Evaluating the Nutritional and Immune Potentiating Characteristics of Unfermented and Fermented Turmeric Camel Milk in Cyclophosphamide-Induced Immunosuppression in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ingredients
2.2. Preparation of Turmeric Camel Milk (TCM) and Fermented Turmeric Camel Milk (FTCM)
2.3. Determination of Total Phenolic Content (TPC), Total Carotenoids (TC), Total Flavonoids (TF), and Total Flavonols (TFL) in TCM and FTCM
2.4. Antioxidant Capacity Determination
2.5. Animals and Experimental Design
2.5.1. Measurement of Hematological Parameters
2.5.2. Immunoglobulin, Pro-Inflammation, and Anti-Inflammation Cytokines Assay
2.5.3. Oxidative Stress Biomarkers
2.6. Statistical Analysis
3. Results
3.1. Phytochemicals and Antioxidant Capacities of TCM and FTCM
3.2. Effect of TCM and FTCM Administration on Weight Gain %, Organs’ Weight, Food Intake, and Feed Efficiency in CYP-Induced Immunosuppression in Rats
3.3. Effect of TCM and FTCM Administration on Hematological Parameters in CYP-Induced Immunosuppression in Rats
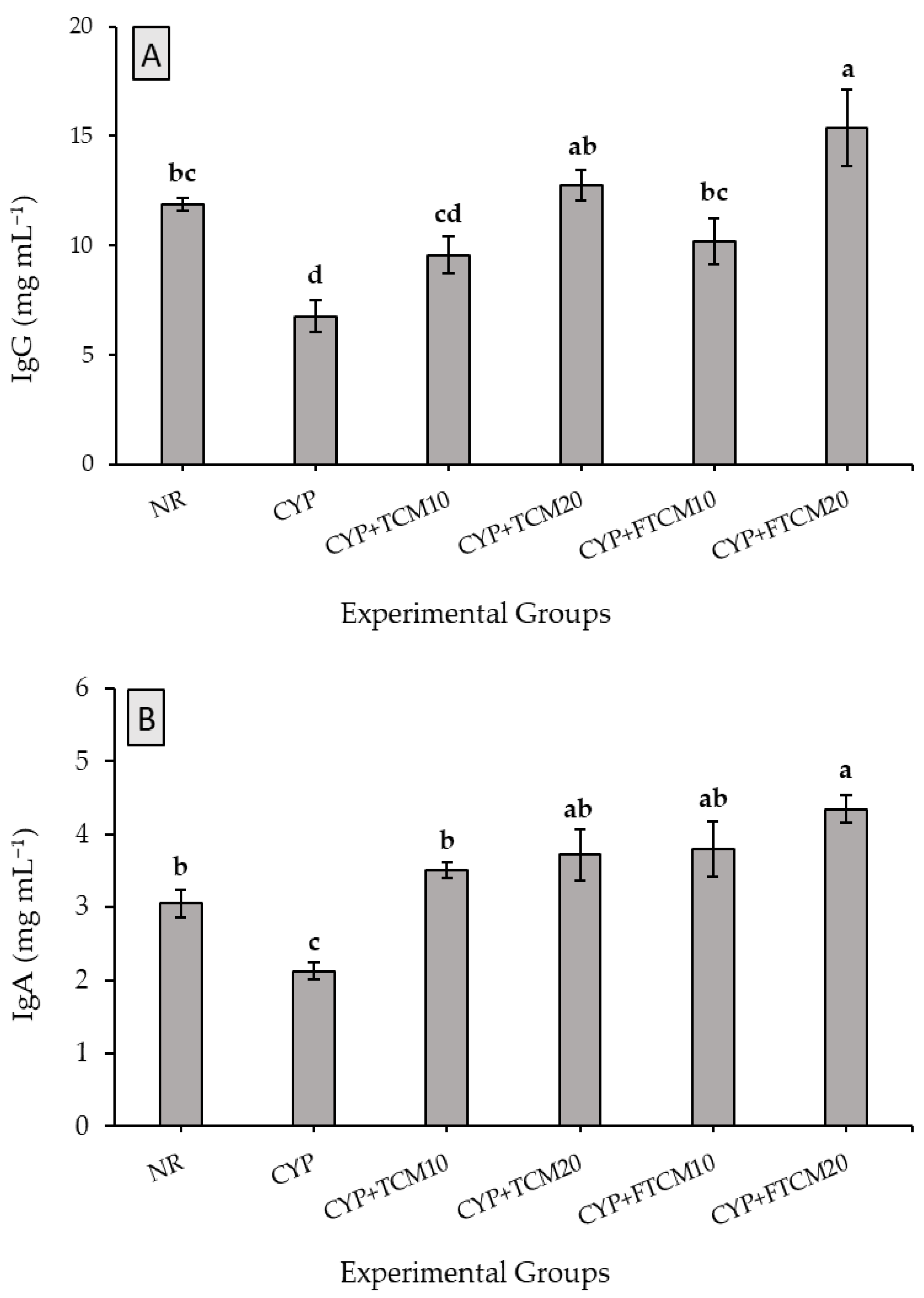
3.4. Effect of TCM and FTCM Administration on Immunoglobulins in CYP-Induced Immunosuppression in Rats
3.5. Effect of TCM and FTCM Administration on Pro-Inflammation and Anti-Inflammation Cytokines in CYP-Induced Immunosuppression in Rats
3.6. Antioxidant Biomarkers
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Roberfroid, M.B. Concepts and Strategy of Functional Food Science: The European Perspective. Am. J. Clin. Nutr. 2000, 71, 1660S–1664S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hilliam, M. Functional Food––How Big is The Market. World Food Ing. 2000, 12, 50–52. [Google Scholar]
- Bigliardi, B.; Galati, F. Innovation trends in the food industry: The case of functional foods. Trends Food Sci. Technol. 2013, 31, 118–129. [Google Scholar] [CrossRef]
- Vanneste, J.; Cornish, D.; Yu, J.; Voyle, M. P10c: A New Biological Control Agent for Control of Fire Blight Which Can be Sprayed or Distributed Using Honey Bees. Acta Hortic. 2002, 590, 231–235. [Google Scholar] [CrossRef]
- Al-Qabba, M.M.; El-Mowafy, M.A.; Althwab, S.A.; Alfheeaid, H.A.; Aljutaily, T.; Barakat, H. Phenolic Profile, Antioxidant Activity, and Ameliorating Efficacy of Chenopodium quinoa Sprouts against CCl4-Induced Oxidative Stress in Rats. Nutrients 2020, 12, 2904. [Google Scholar] [CrossRef]
- Aleid, I.S.; Alfheeaid, H.A.; Aljutaily, T.; Alhomaid, R.M.; Alharbi, H.F.; Althwab, S.A.; Abdel-Rahman, H.A.; Algeffari, M.A.; Barakat, H. Gastroprotective effects of spirulina platensis, golden kiwifruit flesh, and golden kiwifruit peel extracts individually or in combination against indomethacin-induced gastric ulcer in rats. Nutrients 2021, 13, 3499. [Google Scholar] [CrossRef]
