Abstract
Coronary artery bypass graft (CABG) surgery still represents the gold standard for patients with complex multivessel coronary artery disease. However, graft occlusion still occurs in a significant proportion of CABG conduits, and oxidative stress is currently considered to be a potential contributor. Human serum albumin (HSA) represents the main antioxidant in plasma through its reduced amino acid Cys34, which can efficiently scavenge several oxidants. In a nested case–control study including 36 patients with occluded grafts and 38 age- and sex-matched patients without occlusion, we assessed the levels of the native mercaptoalbumin (HSA-SH) and oxidized thiolated form of albumin (Thio-HSA) in relation with graft occlusion within 5 years after CABG. We found that the plasma level of preoperative HSA-SH was significantly lower in patients with occluded graft at 5 years follow-up than in patients with graft patency. Furthermore, low HSA-SH remained independently associated with graft occlusion even after adjusting for preoperative D-dimer, a well-known marker of activated coagulation recently found to be associated with graft occlusion. In conclusion, the preoperative level of HSA-SH is independently associated with graft occlusion in CABG and represents a measurable and potentially druggable predictor.
1. Introduction
According to the US and European guidelines, coronary artery bypass graft (CABG) surgery represents the gold standard of care for patients with complex multivessel coronary artery disease and/or left main disease, diabetes, or reduced left-ventricular function [1,2].
Despite significant improvements in the outcome of patients undergoing CABG procedure, graft occlusion is the ‘Achilles’ heel’ of this procedure, occurring in a significant number of CABG conduits. Biological mechanisms, characteristics of the target vessels, surgical procedure, and type of graft used all are critical factors in determining failure [3,4]. Indeed, many studies have demonstrated that CABG is associated with the activation of different molecular pathways, which lead to a persistent systemic inflammatory state associated with the activation of the hemostatic systems and oxidative perturbations (reviewed in [5]).
Indeed, the increased production of reactive oxygen species (ROS) is considered a potential contributor to the incidence of perioperative or postoperative complications occurring after CABG, causing damage and dysfunction of macromolecules, cells, and tissues by overwhelming the local antioxidant defense mechanisms. Several studies have shown an increase of oxidative stress during the intraoperative period and the very early hours after surgery, with increases in several pro-oxidant markers [5] and a concomitant decrease in the levels of antioxidant molecules (such as tocopherols and reduced glutathione [6,7]).
Beyond the perioperative period, levels of oxidative stress markers at longer periods have not been assessed. To the best of our knowledge, the only study with a follow-up of 1 week after surgery showed that myeloperoxidase levels increased up to the second postoperative day [8].
Human serum albumin (HSA), beyond its crucial physiological functions (i.e., maintenance of the oncotic pressure and microvascular integrity, regulation of metabolic and vascular functions, and transport of biomolecules and drugs) [9], represents the main antioxidant in plasma [10,11]. Albumin exerts its antioxidant activity through its multiple-binding sites and free radical-trapping properties [10,12], with the latter mainly occurring through the presence of the sulfhydryl group of the amino acid Cys34, which can efficiently scavenge several oxidants, including hydroxyl and peroxyl radicals, hydrogen peroxide, and peroxynitrite [10,11,13,14]. The Cys34 sulfhydryl group present on albumin is the largest fraction of all free thiols in plasma (~80%, corresponding to ~500 µmol/L), thus attributing to HSA, due to its abundance in the plasma, a major role as an antioxidant [10]. Indeed, in plasma samples from healthy subjects, the main HSA fraction is represented by mercaptoalbumin, HSA-SH, which contains a reduced free thiol at Cys34, while a small proportion of the Cys34 forms reversible mixed disulfides with low-molecular-weight thiols, generating S-thiolated albumin isoforms. As these oxidized isoforms are not present on albumin after its secretion from hepatocytes but occur after being released into the circulation, they may represent potential biomarkers of oxidative stress in human diseases [11,15].
Therefore, in search of biomarkers that may predict graft occlusion and possibly suggest strategies to reduce graft failure, we assessed preoperative and postoperative levels of native and thiolated forms of albumin in relation to graft occlusion evaluated up to 5 years after CABG.
