Carotenoids: Dietary Sources, Extraction, Encapsulation, Bioavailability, and Health Benefits—A Review of Recent Advancements
Abstract
:1. Introduction
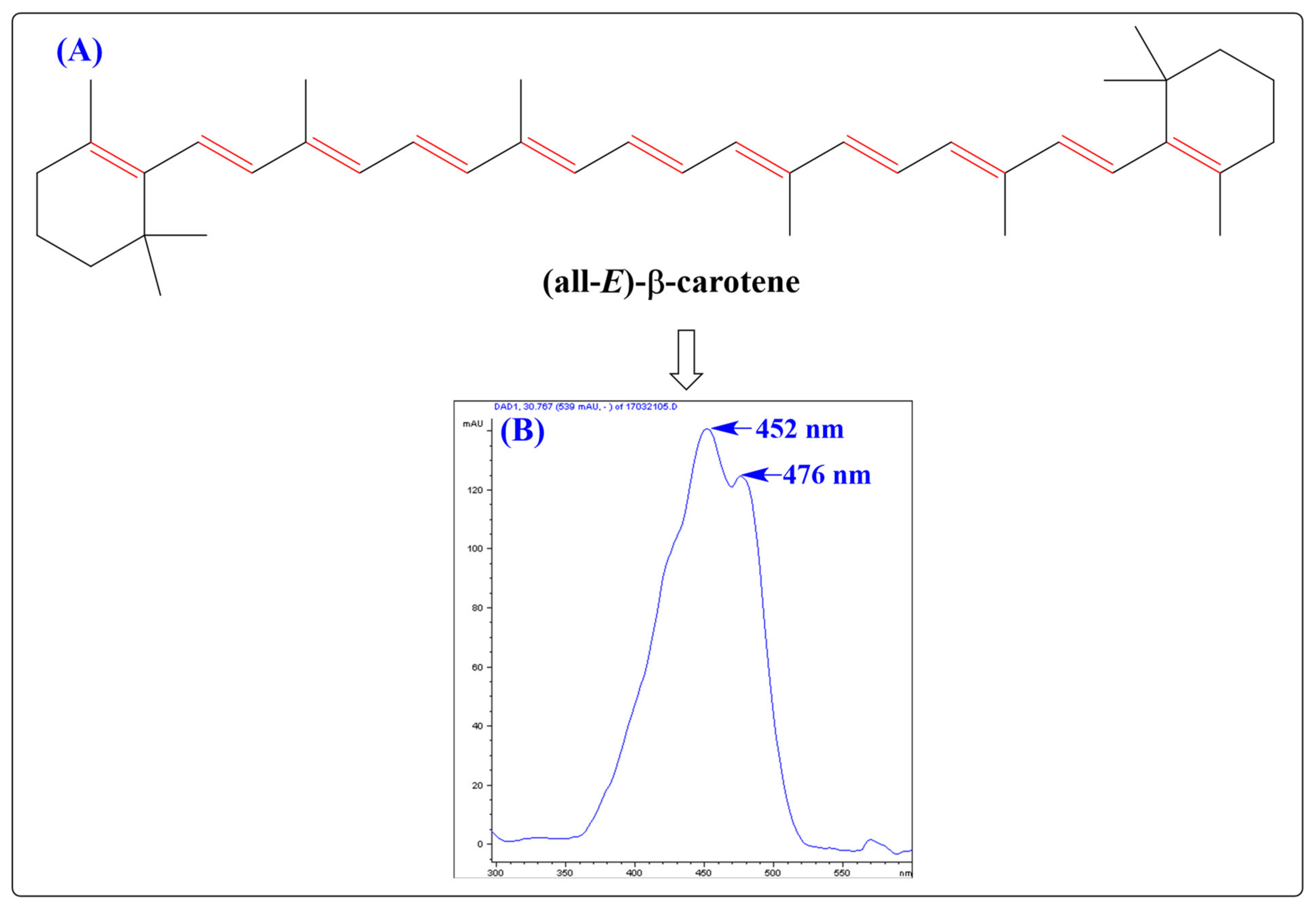
2. Chemistry and Antioxidant Activity of CARs
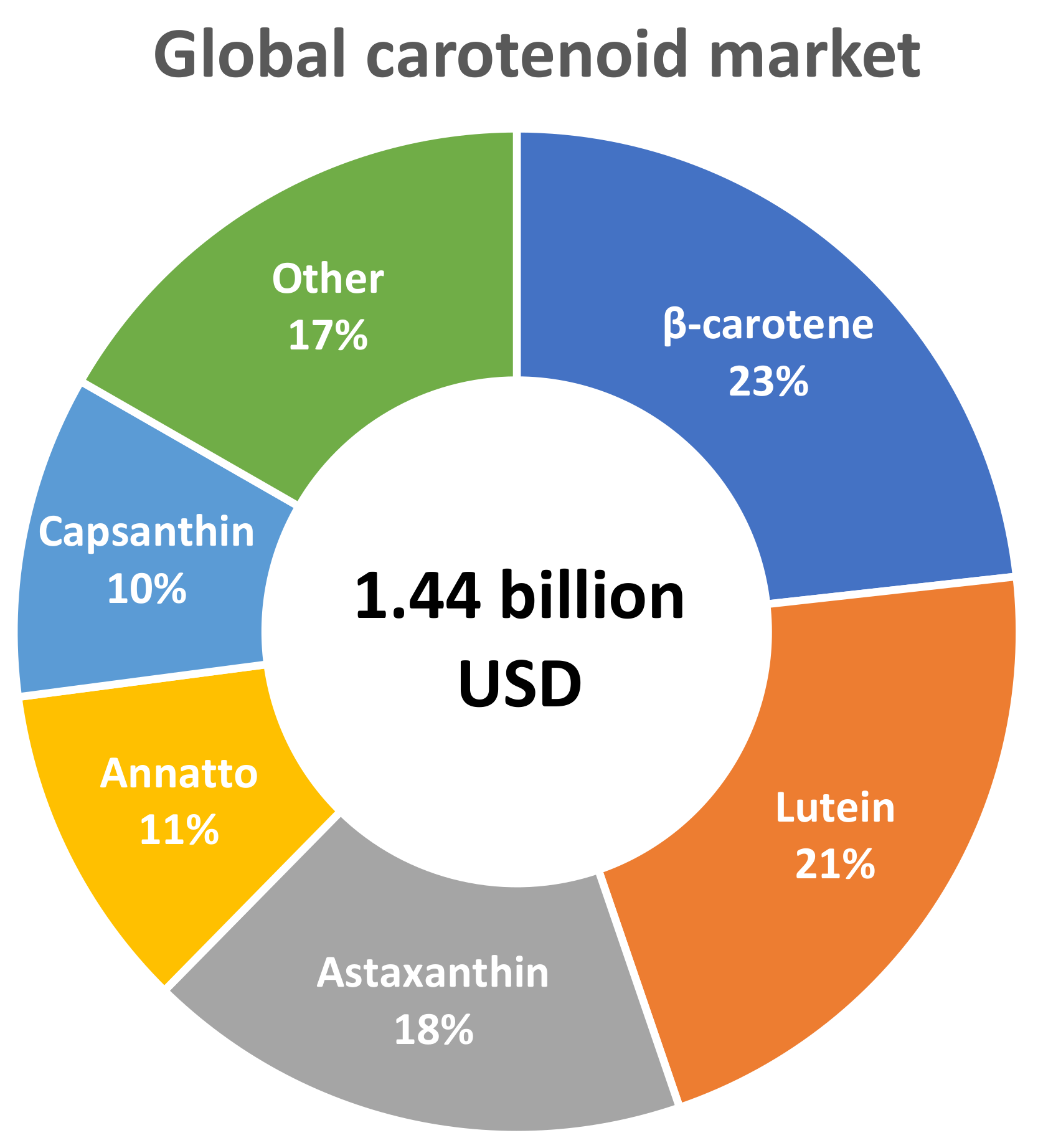
3. Marketing Trends of CARs
4. Source of Natural CARs
4.1. Fruits, Vegetables, Grains and Other Higher Plant-Based Products
4.2. Microalgae, Macroalgae (Seaweeds), and Fungi
4.3. Shellfish Species
4.4. Biofortified Crops and Microbes
5. Extraction of CARs: Pretreatments Improve the Recovery
6. Bioaccessibility and Bioavailability
7. Encapsulation of CARs
8. Normal, Safe, and Desirable Intake of CARs
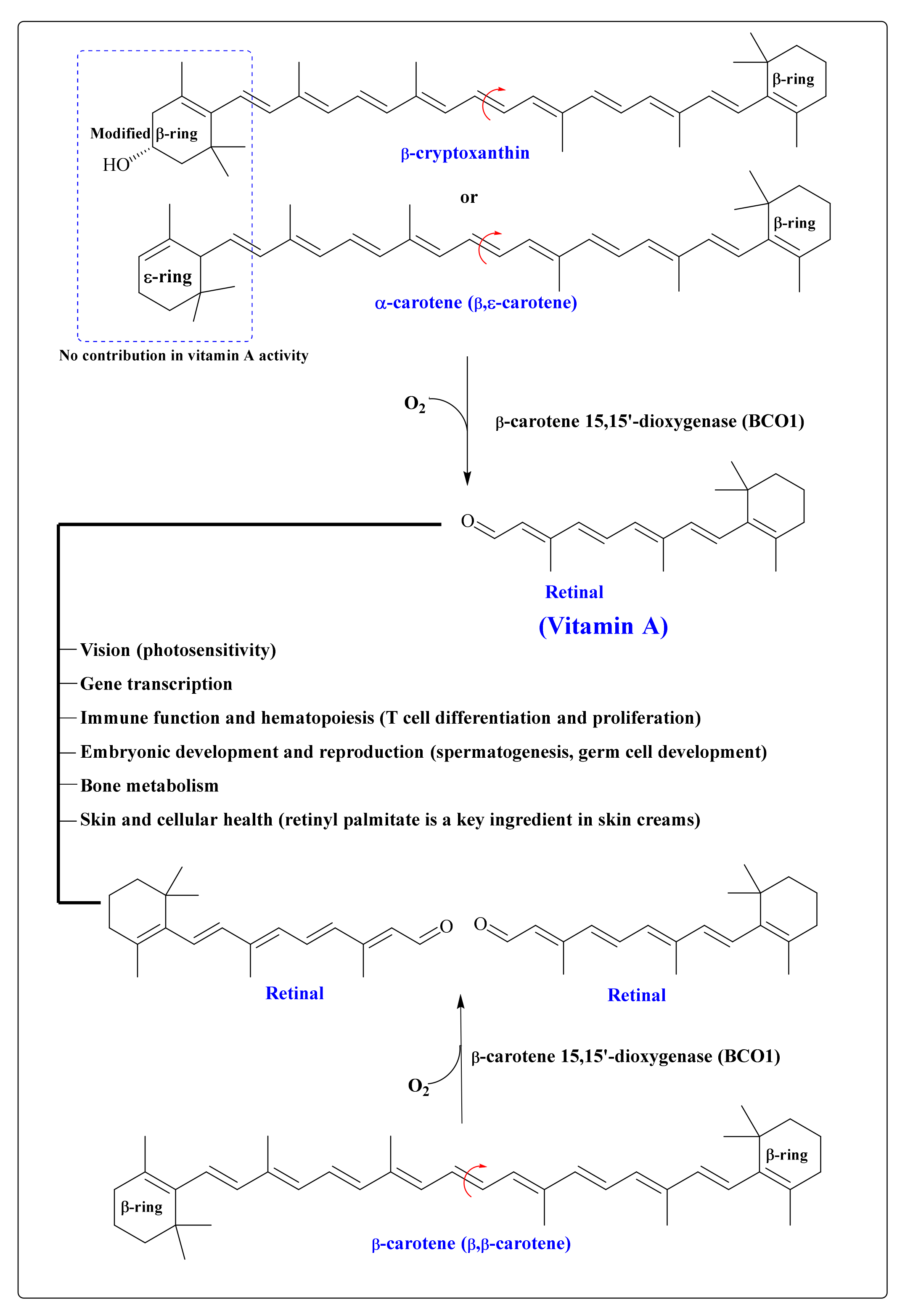
9. CARs in the Human Body
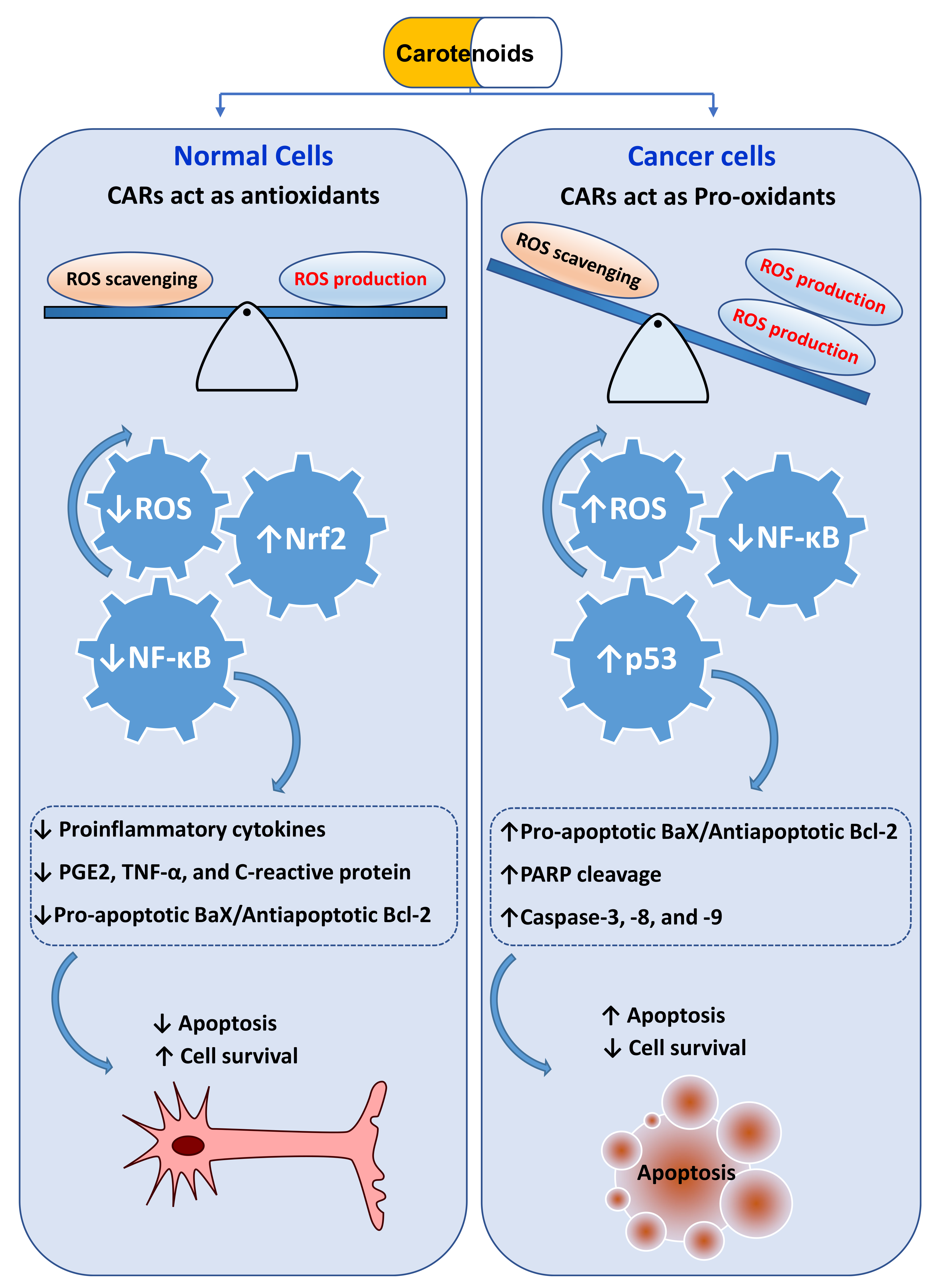
10. Health Benefits of CARs
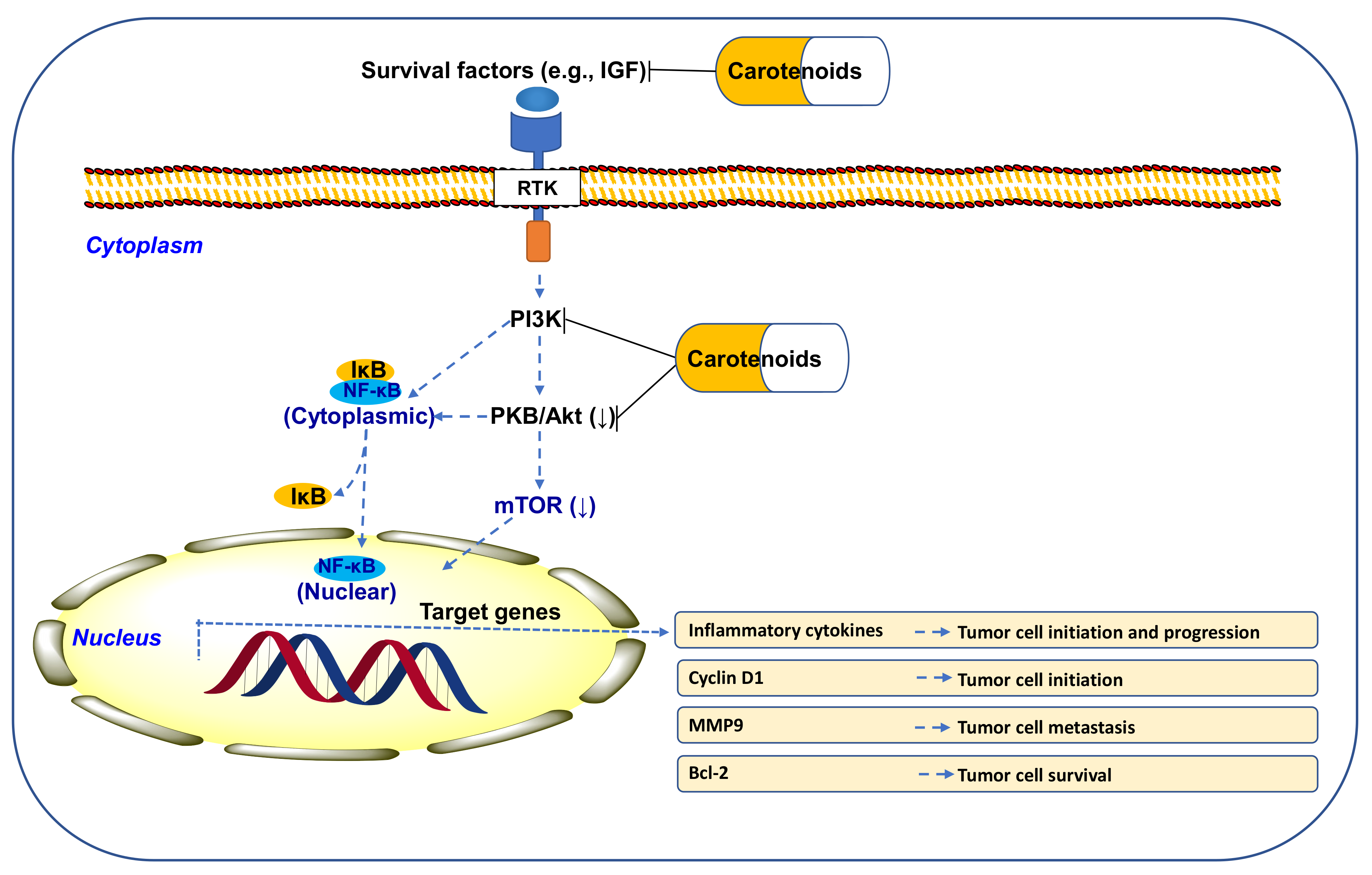
10.1. CARs Regulate PI3K/Akt/mTOR Signaling
10.2. CARs Protect from Cancer
10.3. CARs in Obesity and T2D
10.4. CARs in Cardiovascular Diseases (CVDs)
10.5. CARs in Osteoporosis and Muscle Strength
10.6. CARs in Neurodegenerative Disease and Mental Health
10.7. Eye and Skin Health
10.8. Other Benefits
11. Agroindustrial Waste Valorization, Biorefinery, and Circular Bioeconomy Perspective
12. Conclusions and Future Prospective
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AChE | Acetylcholinesterase |
| AD | Alzheimer’s disease |
| ADIs | Acceptable daily intakes |
| ALG | Alginate |
| AMD | Age-related macular degeneration |
| AQPs | Aquaporins |
| ASK1 | Apoptosis signal-regulating kinase 1 |
| ATBC | α-Tocopherol β-Carotene Cancer Prevention Trial |
| Bax | B-cell lymphoma 2 associated X |
| Bcl-2 | B-cell lymphoma 2 |
| BCO1 | β-carotene oxygenase 1 |
| BHA | Butylated hydroxyanisole |
| BHT | Butylated hydroxytoluene |
| BMD | Bone mineral density |
| BW | Body weight |
| CARET | β-Carotene and Retinol Efficacy Trial |
| CARs | Carotenoids |
| Cas9 | CRISPR-associated protein 9 |
| Cdk | Cyclin-dependent kinase |
| CEH | Cholesterol ester hydrolase |
| COL1A1 | Collagen, type i, alpha 1 |
| COX-2 | Cyclooxygenase-2 |
| CRISPR | Clustered regularly interspaced short palindromic repeats |
| CRP | C-reactive protein |
| CS | Chitosan |
| CVDs | Cardiovascular diseases |
| DBP | Diastolic blood pressure |
| DCW | Dry cell weight |
| DHA | Docosahexaenoic acids |
| DMAPP | Dimethylallyl pyrophosphate |
| DRIs | Dietary reference intakes |
| DW | Dry weight |
| EPA | Eicosapentaenoic acid |
| FFQ | Food Frequency Questionnaire |
| FW | Fresh weight |
| GBM | Astroglioma multiforme |
| Glut4 | Glucose transporter type 4 |
| GM | Genetic modification |
| GPx | Glutathione peroxidase |
| GRADE | Grading of Recommendation Assessment, Development and Evaluation |
| GSK-3β | Glycogen synthase kinase-3β |
| HDL-c | High-density lipoprotein cholesterol |
| HeLa | Human cervical carcinoma |
| HPH | High-pressure homogenization |
| HR | Hazard ratio |
| HUVECs | Human umbilical vein endothelial cells |
| IGF | Insulin-like growth factor |
| IL | Interleukin |
| iNOS | Inducible nitric oxide synthase () |
| IPP | Isopentenyl pyrophosphate |
| IRS-1 | Insulin receptor substrate-1 |
| LC-PUFAs | Long-chain-polyunsaturated fatty acids |
| LP | Low-temperature pasteurization |
| MAE | Microwave-assisted extraction |
| MD | Mammographic density |
| MDA | Malondialdehyde |
| MEP | 2-C-methyld-erythritol 4-phosphate |
| MetS | Metabolic syndromes |
| MMP | Matrix metallopeptidase |
| mTOR | Mechanistic target of rapamycin |
| MVA | Mevalonate |
| n-3 | Omega-3 |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NPs | Nanoparticles |
| Nrf2 | Nuclear factor-erythroid 2-related factor 2 |
| NSMs | Natural small molecules |
| OxLDL | Oxidized phospholipids |
| PARP | Poly (ADP-ribose) polymerase |
| PD | Parkinson’s disease |
| PEF | Pulsed electric field |
| PGE2 | Prostaglandin E2 |
| PI3K | Phosphoinositide 3-kinase |
| PKB | Phosphorylated protein kinase B |
| PPARs | Peroxisome proliferator-activated receptor |
| Prx2 | Peroxiredoxin 2 |
| RCTs | Randomized controlled trials |
| ROS | Reactive oxygen species |
| SBP | Systolic blood pressure |
| SCARB1 | Scavenger receptor class b member 1 |
| SCE | Supercritical CO2 extraction |
| SC-FAs | Short-chain fatty acids |
| SGF | Simulated gastric fluid |
| SIF | Simulated intestinal fluid |
| SIRT1 | Sirtuin 1 |
| SNCA | Alpha-synuclein |
| SNP | Single-nucleotide polymorphism |
| SOD | Superoxide dismutase |
| SWRO | Seawater reverse osmosis |
| T2D | Type 2 diabetes |
| TGF-β | Transforming growth factor-β |
| TNF-α | Tumor necrosis factor-alpha |
| TRAIL | Tumor necrosis factor (TNF)-related apoptosis-inducing ligand |
| UAE | Ultrasonication-assisted extraction |
| UFAs | Unsaturated fatty acids |
| VEGF | Vascular endothelial growth factor |
| WAT | White adipose tissue |
| WMD | Weighted mean difference |
| α-SMA | Alpha-smooth muscle actin |
References
- Wang, P.Y.; Fang, J.C.; Gao, Z.H.; Zhang, C.; Xie, S.Y. Higher intake of fruits, vegetables or their fiber reduces the risk of type 2 diabetes: A meta-analysis. J. Diabetes Investig. 2016, 7, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality—A systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef] [PubMed]
- Medina-Remón, A.; Kirwan, R.; Lamuela-Raventós, R.M.; Estruch, R. Dietary patterns and the risk of obesity, type 2 diabetes mellitus, cardiovascular diseases, asthma, and neurodegenerative diseases. Crit. Rev. Food Sci. Nutr. 2018, 58, 262–296. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wu, X.T.; Zhuang, W.; Xia, L.; Chen, Y.; Wu, C.C.; Rao, Z.Y.; Du, L.; Zhao, R.; Yi, M.S.; et al. Tomato and lycopene and multiple health outcomes: Umbrella review. Food Chem. 2021, 343, 128396. [Google Scholar] [CrossRef] [PubMed]
- Kaulmann, A.; Bohn, T. Carotenoids, inflammation, and oxidative stress—Implications of cellular signaling pathways and relation to chronic disease prevention. Nutr. Res. 2014, 34, 907–929. [Google Scholar] [CrossRef] [PubMed]
- Hajizadeh-Sharafabad, F.; Ghoreishi, Z.; Maleki, V.; Tarighat-Esfanjani, A. Mechanistic insights into the effect of lutein on atherosclerosis, vascular dysfunction, and related risk factors: A systematic review of in vivo, ex vivo and in vitro studies. Pharmacol. Res. 2019, 149, 104477. [Google Scholar] [CrossRef]
- Kohandel, Z.; Farkhondeh, T.; Aschner, M.; Pourbagher-Shahri, A.M.; Samarghandian, S. Anti-inflammatory action of astaxanthin and its use in the treatment of various diseases. Biomed. Pharm. 2022, 145, 112179. [Google Scholar] [CrossRef]
- Zhu, R.Y.; Chen, B.B.; Bai, Y.; Miao, T.Y.; Rui, L.; Zhang, H.; Xia, B.K.; Li, Y.; Gao, S.H.; Wang, X.D.; et al. Lycopene in protection against obesity and diabetes: A mechanistic review. Pharmacol. Res. 2020, 159, 104966. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Carotenoids: How Effective Are They to Prevent Age-Related Diseases? Molecules 2019, 24, 1801. [Google Scholar] [CrossRef] [Green Version]
- Przybylska, S. Lycopene—A bioactive carotenoid offering multiple health benefits: A review. Int. J. Food Sci. Technol. 2020, 55, 11–32. [Google Scholar] [CrossRef]
