Abstract
Diabetic retinopathy is a neurovascular complication of diabetes and the main cause of vision loss in adults. Glial cells have a key role in maintenance of central nervous system homeostasis. In the retina, the predominant element is the Müller cell, a specialized cell with radial morphology that spans all retinal layers and influences the function of the entire retinal circuitry. Müller cells provide metabolic support, regulation of extracellular composition, synaptic activity control, structural organization of the blood–retina barrier, antioxidant activity, and trophic support, among other roles. Therefore, impairments of Müller actions lead to retinal malfunctions. Accordingly, increasing evidence indicates that Müller cells are affected in diabetic retinopathy and may contribute to the severity of the disease. Here, we will survey recently described alterations in Müller cell functions and cellular events that contribute to diabetic retinopathy, especially related to oxidative stress and inflammation. This review sheds light on Müller cells as potential therapeutic targets of this disease.
1. Pathophysiology of Diabetic Retinopathy
Diabetic retinopathy (DR) is a neurovascular disease that ultimately leads to blindness in diabetes mellitus patients [1]. Visual loss impacts the quality of life, daily activities, and reduces individual work capacity [2], contributing to economic burden on health systems [3]. Due to the absence of a cure and the unavailability of efficient treatment for DR, development of therapeutic alternatives should be a priority. Emerging evidence points to the role of Müller glia cells (MGC), not only as crucial players in the retinal physiology, but also involved in many dysfunctional aspects in diabetes. This review aims to discuss the molecular and cellular mechanisms underlying DR, focusing on early retinal alterations in the diabetic condition as a promising therapeutic window to prevent blindness. Here, we will present evidence of the contribution of MGC deficit to DR progression, and we will suggest MGC as alternative therapeutic target to DR.
Glycaemia uncontrolled over a long period of time, dysregulates several metabolic pathways in the retina, leading to a progressive neurovascular impairment characterized by many elements, such as alteration in the levels of growth and neurotrophic factors, oxidative stress, inflammation, hypoxia, adhesion molecules, and genetic factors [4,5,6,7]. Since diagnosis and treatment of DR rely on the detection of microvascular alterations, it is considered a vascular disease, divided into two late stages: non-proliferative diabetic retinopathy and proliferative diabetic retinopathy. A large amount of data gathered over the last two decades has revealed many early modifications in neural aspects of retinal physiology/anatomy, extending the current concept of DR so as to be considered a neurovascular disease. Whether diabetic retinal neuropathy is the cause or effect of microangiopathy under chronic hyperglycemia is still under debate [8,9].
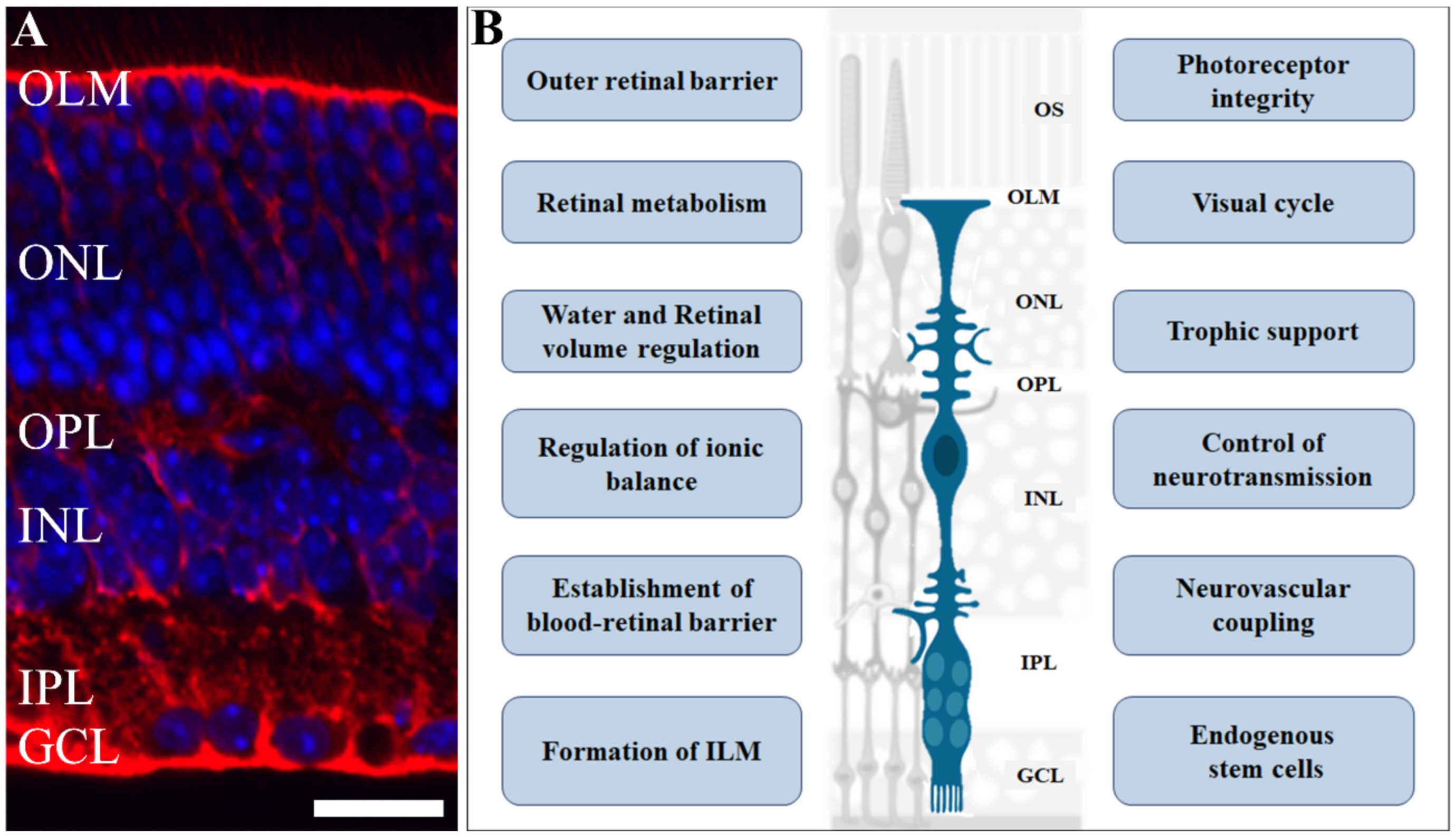
The retina is a special tissue with a highly ordered architecture containing light transducing cells (photoreceptors), interneurons, projection neurons, and three types of glial cells. The cell bodies of retinal cells are organized in three nuclear layers intercalated by plexiform layers, consisting of cell processes and synapses (Figure 1A). Located on the outermost layer, closer to the choroid, are two types of photoreceptor cells, approximately 120 million rods [10] and 4.6 million cones in the human eye [11], responsible for phototransduction, and forming the outer nuclear layer. In addition, around 0.7–1.5 million retinal ganglion cells, found in the ganglion cell layer, transmit electrical activity through the optic nerve to the brain’s visual processing areas [12]. In between these two layers, there is the inner nuclear layer, containing the cell bodies of excitatory bipolar interneurons, controlled by inhibitory horizontal and amacrine neurons, and MGC, a fundamental cell involved with major functions in the retina.
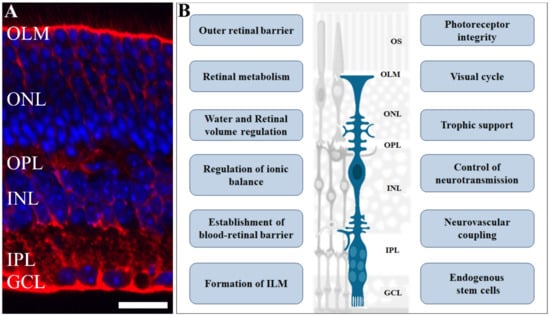
Figure 1.
Role of Müller glia in retinal physiology. Müller glia cells have an elaborate radial morphology, characterized by vertical processes, that span throughout the retina and branches in outer and inner layers: (A) Photomicrograph of retinal section immunolabelled for glutamine synthetase (red), a Müller cell marker, counterstained with DAPI (blue), showing the retinal cells nuclei. (B) A scheme highlighting various functions and events regulated by Müller cells in the retina such as retinal volume and ionic balance; blood–retinal barrier formation and maintenance; neurotransmission control, trophic support for other cells, and are an endogenous source of stem cells. Scale bar= 20 µm. OS: outer segment; OLM: outer limiting membrane; ONL: outer nuclear layer; OPL: outer plexiform layer; INL: inner plexiform layer; IPL: inner plexiform layer; GCL: ganglion cell layer. Adapted from smart.servier.com (accessed on 1 April 2021).
The retina is known to be one of the most energetically demanding tissues in the body [13]. The energy support for the mammalian retina comes from the inner and outer vascular systems. The vascularization of the inner retina consists of three parallel planar vascular plexuses that originate from a branch of the internal carotid artery, the ophthalmic artery. The ophthalmic artery enters through the optical disc and branches into the inner (ganglion cell layer) and outer (inner plexiform layer, inner nuclear layer to outer plexiform layer) intraretinal bed and the peripapillary bed, present in the human and mouse eye but absent in the rat [14]. The blood supply for the outer retina comes from the choroidal vasculature. The intraretinal and the choroid blood vessels consist of, respectively, non-fenestrated and fenestrated endothelial cells, the former surrounded by pericytes. Therefore, the inner, but not the outer, retinal vasculature has a blood–retinal barrier (BRB) that rigorously regulates the molecular traffic between blood and retina.
A hallmark of the initial non-proliferative diabetic retinopathy vascular damage is the induction of basal membrane thickness. This leads to a reduced endothelial adherence and tight junction impairment, resulting in BRB breakdown. Moreover, there is cell loss of pericytes and endothelial dysfunction, resulting in fragile capillaries, formation of microaneurysms, vascular leakage, and small hemorrhages. The accumulation of these vascular lesions results in ischemia in some areas of the retinal tissue. The ischemic condition induces a further increase in the release of pro-angiogenic factors, such as vascular endothelial growth factor (VEGF), promoting neovascularization, a hallmark for the beginning of proliferative diabetic retinopathy [15]. However, these new blood vessels, in most cases, are not functional, aggravating the damage. Neovascularization exacerbates micro-hemorrhages and can also lead to retinal detachment, exacerbating the patient’s visual loss [16].
Although the current diagnosis of DR is based on vascular alterations, and treatment is limited to more advanced stages of the disease, a large amount of data has revealed changes in neural retina before clinical signs of vascular abnormalities in animal models and patients [17,18,19,20]. Indeed, electrophysiological studies have demonstrated that diabetic patients present neuronal alterations even when no vascular (clinical) signs are detected in the examination of the eye fundus [21,22,23].
Neurodegeneration is one of the earliest signs that occurs in experimental models for DR, detected as early as two weeks post-diabetes induction [17,24,25,26]. Accordingly, Carrasco and coworkers (2007) have described that post-mortem diabetic patients with no clinically detectable signs of DR show a significantly higher number of apoptotic cells in all retinal layers [18]. More recently, with the advance of powerful methodological strategies, such as optical coherence tomography, it has been shown that patients with diabetes have thinner inner nuclear–ganglion cell layers and reduced nerve fiber layer, confirming in an in vivo procedure that neurodegeneration is an early event in DR pathology [27,28,29]. Indeed, a thinner nerve fiber layer has been correlated with early changes in the peripapillary vessel morphology and vessel density of the radial peripapillary plexus in patients with diabetes without DR. Earlier changes in superficial vessel density have been documented in the peripapillary rather than in the macular region [30]. Based on these new imaging techniques new biomarkers have been developed that could help in the detection of subclinical disease, before clinically (vascular) detectable changes or visual symptoms are noted [31]. However, this approach is expensive, which may limit the impact in preventing DR worldwide.
In addition to neurons, three types of glia can be found in the retina: astrocytes in the nerve fiber layer; microglia mainly in the plexiform layers; and, the predominant type, the MGC. The optic nerve presents oligodendrocytes besides astrocytes and microglia. We have previously shown that all these glial types are altered in the type I diabetes mice model, and attenuation of inflammation controls this response [32].
Glial cells have key functions in maintaining brain homeostasis and in the control of central nervous system (CNS) in response to insults. Emerging evidence from our research and others supports the concept that reactive glial cells play fundamental roles in neurodegenerative diseases such as Alzheimer’s and Parkinson’s diseases [33,34,35,36,37]. In common, these conditions present a general neuroinflammatory scenario, characterized by production and release of pro- and/or anti-inflammatory cytokines and chemokines, antioxidants, free radicals, and neurotrophic factors by glial cells, such as astrocytes, microglia, and MGC. Whether this response contributes to the development or delay of the disease is still a matter of discussion. Whereas involvement of glial cells in brain diseases is well documented, the contribution of MGC to neurodegenerative diseases such as DR, is less clear. Given the large number of functions performed by MGC, it is expected that alterations of these cells might have a major impact in DR outcome. In the next sections, we will present the roles of MGC in retinal function and homeostasis and discuss accumulating evidence of the involvement of these cells in DR.
2. Role of Müller Cells in Retinal Physiology
MGC is the predominant (90%) glial element of the vertebrate retina. It has been considered for a long time to be a passive element, supporting the structure and function of the architecture of the vertebrate retina. Identified in the middle of the 19th century by the anatomist Heinrich Müller as radial fibers [38,39], these cells span the entire retinal tissue: with the soma in the inner nuclear layer and their radial processes extending from the inner vitreous border to the outer retina in the inner segment of photoreceptors [40,41] (Figure 1A). Due to this unique morphology, the MGC interact with cells in all retinal layers contributing to specify neuron–glia–vascular–epithelium functional domains.
Although a full assessment of MGC morphology and function is beyond the scope of the present review, we will provide an overview of MGC properties in the healthy retina, important to understand the impact of its dysfunction in DR.
MGC play several important roles in the outer retina. These cells regenerate all-trans retinol to 11-cis retinal participating in the cone visual cycle, a crucial step to maintain the response to light, a process formerly attributed exclusively to pigmented epithelium cells [42,43]. Important for visual acuity, is the ability of MGC to function as optical fibers guiding the light [44,45,46,47,48]. Interestingly, the local density of MGC and cones are the same in many species in a way that each Müller cell guides the light to “its own” cone photoreceptor [45,48]. Thus, MGC bypass the light scattering by nerve fibers and synapses allowing minimal light distortion and little light loss to photoreceptors. The strands of microtubules and intermediate filaments in the radial fibers of MGC may also contribute to structural stability for the retina, especially for central fovea [45].
The outer processes of MGC are attached to each other and to the inner segments of photoreceptors by adherens and tight junctions to form the outer limiting membrane (OLM). The OLM plays a role in maintaining the structure of the retina through mechanical strength and as part of the outer retinal barrier [49,50].
The ability of MGC to phagocyte is important to the integrity of photoreceptors and for the rearrangement of new cone outer segments (COS) under physiological [51] and pathological conditions [52]. In addition, there are two types of MGC in the fovea: specialized MGC, which form a structure called the Müller cell cone in the foveola; and the z-shape MGC of the fovea walls and parafovea [40]. The specialized MGC of the foveola contains a high density of macular pigment, protecting photoreceptors by acting as a short-wavelength light filter compensating the absence of blood vessels in this region [45,53].
The inner retina also benefits from MGC [41,54]. The vitreous endfeet of MGC processes, together with an extracellular matrix structure, form the inner limiting membrane (ILM), which is critical in defining the retinal border and stabilizing ocular blood vessels [41,54]. The ILM region is the main location of TRPV4 (Transient Receptor Potential Cation Channel Subfamily V Member 4) in MGC, which seems to exert an important role as an osmosensor, crucial for volume (dys)regulation of the retina under both healthy and pathological conditions [55]. The TRPV4 function in MGC requires arachidonic acid and 5,6-EET [55]. 5,6-Epoxyeicosatrienoic acid (5,6-EET), among other inflammatory signals, activates TRPA1 [56], which is also expressed by MGC [57]. MGC also respond to swelling or shrinkage of neurons or extracellular space, opening potassium and chloride channels and adapting their morphology, regulating cellular volume [58]. Additionally, aquaporin 4 (AQP4), a water channel present in MGC, is also involved in retinal volume regulation [59]. Water in the inner retina is transported by MGC and can be eliminated by pigmented epithelium cells [60,61]. So, MGC and retinal pigment epithelium cells (RPE) actively drain water and osmolytes outside the retina. The potassium channels involved in water removal in MGC are also important for the regulation of ionic balance. MGC take up and redistribute extracellular potassium, arising during neuronal activity especially in the plexiform layers, in a process called potassium spatial buffering [62]. This is very important for maintenance of the neuronal microenvironmental since the increase in extracellular potassium induces neuronal depolarization and is also coupled to drainage function of MGC [50].
MGC also have a huge influence on the vascular function in the retina, from the establishment of BRB [63] to the maintenance and enhancement of its function [50,64]. Retinal arterioles, venules, and capillaries are tightly ensheathed by macroglia. In the retina, the vascular tone is controlled by glial cells, astrocytes, and Müller cells. Since the astrocyte cells are restricted to nerve fiber layers and GCL, Müller cells probably have a predominant action [65,66,67] (Table 1). ATP seems to be a key modulator, by acting as P2X receptors, inducing the production and release of metabolites of arachidonic acid that control vessels constriction [67]. The described coupling between neuronal activity and blood flow is called neurovascular coupling, which is also largely mediated by glial cells in the retina [67].

Table 1.
Müller cell under retinal physiology and diabetic conditions.
In addition, Müller cells primarily metabolize glucose by anaerobic glycolysis [131], and represent the main site of retinal glycogen storage, from which metabolites can be donated to other retinal cells [134]. Thus, oxygen is saved for neurons warranting more resistance to anoxia/hypoxia conditions and the lactate can be donated to neurons, to use in alternative energy pathways to produce ATP, especially for photoreceptors [132,133] (Table 1).
The special and characteristic morphology of MGC enabling them to transverse the retina, together with the ability to uptake and metabolize glutamate and gamma-aminobutyric acid (GABA), the main excitatory and inhibitory neurotransmitters, respectively, underlies another key role in the retina: control of neurotransmission [40]. Glutamate uptake by MGC is converted to glutamine, through glutamine synthetase, the so-called glutamate–glutamine cycle, regulating the driving force of neuronal glutamate uptake and the maintenance of biochemical pathways, especially in the inner retina [131,138]. In addition to the buffering of GABA and glutamate availability, MGC also regulate neuron excitability, especially ganglion cells, neuroprotection, and immune response through gliotransmitters (ATP, glutamate, D-serine, among others), cytokines, neurotrophic factors, antioxidants, and inflammatory signals [41,139,140].
Finally, MGC are considered as endogenous stem cells in the adult retina, and upon being activated by damage or growth factors, they proliferate, de-differentiate, and contribute to neural regeneration [113]. However, the regenerative potential of MGC varies greatly according to vertebrate species, behaving as radial-glial-like neural stem cells in fish and amphibians (regenerating retinal neurons in response to injury) and being extremely limited in mammals and birds [141]. Pathological conditions, such as excitotoxicity, induce MGC gliosis increasing GFAP expression, changing the identity and signaling pattern [113,142].
In the past two decades, various genes and pathways have been studied to support research on the stemness shown by MGC. Postnatal and adult mouse MGC have been genetically reprogrammed through the expression of proneural genes in the Lin28-let7-Ascl1 axis to unlock the neurogenic potential of mammalian MGC [143]. Overexpression of ASCL1 in dissociated mouse MGC in cultures or in intact retinal explants upregulated retinal progenitor-specific genes and downregulated glial genes [144]. MGC-derived progenitors differentiated into cells that exhibited neuronal morphologies, expressed retinal subtype-specific neuronal markers, and displayed neuron-like physiological response. Recent data have shown a generation of retinal neurons from pluripotent stem cells or MGC, thus implying that an in situ cellular reprogramming strategy for restoration of vision might not be so distant [145,146,147,148]. Alternatively, a small subset of miRNAs (highly conserved ~22 nucleotide (nt) RNAs that post-transcriptionally regulate gene expression) involved with development in vertebrates, has been recognized as controlling MGC reprogramming during retina regeneration, behaving as therapeutic targets for treatment of visual disorders and damage [149]. Recent data from the lab of Iqbal Ahmad suggest that in the near future, neurogenic properties of MGC might be revealed by serial exposure to different combinations of small molecules such as a cocktail [150]. For instance, Müller cells exposed to I-bet151, an inhibitor of the binding of BET family of proteins to the cell-type specific activated chromatin domains, forskolin, a diterpene adenylate cyclase activator, isoxazole 9 (ISX9), which induces neuronal differentiation, might silence glia-specific genes, and induce acquired transcriptional signature of neurons, therefore regulating neuronal potential of MGC. Neurons derived from MGC expressed neuronal proteins and showed electrophysiological features of immature neurons [150]. Thus, reprogramming of MGC might represent a possible treatment for regeneration of the retinal neural circuits, restoring visual capacity in patients in advanced stages of DR.
