Efficacy of Antioxidant Supplementation to Non-Surgical Periodontal Therapy on Metabolic Control in Type 2 Diabetes Patients: A Network Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Search Strategy and Eligibility Criteria
- (P) adult patients with diagnosed T2D (controlled or not) under treatment (including diet, exercises, pharmacological therapy or any combination of those) and untreated periodontitis (according to the case definition of the new Periodontal Diseases Classification [37], patients with interdental clinical attachment level (CAL) detectable at ≥2 non-adjacent teeth, or buccal or oral CAL ≥ 3 mm with pocketing > 3 mm detectable at ≥2 teeth);
- (I) NSPT with any type of adjunctive antioxidant supplement ingestion;
- (C) NSPT alone or associated to placebo ingestion;
- (O) Metabolic control evaluated through HbA1c level change from baseline;
- (S) Randomized controlled clinical trials (RCTs). Only RCTs were included once this is the most appropriate type of study to answer interventional questions and constitute the best scientific evidence to support the therapeutic practice.
2.3. Data Extraction and Risk of Bias
2.4. Data Synthesis and Meta-Analysis
2.5. Certainty of Evidence Assessment
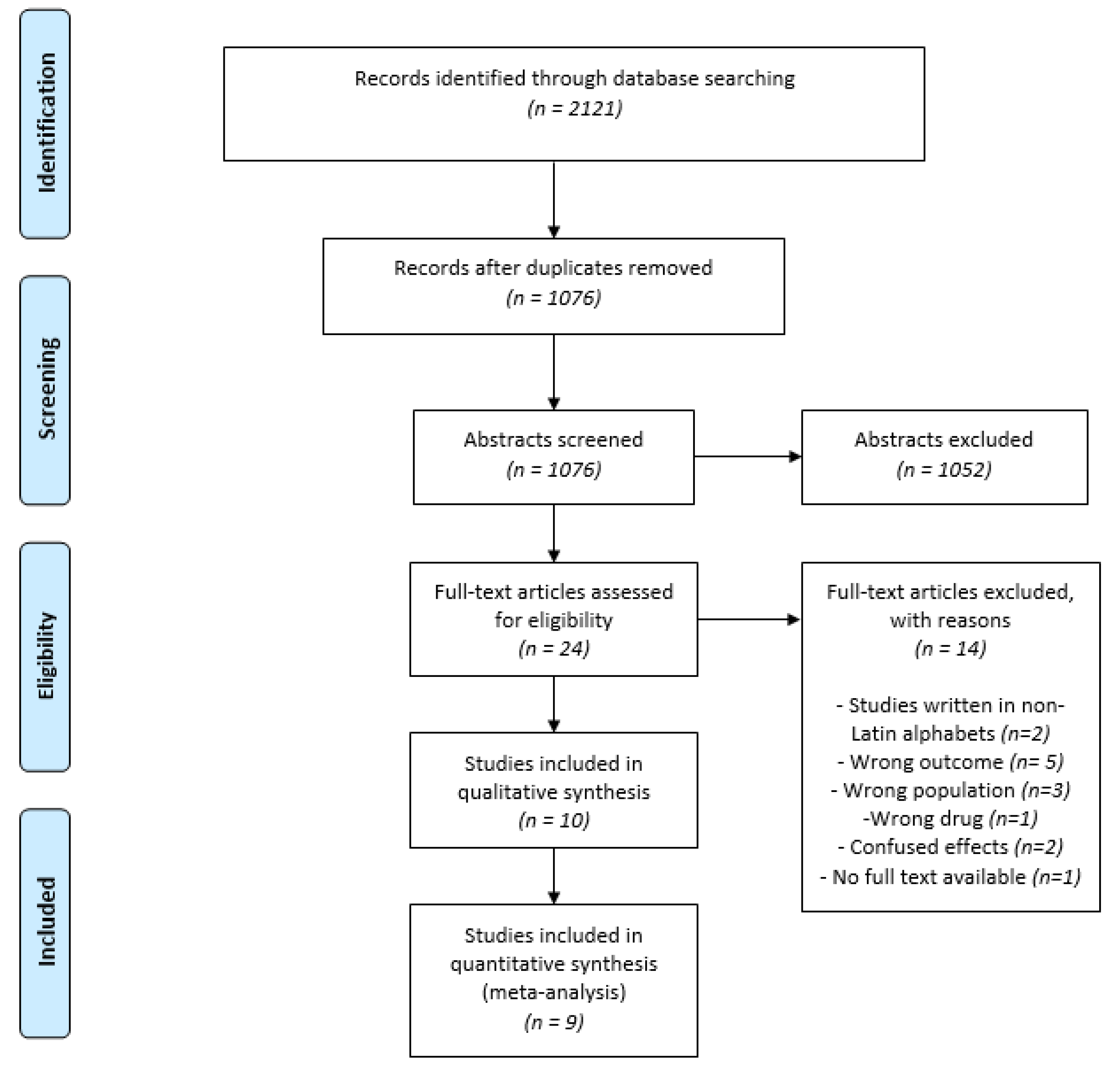
3. Results
3.1. Characteristics of the Included Studies
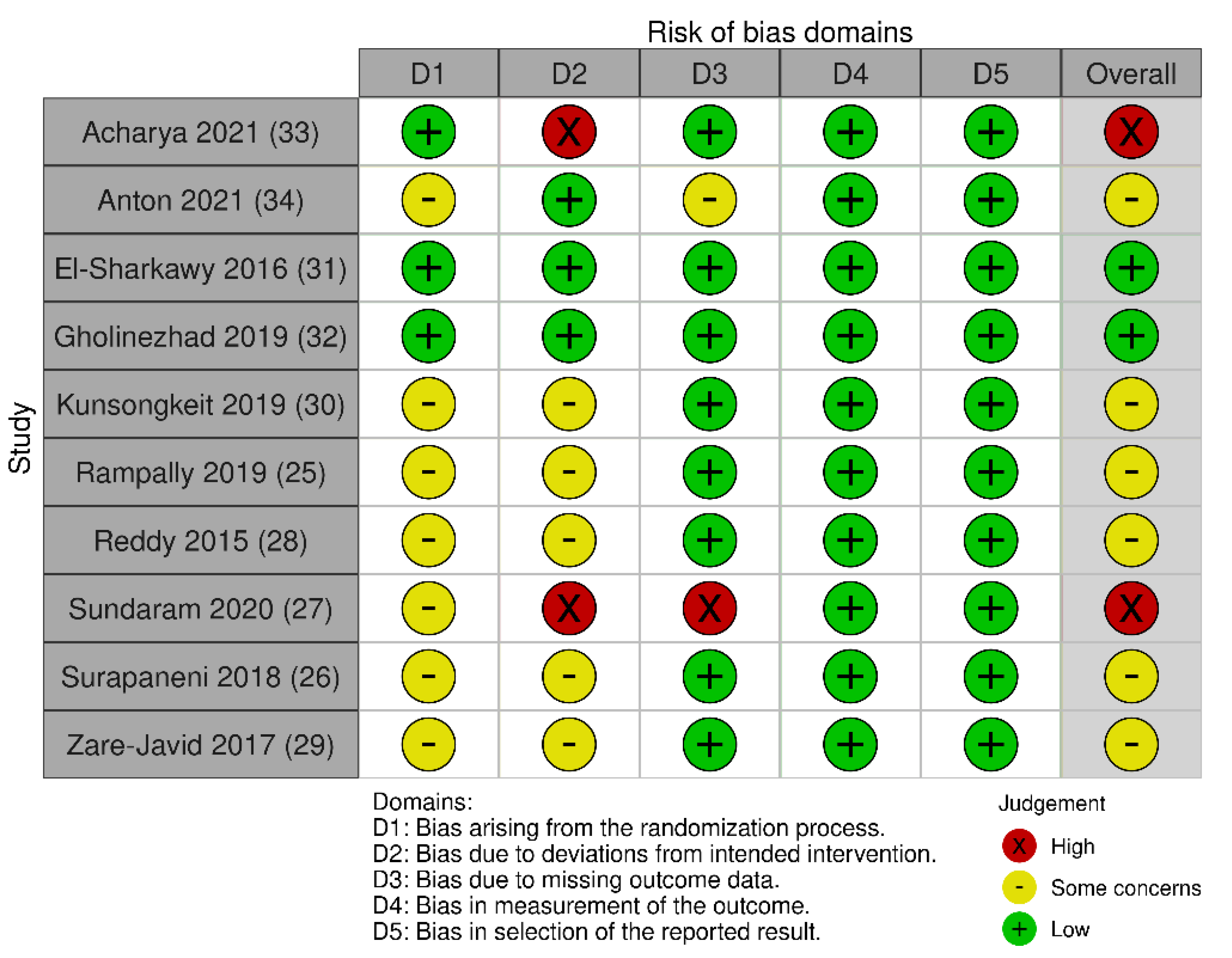
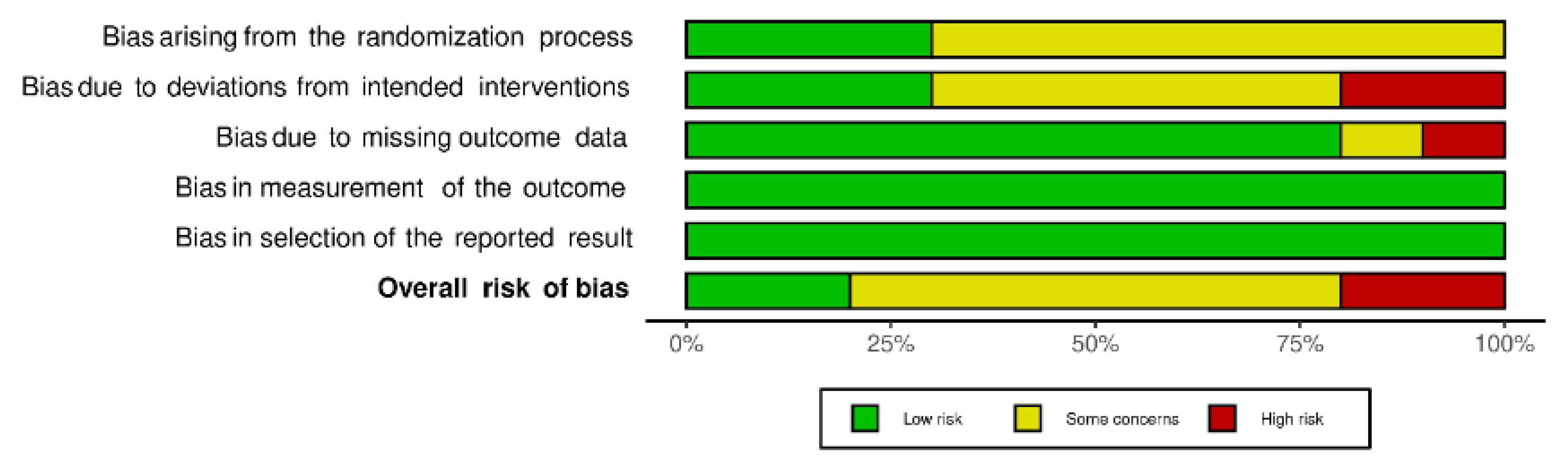
3.2. Risk of Bias
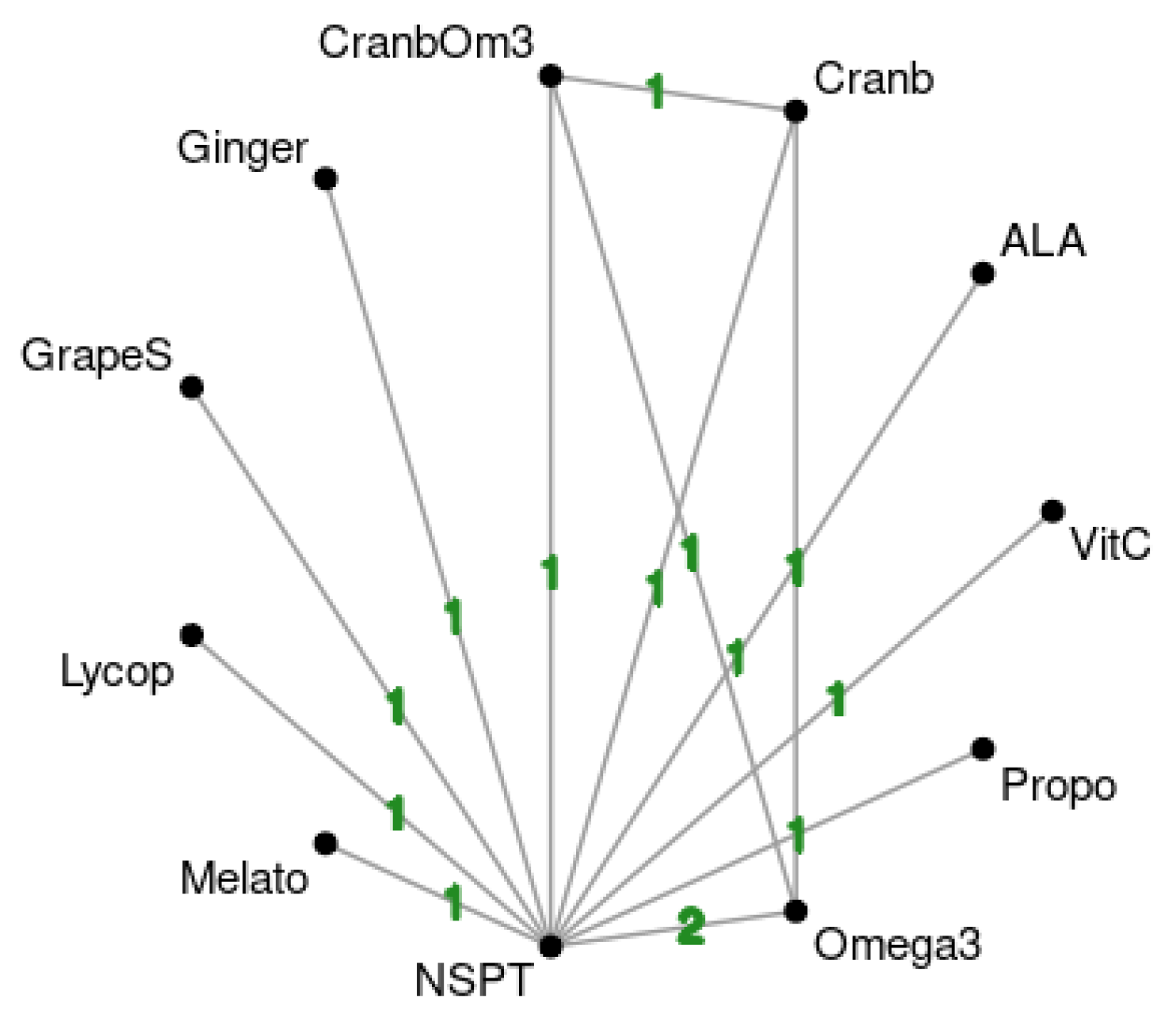
3.3. Network Meta-Analysis Results
3.4. Certainty of Evidence
4. Discussion
4.1. Limitations and Strengths
4.2. Implications for Future Research and Clinical Practice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S173–S182. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2013, 36, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, T.; Wu, X.; Nice, E.C.; Huang, C.; Zhang, Y. Oxidative stress and diabetes: Antioxidative strategies. Front. Med. 2020, 14, 583–600. [Google Scholar] [CrossRef]
- Deshpande, A.D.; Harris-Hayes, M.; Schootman, M. Epidemiology of diabetes and diabetes-related complications. Phys. Ther. 2008, 88, 1254–1264. [Google Scholar] [CrossRef]
- Papatheodorou, K.; Banach, M.; Bekiari, E.; Rizzo, M.; Edmonds, M. Complications of Diabetes 2017. J. Diabetes Res. 2018, 11, 3086167. [Google Scholar] [CrossRef]