2. Materials and Methods
2.1. Study Population
In this study, we used samples from an existing plasma biorepository of a cohort of 330 consecutive patients (age 18 to 89 years) enrolled for elective primary surgical myocardial revascularization between November 2006 and February 2010 at Centro Cardiologico Monzino IRCCS (Milan, Italy) (NCT00755248, CAGE Study) [16]. The CAGE study was approved by the Ethical Committee of Centro Cardiologico Monzino IRCCS (approval number: CCMS90/108) and was conducted according to the Declaration of Helsinki. Informed consent was obtained from all the patients. Patients with concomitant surgery, major end-organ dysfunction, known coagulation disorders, serious undercurrent illness or infection, serum creatinine level greater than 2 mg/dL, and atrial fibrillation were excluded, as previously described [16].
For all patients, postoperative therapy followed guidelines for CAD and surgical myocardial revascularization including always at least single antiplatelet therapy, optimized blood pressure control therapy, and statin. As described in Figure 1, coronary computed tomography angiography (CCTA) and/or coronary angiography were available for 179 patients within a 52 months follow-up for evaluating graft patency.
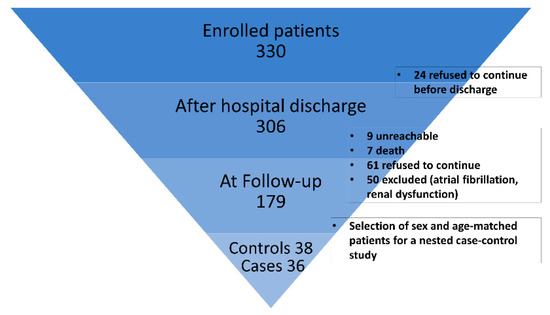
Figure 1.
Study enrollment flow chart.
For this work, a nested case–control study was designed. Suitable samples were available from 36 patients with occluded grafts at 5 years follow-up (cases) and were compared with 38 patients without occlusion (control subjects), frequency-matched for age and sex. Analysis was performed on preoperative plasma samples (T0) and postoperative plasma samples collected at patient discharge (T1), usually between postoperative days 6 and 9 after surgery.
2.2. Quantitation of S-Thiolated Albumin by Mass Spectrometry (MS)
The relative composition of albumin isoforms in human plasma samples was evaluated by direct infusion of diluted plasma (500-fold dilution) using the Xevo TQ-S micro triple-quadrupole mass spectrometer coupled with the ACQUITY UPLC® M-Class system (Waters Corporation, Milford, CT, USA), as previously described [17]. After data deconvolution with the MaxEnt1 function on the MassLynx software (Waters Corporation, Milford, CT, USA), HSA-SH, thiolated albumin (+120 ± 2 Da, Thio-HSA), and glycated albumin (Gly-HSA, +160 ± 2 Da) were detected, and their intensities were used to calculate the relative abundances as previously described [13].
2.3. Statistical Analysis
Quantitative variables were reported as the mean ± SD or median and interquartile range (IQR) according to their distribution. Spearman’s correlation was used to find monotonic associations between variables. Categorical variables were compared between the two groups using the chi-square test or Fisher’s exact test when appropriate, and quantitative variables were compared using Student’s t-test and the Wilcoxon rank sum test when appropriate. Multivariable logistic regression was used to assess whether albumin isoforms measured before surgery were independently associated with graft occlusion. Variables included in the model were selected among potential confounders, i.e., baseline variables that were associated with both the dependent variable (graft occlusion) and the variable under investigation (HSA-SH). The ability of mercaptoalbumin to discriminate between cases and controls was assessed by receiver operating characteristic (ROC) curve analysis. Net reclassification improvement (NRI) was used to check the improvement in risk prediction by adding albumin isoforms to the reference model including D-dimer, logistic EuroSCORE, and extracorporeal circulation (ECC) time. Three risk categories were defined as low (0–33%), middle (33–66%), and high (66–100%). SAS statistical software version 9.4 (SAS Institute, Cary, NC, USA) was used for all analyses, and a p-value < 0.05 was considered statistically significant.
