Influence of Diabetes-Induced Glycation and Oxidative Stress on the Human Rotator Cuff
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Protocol
2.3. Preparation of Human Rotator-Cuff-Derived Cells and Tissue
2.4. Cell Proliferation Assay
2.5. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR) Analysis
2.6. Detection of ROS
2.7. Cell Apoptosis Analysis
2.8. Rotator Cuff Histology and Immunohistochemistry
2.9. Statistical Analysis
3. Results
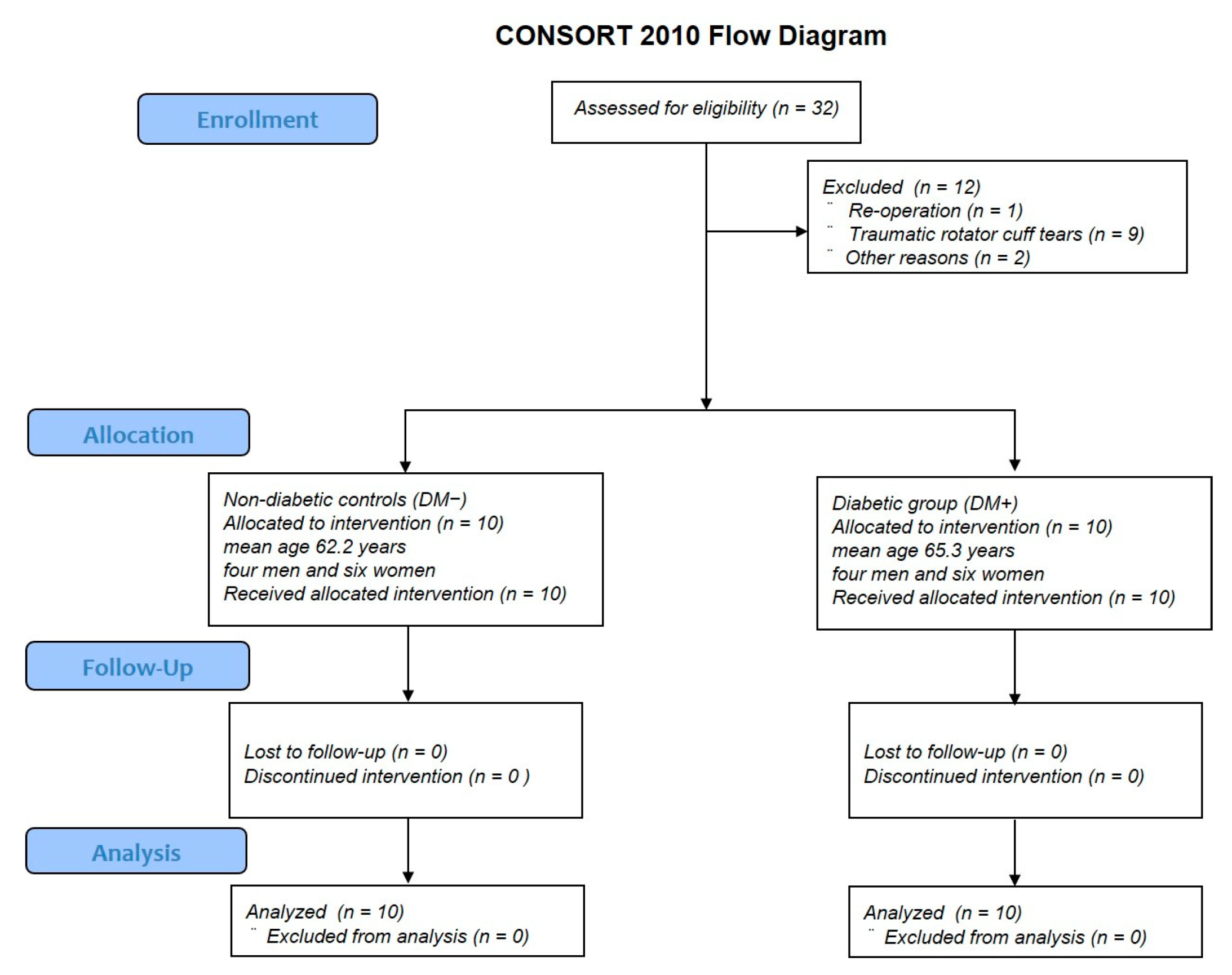
3.1. Patient Background
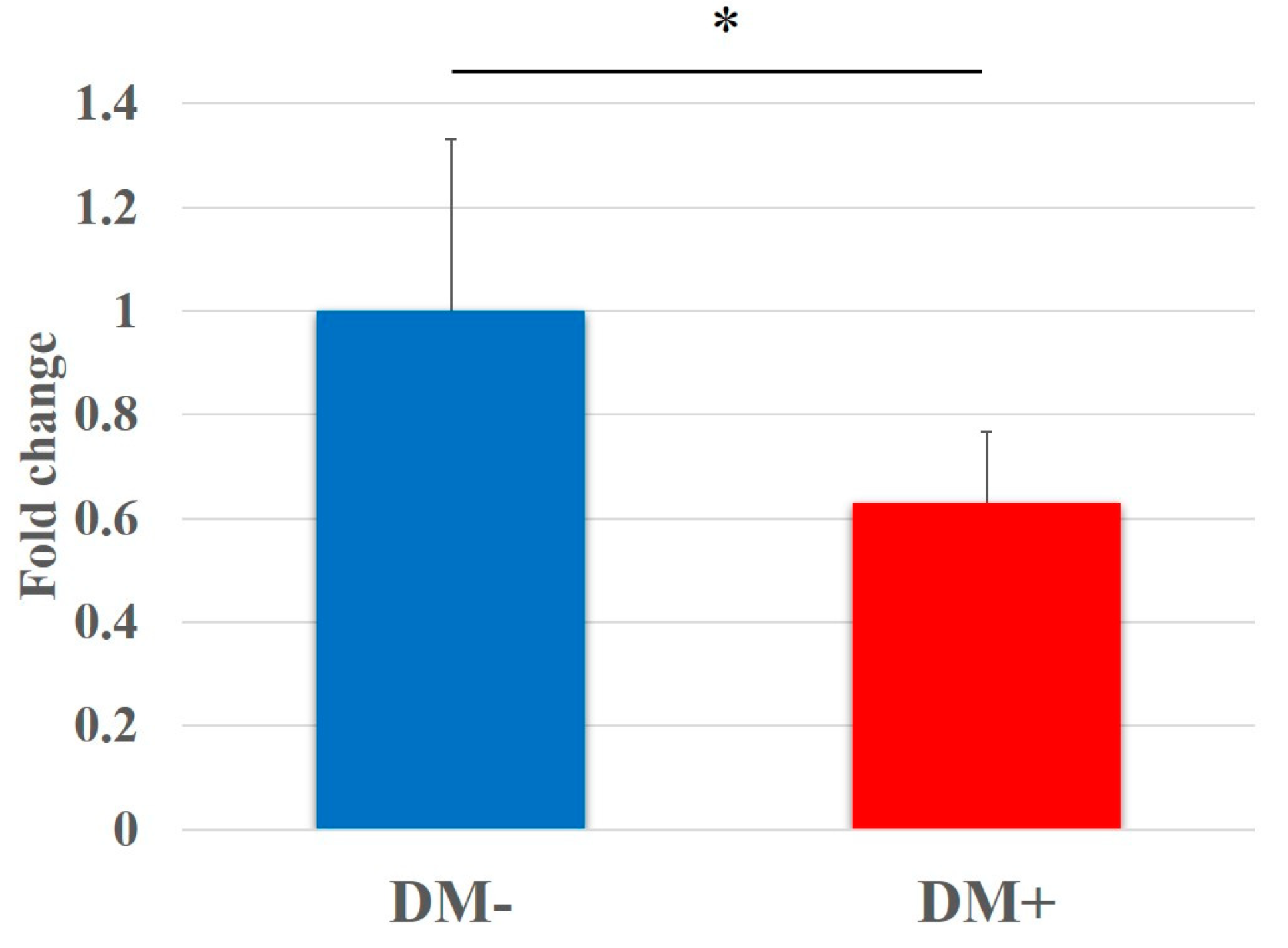
3.2. Cell Proliferation Assay
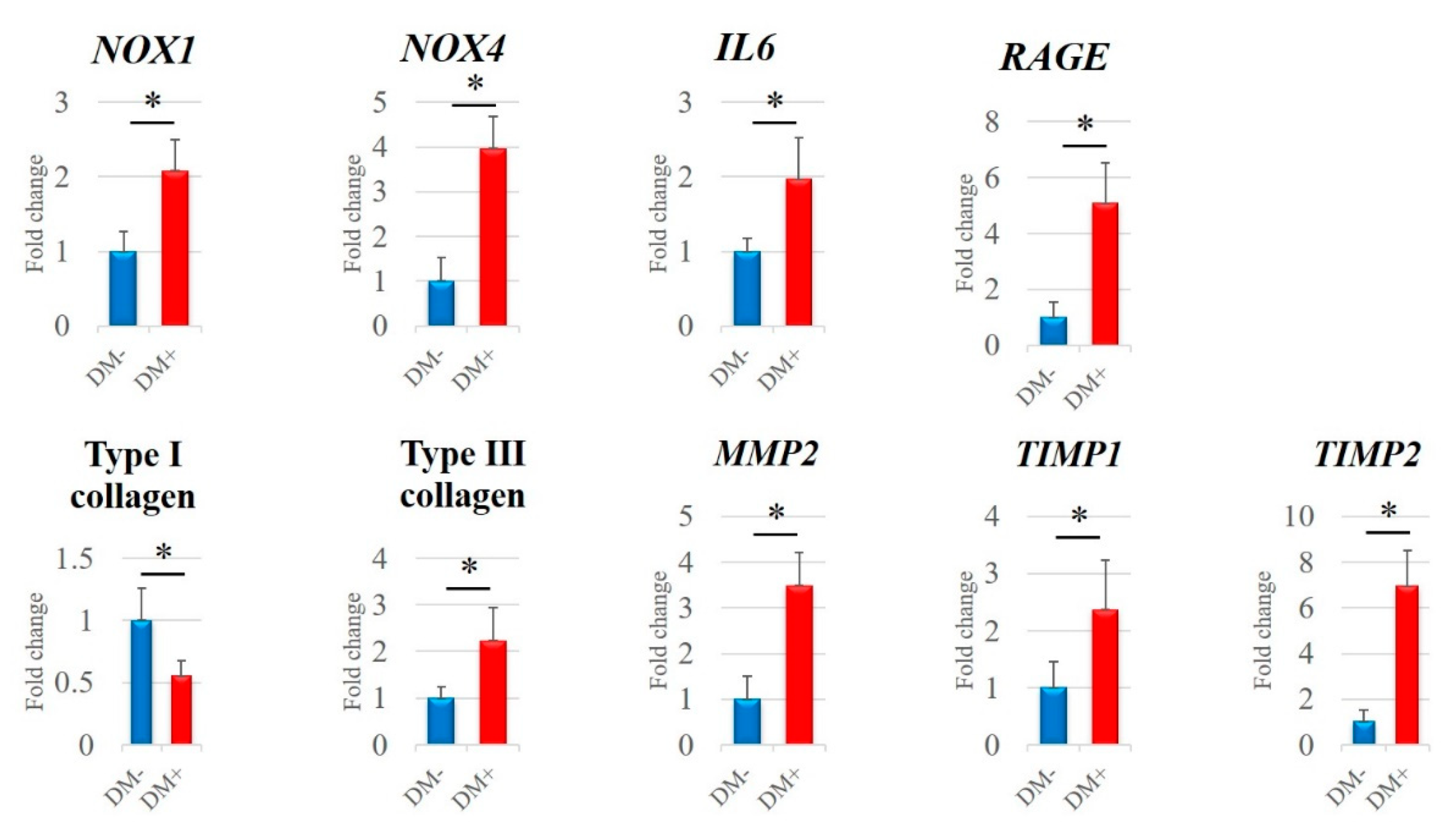
3.3. qRT-PCR Analysis
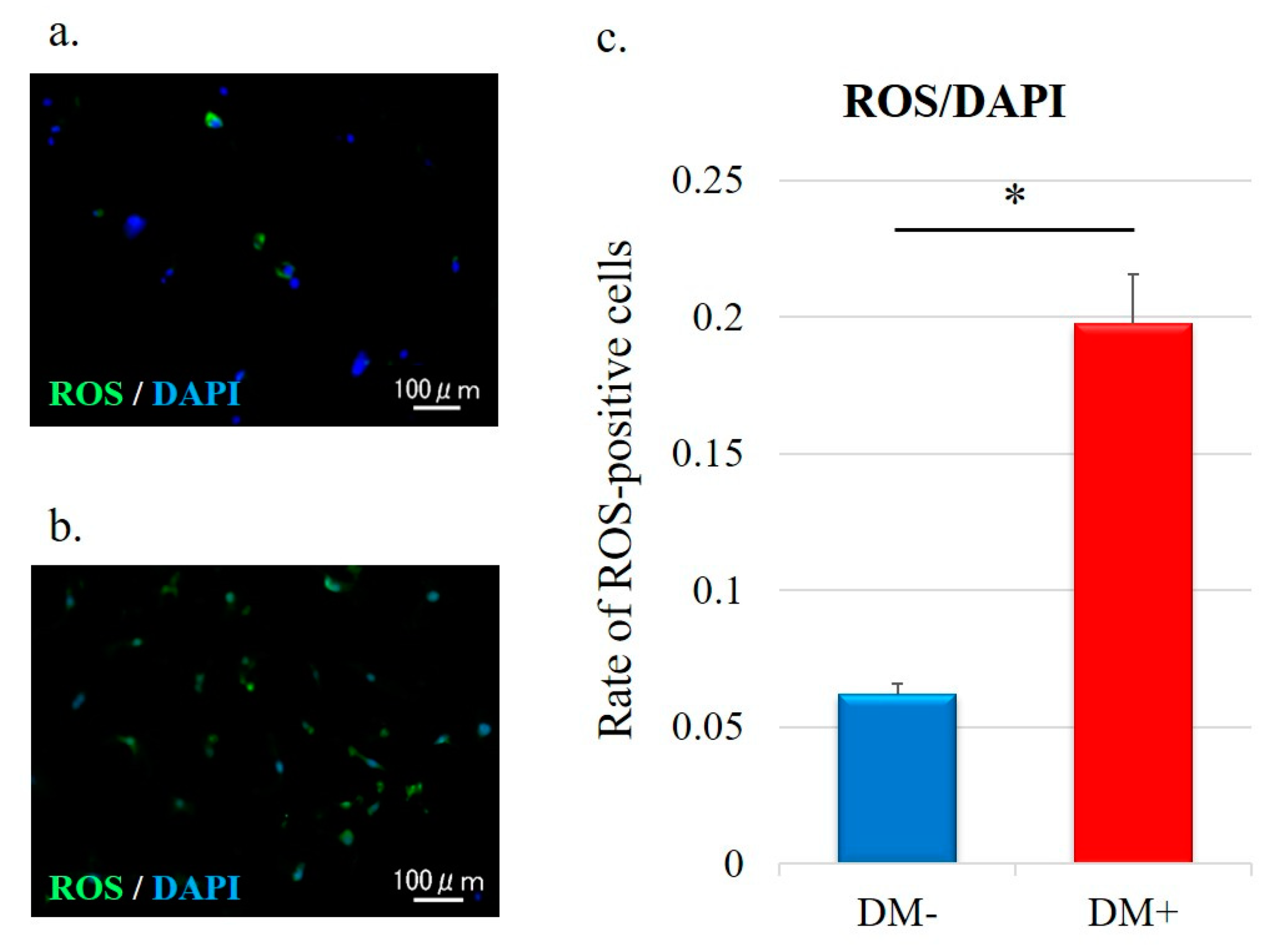
3.4. Detection of ROS
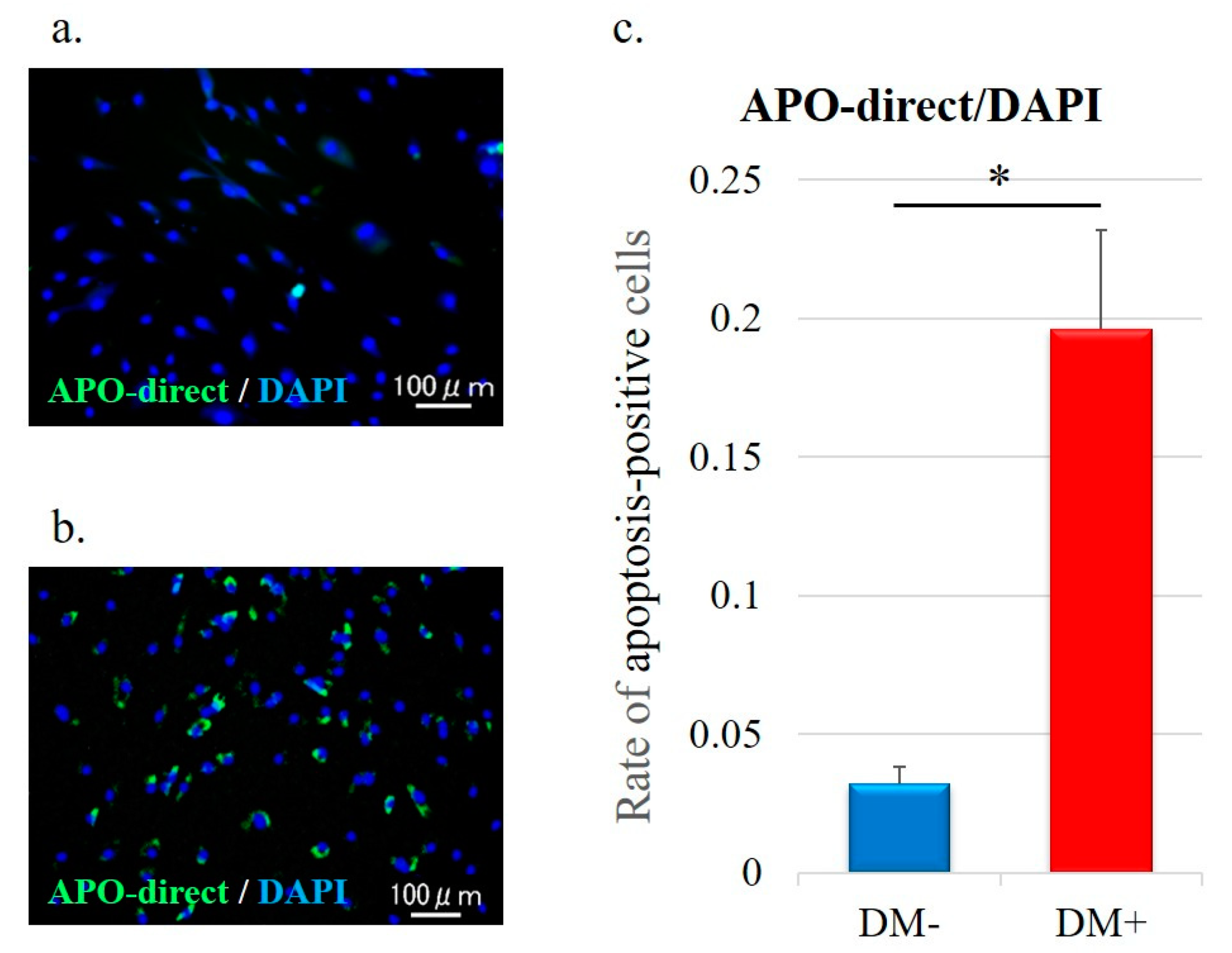
3.5. Cell Apoptosis Analysis
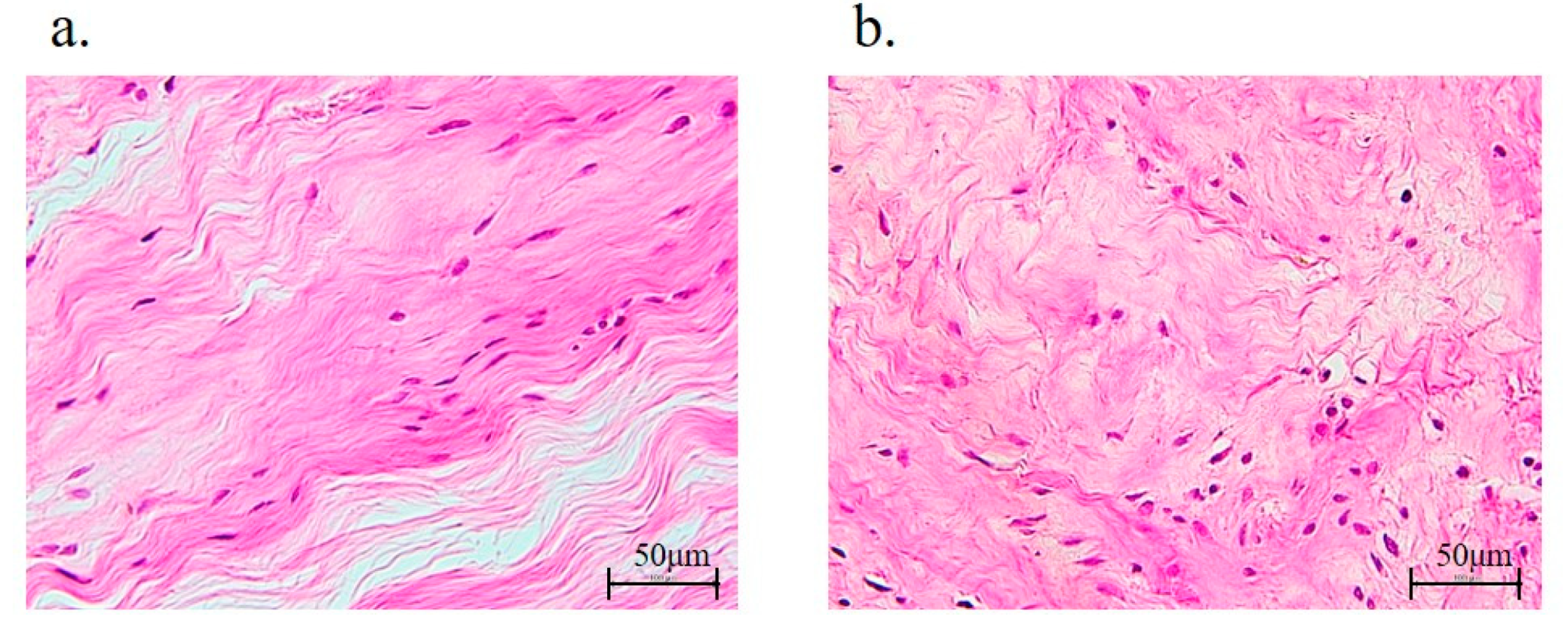
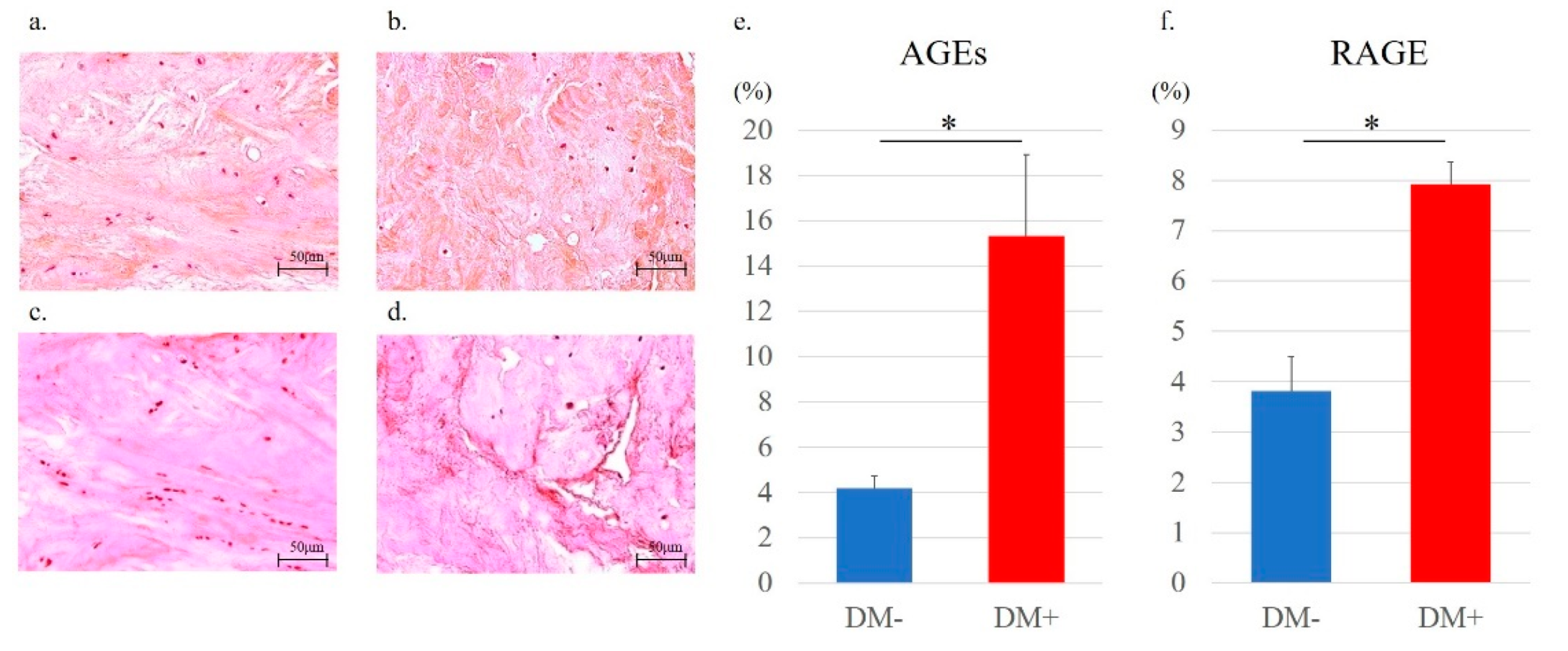
3.6. Rotator Cuff Histology and Immunohistochemistry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hashimoto, T.; Nobuhara, K.; Hamada, T. Pathologic evidence of degeneration as a primary cause of rotator cuff tear. Clin. Orthop. Relat. Res. 2003, 415, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Longo, U.G.; Franceschi, F.; Ruzzini, L.; Spiezia, F.; Maffulli, N.; Denaro, V. Higher fasting plasma glucose levels within the normoglycaemic range and rotator cuff tears. Br. J. Sports Med. 2009, 43, 284–287. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. (2) Classification and diagnosis of diabetes. Diabetes Care 2015, 38 (Suppl. 1), S8–S16. [Google Scholar] [CrossRef] [PubMed]
- Kisic, B.; Miric, D.; Dragojevic, I.; Rasic, J.; Popovic, L. Role of myeloperoxidase in patients with chronic kidney disease. Oxid. Med. Cell. Longev. 2016, 2016, 1069743. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Inui, A.; Mifune, Y.; Sakata, R.; Muto, T.; Harada, Y.; Takase, F.; Kataoka, T.; Kokubu, T.; Kuroda, R. The effects of high glucose condition on rat tenocytes in vitro and rat Achilles tendon in vivo. Bone Jt. Res. 2018, 7, 362–372. [Google Scholar] [CrossRef]
