Evaluation of Phenolic Profile and Antioxidant Activity of Eleven Pistachio Cultivars (Pistacia vera L.) Cultivated in Andalusia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Materials and Sample Preparation
2.3. Extraction of Polyphenols
2.4. Antioxidant Activity and Total Phenolic Content
2.4.1. ABTS Assay
2.4.2. DPPH Assay
2.4.3. ORAC Assay
2.4.4. Total Phenolic Content
2.5. UHPLC-HRMS Polyphenol Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Antioxidant Activity
3.2. Polyphenols by UHPLC
3.3. Multivariate Analysis of Data (PCA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Catalán, L.; Alvarez-Ortí, M.; Pardo-Giménez, A.; Gómez, R.; Rabadán, A.; Pardo, J.E. Pistachio oil: A review on its chemical composition, extraction systems, and uses. Eur. J. Lipid Sci. Technol. 2016, 119, 1600126. [Google Scholar] [CrossRef]
- Grace, M.H.; Esposito, D.; Timmers, M.A.; Xiong, J.; Yousef, G.; Komarnytsky, S.; Lila, M.A. Chemical composition, antioxidant and anti-inflammatory properties of pistachio hull extracts. Food Chem. 2016, 210, 85–95. [Google Scholar] [CrossRef]
- Cardullo, N.; Leanza, M.; Muccilli, V.; Tringali, C. Valorization of agri-food waste from pistachio hard shells: Extraction of polyphenols as natural antioxidants. Resources 2021, 10, 45. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). FAOSTAT 2020. Available online: http://www.fao.org/faostat/es/#data (accessed on 10 February 2022).
- Razavi, S. Pistachio production, Iran vs. the world. Acta Hortic. 2006, 726, 689–694. [Google Scholar] [CrossRef]
- Ministerio de Agricultura, Pesca y Alimentación. Superficies y Producciones Anuales de Cultivos. Available online: https://www.mapa.gob.es/es/estadistica/temas/estadisticas-agrarias/agricultura/superficies-producciones-anuales-cultivos/ (accessed on 14 February 2022).
- Ministerio de Agricultura, Pesca y Alimentación. Informe del Consumo de Alimentación en España 2020. Available online: https://www.mapa.gob.es/es/alimentacion/temas/consumo-tendencias/panel-de-consumo-alimentario/ultimos-datos/ (accessed on 14 February 2022).
- Hormaza, J.I.; Wünsch, A. Pistachio. In Fruits and Nuts. Genome Mapping and Molecular Breeding in Plants; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 4. [Google Scholar]
- Benmoussa, H.; Luedeling, E.; Ghrab, M.; Ben Yahmed, J.; Ben Mimoun, M. Performance of pistachio (Pistacia vera L.) in warming Mediterranean orchards. Environ. Exp. Bot. 2017, 140, 76–85. [Google Scholar] [CrossRef]