3. Results
3.1. Characteristics of the Study Participants and Study Workflow
This study was performed on a cohort of 74 sex- and age-matched patients, selected from the CAGE study population divided into two groups according to graft patency at 5 years follow-up. We analyzed a total of 36 patients with graft occlusion (cases) and 38 patients with graft patency (controls), with no significant differences between the two groups in terms of age, sex, risk factors, graft vessel origin, and medications (Table 1).

Table 1.
Clinical characteristics of the study population.
3.2. Albumin Isoforms Differences between Occluded and Patent Grafts
Albumin isoforms were analyzed in plasma samples collected before surgery (T0) in cases vs. controls by mass spectrometry, to quantify the percentage of HSA-SH and its main modified isoforms: Thio-HSA and Gly-HSA.
At univariable analysis, preoperative HSA-SH resulted significantly lower in cases with occluded graft at 5 years follow-up than in controls (79.6% ± 4.2% and 82.1% ± 2.7%, mean ± SD, p = 0.007 in cases and controls, respectively). On the contrary, Thio-HSA showed an inverse behavior (13.5% ± 3.98% and 10.96% ± 2.78% in cases and controls, respectively, p = 0.002), whereas Gly-HSA, another modification often occurring in diabetes as the result of glycation reactions, was not different between the two groups (Figure 2).
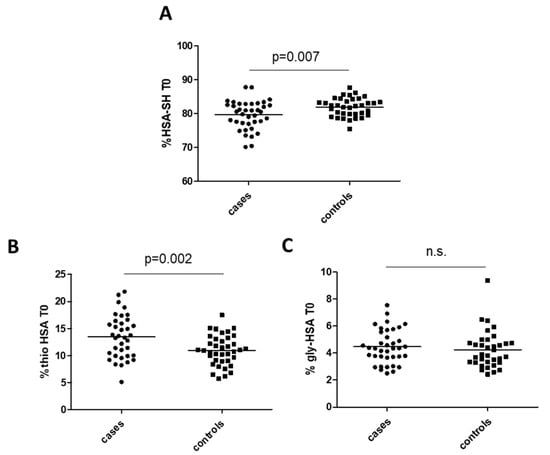
Figure 2.
Albumin isoforms in cases and controls: (A) mercaptoalbumin (HSA-SH), (B) thiolated albumin (Thio-HSA), and (C) glycated albumin (Gly-HSA). p-Values are from Student’s t-test. n.s. means not significant.
In addition, Spearman’s correlation analysis (Table 2) showed significant negative associations between HSA-SH and preoperative plasma D-dimer levels, creatinine, and ECC duration, whereas Thio-HSA was positively associated with these three parameters. HSA-SH showed negative correlations also with logistic EuroSCORE and preoperative fibrinogen. Of note, we found a significant positive correlation between antiplatelet therapy and HSA-SH and a negative correlation with Thio-HSA.

Table 2.
Spearman correlations between preoperative albumin isoforms and baseline clinical characteristics.
Notably, in a multivariable model, adjusting for preoperative D-dimer plasma levels and logistic EuroSCORE, HSA-SH remained independently and negatively associated with graft occlusion within 5 years. This association was still valid even when including ECC duration as a confounding factor (Table 3). Thus, a 19% reduction in the risk of occlusion for a 1% increase in HSA-SH, independent of the confounders considered, was estimated.

Table 3.
Logistic models for HSA-SH association with graft occlusion at 5 years follow-up.
On the other hand, Thio-HSA did not result significantly different between cases and controls after adjustment for the above variables (p = 0.0946).
3.3. HSA-SH as a Predictor of Graft Occlusion
To quantify the ability of HSA-SH to predict graft patency at 5 years follow-up, we employed a ROC curve analysis, which revealed an area under curve (AUC) of 0.7126 (Figure 3). This analysis showed that the best HSA-SH cutoff value, which could identify with sufficient accuracy patients who experienced graft occlusion after 5 years, was 81.16% (sensitivity 66.7%, specificity 63.17%).