- Grossi, S.G.; Genco, R.J. Periodontal Disease and Diabetes Mellitus: A Two-Way Relationship. Ann. Periodontol. 1998, 3, 51–61. [Google Scholar] [CrossRef]
- Preshaw, P.M.; Alba, A.L.; Herrera, D.; Jepsen, S.; Konstantinidis, A.; Makrilakis, K.; Taylor, R. Periodontitis and diabetes: A two-way relationship. Diabetologia 2012, 55, 21–31. [Google Scholar] [CrossRef]
- Taylor, G.W. Bidirectional interrelationships between diabetes and periodontal diseases: An epidemiologic perspective. Ann. Periodontol. 2001, 6, 99–112. [Google Scholar] [CrossRef]
- Sanz, M.; Ceriello, A.; Buysschaert, M.; Chapple, I.; Demmer, R.T.; Graziani, F.; Herrera, D.; Jepsen, S.; Lione, L.; Madianos, P.; et al. Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International diabetes Federation and the European Federation of Periodontology. Diabetes Res. Clin. Pract. 2018, 137, 231–241. [Google Scholar] [CrossRef]
- Loe, H. Periodontal Disease: The sixth complication of diabetes mellitus. Diabetes Care 1993, 16, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1998, 21, S5–S19. [Google Scholar] [CrossRef]
- Patil, V.S.; Patil, V.P.; Gokhale, N.; Acharya, A.; Kangokar, P. Chronic Periodontitis in Type 2 Diabetes Mellitus: Oxidative Stress as a Common Factor in Periodontal Tissue Injury. J. Clin. Diagnostic Res. 2016, 10, BC12. [Google Scholar] [CrossRef] [PubMed]
- Vincent, R.R.; Appukuttan, D.; Victor, D.J.; Balasundaram, A. Oxidative stress in chronic periodontitis patients with type II diabetes mellitus. Eur. J. Dent. 2018, 12, 225–231. [Google Scholar] [CrossRef]
- Arana, C.; Moreno-Fernández, A.M.; Gómez-Moreno, G.; Morales-Portillo, C.; Serrano-Olmedo, I.; de la Cuesta Mayor, M.C.; Hernández, T.M. Incremento de los parámetros de estrés oxidativo salival en pacientes con diabetes tipo 2: Relación con la enfermedad periodontal. Endocrinol. Diabetes Nutr. 2017, 64, 258–264. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Cai, W.; Zhao, S.; Shi, L.; Chen, Y.; Li, X.; Sun, X.; Mao, Y.; He, B.; Hou, Y.; et al. Oxidative stress-related biomarkers in saliva and gingival crevicular fluid associated with chronic periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, 608–622. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.; Rao, A.; Prasad, B.R.; Kumari, S. Serum levels of antioxidants and superoxide dismutase in periodontitis patients with diabetes type 2. J. Indian Soc. Periodontol. 2014, 18, 451–455. [Google Scholar] [CrossRef]
