UHPLC–MS Characterization, and Antioxidant and Nutritional Analysis of Cocoa Waste Flours from the Peruvian Amazon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. HPLC–MS Parameters
2.4. HPLC–DAD Analysis of Catechins and Methylxanthines
2.5. Determination of Proximate Composition
2.6. Mineral Analysis
2.7. Fatty Acid Profile
2.8. Antioxidant Activity
2.8.1. DPPH Scavenging Activity
2.8.2. ABTS Bleaching Capacity
2.8.3. Ferric-Reducing Antioxidant Power Assay (FRAP)
2.8.4. Total Phenolic (TP) Content
2.9. Statistical Analysis
3. Results and Discussion
3.1. UHPLC–MS Analysis of Cocoa Extract
3.1.1. Saturated Organic Acids
3.1.2. Fatty Acids or Derivatives
3.1.3. Procyanidins
3.1.4. Flavonols
3.1.5. Phenolic Acids
3.1.6. Amino Acids
3.1.7. Alkaloids
3.2. Chemical Composition and Nutritional Properties of Flours
3.3. Antioxidant Activity and Total Polyphenol Content
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rojo-Poveda, O.; Zeppa, G.; Ferrocino, I.; Stévigny, C.; Barbosa-Pereira, L. Chemometric Classification of Cocoa Bean Shells Based on Their Polyphenolic Profile Determined by RP-HPLC-PDA Analysis and Spectrophotometric Assays. Antioxidants 2021, 10, 1533. [Google Scholar] [CrossRef] [PubMed]
- Vásquez, Z.S.; Neto, D.P.C.; Pereira, G.V.M.; Vandergberghe, L.P.S.; de Oliveira, P.Z.; Tiburcio, P.B.; Rogez, H.L.G.; Neto, A.G.; Soccol, C.R. Biotechnological approaches for cocoa waste management: A review. Waste Manag. 2019, 90, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Oddoye, E.O.K.; Agyente-Badu, C.K.; Gyedu-Akoto, E. Cocoa and its by-products: Identification and utilization. In Chocolate in Health and Nutrition; Watson, R., Preedy, V., Zibadi, S., Eds.; Humana Press: Totowa, NJ, USA, 2013; Volume 7, pp. 23–37. [Google Scholar] [CrossRef]
- Beg, M.S.; Ahmad, S.; Jan, K.; Bashir, K. Status, supply chain and processing of cocoa—A review. Trends Food Sci. Technol. 2017, 66, 108–116. [Google Scholar] [CrossRef]
- Rojo-Poveda, O.; Barbosa-Pereira, L.; Zeppa, G.; Stévigny, C. Cocoa Bean Shell—A By-Product with Nutritional Properties and Biofunctional Potential. Nutrients 2020, 12, 1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domínguez-Pérez, L.A.; Beltrán-Barrientos, L.M.; González-Córdova, A.F.; Hernández-Mendoza, A.; Vallejo-Cordoba, B. Artisanal cocoa bean fermentation: From cocoa bean proteins to bioactive peptides with potential health benefits. J. Funct. Foods 2020, 73, 104134. [Google Scholar] [CrossRef]
- Keen, C.L.; Holt, R.R.; Oteiza, P.I.; Fraga, C.G.; Schmitz, H.H. Cocoa antioxidants and cardiovascular health. Am. J. Clin. Nutr. 2005, 81, 298S–303S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín, M.Á.; Ramos, S. Health beneficial effects of cocoa phenolic compounds: A mini-review. Curr. Opin. Food Sci. 2017, 14, 20–25. [Google Scholar] [CrossRef] [Green Version]