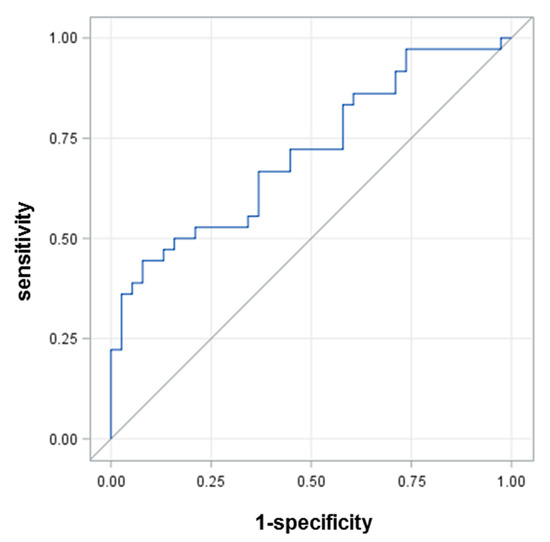
Figure 3.
ROC curve for HSA-SH.
In addition, to quantify the capacity of HSA-SH to properly classify patients undergoing occlusion, on top of the other variables considered as confounders, we performed a reclassification analysis. On the basis of the NRI, HSA-SH was able to better reclassify 30% of the patients in comparison with a model including D-dimer, logistic EuroSCORE, and duration of ECC (p = 0.007, Table S1).
3.4. Thio-HSA Increased Significantly after Surgery but Was Not a Predictor of Graft Occlusion
We found a marked increase in the levels of Thio-HSA at patient discharge (T1) compared with preoperative levels, with a concomitant decrease in HSA-SH (Figure 4). Furthermore, HSA-SH was significantly and negatively associated with C-reactive protein (CRP) at discharge (Table 4). However, at discharge, neither Thio-HSA nor HSA-SH levels (expressed as absolute values or delta vs. baseline) correlated with the graft occlusion at 5 years.
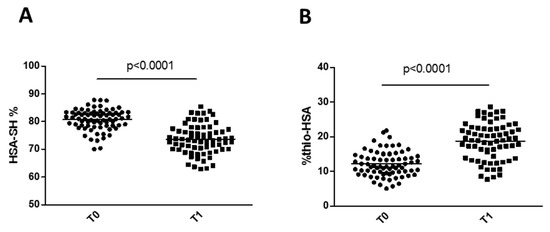
Figure 4.
Changes in HSA-SH (A) and Thio-HSA (B) levels after surgery. p-Values are from Student’s t-test.

Table 4.
Spearman correlation for mercaptoalbumin (HSA-SH) and thiolated albumin (Thio-HSA) after surgery.
4. Discussion
This study provides evidence, for the first time, that the preoperative plasma level of HSA-SH was significantly lower in patients with occluded graft than in patients with graft patency at 5 years follow-up, and that it was independently and negatively associated with graft occlusion at 5 years after CABG. Furthermore, low HSA-SH remained independently associated with graft occlusion even after adjusting for preoperative D-dimer, a well-known marker of activated coagulation recently found to be associated with graft occlusion [16]. Furthermore, by assessing the capacity of HSA-SH to properly classify patients undergoing occlusion, on top of other variables considered as confounders, a reclassification analysis based on the NRI revealed that HSA-SH was able to better reclassify 30% of the patients in comparison with a model including D-dimer, logistic EuroSCORE, and duration of ECC (p = 0.007).
The novelty of these findings is that it is the quality of albumin, rather than its quantity, that is relevant as a disease biomarker in the cardiovascular setting. Until now, the interest in albumin in the cardiovascular field has been mainly limited to its levels in the bloodstream. Hypoalbuminemia has been associated with poor prognosis in many conditions, such as acute coronary syndrome, heart failure (HF), and in patients undergoing CABG [9,18,19,20]. It has been also demonstrated that patients with preoperative hypoalbuminemia had worse long-term survival after CABG [20]. On the other hand, we recently published new insights into the structural alterations of HSA occurring in the plasma of patients with HF [17]. In this setting, the increase in Thio-HSA and the concomitant decrease in the mercaptoalbumin species correlate with an impairment of the plasma antioxidant activity. Moreover, Thio-HSA levels inversely correlate with peak oxygen uptake (VO2), the most objective functional method to assess the cardiopulmonary exercise capacity [17,21]. Furthermore, in HL-1 cardiomyocytes, HSA-SH, but not Thio-HSA, prevented the decrease in cell viability after treatment with hydrogen peroxide [17]. Taken together, these data suggest that albumin thiolation increases in HF patients, provokes alterations in the structure and antioxidant function of HSA, and potentially contributes to HF progression.
