BMP4), which induces Smad1, showed a noticeable reduction in AD samples. Still, they were significantly augmented as a result of training. Smad2 and Smad3 expressions were also altered in the kidneys of AD mice: Smad2 increased, while Smad3 showed a moderate decrease in AD samples, but physical activity can compensate for these alterations [49].
TGFβRII, ERK, p38, JNK, and Smad phosphorylation occur on serine (Ser) amino acid, which raises a possible activation of serine–threonine protein phosphatases (Ser/Thr phosphatases) in AD [139]. Altered PP2A and PP2B expressions and modified protein phosphorylations have been reported in AD [140,141,142]. PP2A can regulate tau phosphorylation with a higher affinity than PP2B [143]. Reduced PP2A, but an increased PP2B expression in kidneys of AD mice was observed, similarly to the CNS, which can be compensated by physical exercise [49]. Increased phosphorylation on Ser residues, as the result of PP2A expression decrease, can induce the hyperphosphorylation of tau protein or APP, as was predicted in AD. In addition, ERK kinase dephosphorylation can happen dominantly through PP2A activation [144]. Subsequently, reduced expression of PP2A can lead to increased ERK phosphorylation in AD [49]. The PP2B can directly regulate the dephosphorylation of JNK and p38 kinases [145]. Therefore, the decreased activation of these kinases is the consequence of elevated PP2B expression in AD (Figure 1).
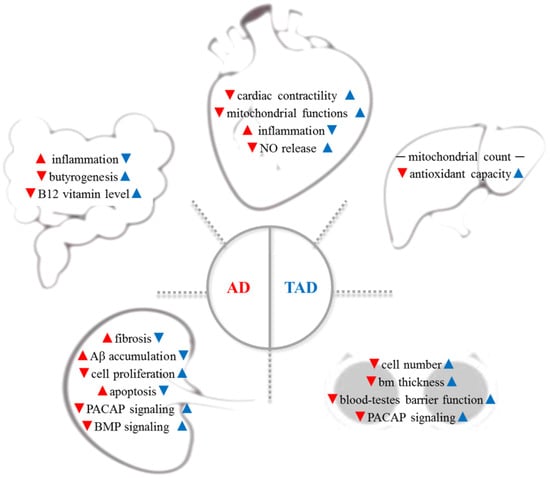
Figure 1.
AD is a complex systemic disorder, which induces the degenerative process in organs distant from the brain: cardiovascular system, gut microbiome, liver, testes, and kidney are involved (red arrows). Therefore, increased physical activity has been reported to have a preventive effect on all organs in AD (blue arrows). Aβ—β-amyloid; AD—Alzheimer’s disease; bm—basement membrane; BMP—bone morphogenetic protein; NO—nitric oxide, PACAP—pituitary--cyclase-activating polypeptide; TAD—trained AD.
9. The Effects of Exercise on Peripheral Organs in Alzheimer’s Disease
AD is a complex systemic disorder, which induces the degenerative process in organs distant from the brain: cardiovascular system, gut microbiome, liver, testes, and kidney are involved (Figure 1, red arrows). Therefore, increased physical activity has been reported to have a preventive effect on all organs in AD (Figure 1, blue arrows).
10. Conclusions
These results further support the hypothesis that AD induces the degenerative process of peripheral organs, showing that AD is a systemic disease. Increased physical activity has been reported to have a preventive and debilitating effect on all organs, emphasizing the potential preventive role of regular exercise in AD. The results further suggest that exercise can also attenuate the progress of AD. However, it is unknown how the involvement of different organs in the pathology of AD affects the progress of neurodegeneration. It is suggested here that the systemic nature of the exercise is one of the most powerful natural tools to fight AD.
Author Contributions
Conceptualization, D.A., P.B. and Z.R.; methodology, D.A., P.B., B.G. and R.P.; writing—original draft preparation, D.A. G.Y. and Z.R.; writing—review and editing, R.B.; supervision, R.P., I.B., G.Y. and Z.R.; funding acquisition, Z.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Innovation and Technology Ministry, Hungary, grant number National Excellence Program (126823), and Scientific Excellence Program TKP2020-NKA-17 and TKP2021-EGA-37, at the University of Physical Education, awarded to Z.R.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to acknowledge the editorial help of Albert W. Taylor from University Western Ontario, Canada.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
Aβ—β-amyloid; AD—Alzheimer’s disease; BMP4—bone morphogenetic protein 4; BMP1R—bone morphogenetic protein receptor, type 1; cAMP—cyclic adenosine monophosphate protein kinase A; CREB—cAMP-response-element-binding protein; CDKN1/p21—cyclin-dependent kinase inhibitor 1; ERK1/2—extracellular-signal-regulated kinase 1/2; JNK—c-Jun N-terminal kinase; MMP9—matrix metalloproteinase 9; NO—nitric oxide; NRF-2—nuclear factor erythroid 2-related factor 2; p38—p38-mitogen-activated protein kinase; PAC1R—pituitary-adenylate-cyclase-activating polypeptide type 1 receptor; PACAP—pituitary-adenylate-cyclase-activating polypeptide; PCNA—proliferating cell nuclear antigen; PKA—protein kinase A; P-PKA—phosphoprotein kinase A; PP2A—protein phosphatase 2 A; PP2B—protein phosphatase 2 B; SOD2—superoxide dismutase 2; TAD—trained AD; TGFβ1—transforming growth factor β1; TGFβRI—transforming growth factor β receptor I; TGFβRII—transforming growth factor β receptor II; VPAC1R—vasoactive intestinal peptide receptor type 1; VPAC2R—vasoactive intestinal peptide receptor type 2.