- Oyeleke, S.A.; Ajayi, A.M.; Umukoro, S.; Aderibigbe, A.O.; Ademowo, O.G. Anti-inflammatory activity of Theobroma cacao L. stem bark ethanol extract and its fractions in experimental models. J. Ethnopharmacol. 2018, 222, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Cambrai, A.; Marchioni, E.; Julien-David, D.; Marcic, C. Discrimination of Cocoa Bean Origin by Chocolate Polyphenol Chromatographic Analysis and Chemometrics. Food Anal. Methods 2017, 10, 1991–2000. [Google Scholar] [CrossRef]
- Peláez, P.; Bardón, I.; Camasca, P. Methylxanthine and catechin content of fresh and fermented cocoa beans, dried cocoa beans, and cocoa liquor. Sci. Agropecu. 2016, 7, 355–365. [Google Scholar] [CrossRef] [Green Version]
- Oracz, J.; Nebesny, E.; Żyżelewicz, D. Identification and quantification of free and bound phenolic compounds contained in the high-molecular weight melanoidin fractions derived from two different types of cocoa beans by UHPLC-DAD-ESI-HR-MSn. Food Res. Int. 2019, 115, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, M.M.; Santos, H.M.; Coutinho, J.P.; Lôbo, I.P.; da Silva Junior, A.L.S.; Santos, A.G.; de Jesus, R.M. Optimization of chromatographic separation and classification of artisanal and fine chocolate based on its bioactive compound content through multivariate statistical techniques. Microchem. J. 2020, 152, 104342. [Google Scholar] [CrossRef]
- Bartella, L.; Di Donna, L.; Napoli, A.; Siciliano, C.; Sindona, G.; Mazzotti, F. A rapid method for the assay of methylxanthines alkaloids: Theobromine, theophylline and caffeine, in cocoa products and drugs by paper spray tandem mass spectrometry. Food Chem. 2019, 278, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, T.; Capuano, E.; Cämmerer, B.; Fogliano, V. Influence of Roasting on the Antioxidant Activity and HMF Formation of a Cocoa Bean Model Systems. J. Agric. Food Chem. 2009, 57, 147–152. [Google Scholar] [CrossRef]
- Del Rosario Brunetto, M.; Gutiérrez, L.; Delgado, Y.; Gallignani, M.; Zambrano, A.; Gómez, Á.; Ramos, G.; Romero, C. Determination of theobromine, theophylline and caffeine in cocoa samples by a high-performance liquid chromatographic method with on-line sample cleanup in a switching-column system. Food Chem. 2007, 100, 459–467. [Google Scholar] [CrossRef]
- Darnet, S.H.; da Silva, L.H.M.; da Rodrigues, A.M.C.; Lins, R.T. Nutritional composition, fatty acid and tocopherol contents of buriti (Mauritia flexuosa) and patawa (Oenocarpus bataua) fruit pulp from the amazon region. Ciência Tecnol. Aliment. 2011, 31, 488–491. [Google Scholar] [CrossRef] [Green Version]