- Saini, R.K.; Rengasamy, K.R.R.; Mahomoodally, F.M.; Keum, Y.S. Protective effects of lycopene in cancer, cardiovascular, and neurodegenerative diseases: An update on epidemiological and mechanistic perspectives. Pharmacol. Res. 2020, 155, 104730. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Nile, S.H.; Park, S.W. Carotenoids from fruits and vegetables: Chemistry, analysis, occurrence, bioavailability and biological activities. Food Res. Int. 2015, 76, 735–750. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.H.; Yuan, H.; Cao, H.B.; Yazdani, M.; Tadmor, Y.; Li, L. Carotenoid Metabolism in Plants: The Role of Plastids. Mol. Plant 2018, 11, 58–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, T.H.; Li, L. Toward the ‘golden’ era: The status in uncovering the regulatory control of carotenoid accumulation in plants. Plant Sci. 2020, 290, 110331. [Google Scholar] [CrossRef]
- Muzzopappa, F.; Kirilovsky, D. Changing Color for Photoprotection: The Orange Carotenoid Protein. Trends. Plant Sci. 2020, 25, 92–104. [Google Scholar] [CrossRef]
- Jia, K.P.; Baz, L.; Al-Babili, S. From carotenoids to strigolactones. J. Exp. Bot. 2018, 69, 2189–2204. [Google Scholar] [CrossRef] [Green Version]
- Saini, R.K.; Keum, Y.S. Significance of Genetic, Environmental, and Pre- and Postharvest Factors Affecting Carotenoid Contents in Crops: A Review. J. Agric. Food Chem. 2018, 66, 5310–5324. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S.; Daglia, M.; Rengasamy, K.R. Dietary carotenoids in cancer chemoprevention and chemotherapy: A review of emerging evidence. Pharm. Res. 2020, 157, 104830. [Google Scholar] [CrossRef]
- Moran, N.A.; Jarvik, T. Lateral transfer of genes from fungi underlies carotenoid production in aphids. Science 2010, 328, 624–627. [Google Scholar] [CrossRef] [Green Version]
- Ambati, R.R.; Phang, S.-M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Nabi, F.; Arain, M.A.; Rajput, N.; Alagawany, M.; Soomro, J.; Umer, M.; Soomro, F.; Wang, Z.Q.; Ye, R.L.; Liu, J. Health benefits of carotenoids and potential application in poultry industry: A review. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.C.; Yusoff, F.M.; Shariff, M.; Kamarudin, M.S. Astaxanthin as feed supplement in aquatic animals. Rev. Aquac. 2018, 10, 738–773. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Li, H.K.; Zou, Y.; Liu, H.; Yang, L.M. Astaxanthin as a microalgal metabolite for aquaculture: A review on the synthetic mechanisms, production techniques, and practical application. Algal. Res. 2021, 54, 102178. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Yabuzaki, J. Carotenoids Database: Structures, chemical fingerprints and distribution among organisms. Database 2017, 2017, bax004. [Google Scholar] [CrossRef] [Green Version]
- Melendez-Martinez, A.J.; Stinco, C.M.; Mapelli-Brahm, P. Skin Carotenoids in Public Health and Nutricosmetics: The Emerging Roles and Applications of the UV Radiation-Absorbing Colourless Carotenoids Phytoene and Phytofluene. Nutrients 2019, 11, 1093. [Google Scholar] [CrossRef] [Green Version]
- Saini, R.K.; Keum, Y.S. Microbial platforms to produce commercially vital carotenoids at industrial scale: An updated review of critical issues. J. Ind. Microbiol. Biotechnol. 2019, 46, 657–674. [Google Scholar] [CrossRef]
- Kim, D.E.; Shang, X.; Assefa, A.D.; Keum, Y.S.; Saini, R.K. Metabolite profiling of green, green/red, and red lettuce cultivars: Variation in health beneficial compounds and antioxidant potential. Food Res. Int. 2018, 105, 361–370. [Google Scholar] [CrossRef]
- Namitha, K.K.; Negi, P.S. Chemistry and biotechnology of carotenoids. Crit. Rev. Food Sci. Nutr. 2010, 50, 728–760. [Google Scholar] [CrossRef]
- Sandmann, G. Antioxidant Protection from UV- and Light-Stress Related to Carotenoid Structures. Antioxidants 2019, 8, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, D.; Freitas, M.; Silva, A.M.S.; Carvalho, F.; Fernandes, E. Antioxidant and pro-oxidant activities of carotenoids and their oxidation products. Food Chem. Toxicol. 2018, 120, 681–699. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Murakami, K.; Osawa, Y.; Kawashima, Y.; Hirasawa, K.; Kuroda, I. Z-Isomers of Astaxanthin Exhibit Greater Bioavailability and Tissue Accumulation Efficiency than the All-E-Isomer. J. Agric. Food Chem. 2021, 69, 3489–3495. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Kageyama, H.; Hibino, T.; Ichihashi, K.; Takada, W.; Goto, M. Isomerization of Commercially Important Carotenoids (Lycopene, beta-Carotene, and Astaxanthin) by Natural Catalysts: Isothiocyanates and Polysulfides. J. Agric. Food Chem. 2020, 68, 3228–3237. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.D.; Huang, W.Y.; Li, D.J.; Song, J.F.; Liu, C.Q.; Wei, Q.Y.; Zhang, M.; Yang, Q.M. Thermal degradation kinetics of all-trans and cis-carotenoids in a light-induced model system. Food Chem. 2018, 239, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; AE, A.B.; Roohinejad, S.; Rengasamy, K.R.R.; Keum, Y.S. Chemical Stability of Lycopene in Processed Products: A Review of the Effects of Processing Methods and Modern Preservation Strategies. J. Agric. Food Chem. 2020, 68, 712–726. [Google Scholar] [CrossRef]
- Semitsoglou-Tsiapou, S.; Meador, T.B.; Peng, B.; Aluwihare, L. Photochemical (UV-vis/H2O2) degradation of carotenoids: Kinetics and molecular end products. Chemosphere 2022, 286, 131697. [Google Scholar] [CrossRef]
- Britton, G. Carotenoid research: History and new perspectives for chemistry in biological systems. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158699. [Google Scholar] [CrossRef]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [Green Version]
- Edge, R.; Truscott, T.G. Singlet Oxygen and Free Radical Reactions of Retinoids and Carotenoids—A Review. Antioxidants 2018, 7, 5. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.; Song, M.H.; Oh, J.W.; Keum, Y.S.; Saini, R.K. Pro-Oxidant Actions of Carotenoids in Triggering Apoptosis of Cancer Cells: A Review of Emerging Evidence. Antioxidants 2020, 9, 532. [Google Scholar] [CrossRef] [PubMed]
- Black, H.S.; Boehm, F.; Edge, R.; Truscott, T.G. The Benefits and Risks of Certain Dietary Carotenoids that Exhibit both Anti- and Pro-Oxidative Mechanisms—A Comprehensive Review. Antioxidants 2020, 9, 264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Cámara, S.; Ibañez, A.; Rubio, S.; Barreiro, C.; Barredo, J.-L. Main Carotenoids Produced by Microorganisms. Encyclopedia 2021, 1, 1223–1245. [Google Scholar] [CrossRef]
- Foong, L.C.; Loh, C.W.L.; Ng, H.S.; Lan, J.C.-W. Recent development in the production strategies of microbial carotenoids. World J. Microbiol. Biotechnol. 2021, 37, 12. [Google Scholar] [CrossRef] [PubMed]
- Melendez-Martinez, A.J.; Mandic, A.I.; Bantis, F.; Bohm, V.; Borge, G.I.A.; Brncic, M.; Bysted, A.; Cano, M.P.; Dias, M.G.; Elgersma, A.; et al. A comprehensive review on carotenoids in foods and feeds: Status quo, applications, patents, and research needs. Crit. Rev. Food Sci. Nutr. 2021, 62, 1999–2049. [Google Scholar] [CrossRef] [PubMed]
- Novoveska, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.F. Microalgal Carotenoids: A Review of Production, Current Markets, Regulations, and Future Direction. Mar. Drugs 2019, 17, 640. [Google Scholar] [CrossRef] [Green Version]