- Gentile, C.; Tesoriere, L.; Butera, D.; Fazzari, M.; Monastero, M.; Allegra, M.; Livrea, M.A. Antioxidant activity of Sicilian pistachio (Pistacia vera L Var. Bronte) nut extract and its bioactive components. J. Agric. Food Chem. 2007, 55, 643–648. [Google Scholar] [CrossRef]
- Dreher, M.L. Pistachio nuts: Composition and potential health benefits. Nutr. Rev. 2012, 70, 234–240. [Google Scholar] [CrossRef]
- D’Evoli, L.; Lucarini, M.; Gabrielli, P.; Aguzzi, A.; Lombardi-Boccia, G. Nutritional Value of Italian Pistachios from Bronte (Pistacia vera, L.), Their Nutrients, Bioactive Compounds and Antioxidant Activity. Food Nutr. Sci. 2015, 6, 1267–1276. [Google Scholar] [CrossRef] [Green Version]
- Kay, C.D.; Gebauer, S.K.; West, S.G.; Kris-Etherton, P.M. Pistachios increase serum antioxidants and lower serum oxidized-LDL in hypercholesterolemic adults. J. Nutr. 2010, 140, 1093–1098. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim Sari, M.D.; Yasemin Baltaci, M.D.; Cahit Bagci, M.D.; Vedat Davutoglu, M.D.; Ozcan Erel, M.D.; Hakim Celik, M.D.; Orhan Ozer, M.D.; Nur Aksoy, M.D.; Mehmet Aksoy, M.D. Effect of pistachio diet on lipid parameters, endothelial function, inflammation, and oxidative status: A prospective study. Nutrition 2010, 26, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Seifzadeh, N.; Ali Sahari, M.; Barzegar, M.; Ahmadi Gavlighi, H.; Calani, L.; Del Rio, D.; Galaverna, G. Evaluation of polyphenolic compounds in membrane concentrated pistachio hull extract. Food Chem. 2019, 277, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Erşan, S.; Güçlü Üstündağ, Ö.; Carle, R.; Schweiggert, R.M. Identification of phenolic compounds in red and green pistachio (Pistacia vera L.) hulls (exo- and mesocarp) by HPLC-DAD-ESI-(HR)- MSn. J. Agric. Food Chem. 2016, 64, 5334–5344. [Google Scholar] [CrossRef]
- Erşan, S.; Güçlü Üstündağ, Ö.; Carle, R.; Schweiggert, R.M. Determination of pistachio (Pistacia vera L.) hull (exo- and mesocarp) phenolics by HPLC-DAD-ESI/MSn and UHPLC-DAD-ELSD after ultrasound- assisted extraction. J. Food Compos. Anal. 2017, 62, 103–114. [Google Scholar] [CrossRef]
- Barreca, D.; Laganà, G.; Leuzzi, U.; Smeriglio, A.; Trombetta, D.; Bellocco, E. Evaluation of the nutraceutical, antioxidant and cytoprotective properties of ripe pistachio (Pistacia vera L., variety Bronte) hulls. Food Chem. 2016, 196, 493–502. [Google Scholar] [CrossRef]
- Özbek, H.N.; Halahlih, F.; Göğüş, F.; Koçak Yanık, D.; Azaizeh, H. Pistachio (Pistacia vera L.) Hull as a Potential Source of Phenolic Compounds: Evaluation of Ethanol–Water Binary Solvent Extraction on Antioxidant Activity and Phenolic Content of Pistachio Hull Extracts. Waste Biomass Valorization 2020, 11, 2101–2110. [Google Scholar] [CrossRef]