- Guiné, R.; Lima, M.; Barroca, M. Role and health benefits of different functional food components. Millenium 2009, 37, 9–36. [Google Scholar]
- Schloerb, P.R. Immune-Enhancing Diets: Products, Components, and their Rationales/Discussion. JPEN J. Parenter Enteral. Nutr. 2001, 25, S3. [Google Scholar] [CrossRef]
- Ebaid, H.; Abdel-Salam, B.; Hassan, I.; Al-Tamimi, J.; Metwalli, A.; Alhazza, I. Camel milk peptide improves wound healing in diabetic rats by orchestrating the redox status and immune response. Lipids Health Dis. 2015, 14, 132. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.A. Immune potentiating and antitoxic effects of camel milk against cyclophosphamide-induced toxicity in BALB/C mice. Int. J. Health Sci. 2017, 11, 18. [Google Scholar]
- Zeng, Y.; Hu, X.; Yu, Z.; Wang, F.; Zhang, Z.; He, K.; Tian, H.; Yu, F. Immune Enhancement and Antioxidant Effects of Low Molecular-Weight Peptides Derived from Nibea Japonica Muscles on Immune-Deficient Mice Induced by Cyclophosphamide. Process Biochem. 2021, 102, 42–50. [Google Scholar] [CrossRef]
- Aljutaily, T.; Huarte, E.; Martinez-Monteagudo, S.; Gonzalez-Hernandez, J.L.; Rovai, M.; Sergeev, I.N. Probiotic-enriched milk and dairy products increase the gut microbiota diversity: A comparative study. Nut. Res. 2020, 82, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Bengmark, S.; Gil, A. Bioecological and Nutritional Control of Disease: Prebiotics, Probiotics and Synbiotics. Nutr. Hosp. 2006, 21, 72–84, 73. [Google Scholar] [PubMed]
- London, C. Functional foods that boost the immune system. Funct. Food Prod. Develop. 2010, 2, 295–321. [Google Scholar]
- Shobana, S.; Akhilender Naidu, K. Antioxidant Activity of Selected Indian Spices. Prostaglandins Leukot. Essent. Fat. Acids 2000, 62, 107–110. [Google Scholar] [CrossRef]
- van Vliet, M.J.; Harmsen, H.J.; de Bont, E.S.; Tissing, W.J. The role of intestinal microbiota in the development and severity of chemotherapy-induced mucositis. PLoS Pathog. 2010, 6, e1000879. [Google Scholar] [CrossRef] [Green Version]
- Berhe, T.; Seifu, E.; Ipsen, R.; Kurtu, M.Y.; Hansen, E.B. Processing Challenges and Opportunities of Camel Dairy Products. Int. J. Food Sci. 2017, 2017, 9061757. [Google Scholar] [CrossRef] [Green Version]
- Elagamy, E.I. Effect of Heat Treatment on Camel Milk Proteins with Respect to Antimicrobial Factors: A Comparison with Cows’ and Buffalo Milk Proteins. Food Chem. 2000, 68, 227–232. [Google Scholar] [CrossRef]
- Solanki, D.; Hati, S. Fermented camel milk: A Review on its Bio-Functional Properties. Emir. J. Food Agric. 2018, 30, 268–274. [Google Scholar]
- Giri, S.S.; Sukumaran, V.; Park, S.C. Effects of Bioactive Substance From Turmeric on Growth, Skin Mucosal Immunity and Antioxidant Factors in Common Carp, Cyprinus carpio. Fish Shellfish Immunol. 2019, 92, 612–620. [Google Scholar] [CrossRef]