- Akalin, A.; Alatas, O.; Colak, O. Relation of plasma homocysteine levels to atherosclerotic vascular disease and inflammation markers in type 2 diabetic patients. Eur. J. Endocrinol. 2008, 158, 47–52. [Google Scholar] [CrossRef]
- Duarte, P.M.; Napimoga, M.H.; Fagnani, E.C.; Santos, V.R.; Bastos, M.F.; Ribeiro, F.V.; Araújo, V.C.; Demasi, A.P. The expression of antioxidant enzymes in the gingivae of type 2 diabetics with chronic periodontitis. Arch. Oral Biol. 2012, 57, 161–168. [Google Scholar] [CrossRef]
- Vats, A.; Gourie-Devi, M.; Verma, M.; Ramachandran, S.; Taneja, B.; Kukreti, R.; Taneja, V. Identification of L84F mutation with a novel nucleotide change c.255G > T in the superoxide dismutase gene in a North Indian family with amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Frontotemporal. Degener. 2016, 17, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Vega, C.M.; Godoy, J.M.; Barrocas, P.R.; Gonçalves, R.A.; De Oliveira, B.F.; Jacobson, L.V.; Mourão, D.S.; Hacon, S.S. Selenium levels in the whole blood of children and teenagers from two riparian communities at the Madeira River Basin in the Western Brazilian Amazon. Biol. Trace Elem. Res. 2017, 175, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45–63. [Google Scholar] [PubMed]
- Żukowski, P.; Maciejczyk, M.; Waszkiel, D. Sources of free radicals and oxidative stress in the oral cavity. Arch. Oral Biol. 2018, 92, 8–17. [Google Scholar] [CrossRef]
- Tunkel, J.; Heinecke, A.; Flemmig, T.F. A systematic review of efficacy of machine-driven and manual subgingival debridement in the treatment of chronic periodontitis. J. Clin. Periodontol. 2002, 29, 72–81. [Google Scholar] [CrossRef]
- Jain, A.; Gupta, J.; Bansal, D.; Sood, S.; Gupta, S.; Jain, A. Effect of scaling and root planing as monotherapy on glycemic control in patients of Type 2 diabetes with chronic periodontitis: A systematic review and meta-analysis. J. Indian Soc. Periodontol. 2019, 23, 303–310. [Google Scholar] [CrossRef]
- Teeuw, W.J.; Gerdes, V.E.A.; Loos, B.G. Effect of periodontal treatment on glycemic control of diabetic patients: A systematic review and meta-analysis. Diabetes Care 2010, 33, 421–427. [Google Scholar] [CrossRef]