- Garavand, F.; Madadlou, A.; Moini, S. Determination of phenolic profile and antioxidant activity of pistachio hull using high-performance liquid chromatography–diode array detector–electro-spray ionization–mass spectrometry as affected by ultrasound and microwave. Int. J. Food Prop. 2017, 20, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Tomaino, A.; Martorana, M.; Arcoraci, T.; Monteleone, D.; Giovinazzo, C.; Saija, A. Antioxidant activity and phenolic profile of pistachio (Pistacia vera L., variety Bronte) seeds and skins. Biochimie 2010, 92, 1115–1122. [Google Scholar] [CrossRef]
- Mannino, G.; Gentile, C.; Maffei, M.E. Chemical partitioning and DNA fingerprinting of some pistachio (Pistacia vera L.) varieties of different geographical origin. Phytochemistry 2019, 160, 40–47. [Google Scholar] [CrossRef]
- Martorana, M.; Arcoraci, T.; Rizza, L.; Cristani, M.; Bonina, F.P.; Saija, A.; Trombetta, D.; Tomaino, A. In vitro antioxidant and in vivo photoprotective effect of pistachio (Pistacia vera L., variety Bronte) seed and skin extracts. Fitoterapia 2013, 85, 41–48. [Google Scholar] [CrossRef]
- Mandalari, G.; Bisignano, C.; Filocamo, A.; Chessa, S.; Sarò, M.; Torre, G.; Faulks, R.M.; Dugo, P. Bioaccessibility of pistachio polyphenols, xanthophylls, and tocopherols during simulated human digestion. Nutrition 2013, 29, 338–344. [Google Scholar] [CrossRef]
- Liu, Y.; Blumberg, J.B.; Chen, C.Y.O. Quantification and bioaccessibility of California pistachio bioactives. J. Agric. Food Chem. 2014, 62, 1550–1556. [Google Scholar] [CrossRef]
- Ballistreri, G.; Arena, E.; Fallico, B. Influence of ripeness and drying process on the polyphenols and tocopherols of Pistacia vera L. Molecules 2009, 14, 4358–4369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Bencomo, J.J.; Kelebek, H.; Sonmezdag, A.S.; Rodríguez-Alcalá, L.M.; Fontecha, J.; Selli, S. Characterization of the Aroma-Active, Phenolic, and Lipid Profiles of the Pistachio (Pistacia vera L.) Nut as Affected by the Single and Double Roasting Process. J. Agric. Food Chem. 2015, 63, 7830–7839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonmezdag, A.S.; Kelebek, H.; Selli, S. Characterization and comparative evaluation of volatile, phenolic and antioxidant properties of pistachio (Pistacia vera L.) hull. J. Essent. Oil Res. 2017, 29, 262–270. [Google Scholar] [CrossRef]