- Mussagy, C.U.; Khan, S.; Kot, A.M. Current developments on the application of microbial carotenoids as an alternative to synthetic pigments. Crit. Rev. Food Sci. Nutr. 2021, 10, 1–15. [Google Scholar] [CrossRef]
- Saini, R.K.; Assefa, A.D.; Keum, Y.S. Fatty acid and carotenoid composition of bitter melon (Momordica charantia L.) seed arils: A potentially valuable source of lycopene. J. Food Meas. Charact. 2017, 11, 1266–1273. [Google Scholar] [CrossRef]
- Rowles, J.L.; Erdman, J.W. Carotenoids and their role in cancer prevention. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158613. [Google Scholar] [CrossRef]
- Dias, M.G.; Olmedilla-Alonso, B.; Hornero-Mendez, D.; Mercadante, A.Z.; Osorio, C.; Vargas-Murga, L.; Melendez-Martinez, A.J. Comprehensive Database of Carotenoid Contents in Ibero-American Foods. A Valuable Tool in the Context of Functional Foods and the Establishment of Recommended Intakes of Bioactives. J. Agric. Food Chem. 2018, 66, 5055–5107. [Google Scholar] [CrossRef] [Green Version]
- Saini, R.K.; Shang, X.M.; Ko, E.Y.; Choi, J.H.; Kim, D.; Keum, Y.S. Characterization of nutritionally important phytoconstituents in minimally processed ready-to-eat baby-leaf vegetables using HPLC-DAD and GC-MS. J. Food Meas. Charact. 2016, 10, 341–349. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S.; Rengasamy, K.R.R. Profiling of nutritionally important metabolites in green/red and green perilla (Perilla frutescens Britt.) cultivars: A comparative study. Ind. Crop. Prod. 2020, 151, 112441. [Google Scholar] [CrossRef]
- Saini, R.K.; Moon, S.H.; Gansukh, E.; Keum, Y.S. An efficient one-step scheme for the purification of major xanthophyll carotenoids from lettuce, and assessment of their comparative anticancer potential. Food Chem. 2018, 266, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.Y.; Saini, R.K.; Keum, Y.S.; An, B.K. Age of Laying Hens Significantly Influences the Content of Nutritionally Vital Lipophilic Compounds in Eggs. Foods 2021, 10, 22. [Google Scholar] [CrossRef]
- Kljak, K.; Carovic-Stanko, K.; Kos, I.; Janjecic, Z.; Kis, G.; Duvnjak, M.; Safner, T.; Bedekovic, D. Plant Carotenoids as Pigment Sources in Laying Hen Diets: Effect on Yolk Color, Carotenoid Content, Oxidative Stability and Sensory Properties of Eggs. Foods 2021, 10, 721. [Google Scholar] [CrossRef]
- Biswas, S.K.; Kim, D.E.; Keum, Y.S.; Saini, R.K. Metabolite profiling and antioxidant activities of white, red, and black rice (Oryza sativa L.) grains. J. Food Meas. Charact. 2018, 12, 2484–2492. [Google Scholar] [CrossRef]
- Becerra, M.O.; Contreras, L.M.; Lo, M.H.; Diaz, J.M.; Herrera, G.C. Lutein as a functional food ingredient: Stability and bioavailability. J. Funct. Foods 2020, 66, 103771. [Google Scholar] [CrossRef]
- Mariutti, L.R.B.; Mercadante, A.Z. Carotenoid esters analysis and occurrence: What do we know so far? Arch. Biochem. Biophys. 2018, 648, 36–43. [Google Scholar] [CrossRef]
- Hassan, N.M.; Yusof, N.A.; Yahaya, A.F.; Rozali, N.N.M.; Othman, R. Carotenoids of Capsicum Fruits: Pigment Profile and Health-Promoting Functional Attributes. Antioxidants 2019, 8, 469. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-S.; An, C.G.; Park, J.-S.; Lim, Y.P.; Kim, S. Carotenoid profiling from 27 types of paprika (Capsicum annuum L.) with different colors, shapes, and cultivation methods. Food Chem. 2016, 201, 64–71. [Google Scholar] [CrossRef]
- Girme, A.; Pawar, S.; Ghule, C.; Shengule, S.; Saste, G.; Balasubramaniam, A.K.; Deshmukh, A.; Hingorani, L. Bioanalytical Method Development and Validation Study of Neuroprotective Extract of Kashmiri Saffron Using Ultra-Fast Liquid Chromatography-Tandem Mass Spectrometry (UFLC-MS/MS): In Vivo Pharmacokinetics of Apocarotenoids and Carotenoids. Molecules 2021, 26, 1815. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.S.; Wang, Y.; Li, S.; Nagarajan, D.; Varjani, S.; Lee, D.J.; Chang, J.S. Recent advances in lutein production from microalgae. Renew. Sustain. Energy Rev. 2022, 153, 111795. [Google Scholar] [CrossRef]
- Ambati, R.R.; Gogisetty, D.; Aswathanarayana, R.G.; Ravi, S.; Bikkina, P.N.; Lei, B.; Su, Y.P. Industrial potential of carotenoid pigments from microalgae: Current trends and future prospects. Crit. Rev. Food Sci. Nutr. 2019, 59, 1880–1902. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Prasad, P.; Sreedhar, R.V.; Akhilender Naidu, K.; Shang, X.; Keum, Y.-S. Omega−3 Polyunsaturated Fatty Acids (PUFAs): Emerging Plant and Microbial Sources, Oxidative Stability, Bioavailability, and Health Benefits—A Review. Antioxidants 2021, 10, 1627. [Google Scholar] [CrossRef]
- Xie, Y.X.; Xiong, X.C.; Chen, S.L. Challenges and Potential in Increasing Lutein Content in Microalgae. Microorganisms 2021, 9, 1068. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Progress in Microbial Carotenoids Production. Indian J. Microbiol. 2017, 57, 129–130. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Update on natural food pigments—A mini-review on carotenoids, anthocyanins, and betalains. Food Res. Int. 2019, 124, 200–205. [Google Scholar] [CrossRef]
- Rammuni, M.N.; Ariyadasa, T.U.; Nimarshana, P.H.V.; Attalage, R.A. Comparative assessment on the extraction of carotenoids from microalgal sources: Astaxanthin from H-pluvialis and beta-carotene from D. salina. Food Chem. 2019, 277, 128–134. [Google Scholar] [CrossRef]
- Liu, C.; Hu, B.; Cheng, Y.L.; Guo, Y.H.; Yao, W.R.; Qian, H. Carotenoids from fungi and microalgae: A review on their recent production, extraction, and developments. Bioresour. Technol. 2021, 337, 125398. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.Q.; Duan, C.L.; Yi, S.S.; Gao, Z.Q.; Xiao, C.W.; Agathos, S.N.; Wang, G.C.; Li, J. Biotechnological production of astaxanthin from the microalga Haematococcus pluvialis. Biotechnol. Adv. 2020, 43, 107602. [Google Scholar] [CrossRef]
- Pereira, H.; Sa, M.; Maia, I.; Rodrigues, A.; Teles, I.; Wijffels, R.H.; Navalho, J.; Barbosa, M. Fucoxanthin production from Tisochrysis lutea and Phaeodactylum tricornutum at industrial scale. Algal. Res. 2021, 56, 102322. [Google Scholar] [CrossRef]
- Bae, M.; Kim, M.B.; Park, Y.K.; Lee, J.Y. Health benefits of fucoxanthin in the prevention of chronic diseases. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158618. [Google Scholar] [CrossRef] [PubMed]
- Leong, Y.K.; Chen, C.Y.; Varjani, S.; Chang, J.S. Producing fucoxanthin from algae—Recent advances in cultivation strategies and downstream processing. Bioresour. Technol. 2022, 344, 126170. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.L.; Zhao, Y.T.; Li, T.; Han, B.Y.; Zhao, P.; Yu, X.Y. Enhancing astaxanthin and lipid coproduction in Haematococcus pluvialis by the combined induction of plant growth regulators and multiple stresses. Bioresour. Technol. 2022, 344, 126225. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Q.; You, J.K.; Qiao, T.S.; Zhong, D.B.; Yu, X.Y. Sodium chloride stimulates the biomass and astaxanthin production by Haematococcus pluvialis via a two-stage cultivation strategy. Bioresour. Technol. 2022, 344, 126214. [Google Scholar] [CrossRef]
- Oslan, S.N.H.; Shoparwe, N.F.; Yusoff, A.H.; Rahim, A.A.; Chang, C.S.; Tan, J.S.; Oslan, S.N.; Arumugam, K.; Bin Ariff, A.; Sulaiman, A.Z.; et al. A Review on Haematococcus pluvialis Bioprocess Optimization of Green and Red Stage Culture Conditions for the Production of Natural Astaxanthin. Biomolecules 2021, 11, 256. [Google Scholar] [CrossRef] [PubMed]