As discussed, MGC play key roles in the retinal functions summarized in Figure 1B [40,138,139,140,151,152]. The current concept of MGC implies that these cells are active players in a healthy or injured retina. Due to the broad actions of MGC in retina homeostasis, it is likely that dysfunctions on these cells can severely impact retinal function. Essentially, Müller cells respond primarily with a proinflammatory phenotype when activated and secretion of cytokines and components of the complement system [153] Authors described mitochondrial dysfunction, implying oxidative stress after treatment with the various cytokines, highlighting the importance of Müller cell signaling in the aggravated retina, indicating an active role in chronic retinal inflammation. A combination of high glucose levels and oxidative stress favor DR in Müller cells, which reduce glutamine synthetase levels, and augment the migration capacity of Müller glia. This suggests that these experimental conditions could induce some degree of dedifferentiation and favor the migration ability. Indeed, a correlation with several expressed genes and transcriptomic alteration related to glucose metabolism, cell migration, development, and pluripotency were found in cells of diabetic animals associated with the histone deacetylase sirtuin 6 (SIRT6), as an epigenetic modulator [154]. One of the transcription factors possibly involved in the differential expression program observed in diabetic MGs is SRY-Box transcription factor 9 (SOX9). Regarding inflammation, it is known that this multi-step process plays a crucial role in retinal regeneration when the model is zebrafish. Several mediators are involved as pattern recognition receptors activated by damage-associated molecular pattern molecules, including the chromatin-associated nuclear protein high mobility group 1, heat shock proteins, purine metabolites, such as adenosine triphosphate (ATP), and uric acid [155]. In the chick retina, nuclear factor kappa B (NF-κB), a critical regulator of inflammation, was shown to inhibit the reprogramming of Müller glia into proliferating progenitor cells [156]. In addition, inflammation-induced mammalian target of rapamycin (mTOR) signaling in the Müller glia was shown to be important for retina regeneration in the adult zebrafish [157] and the chick model [158]. Finally, the innate immune system also plays key roles in inflammation, reprogramming, and tissue regeneration. Indeed, microglia stimulate neurogenesis in avian and fish Müller cells after injury, but they do not normally regenerate; overexpressing the pro-neural achaete-scute family bHLH transcription factor 1 (Ascl1) in Müller glia is sufficient to stimulate regeneration into functional neurons in the adult mouse retina neurogenesis. A combination of Ascl1:Atoh1 (from atonal family bHLH transcription factor 1), increases efficiency at stimulating neurogenesis, even in the absence of retinal injury [159]. Microglia inhibit the Ascl1-mediated retinal regeneration, suggesting that the innate immune system limits the regenerative response to injury [159]. Reactive microglia coordinate signals as NF-κB to suppresses the reprogramming of Müller glia into proliferating progenitor cells [156]. In the next section, we will discuss the implications of the MGC function on the outcome of DR.
3. Müller Cells in DR
As discussed previously, while there are many studies on neurons and vascular cells in DR, less attention has been given to MGC cells in DR research, especially in the early stages of the disease, when no microangiopathy is observed. Accumulating evidence from animal experimental models and human post-mortem samples, as well as patient evaluations, strongly suggests that MGC play an important role in DR outcome. By using two different experimental models, Shen and coworkers have shown that Müller depletion leads to BRB breakdown and vascular alterations, two hallmarks of DR pathology [160]. Analysis of post-mortem samples from diabetic patients with no signs of vascular alterations revealed higher levels of glial reactivity, suggesting that this is an early event in DR development and could precede the vascular clinical symptoms [18,161,162]. Patients with non-proliferative diabetic retinopathy have displayed increased thickness of the inner nuclear layer and outer plexiform layer, which may be a result of activation and Müller cell swelling [163], which involves a number of angiogenic molecules and inflammatory cytokines [164]. According to the idea that MGC are altered in diabetic conditions, hyperglycemia directly upregulates GFAP in MGC in culture, a classic signal of gliosis [165,166]. Indeed, high glucose treatment in MGC cultures results in a wide range of pathological responses. Oxidative stress (increase in reactive oxygen species (ROS) and decrease in antioxidant systems) and activation of inflammatory responses is an early response in MGC to hyperglycemia conditions [165,167,168,169,170,171,172]. These alterations lead to the activation of cell death pathways. Apoptosis is described due to a decrease in Bcl-2 and an increase in Bax (bcl2 associated X) and caspase-3 levels [173,174,175]. Recently, several studies have shown the activation of pyroptosis and mitophagy/autophagy pathways [169,176,177,178,179,180]. Corroborating these findings, various morphological and functional alterations have been described in MGC in diabetes experimental models. Increase in GFAP labelling in MGC, a hallmark of glia stress and reactivity, is detected in diabetes induction models [181,182,183]. Gliosis of the foveolar MGC is recognizable in OCT images by the presence of hyperreflective dots (HRD) [40]. Since inner processes of MGC seem to be important to compensate for the thin basal lamina of the fovea, providing structural stability, the increase in intermediate filaments by MGC may increase the resistance against tractional forces in DME [184]. However, the absence of these filaments in the outer processes of MGC may explain the disruption between inner layer and OLM in DME. Disruption of the foveolar Müller cell layer results in macular hole formation [45]. Further, diabetes also leads to a reduction in and displacement of the Kir4.1 channel and to an increase in aquaporin-4 in MGC, which can be associated with edematous alterations and retinal hyperexcitation [75,82]. Intercellular fluid accumulation in MGC provokes the cyst formation observed in DME, even though it is still in debate whether this is intracellular or extracellular [185]. In DR progression, regulation of neurotransmitters is also impaired. In retinas from diabetic animals, there is a decrease in excitatory amino acid transporter 1 (EAAT1) and glutamine synthetase expression/activity in MGC, which promote cell death by excitotoxicity from early stages of diabetes [85,93,182]. These data agree with the increase in glutamate in the retina of animal models of DR [17,88,89,90] and in diabetic patients [91].
According to the idea that MGC play a crucial role in the outer retina [45,51,52,53], a disruption in OLM and in the distance between OLM and RPE, known as outer retinal layer, observed in OCT, has been correlated with poorer visual acuity [50,185] and to poor responses to anti-VEGF therapy in DME [186,187]. Chronic DME can also lead to structural disorganization of retinal inner layers (DRIL) due to glial and neuronal cell death. DRIL is a good predictor of visual outcome [188]. Another correlation of MGC in DME has been related to the correlation of poor visual acuity and the destruction of Müller cell cones [189]. Altogether, these data indicate that MGC seem to be closely involved with DME.
Further evidence of the involvement of MGC in the development of DR has been found in studies with VEGF, oxidative stress, and retinal inflammation. MGC is the main source of VEGF in the retina [190,191], and the increase in VEGF triggered by diabetes is associated with upregulation of GFAP, a signal of gliosis [75]. Conditional knockout of VEGF in MGC reduced nearly 50% of the total VEGF in the retina of diabetic animal models and greatly prevented vascular dysfunctions and increased permeability [108,192]. Interestingly, the increase in permeability induced by VEGF is inhibited by the pigment epithelium-derived factor (PEDF) produced by MGC [193], indicating that MGC can control retinal vascular permeability. Some of the hallmarks of DR, such as increase in retinal VEGF and tumor necrosis factor alpha (TNF-α), and pericyte loss were decreased by the disruption of β-catenin in MGC of diabetic animals [194]. Oxidative stress upregulates VEGF in several retinal cell types, such as endothelial cells [195], human retinal pigmental epithelial (ARPE-19) cells [196,197], and MGC [198], decreasing cell viability. On the other hand, VEGF increases oxidative stress [199]. Alternatively, VEGF conditional knockout mice also present reduction in nitrosylated proteins [199], NFκB activation [108], intercellular adhesion molecule (ICAM), and TNF-ɑ expression [108,192,199], hallmarks of retinal inflammation. Indeed, VEGF gene polymorphisms have been associated with the risk of DR [200]. Multiple studies have described various pathways that induce an increase in VEGF in MGC [5,198] and recently it was shown that VEGF upregulation is determined by an autocrine loop. Oxidative stress induces VEGF release by MGC, which activates the VEGF receptor 2 in MGC, further strengthening both VEGF expression and oxidative stress [198]. Therefore, MGC VEGF appears to be involved in important pathological features of early stages of DR. Interestingly, VEGF is also important to MGC and all retinal neuronal types after a long period of diabetes. Conditioned knockout of VEGF receptor 2 in MGC leads to a significant decrease in BDNF and GDNF and the loss of MGC and all types of retinal neuronal cells in diabetic mice [128]. Finally, several studies have also shown the crucial correlation of oxidative stress, VEGF and vascular alterations and neovascularization, features of later stages of DR [201,202,203].
Thus, MGC-derived VEGF induces retinal inflammation, oxidative stress, vascular leakage, and neovascularization [108,192]. The temporal course of in vitro changes detected in MGC or in ganglion cells by hyperglycemia corroborates the idea that there is a connection between neural damage and oxidative stress/inflammation response in Müller glia. As early as a few hours in a hyperglycemic medium, MGC show signals of oxidative stress and inflammation [167,171,178,204]. However, the oxidative stress, inflammation, and cell death of ganglion cells appear to occur later on [205,206,207].
Interestingly, recent data have suggested other modulators, such as fibroblast growth factor type 2 (FGF2), important to Müller cell activation, which expand the range of possible therapeutic targets to be investigated [208]. Next, we will present molecular pathways by which MGC control two other essential events for DR progression: oxidative stress and inflammation.
3.1. Oxidative Stress
Today, it is well-recognized that oxidative stress is one of the main causes of cellular imbalance in DR [209]. The hyperglycemic state induces multiple alterations in the biochemical pathways, accumulation of advanced glycation end products, and higher citric acid cycle activity that result in increased reactive oxygen species (ROS) production in the retina [210,211,212].
In animal models, many supplemented natural antioxidants, such as cocoa [213], quercetin [214] -based flavonoids [215], green tea [216], and apocynin [217], have been shown be beneficial, as they inhibit apoptosis, decrease proinflammatory cytokines, and suppress endoplasmic reticulum stress and ROS production in the retina [218,219]. Although the use of a specific antioxidant molecule or a combination of antioxidants might represent a promising therapeutic strategy for DR, the number of clinical trials with general antioxidant molecules for DR treatment are scarce and the results are controversial and disappointing [220,221,222]. For example, in clinical studies investigating combined antioxidant supplementation, it was demonstrated that an eighteen-month antioxidant therapy reduced malondialdehyde (MDA) levels and increased antioxidant capacity in the retina of type 2 diabetic patients with DR [223]. Further, antioxidant therapy slowed the progress of DR stages in the supplemented group in a longer follow up of five years; the visual acuity, however, was not different between the supplemented and non-supplement groups [224]. Another example of conflicting results with clinical trials was reported with the dietary antioxidant, lutein. Lutein and its isomers are carotenoids found in a wide variety of vegetables and fruits, with particularly high concentrations in leafy green vegetables, known to be beneficial for ocular health and vision [225]. In 2017, it was demonstrated that a nine-month therapy with lutein had a beneficial effect on contrast sensitivity in non-proliferative diabetic patients [226]; however, a recent study, with more subjects, did not detect any difference in visual acuity after 4 months of supplementation [227]. Thus, even though a large number of in vitro and animal model investigations have suggested that antioxidants might prevent DR development, clinical studies have not been able to corroborate such efficiency. Whether this controversy is due to differences between humans and rodents or due to different methodological approaches remains to be investigated. A better understanding of the molecular and cellular mechanisms of antioxidants and their targets would be helpful in designing more successful therapies for DR. For example, recently, we have demonstrated that eye drop treatment with the antioxidant α-lipoic acid, an inhibitor of transient potential receptor ankyrin 1 (TRPA1) or its absence in knockout animals, prevents oxidative stress and cell death in retinal ischemic conditions [57]. This kind of molecular target could be useful for alleviating retinal oxidative stress under conditions of diabetes and the impairments associated with these circumstances.
Glial cells have been described as important controllers of oxidative stress in different brain regions. We have shown that hesperidin and casticin, flavonoid compounds, increase the neuroprotective potential of cerebral cortex glial cells in vitro and in mice by modulating the secretion of protective factors [228,229].
MGC play a fundamental role in retinal antioxidant defense. High glucose leads to increase in Heme Oxygenase-1 expression and ROS production in these cells [82,178,230]. Furthermore, MGC is the main cell that contains glutathione (GSH) that is released in response to tissue stress, to support other cells against oxidant insults [231,232,233]. GSH activates calcium shifts in MGC and induces GABA release that could help to decrease excitotoxicity in the retina [234]. GSH is a thiol-containing tripeptide and is one of the main intracellular antioxidant molecules, acting directly by scavenging reactive oxygen and nitrogen species [235]. We have shown that GSH levels are lowered after 3 weeks of hyperglycemia in the rat retina [236] and remain low until later stages [237,238], probably contributing to the oxidative stress status of diabetic retina.
GSH impairment is a multifactorial phenomenon; for example, a reduction in γ-glutamylcysteine synthetase, an enzyme responsible for GSH synthesis, has been reported to be reduced in DR [239]. Since GSH is a tripeptide produced with glutamate, cysteine, and glycine, the levels of intracellular cysteine are also a limiting factor for its production [240]. System xc- is a cystine/glutamate exchanger that mediates cystine uptake. Once inside the cells, cystine is reduced to cysteine [241]. Therefore, this exchanger activity is crucial for maintaining GSH levels in the retina [242]. Our group was pioneer in demonstrating that system xc- is affected in the retina of DR animal models [236]. Both content of the catalytic subunit (xCT) and activity of this transporter are reduced at very early stages of diabetes.
We also have shown that this transport system remains impaired up to 6 months after the diabetes has been diagnosed [237]. Since system xc- seems to be enriched in MGC [243], these alterations could lead to a condition in which MGC could be depleted of one of the main molecules involved in control of oxidative stress under diabetic conditions. Accordingly, in the Müller cell cultures of Sigma 1 receptor in knockout mice, there is an increase in oxidative stress that occurs due to a reduction in system xc-, and decrease in GSH levels [199].
As mentioned before, treatments with antioxidant molecules have failed in clinical trials so far. Although the reasons for the failure are not completely known, one possible explanation is that the action of exogenous antioxidants might be limited by the impaired antioxidant properties of retinal cells. A possible solution for this problem comes from MGC and nuclear factor erythroid 2-related factor 2 (Nrf2), which is a key transcription factor and regulator of cellular antioxidant defense. Upon an increase in cellular ROS levels, Nrf2 is translocated to the nucleus and induces the expression of several genes associated with oxidative stress protection, including the enzymes responsible for GSH production [244,245,246], increasing cellular antioxidant capacity in response to a ROS upsurge. In particular, Nrf2 binds to a specific promoter region, an antioxidant-responsive element (ARE) that increases xCT expression, the catalytic subunit of system xc- [247]. In the retina, Nrf2 is strongly and preferentially expressed by MGC [172]. Nrf2 has been shown to be essential for maintenance of the antioxidant status; knocking out Nrf2 increases oxidative stress directly induced by oxidants in MGC, intensifies oxidative stress and ganglion cell death, worsening retinal dysfunction in diabetic retina [172,248]. The same is seen in other models of retinal neurodegeneration [249,250]. An increase in oxidative stress in MGC is seen when Nrf2 levels and activity are reduced [251]. Hyperglycemia induces a fast decrease in nuclear Nrf2 in cultures of MGC [167,169,252]. Accordingly, hyperglycemia promotes a decrease in antioxidant defense elements (activity of catalase and superoxide dismutase (SOD), GSH, HO-1, and NAD(P)H: quinone oxireductase 1 (NQO-1) levels in MGC, intensifying oxidative stress [167,169,170,204,252] and affecting MGC viability [253].
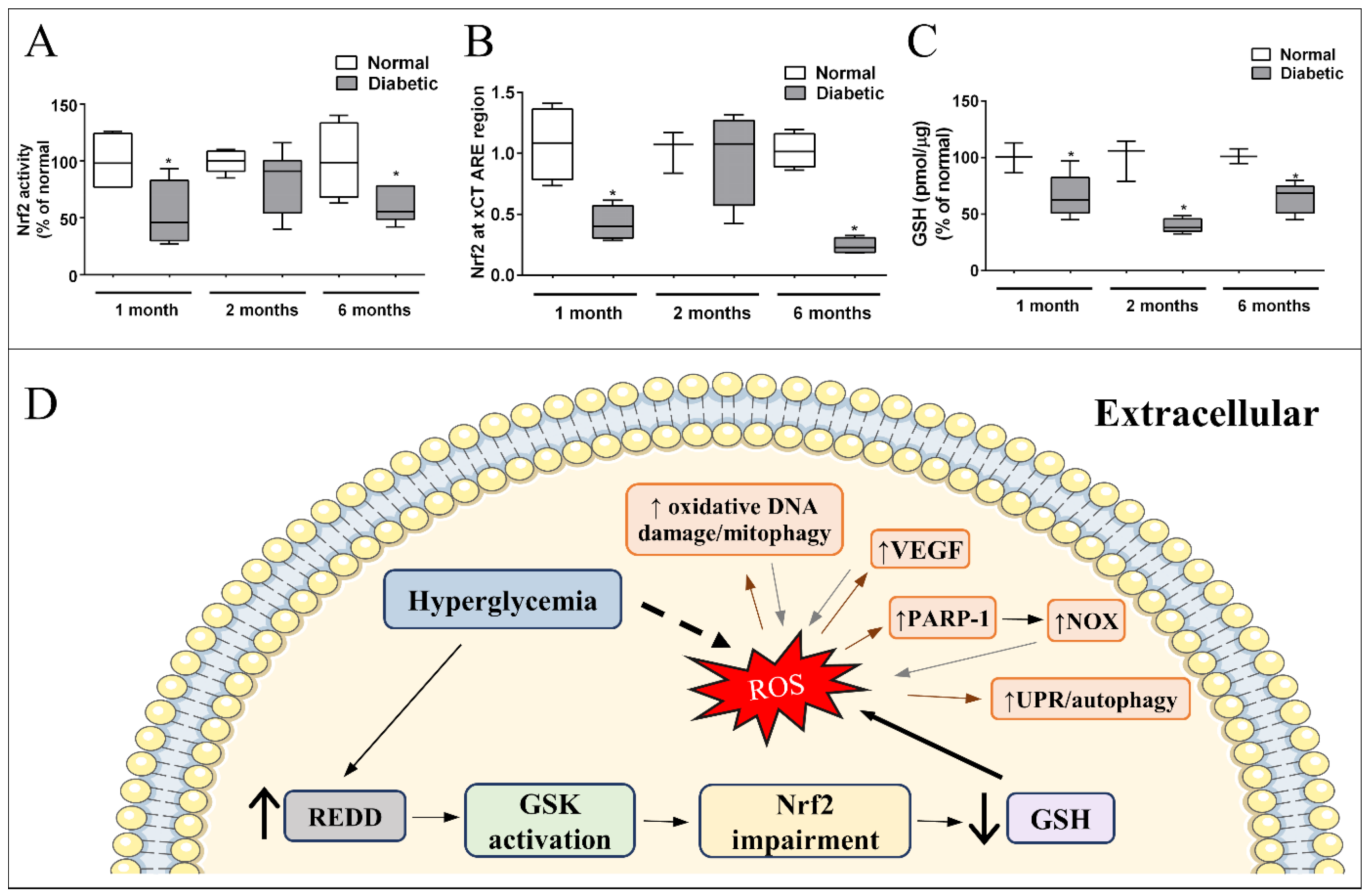
Our group and others have shown that Nrf2 expression, activity, and ARE-binding oscillate with diabetes progression resulting in lower levels of system xc- and GSH in diabetic animals [157,167,237,254]. Nrf2 was reduced after one month in diabetic rat retinas, recovered to normal levels in 2 months, and again decreased after 6 months of diabetes (Figure 2A) [237]. The expression of xCT follows this same pattern [237], and is probably a result of a decrease in xCT-induced expression by Nrf2 (Figure 2B). This suggests that the retinal tissue has a compensatory mechanism to improve the cellular antioxidant defense in response to oxidative stress, a recovery in Nrf2 activity was detected in 2 months, after an initial decrease. However, this was not sufficient to restore proper oxidative status, as seen by continuous high levels of ROS and lower GSH through the time window analyzed (Figure 2C). Upon the continuous stress inflicted by diabetes in the retina, this mechanism is overwhelmed and Nrf2 is impaired after 6 months. Thus, it is possible that we have an early time window for initial treatment with Nrf2 inductors that would be more efficient because of this compensatory response [237,239]. This is an unexpected result because if oxidative stress is a stimulus for Nrf2 activation, why is there Nrf2 impairment in DR models, which present higher oxidative stress status? A recent explanation for this phenomenon has been suggested based on the modulation of the protein, “regulated in development and DNA damage response-1” (REDD1). REDD1 is a stress induced protein, which acts as a negative regulator of Nrf2, and has its expression increased with oxidative stress in diabetic mice retina and in MGC exposed to high glucose (Miller et al., 2019, 2020). High glucose treatment induces oxidative stress in the human retinal Müller cell line (MIO-M1); however, oxidative stress is not observed in REDD1 knocked down cultures, neither is the expression of Nrf2-driven genes detected. Furthermore, it was also shown that REDD1 induces Nrf2 degradation through glycogen synthase kinase 3β (GSK) activation. Diabetic mice treated with a GSK inhibitor had higher levels of Nrf2 activity and lower oxidative stress in their retinas [255]. GSK is classically regulated by Akt, which phosphorylates and inactivates GSK [256]. However, hyperglycemia decreases Akt activity, stimulating GSK [173]. Thus, stimulated GSK increases the Nrf2 extrusion from the nucleus, inducing a decrease in nuclear Nrf2 [157]. Thus, Nrf2 impairment seems to be a crucial step to induce oxidative stress in DR while treatments that boost glial Nrf2 activity might have a benefit effect (Figure 2D). Indeed, a synthetic Nrf2 activator, dihydro-CDDO-trifluoroethyl amide (dh404), increases the antioxidant capacity of MGC exposed to high glucose, and inhibits the upregulation of angiogenic and inflammatory molecules. Further, in diabetic rats, dh404 was able to prevent the increase in oxidative stress, pro-inflammatory molecules, and BRB breakdown, major hallmarks of DR progression [168]. Other molecules that also activate MGC Nrf2 were reported to have beneficial effects in DR, such as fenofibrate [257] and sulforaphane [169]. In addition, MGC are directly affected by hyperglycemia. One of the early alterations observed in cultures of MGC was in the ROS levels [178,204], along with an increase in inflammatory signals (TNF-alpha1, iNOS, NFkB), activation of caspase-1, all observed in the first 24 h [171,178,180]. The data suggested that these changes are followed by stimulation of caspase-3 and -9, cytochrome c release, and MGC death [171,173]. MGC are directly impacted by ROS, with an increasing system xc- activity and decreasing GLAST levels [258]. In addition, MGC also respond to oxidative stress indirectly generated by other stimuli. For instance, MGC show an increase in ROS after 1h of exposure to modified LDL, which triggers endoplasmic reticulum stress and cell death [259]. Therefore, targeting Müller oxidative stress might lead to better therapeutic treatments for prevention of oxidative stress in DR.