After CABG, there is a marked and prolonged activation of many molecular pathways leading to increased inflammation and oxidative stress, hemostasis activation, and unfavorable endothelial milieu. Thus, several biomarkers reflecting the activation of these metabolic systems have been proposed as predictors of early and late graft occlusion [5,16,22,23]. Indeed, the identification of patients who are at risk of graft failure still represents an urgent clinical need and a research challenge.
Considering that oxidized isoforms of albumin are not present in the protein immediately after secretion from the liver, they might represent potential biomarkers of oxidative stress in human diseases [11,15]. Furthermore, as albumin has a half-life of 3 weeks, any modifications of its structure are more stable than the oxidative stress markers usually evaluated so far. For example, a consistent increase in isoprostane 8-iso prostaglandin F2α excretion was observed in CABG patients during surgery, with levels returning to baseline 24 h after surgery. Similarly, malondialdehyde increased significantly during surgery and declined immediately after [6,24]. Of note, in our study, Thio-HSA was still high at patient discharge, thus representing a stable indicator of oxidative stress.
In addition to its role as a potential marker of oxidative stress, it should be emphasized that HSA represents a significant source of antioxidant defense in the plasma, which is constantly exposed to oxidative stress. HSA is involved in scavenging the vast majority of free radicals, thanks to the presence of the free thiol group on Cys34, which constitutes the largest pool of free thiols in circulation and works as a radical scavenger due to its peculiar acidity (pKa = 8.1) and spatial accessibility.
Lastly, we recently showed that Thio-HSA can be restored to HSA-SH by a disulfide-breaking agent, which can, thus, preserve the antioxidant potential of the extracellular milieu [25].
5. Conclusions
Although some limitations should be acknowledged such as the relatively small sample size and lack of validation on a larger cohort of patients, together with the fact that we could not precisely establish the timing of graft failure within the follow-up period, we believe that we identified a measurable and druggable predictor of graft occlusion.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/antiox11040702/s1: Table S1. Results from NRI analysis.
Author Contributions
Conceptualization, C.B.; investigation, M.B. and E.G.; resources, A.P., D.A., M.P. and G.P.; data curation, L.C., S.M. and A.P.; formal analysis, F.V. and C.C.T.; writing—original draft preparation, M.B. and C.B.; writing—review and editing, all authors; visualization, M.B., E.G. and G.I.C.; supervision, C.B.; project administration, C.B. and E.T.; funding acquisition, C.B. and E.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Italian Ministry of Health, Rome, Italy (Ricerca Corrente RC 2019 MPP1A ID 2755301).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Istituto Europeo di Oncologia and Centro Cardiologico Monzino (protocol code Identifier: NCT00755248; date of approval: 28 January 2008).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data collected in the study will be made available using the data repository Zenodo (https://zenodo.org/), doi:10.5281/zenodo.6405086, accessed on 10 February 2022.