- Abo Ghanima, M.M.; Elsadek, M.F.; Taha, A.E.; Abd El-Hack, M.E.; Alagawany, M.; Ahmed, B.M.; Elshafie, M.M.; El-Sabrout, K. Effect of Housing System and Rosemary and Cinnamon Essential Oils on Layers Performance, Egg Quality, Haematological Traits, Blood Chemistry, Immunity, and Antioxidant. Animals 2020, 10, 245. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Varela, S.; Gonzalez-Gross, M.; Marcos, A. Functional Foods and The Immune System: A Review. Eur. J. Clin. Nutr. 2002, 56, S29–S33. [Google Scholar] [CrossRef] [PubMed]
- Boskabady, M.H.; Amin, F.; Shakeri, F. The Effect of Curcuma longa on Inflammatory Mediators and Immunological, Oxidant, and Antioxidant Biomarkers in Asthmatic Rats. Evid. Based Complement. Alternat. Med. 2021, 2021, 4234326. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.; Yadav, K.; Khadka, K.B. Study of antioxidant, ANTIBACTERIAL and Anti-inflammatory Activity of Cinnamon (Cinamomum tamala), GINGER (Zingiber officinale) and Turmeric (Curcuma longa). Am. J. Life Sci. 2013, 1, 273–277. [Google Scholar] [CrossRef] [Green Version]
- Jurenka, J.S. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: A review of preclinical and clinical research. Altern. Med. Rev. 2009, 14, 141–153. [Google Scholar] [PubMed]
- Salehi, B.; Stojanović-Radić, Z.; Matejić, J.; Sharifi-Rad, M.; Anil Kumar, N.V.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of curcumin: A review of clinical trials. Eur. J. Med. Chem. 2019, 163, 527–545. [Google Scholar] [CrossRef]
- Abeysekera, W.; Arachchige, S.P.G.; Abeysekera, W.; Ratnasooriya, W.D.; Medawatta, H. Antioxidant and glycemic regulatory properties potential of different maturity stages of leaf of ceylon cinnamon (Cinnamomum zeylanicum Blume) in vitro. Evid. Based Complement. Alternat. Med. 2019, 2019, 2693795. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Cai, D.; Zhang, L.; Tang, W.; Yan, R.; Guo, H.; Chen, X. Identification of hydrolyzable tannins (punicalagin, punicalin and geraniin) as novel inhibitors of hepatitis B virus covalently closed circular DNA. Antivir. Res. 2016, 134, 97–107. [Google Scholar] [CrossRef] [Green Version]
- Idowu-Adebayo, F.; Fogliano, V.; Linnemann, A. Turmeric-Fortified Cow and Soya Milk: Golden Milk as a Street Food to Support Consumer Health. Foods 2022, 11, 558. [Google Scholar] [CrossRef]
- Wang, K.; Conlon, M.; Ren, W.; Chen, B.B.; Bączek, T. Natural Products as Targeted Modulators of the Immune System. J. Immunol. Res. 2018, 2018, 7862782. [Google Scholar] [CrossRef]
- Yu, F.; He, K.; Dong, X.; Zhang, Z.; Wang, F.; Tang, Y.; Chen, Y.; Ding, G. Immunomodulatory Activity of Low Molecular-Weight Peptides from Nibea japonica Skin in Cyclophosphamide-Induced Immunosuppressed Mice. J. Funct. Foods 2020, 68, 404. [Google Scholar] [CrossRef]