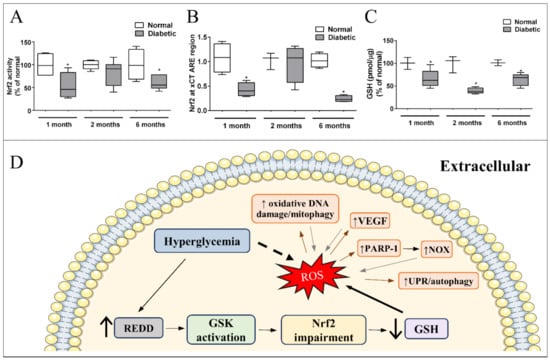
Figure 2.
Signaling pathway underlying impaired Nrf2 function and effectors of excessive ROS in Müller cells in diabetes: (A) Nrf2 activity and (B) Binding to xCT ARE region, are decreased (↓) after 1 and 6 months of diabetes. (C) GSH is reduced in the retina of diabetic rats in early (1 month) and late periods (6 months). Modified from [237]. Copyright 2018 Elsevier. (D) Hyperglycemia induces an increase in REDD1 expression in Müller cells, which activates GSK. Once activated, GSK impairs Nrf2 function. As a result, there is a decrease in GSH levels and antioxidant defense capacity, resulting in higher oxidative stress in Müller glia. Direct effect of excessive ROS in Müller cells is indicated by brown arrows: increase (↑) in oxidative DNA damage/mitophagy, VEGF, poly [ADP-ribose] polymerase 1 (PARP-1), and unfolded protein response (UPR)/autophagy. All these responses intensify the production of ROS (gray arrows) enhancing oxidative stress in Müller cells. * p < 0.05 in comparison to normal.
3.2. Inflammation
DR is a chronic low grade inflammatory disease [211,260], generally associated with microglia/macrophage activation in the retina with the upregulation of various proinflammatory, such as galectin-3, angiogenic factors, and cytokines, such as TNF-α and interleukin 1-beta (IL-1β) [261,262]. The levels of these particular factors, among others, have been shown to be enhanced in the aqueous humor of diabetic patients (with or without retinopathy) compared to non-diabetic controls [263]. Although conflicting results appear from several reports, inflammatory cytokines and chemokines are increased in DR in both serum as well as in the eye [264]. However, local rather than systemic production of proinflammatory cytokines seems to be a hallmark of DR. Inflammatory milieu contributes to the development of retinal damage in DR, such as BRB breakdown, neovascularization, neuronal, and capillary degeneration [163].
Due to the inflammatory profile of DR, treatments that inhibit the pro-inflammatory burden have been suggested as promising alternatives in DR control. In a multicenter randomized controlled clinical trial (1989), it was shown that aspirin, a nonsteroidal anti-inflammatory drug, slows the appearance of microaneurysms in diabetic patients, but had no effect in patients with advanced DR. In addition, in a study to evaluate the efficacy and safety of an IL-1β inhibitor (canakinumab), the systemic administration of the drug prevented the progression of DR and had promising results in reducing DR related macular edema; although, it had no effect on neovascularization [265]. Although these results are encouraging, there is still insufficient clinical evidence to recommend a fully anti-inflammatory treatment for DR patients; so far, the gold standard treatment is based on laser coagulation and intravitreal injections of anti-VEGF agents [266].
Glial cells are the main sources of cytokines in the CNS [33,34,35,37,267,268,269]. MGC, in addition to RPE, are the major sources of proinflammatory cytokines in DR [264]. MGC acquire a complex reactive phenotype in response to diabetes, with different expressions of 78 genes. Of these genes, 33% are associated with inflammation [270]. As reviewed by Coughlin and coworkers in 2017, under high glucose conditions, MGC are a source of damaging cytokines, such as IL-1β, interleukin 6 (IL-6), and TNF-α, which can be an important part of the chronic inflammation status of the diabetic retina [271]. However, what are the cellular mechanisms underlying the upregulation of cytokines in MGC that contribute to a shift to a pro-inflammatory phenotype in DR? Some new studies are focusing on this question to understand the cellular mechanisms that may help to improve the early treatment for DR. NF-κB, a well-known regulator of inflammation, is increased in MGC by hyperglycemia as an early response and has a great contribution to this pro-inflammatory response [167,170,171]. Tu and co-workers suggest that increase in ROS activates NF-κB, and Nrf-2 could block this pathway by controlling MGC oxidative stress [165]. In fact, the stimulation of Nrf2 dumps the augment of cytokines in MGC and diabetic retinas [168]. It has also been shown that advanced glycated elements, a common feature of diabetes, activate their receptors in MGC leading to glial dysfunction and increase in pro-inflammatory cytokines through MAPK signaling during diabetes [166,272]. In addition, hyperglycemia leads to Rho kinase (ROCK) activation, which increases Erk pathway signaling, resulting in an increase in pro-inflammatory production [273]. It was recently shown that C-myc, a protein considered a proto-oncogene, but also associated with inflammation [274], is expressed in MGC [275] and regulates pro-inflammatory cytokines release under hyperglycemic state (Figure 3). Interestingly, rat retinal Müller cells (rMC-1) cultures have increased C-myc expression after 4 h of high glucose exposure, while IL-1β, IL-6, and TNF-α levels augmented only after 24 h. Moreover, silencing C-myc in MGC prevented cytokine upregulation, demonstrating that this transcription factor is essential for initiation of the Müller cell inflammatory response in the diabetic context [276].
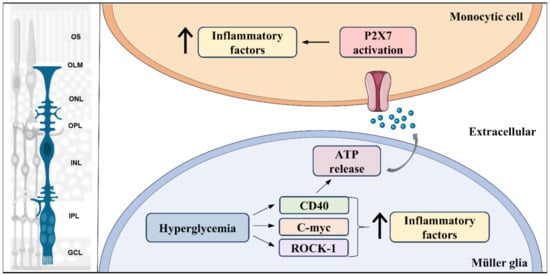
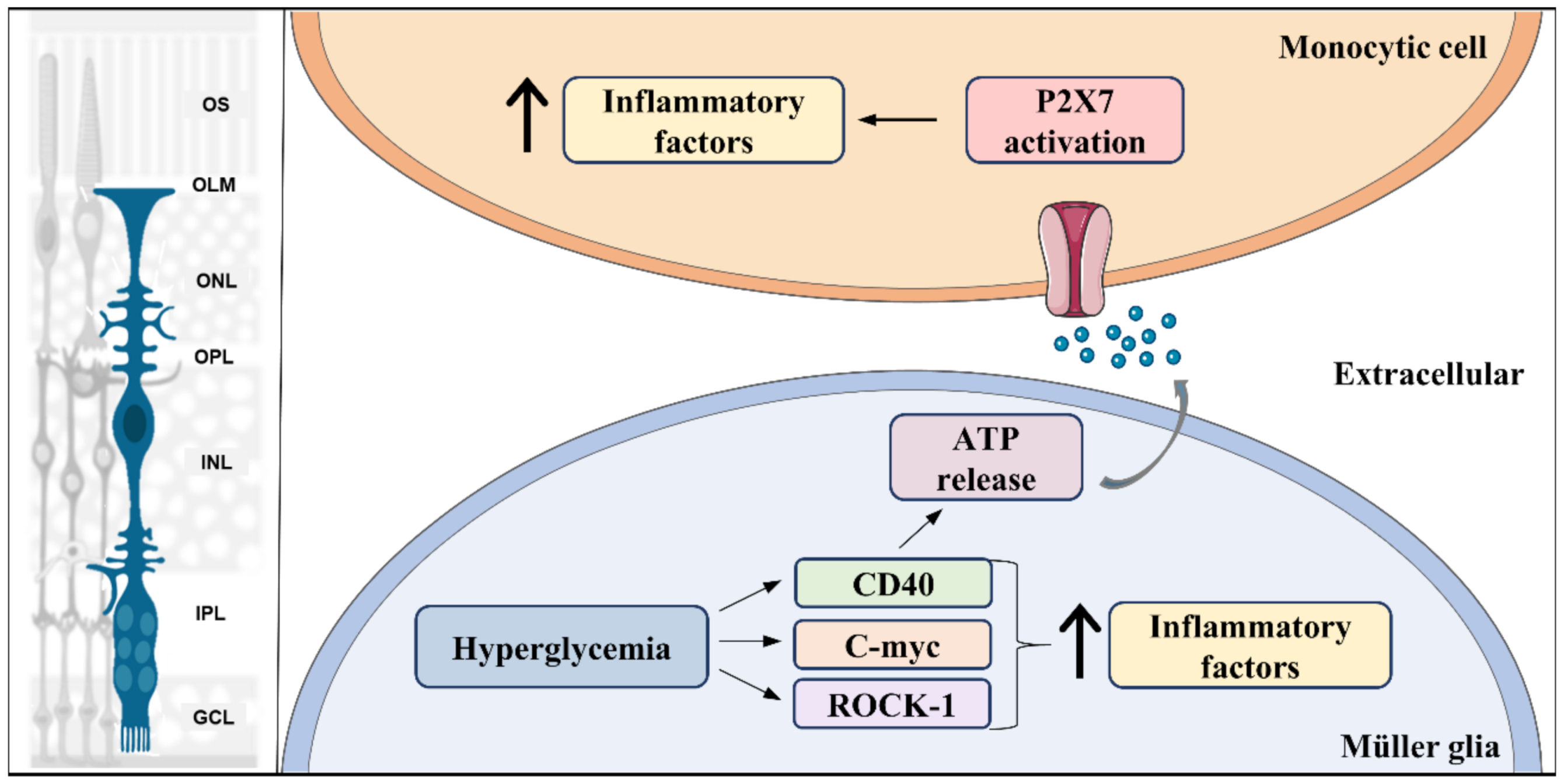
Figure 3.
Pro-inflammatory signal pathways activated in Müller glia in diabetes. Hyperglycemia leads to activation of multiple pro-inflammatory signaling pathways, such as Rock-1, c-myc, and CD40, which results in an initial increase (↑) in production of pro-inflammatory factors in Müller glia. In addition, CD40 activation triggers ATP release by Müller glia, which acts as an inflammatory inducer in monocytic cells through its receptor, P2X7. Left part of the figure was adapted from smart.servier.com (accessed on 1 April 2021).
Likewise, once MGC adopt an inflammatory behavior, they can trigger inflammatory responses in other cells such as microglia/macrophages. CD40, an inflammation inducer, is increased in Müller, microglia, and endothelial cells in the retina of diabetic mice. In MGC, diabetes induced-CD40 activation is responsible for upregulating expression of inflammatory cytokines, such as TNF-α, IL-1β, NOS, and monocyte chemoattractant protein-1 (MCP-1 or chemokine ligand 2, CCL2) (Figure 3). Further, besides increasing these inflammatory mediators, CD40 activation also induces ATP release from MGC, leading to upregulation of TNF-α and IL-1β through P2X7 receptor activation in monocytic cells, which is a trigger for retinal inflammation [277]. Finally, ATP activation of P2X7 receptors forms large pores highly permeated by calcium, both in astrocytes [278] and in MGC [279]. Interestingly, the CD40-ATP-P2X7 pathway is also involved in both inflammation and death of retinal endothelial cells, promoting development of capillary degeneration and retinal ischemia [280]. Altered calcium signaling induced by overactivation of P2X7 receptors is a major step in the induction of neuronal and microvascular cell death under disease conditions such as ischemia-hypoxia and diabetes [281].
Together, these data suggest that MGC participate not only in inflammation amplification but also in triggering inflammatory responses in other cells. Interestingly, in transgenic diabetic mice that are knockout for P2X7 receptor, the upregulation of TNF-α, IL-1β, NOS, and ICAM was prevented [277]. Moreover, treatment with the nucleoside reverse transcriptase inhibitor lamivudine, a P2X7 receptor inhibitor, decreased progression of both neuronal and vascular pathology in DR, avoiding development of acellular capillaries and electrophysiological deficits, and the loss of GABA positive neurons [282]. In agreement with these results, Tu and collaborators demonstrated that melatonin prevented ganglion cell loss in diabetic mice through inhibition of Müller gliosis and prevention of increase in VEGF, TNF-α, IL-1β, and IL-6 in vitro and in vivo [165]. Furthermore, it was recently described that MGC treated with proliferative diabetic retinopathy vitreous humor obtained from diabetic patients had a higher expression of pro-inflammatory cytokines, demonstrating that the vitreous humor of patients with advanced DR had a potential for a proinflammatory response in MGC [208]. Altogether, these studies demonstrated that MGC are strongly associated with DR inflammatory insults and shed light on the manipulation of MGC inflammatory pathways as important therapeutic alternatives (Figure 3).
Altogether, data presented here show that while there is a great deal of evidence for the Müller role in retinal physiology, it is evident that the glial contribution in DR is still a field to be explored. A list of Müller roles in retinal physiology and alteration in diabetes condition is presented in Table 1. Further studies focusing on MGC participation in DR development are needed.
4. Future Directions
DR is a multifactorial progressive disease with accumulating vascular and neural damage that results in BRB breakage, microaneurysms, hemorrhages, edemas, neovascularization, and neurodegeneration. Currently, this disease is one of the major causes of visual impairment and blindness in the world [283,284]. Presently, available treatment consists mainly of intraocular anti-VEGF injections, which have side effects, such as cataracts and endophthalmitis. To further aggravate this situation, due to anti-VEGF short-life, frequent injections are necessary [285,286,287]. Other setbacks are that, although, this treatment slows down DR progression, it does not promote a cure and has limited use when vascular damage is advanced, and the disease is found in a late phase. Thus, it is evident that new alternative treatments are required to counteract DR progression at early stages, when the retinal tissue has not yet become severely compromised.
Given the large number of functions performed by glial cells, it is expected that deficits in these cells have a major impact on nervous system functioning. Thus, modulation of glial function and reactivity state are important venues for the development of new preventive and therapeutic strategies for the care of neurodegenerative diseases [288].
As extensively discussed in this review, MGC play an essential role in retina neurovascular unit function and retinal homeostasis (Table 1; Figure 1B). During DR progression, some of MGC functions are severely impaired (Table 1), such as the ability to maintain BRB [289,290], control of glutamatergic [89,90,93] and ionic homeostasis [82,291], modulation of vascular function and neuronal integrity by VEGF [292], and maintenance of anti- and pro-inflammatory balance profile [108,192,271] (Figure 4).
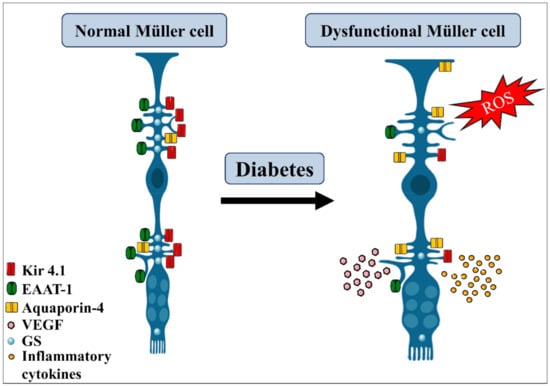
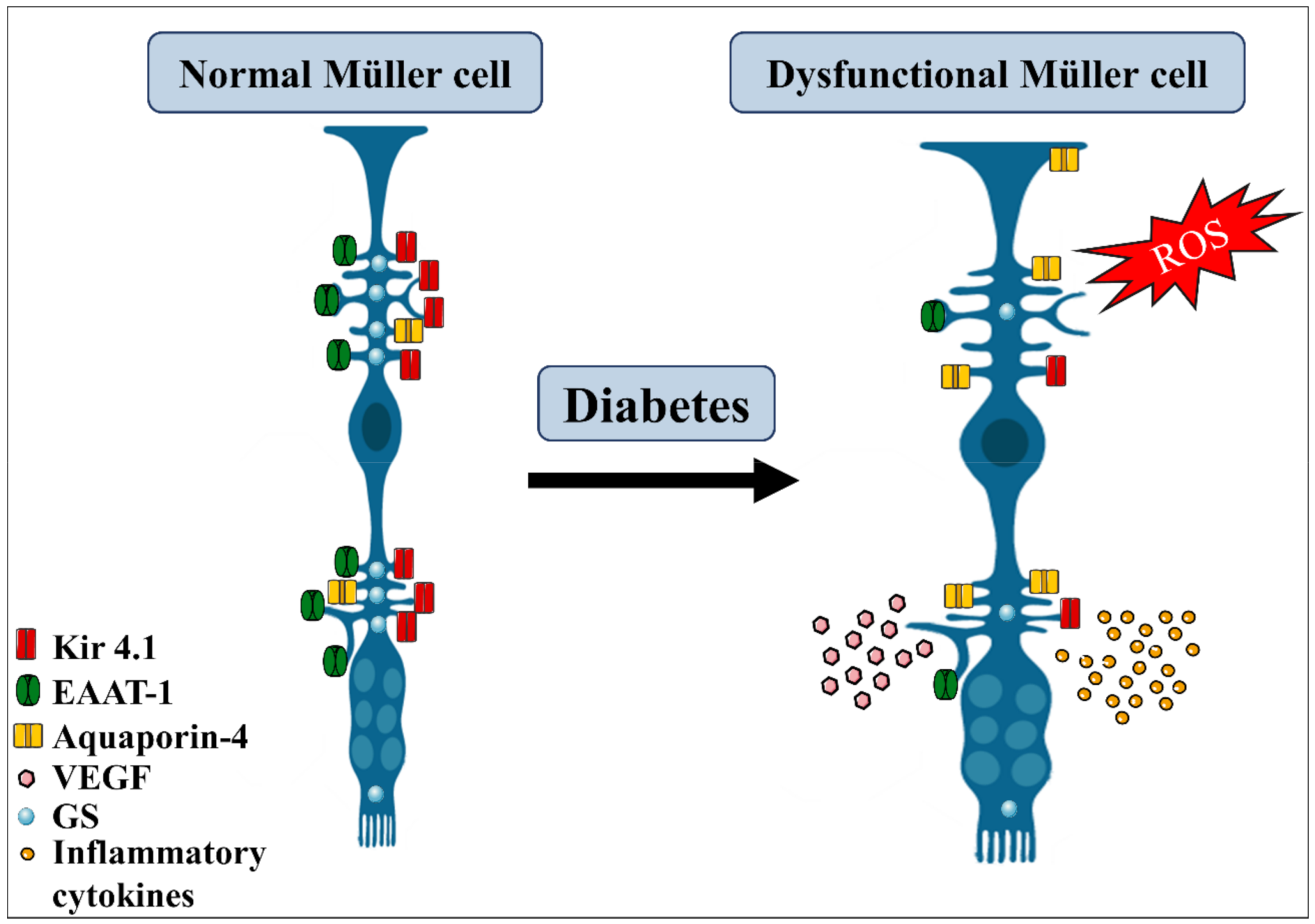
Figure 4.