References
- Alzheimer’s Association. 2016 Alzheimer’s disease facts and figures. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2016, 12, 459–509. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.H.; Oliver, D.M. Amyloid beta and phosphorylated tau-induced defective autophagy and mitophagy in Alzheimer’s disease. Cells 2019, 8, 488. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; Blennow, K.; Breteler, M.M.; de Strooper, B.; Frisoni, G.B.; Salloway, S.; van der Flier, W.M. Alzheimer’s disease. Lancet 2016, 388, 505–517. [Google Scholar] [CrossRef]
- Burns, A.; Iliffe, S. Alzheimer’s disease. BMJ 2009, 338, b158. [Google Scholar] [CrossRef]
- Hill, J.M.; Lukiw, W.J. Microbial-generated amyloids and Alzheimer’s disease (AD). Front. Aging Neurosci. 2015, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Tiraboschi, P.; Hansen, L.A.; Thal, L.J.; Corey-Bloom, J. The importance of neuritic plaques and tangles to the development and evolution of AD. Neurology 2004, 62, 1984–1989. [Google Scholar] [CrossRef]
- Pomilio, C.; Gorojod, R.M.; Riudavets, M.; Vinuesa, A.; Presa, J.; Gregosa, A.; Bentivegna, M.; Alaimo, A.; Alcon, S.P.; Sevlever, G.; et al. Microglial autophagy is impaired by prolonged exposure to β-amyloid peptides: Evidence from experimental models and Alzheimer’s disease patients. GeroScience 2020, 42, 613–632. [Google Scholar] [CrossRef]
- Turner, R.S.; Stubbs, T.; Davies, D.A.; Albensi, B.C. Potential new approaches for diagnosis of Alzheimer’s disease and related dementias. Front. Neurol. 2020, 11, 496. [Google Scholar] [CrossRef]
- Serý, O.; Povová, J.; Míšek, I.; Pešák, L.; Janout, V. Molecular mechanisms of neuropathological changes in Alzheimer’s disease: A review. Folia Neuropathol. 2013, 51, 1–9. [Google Scholar] [CrossRef]
- Tavares, R.S.; Martins, S.; Almeida-Santos, T.; Sousa, A.P.; Ramalho-Santos, J.; da Cruz, E.S.O.A. Alzheimer’s disease-related amyloid-β(1-42) peptide induces the loss of human sperm function. Cell Tissue Res. 2017, 369, 647–651. [Google Scholar] [CrossRef]
- Meng, Q.; Lin, M.S.; Tzeng, I.S. Relationship between exercise and Alzheimer’s disease: A narrative literature review. Front. Neurosci. 2020, 14, 131. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Doody, R.; Kurz, A.; Mohs, R.C.; Morris, J.C.; Rabins, P.V.; Ritchie, K.; Rossor, M.; Thal, L.; Winblad, B. Current concepts in mild cognitive impairment. Arch. Neurol. 2001, 58, 1985–1992. [Google Scholar] [CrossRef] [PubMed]
- Amieva, H.; Jacqmin-Gadda, H.; Orgogozo, J.M.; le Carret, N.; Helmer, C.; Letenneur, L.; Barberger-Gateau, P.; Fabrigoule, C.; Dartigues, J.F. The 9 year cognitive decline before dementia of the Alzheimer type: A prospective population-based study. Brain J. Neurol. 2005, 128, 1093–1101. [Google Scholar] [CrossRef]
- Griffith, C.M.; Eid, T.; Rose, G.M.; Patrylo, P.R. Evidence for altered insulin receptor signaling in Alzheimer’s disease. Neuropharmacology 2018, 136, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Csiszar, A.; Tarantini, S.; Fülöp, G.A.; Kiss, T.; Valcarcel-Ares, M.N.; Galvan, V.; Ungvari, Z.; Yabluchanskiy, A. Hypertension impairs neurovascular coupling and promotes microvascular injury: Role in exacerbation of Alzheimer’s disease. GeroScience 2017, 39, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.K.; Honea, R.A.; Vidoni, E.D.; Swerdlow, R.H.; Burns, J.M. Is Alzheimer’s disease a systemic disease? Biochim. Biophys. Acta 2014, 1842, 1340–1349. [Google Scholar] [CrossRef]
- Levine, M.E.; Lu, A.T.; Quach, A.; Chen, B.H.; Assimes, T.L.; Bandinelli, S.; Hou, L.; Baccarelli, A.A.; Stewart, J.D.; Li, Y.; et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging 2018, 10, 573–591. [Google Scholar] [CrossRef]
- Han, P.; Tang, Z.; Yin, J.; Maalouf, M.; Beach, T.G.; Reiman, E.M.; Shi, J. Pituitary adenylate cyclase-activating polypeptide protects against β-amyloid toxicity. Neurobiol. Aging 2014, 35, 2064–2071. [Google Scholar] [CrossRef]
- Lee, E.H.; Seo, S.R. Neuroprotective roles of pituitary adenylate cyclase-activating polypeptide in neurodegenerative diseases. BMB Rep. 2014, 47, 369–375. [Google Scholar] [CrossRef]
- Reglodi, D.; Atlasz, T.; Szabo, E.; Jungling, A.; Tamas, A.; Juhasz, T.; Fulop, B.D.; Bardosi, A. PACAP deficiency as a model of aging. GeroScience 2018, 40, 437–452. [Google Scholar] [CrossRef]
- Reglodi, D.; Jungling, A.; Longuespée, R.; Kriegsmann, J.; Casadonte, R.; Kriegsmann, M.; Juhasz, T.; Bardosi, S.; Tamas, A.; Fulop, B.D.; et al. Accelerated pre-senile systemic amyloidosis in PACAP knockout mice—A protective role of PACAP in age-related degenerative processes. J. Pathol. 2018, 245, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Liang, W.; Baxter, L.C.; Yin, J.; Tang, Z.; Beach, T.G.; Caselli, R.J.; Reiman, E.M.; Shi, J. Pituitary adenylate cyclase-activating polypeptide is reduced in Alzheimer disease. Neurology 2014, 82, 1724–1728. [Google Scholar] [CrossRef] [PubMed]