- Cai, H.; Griendling, K.K.; Harrison, D.G. The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol. Sci. 2003, 24, 471–478. [Google Scholar] [CrossRef]
- Jin, L.; Burnett, A.L. NADPH oxidase: Recent evidence for its role in erectile dysfunction. Asian J. Androl. 2008, 10, 6–13. [Google Scholar] [CrossRef]
- Jin, L.; Lagoda, G.; Leite, R.; Webb, R.C.; Burnett, A.L. NADPH oxidase activation: A mechanism of hypertension-associated erectile dysfunction. J. Sex Med. 2008, 5, 544–551. [Google Scholar] [CrossRef]
- Bonnefont-Rousselot, D.; Beaudeux, J.L.; Thérond, P.; Peynet, J.; Legrand, A.; Delattre, J. [Diabetes mellitus, oxidative stress and advanced glycation endproducts]. Ann. Pharm. Fr. 2004, 62, 147–157. [Google Scholar] [CrossRef]
- Forbes, J.M.; Thallas, V.; Thomas, M.C.; Founds, H.W.; Burns, W.C.; Jerums, G.; Cooper, M.E. The breakdown of preexisting advanced glycation end products is associated with reduced renal fibrosis in experimental diabetes. FASEB J. 2003, 17, 1762–1764. [Google Scholar] [CrossRef]
- Singh, R.; Barden, A.; Mori, T.; Beilin, L. Advanced glycation end-products: A review. Diabetologia 2001, 44, 129–146. [Google Scholar] [CrossRef] [PubMed]
- Mifune, Y.; Inui, A.; Muto, T.; Nishimoto, H.; Kataoka, T.; Kurosawa, T.; Yamaura, K.; Mukohara, S.; Niikura, T.; Kokubu, T.; et al. Influence of advanced glycation end products on rotator cuff. J. Shoulder Elb. Surg. 2019, 28, 1490–1496. [Google Scholar] [CrossRef] [PubMed]
- Pal, B.; Anderson, J.; Dick, W.C.; Griffiths, I.D. Limitation of joint mobility and shoulder capsulitis in insulin- and non-insulin-dependent diabetes mellitus. Br. J. Rheumatol. 1986, 25, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Gumucio, J.; Mendias, C.; Bedi, A. What is the role of systemic conditions and options for manipulation of bone formation and bone resorption in rotator cuff tendon healing and repair. Tech. Shoulder Elb. Surg. 2017, 18, 113–120. [Google Scholar] [CrossRef]