- D’Aiuto, F.; Gkranias, N.; Bhowruth, D.; Khan, T.; Orlandi, M.; Suvan, J.; Masi, S.; Tsakos, G.; Hurel, S.; Hingorani, A.D.; et al. Systemic effects of periodontitis treatment in patients with type 2 diabetes: A 12 month, single-centre, investigator-masked, randomised trial. Lancet Diabetes Endocrinol. 2018, 6, 954–965. [Google Scholar] [CrossRef]
- Baeza, M.; Morales, A.; Cisterna, C.; Cavalla, F.; Jara, G.; Isamitt, Y.; Pino, P.; Gamonal, J. Effect of periodontal treatment in patients with periodontitis and diabetes: Systematic review and meta-analysis. J. Appl. Oral Sci. 2020, 28, e20190248. [Google Scholar] [CrossRef]
- Correa, F.O.B.; Gonçalves, D.; Figueredo, C.M.S.; Bastos, A.S.; Gustafsson, A.; Orrico, S.R.P. Effect of periodontal treatment on metabolic control, systemic inflammation and cytokines in patients with type 2 diabetes. J. Clin. Periodontol. 2010, 37, 53–58. [Google Scholar] [CrossRef]
- Chapple, I.L. Reactive oxygen species and antioxidants in inflammatory diseases. J. Clin. Periodontol. 1997, 24, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M. Free Radicals in Biology and Medicine, 3rd ed.; Oxford University Press: Midsomer Norton, UK, 1999. [Google Scholar]
- Ravipati, A.S.; Zhang, L.; Koyyalamudi, S.R.; Jeong, S.C.; Reddy, N.; Bartlett, J.; Smith, P.T.; Shanmugam, K.; Münch, G.; Wu, M.J.; et al. Antioxidant and anti-inflammatory activities of selected Chinese medicinal plants and their relation with antioxidant content. BMC Complement. Altern. Med. 2012, 12, 173. [Google Scholar] [CrossRef] [PubMed]
- Gharbi, A.; Hamila, A.; Bouguezzi, A.; Dandana, A.; Ferchichi, S.; Chandad, F.; Guezguez, L.; Miled, A. Biochemical parameters and oxidative stress markers in Tunisian patients with periodontal disease. BMC Oral Health 2019, 19, 225. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, K.; Buranasin, P.; Mikami, R.; Takeda, K.; Kido, D.; Watanabe, K.; Takemura, S.; Nakagawa, K.; Kominato, H.; Saito, N.; et al. Effects of antioxidant in adjunct with periodontal therapy in patients with type 2 diabetes: A systematic review and meta-analysis. Antioxidants 2021, 10, 1304. [Google Scholar] [CrossRef] [PubMed]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89, S159–S172. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan---a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Assessing risk of bias in a randomized trial. In Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Chichester, UK, 2019; Available online: www.training.cochrane.org/handbook (accessed on 20 September 2021).