- Kienlen Campard, P.; Crochemore, C.; René, F.; Monnier, D.; Koch, B.; Loeffler, J.P. PACAP type I receptor activation promotes cerebellar neuron survival through the cAMP/PKA signaling pathway. DNA Cell Biol. 1997, 16, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Juhász, T.; Matta, C.; Katona, É.; Somogyi, C.; Takács, R.; Hajdú, T.; Helgadottir, S.L.; Fodor, J.; Csernoch, L.; Tóth, G.; et al. Pituitary adenylate cyclase-activating polypeptide (PACAP) signalling enhances osteogenesis in UMR-106 cell line. J. Mol. Neurosci. 2014, 54, 555–573. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.S.; Jhanji, N. Natural products as potential anti-Alzheimer agents. Curr. Med. Chem. 2020, 27, 5887–5917. [Google Scholar] [CrossRef]
- Whitehouse, P.J. Ethical issues in early diagnosis and prevention of Alzheimer disease. Dialogues Clin. Neurosci. 2019, 21, 101–108. [Google Scholar] [CrossRef]
- Raggi, A.; Tasca, D.; Ferri, R. A brief essay on non-pharmacological treatment of Alzheimer’s disease. Rev. Neurosci. 2017, 28, 587–597. [Google Scholar] [CrossRef]
- Barnes, D.E.; Yaffe, K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011, 10, 819–828. [Google Scholar] [CrossRef]
- Tanzi, R.E.; George-Hyslop, P.S.; Gusella, J.F. Molecular genetics of Alzheimer disease amyloid. J. Biol. Chem. 1991, 266, 20579–20582. [Google Scholar] [CrossRef]
- Price, D.L.; Borchelt, D.R.; Sisodia, S.S. Alzheimer disease and the prion disorders amyloid beta-protein and prion protein amyloidoses. Proc. Natl. Acad. Sci. USA 1993, 90, 6381–6384. [Google Scholar] [CrossRef]
- Mattson, M.P. Pathways towards and away from Alzheimer’s disease. Nature 2004, 430, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Hart, N.; Sarga, L.; Koltai, E.; Atalay, M.; Ohno, H.; Boldogh, I. Exercise plays a preventive role against Alzheimer’s disease. J. Alzheimer’s Dis. 2010, 20, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Chung, H.Y.; Goto, S. Systemic adaptation to oxidative challenge induced by regular exercise. Free Radic. Biol. Med. 2008, 44, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Torma, F.; Berkes, I.; Goto, S.; Mimura, T.; Posa, A.; Balogh, L.; Boldogh, I.; Suzuki, K.; Higuchi, M.; et al. Exercise effects on physiological function during aging. Free Radic. Biol. Med. 2019, 132, 33–41. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Castillo-García, A.; Morales, J.S.; de la Villa, P.; Hampel, H.; Emanuele, E.; Lista, S.; Lucia, A. Exercise benefits on Alzheimer’s disease: State-of-the-science. Ageing Res. Rev. 2020, 62, 101108. [Google Scholar] [CrossRef]
- Lourenco, M.V.; Frozza, R.L.; de Freitas, G.B.; Zhang, H.; Kincheski, G.C.; Ribeiro, F.C.; Gonçalves, R.A.; Clarke, J.R.; Beckman, D.; Staniszewski, A.; et al. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat. Med. 2019, 25, 165–175. [Google Scholar] [CrossRef]
- Banerjee, A.K.; Mandal, A.; Chanda, D.; Chakraborti, S. Oxidant, antioxidant and physical exercise. Mol. Cell. Biochem. 2003, 253, 307–312. [Google Scholar] [CrossRef]
- Stranahan, A.M.; Martin, B.; Maudsley, S. Anti-inflammatory effects of physical activity in relationship to improved cognitive status in humans and mouse models of Alzheimer’s disease. Curr. Alzheimer Res. 2012, 9, 86–92. [Google Scholar] [CrossRef]
- Liu, H.L.; Zhao, G.; Zhang, H.; Shi, L.D. Long-term treadmill exercise inhibits the progression of Alzheimer’s disease-like neuropathology in the hippocampus of APP/PS1 transgenic mice. Behav. Brain Res. 2013, 256, 261–272. [Google Scholar] [CrossRef]
- Pereira, T.M.C.; Côco, L.Z.; Ton, A.M.M.; Meyrelles, S.S.; Campos-Toimil, M.; Campagnaro, B.P.; Vasquez, E.C. The emerging scenario of the gut-brain axis: The therapeutic actions of the new actor kefir against neurodegenerative diseases. Antioxidants 2021, 10, 1845. [Google Scholar] [CrossRef]
- De Oliveira, J.; Kucharska, E.; Garcez, M.L.; Rodrigues, M.S.; Quevedo, J.; Moreno-Gonzalez, I.; Budni, J. Inflammatory cascade in Alzheimer’s disease pathogenesis: A review of experimental findings. Cells 2021, 10, 2581. [Google Scholar] [CrossRef] [PubMed]
- Van Valkenburgh, J.; Meuret, C.; Martinez, A.E.; Kodancha, V.; Solomon, V.; Chen, K.; Yassine, H.N. Understanding the exchange of systemic HDL particles into the brain and vascular cells has diagnostic and therapeutic implications for neurodegenerative diseases. Front. Physiol. 2021, 12, 700847. [Google Scholar] [CrossRef] [PubMed]