- Mussagy, C.U.; Pereira, J.F.B.; Dufossé, L.; Raghavan, V.; Santos-Ebinuma, V.C.; Pessoa, A. Advances and trends in biotechnological production of natural astaxanthin by Phaffia rhodozyma yeast. Crit. Rev. Food Sci. Nutr. 2021, 1–15. [Google Scholar] [CrossRef]
- Berman, J.; Zorrilla-López, U.; Farré, G.; Zhu, C.; Sandmann, G.; Twyman, R.M.; Capell, T.; Christou, P. Nutritionally important carotenoids as consumer products. Phytochem. Rev. 2015, 14, 727–743. [Google Scholar] [CrossRef]
- Wu, H.L.; Pan, H.J.; Li, Z.J.; Liu, T.F.; Liu, F.L.; Xiu, S.Y.; Wang, J.; Wang, H.Q.; Hou, Y.; Yang, B.; et al. Efficient production of lycopene from CO2 via microbial electrosynthesis. Chem. Eng. J. 2022, 430, 132943. [Google Scholar] [CrossRef]
- Saini, R.K.; Mahomoodally, M.F.; Sadeer, N.B.; Keum, Y.S.; Rr Rengasamy, K. Characterization of nutritionally important lipophilic constituents from brown kelp Ecklonia radiata (C. Ag.) J. Agardh. Food Chem. 2021, 340, 127897. [Google Scholar] [CrossRef]
- Pereira, A.G.; Otero, P.; Echave, J.; Carreira-Casais, A.; Chamorro, F.; Collazo, N.; Jaboui, A.; Lourenco-Lopes, C.; Simal-Gandara, J.; Prieto, M.A. Xanthophylls from the Sea: Algae as Source of Bioactive Carotenoids. Mar. Drugs 2021, 19, 188. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Song, M.-H.; Yu, J.-W.; Ko, E.-Y.; Shang, X.; Oh, J.-W.; Keum, Y.-S.; Saini, R.K. Anticancer Potential of Lipophilic Constituents of Eleven Shellfish Species Commonly Consumed in Korea. Antioxidants 2021, 10, 1629. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Song, M.H.; Rengasamy, K.R.R.; Ko, E.Y.; Keum, Y.S. Red Shrimp Are a Rich Source of Nutritionally Vital Lipophilic Compounds: A Comparative Study among Edible Flesh and Processing Waste. Foods 2020, 9, 1179. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Keum, Y.S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance: A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Moon, S.H.; Keum, Y.S. An updated review on use of tomato pomace and crustacean processing waste to recover commercially vital carotenoids. Food Res. Int. 2018, 108, 516–529. [Google Scholar] [CrossRef]
- Deng, J.J.; Mao, H.H.; Fang, W.; Li, Z.Q.; Shi, D.; Li, Z.W.; Zhou, T.; Luo, X.C. Enzymatic conversion and recovery of protein, chitin, and astaxanthin from shrimp shell waste. J. Clean. Prod. 2020, 271, 122655. [Google Scholar] [CrossRef]
- Ahmadkelayeh, S.; Hawboldt, K. Extraction of lipids and astaxanthin from crustacean by-products: A review on supercritical CO2 extraction. Trends Food Sci. Technol. 2020, 103, 94–108. [Google Scholar] [CrossRef]
- Li, X.; Li, N.; Zhao, L.; Shi, J.X.; Wang, S.Y.; Ning, X.H.; Li, Y.R.; Hu, X.L. Tissue distribution and seasonal accumulation of carotenoids in Yesso scallop (Mizuhopecten yessoensis) with orange adductor muscle. Food Chem. 2022, 367, 130701. [Google Scholar] [CrossRef]
- Zhu, Q.L.; Zeng, D.C.; Yu, S.Z.; Cui, C.J.; Li, J.M.; Li, H.Y.; Chen, J.Y.; Zhang, R.Z.; Zhao, X.C.; Chen, L.T.; et al. From Golden Rice to aSTARice: Bioengineering Astaxanthin Biosynthesis in Rice Endosperm. Mol. Plant 2018, 11, 1440–1448. [Google Scholar] [CrossRef] [Green Version]
- Garg, M.; Sharma, N.; Sharma, S.; Kapoor, P.; Kumar, A.; Chunduri, V.; Arora, P. Biofortified Crops Generated by Breeding, Agronomy, and Transgenic Approaches Are Improving Lives of Millions of People around the World. Front. Nutr. 2018, 5, 12. [Google Scholar] [CrossRef]
- Zheng, X.J.; Giuliano, G.; Al-Babili, S. Carotenoid biofortification in crop plants: Citius, altius, fortius. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.D.; Wang, Y.N.; Chen, S.; Tian, H.Q.; Fu, D.Q.; Zhu, B.Z.; Luo, Y.B.; Zhu, H.L. Lycopene Is Enriched in Tomato Fruit by CRISPR/Cas9-Mediated Multiplex Genome Editing. Front. Plant Sci. 2018, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Zunjare, R.U.; Hossain, F.; Muthusamy, V.; Baveja, A.; Chauhan, H.S.; Bhat, J.S.; Thirunavukkarasu, N.; Saha, S.; Gupta, H.S. Development of Biofortified Maize Hybrids through Marker-Assisted Stacking of beta-Carotene Hydroxylase, Lycopene-epsilon-Cyclase and Opaque2 Genes. Front. Plant Sci. 2018, 9, 178. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Wesseler, J.; Zilberman, D.; Russell, R.M.; Chen, C.; Dubock, A.C. Opinion: Allow Golden Rice to save lives. Proc. Natl. Acad. Sci. USA 2021, 118, e2120901118. [Google Scholar] [CrossRef] [PubMed]
- De Steur, H.; Stein, A.J.; Demont, M. From Golden Rice to Golden Diets: How to turn its recent approval into practice. Glob. Food Secur. 2022, 32, 100596. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.K.; Wang, D.N.; Chen, J.; Liu, Z.J.; Wei, L.J.; Hua, Q. Metabolic engineering of beta-carotene biosynthesis in Yarrowia lipolytica. Biotechnol. Lett. 2020, 42, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Mary Leema, J.T.; Persia Jothy, T.; Dharani, G. Rapid green microwave assisted extraction of lutein from Chlorella sorokiniana (NIOT-2)—Process optimization. Food Chem. 2022, 372, 131151. [Google Scholar] [CrossRef]
- Gheonea, I.; Aprodu, I.; Circiumaru, A.; Rapeanu, G.; Bahrim, G.E.; Stanciuc, N. Microencapsulation of lycopene from tomatoes peels by complex coacervation and freeze-drying: Evidences on phytochemical profile, stability and food applications. J. Food Eng. 2021, 288, 110166. [Google Scholar] [CrossRef]
- Chuyen, H.V.; Nguyen, M.H.; Roach, P.D.; Golding, J.B.; Parks, S.E. Microwave-assisted extraction and ultrasound-assisted extraction for recovering carotenoids from Gac peel and their effects on antioxidant capacity of the extracts. Food Sci. Nutr. 2018, 6, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Lima, M.D.; Kestekoglou, I.; Charalampopoulos, D.; Chatzifragkou, A. Supercritical Fluid Extraction of Carotenoids from Vegetable Waste Matrices. Molecules 2019, 24, 466. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.; Youn Lee, S.; Lakshmi Narasimhan, A.; Kim, S.; Oh, Y.K. Cell disruption and astaxanthin extraction from Haematococcus pluvialis: Recent advances. Bioresour. Technol. 2022, 343, 126124. [Google Scholar] [CrossRef] [PubMed]
- Catalkaya, G.; Kahveci, D. Optimization of enzyme assisted extraction of lycopene from industrial tomato waste. Sep. Purif. Technol. 2019, 219, 55–63. [Google Scholar] [CrossRef]
- Ceron-Garcia, M.C.; Gonzalez-Lopez, C.V.; Camacho-Rodriguez, J.; Lopez-Rosales, L.; Garcia-Camacho, F.; Molina-Grima, E. Maximizing carotenoid extraction from microalgae used as food additives and determined by liquid chromatography (HPLC). Food Chem. 2018, 257, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Wellala, C.K.D.; Bi, J.F.; Liu, X.; Wu, X.Y.; Lyu, J.; Liu, J.N.; Liu, D.Z.; Guo, C.T. Effect of high pressure homogenization on water-soluble pectin characteristics and bioaccessibility of carotenoids in mixed juice. Food Chem. 2022, 371, 131073. [Google Scholar] [CrossRef]
- Arballo, J.; Amengual, J.; Erdman, J.W. Lycopene: A Critical Review of Digestion, Absorption, Metabolism, and Excretion. Antioxidants 2021, 10, 342. [Google Scholar] [CrossRef]