- Vargas-Arana, G.; Merino-Zegarra, C.; Riquelme-Penaherrera, M.; Nonato-Ramirez, L.; Delgado-Wong, H.; Pertino, M.W.; Parra, C.; Simirgiotis, M.J. Antihyperlipidemic and antioxidant capacities, nutritional analysis and uhplc-pda-ms characterization of cocona fruits (Solanum sessiliflorum dunal) from the peruvian amazon. Antioxidants 2021, 10, 1566. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrinia, N.; Proteggente, A.; Pannalaa, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant Activity and Total Phenolics in Selected Fruits, Vegetables, and Grain Products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Juarez-Garcia, E.; Agama-Acevedo, E.; Sáyago-Ayerdi, S.G.; Rodríguez-Ambriz, S.L.; Bello-Pérez, L.A. Composition, digestibility and application in breadmaking of banana flour. Plant Foods Hum. Nutr. 2006, 61, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Bonafaccia, G.; Marocchini, M.; Kreft, I. Composition and technological properties of the flour and bran from common and tartary buckwheat. Food Chem. 2003, 80, 9–15. [Google Scholar] [CrossRef]
- Porter, M.A.; Jones, A.M. Variability in soy flour composition. JAOCS J. Am. Oil Chem. Soc. 2003, 80, 557–562. [Google Scholar] [CrossRef]
- Felker, P.; Takeoka, G.; Dao, L. Pod Mesocarp Flour of North and South American Species of Leguminous Tree Prosopis (Mesquite): Composition and Food Applications. Food Rev. Int. 2013, 29, 49–66. [Google Scholar] [CrossRef]
- Mariatti, F.; Gunjević, V.; Boffa, L.; Cravotto, G. Process intensification technologies for the recovery of valuable compounds from cocoa by-products. Innov. Food Sci. Emerg. Technol. 2021, 68, 102601. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Nieto-Figueroa, K.H.; Oomah, B.D. Cocoa (Theobroma cacao L.) pod husk: Renewable source of bioactive compounds. Trends Food Sci. Technol. 2018, 81, 172–184. [Google Scholar] [CrossRef]
- Delgado-Ospina, J.; Lucas-González, R.; Viuda-Martos, M.; Fernández-López, J.; Pérez-Álvarez, J.A.; Martuscelli, M.; Chaves-López, C. Bioactive compounds and techno-funtional properties of high-fiber co-products of the cacao agro-industrial chain. Heliyon 2021, 7, e06799. [Google Scholar] [CrossRef]
- Yu, L.; Nanguet, A.L.; Beta, T. Comparison of antioxidant properties of refined and whole wheat flour and bread. Antioxidants 2013, 2, 370–383. [Google Scholar] [CrossRef] [Green Version]
- Grillo, G.; Boffa, L.; Binello, A.; Mantegna, S.; Cravotto, G.; Chemat, F.; Dizhbite, T.; Lauberte, L.; Telysheva, G. Cocoa bean shell waste valorisation; extraction from lab to pilot-scale cavitational reactors. Food Res. Int. 2019, 115, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Amin, I.; Yee, C. Antioxidative effects of extracts of cocoa shell, roselle seeds and a combination of both extracts on the susceptibility of cooked beef to lipid oxidation. J. Food Technol. 2006, 4, 10–15. [Google Scholar]