- Kawarabayashi, T.; Younkin, L.H.; Saido, T.C.; Shoji, M.; Ashe, K.H.; Younkin, S.G. Age-dependent changes in brain, CSF, and plasma amyloid (beta) protein in the Tg2576 transgenic mouse model of Alzheimer’s disease. J. Neurosci. Off. J. Soc. Neurosci. 2001, 21, 372–381. [Google Scholar] [CrossRef]
- Kheirbakhsh, R.; Haddadi, M.; Muhammadnejad, A.; Abdollahi, A.; Shahi, F.; Amanpour-Gharaei, B.; Abrahim-Habibi, A.; Barati, T.; Amanpour, S. Long-term behavioral, histological, biochemical and hematological evaluations of amyloid beta-induced Alzheimer’s disease in rat. Acta Neurobiol. Exp. 2018, 78, 51–59. [Google Scholar] [CrossRef]
- Arbor, S. Targeting amyloid precursor protein shuttling and processing—Long before amyloid beta formation. Neural Regen. Res. 2017, 12, 207–209. [Google Scholar] [CrossRef]
- Wang, J.; Gu, B.J.; Masters, C.L.; Wang, Y.J. A systemic view of Alzheimer disease—Insights from amyloid-β metabolism beyond the brain. Nat. Rev. Neurol. 2017, 13, 612–623. [Google Scholar] [CrossRef]
- Téglás, T.; Ábrahám, D.; Jókai, M.; Kondo, S.; Mohammadi, R.; Fehér, J.; Szabó, D.; Wilhelm, M.; Radák, Z. Exercise combined with a probiotics treatment alters the microbiome, but moderately affects signalling pathways in the liver of male APP/PS1 transgenic mice. Biogerontology 2020, 21, 807–815. [Google Scholar] [CrossRef]
- Szegeczki, V.; Horváth, G.; Perényi, H.; Tamás, A.; Radák, Z.; Ábrahám, D.; Zákány, R.; Reglodi, D.; Juhász, T. Alzheimer’s disease mouse as a model of testis degeneration. Int. J. Mol. Sci. 2020, 21, 5726. [Google Scholar] [CrossRef]
- Perényi, H.; Szegeczki, V.; Horváth, G.; Hinnah, B.; Tamás, A.; Radák, Z.; Ábrahám, D.; Zákány, R.; Reglodi, D.; Juhász, T. Physical activity protects the pathological alterations of Alzheimer’s disease kidneys via the activation of PACAP and BMP signaling pathways. Front. Cell. Neurosci. 2020, 14, 243. [Google Scholar] [CrossRef]
- Szegeczki, V.; Perényi, H.; Horváth, G.; Hinnah, B.; Tamás, A.; Radák, Z.; Ábrahám, D.; Zákány, R.; Reglodi, D.; Juhász, T. Physical training inhibits the fibrosis formation in Alzheimer’s disease kidney influencing the TGFβ signaling pathways. J. Alzheimer’s Dis. 2021, 81, 1195–1209. [Google Scholar] [CrossRef]
- Zheng, H.; Cai, A.; Shu, Q.; Niu, Y.; Xu, P.; Li, C.; Lin, L.; Gao, H. Tissue-specific metabolomics analysis identifies the liver as a major organ of metabolic disorders in amyloid precursor protein/presenilin 1 mice of Alzheimer’s disease. J. Proteome Res. 2019, 18, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.S.; Foster, C.G.; Courtney, J.M.; King, N.E.; Howells, D.W.; Sutherland, B.A. Pericytes and neurovascular function in the healthy and diseased brain. Front. Cell. Neurosci. 2019, 13, 282. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.P.; Baum, L. Lipoproteins and related molecules in Alzheimer’s disease. Microsc. Res. Tech. 2000, 50, 259–260. [Google Scholar] [CrossRef]
- Gianni, D.; Li, A.; Tesco, G.; McKay, K.M.; Moore, J.; Raygor, K.; Rota, M.; Gwathmey, J.K.; Dec, G.W.; Aretz, T.; et al. Protein aggregates and novel presenilin gene variants in idiopathic dilated cardiomyopathy. Circulation 2010, 121, 1216–1226. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C. The pathobiology of vascular dementia. Neuron 2013, 80, 844–866. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Shi, J.; Yuan, G.; Shou, X.; Chen, T.; Zhu, X.; Yang, Y.; Hu, Y. Causal association between heart failure and Alzheimer’s disease: A two-sample bidirectional mendelian randomization study. Front. Genet. 2021, 12, 772343. [Google Scholar] [CrossRef]
- Haider, A.W.; Larson, M.G.; Benjamin, E.J.; Levy, D. Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J. Am. Coll. Cardiol. 1998, 32, 1454–1459. [Google Scholar] [CrossRef]
- Haring, B.; Omidpanah, A.; Suchy-Dicey, A.M.; Best, L.G.; Verney, S.P.; Shibata, D.K.; Cole, S.A.; Ali, T.; Howard, B.V.; Buchwald, D.; et al. Left ventricular mass, brain magnetic resonance imaging, and cognitive performance: Results from the strong heart study. Hypertension 2017, 70, 964–971. [Google Scholar] [CrossRef]
- Frenzel, S.; Wittfeld, K.; Bülow, R.; Völzke, H.; Friedrich, N.; Habes, M.; Felix, S.B.; Dörr, M.; Grabe, H.J.; Bahls, M. Cardiac hypertrophy is associated with advanced brain aging in the general population. J. Am. Heart Assoc. 2021, 10, e020994. [Google Scholar] [CrossRef]
- Cho, Y.K.; Lee, J.; Kim, H.S.; Park, J.Y.; Lee, W.J.; Kim, Y.J.; Jung, C.H. The risk of Alzheimer’s disease according to dynamic changes in metabolic health and obesity: A nationwide population-based cohort study. Aging 2021, 13, 16974–16989. [Google Scholar] [CrossRef]