- Feng, H.; Fan, J.; Lin, L.; Liu, Y.; Chai, D.; Yang, J. Immunomodulatory Effects of Phosphorylated Radix Cyathulae officinalis Polysaccharides in Immunosuppressed Mice. Molecules 2019, 24, 4150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shakeri, F.; Boskabady, M.H. Anti-inflammatory, Antioxidant, and Immunomodulatory Effects Of Curcumin in Ovalbumin-Sensitized Rat. Biofactors 2017, 43, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Zhang, R.; Liu, Y.; Xiao, J.; Liu, L.; Wei, Z.; Yi, Y.; Zhang, M.; Liu, D. Dietary litchi pulp polysaccharides could enhance immunomodulatory and antioxidant effects in mice. Int. J. Biol. Macromol. 2016, 92, 1067–1073. [Google Scholar] [CrossRef]
- Shirani, K.; Hassani, F.V.; Razavi-Azarkhiavi, K.; Heidari, S.; Zanjani, B.R.; Karimi, G. Phytotrapy of cyclophosphamide-induced immunosuppression. Environ. Toxicol. Pharmacol. 2015, 39, 1262–1275. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, S.; Li, C.; Shao, Y.; Chen, A. Polysaccharides from Spores of Cordyceps cicadae Protect against Cyclophosphamide-Induced Immunosuppression and Oxidative Stress in Mice. Foods 2022, 11, 515. [Google Scholar] [CrossRef]
- Noh, E.M.; Kim, J.M.; Lee, H.Y.; Song, H.K.; Joung, S.O.; Yang, H.J.; Kim, M.J.; Kim, K.S.; Lee, Y.R. Immuno-enhancement effects of Platycodon grandiflorum extracts in splenocytes and a cyclophosphamide-induced immunosuppressed rat model. BMC Complement. Altern. Med. 2019, 19, 322. [Google Scholar] [CrossRef] [Green Version]
- Yawadio Nsimba, R.; Kikuzaki, H.; Konishi, Y. Antioxidant activity of various extracts and fractions of Chenopodium quinoa and Amaranthus spp. seeds. Food Chem. 2008, 106, 760–766. [Google Scholar] [CrossRef]
- Yuan, G.F.; Sun, J.; Yuan, Q.; Wang, Q.M. Effects of different cooking methods on health-promoting compounds of broccoli. J. Zhejiang Univ. Sci. B 2009, 10, 580–588. [Google Scholar] [CrossRef] [Green Version]
- Mohdaly, A.A.A.; Hassanien, M.F.R.; Mahmoud, A.; Sarhan, M.A.; Smetanska, I. Phenolics Extracted from Potato, Sugar Beet, and Sesame Processing By-Products. Int. J. Food Prop. 2012, 16, 1148–1168. [Google Scholar] [CrossRef]
- Zhao, H.; Dong, J.; Lu, J.; Chen, J.; Li, Y.; Shan, L.; Lin, Y.; Fan, W.; Gu, G. Effects of Extraction Solvent Mixtures on Antioxidant Activity Evaluation and Their Extraction Capacity and Selectivity for Free Phenolic Compounds in Barley (Hordeum vulgare L.). J. Agri. Food Chem. 2006, 54, 7277–7286. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose Translation from Animal to Human Studies Revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, J.-H.; Yun, S.-I.; Park, H.-O. Effects of Lactobacillus gasseri BNR17 on body weight and adipose tissue mass in diet-induced overweight rats. J. Microbiol. 2010, 48, 712–714. [Google Scholar] [CrossRef]