- Owen, R.K.; Bradbury, N.; Xin, Y.; Cooper, N.; Sutton, A. MetaInsight: An interactive web-based tool for analyzing, interrogating, and visualizing network meta-analyses using R-shiny and netmeta. Res. Synth. Methods 2019, 10, 569–581. [Google Scholar] [CrossRef]
- Puhan, M.A.; Schünemann, H.J.; Murad, M.H.; Li, T.; Brignardello-Petersen, R.; Singh, J.A.; Kessels, A.G.; Guyatt, G.H.; GRADE Working Group. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 2014, 349, g5630. [Google Scholar] [CrossRef]
- Brignardello-Petersen, R.; Bonner, A.; Alexander, P.E.; Siemieniuk, R.A.; Furukawa, T.A.; Rochwerg, B.; Hazlewood, G.S.; Alhazzani, W.; Mustafa, R.A.; Murad, M.H.; et al. Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. J. Clin. Epidemiol. 2018, 93, 36–44. [Google Scholar] [CrossRef]
- Brignardello-Petersen, R.; Izcovich, A.; Rochwerg, B.; Florez, I.D.; Hazlewood, G.; Alhazanni, W.; Yepes-Nuñez, J.; Santesso, N.; Guyatt, G.H.; Schünemann, H.J.; et al. GRADE approach to drawing conclusions from a network meta-analysis using a partially contextualised framework. BMJ 2020, 371, m3907. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; LEA: New York, NY, USA, 1988; p. 40. [Google Scholar]
- Acharya, S.; Gujjari, S.K.; Murthy, K.S.; Battula, R. Evaluation of grape seed formulation as an adjunct to scaling and root planing on oxidative stress, inflammatory status and glycaemic control in Type-2 diabetic patients with chronic periodontitis: A randomised controlled trial. J. Clin. Diagn. Res. 2021, 15, ZC20–ZC25. [Google Scholar] [CrossRef]
- Rampally, P.; Koduganti, R.R.; Ganapathi, S.N.; Panthula, V.R.; Surya, P.J. Comparison of effectiveness of low-dose aspirin versus omega-3 fatty acids as adjuvants to nonsurgical periodontal therapy in Type II diabetic patients with chronic periodontitis. J. Indian Soc. Periodontol. 2019, 23, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.V.N.; Ambati, M.; Koduganti, R. Systemic lycopene as an adjunct to scaling and root planing in chronic periodontitis patients with type 2 diabetes mellitus. J. Int. Soc. Prev. Community Dent. 2015, 5, S25–S31. [Google Scholar] [CrossRef]
- Sundaram, G.; Theagarajan, R.; Gopalakrishnan, K.; Babu, G.R.; Murthy, G.D. Effect of fenugreek consumption with metformin treatment in improving plaque index in diabetic patients. J. Nat. Sci. Biol. Med. 2020, 11, 55–60. [Google Scholar] [CrossRef]
- Surapaneni, K.; Koduganti, R.R.; Ganapathi, S.N.; Panthula, V.N.R.; Jammula, S.P.; Dasari, R.; Gireddy, H.; Ambati, M. Efficacy of systemic administration of alpha lipoic acid and scaling and root planning in patients with chronic periodontitis and type 2 diabetes mellitus-A randomised controlled trial. J. Clin. Diagnostic Res. 2018, 12, ZC01–ZC05. [Google Scholar] [CrossRef]
- Gholinezhad, H.; Bazyar, H.; Rashidi, H.; Salehi, P.; Haghighi-zadeh, M.H.; Zare Javid, A. Using ginger supplement in adjunct with non-surgical periodontal therapy improves metabolic and periodontal parameters in patients with type 2 diabetes mellitus and chronic periodontitis: A double-blind, placebo-controlled trial. J. Herb. Med. 2020, 20, 100315. [Google Scholar] [CrossRef]
- Zare Javid, A.; Maghsoumi-Norouzabad, L.; Ashrafzadeh, E.; Yousefimanesh, H.A.; Zakerkish, M.; Angali, K.A.; Ravanbakhsh, M.; Babaei, H. Brignardello-Petersen Treatment on Metabolic Control and Periodontal Status in Type 2 Patients with Diabetes with Periodontal Disease. J. Am. Coll. Nutr. 2017, 37, 71–79. [Google Scholar] [CrossRef]
- Kunsongkeit, P.; Okuma, N.; Rassameemasmaung, S.; Chaivanit, P. Effect of Vitamin C as an Adjunct in Nonsurgical Periodontal Therapy in Uncontrolled Type 2 Diabetes Mellitus Patients. Eur. J. Dent. 2019, 13, 444–449. [Google Scholar] [CrossRef][Green Version]
- El-Sharkawy, H.M.; Anees, M.M.; Van Dyke, T.E. Propolis Improves Periodontal Status and Glycemic Control in Patients with Type 2 Diabetes Mellitus and Chronic Periodontitis: A Randomized Clinical Trial. J. Periodontol. 2016, 87, 1418–1426. [Google Scholar] [CrossRef]
- Anton, D.-M.; Martu, M.-A.; Maris, M.; Maftei, G.-A.; Sufaru, I.-G.; Tatarciuc, D.; Luchian, I.; Ioanid, N.; Martu, S. Study on the Effects of Melatonin on Glycemic Control and Periodontal Parameters in Patients with Type II Diabetes Mellitus and Periodontal Disease. Medicina 2021, 57, 140. [Google Scholar] [CrossRef] [PubMed]