- Singh, S.; Schwarz, K.; Horowitz, J.; Frenneaux, M. Cardiac energetic impairment in heart disease and the potential role of metabolic modulators: A review for clinicians. Circ. Cardiovasc. Genet. 2014, 7, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Bonomini, F.; Rodella, L.F.; Rezzani, R. Metabolic syndrome, aging and involvement of oxidative stress. Aging Dis. 2015, 6, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.; Le, T.N.V.; Fedorova, J.; Yang, Y.; Krause-Hauch, M.; Davitt, K.; Zoungrana, L.I.; Fatmi, M.K.; Lesnefsky, E.J.; Li, J.; et al. The cardiac dysfunction caused by metabolic alterations in Alzheimer’s disease. Front. Cardiovasc. Med. 2022, 9, 850538. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, V.A.; Fagard, R.H. Effects of endurance training on blood pressure, blood pressure-regulating mechanisms, and cardiovascular risk factors. Hypertension 2005, 46, 667–675. [Google Scholar] [CrossRef]
- Iadecola, C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat. Rev. Neurosci. 2004, 5, 347–360. [Google Scholar] [CrossRef]
- Koike, M.A.; Green, K.N.; Blurton-Jones, M.; Laferla, F.M. Oligemic hypoperfusion differentially affects tau and amyloid-β. Am. J. Pathol. 2010, 177, 300–310. [Google Scholar] [CrossRef]
- Atochin, D.N.; Huang, P.L. Endothelial nitric oxide synthase transgenic models of endothelial dysfunction. Pflug. Archiv.-Eur. J. Physiol. 2010, 460, 965–974. [Google Scholar] [CrossRef]
- Moncada, S. Nitric oxide in the vasculature: Physiology and pathophysiology. Ann. N. Y. Acad. Sci. 1997, 811, 60–69, 60–67; discussion 67–69. [Google Scholar] [CrossRef]
- Katusic, Z.S.; Austin, S.A. Endothelial nitric oxide: Protector of a healthy mind. Eur. Heart J. 2014, 35, 888–894. [Google Scholar] [CrossRef]
- Westfall, S.; Lomis, N.; Kahouli, I.; Dia, S.Y.; Singh, S.P.; Prakash, S. Microbiome, probiotics and neurodegenerative diseases: Deciphering the gut brain axis. Cell. Mol. Life Sci. 2017, 74, 3769–3787. [Google Scholar] [CrossRef]
- Kim, B.S.; Jeon, Y.S.; Chun, J. Current status and future promise of the human microbiome. Pediatric Gastroenterol. Hepatol. Nutr. 2013, 16, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.M.; Clement, C.; Pogue, A.I.; Bhattacharjee, S.; Zhao, Y.; Lukiw, W.J. Pathogenic microbes, the microbiome, and Alzheimer’s disease (AD). Front. Aging Neurosci. 2014, 6, 127. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Mahony, S.M. The microbiome-gut-brain axis: From bowel to behavior. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2011, 23, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.M.; Surette, M.; Bercik, P. The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol. 2012, 10, 735–742. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Lukiw, W.J. Alzheimer’s disease and the microbiome. Front. Cell. Neurosci. 2013, 7, 153. [Google Scholar] [CrossRef]
- Bell, J.S.; Spencer, J.I.; Yates, R.L.; Yee, S.A.; Jacobs, B.M.; DeLuca, G.C. Invited review: From nose to gut—The role of the microbiome in neurological disease. Neuropathol. Appl. Neurobiol. 2019, 45, 195–215. [Google Scholar] [CrossRef]
- Hu, X.; Wang, T.; Jin, F. Alzheimer’s disease and gut microbiota. Sci. China Life Sci. 2016, 59, 1006–1023. [Google Scholar] [CrossRef]
- Szablewski, L. Human gut microbiota in health and Alzheimer’s disease. J. Alzheimer’s Dis. 2018, 62, 549–560. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. The microbiome-gut-brain axis in health and disease. Gastroenterol. Clin. North Am. 2017, 46, 77–89. [Google Scholar] [CrossRef]
- Parker, A.P.; Dean, D.H. Temperate Bacillus bacteriophage SP16 genome is circularly permuted and terminally redundant. J. Bacteriol. 1986, 167, 719–721. [Google Scholar] [CrossRef]
- Nho, K.; Kueider-Paisley, A.; MahmoudianDehkordi, S.; Arnold, M.; Risacher, S.L.; Louie, G.; Blach, C.; Baillie, R.; Han, X.; Kastenmüller, G.; et al. Altered bile acid profile in mild cognitive impairment and Alzheimer’s disease: Relationship to neuroimaging and CSF biomarkers. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2019, 15, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Abraham, D.; Feher, J.; Scuderi, G.L.; Szabo, D.; Dobolyi, A.; Cservenak, M.; Juhasz, J.; Ligeti, B.; Pongor, S.; Gomez-Cabrera, M.C.; et al. Exercise and probiotics attenuate the development of Alzheimer’s disease in transgenic mice: Role of microbiome. Exp. Gerontol. 2019, 115, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.M.; Leonel, A.J.; Melo, M.A.; Santos, R.R.; Cara, D.C.; Cardoso, V.N.; Correia, M.I.; Alvarez-Leite, J.I. Oral supplementation of butyrate reduces mucositis and intestinal permeability associated with 5-Fluorouracil administration. Lipids 2012, 47, 669–678. [Google Scholar] [CrossRef]