- Arena, K.; Cacciola, F.; Mangraviti, D.; Zoccali, M.; Rigano, F.; Marino, N.; Dugo, P.; Mondello, L. Determination of the polyphenolic fraction of Pistacia vera L. kernel extracts by comprehensive two-dimensional liquid chromatography coupled to mass spectrometry detection. Anal. Bioanal. Chem. 2019, 411, 4819–4829. [Google Scholar] [CrossRef] [PubMed]
- Noguera-Artiaga, L.; Salvador, M.D.; Fregapane, G.; Collado-González, J.; Wojdyło, A.; López-Lluch, D.; Carbonell-Barrachina, Á.A. Functional and sensory properties of pistachio nuts as affected by cultivar. J. Sci. Food Agric. 2019, 99, 6696–6705. [Google Scholar] [CrossRef]
- Garcia-Moreno, P.J.; de la Rosa, L.A.; Stevens-Barron, J.C.; Rodríguez-Ramirez, R.; Corral-Diaz, B.; Alvarez-Parrilla, E.; Olivas-Aguirre, F.J.; Wall-Medrano, A. Dehiscence and prolonged storage of ‘Kerman’ Pistachios: Effects on morphometry and nutraceutical value. J. Food Sci. Technol. 2021, 58, 1958–1968. [Google Scholar] [CrossRef]
- Alinezhad, M.; Hojjati, M.; Barzegar, H.; Shahbazi, S.; Askari, H. Effect of gamma irradiation on the physicochemical properties of pistachio (Pistacia vera L.) nuts. J. Food Meas. Charact. 2021, 15, 199–209. [Google Scholar] [CrossRef]
- Ordoñez-Díaz, J.L.; Moreno-Ortega, A.; Roldán-Guerra, F.J.; Ortíz-Somovilla, V.; Moreno-Rojas, J.M.; Pereira-Caro, G. In vitro gastrointestinal digestion and colonic catabolism of mango (Mangifera indica L.) pulp polyphenols. Foods 2020, 9, 1836. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Pereira-Caro, G.; Madrona, A.; Bravo, L.; Espartero, J.L.; Alcudia, F.; Cert, A.; Mateos, R. Antioxidant activity evaluation of alkyl hydroxytyrosyl ethers, a new class of hydroxytyrosol derivatives. Food Chem. 2009, 115, 86–91. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef] [PubMed]
- Slinkard, K.; Singleton, V.L. Total Phenol Analysis: Automation and Comparison with Manual Methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar]
- Hervalejo, A.; Arjona-López, J.M.; Ordóñez-Díaz, J.L.; Romero-Rodríguez, E.; Calero-Velázquez, R.; Moreno-Rojas, J.M.; Arenas-Arenas, F.J. Influence of harvesting season on morphological and sensory quality, bioactive compounds and antioxidant activity of three late-season orange cultivars ‘barberina’, ‘valencia midknight’ and ‘valencia delta seedless’. Agronomy 2021, 11, 673. [Google Scholar] [CrossRef]