- Thomas, S.J.; McDougall, C.; Brown, I.D.; Jaberoo, M.C.; Stearns, A.; Ashraf, R.; Fisher, M.; Kelly, I.G. Prevalence of symptoms and signs of shoulder problems in people with diabetes mellitus. J. Shoulder Elb. Surg. 2007, 16, 748–751. [Google Scholar] [CrossRef]
- Ranger, T.A.; Wong, A.M.; Cook, J.L.; Gaida, J.E. Is there an association between tendinopathy and diabetes mellitus? A systematic review with meta-analysis. Br. J. Sports Med. 2016, 50, 982–989. [Google Scholar] [CrossRef]
- Balci, N.; Balci, M.K.; Tüzüner, S. Shoulder adhesive capsulitis and shoulder range of motion in type II diabetes mellitus: Association with diabetic complications. J. Diabetes Complicat. 1999, 13, 135–140. [Google Scholar] [CrossRef]
- Rosenbloom, A.L. Limitation of finger joint mobility in diabetes mellitus. J. Diabet. Complicat. 1989, 3, 77–87. [Google Scholar] [CrossRef]
- Batista, F.; Nery, C.; Pinzur, M.; Monteiro, A.C.; de Souza, E.F.; Felippe, F.H.; Alcântara, M.C.; Campos, R.S. Achilles tendinopathy in diabetes mellitus. Foot Ankle Int. 2008, 29, 498–501. [Google Scholar] [CrossRef]
- Akturk, M.; Karaahmetoglu, S.; Kacar, M.; Muftuoglu, O. Thickness of the supraspinatus and biceps tendons in diabetic patients. Diabetes Care 2002, 25, 408. [Google Scholar] [CrossRef][Green Version]
- Mavrikakis, M.E.; Drimis, S.; Kontoyannis, D.A.; Rasidakis, A.; Moulopoulou, E.S.; Kontoyannis, S. Calcific shoulder periarthritis (tendinitis) in adult onset diabetes mellitus: A controlled study. Ann. Rheum. Dis. 1989, 48, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, M.H.; Davis, W.A.; Davis, T.M. Incidence and predictors of hospitalization for tendon rupture in type 2 diabetes: The Fremantle diabetes study. Diabet. Med. 2014, 31, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Cofield, R.H. Subscapular muscle transposition for repair of chronic rotator cuff tears. Surg. Gynecol. Obstet. 