Diabetes effects on Müller glia. Diabetic condition triggers several morphological and metabolic alterations in Müller glia cells. They become hypertrophic, suffer increase in aquaporin-4, decrease the expression of Kir4.1 channel, glutamine synthetase (GS), and EAAT-1 transporters, thus affecting the regulation of the ionic balance and glutamate uptake. Under this condition, Müller glia also increase the production of reactive oxidative species (ROS) and the release of pro-inflammatory cytokines and VEGF, contributing to retinal damage. EAAT, excitatory amino acid transporter. Adapted from smart.servier.com (accessed on 1 April 2021).
Within the context of DR, different cocktails of molecules and compounds as potential drugs, both natural and synthetic, have been used to control MGC reactivity and improve retinal function. General antioxidant molecules have been tested as candidates for DR treatment in human trials, although with unsatisfactory outcomes. Molecules that could improve a specific cellular antioxidant response, such as Nrf2 signaling in MGC, for example, dh 404 or GSK inhibitors, might be therapeutic candidates for DR treatment [168]. Further, strong evidence shows that the Müller cell is an initial player in the pro-inflammatory retinal environment in DR [276,277]. Thus, control of the molecular pathways triggered by MGC could lead to new ways for preventing the early deficits found in DR, thus slowing down its progression.
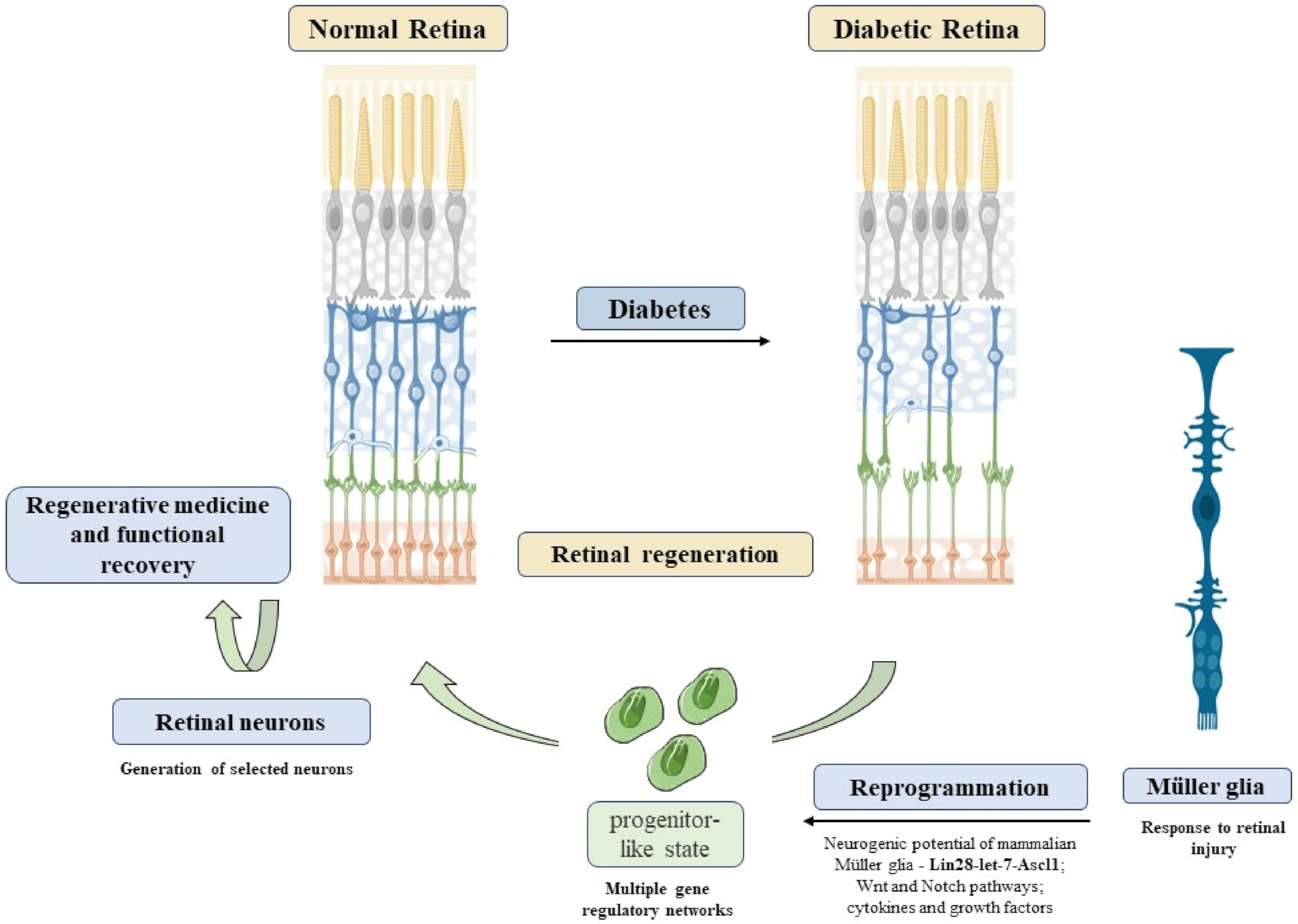
The next frontier of personalized medicine will be to understand the triggering mechanisms of how MGC might generate functional neurons to restore vision in mammals through reprogramming as a promising therapeutic strategy for human retinal diseases [293]. As for most neurodegenerative diseases, the main challenge of DR is to rescue the neuronal loss that usually occurs at a late stage of the disease. MGC may represent a potential candidate for this problem (Figure 5). The regenerative potential of MGC is well known in zebrafish, where retinal injury induces a proliferative response that could reconstitute all major retinal cell types and restore vision, a process named as reprogramming [294]. Previous studies have shown that MGC are closely related to retinal progenitors and act as latent neural stem cells that proliferate in response to injury and create a neurogenic environment, which possibly induces a response by novel retinal neurons. Uncovering this hidden potential could lead to new approaches to degenerative retinopathies, which are leading causes of irreversible visual impairment such as DR.
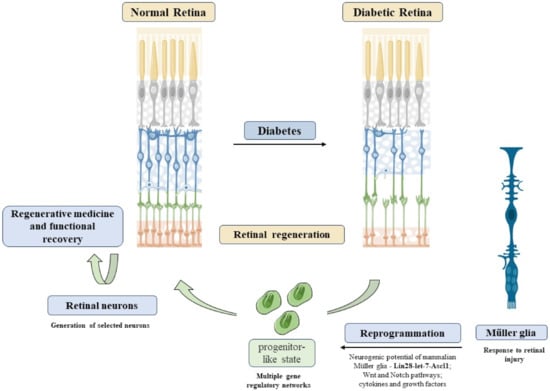
Figure 5.
Reprogramming Müller cells in diabetic retinopathy. DR induces retinal neurodegeneration, an event difficult to recover. Alternatively, Müller reprogramming could act as a possible therapeutic option to restore visual capacity in DR patients. MGC reprogramming through activation of Lin28-let-7-Ascl1 genes, Wnt, and Notch pathways could lead to a progenitor-like state in these cells. Thus, these progenitors could be used to generate selected neurons, leading to retinal regeneration. Refs. [143,295,296,297] was adapted from smart.servier.com (accessed on 1 April 2021). Copyright 2016 Elsevier.
5. Conclusions
The main goal of this review was to highlight the need to consider MGC involvement in the progression of DR and potentially include these cells as targets for therapy development of DR. Either the identification of molecular pathways that control the function of MGC or the successful reprogramming of the fate of MGC should become a keystone for the development of new therapies addressing the progression of DR.
Author Contributions
R.C.-S. and K.C.C.: conceptualization, R.C.-S. and K.C.C.: writing—original draft; R.C.-S., R.A.d.M.R., F.C.A.G. and K.C.C.: writing—review and editing; R.C.-S. and K.C.C.: visualization; K.C.C.: Project administration; R.A.d.M.R., F.C.A.G. and K.C.C.: funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
Work in the laboratories of the authors (RAMR, FCAG, and KCC) was supported by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), CNPq/INCT-INNT (National Institute for Translational Neuroscience), CNPq (305562/2017-7 and 312157/2016-9); Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) (: E-26/202.977/2017; E-26/202.832/2018; E-26/ 210.055/2020; E-26/202.668/2018, E-26/010.002215/2019, 426342/2018-6; E-26/201.025/2021), and INCT-INNT (E-26/010.00080/2018); Ministério da Saúde/Departamento de Ciência e Tecnologia (MS-Decit) (TC 079/17) (FCAG/RC).
Acknowledgments
Work in the laboratories of the authors was supported by grants from the CNPq (RAMR, FCAG, and KCC), FAPERJ (RAMR, FCAG, and KCC), Ministério da Saúde (MS-Decit) (RCS and FCAG) and INCT-INNT (RAMR, FCAG, and KCC). The authors thank BSc Amanda Alves Nascimento for the excellent help in the production of some figures. The English text of this paper has been revised by Sidney Pratt, Canadian, MAT (The Johns Hopkins University), RSAdip-TESL (Cambridge University). KCC, FCAG, and RAMR thanks CNPq and FAPERJ for individual research fellowship.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global Estimates of Diabetes Prevalence for 2017 and Projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- Prevention of Diabetes Mellitus. Report of a WHO Study Group; WHO: Geneva, Switzerland, 1994; Volume 844.
- Pereira-Figueiredo, D.; Brito, R.; Araújo, D.S.M.; Nascimento, A.A.; Lyra, E.S.B.; Cheibub, A.M.S.S.; Pereira Netto, A.D.; Ventura, A.L.M.; Paes-de-Carvalho, R.C.K.; Calaza, K.C. Caffeine Exposure Ameliorates Acute Ischemic Cell Death in Avian Developing Retina. Purinergic Signal. 2020, 16, 41–59. [Google Scholar] [CrossRef]
- Lin, A.D.; Lee, A.Y.; Zhang, Q.; Rezaei, K.A.; Kinyoun, J.; Wang, R.K.; Lee, C.S. Association Between OCT-based Microangiography Perfusion Indices and Diabetic Retinopathy Severity. Br. J. Ophthalmol. 2018, 101, 960–964. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Gong, C.; Chen, X.; Zhou, H.; Yan, J.; Hong, W. Associations between Vascular Endothelial Growth Factor Gene Polymorphisms and Different Types of Diabetic Retinopathy Susceptibility: A Systematic Review and Meta-Analysis. J. Diabetes Res. 2021, 2021, 7059139. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Ripps, H. Neurovascular Interaction and the Pathophysiology of Diabetic Retinopathy. Exp. Diabetes Res. 2011, 2011, 693426. [Google Scholar] [CrossRef]
- Tarr, J.M.; Kaul, K.; Chopra, M.; Kohner, E.M.; Chibber, R. Pathophysiology of Diabetic Retinopathy. ISRN Ophthalmol. 2013, 2013, 343560. [Google Scholar] [CrossRef]
- Barber, A.J. A New View of Diabetic Retinopathy: A Neurodegenerative Disease of the Eye. Prog. Neuro-Psychopharmacology Biol. Psychiatry 2003, 27, 283–290. [Google Scholar] [CrossRef]
- Barber, A.J.; Gardner, T.W.; Abcouwer, S.F. The Significance of Vascular and Neural Apoptosis to the Pathology of Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1156–1163. [Google Scholar] [CrossRef]
- Jonas, J.B.; Schneider, U.; Naumann, G.O. Count and Density of Human Retinal Photoreceptors. Graefe’s Arch. Clin. Exp. Ophthalmol. = Albr. von Graefes Arch. fur Klin. und Exp. Ophthalmol. 1992, 230, 505–510. [Google Scholar] [CrossRef]
- Curcio, C.A.; Sloan, K.R.; Kalina, R.E.; Hendrickson, A.E. Human Photoreceptor Topography. J. Comp. Neurol. 1990, 292, 497–523. [Google Scholar] [CrossRef]
- Watson, A.B. A Formula for Human Retinal Ganglion Cell Receptive Field Density as a Function of Visual Field Location. J. Vis. 2014, 14, 15. [Google Scholar] [CrossRef] [PubMed]
- Wong-Riley, M.T.T. Energy Metabolism of the Visual System. Eye Brain 2010, 2, 99–116. [Google Scholar] [CrossRef] [PubMed]
- de Campos, V.S.; Calaza, K.C.; Adesse, D. Implications of TORCH Diseases in Retinal Development—Special Focus on Congenital Toxoplasmosis. Front. Cell. Infect. Microbiol. 2020, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Cheung, C.M.G.; Larsen, M.; Sharma, S.; Simó, R. Diabetic Retinopathy. Nat. Rev. Dis. Prim. 2016, 17, 16012. [Google Scholar] [CrossRef]
- Semeraro, F.; Cancarini, A.; dell’Omo, R.; Rezzola, S.; Romano, M.R.; Costagliola, C. Diabetic Retinopathy: Vascular and Inflammatory Disease. J. Diabetes Res. 2015, 2015, 582060. [Google Scholar] [CrossRef]
- Barber, A.J.; Lieth, E.; Khin, S.A.; Antonetti, D.A.; Buchanan, A.G.; Gardner, T.W. Neural Apoptosis in the Retina during Experimental and Human Diabetes. Early Onset and Effect of Insulin. J. Clin. Investig. 1998, 102, 783–791. [Google Scholar] [CrossRef]
- Carrasco, E.; Hernández, C.; Miralles, A.; Huguet, P.; Farrés, J.; Simó, R. Lower Somatostatin Expression Is an Early Event in Diabetic Retinopathy and Is Associated with Retinal Neurodegeneration. Diabetes Care 2007, 30, 2902–2908. [Google Scholar] [CrossRef]
- Martin, P.M.; Roon, P.; Van Ells, T.K.; Ganapathy, V.; Smith, S.B. Death of Retinal Neurons in Streptozotocin-Induced Diabetic Mice. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3330–3336. [Google Scholar] [CrossRef]
- Olivares, A.M.; Althoff, K.; Chen, G.F.; Wu, S.; Morrisson, M.A.; DeAngelis, M.M.; Haider, N. Animal Models of Diabetic Retinopathy. Curr. Diab. Rep. 2017, 17, 93. [Google Scholar] [CrossRef]
- Han, Y.; Bearse, M.A.J.; Schneck, M.E.; Barez, S.; Jacobsen, C.H.; Adams, A.J. Multifocal Electroretinogram Delays Predict Sites of Subsequent Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2004, 45, 948–954. [Google Scholar] [CrossRef]
- Juen, S.; Kieselbach, G.F. Electrophysiological Changes in Juvenile Diabetics without Retinopathy. Arch. Ophthalmol. 1990, 108, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Shirao, Y.; Kawasaki, K. Electrical Responses from Diabetic Retina. Prog. Retin. Eye Res. 1998, 17, 59–76. [Google Scholar] [CrossRef]
- Gastinger, M.J.; Singh, R.S.J.; Barber, A.J. Loss of Cholinergic and Dopaminergic Amacrine Cells in Streptozotocin- Diabetic Rat and Ins2Akita-Diabetic Mouse Retinas. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3143–3150. [Google Scholar] [CrossRef] [PubMed]
- Roufail, E.; Soulis, T.; Boel, E.; Cooper, M.E.; Rees, S. Depletion of Nitric Oxide Synthase-Containing Neurons in the Diabetic Retina: Reversal by Aminoguanidine. Diabetologia 1998, 41, 1419–1425. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Seki, M.; Tanaka, T.; Nawa, H.; Usui, T.; Fukuchi, T.; Ikeda, K.; Abe, H.; Takei, N. Early Retinal Neuropathy of Streptozotocin-Induced. Am. Diabetes Assoc. 2004, 53, 1–8. [Google Scholar]
- Carpineto, P.; Toto, L.; Aloia, R.; Ciciarelli, V.; Borrelli, E.; Vitacolonna, E.; Nicola, M.D.; Antonio, L.D.; Mastropasqua, R. Neuroretinal Alterations in the Early Stages of Diabetic Retinopathy in Patients with Type 2 Diabetes Mellitus. Eye 2016, 30, 673–679. [Google Scholar] [CrossRef]
- Ng, D.S.K.; Chiang, P.P.C.; Tan, G.; Cheung, C.M.G.; Cheng, C.Y.; Cheung, C.Y.; Wong, T.Y.; Lamoureux, E.L.; Ikram, M.K. Retinal Ganglion Cell Neuronal Damage in Diabetes and Diabetic Retinopathy. Clin. Exp. Ophthalmol. 2016, 44, 243–250. [Google Scholar] [CrossRef]
- Sohn, E.H.; Van Dijk, H.W.; Jiao, C.; Kok, P.H.B.; Jeong, W.; Demirkaya, N.; Garmager, A.; Wit, F.; Kucukevcilioglu, M.; Van Velthoven, M.E.J.; et al. Retinal Neurodegeneration May Precede Microvascular Changes Characteristic of Diabetic Retinopathy in Diabetes Mellitus. Proc. Natl. Acad. Sci. USA 2016, 113, E2655–E2664. [Google Scholar] [CrossRef]
- Vujosevic, S.; Muraca, A.; Gatti, V.; Masoero, L.; Brambilla, M.; Cannillo, B.; Villani, E.; Nucci, P.; De Cillà, S. Peripapillary Microvascular and Neural Changes in Diabetes Mellitus: An OCT-Angiography Study. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5074–5081. [Google Scholar] [CrossRef]
- Markan, A.; Agarwal, A.; Arora, A.; Bazgain, K.; Rana, V.; Gupta, V. Novel Imaging Biomarkers in Diabetic Retinopathy and Diabetic Macular Edema. Ther. Adv. Ophthalmol. 2020, 12, 2515841420950513. [Google Scholar] [CrossRef]
- Mendonça, H.R.; Carvalho, J.N.A.; Abreu, C.A.; Mariano de Souza Aguiar dos Santos, D.; Carvalho, J.R.; Marques, S.A.; da Costa Calaza, K.; Martinez, A.M.B. Lack of Galectin-3 Attenuates Neuroinflammation and Protects the Retina and Optic Nerve of Diabetic Mice. Brain Res. 2018, 1700, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Diniz, L.P.; Araujo, A.P.B.; Matias, I.; Garcia, M.N.; Barros-Aragão, F.G.Q.; de Melo Reis, R.A.; Foguel, D.; Braga, C.; Figueiredo, C.P.; Romão, L.; et al. Astrocyte Glutamate Transporters Are Increased in an Early Sporadic Model of Synucleinopathy. Neurochem. Int. 2020, 138, 104758. [Google Scholar] [CrossRef] [PubMed]
- Diniz, L.P.; Matias, I.; Siqueira, M.; Stipursky, J.; Gomes, F.C.A. Astrocytes and the TGF-Β1 Pathway in the Healthy and Diseased Brain: A Double-Edged Sword. Mol. Neurobiol. 2019, 56, 4653–4679. [Google Scholar] [CrossRef] [PubMed]
- Diniz, L.P.; Tortelli, V.; Matias, I.; Morgado, J.; Bérgamo Araujo, A.P.; Melo, H.M.; Seixas da Silva, G.S.; Alves-Leon, S.V.; de Souza, J.M.; Ferreira, S.T.; et al. Astrocyte Transforming Growth Factor Beta 1 Protects Synapses against Aβ Oligomers in Alzheimer’s Disease Model. J. Neurosci. 2017, 37, 6797–6809. [Google Scholar] [CrossRef]
- Amaral, R.F.; Geraldo, L.H.M.; Einicker-Lamas, M.; E Spohr, T.C.L. de S.; Mendes, F.; Lima, F.R.S. Microglial Lysophosphatidic Acid Promotes Glioblastoma Proliferation and Migration via LPA(1) Receptor. J. Neurochem. 2021, 156, 499–512. [Google Scholar] [CrossRef]
- Moraes, C.A.; Santos, G.; de Sampaio e Spohr, T.C.L.; D’Avila, J.C.; Lima, F.R.S.; Benjamim, C.F.; Bozza, F.A.; Gomes, F.C.A. Activated Microglia-Induced Deficits in Excitatory Synapses Through IL-1β: Implications for Cognitive Impairment in Sepsis. Mol. Neurobiol. 2015, 52, 653–663. [Google Scholar] [CrossRef]
- Bringmann, A.; Pannicke, T.; Grosche, J.; Francke, M.; Wiedemann, P.; Skatchkov, S.N.; Osborne, N.N.; Reichenbach, A. Müller Cells in the Healthy and Diseased Retina. Prog. Retin. Eye Res. 2006, 25, 397–424. [Google Scholar] [CrossRef]
- Hamon, A.; Roger, J.E.; Yang, X.-J.; Perron, M. Müller Glial Cell-Dependent Regeneration of the Neural Retina: An Overview across Vertebrate Model Systems. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2016, 245, 727–738. [Google Scholar] [CrossRef]
- Reichenbach, A.; Bringmann, A. Glia of the Human Retina. Glia 2020, 68, 768–796. [Google Scholar] [CrossRef]
- Vecino, E.; Rodriguez, F.D.; Ruzafa, N.; Pereiro, X.; Sharma, S.C. Glia-Neuron Interactions in the Mammalian Retina. Prog. Retin. Eye Res. 2016, 51, 1–40. [Google Scholar] [CrossRef]
- Wang, J.S.; Kefalov, V.J. The Cone-Specific Visual Cycle. Prog. Retin. Eye Res. 2011, 30, 115–128. [Google Scholar] [CrossRef]
- Morshedian, A.; Kaylor, J.J.; Ng, S.Y.; Tsan, A.; Frederiksen, R.; Xu, T.; Yuan, L.; Sampath, A.P.; Radu, R.A.; Fain, G.L.; et al. Light-Driven Regeneration of Cone Visual Pigments through a Mechanism Involving RGR Opsin in Müller Glial Cells. Neuron 2019, 102, 1172–1183.e5. [Google Scholar] [CrossRef] [PubMed]
- Franze, K.; Grosche, J.; Skatchkov, S.N.; Schinkinger, S.; Foja, C.; Schild, D.; Uckermann, O.; Travis, K.; Reichenbach, A.; Guck, J. Müller Cells Are Living Optical Fibers in the Vertebrate Retina. Proc. Natl. Acad. Sci. USA 2007, 104, 8287–8292. [Google Scholar] [CrossRef] [PubMed]