- Ojeda-Amador, R.M.; Fregapane, G.; Salvador, M.D. Composition and properties of virgin pistachio oils and their by-products from different cultivars. Food Chem. 2018, 240, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Erşan, S.; Güçlü Üstündağ, Ö.; Carle, R.; Schweiggert, R.M. Subcritical water extraction of phenolic and antioxidant constituents from pistachio (Pistacia vera L.) hulls. Food Chem. 2018, 253, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Kilic, I.H.; Sarikurkcu, C.; Karagoz, I.D.; Uren, M.C.; Kocak, M.S.; Cilkiz, M.; Tepe, B. A significant by-product of the industrial processing of pistachios: Shell skin–RP-HPLC analysis, and antioxidant and enzyme inhibitory activities of the methanol extracts of Pistacia vera L. shell skins cultivated in Gaziantep, Turkey. RSC Adv. 2016, 6, 1203–1209. [Google Scholar] [CrossRef]
- Fabani, M.P.; Luna, L.; Baroni, M.V.; Monferran, M.V.; Ighani, M.; Tapia, A.; Wunderlin, D.A.; Feresin, G.E. Pistachio (Pistacia vera var Kerman) from Argentinean cultivars. A natural product with potential to improve human health. J. Funct. Foods 2013, 5, 1347–1356. [Google Scholar] [CrossRef]
- Bellomo, M.G.; Fallico, B. Anthocyanins, chlorophylls and xanthophylls in pistachio nuts (Pistacia vera) of different geographic origin. J. Food Compos. Anal. 2007, 20, 352–359. [Google Scholar] [CrossRef]
- Saitta, M.; La Torre, G.L.; Potortì, A.G.; Di Bella, G.; Dugo, G. Polyphenols of pistachio (Pistacia vera L.) oil samples and geographical differentiation by principal component analysis. JAOCS J. Am. Oil Chem. Soc. 2014, 91, 1595–1603. [Google Scholar] [CrossRef]
- Al Juhaimi, F.; Özcan, M.M.; Ghafoor, K.; Babiker, E.E.; Hussain, S. Comparison of cold-pressing and soxhlet extraction systems for bioactive compounds, antioxidant properties, polyphenols, fatty acids and tocopherols in eight nut oils. J. Food Sci. Technol. 2018, 55, 3163–3173. [Google Scholar] [CrossRef] [PubMed]
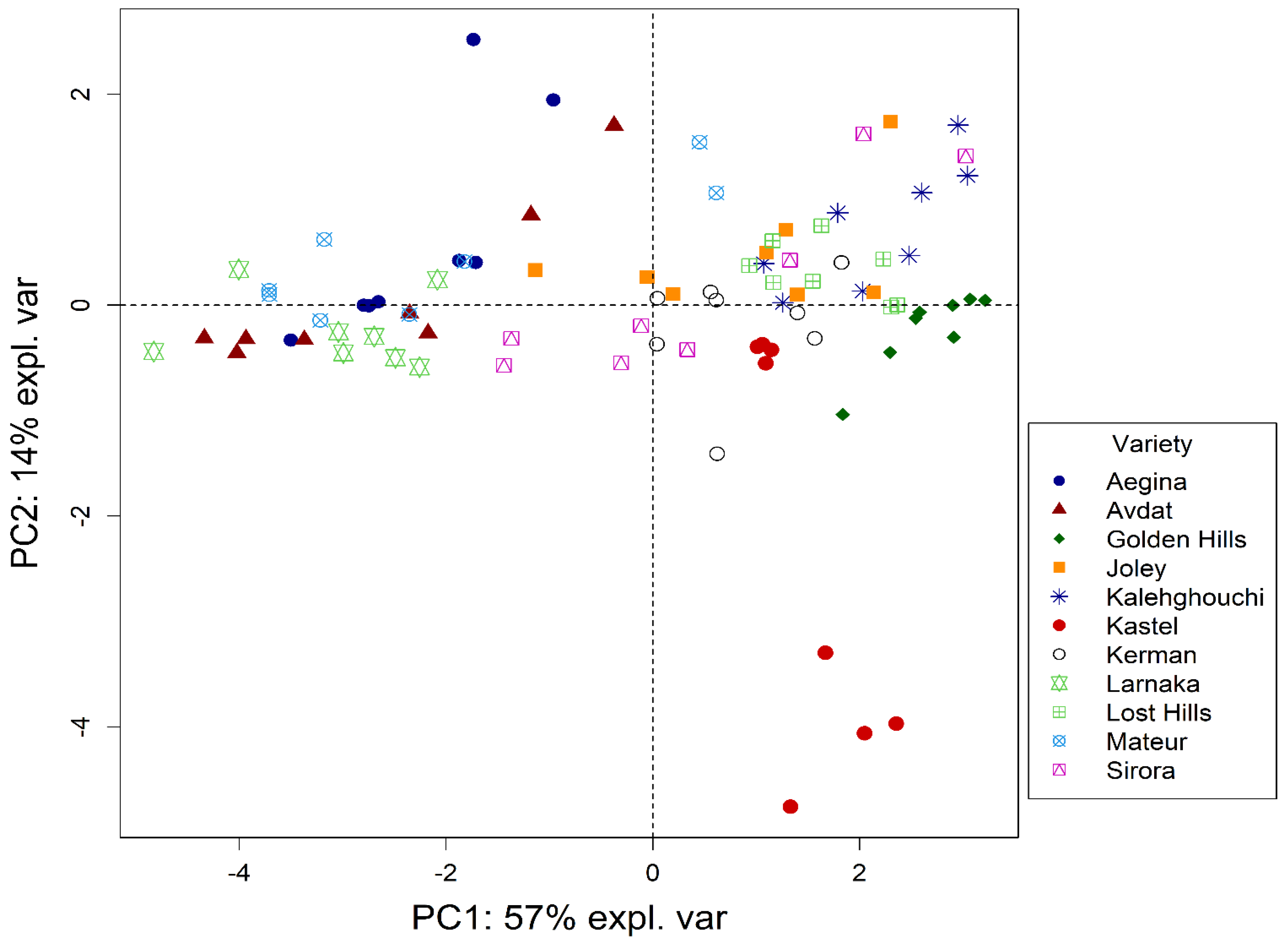
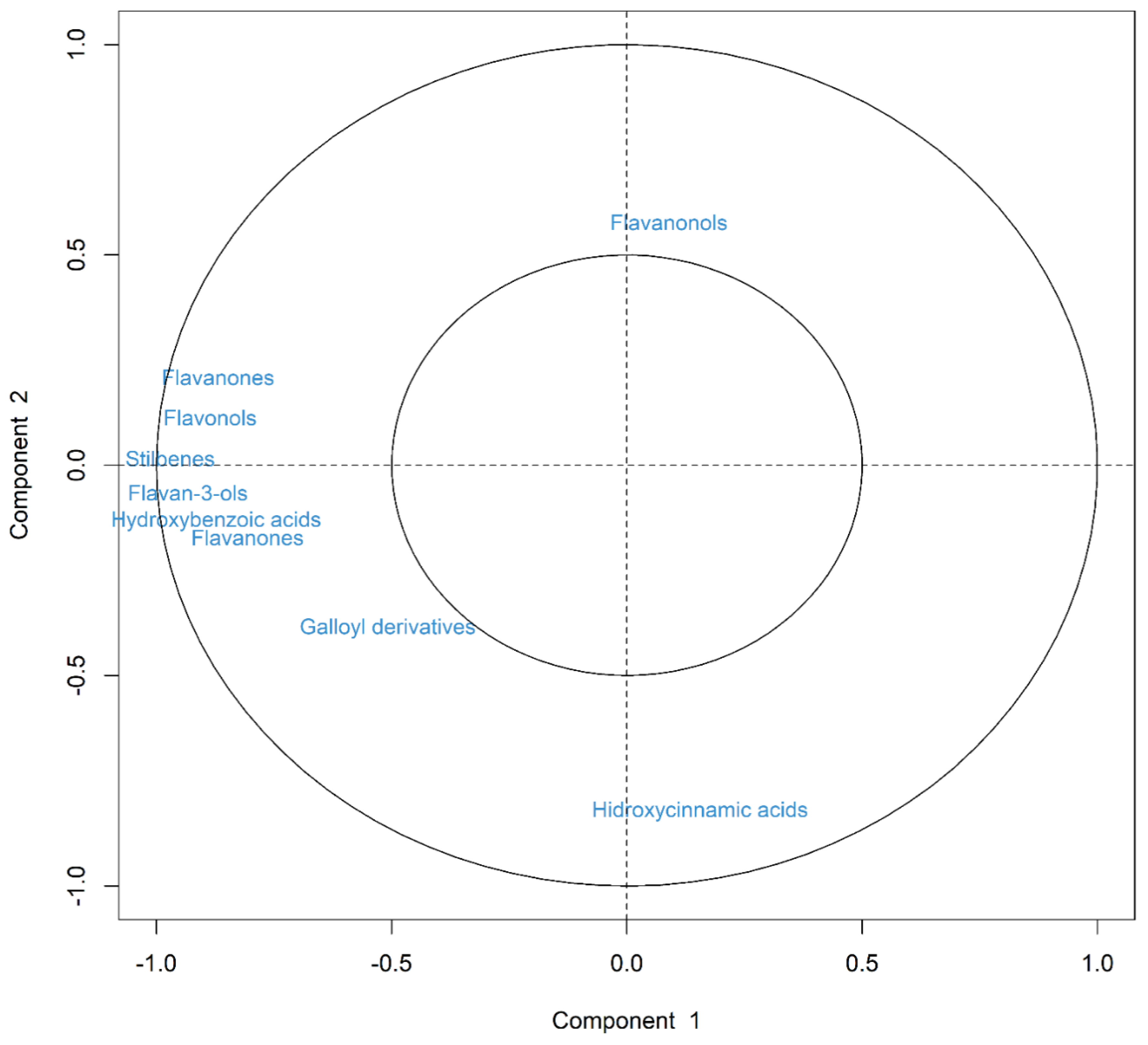
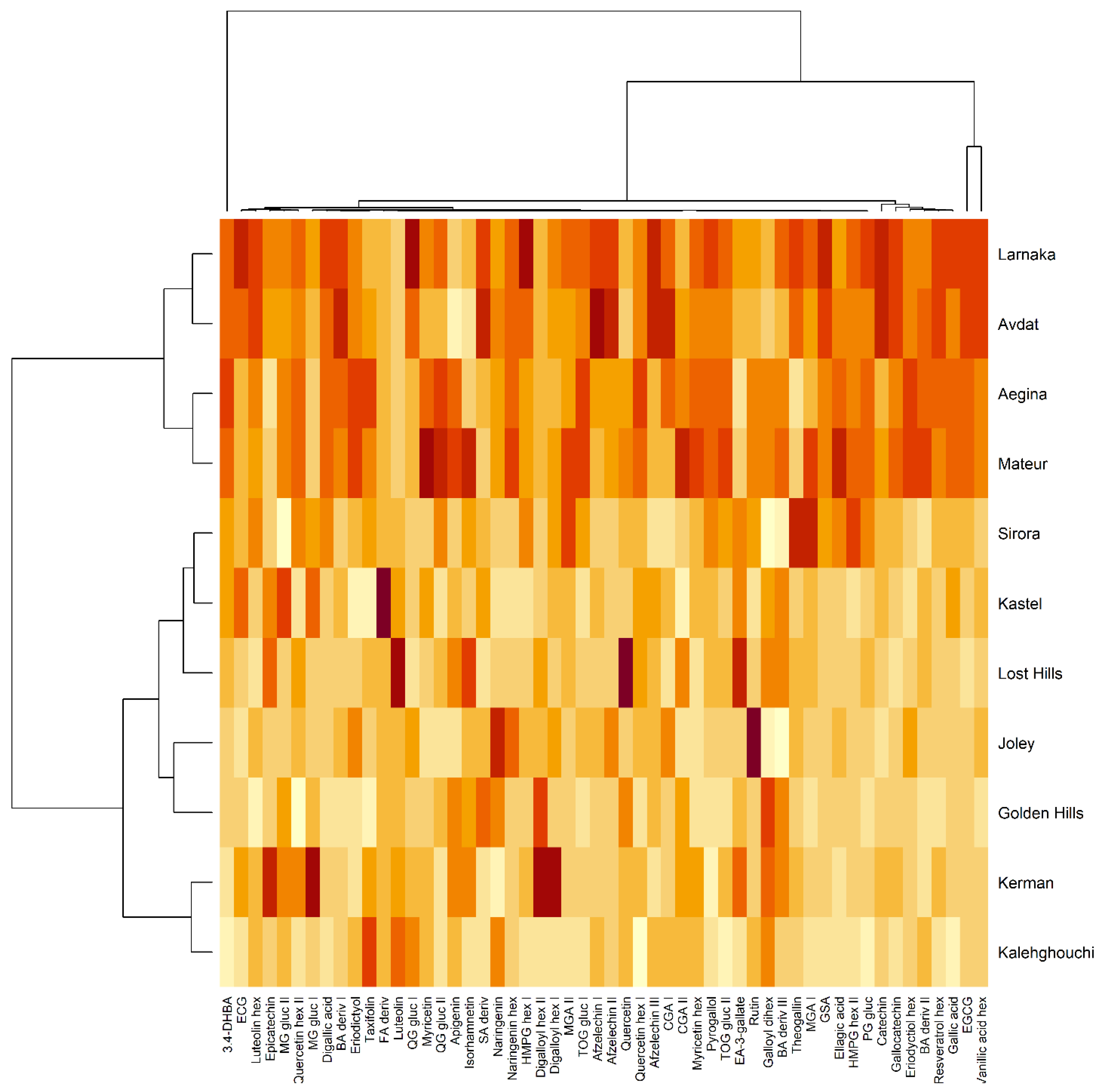
| ABTS | DPPH | ORAC | TPC | |
|---|---|---|---|---|
| Values a | ||||
| Variety | ||||
| Aegina | 3.17 b | 2.82 b | 7.9 c | 2.96 b |
| Avdat | 3.20 ab | 2.78 b | 10.3 b | 2.70 b |
| Golden Hills | 1.28 f | 0.86 e | 3.6 fg | 1.35 d |
| Joley | 1.48 ef | 1.09 de | 3.5 g | 1.57 cd |
| Kalehghouchi | 1.55 def | 0.93 e | 3.5 g | 1.45 d |
| Kastel | 1.87 cd | 1.22 cd | 4.9 e | 1.56 cd |
| Kerman | 2.00 c | 1.25 cd | 5.3 e | 1.91 c |
| Larnaka | 3.53 a | 3.22 a | 15.8 a | 3.51 a |
| Lost Hills | 1.71 cde | 1.22 cd | 4.5 ef | 1.63 cd |
| Mateur | 3.33 ab | 2.72 b | 11.0 b | 2.80 b |
| Sirora | 1.97 c | 1.48 c | 6.4 d | 1.31 d |
| Season | ||||
| 2019 | 2.06 b | 1.59 b | 7.1 | 1.89 b |
| 2020 | 2.51 a | 1.97 a | 6.9 | 2.25 a |
| Significance b | ||||