- Karimian, J.; Hadi, A.; Pourmasoumi, M.; Najafgholizadeh, A.; Ghavami, A. The efficacy of propolis on markers of glycemic control in adults with type 2 diabetes mellitus: A systematic review and meta-analysis. Phytother. Res. 2019, 33, 1616–1626. [Google Scholar] [CrossRef]
- López-Valverde, N.; Pardal-Peláez, B.; López-Valverde, A.; Flores-Fraile, J.; Herrero-Hernández, S.; Macedo-de-Sousa, B.; Herrero-Payo, J.; Ramírez, J.M. Effectiveness of propolis in the treatment of periodontal disease: Updated systematic review with meta-analysis. Antioxidants 2021, 10, 269. [Google Scholar] [CrossRef] [PubMed]
- Hallajzadeh, J.; Milajerdi, A.; Amirani, E.; Attari, V.E.; Maghsoudi, H.; Mirhashemi, S.M. Effects of propolis supplementation on glycemic status, lipid profiles, inflammation and oxidative stresss, liver enzymes, and body weight: A systematic review and meta-analysis of randomized controlled clinical trials. J. Diabetes Metab. Disord. 2021, 20, 831–843. [Google Scholar] [CrossRef]
- Sanghani, N.N.; Shivaprasad, B.M.; Savita, S. Health from the Hive: Propolis as an adjuvant in the treatment of chronic periodontitis—A clinicomicrobiologic study. J. Clin. Diagn. Res. 2014, 8, ZC41–ZC44. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Ou, J.; Chen, L.; Zhang, Y.; Szkudelski, T.; Delmas, D.; Daglia, M.; Xiao, J. Dietary polyphenols and type 2 diabetes: Human Study and Clinical Trial. Crit. Rev. Food Sci. Nutr. 2019, 59, 3371–3379. [Google Scholar] [CrossRef] [PubMed]
- Afsharpour, F.; Javadi, M.; Hashemipour, S.; Koushan, Y.; Haghighian, H.K. Propolis supplementation improves glycemic and antioxidant status in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled study. Complement. Ther. Med. 2019, 43, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Pu, L.; Wei, J.; Li, J.; Wu, J.; Xin, Z.; Gao, W.; Guo, C. Brazilian green propolis improves antioxidant function in patients with type 2 diabetes mellitus. Int. J. Environ. Res. Public Health 2016, 13, 498. [Google Scholar] [CrossRef]
- Gao, W.; Pu, L.; Wei, J.; Yao, Z.; Wang, Y.; Shi, T.; Zhao, L.; Jiao, C.; Guo, C. Serum Antioxidant Parameters are Significantly Increased in Patients with Type 2 Diabetes Mellitus after Consumption of Chinese Propolis: A Randomized Controlled Trial Based on Fasting Serum Glucose Level. Diabetes Ther. 2018, 9, 101–111. [Google Scholar] [CrossRef]
- Toreti, V.C.; Sato, H.H.; Pastore, G.M.; Park, Y.K. Recent progress of propolis for its biological and chemical compositions and its botanical origin. Evid. Based Complement. Altern. Med. 2013, 2013, 697390. [Google Scholar] [CrossRef]
- Ekeuku, S.O.; Chin, K.-Y. Application of Propolis in Protecting Skeletal and Periodontal Health-A Systematic Review. Molecules 2021, 26, 3156. [Google Scholar] [CrossRef] [PubMed]
- Lakhtin, Y. Comparative evaluation of short- and long-term treatment of periodontitis with alpha-lipoic acid. Georgian Med. News. 2013, 5, 19–22. [Google Scholar]
- Golbidi, S.; Badran, M.; Laher, I. Diabetes and alpha lipoic acid. Front. Pharmacol. 2011, 2, 69. [Google Scholar] [CrossRef] [PubMed]
- Bazyar, H.; Gholinezhad, H.; Moradi, L.; Salehi, P.; Abadi, F.; Ravanbakhsh, M.; Zare Javid, A. The effects of melatonin supplementation in adjunct with nonsurgical periodontal therapy on periodontal status, serum melatonin and inflammatory markers in type2 diabetes mellitus patients with chronic periodontitis:a double-blind, placebo-controlled trial. Inflammopharmacology 2018, 27, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Singh, H.; Ahmad, N.; Mishra, P.; Tiwari, A. The role of melatonin in diabetes: Therapeutic implications. Arch. Endocrinol. Metab. 2015, 59, 391–399. [Google Scholar] [CrossRef]
- Ostadmohammadi, V.; Soleimani, A.; Bahmani, F.; Aghadavod, E.; Ramezani, R.; Reiter, R.J.; Mansournia, M.A.; Banikazemi, Z.; Soleimani, M.; Zaroudi, M.; et al. The Effects of Melatonin Supplementation on Parameters of Mental Health, Glycemic Control, Markers of Cardiometabolic Risk, and Oxidative Stress in Diabetic Hemodialysis Patients: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Ren. Nutr. 2020, 30, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, A.; Higgins, J.P.T.; Geddes, J.R.; Salanti, G. Conceptual and Technical Challenges in Network Meta-analysis. Ann. Intern Med. 2013, 159, 130–137. [Google Scholar] [CrossRef]