- Zhang, S.; Gai, Z.; Gui, T.; Chen, J.; Chen, Q.; Li, Y. Antioxidant Effects of Protocatechuic Acid and Protocatechuic Aldehyde: Old Wine in a New Bottle. Evid.-Based Complement. Alternat. Med. 2021, 2021, 6139308. [Google Scholar] [CrossRef]
- Gu, L.; House, S.E.; Wu, X.; Ou, B.; Prior, R.L. Procyanidin and catechin contents and antioxidant capacity of cocoa and chocolate products. J. Agric. Food Chem. 2006, 54, 4057–4061. [Google Scholar] [CrossRef] [PubMed]
- Chin, E.; Miller, K.B.; Payne, M.J.; Hurst, W.J.; Stuart, D.A. Comparison of antioxidant activity and flavanol content of cacao beans processed by modern and traditional Mesoamerican methods. Herit. Sci. 2013, 1, 1–7. [Google Scholar] [CrossRef] [Green Version]
- De Melo, C.W.B.; de Bandeira, M.J.; Maciel, L.F.; da Bispo, E.S.; de Souza, C.O.; Soares, S.E. Chemical composition and fatty acids profile of chocolates produced with different cocoa (Theobroma cacao L.) cultivars. Food Sci. Technol. 2020, 40, 326–333. [Google Scholar] [CrossRef] [Green Version]
- Shramko, V.S.; Polonskaya, Y.V.; Kashtanova, E.V.; Stakhneva, E.M.; Ragino, Y.I. The Short Overview on the Relevance of Fatty Acids for Human Cardiovascular Disorders. Biomolecules 2020, 10, 1127. [Google Scholar] [CrossRef]


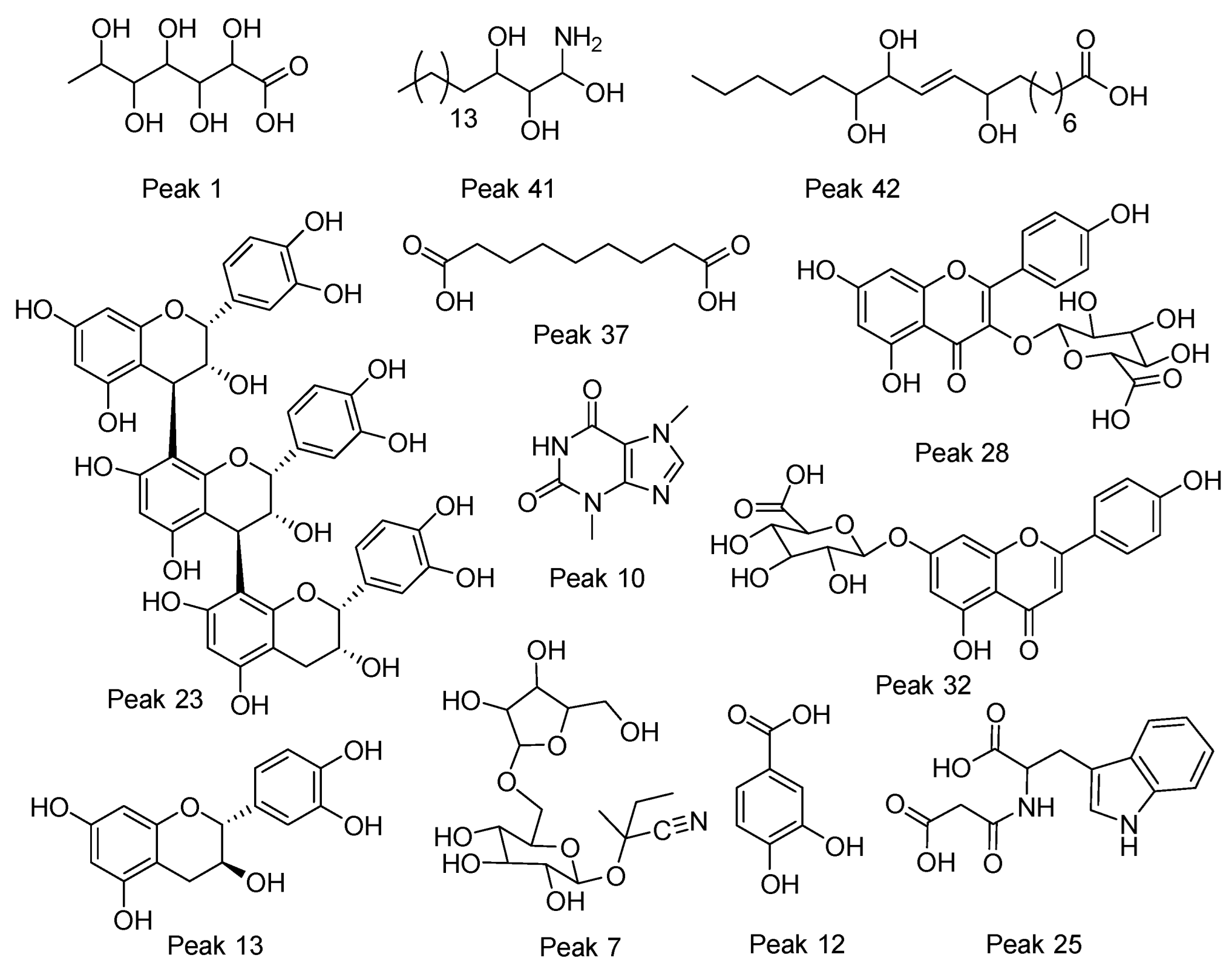
| Peak # | Rt | MS–ESI− or MS–ESI+ | MSn | Error (ppm) | Molecular Formula | Tentative Identification |
|---|---|---|---|---|---|---|
| 1 | 1.62 | 195.0507 | 195.0507, 159.0293, 129.0186, 75.0078 | 4.16 | C6H12O7 | Gluconic acid |
| 2 | 1.70 | 149.0086 | 130.9978, 103.0028, 87.0078, 72.9921 | 3.33 | C4H6O6 | Tartaric acid |
| 3 | 3.57 | 329.0883 | 167.0345, 152.0109, 123.0444 | 3.41 | C14H18O9 | Vanillic acid 4-hexoside |
| 4 | 4.38 | 359.0989 | 197.0453, 182.0215, 153.0551, 138.0315 | 3.12 | C15H20O10 | 1-O-Syringoyl-glucopyranose |
| 5 | 4.41 | 117.0186 | 99.0986, 73.0285 | 2.78 | C4H6O4 | Succinic acid * |
| 6 | 4.54 | 367.1614 | 235.1187, 161.0450, 143.0348, 113.0235, 101.0235, 71.0128 | 2.61 | C17H28O10 | Unknown |
| 7 | 5.24 | 438.1624 | 392.1567, 293.0884, 233.0671, 191.0560, 161.0449, 125.0237, 89.0235 | 4.13 | C16H27NO10 | 6’-Apiosyllotaustralin |
| 8 | 5.73 | 433.1930 | 281.1397, 219.1388, 161.0452, 119.0342101.0235, 89.0235 | 4.17 | C21H31O10 | Unknown |
| 9 | 6.21 | 175.0609 | 157.0502, 146.9608, 131.0707, 115.0392, 113.0600, 85.0649 | 4.46 | C7H12O5 | 2-Isopropylmalic acid |
| 10 | 8.02 | 181.07197 | 67.0282 | 2.83 | C7H8N4O2 | Theobromine * |