- Bringmann, A.; Syrbe, S.; Görner, K.; Kacza, J.; Francke, M.; Wiedemann, P.; Reichenbach, A. The Primate Fovea: Structure, Function and Development. Prog. Retin. Eye Res. 2018, 66, 49–84. [Google Scholar] [CrossRef] [PubMed]
- Khmelinskii, I.; Makarov, V. Intermediate Filaments in the Retinal Müller Cells as Natural Light Energy Guides. J. Photochem. Photobiol. B Biol. 2019, 200, 111641. [Google Scholar] [CrossRef] [PubMed]
- Makarov, V.; Zueva, L.; Golubeva, T.; Korneeva, E.; Khmelinskii, I.; Inyushin, M. Errata: Quantum Mechanism of Light Transmission by the Intermediate Filaments in Some Specialized Optically Transparent Cells. Neurophotonics 2017, 4, 19801. [Google Scholar] [CrossRef][Green Version]
- Lindenau, W.; Kuhrt, H.; Ulbricht, E.; Körner, K.; Bringmann, A.; Reichenbach, A. Cone-to-Müller Cell Ratio in the Mammalian Retina: A Survey of Seven Mammals with Different Lifestyle. Exp. Eye Res. 2019, 181, 38–48. [Google Scholar] [CrossRef]
- Omri, S.; Omri, B.; Savoldelli, M.; Jonet, L.; Thillaye-Goldenberg, B.; Thuret, G.; Gain, P.; Jeanny, J.C.; Crisanti, P.; Behar-Cohen, F. The Outer Limiting Membrane (OLM) Revisited: Clinical Implications. Clin. Ophthalmol. 2010, 4, 183–195. [Google Scholar] [CrossRef]
- Daruich, A.; Matet, A.; Moulin, A.; Kowalczuk, L.; Nicolas, M.; Sellam, A.; Rothschild, P.R.; Omri, S.; Gélizé, E.; Jonet, L.; et al. Mechanisms of Macular Edema: Beyond the Surface. Prog. Retin. Eye Res. 2018, 63, 20–68. [Google Scholar] [CrossRef]
- Bejarano-Escobar, R.; Sánchez-Calderón, H.; Otero-Arenas, J.; Martín-Partido, G.; Francisco-Morcillo, J. Müller Glia and Phagocytosis of Cell Debris in Retinal Tissue. J. Anat. 2017, 231, 471–483. [Google Scholar] [CrossRef]
- Sakami, S.; Imanishi, Y.; Palczewski, K. Müller Glia Phagocytose Dead Photoreceptor Cells in a Mouse Model of Retinal Degenerative Disease. FASEB J. 2019, 33, 3680–3692. [Google Scholar] [CrossRef] [PubMed]
- Balaratnasingam, C.; Chae, B.; Remmer, M.H.; Gomez, E.; Suzuki, M.; Engelbert, M.; Spaide, R.F. The Spatial Profile of Macular Pigments Is Related to the Topological Characteristics of the Foveal Avascular Zone. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7859–7865. [Google Scholar] [CrossRef]
- Candiello, J.; Balasubramani, M.; Schreiber, E.M.; Cole, G.J.; Mayer, U.; Halfter, W.; Lin, H. Biomechanical Properties of Native Basement Membranes. FEBS J. 2007, 274, 2897–2908. [Google Scholar] [CrossRef] [PubMed]
- Ryskamp, D.A.; Jo, A.O.; Frye, A.M.; Vazquez-Chona, F.; MaCaulay, N.; Thoreson, W.B.; Križaj, D. Swelling and Eicosanoid Metabolites Differentially Gate TRPV4 Channels in Retinal Neurons and Glia. J. Neurosci. 2014, 34, 15689–15700. [Google Scholar] [CrossRef] [PubMed]
- Sisignano, M.; Park, C.K.; Angioni, C.; Zhang, D.D.; von Hehn, C.; Cobos, E.J.; Ghasemlou, N.; Xu, Z.Z.; Kumaran, V.; Lu, R.; et al. 5,6-EET Is Released upon Neuronal Activity and Induces Mechanical Pain Hypersensitivity via TRPA1 on Central Afferent Terminals. J. Neurosci. 2012, 32, 6364–6372. [Google Scholar] [CrossRef] [PubMed]
- Souza Monteiro de Araújo, D.; De Logu, F.; Adembri, C.; Rizzo, S.; Janal, M.N.; Landini, L.; Magi, A.; Mattei, G.; Cini, N.; Pandolfo, P.; et al. TRPA1 Mediates Damage of the Retina Induced by Ischemia and Reperfusion in Mice. Cell Death Dis. 2020, 11, 633. [Google Scholar] [CrossRef]
- Uckermann, O.; Wolf, A.; Kutzera, F.; Kalisch, F.; Beck-Sickinger, A.G.; Wiedemann, P.; Reichenbach, A.; Bringmann, A. Glutamate Release by Neurons Evokes a Purinergic Inhibitory Mechanism of Osmotic Glial Cell Swelling in the Rat Retina: Activation by Neuropeptide Y. J. Neurosci. Res. 2006, 83, 538–550. [Google Scholar] [CrossRef]
- Pannicke, T.; Wurm, A.; Iandiev, I.; Hollborn, M.; Linnertz, R.; Binder, D.K.; Kohen, L.; Wiedemann, P.; Steinhäuser, C.; Reichenbach, A.; et al. Deletion of Aquaporin-4 Renders Retinal Glial Cells More Susceptible to Osmotic Stress. J. Neurosci. Res. 2010, 88, 2877–2888. [Google Scholar] [CrossRef]
- Moseley, H.; Foulds, W.S.; Allan, D.; Kyle, P.M. Routes of Clearance of Radioactive Water from the Rabbit Vitreous. Br. J. Ophthalmol. 1984, 68, 145–151. [Google Scholar] [CrossRef]
- Strauss, O. The Retinal Pigment Epithelium in Visual Function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef]
- Karwoski, C.J.; Lu, H.-K.; Newman, E.A. Spatial Buffering of Light-Evoked Potassium Increases by Retinal Müller (Glial) Cells. Science (80-) 1989, 244, 578–580. [Google Scholar] [CrossRef] [PubMed]
- Tout, S.; Chan-Ling, T.; Holländer, H.; Stone, J. The Role of Müller Cells in the Formation of the Blood-Retinal Barrier. Neuroscience 1993, 55, 291–301. [Google Scholar] [CrossRef]
- Shen, W.; Fruttiger, M.; Zhu, L.; Chung, S.H.; Barnett, N.L.; Kirk, J.K.; Lee, S.R.; Coorey, N.J.; Killingsworth, M.; Sherman, L.S.; et al. Conditional Müller Cell Ablation Causes Independent Neuronal and Vascular Pathologies in a Novel Transgenic Model. J. Neurosci. 2012, 32, 15715–15727. [Google Scholar] [CrossRef] [PubMed]
- Metea, M.R.; Newman, E.A. Glial Cells Dilate and Constrict Blood Vessels: A Mechanism of Neurovascular Coupling. J. Neurosci. 2006, 26, 2862–2870. [Google Scholar] [CrossRef]
- Newman, E.A. Functional Hyperemia and Mechanisms of Neurovascular Coupling in the Retinal Vasculature. J. Cereb. Blood Flow Metab. 2013, 33, 1685–1695. [Google Scholar] [CrossRef]
- Newman, E.A. Glial Cell Regulation of Neuronal Activity and Blood Flow in the Retina by Release of Gliotransmitters. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 1–9. [Google Scholar] [CrossRef]
- Amann, B.; Kleinwort, K.J.H.; Hirmer, S.; Sekundo, W.; Kremmer, E.; Hauck, S.M.; Deeg, C.A. Expression and Distribution Pattern of Aquaporin 4, 5 and 11 in Retinas of 15 Different Species. Int. J. Mol. Sci. 2016, 17, 1145. [Google Scholar] [CrossRef]
- Vujosevic, S.; Midena, E. Retinal Layers Changes in Human Preclinical and Early Clinical Diabetic Retinopathy Support Early Retinal Neuronal and Müller Cells Alterations. J. Diabetes Res. 2013, 2013, 905058. [Google Scholar] [CrossRef]
- Orduña Ríos, M.; Noguez Imm, R.; Hernández Godínez, N.M.; Bautista Cortes, A.M.; López Escalante, D.D.; Liedtke, W.; Martínez Torres, A.; Concha, L.; Thébault, S. TRPV4 Inhibition Prevents Increased Water Diffusion and Blood-Retina Barrier Breakdown in the Retina of Streptozotocin-Induced Diabetic Mice. PLoS ONE 2019, 14, e0212158. [Google Scholar] [CrossRef]
- Arredondo Zamarripa, D.; Noguez Imm, R.; Bautista Cortés, A.M.; Vázquez Ruíz, O.; Bernardini, M.; Fiorio Pla, A.; Gkika, D.; Prevarskaya, N.; López-Casillas, F.; Liedtke, W.; et al. Dual Contribution of TRPV4 Antagonism in the Regulatory Effect of Vasoinhibins on Blood-Retinal Barrier Permeability: Diabetic Milieu Makes a Difference. Sci. Rep. 2017, 7, 13094. [Google Scholar] [CrossRef]
- Li, H.; Chen, D.; Sun, W.; Chen, J.; Luo, C.; Xu, H.; Ma, J.H.; Tang, S. Katp Opener Attenuates Diabetic-Induced Müller Gliosis and Inflammation by Modulating Kir6.1 in Microglia. Investig. Ophthalmol. Vis. Sci. 2021, 62, 3. [Google Scholar] [CrossRef] [PubMed]
- Hollborn, M.; Dukic-Stefanovic, S.; Pannicke, T.; Ulbricht, E.; Reichenbach, A.; Wiedemann, P.; Bringmann, A.; Kohen, L. Expression of Aquaporins in the Retina of Diabetic Rats. Curr. Eye Res. 2011, 36, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Pannicke, T.; Iandiev, I.; Wurm, A.; Uckermann, O.; Vom Hagen, F.; Reichenbach, A.; Wiedemann, P.; Hammes, H.P.; Bringmann, A. Diabetes Alters Osmotic Swelling Characteristics and Membrane Conductance of Glial Cells in Rat Retina. Diabetes 2006, 55, 633–639. [Google Scholar] [CrossRef]
- Oosuka, S.; Kida, T.; Oku, H.; Horie, T.; Morishita, S.; Fukumoto, M.; Sato, T.; Ikeda, T. Effects of an Aquaporin 4 Inhibitor, TGN-020, on Murine Diabetic Retina. Int. J. Mol. Sci. 2020, 21, 2324. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Sun, J.-H.; Xiang, F.-F.; Liu, L.; Li, W.-J. Aquaporin 4 Knockdown Exacerbates Streptozotocin-Induced Diabetic Retinopathy through Aggravating Inflammatory Response. Exp. Eye Res. 2012, 98, 37–43. [Google Scholar] [CrossRef]
- Picconi, F.; Parravano, M.; Sciarretta, F.; Fulci, C.; Nali, M.; Frontoni, S.; Varano, M.; Caccuri, A.M. Activation of Retinal Müller Cells in Response to Glucose Variability. Endocrine 2019, 65, 542–549. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, G.; Ling, Q.; Da, C. Expression of Aquaporin 4 and Kir4.1 in Diabetic Rat Retina: Treatment with Minocycline. J. Int. Med. Res. 2011, 39, 464–479. [Google Scholar] [CrossRef]
- Kida, T.; Oku, H.; Horie, T.; Fukumoto, M.; Okuda, Y.; Morishita, S.; Ikeda, T. Implication of VEGF and Aquaporin 4 Mediating Müller Cell Swelling to Diabetic Retinal Edema. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 1149–1157. [Google Scholar] [CrossRef]
- Reichenbach, A.; Bringmann, A. New Functions of Müller Cells. Glia 2013, 61, 651–678. [Google Scholar] [CrossRef]
- Fletcher, E.; Phipps, J.; Ward, M.; Puthussery, T.; Wilkinson-Berka, J. Neuronal and Glial Cell Abnormality as Predictors of Progression of Diabetic Retinopathy. Curr. Pharm. Des. 2007, 13, 2699–2712. [Google Scholar] [CrossRef]
- Curtis, T.M.; Hamilton, R.; Yong, P.H.; McVicar, C.M.; Berner, A.; Pringle, R.; Uchida, K.; Nagai, R.; Brockbank, S.; Stitt, A.W. Müller Glial Dysfunction during Diabetic Retinopathy in Rats Is Linked to Accumulation of Advanced Glycation End-Products and Advanced Lipoxidation End-Products. Diabetologia 2011, 54, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Chavira-Suárez, E.; Sandoval, A.; Felix, R.; Lamas, M. Expression and High Glucose-Mediated Regulation of K+ Channel Interacting Protein 3 (KChIP3) and KV4 Channels in Retinal Müller Glial Cells. Biochem. Biophys. Res. Commun. 2011, 404, 678–683. [Google Scholar] [CrossRef] [PubMed]
- McDowell, R.E.; Barabas, P.; Augustine, J.; Chevallier, O.; McCarron, P.; Chen, M.; McGeown, J.G.; Curtis, T.M. Müller Glial Dysfunction during Diabetic Retinopathy in Rats Is Reduced by the Acrolein-Scavenging Drug, 2-Hydrazino-4,6-Dimethylpyrimidine. Diabetologia 2018, 61, 2654–2667. [Google Scholar] [CrossRef]
- Ishikawa, M. Abnormalities in Glutamate Metabolism and Excitotoxicity in the Retinal Diseases. Scientifica 2013, 2013, 528940. [Google Scholar] [CrossRef]
- Lieth, E.; Barber, A.J.; Xu, B.; Dice, C.; Ratz, M.J.; Tanase, D.; Strother, J.M. Glial Reactivity and Impaired Glutamate Metabolism in Short-Term Experimental Diabetic Retinopathy. Penn State Retina Research Group. Diabetes 1998, 47, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Qiu, A.W.; Liu, Q.H.; Wang, J.L. Blocking IL-17A Alleviates Diabetic Retinopathy in Rodents. Cell. Physiol. Biochem. 2017, 41, 960–972. [Google Scholar] [CrossRef]
- Ma, M.; Zhao, S.; Zhang, J.; Sun, T.; Fan, Y.; Zheng, Z. High Glucose-Induced TRPC6 Channel Activation Decreases Glutamate Uptake in Rat Retinal Müller Cells. Front. Pharmacol. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Gu, L.; Xu, H.; Wang, F.; Xu, G.; Sinha, D.; Wang, J.; Xu, J.Y.; Tian, H.; Gao, F.; Li, W.; et al. Erythropoietin Exerts a Neuroprotective Function against Glutamate Neurotoxicity in Experimental Diabetic Retina. Investig. Ophthalmol. Vis. Sci. 2014, 55, 8208–8222. [Google Scholar] [CrossRef]
- Solà-Adell, C.; Bogdanov, P.; Hernández, C.; Sampedro, J.; Valeri, M.; Garcia-Ramirez, M.; Pasquali, C.; Simó, R. Calcium Dobesilate Prevents Neurodegeneration and Vascular Leakage in Experimental Diabetes. Curr. Eye Res. 2017, 42, 1273–1286. [Google Scholar] [CrossRef]
- Ambati, J.; Chalam, K.V.; Chawla, D.K.; D’Angio, C.T.; Guillet, E.G.; Rose, S.J.; Vanderlinde, R.E.; Ambati, B.K. Elevated Gamma-Aminobutyric Acid, Glutamate, and Vascular Endothelial Growth Factor Levels in the Vitreous of Patients with Proliferative Diabetic Retinopathy. Arch. Ophthalmol. 1997, 115, 1161–1166. [Google Scholar] [CrossRef]
- Deng, J.; Wu, D.Z.; Gao, R. Detection of Glutamate and Gamma-Aminobutyric Acid in Vitreous of Patients with Proliferative Diabetic Retinopathy. Yan ke xue bao 2000, 16, 199–202. [Google Scholar]
- Li, Q.; Puro, D.G. Diabetes-Induced Dysfunction of the Glutamate Transporter in Retinal Müller Cells. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3109–3116. [Google Scholar]
- Gowda, K.; Zinnantif, W.J.; LaNoue, K.F. The Influence of Diabetes on Glutamate Metabolism in Retinas. J. Neurochem. 2011, 117, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Miya-Coreixas, V.S.; Maggesissi Santos, R.; Carpi Santos, R.; Gardino, P.F.; Calaza, K. Regulation of GABA Content by Glucose in the Chick Retina. Exp. Eye Res. 2013, 115, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Carpi-Santos, R.; Maggesissi, R.S.; von Seehausen, M.P.; Calaza, K.C. Retinal Exposure to High Glucose Condition Modifies the GABAergic System: Regulation by Nitric Oxide. Exp. Eye Res. 2017, 162, 116–125. [Google Scholar] [CrossRef]
- Ishikawa, A.; Ishiguro, S.I.; Tamai, M. Changes in GABA Metabolism in Streptozotocin-Induced Diabetic Rat Retinas. Curr. Eye Res. 1996, 15, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Ruzafa, N.; Pereiro, X.; Lepper, M.F.; Hauck, S.M.; Vecino, E. A Proteomics Approach to Identify Candidate Proteins Secreted by Müller Glia That Protect Ganglion Cells in the Retina. Proteomics 2018, 18, 1700321. [Google Scholar] [CrossRef]
- Eastlake, K.; Wang, W.; Jayaram, H.; Murray-Dunning, C.; Carr, A.J.F.; Ramsden, C.M.; Vugler, A.; Gore, K.; Clemo, N.; Stewart, M.; et al. Phenotypic and Functional Characterization of Müller Glia Isolated from Induced Pluripotent Stem Cell-Derived Retinal Organoids: Improvement of Retinal Ganglion Cell Function upon Transplantation. Stem Cells Transl. Med. 2019, 8, 775–784. [Google Scholar] [CrossRef]
- Matteucci, A.; Gaddini, L.; Villa, M.; Varano, M.; Parravano, M.; Monteleone, V.; Cavallo, F.; Leo, L.; Mallozzi, C.; Malchiodi-Albedi, F.; et al. Neuroprotection by Rat Müller Glia against High Glucose-Induced Neurodegeneration through a Mechanism Involving ERK1/2 Activation. Exp. Eye Res. 2014, 125, 20–29. [Google Scholar] [CrossRef]
- Fu, Z.; Wang, Z.; Liu, C.H.; Gong, Y.; Cakir, B.; Liegl, R.; Sun, Y.; Meng, S.S.; Burnim, S.B.; Arellano, I.; et al. Fibroblast Growth Factor 21 Protects Photoreceptor Function in Type 1 Diabetic Mice. Diabetes 2018, 67, 974–985. [Google Scholar] [CrossRef]
- Boss, J.D.; Singh, P.K.; Pandya, H.K.; Tosi, J.; Kim, C.; Tewari, A.; Juzych, M.S.; Abrams, G.W.; Kumar, A. Assessment of Neurotrophins and Inflammatory Mediators in Vitreous of Patients with Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5594–5603. [Google Scholar] [CrossRef] [PubMed]
- Coorey, N.J.; Shen, W.; Chung, S.H.; Zhu, L.; Gillies, M.C. The Role of Glia in Retinal Vascular Disease. Clin. Exp. Optom. 2012, 95, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Nakagawa, K.; Khalil, A.; Ishibashi, T.; Inomata, H.; Sueishi, K. The Relation between Expression of Vascular Endothelial Growth Factor and Breakdown of the Blood-Retinal Barrier in Diabetic Rat Retinas. Lab. Investig. 1996, 74, 819–825. [Google Scholar] [PubMed]
- Joussen, A.M.; Murata, T.; Tsujikawa, A.; Kirchhof, B.; Bursell, S.E.; Adamis, A.P. Leukocyte-Mediated Endothelial Cell Injury and Death in the Diabetic Retina. Am. J. Pathol. 2001, 158, 147–152. [Google Scholar] [CrossRef]