| Variety (V) | *** | *** | *** | *** |
| Season (S) | *** | *** | ns | *** |
| V × S | *** | *** | *** | *** |
| Hydroxy Benzoic Acids | Galloyl Derivatives | Hydroxy Cinnamic Acids | Flavones | Flavonols | Flavan-3-ols | Flavanones | Flavanonols | Stilbenes | Total | |
|---|---|---|---|---|---|---|---|---|---|---|
| Values a | ||||||||||
| Variety | ||||||||||
| Aegina | 380 a | 5.0 abc | 0.34 b | 3.31 ab | 4.31 a | 105 bc | 8.10 a | 1.15 | 4.20 b | 512 a |
| Avdat | 406 a | 5.0 abc | 0.40 b | 4.12 a | 3.95 abcd | 137 ab | 6.24 b | 0.90 | 5.22 a | 569 a |
| Golden Hills | 152 bcd | 4.2 bcd | 0.36 b | 0.44 f | 1.89 f | 17 d | 1.34 cd | 0.44 | 1.40 d | 179 bc |
| Joley | 205 bcd | 3.4 d | 0.33 b | 2.04 cde | 3.14 de | 29 d | 5.22 b | 0.68 | 2.12 cd | 252 bc |
| Kalehghouchi | 116 d | 3.2 d | 0.31 b | 2.32 bcde | 2.49 ef | 17 d | 1.60 cd | 1.23 | 1.66 cd | 146 c |
| Kastel | 242 b | 5.3 ab | 1.87 a | 1.96 de | 2.49 ef | 34 d | 1.05 d | 0.46 | 2.00 cd | 290 b |
| Kerman | 148 cd | 6.2 a | 0.31 b | 2.03 cde | 3.42 bcd | 35 d | 1.72 cd | 0.83 | 2.47 c | 200 bc |
| Larnaka | 422 a | 6.0 a | 0.38 b | 4.36 a | 4.22 abc | 144 a | 6.04 b | 0.76 | 4.92 ab | 592 a |
| Lost Hills | 199 bcd | 3.6 cd | 0.31 b | 1.40 ef | 3.15 de | 19 d | 1.70 cd | 0.78 | 1.88 cd | 231 bc |
| Mateur | 366 a | 5.3 ab | 0.33 b | 2.91 bcd | 4.30 ab | 101 c | 8.31 a | 0.95 | 4.08 b | 494 a |
| Sirora | 227 bc | 4.3 bcd | 0.33 b | 3.18 abc | 3.38 cd | 49 d | 2.42 c | 0.88 | 2.41 c | 292 b |
| Season | ||||||||||
| 2019 | 286 a | 4.5 | 0.633 a | 2.03 b | 3.16 b | 51 b | 3.73 b | 1.11 a | 2.81 b | 355 |
| 2020 | 235 b | 4.9 | 0.326 b | 3.08 a | 3.52 a | 74 a | 4.22 a | 0.54 b | 3.08 a | 328 |
| Significance b | ||||||||||
| Variety (V) | *** | *** | *** | *** | *** | *** | *** | ns | *** | *** |
| Season (S) | *** | ns | *** | *** | ** | *** | ** | *** | * | ns |
| V × S | *** | * | *** | ** | ** | * | *** | ns | * | ** |
| Gallic Acid | Pyro- Gallol | Theogallin (3-Galloyl-Quinic Acid) | 3, 4-DHB | GSA | Digallic Acid | MGA I | MGA II | Vanillic Acid Hexoside | Ellagic Acid | BA Deriv I | BA Deriv II | BA Deriv III | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values a | ||||||||||||||
| Variety | ||||||||||||||
| Aegina | 4.98 a | 0.66 ab | 0.11 b | 238 a | 0.40 b | 1.23 ab | 0.32 cde | 0.123 b | 128 c | 0.48 ab | 1.16 bc | 4.7 ab | 0.40 | 380 a |
| Avdat | 4.31 ab | 0.62 ab | 0.59 a | 215 ab | 0.56 a | 1.30 ab | 0.37 c | 0.120 bc | 176 a | 0.46 ab | 1.41 a | 4.9 ab | 0.36 | 406 a |
| Golden Hills | 2.12 e | 0.27 de | 0.18 b | 101 cde | 0.13 c | 0.23 c | 0.16 ef | 0.093 cd | 44 e | 0.21 b | 0.62 f | 2.4 d | 0.40 | 152 bcd |