| 11 | 8.32 | 191.0194 | 173.0087, 129.0186, 111.0079, 87.0078, 85.0285 | 3.98 | C6H8O7 | Citrate |
| 12 | 8.46 | 153.0188 | 109.0287 | 0.30 | C7H6O4 | Protocatechuic acid * |
| 13 | 8.72 | 289.0722 | 245.0821, 205.0506, 203.0711, 179.0342, 165.0180, 151.0392, 125.0233 | 2.79 | C15H14O6 | (+)-Catechin * |
| 14 | 8.84 | 447.1513 [M+FA-H] | 401.1456, 269.1032, 233.0673, 161.0449, 113.0236, 101.0235 | 2.37 | C18H26O10 | Benzyl O-pentosyl-hexoside |
| 15 | 9.05 | 427.1826 [M+FA-H] | 381.1770, 249.1345, 161.0449, 101.0235, 71.0129 | 2.57 | C16H30O10 | Everlastoside C |
| 16 | 9.35 | 195.08832 | 195.0832 | 1.45 | C8H10N4O2 | Caffeine * |
| 17 | 9.50 | 427.1824 [M+FA-H] | 381.1768, 249.1344, 161.0450, 101.0235, 71.0128 | 1.99 | C16H30O10 | Everlastoside C isomer |
| 18 | 9.59 | 210.0770 | 210.0769, 124.0396, 94.0288 | 1.89 | C10H13NO4 | Methoxytyrosine |
| 19 | 9.90 | 593.1520 | 503.1200, 473.1094, 383.0772, 353.0670 | 2.25 | C27H30O15 | 6,8-C-Dihexosylapigenin |
| 20 | 10.01 | 289.0720 | 245.0820, 205.0504, 203.0710, 179.0345, 165.0188, 151.0394, 125.0236 | 2.79 | C15H14O6 | (-)-Epicatechin * |
| 21 | 10.41 | 563.1413 565.1548 | 545.1313, 503.1196, 473.1091, 443.0986, 425.0883, 383.0777, 353.0369, 529.1335, 511.1228, 481.1122, 427.1020, 409.0912, 379.0805, 325.0700 | 2.27 –1.56 | C26H28O14 | 8-C-Glucosyl-6-C-arabinosylapigenin |
| 22 | 10.61 | 563.1412 565.1548 | 545.1301, 503.1197, 473.1091, 443.0986, 425.0880, 383.0773, 353.0669, 529.1330, 511.1226, 481.1121, 427.1018, 409.0911, 379.0805, 325.0699 | 2.06 –1.56 | C26H28O14 | 6-C-Glucosyl-8-C-arabinosylapigenin |
| 23 | 10.52 | 865.1994 | 695.1393, 577.1351, 407.0774, 289.0720, 243.0398, 161.0283, 125.0236 | 1.63 | C45H38O18 | Procyanidin C1 |
| 24 | 10.91 | 563.1414 565.1075 | 545.1304, 503.1186, 473.1091, 443.0986, 425.0896, 529.1330, 511.1226, 481.1121, 427.1018, 409.0911 | 2.27 –0.82 | C26H28O14 | 8-C-Galactosyl-6-C-arabinosylapigenin |
| 25 | 11.90 | 289.0832 291.0973 | 245.0934, 203.0823, 135.1548, 116.0344, 98.0238, 74.0236, 273.0863, 245.0917, 227.0810, 201.1018, 188.0703, 159.0914, 130.0650 | 4.37 –0.99 | C14H14N2O5 | Malonyltryptophan |
| 26 | 11.90 | 245.0932 | 203.0822, 160.0246, 116.0344, 98.0238, 74.0237, 70.0288 | 4.49 | C13H14N2O3 | N-Acetyltryptophan |
| 27 | 11.91 | 577.1361 579.1495 | 407.0735, 289.0720, 245.0820,161.0238, 125.0236, 409.0910, 287.0544, 275.0544 | 2.62 –1.33 | C30H26O12 | Procyanidin type B isomer 1 |
| 28 | 11.99 | 461.0730 | 285.0406, 113.0285, 85.0285 | 2.10 | C27H18O12 | Kaempferol 3-glucuronide |
| 29 | 12.45 | 433.0781 | 300.0278, 178.9981, 151.0028 | 2.41 | C20H18O11 | Quercetin 3-O-Arabinose |
| 30 | 12.53 | 439.1073 | 409.0967, 260.0360, 110.9749 | –2.05 | C16H24O14 | Unknown |