- Miyamoto, K.; Khosrof, S.; Bursell, S.E.; Rohan, R.; Murata, T.; Clermont, A.C.; Aiello, L.P.; Ogura, Y.; Adamis, A.P. Prevention of Leukostasis and Vascular Leakage in Streptozotocin-Induced Diabetic Retinopathy via Intercellular Adhesion Molecule-1 Inhibition. Proc. Natl. Acad. Sci. USA 1999, 96, 10836–10841. [Google Scholar] [CrossRef]
- Xu, H.-Z.; Le, Y.-Z. Significance of Outer Blood-Retina Barrier Breakdown in Diabetes and Ischemia. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2160–2164. [Google Scholar] [CrossRef]
- Wang, J.; Xu, X.; Elliott, M.H.; Zhu, M.; Le, Y.-Z. Müller Cell-Derived VEGF Is Essential for Diabetes-Induced Retinal Inflammation and Vascular Leakage. Diabetes 2010, 59, 2297–2305. [Google Scholar] [CrossRef]
- Li, Y.; Busoy, J.M.; Zaman, B.A.A.; Tan, Q.S.W.; Tan, G.S.W.; Barathi, V.A.; Cheung, N.; Wei, J.J.Y.; Hunziker, W.; Hong, W.; et al. A Novel Model of Persistent Retinal Neovascularization for the Development of Sustained Anti-VEGF Therapies. Exp. Eye Res. 2018, 174, 98–106. [Google Scholar] [CrossRef]
- Zhou, Y.; Danbolt, N.C. Glutamate as a Neurotransmitter in the Healthy Brain. J. Neural Transm. 2014, 121, 799–817. [Google Scholar] [CrossRef]
- Yafai, Y.; Iandiev, I.; Lange, J.; Unterlauft, J.D.; Wiedemann, P.; Bringmann, A.; Reichenbach, A.; Eichler, W. Müller Glial Cells Inhibit Proliferation of Retinal Endothelial Cells via TGF-Β2 and Smad Signaling. Glia 2014, 62, 1476–1485. [Google Scholar] [CrossRef]
- Mao, X.-B.; Cheng, Y.-H.; Peng, K.-S.; You, Z.-P. Sirtuin (Sirt) 3 Overexpression Prevents Retinopathy in Streptozotocin-Induced Diabetic Rats. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e920883. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.J.; Reh, T.A. Müller Glia Are a Potential Source of Neural Regeneration in the Postnatal Chicken Retina. Nat. Neurosci. 2001, 4, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Mendel, T.A.; Clabough, E.B.D.; Kao, D.S.; Demidova-Rice, T.N.; Durham, J.T.; Zotter, B.C.; Seaman, S.A.; Cronk, S.M.; Rakoczy, E.P.; Katz, A.J.; et al. Pericytes Derived from Adipose-Derived Stem Cells Protect against Retinal Vasculopathy. PLoS ONE 2013, 8, e65691. [Google Scholar] [CrossRef]
- Ezquer, M.; Urzua, C.A.; Montecino, S.; Leal, K.; Conget, P.; Ezquer, F. Intravitreal Administration of Multipotent Mesenchymal Stromal Cells Triggers a Cytoprotective Microenvironment in the Retina of Diabetic Mice. Stem Cell Res. Ther. 2016, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Lieth, E.; Gardner, T.W.; Barber, A.J.; Antonetti, D.A. Retinal Neurodegeneration: Early Pathology in Diabetes. Clin. Exp. Ophthalmol. 2000, 28, 3–8. [Google Scholar] [CrossRef]
- Mylonas, G.; Sacu, S.; Deák, G.; Dunavoelgyi, R.; Buehl, W.; Georgopoulos, M.; Schmidt-Erfurth, U. Macular Edema Following Cataract Surgery in Eyes with Previous 23-Gauge Vitrectomy and Peeling of the Internal Limiting Membrane. Am. J. Ophthalmol. 2013, 155, 253–259.e2. [Google Scholar] [CrossRef]
- Uji, A.; Murakami, T.; Unoki, N.; Ogino, K.; Horii, T.; Yoshitake, S.; Dodo, Y.; Yoshimura, N. Parallelism for Quantitative Image Analysis of Photoreceptor-Retinal Pigment Epithelium Complex Alterations in Diabetic Macular Edema. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3361–3367. [Google Scholar] [CrossRef]
- Kang, M.-K.; Lee, E.-J.; Kim, Y.-H.; Kim, D.Y.; Oh, H.; Kim, S.-I.; Kang, Y.-H. Chrysin Ameliorates Malfunction of Retinoid Visual Cycle through Blocking Activation of AGE-RAGE-ER Stress in Glucose-Stimulated Retinal Pigment Epithelial Cells and Diabetic Eyes. Nutrients 2018, 10, 1046. [Google Scholar] [CrossRef]
- Patrick, A.T.; He, W.; Madu, J.; Sripathi, S.R.; Choi, S.; Lee, K.; Samson, F.P.; Powell, F.L.; Bartoli, M.; Jee, D.; et al. Mechanistic Dissection of Diabetic Retinopathy Using the Protein-Metabolite Interactome. J. Diabetes Metab. Disord. 2020, 19, 829–848. [Google Scholar] [CrossRef]
- Tonade, D.; Kern, T.S. Photoreceptor Cells and RPE Contribute to the Development of Diabetic Retinopathy. Prog. Retin. Eye Res. 2021, 83, 100919. [Google Scholar] [CrossRef]
- Park, S.H.; Park, J.W.; Park, S.J.; Kim, K.Y.; Chung, J.W.; Chun, M.H.; Oh, S.J. Apoptotic Death of Photoreceptors in the Streptozotocin-Induced Diabetic Rat Retina. Diabetologia 2003, 46, 1260–1268. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Veenstra, A.; Palczewski, K.; Kern, T.S. Photoreceptor Cells Are Major Contributors to Diabetes-Induced Oxidative Stress and Local Inflammation in the Retina. Proc. Natl. Acad. Sci. USA 2013, 110, 16586–16591. [Google Scholar] [CrossRef] [PubMed]
- Szabadfi, K.; Atlasz, T.; Kiss, P.; Reglodi, D.; Szabo, A.; Kovacs, K.; Szalontai, B.; Setalo, G.; Banki, E.; Csanaky, K.; et al. Protective Effects of the Neuropeptide PACAP in Diabetic Retinopathy. Cell Tissue Res. 2012, 348, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Énzsöly, A.; Szabó, A.; Kántor, O.; Dávid, C.; Szalay, P.; Szabó, K.; Szél, Á.; Németh, J.; Lukáts, Á. Pathologic Alterations of the Outer Retina in Streptozotocin-Induced Diabetes. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3686–3699. [Google Scholar] [CrossRef]
- Bavinger, J.C.; Dunbar, G.E.; Stem, M.S.; Blachley, T.S.; Kwark, L.; Farsiu, S.; Jackson, G.R.; Gardner, T.W. The Effects of Diabetic Retinopathy and Pan-Retinal Photocoagulation on Photoreceptor Cell Function as Assessed by Dark Adaptometry. Investig. Ophthalmol. Vis. Sci. 2016, 57, 208–217. [Google Scholar] [CrossRef]
- Tavares Ferreira, J.; Alves, M.; Dias-Santos, A.; Costa, L.; Santos, B.O.; Cunha, J.P.; Papoila, A.L.; Abegão Pinto, L. Retinal Neurodegeneration in Diabetic Patients without Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6455–6460. [Google Scholar] [CrossRef]
- Fu, S.; Dong, S.; Zhu, M.; Sherry, D.M.; Wang, C.; You, Z.; Haigh, J.J.; Le, Y.Z. Müller Glia Are a Major Cellular Source of Survival Signals for Retinal Neurons in Diabetes. Diabetes 2015, 64, 3554–3563. [Google Scholar] [CrossRef]
- Nesper, P.L.; Scarinci, F.; Fawzi, A.A. Adaptive Optics Reveals Photoreceptor Abnormalities in Diabetic Macular Ischemia. PLoS ONE 2017, 12, 1–16. [Google Scholar] [CrossRef]
- Lima, V.C.; Rosen, R.B.; Maia, M.; Prata, T.S.; Dorairaj, S.; Farah, M.E.; Sallum, J. Macular Pigment Optical Density Measured by Dual-Wavelength Autofluorescence Imaging in Diabetic and Nondiabetic Patients: A Comparative Study. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5840–5845. [Google Scholar] [CrossRef]
- Hurley, J.B.; Lindsay, K.J.; Du, J. Glucose, Lactate, and Shuttling of Metabolites in Vertebrate Retinas. J. Neurosci. Res. 2015, 93, 1079–1092. [Google Scholar] [CrossRef]
- Poitry-Yamate, C.L.; Poitry, S.; Tsacopoulos, M. Lactate Released by Müller Glial Cells Is Metabolized by Photoreceptors from Mammalian Retina. J. Neurosci. 1995, 15, 5179–5191. [Google Scholar] [CrossRef] [PubMed]
- Hurley, J.B.; Chao, J.R. It’s Never Too Late to Save a Photoreceptor. J. Clin. Invest. 2015, 125, 3424–3426. [Google Scholar] [CrossRef] [PubMed]
- Winkler, B.S.; Arnold, M.J.; Brassell, M.A.; Puro, D.G. Energy Metabolism in Human Retinal Müller Cells. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3183–3190. [Google Scholar] [PubMed]
- Salceda, R.; Vilchis, C.; Coffe, V.; Hernández-Muñoz, R. Changes in the Redox State in the Retina and Brain during the Onset of Diabetes in Rats. Neurochem. Res. 1998, 23, 893–897. [Google Scholar] [CrossRef]
- Santiago, A.R.; Garrido, M.J.; Cristóvão, A.J.; Duarte, J.M.N.; Carvalho, R.A.; Ambrósio, A.F. Evaluation of the Impact of Diabetes on Retinal Metabolites by NMR Spectroscopy. Curr. Eye Res. 2010, 35, 992–1001. [Google Scholar] [CrossRef]
- Ramírez-Pérez, G.; Sánchez-Chávez, G.; Salceda, R. Mitochondrial Bound Hexokinase Type I in Normal and Streptozotocin Diabetic Rat Retina. Mitochondrion 2020, 52, 212–217. [Google Scholar] [CrossRef]
- Pfeiffer, R.L.; Marc, R.E.; Jones, B.W. Müller Cell Metabolic Signatures: Evolutionary Conservation and Disruption in Disease. Trends Endocrinol. Metab. 2020, 31, 320–329. [Google Scholar] [CrossRef]
- Eastlake, K.; Banerjee, P.J.; Angbohang, A.; Charteris, D.G.; Khaw, P.T.; Limb, G.A. Müller Glia as an Important Source of Cytokines and Inflammatory Factors Present in the Gliotic Retina during Proliferative Vitreoretinopathy. Glia 2016, 64, 495–506. [Google Scholar] [CrossRef]
- Subirada, P.V.; Paz, M.C.; Ridano, M.E.; Lorenc, V.E.; Vaglienti, M.V.; Barcelona, P.F.; Luna, J.D.; Sánchez, M.C. A Journey into the Retina: Müller Glia Commanding Survival and Death. Eur. J. Neurosci. 2018, 47, 1429–1443. [Google Scholar] [CrossRef]
- Eymann, J.; Di-Poï, N. Glia-Mediated Regenerative Response Following Acute Excitotoxic Damage in the Postnatal Squamate Retina. Front. Cell Dev. Biol. 2020, 8, 406. [Google Scholar] [CrossRef]
- Schitine, C.S.; Mendez-Flores, O.G.; Santos, L.E.; Ornelas, I.; Calaza, K.C.; Pérez-Toledo, K.; López-Bayghen, E.; Ortega, A.; Gardino, P.F.; De Mello, F.G.; et al. Functional Plasticity of GAT-3 in Avian Müller Cells Is Regulated by Neurons via a Glutamatergic Input. Neurochem. Int. 2015, 82, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Ahmad, I. Let-7 MicroRNA Regulates Neurogliogenesis in the Mammalian Retina through Hmga2. Dev. Biol. 2016, 410, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Pollak, J.; Wilken, M.S.; Ueki, Y.; Cox, K.E.; Sullivan, J.M.; Taylor, R.J.; Levine, E.M.; Reh, T.A. ASCL1 Reprograms Mouse Muller Glia into Neurogenic Retinal Progenitors. Development 2013, 140, 2619–2631. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Zhu, J.; Duan, Y.; Li, G.; Cai, H.; Zheng, L.; Qian, H.; Zhang, C.; Jin, Z.; Fu, X.-D.; et al. Visual Function Restoration in Genetically Blind Mice via Endogenous Cellular Reprogramming. bioRxiv 2020. [Google Scholar] [CrossRef]
- Roska, B.; Sahel, J.-A. Restoring Vision. Nature 2018, 557, 359–367. [Google Scholar] [CrossRef]
- Stower, H. Restoring Sight with Native Cell Reprogramming. Nat. Med. 2018, 24, 1303. [Google Scholar] [CrossRef]
- Yao, K.; Qiu, S.; Wang, Y.V.; Park, S.J.H.; Mohns, E.J.; Mehta, B.; Liu, X.; Chang, B.; Zenisek, D.; Crair, M.C.; et al. Restoration of Vision after de Novo Genesis of Rod Photoreceptors in Mammalian Retinas. Nature 2018, 560, 484–488. [Google Scholar] [CrossRef]
- Konar, G.J.; Ferguson, C.; Flickinger, Z.; Kent, M.R.; Patton, J.G. MiRNAs and Müller Glia Reprogramming During Retina Regeneration. Front. Cell Dev. Biol. 2020, 8, 632632. [Google Scholar] [CrossRef]
- Xia, X.; Teotia, P.; Patel, H.; Van Hook, M.J.; Ahmad, I. Chemical Induction of Neurogenic Properties in Mammalian Müller Glia. Stem Cells 2021, 39, 1081–1090. [Google Scholar] [CrossRef]
- de Melo Reis, R.A.; Ventura, A.L.M.; Schitine, C.S.; de Mello, M.C.F.; de Mello, F.G. Müller Glia as an Active Compartment Modulating Nervous Activity in the Vertebrate Retina: Neurotransmitters and Trophic Factors. Neurochem. Res. 2008, 33, 1466–1474. [Google Scholar] [CrossRef]
- Devoldere, J.; Peynshaert, K.; De Smedt, S.C.; Remaut, K. Müller Cells as a Target for Retinal Therapy. Drug Discov. Today 2019, 24, 1483–1498. [Google Scholar] [CrossRef] [PubMed]
- Schmalen, A.; Lorenz, L.; Grosche, A.; Pauly, D.; Deeg, C.A.; Hauck, S.M. Proteomic Phenotyping of Stimulated Müller Cells Uncovers Profound Pro-In Fl Ammatory Signaling and Antigen-Presenting Capacity. Front. Pharmacol. 2021, 12, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Sanhueza Salas, L.F.; García-Venzor, A.; Beltramone, N.; Capurro, C.; Toiber, D.; Silberman, D.M. Metabolic Imbalance Effect on Retinal Müller Glial Cells Reprogramming Capacity: Involvement of Histone Deacetylase SIRT6. Front. Genet. 2021, 12, 769723. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, M.; Hitchcock, P.F. Inflammation Regulates the Multi-Step Process of Retinal Regeneration in Zebrafish. Cells 2021, 10, 783. [Google Scholar] [CrossRef]
- Palazzo, I.; Deistler, K.; Hoang, T.V.; Blackshaw, S.; Fischer, A.J. NF-ΚB Signaling Regulates the Formation of Proliferating Müller Glia-Derived Progenitor Cells in the Avian Retina. Development 2020, 147, dev183418. [Google Scholar] [CrossRef]
- Zhang, Z.; Hou, H.; Yu, S.; Zhou, C.; Zhang, X.; Li, N.; Zhang, S.; Song, K.; Lu, Y.; Liu, D.; et al. Inflammation-Induced Mammalian Target of Rapamycin Signaling Is Essential for Retina Regeneration. Glia 2020, 68, 111–127. [Google Scholar] [CrossRef]
- Zelinka, C.P.; Volkov, L.; Goodman, Z.A.; Todd, L.; Palazzo, I.; Bishop, W.A.; Fischer, A.J. MTor Signaling Is Required for the Formation of Proliferating Müller Glia-Derived Progenitor Cells in the Chick Retina. Development 2016, 143, 1859–1873. [Google Scholar] [CrossRef]
- Todd, L.; Finkbeiner, C.; Wong, C.K.; Hooper, M.J.; Reh, T.A. Microglia Suppress Ascl1-Induced Retinal Regeneration in Mice. Cell Rep. 2020, 33, 108507. [Google Scholar] [CrossRef]
- Shen, W.; Li, S.; Chung, S.H.; Gillies, M.C. Retinal Vascular Changes after Glial Disruption in Rats. J. Neurosci. Res. 2010, 88, 1485–1499. [Google Scholar] [CrossRef]
- Garcia-Ramírez, M.; Hernández, C.; Villarroel, M.; Canals, F.; Alonso, M.A.; Fortuny, R.; Masmiquel, L.; Navarro, A.; García-Arumí, J.; Simó, R. Interphotoreceptor Retinoid-Binding Protein (IRBP) Is Downregulated at Early Stages of Diabetic Retinopathy. Diabetologia 2009, 52, 2633–2641. [Google Scholar] [CrossRef]
- Mendonca, H.; Carpi-Santos, R.; Da Costa Calaza, K.; Blanco Martinez, A. Neuroinflammation and Oxidative Stress Act in Concert to Promote Neurodegeneration in the Diabetic Retina and Optic Nerve: Galectin-3 Participation. Neural Regen. Res. 2020, 15, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Rübsam, A.; Parikh, S.; Fort, P.E. Role of Inflammation in Diabetic Retinopathy. Int. J. Mol. Sci. 2018, 19, 942. [Google Scholar] [CrossRef] [PubMed]
- Chalam, K.V.; Grover, S.; Sambhav, K.; Balaiya, S.; Murthy, R.K. Aqueous Interleukin-6 Levels Are Superior to Vascular Endothelial Growth Factor in Predicting Therapeutic Response to Bevacizumab in Age-Related Macular Degeneration. J. Ophthalmol. 2014, 2014, 502174. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Li, L.; Zhu, L.; Guo, Y.; Du, S.; Zhang, Y.; Wang, Z.; Zhang, Y.; Zhu, M. Geniposide Attenuates Hyperglycemia-Induced Oxidative Stress and Inflammation by Activating the Nrf2 Signaling Pathway in Experimental Diabetic Retinopathy. Oxid. Med. Cell. Longev. 2021, 2021, 9247947. [Google Scholar] [CrossRef]
- Zong, H.; Ward, M.; Madden, A.; Yong, P.H.; Limb, G.A.; Curtis, T.M.; Stitt, A.W. Hyperglycaemia-Induced pro-Inflammatory Responses by Retinal Müller Glia Are Regulated by the Receptor for Advanced Glycation End-Products (RAGE). Diabetologia 2010, 53, 2656–2666. [Google Scholar] [CrossRef] [PubMed]
- Albert-Garay, J.S.; Riesgo-Escovar, J.; Salceda, R. High Glucose Concentrations Induce Oxidative Stress by Inhibiting Nrf2 Expression in Rat Müller Retinal Cells in Vitro. Sci. Rep. 2022, 12, 1261. [Google Scholar] [CrossRef] [PubMed]
- Deliyanti, D.; Alrashdi, S.F.; Tan, S.M.; Meyer, C.; Ward, K.W.; De, J.B.; Wilkinson-berka, J.L. Nrf2 Activation Is a Potential Therapeutic Approach to Attenuate Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2018, 59, 815–825. [Google Scholar] [CrossRef]