1982, 154, 667–672. [Google Scholar] [PubMed]
- Goutallier, D.; Postel, J.M.; Bernageau, J.; Lavau, L.; Voisin, M.C. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin. Orthop. Relat. Res. 1994, 304, 78–83. [Google Scholar] [CrossRef]
- Maffulli, N.; Barrass, V.; Ewen, S.W. Light microscopic histology of achilles tendon ruptures. A comparison with unruptured tendons. Am. J. Sports Med. 2000, 28, 857–863. [Google Scholar] [CrossRef]
- Mane, D.R.; Kale, A.D.; Belaldavar, C. Validation of immunoexpression of tenascin-C in oral precancerous and cancerous tissues using ImageJ analysis with novel immunohistochemistry profiler plugin: An immunohistochemical quantitative analysis. J. Oral Maxillofac. Pathol. 2017, 21, 211–217. [Google Scholar] [CrossRef]
- Lin, T.T.; Lin, C.H.; Chang, C.L.; Chi, C.H.; Chang, S.T.; Sheu, W.H. The effect of diabetes, hyperlipidemia, and statins on the development of rotator cuff disease: A nationwide, 11-year, longitudinal, population-based follow-up study. Am. J. Sports Med. 2015, 43, 2126–2132. [Google Scholar] [CrossRef]
- Makita, Z.; Radoff, S.; Rayfield, E.J.; Yang, Z.; Skolnik, E.; Delaney, V.; Friedman, E.A.; Cerami, A.; Vlassara, H. Advanced glycosylation end products in patients with diabetic nephropathy. N. Engl. J. Med. 1991, 325, 836–842. [Google Scholar] [CrossRef]
- Murata, T.; Nagai, R.; Ishibashi, T.; Inomuta, H.; Ikeda, K.; Horiuchi, S. The relationship between accumulation of advanced glycation end products and expression of vascular endothelial growth factor in human diabetic retinas. Diabetologia 1997, 40, 764–769. [Google Scholar] [CrossRef]