- Lee, H.Y.; Park, Y.M.; Lee, Y.H.; Kang, Y.G.; Lee, H.M.; Park, D.S.; Yang, H.J.; Kim, M.J.; Lee, Y.-R. Immunostimulatory Effect of Zanthoxylum schinifolium-Based Complex Oil Prepared by Supercritical Fluid Extraction in Splenocytes and Cyclophosphamide-Induced Immunosuppressed Rats. Evid. Based Complement. Alternat. Med. 2018, 2018, 8107326. [Google Scholar] [CrossRef] [PubMed]
- Beutler, E. Improved Method for the Determination of Blood Glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide Dismutases: I. Occurrence in Higher Plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Aebi, H. [13] Catalase in vitro. Meth. Enzymol. 1984, 105, 121–126. [Google Scholar]
- Steel, R.G. Pinciples and Procedures of Statistics a Biometrical Approach, 3rd ed.; McGraw-Hill: Boston, MA, USA, 1997. [Google Scholar]
- Hsu, E. Immune System Receptors in Vertebrates: Immunoglobulins. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Millán, O.; Brunet, M. Cytokine-based immune monitoring. Clin. Biochem. 2016, 49, 338–346. [Google Scholar] [CrossRef]
- Tosun, M.; Ercisli, S.; Sengul, M.; Ozer, H.; Polat, T.; Ozturk, E. Antioxidant properties and total phenolic content of eight Salvia species from Turkey. Biol. Res 2009, 42, 175–181. [Google Scholar] [CrossRef]
- Ollanketo, M.; Peltoketo, A.; Hartonen, K.; Hiltunen, R.; Riekkola, M.-L. Extraction of Sage (Salvia officinalis L.) by Pressurized Hot Water and Conventional Methods: Antioxidant Activity of the Extracts. Eur. Food Res. Technol. 2002, 215, 158–163. [Google Scholar] [CrossRef]
- Farhat, M.B.; Chaouch-Hamada, R.; Sotomayor, J.A.; Landoulsi, A.; Jordán, M.J. Antioxidant potential of Salvia officinalis L. residues as affected by the harvesting time. Ind. Crops Prod. 2014, 54, 78–85. [Google Scholar] [CrossRef]
- Roby, M.H.H.; Sarhan, M.A.; Selim, K.A.-H.; Khalel, K.I. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Ind. Crops Prod. 2013, 43, 827–831. [Google Scholar] [CrossRef]
- Ebrahimzadeh, M.A.; Nabavi, S.M.; Nabavi, S.F.; Bahramian, F.; Bekhradnia, A.R. Antioxidant and free radical scavenging activity of H. officinalis L. var. angustifolius, V. odorata, B. hyrcana and C. speciosum. Pak. J. Pharm. Sci. 2010, 23, 29–34. [Google Scholar]
- Hussain, A.; Shadma, W.; Maksood, A.; Ansari, S.H. Protective effects of Picrorhiza kurroa on cyclophosphamide-induced immunosuppression in mice. Pharmacogn. Res. 2013, 5, 30–35. [Google Scholar] [CrossRef] [Green Version]
- Yu, F.; Zhang, Z.; Ye, S.; Hong, X.; Jin, H.; Huang, F.; Yang, Z.; Tang, Y.; Chen, Y.; Ding, G. Immunoenhancement effects of pentadecapeptide derived from Cyclina sinensis on immune-deficient mice induced by Cyclophosphamide. J. Funct. Foods 2019, 60, 103408. [Google Scholar] [CrossRef]