- Peng, L.; Li, Z.R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Tabat, M.W.; Marques, T.M.; Markgren, M.; Löfvendahl, L.; Brummer, R.J.; Wall, R. Acute effects of butyrate on induced hyperpermeability and tight junction protein expression in human colonic tissues. Biomolecules 2020, 10, 766. [Google Scholar] [CrossRef] [PubMed]
- Leblhuber, F.; Steiner, K.; Schuetz, B.; Fuchs, D.; Gostner, J.M. Probiotic supplementation in patients with Alzheimer’s dementia—An explorative intervention study. Curr. Alzheimer Res. 2018, 15, 1106–1113. [Google Scholar] [CrossRef]
- O’Sullivan, O.; Cronin, O.; Clarke, S.F.; Murphy, E.F.; Molloy, M.G.; Shanahan, F.; Cotter, P.D. Exercise and the microbiota. Gut Microbes 2015, 6, 131–136. [Google Scholar] [CrossRef]
- Allen, J.M.; Mailing, L.J.; Niemiro, G.M.; Moore, R.; Cook, M.D.; White, B.A.; Holscher, H.D.; Woods, J.A. Exercise alters gut microbiota composition and function in lean and obese humans. Med. Sci. Sports Exerc. 2018, 50, 747–757. [Google Scholar] [CrossRef]
- Pan, G.Z.; Chen, S.P.; Mai, C.R. Ranitidine in acute duodenal ulcer. Double blind controlled trial. Chin. Med. J. 1988, 101, 277–279. [Google Scholar]
- Greenhill, C. Gut microbiome influences exercise response. Nat. Rev. Endocrinol. 2020, 16, 68–69. [Google Scholar] [CrossRef]
- Grosicki, G.J.; Durk, R.P.; Bagley, J.R. Rapid gut microbiome changes in a world-class ultramarathon runner. Physiol. Rep. 2019, 7, e14313. [Google Scholar] [CrossRef] [PubMed]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; de Vuyst, L. Bifidobacteria and butyrate-producing colon bacteria: Importance and strategies for their stimulation in the human gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, J.G.; Milani, C.; de Giori, G.S.; Sesma, F.; van Sinderen, D.; Ventura, M. Bacteria as vitamin suppliers to their host: A gut microbiota perspective. Curr. Opin. Biotechnol. 2013, 24, 160–168. [Google Scholar] [CrossRef]
- Quadri, P.; Fragiacomo, C.; Pezzati, R.; Zanda, E.; Forloni, G.; Tettamanti, M.; Lucca, U. Homocysteine, folate, and vitamin B-12 in mild cognitive impairment, Alzheimer disease, and vascular dementia. Am. J. Clin. Nutr. 2004, 80, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Mielech, A.; Puścion-Jakubik, A.; Markiewicz-Żukowska, R.; Socha, K. Vitamins in Alzheimer’s disease—Review of the latest reports. Nutrients 2020, 12, 3458. [Google Scholar] [CrossRef]
- King, B.C.; Vavitsas, K.; Ikram, N.K.; Schrøder, J.; Scharff, L.B.; Bassard, J.; Hamberger, B.; Jensen, P.E.; Simonsen, H.T. Corrigendum: In vivo assembly of DNA-fragments in the moss, Physcomitrella patens. Sci. Rep. 2016, 6, 31261. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Wang, Q. Towards understanding brain-gut-microbiome connections in Alzheimer’s disease. BMC Syst. Biol. 2016, 10 (Suppl. 3), 63. [Google Scholar] [CrossRef]
- Estrada, L.D.; Ahumada, P.; Cabrera, D.; Arab, J.P. Liver dysfunction as a novel player in Alzheimer’s progression: Looking outside the brain. Front. Aging Neurosci. 2019, 11, 174. [Google Scholar] [CrossRef]
- Nho, K.; Kueider-Paisley, A.; Ahmad, S.; MahmoudianDehkordi, S.; Arnold, M.; Risacher, S.L.; Louie, G.; Blach, C.; Baillie, R.; Han, X.; et al. Association of altered liver enzymes with Alzheimer disease diagnosis, cognition, neuroimaging measures, and cerebrospinal fluid biomarkers. JAMA Netw. Open 2019, 2, e197978. [Google Scholar] [CrossRef]
- Torma, F.; Koltai, E.; Nagy, E.; Ziaaldini, M.M.; Posa, A.; Koch, L.G.; Britton, S.L.; Boldogh, I.; Radak, Z. Exercise increases markers of spermatogenesis in rats selectively bred for low running capacity. PLoS ONE 2014, 9, e114075. [Google Scholar] [CrossRef]
- Vaudry, D.; Falluel-Morel, A.; Bourgault, S.; Basille, M.; Burel, D.; Wurtz, O.; Fournier, A.; Chow, B.K.; Hashimoto, H.; Galas, L.; et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol. Rev. 2009, 61, 283–357. [Google Scholar] [CrossRef] [PubMed]
- Prisco, M.; Rosati, L.; Morgillo, E.; Mollica, M.P.; Agnese, M.; Andreuccetti, P.; Valiante, S. Pituitary adenylate cyclase-activating peptide (PACAP) and its receptors in Mus musculus testis. Gen. Comp. Endocrinol. 2020, 286, 113297. [Google Scholar] [CrossRef] [PubMed]
- Spinedi, E.; Cardinali, D.P. The polycystic ovary syndrome and the metabolic syndrome: A possible chronobiotic-cytoprotective adjuvant therapy. Int. J. Endocrinol. 2018, 2018, 1349868. [Google Scholar] [CrossRef] [PubMed]