- Stinco, C.M.; Sentandreu, E.; Mapelli-Brahm, P.; Navarro, J.L.; Vicario, I.M.; Melendez-Martinez, A.J. Influence of high pressure homogenization and pasteurization on the in vitro bioaccessibility of carotenoids and flavonoids in orange juice. Food Chem. 2020, 331, 127259. [Google Scholar] [CrossRef]
- Kopec, R.E.; Failla, M.L. Recent advances in the bioaccessibility and bioavailability of carotenoids and effects of other dietary lipophiles. J. Food Compos. Anal. 2018, 68, 16–30. [Google Scholar] [CrossRef]
- Bernaerts, T.M.M.; Verstreken, H.; Dejonghe, C.; Gheysen, L.; Foubert, I.; Grauwet, T.; Van Loey, A.M. Cell disruption of Nannochloropsis sp. improves in vitro bioaccessibility of carotenoids and omega 3-LC-PUFA. J. Funct. Foods 2020, 65, 103770. [Google Scholar] [CrossRef]
- Olmedilla-Alonso, B.; Granado-Lorencio, F.; de Ancos, B.; Sanchez-Moreno, C.; Martin-Belloso, O.; Blanco, I.; Herrero-Barbudo, C.; Elez-Martinez, P.; Plaza, L.; Cano, M.P. Greater bioavailability of xanthophylls compared to carotenes from orange juice (high-pressure processed, pulsed electric field treated, low-temperature pasteurised, and freshly squeezed) in a crossover study in healthy individuals. Food Chem. 2022, 371, 130821. [Google Scholar] [CrossRef]
- Sorasitthiyanukarn, F.N.; Muangnoi, C.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan oligosaccharide/alginate nanoparticles as an effective carrier for astaxanthin with improving stability, in vitro oral bioaccessibility, and bioavailability. Food Hydrocoll. 2022, 124, 107246. [Google Scholar] [CrossRef]
- Yang, L.; Qiao, X.; Gu, J.Y.; Li, X.M.; Cao, Y.R.; Xu, J.; Xue, C.H. Influence of molecular structure of astaxanthin esters on their stability and bioavailability. Food Chem. 2021, 343, 128497. [Google Scholar] [CrossRef] [PubMed]
- Reboul, E. Mechanisms of Carotenoid Intestinal Absorption: Where Do We Stand? Nutrients 2019, 11, 838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Q.; Yang, L.; Xu, J.; Qiao, X.; Li, Z.; Wang, Y.; Xue, C. Evaluation of the physicochemical stability and digestibility of microencapsulated esterified astaxanthins using in vitro and in vivo models. Food Chem. 2018, 260, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Soukoulis, C.; Bohn, T. A comprehensive overview on the micro- and nano-technological encapsulation advances for enhancing the chemical stability and bioavailability of carotenoids. Crit. Rev. Food Sci. Nutr. 2018, 58, 1–36. [Google Scholar] [CrossRef]
- Silva, K.C.G.; Feltre, G.; Hubinger, M.D.; Sato, A.C.K. Protection and targeted delivery of beta-carotene by starch-alginate-gelatin emulsion-filled hydrogels. J. Food Eng. 2021, 290, 110205. [Google Scholar] [CrossRef]
- Boonlao, N.; Ruktanonchai, U.R.; Anal, A.K. Enhancing bioaccessibility and bioavailability of carotenoids using emulsion-based delivery systems. Colloids Surf. B Biointerfaces 2022, 209, 112211. [Google Scholar] [CrossRef]
- Zare, M.; Roshan, Z.N.; Assadpour, E.; Jafari, S.M. Improving the cancer prevention/treatment role of carotenoids through various nano-delivery systems. Crit. Rev. Food Sci. Nutr. 2021, 61, 522–534. [Google Scholar] [CrossRef]
- Rostamabadi, H.; Falsafi, S.R.; Jafari, S.M. Nanoencapsulation of carotenoids within lipid-based nanocarriers. J. Control. Release 2019, 298, 38–67. [Google Scholar] [CrossRef]
- Rehman, A.; Tong, Q.Y.; Jafari, S.M.; Assadpour, E.; Shehzad, Q.; Aadil, R.M.; Iqbal, M.W.; Rashed, M.M.A.; Mushtaq, B.S.; Ashraf, W. Carotenoid-loaded nanocarriers: A comprehensive review. Adv. Colloid Interface Sci. 2020, 275, 102048. [Google Scholar] [CrossRef]
- Liu, S.Q.; Zhang, J.; Fu, R.; Feng, H.; Chu, Y.B.; Huang, D.; Liu, H.; Li, C.N.; Ma, C.; Abd El-Aty, A.M. Improved stability and aqueous solubility of 0-carotene via encapsulation in self-assembled bioactive oleanolic acid nanoparticles. Food Chem. 2022, 373, 131498. [Google Scholar] [CrossRef]
- Yang, X.; Ma, C.; Chen, Z.; Liu, J.; Liu, F.; Xie, R.; Zhao, H.; Deng, G.; Chen, A.T.; Gong, N.; et al. Single small molecule-assembled nanoparticles mediate efficient oral drug delivery. Nano Res. 2019, 12, 2468–2476. [Google Scholar] [CrossRef]
- Zhao, R.; Zheng, G.; Fan, L.; Shen, Z.; Jiang, K.; Guo, Y.; Shao, J.-W. Carrier-free nanodrug by co-assembly of chemotherapeutic agent and photosensitizer for cancer imaging and chemo-photo combination therapy. Acta Biomater. 2018, 70, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, D.H.; Ma, Y.; Liu, Y.X.; Liu, G.M.; Wang, Y.B.; Tan, B. Dietary fatty acid-mediated protein encapsulation simultaneously improving the water-solubility, storage stability, and oral absorption of astaxanthin. Food Hydrocoll. 2022, 123, 107152. [Google Scholar] [CrossRef]
- Liu, X.; Wang, P.; Zou, Y.X.; Luo, Z.G.; Tamer, T.M. Co-encapsulation of Vitamin C and beta-Carotene in liposomes: Storage stability, antioxidant activity, and in vitro gastrointestinal digestion. Food Res. Int. 2020, 136, 109587. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Hu, S.; Fleming, E.; Lee, J.Y.; Luo, Y. Chitosan-caseinate-dextran ternary complex nanoparticles for potential oral delivery of astaxanthin with significantly improved bioactivity. Int. J. Biol. Macromol. 2020, 151, 747–756. [Google Scholar] [CrossRef]
- Bohm, V.; Lietz, G.; Olmedilla-Alonso, B.; Phelan, D.; Reboul, E.; Banati, D.; Borel, P.; Corte-Real, J.; de Lera, A.R.; Desmarchelier, C.; et al. From carotenoid intake to carotenoid blood and tissue concentrations—Implications for dietary intake recommendations. Nutr. Rev. 2021, 79, 544–573. [Google Scholar] [CrossRef]
- Harrison, E.H. Mechanisms of Transport and Delivery of Vitamin A and Carotenoids to the Retinal Pigment Epithelium. Mol. Nutr. Food Res. 2019, 63, e1801046. [Google Scholar] [CrossRef] [Green Version]
- Melendez-Martinez, A.J.; Bohm, V.; Borge, G.I.A.; Cano, M.P.; Fikselova, M.; Gruskiene, R.; Lavelli, V.; Loizzo, M.R.; Mandic, A.I.; Brahm, P.M.; et al. Carotenoids: Considerations for Their Use in Functional Foods, Nutraceuticals, Nutricosmetics, Supplements, Botanicals, and Novel Foods in the Context of Sustainability, Circular Economy, and Climate Change. In Annual Review of Food Science and Technology; Doyle, M., McClements, D.J., Eds.; Annual Reviews: Palo Alto, CA, USA, 2021; Volume 12, pp. 433–460. [Google Scholar]
- Melendez-Martinez, A.J. An Overview of Carotenoids, Apocarotenoids, and Vitamin A in Agro-Food, Nutrition, Health, and Disease. Mol. Nutr. Food Res. 2019, 63, 1801045. [Google Scholar] [CrossRef] [Green Version]
- Elvira-Torales, L.I.; Garcia-Alonso, J.; Periago-Caston, M.J. Nutritional Importance of Carotenoids and Their Effect on Liver Health: A Review. Antioxidants 2019, 8, 229. [Google Scholar] [CrossRef] [Green Version]
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Brendler, T.; Williamson, E.M. Astaxanthin: How much is too much? A safety review. Phytother. Res. 2019, 33, 3090–3111. [Google Scholar] [CrossRef] [PubMed]
- Maiani, G.; Caston, M.J.; Catasta, G.; Toti, E.; Cambrodon, I.G.; Bysted, A.; Granado-Lorencio, F.; Olmedilla-Alonso, B.; Knuthsen, P.; Valoti, M.; et al. Carotenoids: Actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol. Nutr. Food Res. 2009, 53 (Suppl. 2), S194–S218. [Google Scholar] [CrossRef] [PubMed]