- Zhu, G.; Luo, J.; Du, H.; Jiang, Y.; Tu, Y.; Yao, Y.; Xu, M. Ovotransferrin enhances intestinal immune response in cyclophosphamide-induced immunosuppressed mice. Int. J. Biol. Macromol. 2018, 120, 1–9. [Google Scholar] [CrossRef]
- Meulenbroek, A. Human IgG Subclasses: Useful Diagnostic Markers for Immunocompetence, 3rd ed.; Sanquin: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Mantis, N.J.; Rol, N.; Corthésy, B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011, 4, 603–611. [Google Scholar] [CrossRef]
- Heyman, B.; Shulman, M.J. Structure, Function, and Production of Immunoglobulin M (IgM). In Encyclopedia of Immunobiology; Ratcliffe, M.J.H., Ed.; Academic Press: Oxford, UK, 2016; pp. 1–14. [Google Scholar]
- Mottet, M.; Goffinet, L.; Beckers, A.; Bodart, G.; Morrhaye, G.; Kermani, H.; Renard, C.; Martens, H.; Geenen, V. The Role of the Thymus in the Integrated Evolution of the Recombinase-Dependent Adaptive Immune Response and the Neuroendocrine System. Neuroimmunomodulation 2011, 18, 314–319. [Google Scholar] [CrossRef]
- Valdez, Y.; Brown, E.M.; Finlay, B.B. Influence of the microbiota on vaccine effectiveness. Trends Immunol. 2014, 35, 526–537. [Google Scholar] [CrossRef]
- Brocker, C.; Thompson, D.; Matsumoto, A.; Nebert, D.W.; Vasiliou, V. Evolutionary divergence and functions of the human interleukin (IL) gene family. Hum. Genom. 2010, 5, 30. [Google Scholar] [CrossRef] [PubMed]
- van der Poll, T.; Opal, S.M. Host–pathogen interactions in sepsis. Lancet Infect. Dis. 2008, 8, 32–43. [Google Scholar] [CrossRef]
- Huang, M.; Yang, D.; Xiang, M.; Wang, J. Role of interleukin-6 in regulation of immune responses to remodeling after myocardial infarction. Heart Fail. Rev. 2015, 20, 25–38. [Google Scholar] [CrossRef]
- Dillinger, B.; Ahmadi-Erber, S.; Lau, M.; Hoelzl, M.A.; Erhart, F.; Juergens, B.; Fuchs, D.; Heitger, A.; Ladisch, S.; Dohnal, A.M. IFN-γ and tumor gangliosides: Implications for the tumor microenvironment. Cell. Immunol. 2018, 325, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.; Nichol, A. Inflammation, Immunity and Allergy. Anaesth. Intensive Care 2021, 22, 488–493. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Huang, X.; Nie, S.; Cai, H.; Zhang, G.; Cui, S.W.; Xie, M.; Phillips, G.O. Study on Dendrobium officinale O-acetyl-glucomannan (Dendronan®): Part VI. Protective Effects Against Oxidative Stress in Immunosuppressed Mice. Food Res. Int. 2015, 72, 168–173. [Google Scholar] [CrossRef]