- Dunn, J.A.; Patrick, J.S.; Thorpe, S.R.; Baynes, J.W. Oxidation of glycated proteins: Age-dependent accumulation of N epsilon-(carboxymethyl)lysine in lens proteins. Biochemistry 1989, 28, 9464–9468. [Google Scholar] [CrossRef]
- Baynes, J.W.; Thorpe, S.R. Role of oxidative stress in diabetic complications: A new perspective on an old paradigm. Diabetes 1999, 48, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Jhun, B.S.; Yoon, Y. High-glucose stimulation increases reactive oxygen species production through the calcium and mitogen-activated protein kinase-mediated activation of mitochondrial fission. Antioxid. Redox Signal. 2011, 14, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Opara, E.C. Oxidative stress, micronutrients, diabetes mellitus and its complications. J. R. Soc. Promot. Health 2002, 122, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Xing, M. Oxidative stress: A new risk factor for thyroid cancer. Endocr. Relat. Cancer 2012, 19, C7–C11. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Manea, S.A.; Constantin, A.; Manda, G.; Sasson, S.; Manea, A. Regulation of Nox enzymes expression in vascular pathophysiology: Focusing on transcription factors and epigenetic mechanisms. Redox Biol. 2015, 5, 358–366. [Google Scholar] [CrossRef]
- Kurosawa, T.; Mifune, Y.; Inui, A.; Nishimoto, H.; Ueda, Y.; Kataoka, T.; Yamaura, K.; Mukohara, S.; Kuroda, R. Evaluation of apocynin in vitro on high glucose-induced oxidative stress on tenocytes. Bone Jt. Res. 2020, 9, 23–28. [Google Scholar] [CrossRef]
- Tang, X.Y.; Zhang, Q.; Dai, D.Z.; Ying, H.J.; Wang, Q.J.; Dai, Y. Effects of strontium fructose 1,6-diphosphate on expression of apoptosis-related genes and oxidative stress in testes of diabetic rats. Int. J. Urol. 2008, 15, 251–256. [Google Scholar] [CrossRef]