- González, F. Inflammation in polycystic ovary syndrome: Underpinning of insulin resistance and ovarian dysfunction. Steroids 2012, 77, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Sarahian, N.; Sarvazad, H.; Sajadi, E.; Rahnejat, N.; Eskandari Roozbahani, N. Investigation of common risk factors between polycystic ovary syndrome and Alzheimer’s disease: A narrative review. Reprod. Health 2021, 18, 156. [Google Scholar] [CrossRef]
- Woodward, A.; Klonizakis, M.; Broom, D. Exercise and polycystic ovary syndrome. Adv. Exp. Med. Biol. 2020, 1228, 123–136. [Google Scholar] [CrossRef]
- Davey, D.A. Alzheimer’s disease, dementia, mild cognitive impairment and the menopause: A ‘window of opportunity’? Women’s Health 2013, 9, 279–290. [Google Scholar] [CrossRef]
- Thomas, A.; Daley, A.J. Women’s views about physical activity as a treatment for vasomotor menopausal symptoms: A qualitative study. BMC Women’s Health 2020, 20, 203. [Google Scholar] [CrossRef]
- Davey, D.A. Prevention of Alzheimer’s disease, cerebrovascular disease and dementia in women: The case for menopause hormone therapy. Neurodegener. Dis. Manag. 2017, 7, 85–94. [Google Scholar] [CrossRef]
- Ghiso, J.; Calero, M.; Matsubara, E.; Governale, S.; Chuba, J.; Beavis, R.; Wisniewski, T.; Frangione, B. Alzheimer’s soluble amyloid beta is a normal component of human urine. FEBS Lett. 1997, 408, 105–108. [Google Scholar] [CrossRef]
- Kiuchi, Y.; Isobe, Y.; Fukushima, K. Type IV collagen prevents amyloid beta-protein fibril formation. Life Sci. 2002, 70, 1555–1564. [Google Scholar] [CrossRef]
- Kajdaniuk, D.; Marek, B.; Borgiel-Marek, H.; Kos-Kudła, B. Transforming growth factor β1 (TGFβ1) in physiology and pathology. Endokrynol. Pol. 2013, 64, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, T.; Lu, D.W.; Zhao, H.; Feng, Y.L.; Chen, H.; Chen, D.Q.; Vaziri, N.D.; Zhao, Y.Y. Central role of dysregulation of TGF-β/Smad in CKD progression and potential targets of its treatment. Biomed. Pharmacother. 2018, 101, 670–681. [Google Scholar] [CrossRef] [PubMed]
- Grammas, P.; Ovase, R. Cerebrovascular transforming growth factor-beta contributes to inflammation in the Alzheimer’s disease brain. Am. J. Pathol. 2002, 160, 1583–1587. [Google Scholar] [CrossRef]
- Lian, H.; Zheng, H. Signaling pathways regulating neuron-glia interaction and their implications in Alzheimer’s disease. J. Neurochem. 2016, 136, 475–491. [Google Scholar] [CrossRef] [PubMed]
- Browne, J.A.; Liu, X.; Schnaper, H.W.; Hayashida, T. Serine-204 in the linker region of Smad3 mediates the collagen-I response to TGF-β in a cell phenotype-specific manner. Exp. Cell Res. 2013, 319, 2928–2937. [Google Scholar] [CrossRef]
- Ma, F.Y.; Sachchithananthan, M.; Flanc, R.S.; Nikolic-Paterson, D.J. Mitogen activated protein kinases in renal fibrosis. Front. Biosci. 2009, 1, 171–187. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, L.; Deng, D.; Zhang, Q.; Liu, W. Renalase protects against renal fibrosis by inhibiting the activation of the ERK signaling pathways. Int. J. Mol. Sci. 2017, 18, 855. [Google Scholar] [CrossRef]
- Lee, J.; An, J.N.; Hwang, J.H.; Lee, H.; Lee, J.P.; Kim, S.G. p38 MAPK activity is associated with the histological degree of interstitial fibrosis in IgA nephropathy patients. PLoS ONE 2019, 14, e0213981. [Google Scholar] [CrossRef]
- Stambe, C.; Atkins, R.C.; Tesch, G.H.; Masaki, T.; Schreiner, G.F.; Nikolic-Paterson, D.J. The role of p38alpha mitogen-activated protein kinase activation in renal fibrosis. J. Am. Soc. Nephrol. 2004, 15, 370–379. [Google Scholar] [CrossRef]
- Grynberg, K.; Ma, F.Y.; Nikolic-Paterson, D.J. The JNK signaling pathway in renal fibrosis. Front. Physiol. 2017, 8, 829. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.Y.; Flanc, R.S.; Tesch, G.H.; Han, Y.; Atkins, R.C.; Bennett, B.L.; Friedman, G.C.; Fan, J.H.; Nikolic-Paterson, D.J. A pathogenic role for c-Jun amino-terminal kinase signaling in renal fibrosis and tubular cell apoptosis. J. Am. Soc. Nephrol. 2007, 18, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Duffield, J.S. Cellular and molecular mechanisms in kidney fibrosis. J. Clin. Investig. 2014, 124, 2299–2306. [Google Scholar] [CrossRef] [PubMed]
- Giannandrea, M.; Parks, W.C. Diverse functions of matrix metalloproteinases during fibrosis. Dis. Models Mech. 2014, 7, 193–203. [Google Scholar] [CrossRef]
- Zhao, H.; Dong, Y.; Tian, X.; Tan, T.K.; Liu, Z.; Zhao, Y.; Zhang, Y.; Harris, D.; Zheng, G. Matrix metalloproteinases contribute to kidney fibrosis in chronic kidney diseases. World J. Nephrol. 2013, 2, 84–89. [Google Scholar] [CrossRef]
- Liu, Y.; Shang, D. Transforming growth factor-β1 enhances proliferative and metastatic potential by up-regulating lymphoid enhancer-binding factor 1/integrin αMβ2 in human renal cell carcinoma. Mol. Cell. Biochem. 2020, 465, 165–174. [Google Scholar] [CrossRef]