| Joley | 2.16 e | 0.31 de | 0.22 b | 111 cde | 0.16 c | 0.32 c | 0.20 def | 0.097 bcd | 87 d | 0.30 ab | 0.89 de | 2.8 cd | 0.29 | 205 bcd |
| Kalehghouchi | 1.28 e | 0.28 de | 0.19 b | 50 e | 0.10 c | 0.14 c | 0.13 f | 0.082 d | 59 de | 0.13 b | 0.68 f | 3.4 bcd | 0.35 | 116 d |
| Kastel | 3.48b c | 0.47 bcd | 0.20 b | 154 bc | 0.16 c | 0.36 c | 0.34 cd | 0.107 bcd | 78 de | 0.23 b | 0.78 ef | 3.3 bcd | 0.41 | 242 b |
| Kerman | 2.23 de | 0.18 e | 0.27 b | 67 de | 0.13 c | 0.34 c | 0.23 cdef | 0.100 bcd | 74 de | 0.17 b | 0.76 ef | 2.1 d | 0.40 | 148 cd |
| Larnaka | 5.37 a | 0.76 a | 0.63 a | 237 a | 0.62 a | 1.56 a | 0.56 b | 0.152 a | 169 ab | 0.39 ab | 1.28 ab | 4.4 abc | 0.41 | 422 a |
| Lost Hills | 2.32 de | 0.40 cd | 0.26 b | 133 cd | 0.17 c | 0.34 c | 0.29 cde | 0.106 bcd | 58 de | 0.23 b | 0.67 f | 3.4 bcd | 0.40 | 199 bcd |
| Mateur | 4.94 a | 0.70 a | 0.11 b | 217 ab | 0.38 b | 1.24 ab | 0.67 ab | 0.159 a | 134 bc | 0.61 a | 1.07 cd | 5.4 a | 0.42 | 366 a |
| Sirora | 3.25 cd | 0.60 abc | 0.78 a | 153 bc | 0.31 b | 1.07 b | 0.81 a | 0.160 a | 63 de | 0.44 ab | 0.69 f | 2.2 d | 0.31 | 227 bc |
| Season | ||||||||||||||
| 2019 | 4.07 a | 0.29 b | 0.31 | 189 a | 0.29 | 0.78 | 0.26 b | 0.100 b | 87 b | 0.53 a | 0.91 | 2.1 b | 0.35 b | 286 a |
| 2020 | 2.56 b | 0.66 a | 0.33 | 115 b | 0.28 | 0.70 | 0.48 a | 0.137 a | 107 a | 0.14b | 0.91 | 5.0 a | 0.40 a | 235 b |
| Significance b | ||||||||||||||
| Variety (V) | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | ns | *** |
| Season (S) | *** | *** | ns | *** | ns | ns | *** | *** | *** | *** | ns | *** | * | *** |
| V × S | *** | *** | * | *** | ** | *** | *** | *** | * | ** | ** | *** | ns | *** |
| Gallo Catechin | Catechin | Epi Catechin | Epigallo Catechin Gallate | Epi Catechin Gallate | Epiafzelechin3-Gallate | Afzelechin I | Afzelechin II | Afzelechin III | Total | |
|---|---|---|---|---|---|---|---|---|---|---|
| Values a | ||||||||||
| Variety | ||||||||||
| Aegina | 7.43 b | 7.0 bc | 0.98 e | 86.8 ab | 2.12 bc | 0.30 e | 0.110 b | 0.1108 bc | 0.16 bc | 105 bc |
| Avdat | 10.34 a | 15.5 a | 2.25 bcd | 104.6 ab | 3.60 a | 0.40 cde | 0.119 a | 0.1198 a | 0.23 a | 137 ab |
| Golden Hills | 1.13 d | 3.3 de | 1.41 cde | 8.9 c | 1.70 bcd | 0.40 cde | 0.105 d | 0.1059 de | 0.17 b | 17 d |
| Joley | 2.00 cd | 2.8 e | 1.16 de | 21.2 c | 1.37 cd | 0.39 cde | 0.107 bcd | 0.1131 b | 0.16 bc | 29 d |
| Kalehghouchi | 0.95 d | 3.5 de | 1.16 de | 9.3 c | 1.08 d | 0.29 e | 0.107 bcd | 0.1058 e | 0.17 b | 17 d |