- Poulsen, R.C.; Knowles, H.J.; Carr, A.J.; Hulley, P.A. Cell differentiation versus cell death: Extracellular glucose is a key determinant of cell fate following oxidative stress exposure. Cell Death Dis. 2014, 5, e1074. [Google Scholar] [CrossRef]
- Park, H.B.; Hah, Y.S.; Yang, J.W.; Nam, J.B.; Cho, S.H.; Jeong, S.T. Antiapoptotic effects of anthocyanins on rotator cuff tenofibroblasts. J. Orthop. Res. 2010, 28, 1162–1169. [Google Scholar] [CrossRef]
- Chen, J.; Wang, A.; Xu, J.; Zheng, M. In chronic lateral epicondylitis, apoptosis and autophagic cell death occur in the extensor carpi radialis brevis tendon. J. Shoulder Elb. Surg. 2010, 19, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Duraisamy, Y.; Slevin, M.; Smith, N.; Bailey, J.; Zweit, J.; Smith, C.; Ahmed, N.; Gaffney, J. Effect of glycation on basic fibroblast growth factor induced angiogenesis and activation of associated signal transduction pathways in vascular endothelial cells: Possible relevance to wound healing in diabetes. Angiogenesis 2001, 4, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.D.; Gallant, M.A.; Burr, D.B.; Wallace, J.M. Multiscale analysis of morphology and mechanics in tail tendon from the ZDSD rat model of type 2 diabetes. J. Biomech. 2014, 47, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.F.; Wang, H.K.; Chang, H.W.; Sun, J.; Sun, J.S.; Chao, Y.H. High glucose alters tendon homeostasis through downregulation of the AMPK/Egr1 pathway. Sci. Rep. 2017, 7, 44199. [Google Scholar] [CrossRef] [PubMed]
- Amiel, D.; Frank, C.; Harwood, F.; Fronek, J.; Akeson, W. Tendons and ligaments: A morphological and biochemical comparison. J. Orthop. Res. 1984, 1, 257–265. [Google Scholar] [CrossRef]
- Lin, Y.C.; Li, Y.J.; Rui, Y.F.; Dai, G.C.; Shi, L.; Xu, H.L.; Ni, M.; Zhao, S.; Chen, H.; Wang, C.; et al. The effects of high glucose on tendon-derived stem cells: Implications of the pathogenesis of diabetic tendon disorders. Oncotarget 2017, 8, 17518–17528. [Google Scholar] [CrossRef]