- Alqahtani, S.; Mahmoud, A.M. Gamma-Glutamylcysteine Ethyl Ester Protects against Cyclophosphamide-Induced Liver Injury and Hematologic Alterations via Upregulation of PPARγ and Attenuation of Oxidative Stress, Inflammation, and Apoptosis. Oxidative Med. Cell. Longev. 2016, 2016, 4016209. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Gao, D.-X.; Song, J.-J.; Ren, F.-Z.; Mao, X.-Y. Casein glycomacropeptide hydrolysate exerts cytoprotection against H2O2-induced oxidative stress in RAW 264.7 macrophages via ROS-dependent heme oxygenase-1 expression. RSC Adv. 2015, 5, 4511–4523. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free Radicals, Metals and Antioxidants in Oxidative Stress-Induced Cancer. Chem.-Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Dringen, R.; Pawlowski, P.G.; Hirrlinger, J. Peroxide Detoxification by Brain Cells. J. Neurosci. Res. 2005, 79, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.-C.; Anderson, A.; Coker, J.; Ondrus, M. Characterization of Lipid Oxidation Products in Quinoa (Chenopodium quinoa). Food Chem. 2007, 101, 185–192. [Google Scholar] [CrossRef]
- Puertollano, M.A.; Puertollano, E.; Alvarez de Cienfuegos, G.; de Pablo, M.A. Dietary antioxidants: Immunity and host defense. Curr. Top. Med. Chem. 2011, 11, 1752–1766. [Google Scholar] [CrossRef] [PubMed]

| Item | TCM | FTCM |
|---|---|---|
| TPC (mg GAE 100 mL−1) | 167.25 ± 3.15 a | 171.21 ± 4.19 a |
| TC (µg 100 mL−1) | 11.28 ± 1.15 a | 11.98 ± 1.93 a |
| TF (mg QE 100 mL−1) | 15.85 ± 1.17 a | 15.98 ± 1.38 a |
| TFL (mg QE 100 mL−1) | 9.25 ± 0.67 a | 10.02 ± 1.12 a |
| DPPH (µmol of TE 100 mL−1) | 215.68 ± 6.35 a | 208.91 ± 7.09 a |
| ABTS (µmol of TE 100 mL−1) | 298.27 ± 9.12 a | 301.28 ± 6.28 a |
| CA (mg 100 mL−1) | 89.37 ± 3.27 a | 92.09 ± 2.24 a |
| Items | Experimental Groups * | |||||
|---|---|---|---|---|---|---|
| NR | CYP | CYP + TCM10 | CYP + TCM20 | CYP + FTCM10 | CYP + FTCM20 | |
| Initial BW (g) | 195.33 ± 4.48 | 191.33 ± 3.76 | 181.83 ± 8.99 | 187.00 ± 7.80 | 182.17 ± 4.99 | 180.17 ± 4.41 |
| Final BW (g) | 224.83 ± 3.94 | 182.67 ± 6.19 | 192.00 ± 7.45 | 201.17 ± 7.98 | 193.67 ± 4.74 | 195.83 ± 3.46 |
| BW gain % | 15.19 a ± 0.91 | −4.62 ± 1.94 c | 6.06 ± 2.11 b | 7.83 ± 1.26 b | 6.43 ± 1.80 b | 8.83 ± 1.63 b |
| Liver weight % | 3.12 ± 0.12 a | 2.43 ± 0.23 c | 2.91 ± 0.21 a,b | 3.17 ± 0.26 a | 2.89 ± 0.19 a,b | 3.29 ± 0.26 a |
| Kidneys weight % | 0.84 ± 0.04 a | 0.57 ± 0.00 c | 0.59 ± 0.02 c | 0.68 ± 0.03 b,c | 0.68 ± 0.02 b,c | 0.74 ± 0.05 a,b |
| Spleen weight % | 0.68 ± 0.02 a | 0.36 ± 0.11 b | 0.37 ± 0.06 b | 0.57 ± 0.09 a | 0.37 ± 0.05 b | 0.67 ± 0.09 a |
| Food intake (g day−1) # | 17.28 ± 1.54 a | 11.01 ± 1.15 d | 13.2 ± 1.48 c | 16.85 ± 1.58 b,c | 14.85 ± 1.67 b | 16.91 ± 1.75 c |
| Feed efficiency ## | 0.165 ± 0.017 a | 0.085 ± 0.006 e | 0.125 ± 0.019 d | 0.156 ± 0.016 b,c | 0.135 ± 0.014 a | 0.161 ± 0.011 b,c |
| Hematological Parameters | Experimental Groups * | |||||
|---|---|---|---|---|---|---|
| NR | CYP | CYP + TCM10 | CYP + TCM20 | CYP + FTCM10 | CYP + FTCM20 | |
| WBCs [109 L−1] | 8.82 ± 0.07 b | 5.90 ± 0.17 e | 7.50 ± 0.10 d | 9.43 ± 0.20 a | 8.15 ± 0.15 c | 9.57 ± 0.18 a |