- Li, S.; Yang, H.; Chen, X. Protective Effects of Sulforaphane on Diabetic Retinopathy: Activation of the Nrf2 Pathway and Inhibition of NLRP3 Inflammasome Formation. Exp. Anim. 2019, 68, 221–231. [Google Scholar] [CrossRef]
- Nonarath, H.J.; Hall, A.E.; SenthilKumar, G.; Abroe, B.; Eells, J.T.; Liedhegner, E.S. 670nm Photobiomodulation Modulates Bioenergetics and Oxidative Stress, in Rat Müller Cells Challenged with High Glucose. PLoS ONE 2021, 16, e0260968. [Google Scholar] [CrossRef]
- Walker, R.J.; Steinle, J.J. Role of Beta-Adrenergic Receptors in Inflammatory Marker Expression in Müller Cells. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5276–5281. [Google Scholar] [CrossRef]
- Xu, Z.; Wei, Y.; Gong, J.; Cho, H.; Park, J.K.; Sung, E.-R.; Huang, H.; Wu, L.; Eberhart, C.; Handa, J.T.; et al. NRF2 Plays a Protective Role in Diabetic Retinopathy in Mice. Diabetologia 2014, 57, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Xi, X.; Gao, L.; Hatala, D.A.; Smith, D.G.; Codispoti, M.C.; Gong, B.; Kern, T.S.; Zhang, J.-Z. Chronically Elevated Glucose-Induced Apoptosis Is Mediated by Inactivation of Akt in Cultured Müller Cells. Biochem. Biophys. Res. Commun. 2005, 326, 548–553. [Google Scholar] [CrossRef]
- Jiang, Y.; Pagadala, J.; Miller, D.; Steinle, J.J. Reduced Insulin Receptor Signaling in Retinal Müller Cells Cultured in High Glucose. Mol. Vis. 2013, 19, 804–811. [Google Scholar] [PubMed]
- Han, N.; Yu, L.; Song, Z.; Luo, L.; Wu, Y. Agmatine Protects Müller Cells from High-Concentration Glucose-Induced Cell Damage via N-Methyl-D-Aspartic Acid Receptor Inhibition. Mol. Med. Rep. 2015, 12, 1098–1106. [Google Scholar] [CrossRef]
- Ao, H.; Li, H.; Zhao, X.; Liu, B.; Lu, L. TXNIP Positively Regulates the Autophagy and Apoptosis in the Rat Müller Cell of Diabetic Retinopathy. Life Sci. 2021, 267, 118988. [Google Scholar] [CrossRef]
- Duarte, D.A.; Papadimitriou, A.; Gilbert, R.E.; Thai, K.; Zhang, Y.; Rosales, M.A.B.; De Faria, J.B.L.; De Faria, J.M.L. Conditioned Medium from Early-Outgrowth Bone Marrow Cells Is Retinal Protective in Experimental Model of Diabetes. PLoS ONE 2016, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Devi, T.S.; Lee, I.; Hüttemann, M.; Kumar, A.; Nantwi, K.D.; Singh, L.P. TXNIP Links Innate Host Defense Mechanisms to Oxidative Stress and Inflammation in Retinal Muller Glia under Chronic Hyperglycemia: Implications for Diabetic Retinopathy. Exp. Diabetes Res. 2012, 2012, 438238. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.P.; Devi, T.S. Potential Combination Drug Therapy to Prevent Redox Stress and Mitophagy Dysregulation in Retinal Müller Cells under High Glucose Conditions: Implications for Diabetic Retinopathy. Diseases 2021, 9, 91. [Google Scholar] [CrossRef]
- Trueblood, K.E.; Mohr, S.; Dubyak, G.R. Purinergic Regulation of High-Glucose-Induced Caspase-1 Activation in the Rat Retinal Müller Cell Line RMC-1. Am. J. Physiol. Cell Physiol. 2011, 301, 1–22. [Google Scholar] [CrossRef]
- Hernández-Ramírez, E.; Sánchez-Chávez, G.; Estrella-Salazar, L.A.; Salceda, R. Nitrosative Stress in the Rat Retina at the Onset of Streptozotocin-Induced Diabetes. Cell. Physiol. Biochem. 2017, 42, 2353–2363. [Google Scholar] [CrossRef]
- Lieth, E.; LaNoue, K.F.; Antonetti, D.A.; Ratz, M. Diabetes Reduces Glutamate Oxidation and Glutamine Synthesis in the Retina. The Penn State Retina Research Group. Exp. Eye Res. 2000, 70, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Rungger-Brändle, E.; Dosso, A.A.; Leuenberger, P.M. Glial Reactivity, an Early Feature of Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1971–1980. [Google Scholar]
- Syrbe, S.; Kuhrt, H.; Gärtner, U.; Habermann, G.; Wiedemann, P.; Bringmann, A.; Reichenbach, A. Müller Glial Cells of the Primate Foveola: An Electron Microscopical Study. Exp. Eye Res. 2018, 167, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Udaondo, P.; Adan, A.; Arias-Barquet, L.; Ascaso, F.J.; Cabrera-López, F.; Castro-Navarro, V.; Donate-López, J.; García-Layana, A.; Lavid, F.J.; Rodríguez-Maqueda, M.; et al. Challenges in Diabetic Macular Edema Management: An Expert Consensus Report. Clin. Ophthalmol. 2021, 15, 3183–3195. [Google Scholar] [CrossRef]
- Ashraf, M.; Souka, A.; Adelman, R. Predicting Outcomes to Anti-Vascular Endothelial Growth Factor (VEGF) Therapy in Diabetic Macular Oedema: A Review of the Literature. Br. J. Ophthalmol. 2016, 100, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Muftuoglu, I.K.; Mendoza, N.; Gaber, R.; Alam, M.; You, Q.; Freeman, W.R. Integrity of outer retinal layers after resolution of central involved diabetic macular edema. Retina 2017, 37, 2015–2024. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Ramasamy, K.; Sivaprasad, S. Indicators of Visual Prognosis in Diabetic Macular Oedema. J. Pers. Med. 2021, 11, 449. [Google Scholar] [CrossRef]
- Choi, M.; Yun, C.; Oh, J.-H.; Kim, S.-W. Foveal Müller Cell Cone as a Prognostic OCT Biomarker for Initial Response to Anti-VEGF Treatment in Cystoid Diabetic Macular Edema. Retina 2021. [Google Scholar] [CrossRef]
- Pierce, E.A.; Avery, R.L.; Foley, E.D.; Aiello, L.P.; Smith, L.E. Vascular Endothelial Growth Factor/Vascular Permeability Factor Expression in a Mouse Model of Retinal Neovascularization. Proc. Natl. Acad. Sci. USA 1995, 92, 905–909. [Google Scholar] [CrossRef]
- Robbins, S.G.; Conaway, J.R.; Ford, B.L.; Roberto, K.A.; Penn, J.S. Detection of Vascular Endothelial Growth Factor (VEGF) Protein in Vascular and Non-Vascular Cells of the Normal and Oxygen-Injured Rat Retina. Growth Factors 1997, 14, 229–241. [Google Scholar] [CrossRef]
- Bai, Y.; Ma, J.; Guo, J.; Wang, J.; Zhu, M.; Chen, Y.; Le, Y.-Z. Müller Cell-Derived VEGF Is a Significant Contributor to Retinal Neovascularization. J. Pathol. 2009, 219, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Hauck, S.M.; von Toerne, C.; Ueffing, M. The Neuroprotective Potential of Retinal Müller Glial Cells. Adv. Exp. Med. Biol. 2014, 801, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.K.; Benyajati, S.; Le, Y.; Cheng, R.; Zhang, W.; Ma, J. Interruption of Wnt Signaling in Müller Cells Ameliorates Ischemia-Induced Retinal Neovascularization. PLoS ONE 2014, 9, e108454. [Google Scholar] [CrossRef] [PubMed]
- Nie, F.; Yan, J.; Ling, Y.; Liu, Z.; Fu, C.; Li, X.; Qin, Y. Effect of Shuangdan Mingmu Capsule, a Chinese Herbal Formula, on Oxidative Stress- Induced Apoptosis of Pericytes through PARP/GAPDH Pathway. BMC Complementary Med. Ther. 2021, 21, 118. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; An, Y.; He, X.; Zhang, D.; He, W. Protection of Kaempferol on Oxidative Stress-Induced Retinal Pigment Epithelial Cell Damage. Oxidative Med. Cell. Longev. 2018, 2018, 438238. [Google Scholar] [CrossRef]
- Haque, R.; Iuvone, P.M.; He, L.; Hur, E.H.; Su, K.; Choi, C.; Park, D.; Farrell, A.N.; Ngo, A.; Gokhale, S.; et al. Prorenin receptor (PRR)-mediated NADPH oxidase (Nox) signaling regulates VEGF synthesis under hyperglycemic condition in ARPE-19 cells. J. Recept. Signal Transduct. 2017, 37, 560–568. [Google Scholar] [CrossRef]
- Rossino, M.G.; Lulli, M.; Amato, R.; Cammalleri, M.; Monte, M.D.; Casini, G. Oxidative Stress Induces a VEGF Autocrine Loop in the Retina: Relevance for Diabetic Retinopathy. Cells 2020, 9, 1452. [Google Scholar] [CrossRef]
- Wang, J.; Shanmugam, A.; Markand, S.; Zorrilla, E.; Ganapathy, V.; Smith, S.B. Sigma 1 Receptor Regulates the Oxidative Stress Response in Primary Retinal Müller Glial Cells via NRF2 Signaling and System Xc(-), the Na(+)-Independent Glutamate-Cystine Exchanger. Free Radic. Biol. Med. 2015, 86, 25–36. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, J.; Chen, L. The Cells Involved in the Pathological Process of Diabetic Retinopathy. Biomed. Pharmacother. 2020, 132, 110818. [Google Scholar] [CrossRef]
- Chalke, S.D.; Kale, P.P. Combinational Approaches Targeting Neurodegeneration, Oxidative Stress, and Inflammation in the Treatment of Diabetic Retinopathy. Curr. Drug Targets 2021, 22, 1810–1824. [Google Scholar] [CrossRef]
- Fukai, T.; Ushio-fukai, M. Cross-Talk between NADPH Oxidase and Mitochondria: Role in ROS Signaling and Angiogenesis. Cells 2020, 9, 1849. [Google Scholar] [CrossRef] [PubMed]
- Laddha, A.P.; Kulkarni, Y.A. NADPH Oxidase: A Membrane-Bound Enzyme and Its Inhibitors in Diabetic Complications. Eur. J. Pharmacol. 2020, 881, 173206. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ai, S.; Chen, Z.; Li, C. Probucol Promotes High Glucose-Induced Proliferation and Inhibits Apoptosis by Reducing Reactive Oxygen Species Generation in Müller Cells. Int. Ophthalmol. 2019, 39, 2833–2842. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Y.; Ho, Y.-J.; Chou, H.-C.; Liao, E.-C.; Tsai, Y.-T.; Wei, Y.-S.; Lin, L.-H.; Lin, M.-W.; Wang, Y.-S.; Ko, M.-L.; et al. The Role of Transforming Growth Factor-Beta in Retinal Ganglion Cells with Hyperglycemia and Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 6482. [Google Scholar] [CrossRef]
- Chen, N.; Li, Y.; Huang, N.; Yao, J.; Luo, W.-F.; Jiang, Q. The Nrf2 Activator MIND4-17 Protects Retinal Ganglion Cells from High Glucose-Induced Oxidative Injury. J. Cell. Physiol. 2020, 235, 7204–7213. [Google Scholar] [CrossRef]
- Sun, W.; Yu, J.; Kang, Q. Upregulation of Heme Oxygenase-1 by Brahma-Related Gene 1 through Nrf2 Signaling Confers Protective Effect against High Glucose-Induced Oxidative Damage of Retinal Ganglion Cells. Eur. J. Pharmacol. 2020, 875, 173038. [Google Scholar] [CrossRef]
- Rezzola, S.; Guerra, J.; Chandran, A.M.K.; Loda, A.; Cancarini, A.; Sacristani, P.; Semeraro, F.; Presta, M. Vegf-independent Activation of Müller Cells by the Vitreous from Proliferative Diabetic Retinopathy Patients. Int. J. Mol. Sci. 2021, 22, 2179. [Google Scholar] [CrossRef]
- Hammes, H.-P. Diabetic Retinopathy: Hyperglycaemia, Oxidative Stress and Beyond. Diabetologia 2018, 61, 29–38. [Google Scholar] [CrossRef]
- Brownlee, M. The Pathobiology of Diabetic Complications: A Unifying Mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef]
- Gui, F.; You, Z.; Fu, S.; Wu, H.; Zhang, Y. Endothelial Dysfunction in Diabetic Retinopathy. Front. Endocrinol. 2020, 11, 591. [Google Scholar] [CrossRef]
- Trumpower, B.L. The Protonmotive Q Cycle. Energy Transduction by Coupling of Proton Translocation to Electron Transfer by the Cytochrome Bc1 Complex. J. Biol. Chem. 1990, 265, 11409–11412. [Google Scholar] [CrossRef]
- Duarte, D.A.; Rosales, M.A.B.; Papadimitriou, A.; Silva, K.C.; Amancio, V.H.O.; Mendonça, J.N.; Lopes, N.P.; Lopes de Faria, J.B.; Lopes de Faria, J.M. Polyphenol-Enriched Cocoa Protects the Diabetic Retina from Glial Reaction through the Sirtuin Pathway. J. Nutr. Biochem. 2015, 26, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Gupta, S.K.; Nag, T.C.; Srivastava, S.; Saxena, R.; Jha, K.A.; Srinivasan, B.P. Retinal Neuroprotective Effects of Quercetin in Streptozotocin-Induced Diabetic Rats. Exp. Eye Res. 2014, 125, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Al-Dosary, D.I.; Alhomida, A.S.; Ola, M.S. Protective Effects of Dietary Flavonoids in Diabetic Induced Retinal Neurodegeneration. Curr. Drug Targets 2017, 18, 1468–1476. [Google Scholar] [CrossRef]
- Silva, K.C.; Rosales, M.A.B.; Hamassaki, D.E.; Saito, K.C.; Faria, A.M.; Ribeiro, P.A.O.; de Faria, J.B.L.; de Faria, J.M.L. Green Tea Is Neuroprotective in Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1325–1336. [Google Scholar] [CrossRef]
- Wang, Y.; Tao, J.; Jiang, M.; Yao, Y. Apocynin Ameliorates Diabetic Retinopathy in Rats: Involvement of TLR4/NF-ΚB Signaling Pathway. Int. Immunopharmacol. 2019, 73, 49–56. [Google Scholar] [CrossRef]
- Matos, A.L.; Bruno, D.F.; Ambr, F. The Benefits of Flavonoids in Diabetic Retinopathy. Nutrients 2020, 12, 3169. [Google Scholar] [CrossRef]
- Rossino, M.G.; Casini, G. Nutraceuticals for the Treatment of Diabetic Retinopathy. Nutrients 2019, 11, 771. [Google Scholar] [CrossRef]
- Domanico, D.; Fragiotta, S.; Cutini, A.; Carnevale, C.; Zompatori, L.; Vingolo, E.M. Circulating Levels of Reactive Oxygen Species in Patients with Nonproliferative Diabetic Retinopathy and the Influence of Antioxidant Supplementation: 6-Month Follow-Up. Indian J. Ophthalmol. 2015, 63, 9–14. [Google Scholar] [CrossRef]
- Garcia-Medina, J.J.; Rubio-Velazquez, E.; Foulquie-Moreno, E.; Casaroli-Marano, R.P.; Pinazo-Duran, M.D.; Zanon-Moreno, V.; Del-Rio-vellosillo, M. Update on the Effects of Antioxidants on Diabetic Retinopathy: In Vitro Experiments, Animal Studies and Clinical Trials. Antioxidants 2020, 9, 561. [Google Scholar] [CrossRef]
- Tabatabaei-Malazy, O.; Ardeshirlarijani, E.; Namazi, N.; Nikfar, S.; Jalili, R.B.; Larijani, B. Dietary Antioxidative Supplements and Diabetic Retinopathy; a Systematic Review. J. Diabetes Metab. Disord. 2019, 18, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Roig-Revert, M.J.; Lleó-Pérez, A.; Zanón-Moreno, V.; Vivar-Llopis, B.; Marín-Montiel, J.; Dolz-Marco, R.; Alonso-Muñoz, L.; Albert-Fort, M.; López-Gálvez, M.I.; Galarreta-Mira, D.; et al. Enhanced Oxidative Stress and Other Potential Biomarkers for Retinopathy in Type 2 Diabetics: Beneficial Effects of the Nutraceutic Supplements. Biomed Res. Int. 2015, 2015, 408180. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Medina, J.J.; Pinazo-Duran, M.D.; Garcia-Medina, M.; Zanon-Moreno, V.; Pons-Vazquez, S. A 5-Year Follow-up of Antioxidant Supplementation in Type 2 Diabetic Retinopathy. Eur. J. Ophthalmol. 2011, 21, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Mares, J. Lutein and Zeaxanthin Isomers in Eye Health and Disease. Annu. Rev. Nutr. 2016, 36, 571–602. [Google Scholar] [CrossRef]
- Zhang, P.-C.; Wu, C.-R.; Wang, Z.-L.; Wang, L.-Y.; Han, Y.; Sun, S.-L.; Li, Q.-S.; Ma, L. Effect of Lutein Supplementation on Visual Function in Nonproliferative Diabetic Retinopathy. Asia Pac. J. Clin. Nutr. 2017, 26, 406–411. [Google Scholar] [CrossRef]
- Ren, Y.-B.; Qi, Y.-X.; Su, X.-J.; Luan, H.-Q.; Sun, Q. Therapeutic Effect of Lutein Supplement on Non-Proliferative Diabetic Retinopathy: A Retrospective Study. Medicine 2019, 98, e15404. [Google Scholar] [CrossRef]
- de Sampaio e Spohr, T.C.L.; Stipursky, J.; Sasaki, A.C.; Barbosa, P.R.; Martins, V.; Benjamim, C.F.; Roque, N.F.; Costa, S.L.; Gomes, F.C.A. Effects of the Flavonoid Casticin from Brazilian Croton Betulaster in Cerebral Cortical Progenitors in Vitro: Direct and Indirect Action through Astrocytes. J. Neurosci. Res. 2010, 88, 530–541. [Google Scholar] [CrossRef]
- Nones, J.; de Sampaio Spohr, T.C.L.; Gomes, F.C.A. Effects of the Flavonoid Hesperidin in Cerebral Cortical Progenitors in Vitro: Indirect Action through Astrocytes. Int. J. Dev. Neurosci. Off. J. Int. Soc. Dev. Neurosci. 2012, 30, 303–313. [Google Scholar] [CrossRef]
- Du, Y.; Miller, C.M.; Kern, T.S. Hyperglycemia Increases Mitochondrial Superoxide in Retina and Retinal Cells. Free Radic. Biol. Med. 2003, 35, 1491–1499. [Google Scholar] [CrossRef]
- Frenzel, J.; Richter, J.; Eschrich, K. Pyruvate Protects Glucose-Deprived Müller Cells from Nitric Oxide-Induced Oxidative Stress by Radical Scavenging. Glia 2005, 52, 276–288. [Google Scholar] [CrossRef]
- Huster, D.; Reichenbach, A.; Reichelt, W. The Glutathione Content of Retinal Müller (Glial) Cells: Effect of Pathological Conditions. Neurochem. Int. 2000, 36, 461–469. [Google Scholar] [CrossRef]