| 31 | 12.53 | 439.1071 | 409.0964, 260.0364, 110.9748, 96.9591 | –2.53 | C16H24O14 | Unknown |
| 32 | 12.89 | 445.0779 | 269.0456, 175.0242, 113.0235, 85.0284 | 1.85 | C21H18O11 | Apigenin-7-O-glucuronide |
| 33 | 12.99 | 417.0830 | 284.0327, 255.0297, 209.0455, 151.0030 | 1.94 | C20H18O10 | Kaempferol 3-O-pentoside |
| 34 | 13.00 | 463.0863 | 300.0276, 178.9982, 161.0450 | 2.03 | C21H20O12 | Quercetin 3-O-glucoside |
| 35 | 9.43 | 577.1361 579.1495 | 407.0775, 289.0720, 245.0821, 161.0238, 125.0236, 409.0910, 287.0544, 275.0544, 247.0597, 163.0387, 127.0389 | 3.46 –1.33 | C30H26O12 | Procyanidin type B |
| 36 | 13.26 | 315.0727 | 165.0187, 152.0109, 108.0208, 85.0285 | 5.18 | C13H16O9 | Gentisic acid 5-O-hexoside |
| 37 | 13.10 | 187.0971 | 169.0864, 143.1071, 125.0963 | C9H16O4 | Azelaic acid | |
| 38 | 17.25 | 373.1870 | 331.1780, 177.0552, 165.0550, 59.0128 | 3.46 | C18H30O8 | Unknown |
| 39 | 18.04 | 288.2894 | 106.0865, 88.0861 | 0.96 | C17H37NO2 | Heptadecasphinganine isomer 1 |
| 40 | 18.29 | 288.2895 | 270.2783, 106.0864, 88.0760 | –0.85 | C17H37NO2 | Heptadecasphinganine isomer 2 |
| 41 | 19.07 | 318.2999 | 300.2891, 282.2785, 270.2785, 252.2680, 60.0450 | –1.07 | C18H39NO3 | Phytosphingosine |
| 42 | 19.30 | 329.2335 | 229.1443, 211.1336, 171.1020, 139.1120 | 3.67 | C18H34O5 | 9,12,13-Trihydroxyoctadecenoic acid |
| 43 | 493.2294 [M+FA-H] | 447.2236, 315.1813, 161.0449, 143.0340, 113.0235, 101.0234, 71.0128 | 3.00 | C21H36O10 | Unknown | |
| 44 | 17.35 | 274.2737 | 256.2629, 106.0864, 88.0761 | –1.26 | C16H37NO2 | Unknown |
| 45 | 17.96 | 329.2336 | 311.2228, 201.1127, 171.1020, 129.0917 | 4.04 | C18H34O5 | Trihydroxyoctadecenoic acid isomer 3 |
| 46 | 19.87 | 285.24893 | 155.11879 | C18H33O2 | Stearic acid * | |
| 47 | 22.63 | 281.24887 | 152.99487 | 4.85 | C18H34O2 | Oleic acid * |
| 48 | 23.39 | 303.23272, 305.23784 | 259.2525, 231.2116 | C20H32O2 | Araquidonic acid * | |
| 49 | 21.36 | 277.21603 | 220.11197, 181.01431 | –0.62 | C18H30O2 | Linolenic acid * |
| 50 | 279.23186, 281.23198 | 259.2436, 231.2112 | 4.31 | C18H32O2 | Linoleic acid * | |
| 51 | 269.24899, 271.24878 | 143.03407 | 5.51 | C17H34O2 | Margaric acid |
| Cocoa Waste | Humidity | Ashes | Total Lipids | Crude Protein | Crude Fiber | Carbohydrates |
|---|---|---|---|---|---|---|
| Vein flour | 15.2 ± 0.10 a | 6.42 ± 0.26 a | 0.42 ± 0.02 a | 12.74 ± 0.01 a | 7.26 ± 0.17 a | 57.96 |
| Pod husk flour | 5.00 ± 0.06 b | 8.62 ± 0.07 b | 0.51 ± 0.02 b | 8.50 ± 0.00 b | 35.48 ± 1.47 b | 41.89 |
| Cocoa Waste | Fe | Zn | Mn | Cu | Mg | K | Na | Ca |
|---|---|---|---|---|---|---|---|---|
| Vein flour | 4.49 ± 0.18 a | 2.97 ± 0.04 a | 1.02 ± 0.02 a | 0.76 ± 0.03 a | 7.60 ± 0.05 a | 54.03 ± 0.11 a | 0.09 ± 0.00 a | 1.38 ± 0.02 a |