| Lymphocytes [109 L−1] | 7.47 ± 0.07 b | 4.82 ± 0.20 e | 6.50 ± 0.14 d | 7.84 ± 0.07 a | 7.01 ± 0.09 c | 7.94 ± 0.12 a |
| Neutrophils [109 L−1] | 1.03 ± 0.03 b | 0.95 ± 0.07 b,c | 0.80 ± 0.04 c | 1.39 ± 0.13 a | 0.89 ± 0.04 b,c | 1.49 ± 0.04 a |
| Cytokines | Experimental Groups * | |||||
|---|---|---|---|---|---|---|
| NR | CYP | CYP + TCM10 | CYP + TCM20 | CYP + FTCM10 | CYP + FTCM20 | |
| IL-1 β (ng mL−1) | 58.73 ± 4.62 b,c | 33.60 ± 4.78 d | 49.78 ± 4.32 c | 69.70 ± 6.53 b | 66.35 ± 3.44 b | 93.63 ± 2.27 a |
| IL-6 (ng mL−1) | 196.00 ± 3.58 c | 126.67 ± 6.67 d | 212.67 ± 9.09 b,c | 244.00 ± 4.38 a,b | 223.33 ± 5.70 b,c | 268.00 ± 22.41 a |
| IL-10 (ng mL−1) | 33.72 ± 3.72 a,b | 19.94 ± 2.60 c | 27.41 ± 2.69 b,c | 38.89 ± 2.32 a | 33.89 ± 3.55 a,b | 42.64 ± 4.14 a |
| IL-13 (ng mL−1) | 79.27 ± 1.82 c | 52.48 ± 0.47 e | 66.07 ± 1.48 d | 91.98 ± 1.35 b | 78.17 ± 1.04 c | 95.93 ± 1.61 a |
| IL-TNF-α (ng mL−1) | 95.93 ± 1.61 a | 12.41 ± 1.03 d | 15.91 ± 1.97 c,d | 15.91 ± 1.97 c,d | 21.12 ± 2.56 b,c | 29.65 ± 2.83 a |
| Experimental Groups * | Antioxidant Biomarkers | |||
|---|---|---|---|---|
| GSH (µg dL−1) | CAT (U L−1) | SOD (U L−1) | MDA (µ mol mL−1) | |
| NR | 75.02 ± 7.43 a,b | 85.44 ± 11.96 b | 117.08 ± 3.66 b | 25.50 ± 2.25 b |
| CYP | 49.40 ± 4.14 d | 59.29 ± 6.20 d | 72.98 ± 0.94 d | 47.36 ± 4.04 a |
| CYP + TCM10 | 60.49 ± 2.32 c | 75.32 ± 9.73 b,c | 98.24 ± 1.27 c | 29.46 ± 3.26 b |
| CYP + TCM20 | 74.82 ± 10.18 a,b | 97.38 ± 9.13 b | 118.96 ± 1.77 b | 23.57 ± 3.09 b,c |
| CYP + FTCM10 | 71.64 ± 6.98 a,b | 72.22 ± 9.12 b,c | 118.70 ± 1.46 b | 28.60 ± 2.85 b |
| CYP + FTCM20 | 86.32 ± 8.98 a | 112.62 ± 11.56 a | 137.30 ± 1.67 a | 22.32 ± 3.56 b,c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aljutaily, T. Evaluating the Nutritional and Immune Potentiating Characteristics of Unfermented and Fermented Turmeric Camel Milk in Cyclophosphamide-Induced Immunosuppression in Rats. Antioxidants 2022, 11, 792. https://doi.org/10.3390/antiox11040792
Aljutaily T. Evaluating the Nutritional and Immune Potentiating Characteristics of Unfermented and Fermented Turmeric Camel Milk in Cyclophosphamide-Induced Immunosuppression in Rats. Antioxidants. 2022; 11(4):792. https://doi.org/10.3390/antiox11040792
Chicago/Turabian StyleAljutaily, Thamer. 2022. "Evaluating the Nutritional and Immune Potentiating Characteristics of Unfermented and Fermented Turmeric Camel Milk in Cyclophosphamide-Induced Immunosuppression in Rats" Antioxidants 11, no. 4: 792. https://doi.org/10.3390/antiox11040792
APA StyleAljutaily, T. (2022). Evaluating the Nutritional and Immune Potentiating Characteristics of Unfermented and Fermented Turmeric Camel Milk in Cyclophosphamide-Induced Immunosuppression in Rats. Antioxidants, 11(4), 792. https://doi.org/10.3390/antiox11040792