- Rice, M.E.; Russo-Menna, I. Differential Compartmentalization of Brain Ascorbate and Glutathione between Neurons and Glia. Neuroscience 1998, 82, 1213–1223. [Google Scholar] [CrossRef]
- Freitas, H.R.; Ferraz, G.; Ferreira, G.C.; Ribeiro-Resende, V.T.; Chiarini, L.B.; Nascimento, J.L.M.D.; Oliveira, K.R.H.M.; De Pereira, T.L.; Ferreira, L.G.B.; Kubrusly, R.C.; et al. Glutathione-Induced Calcium Shifts in Chick Retinal Glial Cells. PLoS ONE 2016, 11, 1–20. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Carpi-Santos, R.; Ferreira, M.J.; Pereira Netto, A.D.; Giestal-de-Araujo, E.; Ventura, A.L.M.; Cossenza, M.; Calaza, K.C. Early Changes in System [Formula: See Text] and Glutathione in the Retina of Diabetic Rats. Exp. Eye Res. 2016, 146, 35–42. [Google Scholar] [CrossRef]
- Carpi-Santos, R.; Calaza, K.C. Alterations in System x(c)(-) Expression in the Retina of Type 1 Diabetic Rats and the Role of Nrf2. Mol. Neurobiol. 2018, 55, 7941–7948. [Google Scholar] [CrossRef]
- Kern, T.S.; Kowluru, R.A.; Engerman, R.L. Abnormalities of Retinal Metabolism in Diabetes or Galactosemia: ATPases and Glutathione. Investig. Ophthalmol. Vis. Sci. 1994, 35, 2962–2967. [Google Scholar]
- Zhong, Q.; Mishra, M.; Kowluru, R.A. Transcription Factor Nrf2-Mediated Antioxidant Defense System in the Development of Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3941–3948. [Google Scholar] [CrossRef]
- Lu, S.C. Regulation of Glutathione Synthesis. Mol. Aspects Med. 2009, 30, 42–59. [Google Scholar] [CrossRef]
- Bannai, S. Exchange of Cystine and Glutamate across Plasma Membrane of Human Fibroblasts. J. Biol. Chem. 1986, 261, 2256–2263. [Google Scholar] [CrossRef]
- Martis, R.M.; Knight, L.J.; Donaldson, P.J.; Lim, J.C. Identification, Expression, and Roles of the Cystine/Glutamate Antiporter in Ocular Tissues. Oxid. Med. Cell. Longev. 2020, 2020, 4594606. [Google Scholar] [CrossRef]
- Kato, S.; Ishita, S.; Sugawara, K.; Mawatari, K. Cystine/Glutamate Antiporter Expression in Retinal Müller Glial Cells: Implications for DL-Alpha-Aminoadipate Toxicity. Neuroscience 1993, 57, 473–482. [Google Scholar] [CrossRef]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Keap1 Represses Nuclear Activation of Antioxidant Responsive Elements by Nrf2 through Binding to the Amino-Terminal Neh2 Domain. Genes Dev. 1999, 13, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Kensler, T.W.; Wakabayashi, N.; Biswal, S. Cell Survival Responses to Environmental Stresses via the Keap1-Nrf2-ARE Pathway. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 89–116. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-Antioxidant Response Element Signaling Pathway and Its Activation by Oxidative Stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef]
- Shih, A.Y.; Johnson, D.A.; Wong, G.; Kraft, A.D.; Jiang, L.; Erb, H.; Johnson, J.A.; Murphy, T.H. Coordinate Regulation of Glutathione Biosynthesis and Release by Nrf2-Expressing Glia Potently Protects Neurons from Oxidative Stress. J. Neurosci. 2003, 23, 3394–3406. [Google Scholar] [CrossRef]
- Shanab, A.Y.; Nakazawa, T.; Ryu, M.; Tanaka, Y.; Himori, N.; Taguchi, K.; Yasuda, M.; Watanabe, R.; Takano, J.; Saido, T.; et al. Metabolic Stress Response Implicated in Diabetic Retinopathy: The Role of Calpain, and the Therapeutic Impact of Calpain Inhibitor. Neurobiol. Dis. 2012, 48, 556–567. [Google Scholar] [CrossRef]
- Larabee, C.M.; Desai, S.; Agasing, A.; Georgescu, C.; Wren, J.D.; Axtell, R.C.; Plafker, S.M. Loss of Nrf2 Exacerbates the Visual Deficits and Optic Neuritis Elicited by Experimental Autoimmune Encephalomyelitis. Mol. Vis. 2016, 22, 1503–1513. [Google Scholar]
- Qin, X.; Li, N.; Zhang, M.; Lin, S.; Zhu, J.; Xiao, D.; Cui, W.; Zhang, T.; Lin, Y.; Cai, X. Tetrahedral Framework Nucleic Acids Prevent Retina Ischemia-Reperfusion Injury from Oxidative Stress via Activating the Akt/Nrf2 Pathway. Nanoscale 2019, 11, 20667–20675. [Google Scholar] [CrossRef]
- Wang, J.-J. Functions of Müller Cell-Derived Vascular Endothelial Growth Factor in Diabetic Retinopathy. World J. Diabetes 2015, 6, 726. [Google Scholar] [CrossRef]
- Tu, Y.; Zhu, M.; Wang, Z.; Wang, K.; Chen, L.; Liu, W.; Shi, Q.; Zhao, Q.; Sun, Y.; Wang, X.; et al. Melatonin Inhibits Müller Cell Activation and Pro-Inflammatory Cytokine Production via Upregulating the MEG3/MiR-204/Sirt1 Axis in Experimental Diabetic Retinopathy. J. Cell. Physiol. 2020, 235, 8724–8735. [Google Scholar] [CrossRef] [PubMed]
- Lopes de Faria, J.M.; Duarte, D.A.; Montemurro, C.; Papadimitriou, A.; Consonni, S.R.; Lopes de Faria, J.B. Defective Autophagy in Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4356–4366. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Chen, X.; Chen, W.; Huang, S.; Peng, X.; Tian, L.; Wu, X.; Huang, Y. Curcumin Is a Potential Adjuvant to Alleviates Diabetic Retinal Injury via Reducing Oxidative Stress and Maintaining Nrf2 Pathway Homeostasis. Front. Pharmacol. 2021, 12, 796565. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.P.; Sunilkumar, S.; Giordano, J.F.; Toro, A.L.; Barber, A.J.; Dennis, M.D. The Stress Response Protein REDD1 Promotes Diabetes-Induced Oxidative Stress in the Retina by Keap1-Independent Nrf2 Degradation. J. Biol. Chem. 2020, 295, 7350–7361. [Google Scholar] [CrossRef] [PubMed]
- Bryan, H.K.; Olayanju, A.; Goldring, C.E.; Park, B.K. The Nrf2 Cell Defence Pathway: Keap1-Dependent and -Independent Mechanisms of Regulation. Biochem. Pharmacol. 2013, 85, 705–717. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, F.; Zhang, X.; Cheng, R.; Ma, J.X.; Yi, J.; Li, J. Fenofibrate Ameliorates Diabetic Retinopathy by Modulating Nrf2 Signaling and NLRP3 Inflammasome Activation. Mol. Cell. Biochem. 2018, 445, 105–115. [Google Scholar] [CrossRef]
- Mysona, B.; Dun, Y.; Duplantier, J.; Ganapathy, V.; Smith, S.B. Effects of Hyperglycemia and Oxidative Stress on the Glutamate Transporters GLAST and System Xc− in Mouse Retinal Müller Glial Cells. Cell Tissue Res. 2009, 335, 477–488. [Google Scholar] [CrossRef]
- Wu, M.; Yang, S.; Elliott, M.H.; Fu, D.; Wilson, K.; Zhang, J.; Du, M.; Chen, J.; Lyons, T. Oxidative and Endoplasmic Reticulum Stresses Mediate Apoptosis Induced by Modified LDL in Human Retinal Müller Cells. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4595–4604. [Google Scholar] [CrossRef]
- Yang, L.; Sun, H.; Wu, L.; Guo, X.; Dou, H.; Tso, M.O.M.; Zhao, L.; Li, S. Baicalein Reduces Inflammatory Process in a Rodent Model of Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2319–2327. [Google Scholar] [CrossRef]
- Canning, P.; Glenn, J.V.; Hsu, D.K.; Liu, F.T.; Gardiner, T.A.; Stitt, A.W. Inhibition of Advanced Glycation and Absence of Galectin-3 Prevent Blood-Retinal Barrier Dysfunction during Short-Term Diabetes. Exp. Diabesity Res. 2007, 2007, 3169. [Google Scholar] [CrossRef]
- Grigsby, J.G.; Cardona, S.M.; Pouw, C.E.; Muniz, A.; Mendiola, A.S.; Tsin, A.T.C.; Allen, D.M.; Cardona, A.E. The Role of Microglia in Diabetic Retinopathy. J. Ophthalmol. 2014, 2014, 705783. [Google Scholar] [CrossRef] [PubMed]
- Cheung, B.M.Y.; Li, C. Diabetes and Hypertension: Is There a Common Metabolic Pathway? Curr. Atheroscler. Rep. 2012, 14, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Vujosevic, S.; Simó, R. Local and Systemic Inflammatory Biomarkers of Diabetic Retinopathy: An Integrative Approach. Investig. Ophthalmol. Vis. Sci. 2017, 58, BIO68–BIO75. [Google Scholar] [CrossRef] [PubMed]
- Stahel, M.; Becker, M.; Graf, N.; Michels, S. SYSTEMIC INTERLEUKIN 1β INHIBITION IN PROLIFERATIVE DIABETIC RETINOPATHY: A Prospective Open-Label Study Using Canakinumab. Retina 2016, 36, 385–391. [Google Scholar] [CrossRef]
- Wong, T.Y.; Sun, J.; Kawasaki, R.; Ruamviboonsuk, P.; Gupta, N.; Lansingh, V.C.; Maia, M.; Mathenge, W.; Moreker, S.; Muqit, M.M.K.; et al. Guidelines on Diabetic Eye Care: The International Council of Ophthalmology Recommendations for Screening, Follow-up, Referral, and Treatment Based on Resource Settings. Ophthalmology 2018, 125, 1608–1622. [Google Scholar] [CrossRef]
- Diniz, L.P.; Tortelli, V.; Garcia, M.N.; Araújo, A.P.B.; Melo, H.M.; da Silva, G.S.S.; De Felice, F.G.; Alves-Leon, S.V.; de Souza, J.M.; Romão, L.F.; et al. Astrocyte Transforming Growth Factor Beta 1 Promotes Inhibitory Synapse Formation via CaM Kinase II Signaling. Glia 2014, 62, 1917–1931. [Google Scholar] [CrossRef]
- Matias, I.; Diniz, L.P.; Buosi, A.; Neves, G.; Stipursky, J.; Gomes, F.C.A. Flavonoid Hesperidin Induces Synapse Formation and Improves Memory Performance through the Astrocytic TGF-Β1. Front. Aging Neurosci. 2017, 9, 184. [Google Scholar] [CrossRef]
- Matias, I.; Morgado, J.; Gomes, F.C.A. Astrocyte Heterogeneity: Impact to Brain Aging and Disease. Front. Aging Neurosci. 2019, 11, 59. [Google Scholar] [CrossRef]
- Gerhardinger, C.; Costa, M.B.; Coulombe, M.C.; Toth, I.; Hoehn, T.; Grosu, P. Expression of Acute-Phase Response Proteins in Retinal Müller Cells in Diabetes. Investig. Ophthalmol. Vis. Sci. 2005, 46, 349–357. [Google Scholar] [CrossRef]
- Coughlin, B.A.; Feenstra, D.J.; Mohr, S. Müller Cells and Diabetic Retinopathy. Vision Res. 2017, 139, 93–100. [Google Scholar] [CrossRef]
- Liu, X.; Ye, F.; Xiong, H.; Hu, D.-N.; Limb, G.A.; Xie, T.; Peng, L.; Zhang, P.; Wei, Y.; Zhang, W.; et al. IL-1β Induces IL-6 Production in Retinal Müller Cells Predominantly through the Activation of P38 MAPK/NF-ΚB Signaling Pathway. Exp. Cell Res. 2015, 331, 223–231. [Google Scholar] [CrossRef]
- Mohammad, G.; AlSharif, H.M.; Siddiquei, M.M.; Ahmad, A.; Alam, K.; El-Asrar, A.M.A. Rho-Associated Protein Kinase-1 Mediates the Regulation of Inflammatory Markers in Diabetic Retina and in Retinal Müller Cells. Ann. Clin. Lab. Sci. 2018, 48, 137–145. [Google Scholar] [PubMed]
- Sipos, F.; Firneisz, G.; Műzes, G. Therapeutic Aspects of C-MYC Signaling in Inflammatory and Cancerous Colonic Diseases. World J. Gastroenterol. 2016, 22, 7938–7950. [Google Scholar] [CrossRef] [PubMed]
- Naskar, R.; Thanos, S. Retinal Gene Profiling in a Hereditary Rodent Model of Elevated Intraocular Pressure. Mol. Vis. 2006, 12, 1199–1210. [Google Scholar] [PubMed]
- Zhang, J.; Chen, C.; Wu, L.; Wang, Q.; Chen, J.; Zhang, S.; Chen, Z. C-Myc Contributes to the Release of Müller Cells-Derived Proinflammatory Cytokines by Regulating LncRNA MIAT/XNIP Pathway. Int. J. Biochem. Cell Biol. 2019, 114, 105574. [Google Scholar] [CrossRef] [PubMed]
- Portillo, J.-A.C.; Lopez Corcino, Y.; Miao, Y.; Tang, J.; Sheibani, N.; Kern, T.S.; Dubyak, G.R.; Subauste, C.S. CD40 in Retinal Müller Cells Induces P2X7-Dependent Cytokine Expression in Macrophages/Microglia in Diabetic Mice and Development of Early Experimental Diabetic Retinopathy. Diabetes 2017, 66, 483–493. [Google Scholar] [CrossRef]
- Faria, R.X.; Reis, R.A.M.; Ferreira, L.G.B.; Cezar-de-Mello, P.F.T.; Moraes, M.O. P2X7R Large Pore Is Partially Blocked by Pore Forming Proteins Antagonists in Astrocytes. J. Bioenerg. Biomembr. 2016, 48, 309–324. [Google Scholar] [CrossRef]
- Faria, R.X.; Freitas, H.R.; Reis, R.A.M. P2X7 Receptor Large Pore Signaling in Avian Müller Glial Cells. J. Bioenerg. Biomembr. 2017, 49, 215–229. [Google Scholar] [CrossRef]
- Subauste, C.S. The CD40-ATP-P2X (7) Receptor Pathway: Cell to Cell Cross-Talk to Promote Inflammation and Programmed Cell Death of Endothelial Cells. Front. Immunol. 2019, 10, 2958. [Google Scholar] [CrossRef]
- Reichenbach, A.; Bringmann, A. Purinergic Signaling in Retinal Degeneration and Regeneration. Neuropharmacology 2016, 104, 194–211. [Google Scholar] [CrossRef]
- Pavlou, S.; Augustine, J.; Cunning, R.; Harkin, K.; Stitt, A.W.; Xu, H.; Chen, M. Attenuating Diabetic Vascular and Neuronal Defects by Targeting P2rx7. Int. J. Mol. Sci. 2019, 20, 2101. [Google Scholar] [CrossRef]
- Leasher, J.L.; Bourne, R.R.A.; Flaxman, S.R.; Jonas, J.B.; Keeffe, J.; Naidoo, N.; Pesudovs, K.; Price, H.; White, R.A.; Wong, T.Y.; et al. Erratum. Global Estimates on the Number of People Blind or Visually Impaired by Diabetic Retinopathy: A Meta-Analysis From 1990-2010. Diabetes Care 2016;39:1643-1649. Diabetes Care 2016, 39, 2096. [Google Scholar] [CrossRef] [PubMed]
- Pantalone, K.M.; Hobbs, T.M.; Wells, B.J.; Kong, S.X.; Kattan, M.W.; Bouchard, J.; Yu, C.; Sakurada, B.; Milinovich, A.; Weng, W.; et al. Clinical Characteristics, Complications, Comorbidities and Treatment Patterns among Patients with Type 2 Diabetes Mellitus in a Large Integrated Health System. BMJ Open Diabetes Res. Care 2015, 3, e000093. [Google Scholar] [CrossRef] [PubMed]
- Duh, E.J.; Sun, J.K.; Stitt, A.W. Diabetic Retinopathy: Current Understanding, Mechanisms, and Treatment Strategies. JCI Insight 2017, 2, e93751. [Google Scholar] [CrossRef] [PubMed]
- Simó, R.; Sundstrom, J.M.; Antonetti, D.A. Ocular Anti-VEGF Therapy for Diabetic Retinopathy: The Role of VEGF in the Pathogenesis of Diabetic Retinopathy. Diabetes Care 2014, 37, 893–899. [Google Scholar] [CrossRef]
- Zhao, Y.; Singh, R.P. The Role of Anti-Vascular Endothelial Growth Factor (Anti-VEGF) in the Management of Proliferative Diabetic Retinopathy. Drugs Context 2018, 7, 212532. [Google Scholar] [CrossRef]
- Yun, S.P.; Kam, T.-I.; Panicker, N.; Kim, S.; Oh, Y.; Park, J.-S.; Kwon, S.-H.; Park, Y.J.; Karuppagounder, S.S.; Park, H.; et al. Block of A1 Astrocyte Conversion by Microglia Is Neuroprotective in Models of Parkinson’s Disease. Nat. Med. 2018, 24, 931–938. [Google Scholar] [CrossRef]
- Roy, S.; Kern, T.S.; Song, B.; Stuebe, C. Mechanistic Insights into Pathological Changes in the Diabetic Retina Implications for Targeting Diabetic Retinopathy. Am. J. Pathol. 2017, 187, 9–19. [Google Scholar] [CrossRef]
- Roy, S.; Jiang, J.X.; Li, A.-F.; Kim, D. Connexin Channel and Its Role in Diabetic Retinopathy. Prog. Retin. Eye Res. 2017, 61, 35–59. [Google Scholar] [CrossRef]
- Iandiev, I.; Tenckhoff, S.; Pannicke, T.; Biedermann, B.; Hollborn, M.; Wiedemann, P.; Reichenbach, A.; Bringmann, A. Differential Regulation of Kir4.1 and Kir2.1 Expression in the Ischemic Rat Retina. Neurosci. Lett. 2006, 396, 97–101. [Google Scholar] [CrossRef]
- Le, Y.-Z. VEGF Production and Signaling in Müller Glia Are Critical to Modulating Vascular Function and Neuronal Integrity in Diabetic Retinopathy and Hypoxic Retinal Vascular Diseases. Vision Res. 2017, 139, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Huang, X.; Chen, H.; Xu, H. Müller Glia-Mediated Retinal Regeneration. Mol. Neurobiol. 2021, 58, 2342–2361. [Google Scholar] [CrossRef] [PubMed]
- Goldman, D. Müller Glial Cell Reprogramming and Retina Regeneration. Nat. Rev. Neurosci. 2014, 15, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, R.; Fausett, B.V.; Goldman, D. Ascl1a Regulates Müller Glia Dedifferentiation and Retinal Regeneration through a Lin-28-Dependent, Let-7 MicroRNA Signalling Pathway. Nat. Cell Biol. 2015, 17, 532. [Google Scholar] [CrossRef]
- Hoang, T.; Wang, J.; Boyd, P.; Wang, F.; Santiago, C.; Jiang, L.; Yoo, S.; Lahne, M.; Todd, L.J.; Jia, M.; et al. Gene Regulatory Networks Controlling Vertebrate Retinal Regeneration. Science 2020, 370, eabb8598. [Google Scholar] [CrossRef]
- Lahne, M.; Nagashima, M.; Hyde, D.R.; Hitchcock, P.F. Reprogramming Müller Glia to Regenerate Retinal Neurons. Annu. Rev. Vis. Sci. 2020, 6, 171–193. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).