| Author, Year Country | Age in Years Mean ± SD and/or Range | Case Definitions | Groups (N) | Treatments TG CG | Baseline HbA1c % Mean ± SD | Follow-Up (in Months) | Final HbA1c % Mean ± SD (p Value) | Main Conclusions |
|---|---|---|---|---|---|---|---|---|
| Acharya et al., 2021, India | NA | Periodontitis: CP with PPD ≥ 5 mm Diabetes: HbA1c in the range 6%–8% and FBS in the range 135–205 mg/dL, for 5 to 10 years | TG (n = 24) CG (n = 24) | TG: 200 mg of Grape Seed extract for 12 weeks + NSPT CG: placebo for 12 weeks + NSPT | TG 7.33 ± 0.73 CG 7.3 ± 0.71 | 12 weeks 24 weeks | TG 6.38 ± 0.51 (p < 0.01) CG 6.81 ± 0.55 (p < 0.01) CBG: NS TG 6.68 ± 0.59 (p < 0.01) CG 6.76 ± 0.54 (p < 0.01) CBG: NS | This study shows a promising result in using grape seed formulation as an adjunct to scaling and root planing to reduce the oxidative stress, decreasing the inflammation and achieving the glycaemic control in diabetic patients with CP. |
| Anton et al., 2021, Romania | TG 53.24 ± 3.4 CG 52.21 ± 3.1 | Periodontitis: CAL ≥ 5 mm Diabetes: FBS > 126 mg/dL and HbA1c > 6.5% | TG (n= 27) CG (n= 27) | TG: two tablets containing 3 mg of melatonin daily for 8 weeks + NSPT CG: placebo for 8 weeks + NSPT | TG 7.62 ± 0.71 CG 7.61 ± 0.62 | 8 weeks | TG 6.28± 0.31 p < 0.001 CG 7.58 ± 0.57 (NS) CBG: p < 0.001 | Combined NSPT and systemic treatment with melatonin provided additional improvements to severe periodontal condition (improve PPD and CAL) and the glycemic control of patients with type 2 diabetes. |
| El-Sharkawy et al., 2016, Egypt | TG 48.9 ± 8.3 Age range: 38–63 CG 51.2 ± 6.5 Age range: 40–61 | Periodontitis: PPD and CAL ≥ 5 mm with BOP in at least one site in each sextant Diabetes: History T2D > 5 years | TG (n = 24) CG (n = 26) | TG: 400 mg propolis capsule orally daily for 24 weeks + NSPT CG: placebo for 24 weeks + NSPT | TG 8.73 ± 0.55 CG 8.59 ± 0.91 | 12 weeks 24 weeks | TG: 8.71 ± 0.56 (p < 0.01) CG: 8.58 ± 0.82 (NS) CBG: NA TG 7.75 ± 0.48 (p < 0.01) CG 8.5 ± 0.73 (NS) CBG: NA | A 6-month regimen of 400 mg daily propolis + SRP significantly reduces HbA1c levels and improves periodontal therapy outcomes (PPD and CAL gain). |
| Gholinezhad et al., 2019, Iran | TG 52.81 ± 6.44 CG 51.62 ± 5.95 | Periodontitis: PPD ≥ 4 mm and CAL = 1–4 mm Diabetes: FBS ≥ 126 mg/dL and HbA1c ≥ 6.5% > 5 years | TG (n = 21) CG (n = 21) | TG: two tablets with 1 g ginger supplement twice daily for 8 weeks + NSPT CG: placebo for 8 weeks + NSPT | TG 8.60 ± 1.37 CG 8.35 ± 1.01 | 8 weeks | TG 7.84 ± 1.48 (p = 0.008) CG 8.18 ± 1.02 (NS) CBG: NS | Ginger + NSPT may be effective in control of the glycemic, lipid, antioxidant, and periodontal status (PPD, CAL, PI and BOP levels) in T2DM patients with CP. |
| Kunsongkeit et al., 2019, Thailand | TG 59.87 ± 11.3 CG 57.94 ± 14.0 | Periodontitis: CAL ≥ 3 mm and PD ≥ 5 mm at least in one tooth Diabetes: FBS > 150 mg/dL and HbA1c > 7% | TG (n = 15) CG (n = 16) | TG: 500 mg/day vitamin C for 8 weeks + NSPT CG: placebo for 8 weeks + NSPT | TG 7.53 ± 0.79 CG 8.39 ± 1.50 | 8 weeks | TG 7.27 ± 0.88 (NS) CG 7.98 ± 1.85 (NS) CBG: NS | Supplementation of 500 mg/day vitamin C did not give an additional benefit, HbA1c were not significantly different compared with baseline in the test group. All periodontal parameters were significantly improved in both groups. |
| Rampally et al., 2019, India | Age range: 30–65 | Periodontitis: at least four teeth with one or more sites with PD ≥ 5 mm and CAL ≥ 4 mm Diabetes: HbA1c ≥ 6.5% | TG1 (n = 14) TG2 (n = 14) CG (n = 14) | TG1 75 mg of aspirin orally once a day for 12 weeks NSPT TG2 500 mg of O3FAs orally twice a day for 12 weeks + NSPT CG placebo for 12 weeks + NSPT | TG1 8.97 ± 1.46 TG2 8.079 ± 1.15 CG 7.54 ± 0.82 | 12 weeks | TG1 6.98 ± 0.88 (p < 0.001) TG2 7.136 ± 1.21 (p < 0.001) CG 7.25 ± 0.81 (p < 0.001) CBG: NS | All groups showed statistically significant results after 3 months for HbA1c and periodontal clinical parameters (GI, PPD and CAL). However, the difference between the groups was not significant for those parameters. |
| Reddy et al., 2015, India | Age range: 35–50 | Periodontitis: at least four teeth with one or more sites with PPD ≥ 5 mm, CAL ≥ 4 mm and BOP Diabetes: FPG >126 mg/dL | TG (n = 20) CG (n = 20) | TG: 8 mg Lycopene soft gels daily for 8 weeks + NSPT CG: NSPT | TG 7.58 ± 0.88 CG 7.80 ± 0.98 | 8 weeks 24 weeks | TG 6.10 ± 0.56 (p < 0.001) CG 6.84 ± 0.65 (p < 0.001) CBG: p < 0.001 TG 6.82 ± 0.61 (NS) CG 7.12 ± 0.41 (NS) CBG: NS | Lycopene along NSPT was effective in restoring altered glycemic control and in reducing the PPD in diabetic patients. |