Acknowledgments
We thank Chiara Centenaro for the project and data management.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Fihn, S.D.; Blankenship, J.C.; Alexander, K.P.; Bittl, J.A.; Byrne, J.G.; Fletcher, B.J.; Fonarow, G.C.; Lange, R.A.; Levine, G.N.; Maddox, T.M.; et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 2014, 64, 1929–1949. [Google Scholar] [PubMed] [Green Version]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. The Task Force on myocardial revascularization of the European Society of Cardiology (ESC) and European Association for Cardio-Thoracic Surgery (EACTS). G. Ital. Cardiol. (Rome) 2019, 20, 1S–61S. [Google Scholar]
- Gaudino, M.; Antoniades, C.; Benedetto, U.; Deb, S.; Di Franco, A.; Di Giammarco, G.; Fremes, S.; Glineur, D.; Grau, J.; He, G.W.; et al. Mechanisms, Consequences, and Prevention of Coronary Graft Failure. Circulation 2017, 136, 1749–1764. [Google Scholar] [CrossRef] [PubMed]
- Caliskan, E.; de Souza, D.R.; Boning, A.; Liakopoulos, O.J.; Choi, Y.H.; Pepper, J.; Gibson, C.M.; Perrault, L.P.; Wolf, R.K.; Kim, K.B.; et al. Saphenous vein grafts in contemporary coronary artery bypass graft surgery. Nat. Rev. Cardiol. 2020, 17, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Parolari, A.; Poggio, P.; Myasoedova, V.; Songia, P.; Pilozzi, A.; Alamanni, F.; Tremoli, E. Molecular pathways activation in coronary artery bypass surgery: Which role for pump avoidance? J. Cardiovasc. Med. (Hagerstown) 2016, 17, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Veglia, F.; Werba, J.P.; Tremoli, E.; Squellerio, I.; Sisillo, E.; Parolari, A.; Minardi, F.; Cavalca, V. Assessment of oxidative stress in coronary artery bypass surgery: Comparison between the global index OXY-SCORE and individual biomarkers. Biomarkers 2009, 14, 465–472. [Google Scholar] [CrossRef]
- Akila; D’souza, B.; Vishwanath, P.; D’souza, V. Oxidative injury and antioxidants in coronary artery bypass graft surgery: Off-pump CABG significantly reduces oxidative stress. Clin. Chim. Acta 2007, 375, 147–152. [Google Scholar] [CrossRef]
- Karu, I.; Taal, G.; Zilmer, K.; Pruunsild, C.; Starkopf, J.; Zilmer, M. Inflammatory/oxidative stress during the first week after different types of cardiac surgery. Scand. Cardiovasc. J. 2010, 44, 119–124. [Google Scholar] [CrossRef]
- Chien, S.C.; Chen, C.Y.; Lin, C.F.; Yeh, H.I. Critical appraisal of the role of serum albumin in cardiovascular disease. Biomark. Res. 2017, 5, 31. [Google Scholar] [CrossRef]
- Roche, M.; Rondeau, P.; Singh, N.R.; Tarnus, E.; Bourdon, E. The antioxidant properties of serum albumin. FEBS Lett. 2008, 582, 1783–1787. [Google Scholar] [CrossRef]
- Colombo, G.; Clerici, M.; Giustarini, D.; Rossi, R.; Milzani, A.; Dalle-Donne, I. Redox albuminomics: Oxidized albumin in human diseases. Antioxid. Redox Signal. 2012, 17, 1515–1527. [Google Scholar] [CrossRef] [PubMed]
- Laussac, J.P.; Sarkar, B. Characterization of the copper(II)- and nickel(II)-transport site of human serum albumin. Studies of copper(II) and nickel(II) binding to peptide 1-24 of human serum albumin by 13C and 1H NMR spectroscopy. Biochemistry 1984, 23, 2832–2838. [Google Scholar] [CrossRef] [PubMed]
- Regazzoni, L.; Del Vecchio, L.; Altomare, A.; Yeum, K.J.; Cusi, D.; Locatelli, F.; Carini, M.; Aldini, G. Human serum albumin cysteinylation is increased in end stage renal disease patients and reduced by hemodialysis: Mass spectrometry studies. Free Radic. Res. 2013, 47, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Zoanni, B.; Brioschi, M.; Mallia, A.; Gianazza, E.; Eligini, S.; Carini, M.; Aldini, G.; Banfi, C. Novel insights about albumin in cardiovascular diseases: Focus on heart failure. Mass Spectrom. Rev. 2021, e21743. [Google Scholar] [CrossRef] [PubMed]