| Pod husk flour | 5.04 ± 0.10 b | 7.22 ± 0.12 b | 7.32 ± 0.12 b | 8.55 ± 0.28 b | 21.23 ± 0.22 b | 112.04 ± 0.18 b | 0.47 ± 0.02 b | 6.15 ± 0.04 b |
| Fatty Acid Profile | Cocoa Waste | |
|---|---|---|
| Vein Flour | Pod Husk Flour | |
| c12:0 (lauric acid) | 0.685 ± 0.01 a | 0.564 ± 0.01 b |
| c14:0 (miristic acid) | 0.634 ± 0.02 a | 1.366 ± 0.01 b |
| c16:0 (palmitic acid) | 26.820 ± 0.11 a | 34.324 ± 0.11 b |
| c16:1 (palmitoleic acid) | 0.291 ± 0.01 a | 0.430 ± 0.00 b |
| c17:0 (margaric acid) | 0.374 ± 0.01 | - |
| c18:0 (stearic acid) | 15.784 ± 0.40 a | 3.143 ± 0.03 b |
| c18:1 (oleic acid) | 36.822 ± 0.56 a | 7.635 ± 0.12 b |
| c18:2 (linoleic acid) | 15.690 ± 0.19 a | 48.924 ± 0.06 b |
| c18:3 (linolenic acid) | 2.212 ± 0.04 a | 3.614 ± 0.03 b |
| c20:0 (arachidonic acid) | 0.688 ± 0.05 | - |
| Saturated FAs | 44.99% | 39.40% |
| Mono-UFAs | 37.11% | 8.07% |
| Poly-UFAs | 17.90% | 52.54% |
| Cocoa Waste | Theobromine (µg/g) | Caffeine (µg/g) | Catechin (mg/g) | Epicatechin (µg/g) |
|---|---|---|---|---|
| Vein flour | 6.32 ± 0.07 a | 1.11 ± 0.04 a | 3.09 ± 0.15 a | 3.40 ± 0.16 a |
| Pod husk flour | 10.21 ± 0.18 b | 0.22 ± 0.00 b | 2.93 ± 0.15 a | 1.14 ± 0.02 b |
| Cocoa Waste | DPPH (µmol Trolox/g) | ABTS (µmol Trolox/g) | FRAP (µmol Trolox/g) | Total Phenolics (mg AG/g) |
|---|---|---|---|---|
| Vein fluor | 46.51 ± 1.13 a | 104.07 ± 4.25 a | 73.77 ± 1.59 a | 86.90 ± 0.81 a |
| Pod husk flour | 87.42 ± 1.22 b | 155.38 ± 2.96 b | 127.44 ± 3.86 b | 111.05 ± 1.34 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas-Arana, G.; Merino-Zegarra, C.; Tang, M.; Pertino, M.W.; Simirgiotis, M.J. UHPLC–MS Characterization, and Antioxidant and Nutritional Analysis of Cocoa Waste Flours from the Peruvian Amazon. Antioxidants 2022, 11, 595. https://doi.org/10.3390/antiox11030595
Vargas-Arana G, Merino-Zegarra C, Tang M, Pertino MW, Simirgiotis MJ. UHPLC–MS Characterization, and Antioxidant and Nutritional Analysis of Cocoa Waste Flours from the Peruvian Amazon. Antioxidants. 2022; 11(3):595. https://doi.org/10.3390/antiox11030595
Chicago/Turabian StyleVargas-Arana, Gabriel, Claudia Merino-Zegarra, Miguel Tang, Mariano Walter Pertino, and Mario J. Simirgiotis. 2022. "UHPLC–MS Characterization, and Antioxidant and Nutritional Analysis of Cocoa Waste Flours from the Peruvian Amazon" Antioxidants 11, no. 3: 595. https://doi.org/10.3390/antiox11030595
APA StyleVargas-Arana, G., Merino-Zegarra, C., Tang, M., Pertino, M. W., & Simirgiotis, M. J. (2022). UHPLC–MS Characterization, and Antioxidant and Nutritional Analysis of Cocoa Waste Flours from the Peruvian Amazon. Antioxidants, 11(3), 595. https://doi.org/10.3390/antiox11030595