- Gulbis, J.M.; Kelman, Z.; Hurwitz, J.; O’Donnell, M.; Kuriyan, J. Structure of the C-terminal region of p21(WAF1/CIP1) complexed with human PCNA. Cell 1996, 87, 297–306. [Google Scholar] [CrossRef]
- Park, G.; Nhan, H.S.; Tyan, S.H.; Kawakatsu, Y.; Zhang, C.; Navarro, M.; Koo, E.H. Caspase activation and caspase-mediated cleavage of APP is associated with amyloid β-protein-induced synapse loss in Alzheimer’s disease. Cell Rep. 2020, 31, 107839. [Google Scholar] [CrossRef]
- Horvath, G.; Opper, B.; Reglodi, D. The neuropeptide pituitary adenylate cyclase-activating polypeptide (PACAP) is protective in inflammation and oxidative stress-induced damage in the kidney. Int. J. Mol. Sci. 2019, 20, 4944. [Google Scholar] [CrossRef]
- Li, M.; Maderdrut, J.L.; Lertora, J.J.; Arimura, A.; Batuman, V. Renoprotection by pituitary adenylate cyclase-activating polypeptide in multiple myeloma and other kidney diseases. Regul. Pept. 2008, 145, 24–32. [Google Scholar] [CrossRef]
- Mayr, B.; Montminy, M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat. Rev. Mol. Cell Biol. 2001, 2, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Pavelock, K.A.; Girard, B.M.; Schutz, K.C.; Braas, K.M.; May, V. Bone morphogenetic protein down-regulation of neuronal pituitary adenylate cyclase-activating polypeptide and reciprocal effects on vasoactive intestinal peptide expression. J. Neurochem. 2007, 100, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.; Yadav, P.S.; Prashar, P. BMP signaling in development and diseases: A pharmacological perspective. Biochem. Pharmacol. 2013, 85, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Józsa, G.; Fülöp, B.D.; Kovács, L.; Czibere, B.; Szegeczki, V.; Kiss, T.; Hajdú, T.; Tamás, A.; Helyes, Z.; Zákány, R.; et al. Lack of pituitary adenylate cyclase-activating polypeptide (PACAP) disturbs callus formation. J. Mol. Neurosci. 2021, 71, 1543–1555. [Google Scholar] [CrossRef]
- Laszlo, E.; Juhasz, T.; Varga, A.; Czibere, B.; Kovacs, K.; Degrell, P.; Horvath, G.; Jancso, G.; Szakaly, P.; Tamas, A.; et al. Protective effect of PACAP on ischemia/reperfusion-induced kidney injury of male and female rats: Gender differences. J. Mol. Neurosci. 2019, 68, 408–419. [Google Scholar] [CrossRef]
- Józsa, G.; Szegeczki, V.; Pálfi, A.; Kiss, T.; Helyes, Z.; Fülöp, B.; Cserháti, C.; Daróczi, L.; Tamás, A.; Zákány, R.; et al. Signalling alterations in bones of pituitary adenylate cyclase activating polypeptide (PACAP) gene deficient mice. Int. J. Mol. Sci. 2018, 19, 2538. [Google Scholar] [CrossRef]
- Von Bubnoff, A.; Cho, K.W. Intracellular BMP signaling regulation in vertebrates: Pathway or network? Dev. Biol. 2001, 239, 1–14. [Google Scholar] [CrossRef]
- Matsubara, T.; Araki, M.; Abe, H.; Ueda, O.; Jishage, K.; Mima, A.; Goto, C.; Tominaga, T.; Kinosaki, M.; Kishi, S.; et al. Bone morphogenetic protein 4 and Smad1 mediate extracellular matrix production in the development of diabetic nephropathy. Diabetes 2015, 64, 2978–2990. [Google Scholar] [CrossRef][Green Version]
- Heldin, C.H.; Moustakas, A. Signaling receptors for TGF-β family members. Cold Spring Harb. Perspect. Biol. 2016, 8, a022053. [Google Scholar] [CrossRef]
- Qian, W.; Yin, X.; Hu, W.; Shi, J.; Gu, J.; Grundke-Iqbal, I.; Iqbal, K.; Gong, C.X.; Liu, F. Activation of protein phosphatase 2B and hyperphosphorylation of tau in Alzheimer’s disease. J. Alzheimer’s Dis. 2011, 23, 617–627. [Google Scholar] [CrossRef]
- Oliveira, J.M.; Henriques, A.G.; Martins, F.; Rebelo, S.; da Cruz e Silva, O.A. Amyloid-β modulates both AβPP and tau phosphorylation. J. Alzheimer’s Dis. 2015, 45, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Leong, W.; Xu, W.; Wang, B.; Gao, S.; Zhai, X.; Wang, C.; Gilson, E.; Ye, J.; Lu, Y. PP2A subunit PPP2R2C is downregulated in the brains of Alzheimer’s transgenic mice. Aging 2020, 12, 6880–6890. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.X.; Singh, T.J.; Grundke-Iqbal, I.; Iqbal, K. Phosphoprotein phosphatase activities in Alzheimer disease brain. J. Neurochem. 1993, 61, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.G.; Coffee, R.L., Jr.; Zhang, H.; Pelech, S.; Strack, S.; Wadzinski, B.E. Positive regulation of Raf1-MEK1/2-ERK1/2 signaling by protein serine/threonine phosphatase 2A holoenzymes. J. Biol. Chem. 2005, 280, 42644–42654. [Google Scholar] [CrossRef]
- Molkentin, J.D. Calcineurin-NFAT signaling regulates the cardiac hypertrophic response in coordination with the MAPKs. Cardiovasc. Res. 2004, 63, 467–475. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).