- Al-Delaimy, W.K.; van Kappel, A.L.; Ferrari, P.; Slimani, N.; Steghens, J.P.; Bingham, S.; Johansson, I.; Wallstrom, P.; Overvad, K.; Tjonneland, A.; et al. Plasma levels of six carotenoids in nine European countries: Report from the European Prospective Investigation into Cancer and Nutrition (EPIC). Public Health Nutr. 2004, 7, 713–722. [Google Scholar] [CrossRef]
- Miyashita, K.; Beppu, F.; Hosokawa, M.; Liu, X.Y.; Wang, S.Z. Nutraceutical characteristics of the brown seaweed carotenoid fucoxanthin. Arch. Biochem. Biophys. 2020, 686, 108364. [Google Scholar] [CrossRef]
- Stiefvatter, L.; Lehnert, K.; Frick, K.; Montoya-Arroyo, A.; Frank, J.; Vetter, W.; Schmid-Staiger, U.; Bischoff, S.C. Oral Bioavailability of Omega-3 Fatty Acids and Carotenoids from the Microalgae Phaeodactylum tricornutum in Healthy Young Adults. Mar. Drugs 2021, 19, 700. [Google Scholar] [CrossRef]
- Marti, R.; Rosello, S.; Cebolla-Cornejo, J. Tomato as a Source of Carotenoids and Polyphenols Targeted to Cancer Prevention. Cancers 2016, 8, 58. [Google Scholar] [CrossRef] [Green Version]
- Koklesova, L.; Liskova, A.; Samec, M.; Buhrmann, C.; Samuel, S.M.; Varghese, E.; Ashrafizadeh, M.; Najafi, M.; Shakibaei, M.; Busselberg, D.; et al. Carotenoids in Cancer Apoptosis-The Road from Bench to Bedside and Back. Cancers 2020, 12, 2425. [Google Scholar] [CrossRef]
- Bohn, T.; Desmarchelier, C.; Dragsted, L.O.; Nielsen, C.S.; Stahl, W.; Ruhl, R.; Keijer, J.; Borel, P. Host-related factors explaining interindividual variability of carotenoid bioavailability and tissue concentrations in humans. Mol. Nutr. Food Res. 2017, 61, 61. [Google Scholar] [CrossRef]
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Melendez-Martinez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.Y.; Wang, X.D. Mechanistic understanding of beta-cryptoxanthin and lycopene in cancer prevention in animal models. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158652. [Google Scholar] [CrossRef] [PubMed]
- Kohandel, Z.; Farkhondeh, T.; Aschner, M.; Samarghandian, S. Nrf2 a molecular therapeutic target for Astaxanthin. Biomed. Pharm. 2021, 137, 111374. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Ghorat, F.; Ul-Haq, I.; Ur-Rehman, H.; Aslam, F.; Heydari, M.; Shariati, M.A.; Okuskhanova, E.; Yessimbekov, Z.; Thiruvengadam, M.; et al. Lycopene as a Natural Antioxidant Used to Prevent Human Health Disorders. Antioxidants 2020, 9, 706. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Abbaszadeh, F.; Dargahi, L.; Jorjani, M. Astaxanthin: A mechanistic review on its biological activities and health benefits. Pharmacol. Res. 2018, 136, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T. Carotenoids and Markers of Oxidative Stress in Human Observational Studies and Intervention Trials: Implications for Chronic Diseases. Antioxidants 2019, 8, 179. [Google Scholar] [CrossRef] [Green Version]
- Hajizadeh-Sharafabad, F.; Zahabi, E.S.; Malekahmadi, M.; Zarrin, R.; Alizadeh, M. Carotenoids supplementation and inflammation: A systematic review and meta-analysis of randomized clinical trials. Crit. Rev. Food Sci. Nutr. 2021, 1–17. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Chen, X.L.; Jha, K.; Beydoun, H.A.; Zonderman, A.B.; Canas, J.A. Carotenoids, vitamin A, and their association with the metabolic syndrome: A systematic review and meta-analysis. Nutr. Rev. 2019, 77, 32–45. [Google Scholar] [CrossRef]
- Wu, D.; Xu, H.; Chen, J.Y.; Zhang, L.S. Effects of Astaxanthin Supplementation on Oxidative Stress A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Vitam. Nutr. Res. 2020, 90, 179–194. [Google Scholar] [CrossRef]
- Zarneshan, S.N.; Fakhri, S.; Farzaei, M.H.; Khan, H.; Saso, L. Astaxanthin targets PI3K/Akt signaling pathway toward potential therapeutic applications. Food Chem. Toxicol. 2020, 145, 17. [Google Scholar] [CrossRef]
- Ming, J.X.; Wang, Z.C.; Huang, Y.; Ohishi, H.; Wu, R.J.; Shao, Y.; Wang, H.; Qin, M.Y.; Wu, Z.L.; Li, Y.Y.; et al. Fucoxanthin extracted from Laminaria Japonica inhibits metastasis and enhances the sensitivity of lung cancer to Gefitinib. J. Ethnopharmacol. 2021, 265, 14. [Google Scholar] [CrossRef]
- Faraone, I.; Sinisgalli, C.; Ostuni, A.; Armentano, M.F.; Carmosino, M.; Milella, L.; Russo, D.; Labanca, F.; Khan, H. Astaxanthin anticancer effects are mediated through multiple molecular mechanisms: A systematic review. Pharmacol. Res. 2020, 155, 111741. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, J. The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study in Finland. Nutr. Rev. 1994, 52, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Omenn, G.S.; Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Glass, A.; Keogh, J.P.; Meyskens, F.L.; Valanis, B.; Williams, J.H.; et al. Risk Factors for Lung Cancer and for Intervention Effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. J. Natl. Cancer Inst. 1996, 88, 1550–1559. [Google Scholar] [CrossRef]
- Dulinska-Litewka, J.; Halubiec, P.; Lazarczyk, A.; Szafranski, O.; Sharoni, Y.; McCubrey, J.A.; Gasiorkiewicz, B.; Bohn, T. Recent Progress in Discovering the Role of Carotenoids and Metabolites in Prostatic Physiology and Pathology—A Review-Part II: Carotenoids in the Human Studies. Antioxidants 2021, 10, 319. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Gao, C.; Lu, D.H.; Rosner, B.A.; Zeleznik, O.; Hankinson, S.E.; Kraft, P.; Eliassen, A.H.; Tamimi, R.M. Circulating carotenoids and breast cancer among high-risk individuals. Am. J. Clin. Nutr. 2021, 113, 525–533. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Gu, Y.; Zhang, S. Vitamin A and Breast Cancer Survival: A Systematic Review and Meta-analysis. Clin. Breast Cancer 2018, 18, e1389–e1400. [Google Scholar] [CrossRef] [PubMed]
- Gansukh, E.; Nile, A.; Sivanesan, I.; Rengasamy, K.R.R.; Kim, D.H.; Keum, Y.S.; Saini, R.K. Chemopreventive Effect of beta-Cryptoxanthin on Human Cervical Carcinoma (HeLa) Cells Is Modulated through Oxidative Stress-Induced Apoptosis. Antioxidants 2019, 9, 28. [Google Scholar] [CrossRef] [Green Version]
- Gansukh, E.; Mya, K.K.; Jung, M.; Keum, Y.S.; Kim, D.H.; Saini, R.K. Lutein derived from marigold (Tagetes erecta) petals triggers ROS generation and activates Bax and caspase-3 mediated apoptosis of human cervical carcinoma (HeLa) cells. Food Chem. Toxicol. 2019, 127, 11–18. [Google Scholar] [CrossRef]
- Shin, J.; Saini, R.K.; Oh, J.W. Low Dose Astaxanthin Treatments Trigger the Hormesis of Human Astroglioma Cells by Up-Regulating the Cyclin-Dependent Kinase and Down-Regulated the Tumor Suppressor Protein P53. Biomedicines 2020, 8, 434. [Google Scholar] [CrossRef]
- Shin, J.; Nile, A.; Saini, R.K.; Oh, J.-W. Astaxanthin Sensitizes Low SOD2-Expressing GBM Cell Lines to TRAIL Treatment via Pathway Involving Mitochondrial Membrane Depolarization. Antioxidants 2022, 11, 375. [Google Scholar] [CrossRef]
- Mounien, L.; Tourniaire, F.; Landrier, J.F. Anti-Obesity Effect of Carotenoids: Direct Impact on Adipose Tissue and Adipose Tissue-Driven Indirect Effects. Nutrients 2019, 11, 1562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Zhang, Y.J.; Zeng, X.; Cheng, Y.H.; Tang, L.; Hong, D.; Yang, X.L. Lycopene ameliorates insulin resistance and increases muscle capillary density in aging via activation of SIRT1. J. Nutr. Biochem. 2022, 99, 108862. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.W.; Zhao, H.L.; Liu, Z.L.; Sun, X.; Zhang, D.D.; Wang, S.H.; Xu, Y.; Zhang, G.F.; Wang, D.F. Modulation of Gut Microbiota by Fucoxanthin During Alleviation of Obesity in High-Fat Diet-Fed Mice. J. Agric. Food Chem. 2020, 68, 5118–5128. [Google Scholar] [CrossRef] [PubMed]