| Sundaram et.al. 2020, India | NA | Periodontitis: at least 30% of the sites with CAL ≥ 4 mm, PD ≥ 5 mm and BOP Diabetes: HbA1c > 8% and history T2D > 5 years | TG (n = 40) CG (n = 40) | TG: 12,5 mg fenugreek powder twice daily for 4 weeks + NSPT CG: NSPT | CG 8.5 ± 0.9 TG 8.90 ± 1.1 | 4 weeks | TG 6.7 ± 0.5 (p < 0.001) CG 7.3 ± 0.6 (NS) CBG: NS | Fenugreek + NSPT might have added additional benefit in reducing the glycemic status There was also a significant reduction in the PI. |
| Surapaneni et al., 2018, India | 35–60 (mean age 50.3) | Periodontitis: at least 4 teeth with PPD ≥ 5 mm, CAL ≥ 4 mm and BOP Diabetes: HbA1c ≥ 6.5% up to 10%, recently diagnosed (<1 month) | TG (n = 20) CG (n = 20) | TG: Alpha Lipoic Acid 600 mg thrice a day for 12 weeks + NSPT CG: NSPT | TG 9.9 ± 0.3 CG 8.6 ± 1.1 | 12 weeks | TG 6.3 ± 0.3 (p < 0.001) CG 7.4 ± 0.7 (p < 0.001) CBG: p < 0.001 | Alpha Lipoic Acid + NSPT proved to be efficacious in improving the clinical parameters (GI, PPD and CAL), and glycemic control in patients with CP and T2DM. |
| Zare Javid et al., 2017, Iran | TG1: 57,75 ± 8,58 TG2: 57,88 ± 6,03 TG3: 53,14 ± 6,91 CG: 53,60 ± 6,23 | Periodontitis: ten selected sites PPD ≥ 4 mm from at least 3 of the quadrants Diabetes: History T2D > 5 years | TG1 (n = 10) TG2 (n = 9) TG3 (n = 10) CG (n = 12) | TG1: 1 g O3FA capsule twice daily, for 8 weeks + NSPT TG2: 200 mL cranberry juice twice daily for 8 weeks + NSPT TG3: 200 mL cranberry juice enriched with 1 g O3FA twice daily for 8 weeks + NSPT CG: NSPT | TG1 6.82 ± 1.31 TG2 6.17 ± 0.53 TG3 6.32 ± 0.40 CG 6.64 ± 0.72 | 8 weeks | TG1 5.95 ± 0.60 (p = 0.025) TG2 5.92 ± 0.65 (NS) TG3 5.92 ± 0.19 (p= 0.047) CG 6.35 ± 0.76 (NS) CBG: NS | Cranberry juice enriched with O3FA can be beneficial in decreasing HbA1c and improving periodontal status in patients with diabetes and periodontal disease. |
| Cohen’s Classification 1 | Intervention 2 | Intervention versus NSPT SMD 3 [95% CI] | Intervention versus NSPT MD 4 [95% CI] | Certainty |
|---|---|---|---|---|
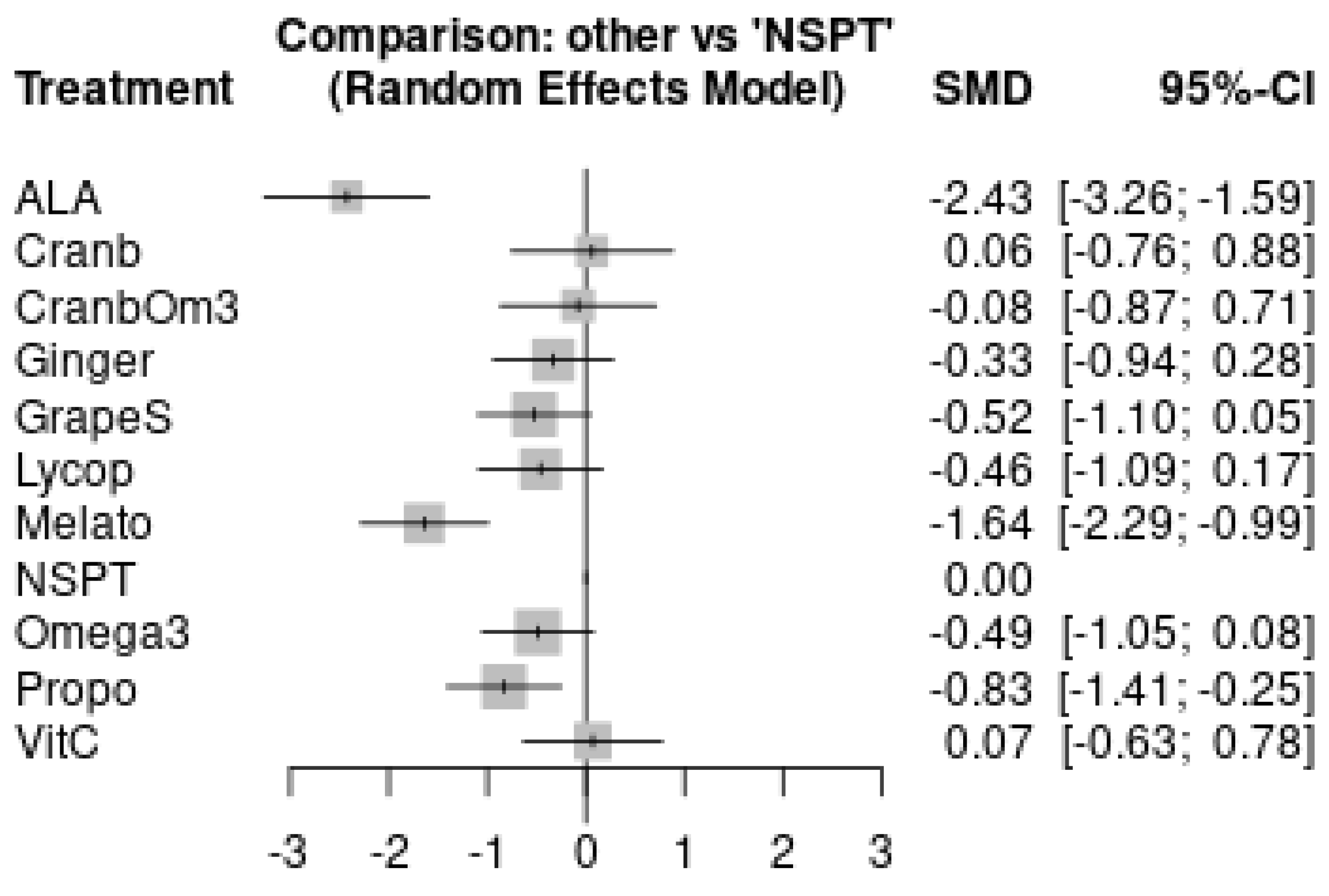
| Large effect | Propolis | −0.83 [−1.41; −0.25] | −0.74 [−1.22; −0.26] | Moderate |
| Large effect | ALA | −2.43 [−3.26; −1.59] | −2.40 [−3.00; −1.80] | Low |
| Melatonin | −1.64 [−2.29; −0.99] | −1.31 [−1.75; −0.87] | Low | |
| Moderate effect | Grape Seeds | −0.52 [−1.10; 0.05] | −0.46 [−0.95; 0.03] | Very Low |
| Small effect | Omega-3 | −0.49 [−1.05; 0.08] | −0.62 [−1.37; 0.14] | Very Low |
| Lycopene | −0.46 [−1.09; 0.17] | −0.52 [−1.21; 0.17] | Very Low | |
| Ginger | −0.33 [−0.94; 0.28] | −0.59 [−1.65; 0.47] | Very Low | |
| Trivial/No effect | Cranberry + Omega-3 | −0.10 [−0.94; 0.74] | −0.11 [−0.77; 0.55] | Very Low |
| Cranberry | 0.04 [−0.83; 0.90] | 0.04 [−0.77; 0.85] | Very Low | |
| Vitamin C | 0.07 [−0.63; 0.78] | 0.15 [−1.28; 1.58] | Very Low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araújo, E.G.; Oliveira, D.M.S.L.d.; Martins, C.C.; Stefani, C.M. Efficacy of Antioxidant Supplementation to Non-Surgical Periodontal Therapy on Metabolic Control in Type 2 Diabetes Patients: A Network Meta-Analysis. Antioxidants 2022, 11, 621. https://doi.org/10.3390/antiox11040621
Araújo EG, Oliveira DMSLd, Martins CC, Stefani CM. Efficacy of Antioxidant Supplementation to Non-Surgical Periodontal Therapy on Metabolic Control in Type 2 Diabetes Patients: A Network Meta-Analysis. Antioxidants. 2022; 11(4):621. https://doi.org/10.3390/antiox11040621
Chicago/Turabian StyleAraújo, Elisa Grillo, Domitilla Marchiori Sant’Anna Leal de Oliveira, Carolina Castro Martins, and Cristine Miron Stefani. 2022. "Efficacy of Antioxidant Supplementation to Non-Surgical Periodontal Therapy on Metabolic Control in Type 2 Diabetes Patients: A Network Meta-Analysis" Antioxidants 11, no. 4: 621. https://doi.org/10.3390/antiox11040621
APA StyleAraújo, E. G., Oliveira, D. M. S. L. d., Martins, C. C., & Stefani, C. M. (2022). Efficacy of Antioxidant Supplementation to Non-Surgical Periodontal Therapy on Metabolic Control in Type 2 Diabetes Patients: A Network Meta-Analysis. Antioxidants, 11(4), 621. https://doi.org/10.3390/antiox11040621