- Aimes, R.T.; Quigley, J.P. Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific 3/4- and 1/4-length fragments. J. Biol. Chem. 1995, 270, 5872–5876. [Google Scholar] [CrossRef]
- Lee, S.J.; Bae, S.S.; Kim, K.H.; Lee, W.S.; Rhim, B.Y.; Hong, K.W.; Kim, C.D. High glucose enhances MMP-2 production in adventitial fibroblasts via Akt1-dependent NF-kappaB pathway. FEBS Lett. 2007, 581, 4189–4194. [Google Scholar] [CrossRef]
- Abate, M.; Salini, V.; Schiavone, C. Achilles tendinopathy in elderly subjects with type II diabetes: The role of sport activities. Aging Clin. Exp. Res. 2016, 28, 355–358. [Google Scholar] [CrossRef]

| Gene | Oligonucleotide Sequence |
|---|---|
| NOX1 | Forward 5′ GGTTTTACCGCTCCCAGCAGAA 3′ Reverse 5′ CTTCCATGCTGAAGCCACGCTT 3′ |
| NOX4 | Forward 5′ GCCAGAGTATCACTACCTCCAC 3′ Reverse 5′ CTCGGAGGTAAGCCAAGAGTGT 3′ |
| IL6 | Forward 5′ AGACAGCCACTCACCTCTTCAG 3′ Reverse 5′ TTCTGCCAGTGCCTCTTTGCTG 3′ |
| RAGE | Forward 5′ CACCTTCTCCTGTAGCTTCAGC 3′ Reverse 5′ AGGAGCTACTGCTCCACCTTCT 3′ |
| Type I collagen | Forward 5′ AGGAATTCGGCTTCGACGTT 3′ Reverse 5′ GGTTCAGTTTGGGTTGCTTG 3′ |
| Type III collagen | Forward 5′ GGGAACAACTTGATGGTGCT 3′ Reverse 5′ CCTCCTTCAACAGCTTCCTG 3′ |
| MMP2 | Forward 5′ GTGCTGAAGGACACACTAAAGAAGA 3′ Reverse 5′ TTGCCATCCTTCTCAAAGTTGTAGG 3′ |
| TIMP1 | Forward 5′ CCAAGATGTATAAAGGGTTCCAA 3′ Reverse 5′ TTTCCAGCAATGAGAAACTCCT 3′ |
| TIMP2 | Forward 5′ GAGCCTGAACCACAGGTACCA 3′ Reverse 5′ AGGAGATGTAGCACGGGATCA 3′ |
| GAPDH | Forward 5′ GTCTCCTCTGACTTCAACAGCG 3′ Reverse 5′ ACCACCCTGTTGCTGTAGCCAA 3′ |
| DM− | DM+ | p-Value | |
|---|---|---|---|
| Mean age | 62.2 ± 6.4 | 65.3 ± 5.5 | 0.26 |
| Sex | Men: 4; Women: 6 | Men: 4; Women: 6 | 1 |
| Mean HbA1c level (%) | 5.7 ± 0.26 | 6.9 ± 0.5 | <0.001 * |
| Duration of diabetes (years) | - | 5.5 ± 4.4 | - |
| Current pharmacotherapy | - | insulin: 2; oral medications: 4; insulin and oral medications: 2; none: 2 | - |