- Hajizadeh-Sharafabad, F.; Tarighat-Esfanjani, A.; Ghoreishi, Z.; Sarreshtedari, M. Lutein supplementation combined with a low-calorie diet in middle-aged obese individuals: Effects on anthropometric indices, body composition and metabolic parameters. Br. J. Nutr. 2021, 126, 1028–1039. [Google Scholar] [CrossRef]
- Yao, N.; Yan, S.M.; Guo, Y.P.; Wang, H.; Li, X.T.; Wang, L.; Hu, W.Y.; Li, B.; Cui, W.W. The association between carotenoids and subjects with overweight or obesity: A systematic review and meta-analysis. Food Funct. 2021, 12, 4768–4782. [Google Scholar] [CrossRef]
- Harari, A.; Coster, A.C.F.; Jenkins, A.; Xu, A.M.; Greenfield, J.R.; Harats, D.; Shaish, A.; Samocha-Bonet, D. Obesity and Insulin Resistance Are Inversely Associated with Serum and Adipose Tissue Carotenoid Concentrations in Adults. J. Nutr. 2020, 150, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Shokri-Mashhadi, N.; Tahmasebi, M.; Mohammadi-Asl, J.; Zakerkish, M.; Mohammadshahi, M. The antioxidant and anti-inflammatory effects of astaxanthin supplementation on the expression of miR-146a and miR-126 in patients with type 2 diabetes mellitus: A randomised, double-blind, placebo-controlled clinical trial. Int. J. Clin. Pract. 2021, 75, e14062. [Google Scholar] [CrossRef]
- Kelishadi, M.R.; Asbaghi, O.; Nazarian, B.; Naeini, F.; Kaviani, M.; Moradi, S.; Askari, G.; Nourian, M.; Ashtary-Larky, D. Lycopene Supplementation and Blood Pressure: Systematic review and meta-analyses of randomized trials. J. Herb. Med. 2022, 31, 100521. [Google Scholar] [CrossRef]
- Cheng, H.M.; Koutsidis, G.; Lodge, J.K.; Ashor, A.W.; Siervo, M.; Lara, J. Lycopene and tomato and risk of cardiovascular diseases: A systematic review and meta-analysis of epidemiological evidence. Crit. Rev. Food Sci. Nutr. 2019, 59, 141–158. [Google Scholar] [CrossRef]
- Kan, B.; Guo, D.J.; Yuan, B.M.; Vuong, A.M.; Jiang, D.P.; Zhang, M.M.; Cheng, H.T.; Zhao, Q.Q.; Li, B.B.; Feng, L.J.; et al. Dietary carotenoid intake and osteoporosis: The National Health and Nutrition Examination Survey, 2005–2018. Arch. Osteoporos. 2022, 17, 1–8. [Google Scholar] [CrossRef]
- Sahni, S.; Dufour, A.B.; Fielding, R.A.; Newman, A.B.; Kiel, D.P.; Hannan, M.T.; Jacques, P.F. Total carotenoid intake is associated with reduced loss of grip strength and gait speed over time in adults: The Framingham Offspring Study. Am. J. Clin. Nutr. 2021, 113, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.Z.; Chen, H.; Wang, Y.M.; Schneider, J.A.; Willett, W.C.; Morris, M.C. Dietary carotenoids related to risk of incident Alzheimer dementia (AD) and brain AD neuropathology: A community-based cohort of older adults. Am. J. Clin. Nutr. 2021, 113, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Galasso, C.; Orefice, I.; Pellone, P.; Cirino, P.; Miele, R.; Ianora, A.; Brunet, C.; Sansone, C. On the Neuroprotective Role of Astaxanthin: New Perspectives? Mar. Drugs 2018, 16, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.J.; Huang, C.; Chen, Z. A review for the pharmacological effect of lycopene in central nervous system disorders. Biomed. Pharmacother. 2019, 111, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Mahmassani, H.A.; Switkowski, K.M.; Scott, T.M.; Johnson, E.J.; Rifas-Shiman, S.L.; Oken, E.; Jacques, P.F. Maternal Intake of Lutein and Zeaxanthin during Pregnancy Is Positively Associated with Offspring Verbal Intelligence and Behavior Regulation in Mid-Childhood in the Project Viva Cohort. J. Nutr. 2021, 151, 615–627. [Google Scholar] [CrossRef]
- Davinelli, S.; Ali, S.; Solfrizzi, V.; Scapagnini, G.; Corbi, G. Carotenoids and Cognitive Outcomes: A Meta-Analysis of Randomized Intervention Trials. Antioxidants 2021, 10, 223. [Google Scholar] [CrossRef]
- Zuniga, K.E.; Bishop, N.J.; Turner, A.S. Dietary lutein and zeaxanthin are associated with working memory in an older population. Public Health Nutr. 2021, 24, 1708–1715. [Google Scholar] [CrossRef]
- Zhang, X.S.; Lu, Y.; Li, W.; Tao, T.; Peng, L.; Wang, W.H.; Gao, S.; Liu, C.; Zhuang, Z.; Xia, D.Y.; et al. Astaxanthin ameliorates oxidative stress and neuronal apoptosis via SIRT1/NRF2/Prx2/ASK1/p38 after traumatic brain injury in mice. Br. J. Pharmacol. 2021, 178, 1114–1132. [Google Scholar] [CrossRef]
- Farouk, S.M.; Gad, F.A.; Almeer, R.; Abdel-Daim, M.M.; Emam, M.A. Exploring the possible neuroprotective and antioxidant potency of lycopene against acrylamide-induced neurotoxicity in rats’ brain. Biomed. Pharmacother. 2021, 138, 111458. [Google Scholar] [CrossRef]
- Shen, D.F.; Qi, H.P.; Ma, C.; Chang, M.X.; Zhang, W.N.; Song, R.R. Astaxanthin suppresses endoplasmic reticulum stress and protects against neuron damage in Parkinson’s disease by regulating miR7/SNCA axis. Neurosci. Res. 2021, 165, 51–60. [Google Scholar] [CrossRef]
- Rahman, S.O.; Panda, B.P.; Parvez, S.; Kaundal, M.; Hussain, S.; Akhtar, M.; Najmi, A.K. Neuroprotective role of astaxanthin in hippocampal insulin resistance induced by A beta peptides in animal model of Alzheimer’s disease. Biomed. Pharmacother. 2019, 110, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Previn, R.; Lu, L.; Liao, R.-F.; Jin, Y.; Wang, R.-K. Crocin, a natural product attenuates lipopolysaccharide-induced anxiety and depressive-like behaviors through suppressing NF-kB and NLRP3 signaling pathway. Brain Res. Bull. 2018, 142, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, R.; Gorusupudi, A.; Bernstein, P.S. The macular carotenoids: A biochemical overview. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158617. [Google Scholar] [CrossRef] [PubMed]
- Melendez-Martinez, A.J.; Mapelli-Brahm, P.; Stinco, C.M. The colourless carotenoids phytoene and phytofluene: From dietary sources to their usefulness for the functional foods and nutricosmetics industries. J. Food Compos. Anal. 2018, 67, 91–103. [Google Scholar] [CrossRef]
- Donoso, A.; Gonzalez-Duran, J.; Munoz, A.A.; Gonzalez, P.A.; Agurto-Munoz, C. Therapeutic uses of natural astaxanthin: An evidence-based review focused on human clinical trials. Pharmacol. Res. 2021, 166, 105479. [Google Scholar] [CrossRef]
- Ng, Q.X.; De Deyn, M.; Loke, W.; Foo, N.X.; Chan, H.W.; Yeo, W.S. Effects of Astaxanthin Supplementation on Skin Health: A Systematic Review of Clinical Studies. J. Diet. Suppl. 2021, 18, 169–182. [Google Scholar] [CrossRef]
- Guo, J.Y.; Lin, J.; Huang, Y.Q.; Talukder, M.; Yu, L.; Li, J.L. AQP2 as a target of lycopene protects against atrazine-induced renal ionic homeostasis disturbance. Food Funct. 2021, 12, 4855–4863. [Google Scholar] [CrossRef]
- Saini, A.; Panesar, P.S.; Bera, M.B. Valuation of Citrus reticulata (kinnow) peel for the extraction of lutein using ultrasonication technique. Biomass Convers. Biorefin. 2021, 11, 2157–2165. [Google Scholar] [CrossRef]
- Costanzo, G.; Iesce, M.R.; Naviglio, D.; Ciaravolo, M.; Vitale, E.; Arena, C. Comparative Studies on Different Citrus Cultivars: A Revaluation of Waste Mandarin Components. Antioxidants 2020, 9, 517. [Google Scholar] [CrossRef]