| Kastel | 1.71 cd | 5.8 bcde | 2.46 bc | 19.2 c | 3.57 a | 0.59 abc | 0.107 bcd | 0.1069 cde | 0.17 b | 34 d |
| Kerman | 2.85 cd | 6.9 bc | 3.66 a | 17.7 c | 2.51 b | 0.62 ab | 0.106 cd | 0.1049 e | 0.13 d | 35 d |
| Larnaka | 10.43 a | 16.0 a | 2.51 bc | 110.0 a | 4.10 a | 0.46 bcde | 0.116 a | 0.1190 a | 0.25 a | 144 a |
| Lost Hills | 1.09 d | 4.2 cde | 3.08 ab | 7.5 c | 1.91 bc | 0.78 a | 0.106 cd | 0.1061 de | 0.16 bc | 19 d |
| Mateur | 8.14 ab | 8.0 b | 1.16 de | 80.9 b | 2.12 bc | 0.32 de | 0.108 bc | 0.1100 bcd | 0.17 b | 101 c |
| Sirora | 4.12 c | 6.2 bcd | 1.48 cde | 33.9 c | 2.14 bc | 0.52 bcd | 0.106 cd | 0.1059 e | 0.14 cd | 49 d |
| Season | ||||||||||
| 2019 | 3.22 b | 5.9 b | 1.10 b | 37.9 b | 2.26 b | 0.39 b | 0.1086 | 0.1087 b | 0.16 b | 51 b |
| 2020 | 5.90 a | 8.5 a | 2.77 a | 53.0 a | 2.50 a | 0.53 a | 0.1088 | 0.1109 a | 0.18 a | 74 a |
| Significance b | ||||||||||
| Variety (V) | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Season (S) | *** | *** | *** | *** | * | *** | ns | *** | *** | *** |
| V × S | ** | ns | ** | ** | ns | ns | * | * | *** | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Rojas, J.M.; Velasco-Ruiz, I.; Lovera, M.; Ordoñez-Díaz, J.L.; Ortiz-Somovilla, V.; De Santiago, E.; Arquero, O.; Pereira-Caro, G. Evaluation of Phenolic Profile and Antioxidant Activity of Eleven Pistachio Cultivars (Pistacia vera L.) Cultivated in Andalusia. Antioxidants 2022, 11, 609. https://doi.org/10.3390/antiox11040609
Moreno-Rojas JM, Velasco-Ruiz I, Lovera M, Ordoñez-Díaz JL, Ortiz-Somovilla V, De Santiago E, Arquero O, Pereira-Caro G. Evaluation of Phenolic Profile and Antioxidant Activity of Eleven Pistachio Cultivars (Pistacia vera L.) Cultivated in Andalusia. Antioxidants. 2022; 11(4):609. https://doi.org/10.3390/antiox11040609
Chicago/Turabian StyleMoreno-Rojas, José Manuel, Isabel Velasco-Ruiz, María Lovera, José Luis Ordoñez-Díaz, Víctor Ortiz-Somovilla, Elsy De Santiago, Octavio Arquero, and Gema Pereira-Caro. 2022. "Evaluation of Phenolic Profile and Antioxidant Activity of Eleven Pistachio Cultivars (Pistacia vera L.) Cultivated in Andalusia" Antioxidants 11, no. 4: 609. https://doi.org/10.3390/antiox11040609
APA StyleMoreno-Rojas, J. M., Velasco-Ruiz, I., Lovera, M., Ordoñez-Díaz, J. L., Ortiz-Somovilla, V., De Santiago, E., Arquero, O., & Pereira-Caro, G. (2022). Evaluation of Phenolic Profile and Antioxidant Activity of Eleven Pistachio Cultivars (Pistacia vera L.) Cultivated in Andalusia. Antioxidants, 11(4), 609. https://doi.org/10.3390/antiox11040609