- Bonanata, J.; Turell, L.; Antmann, L.; Ferrer-Sueta, G.; Botasini, S.; Mendez, E.; Alvarez, B.; Coitino, E.L. The thiol of human serum albumin: Acidity, microenvironment and mechanistic insights on its oxidation to sulfenic acid. Free Radic. Biol. Med. 2017, 108, 952–962. [Google Scholar] [CrossRef] [PubMed]
- Parolari, A.; Cavallotti, L.; Andreini, D.; Myasoedova, V.; Banfi, C.; Camera, M.; Poggio, P.; Barili, F.; Pontone, G.; Mussoni, L.; et al. D-dimer is associated with arterial and venous coronary artery bypass graft occlusion. J. Thorac. Cardiovasc. Surg. 2018, 155, 200–207e3. [Google Scholar] [CrossRef] [Green Version]
- Brioschi, M.; Gianazza, E.; Mallia, A.; Zoanni, B.; Altomare, A.; Martinez Fernandez, A.; Agostoni, P.; Aldini, G.; Banfi, C. S-Thiolation Targets Albumin in Heart Failure. Antioxidants 2020, 9, 763. [Google Scholar] [CrossRef]
- Liu, M.; Chan, C.P.; Yan, B.P.; Zhang, Q.; Lam, Y.Y.; Li, R.J.; Sanderson, J.E.; Coats, A.J.; Sun, J.P.; Yip, G.W.; et al. Albumin levels predict survival in patients with heart failure and preserved ejection fraction. Eur. J. Heart Fail. 2012, 14, 39–44. [Google Scholar] [CrossRef]
- Hartopo, A.B.; Gharini, P.P.; Setianto, B.Y. Low serum albumin levels and in-hospital adverse outcomes in acute coronary syndrome. Int. Heart J. 2010, 51, 221–226. [Google Scholar] [CrossRef] [Green Version]
- de la Cruz, K.I.; Bakaeen, F.G.; Wang, X.L.; Huh, J.; LeMaire, S.A.; Coselli, J.S.; Chu, D. Hypoalbuminemia and long-term survival after coronary artery bypass: A propensity score analysis. Ann. Thorac. Surg. 2011, 91, 671–675. [Google Scholar] [CrossRef]
- Malhotra, R.; Bakken, K.; D’Elia, E.; Lewis, G.D. Cardiopulmonary Exercise Testing in Heart Failure. JACC Heart Fail. 2016, 4, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Camera, M.; Brambilla, M.; Canzano, P.; Cavallotti, L.; Parolari, A.; Tedesco, C.C.; Zara, C.; Rossetti, L.; Tremoli, E. Association of Microvesicles With Graft Patency in Patients Undergoing CABG Surgery. J. Am. Coll. Cardiol. 2020, 75, 2819–2832. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulos, A.S.; Odutayo, A.; Oikonomou, E.K.; Trivella, M.; Petrou, M.; Collins, G.S.; Antoniades, C.; SAFINOUS-CABG (Saphenous Vein Graft Failure—An Outcomes Study in Coronary Artery Bypass Grafting) Group. Development of a risk score for early saphenous vein graft failure: An individual patient data meta-analysis. J. Thorac. Cardiovasc. Surg. 2020, 160, 116–127.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vukicevic, P.; Klisic, A.; Neskovic, V.; Babic, L.; Mikic, A.; Bogavac-Stanojevic, N.; Matkovic, M.; Milicevic, V.; Aleksic, N.; Kotur-Stevuljevic, J. Oxidative Stress in Patients before and after On-Pump and Off-Pump Coronary Artery Bypass Grafting: Relationship with Syntax Score. Oxid. Med. Cell Longev. 2021, 2021, 3315951. [Google Scholar] [CrossRef] [PubMed]
- Altomare, A.; Baron, G.; Brioschi, M.; Longoni, M.; Butti, R.; Valvassori, E.; Tremoli, E.; Carini, M.; Agostoni, P.; Vistoli, G.; et al. N-Acetyl-Cysteine Regenerates Albumin Cys34 by a Thiol-Disulfide Breaking Mechanism: An Explanation of Its Extracellular Antioxidant Activity. Antioxidants 2020, 9, 367. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).