| Prevalence of hypertension (%) | 40 | 30 | 0.66 |
| BMI (kg/m2) | 24.5 ± 3.7 | 25.8 ± 3.5 | 0.44 |
| Rotator cuff tear size (Cofield classification) | Small: 6; Medium: 3; Large: 1 | Small: 6; Medium: 3; Large: 1 | 1 |
| Fatty degeneration (Goutallier classification) | Stage 1: 5; Stage 2: 4; Stage 3: 1 | Stage 1: 5; Stage 2: 4; Stage 3: 1 | 1 |
| DM− Mean (SD) | DM+ Mean (SD) | p-Value | |
|---|---|---|---|
| Fiber structure | 0.40 (0.49) | 0.48 (0.50) | 0.43 |
| Fiber arrangement | 0.42 (0.50) | 0.98 (0.79) | <0.001 * |
| Nuclear morphology (rounding) | 0.46 (0.50) | 0.60 (0.57) | 0.20 |
| Regional variations in cellularity | 0.28 (0.45) | 0.36 (0.48) | 0.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshikawa, T.; Mifune, Y.; Inui, A.; Nishimoto, H.; Yamaura, K.; Mukohara, S.; Shinohara, I.; Kuroda, R. Influence of Diabetes-Induced Glycation and Oxidative Stress on the Human Rotator Cuff. Antioxidants 2022, 11, 743. https://doi.org/10.3390/antiox11040743
Yoshikawa T, Mifune Y, Inui A, Nishimoto H, Yamaura K, Mukohara S, Shinohara I, Kuroda R. Influence of Diabetes-Induced Glycation and Oxidative Stress on the Human Rotator Cuff. Antioxidants. 2022; 11(4):743. https://doi.org/10.3390/antiox11040743
Chicago/Turabian StyleYoshikawa, Tomoya, Yutaka Mifune, Atsuyuki Inui, Hanako Nishimoto, Kohei Yamaura, Shintaro Mukohara, Issei Shinohara, and Ryosuke Kuroda. 2022. "Influence of Diabetes-Induced Glycation and Oxidative Stress on the Human Rotator Cuff" Antioxidants 11, no. 4: 743. https://doi.org/10.3390/antiox11040743
APA StyleYoshikawa, T., Mifune, Y., Inui, A., Nishimoto, H., Yamaura, K., Mukohara, S., Shinohara, I., & Kuroda, R. (2022). Influence of Diabetes-Induced Glycation and Oxidative Stress on the Human Rotator Cuff. Antioxidants, 11(4), 743. https://doi.org/10.3390/antiox11040743