- Murador, D.C.; Salafia, F.; Zoccali, M.; Martins, P.L.G.; Ferreira, A.G.; Dugo, P.; Mondello, L.; De Rosso, V.V.; Giuffrida, D. Green Extraction Approaches for Carotenoids and Esters: Characterization of Native Composition from Orange Peel. Antioxidants 2019, 8, 613. [Google Scholar] [CrossRef] [Green Version]
- Scaglia, B.; D’Incecco, P.; Squillace, P.; Dell’Orto, M.; De Nisi, P.; Pellegrino, L.; Botto, A.; Cavicchi, C.; Adani, F. Development of a tomato pomace biorefinery based on a CO2-supercritical extraction process for the production of a high value lycopene product, bioenergy and digestate. J. Clean. Prod. 2020, 243, 118650. [Google Scholar] [CrossRef]
- Saini, R.K.; Ranjit, A.; Sharma, K.; Prasad, P.; Shang, X.; Gowda, K.G.M.; Keum, Y.-S. Bioactive Compounds of Citrus Fruits: A Review of Composition and Health Benefits of Carotenoids, Flavonoids, Limonoids, and Terpenes. Antioxidants 2022, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Long, X.Y.; Zeng, X.G.; Yan, H.T.; Xu, M.J.; Zeng, Q.T.; Xu, C.; Xu, Q.M.; Liang, Y.; Zhang, J. Flavonoids composition and antioxidant potential assessment of extracts from Gannanzao Navel Orange (Citrus sinensis Osbeck Cv. Gannanzao) peel. Nat. Prod. Res. 2021, 35, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Li, X.; Zhao, P.; Zhang, X.Q.; Qiao, O.; Huang, L.Q.; Guo, L.P.; Gao, W.Y. A review of chemical constituents and health-promoting effects of citrus peels. Food Chem. 2021, 365, 130585. [Google Scholar] [CrossRef]
- Gogoi, M.; Boruah, J.L.H.; Bora, P.K.; Das, D.J.; Famhawite, V.; Biswas, A.; Puro, N.; Kalita, J.; Haldar, S.; Baishya, R. Citrus macroptera induces apoptosis via death receptor and mitochondrial mediated pathway as prooxidant in human non-small cell lung cancer cells. Food Biosci. 2021, 43, 101293. [Google Scholar] [CrossRef]
- Abdelghffar, E.A.; El-Nashar, H.A.S.; Al-Mohammadi, A.G.A.; Eldahshan, O.A. Orange fruit (Citrus sinensis) peel extract attenuates chemotherapy-induced toxicity in male rats. Food Funct. 2021, 12, 9443–9455. [Google Scholar] [CrossRef]
- Mahato, N.; Sharma, K.; Sinha, M.; Cho, M.H. Citrus waste derived nutra-/pharmaceuticals for health benefits: Current trends and future perspectives. J. Funct. Foods 2018, 40, 307–316. [Google Scholar] [CrossRef]
- Sharma, M.; Hussain, S.; Shalima, T.; Aav, R.; Bhat, R. Valorization of seabuckthorn pomace to obtain bioactive carotenoids: An innovative approach of using green extraction techniques (ultrasonic and microwave-assisted extractions) synergized with green solvents (edible oils). Ind. Crop. Prod. 2022, 175, 114257. [Google Scholar] [CrossRef]
- Roy, V.C.; Ho, T.C.; Lee, H.J.; Park, J.S.; Nam, S.Y.; Lee, H.; Getachew, A.T.; Chun, B.S. Extraction of astaxanthin using ultrasound-assisted natural deep eutectic solvents from shrimp wastes and its application in bioactive films. J. Clean. Prod. 2021, 284, 125417. [Google Scholar] [CrossRef]
- Murador, D.C.; Mesquita, L.M.D.; Neves, B.V.; Braga, A.R.; Martins, P.L.G.; Zepka, L.Q.; De Rosso, V.V. Bioaccessibility and cellular uptake by Caco-2 cells of carotenoids and chlorophylls from orange peels: A comparison between conventional and ionic liquid mediated extractions. Food Chem. 2021, 339, 127818. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Factories 2018, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Monte, J.; Ribeiro, C.; Parreira, C.; Costa, L.; Brive, L.; Casal, S.; Brazinha, C.; Crespo, J.G. Biorefinery of Dunaliella salina: Sustainable recovery of carotenoids, polar lipids and glycerol. Bioresour. Technol. 2020, 297, 122509. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, O.; Tunay, D.; Ozkaya, B. Reuse of sea water reverse osmosis brine to produce Dunaliella salina based β-carotene as a valuable bioproduct: A circular bioeconomy perspective. J. Environ. Manag. 2022, 302, 114024. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, S.; Yusoff, M.M.; Rahim, M.H.A.; Maniam, G.P.; Govindan, N. The prospect of microalgal biodiesel using agro-industrial and industrial wastes in Malaysia. Renew. Sustain. Energy Rev. 2017, 72, 33–47. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.; Ghoshal, G. Optimization of carotenoids production by Rhodotorula mucilaginosa (MTCC-1403) using agro-industrial waste in bioreactor: A statistical approach. Biotechnol. Rep. 2020, 25, e00407. [Google Scholar] [CrossRef]
- Burton-Freeman, B.M.; Sesso, H.D. Whole Food versus Supplement: Comparing the Clinical Evidence of Tomato Intake and Lycopene Supplementation on Cardiovascular Risk Factors. Adv. Nutr. 2014, 5, 457–485. [Google Scholar] [CrossRef] [Green Version]
- Desmarchelier, C.; Landrier, J.-F.; Borel, P. Genetic factors involved in the bioavailability of tomato carotenoids. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 489–497. [Google Scholar] [CrossRef]
- Dewett, D.; Lam-Kamath, K.; Poupault, C.; Khurana, H.; Rister, J. Mechanisms of vitamin A metabolism and deficiency in the mammalian and fly visual system. Dev. Biol. 2021, 476, 68–78. [Google Scholar] [CrossRef]
- Khoo, K.S.; Lee, S.Y.; Ooi, C.W.; Fu, X.; Miao, X.; Ling, T.C.; Show, P.L. Recent advances in biorefinery of astaxanthin from Haematococcus pluvialis. Bioresour. Technol. 2019, 288, 121606. [Google Scholar] [CrossRef]


| Limitations | Possible Solution | Reference |
|---|---|---|
| Low contents of CARs in staple foods crops | Biofortification of crops | [89,90,91,92] |
| Solvent residues in extracted CARs | Green-solvent-assisted extraction | [199] |
| Water insolubility, low storage stability, bioaccessibility, bioavailability, and bioactivity of CARs | Encapsulation of CARs | [110,117,120,207] |
| Low bioaccessibility and bioavailability | Mechanical processing of foods | [104] |
| Food waste (e.g., fruit peel) contains more CARs than edible parts, or loss of CARs in food waste | Waste valorization for recovery of CARs | [199] |
| High cost of microbial CAR production | Use of a biorefinery and a circular bioeconomy approach | [202,204,205,206,210] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saini, R.K.; Prasad, P.; Lokesh, V.; Shang, X.; Shin, J.; Keum, Y.-S.; Lee, J.-H. Carotenoids: Dietary Sources, Extraction, Encapsulation, Bioavailability, and Health Benefits—A Review of Recent Advancements. Antioxidants 2022, 11, 795. https://doi.org/10.3390/antiox11040795
Saini RK, Prasad P, Lokesh V, Shang X, Shin J, Keum Y-S, Lee J-H. Carotenoids: Dietary Sources, Extraction, Encapsulation, Bioavailability, and Health Benefits—A Review of Recent Advancements. Antioxidants. 2022; 11(4):795. https://doi.org/10.3390/antiox11040795
Chicago/Turabian StyleSaini, Ramesh Kumar, Parchuri Prasad, Veeresh Lokesh, Xiaomin Shang, Juhyun Shin, Young-Soo Keum, and Ji-Ho Lee. 2022. "Carotenoids: Dietary Sources, Extraction, Encapsulation, Bioavailability, and Health Benefits—A Review of Recent Advancements" Antioxidants 11, no. 4: 795. https://doi.org/10.3390/antiox11040795
APA StyleSaini, R. K., Prasad, P., Lokesh, V., Shang, X., Shin, J., Keum, Y.-S., & Lee, J.-H. (2022). Carotenoids: Dietary Sources, Extraction, Encapsulation, Bioavailability, and Health Benefits—A Review of Recent Advancements. Antioxidants, 11(4), 795. https://doi.org/10.3390/antiox11040795








