COVID-19, Oxidative Stress and Male Reproduction: Possible Role of Antioxidants
Abstract
:1. Introduction
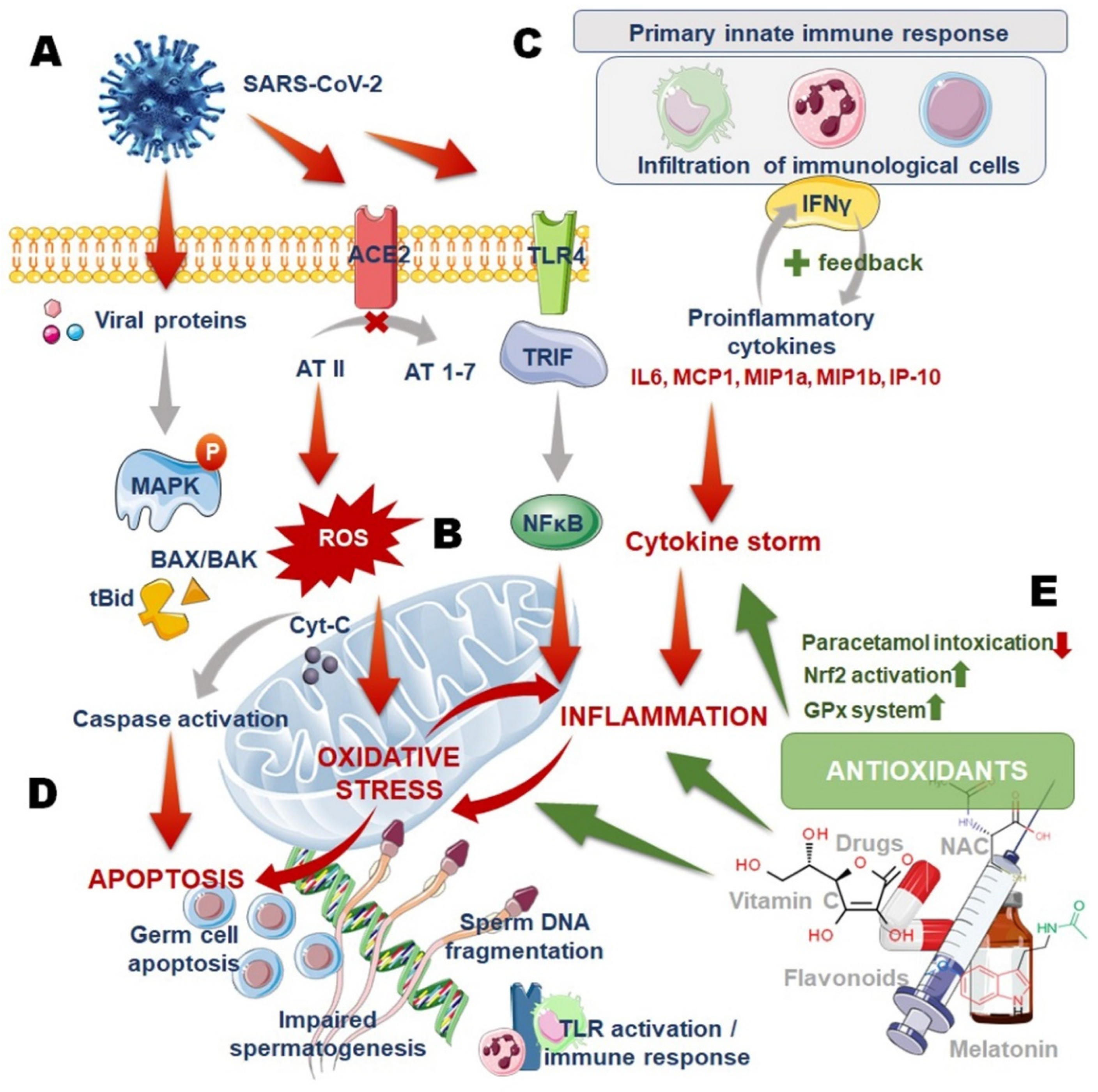
2. COVID-19 and Male Infertility: Oxidative Stress (OS)–Inflammation Vicious Cycle
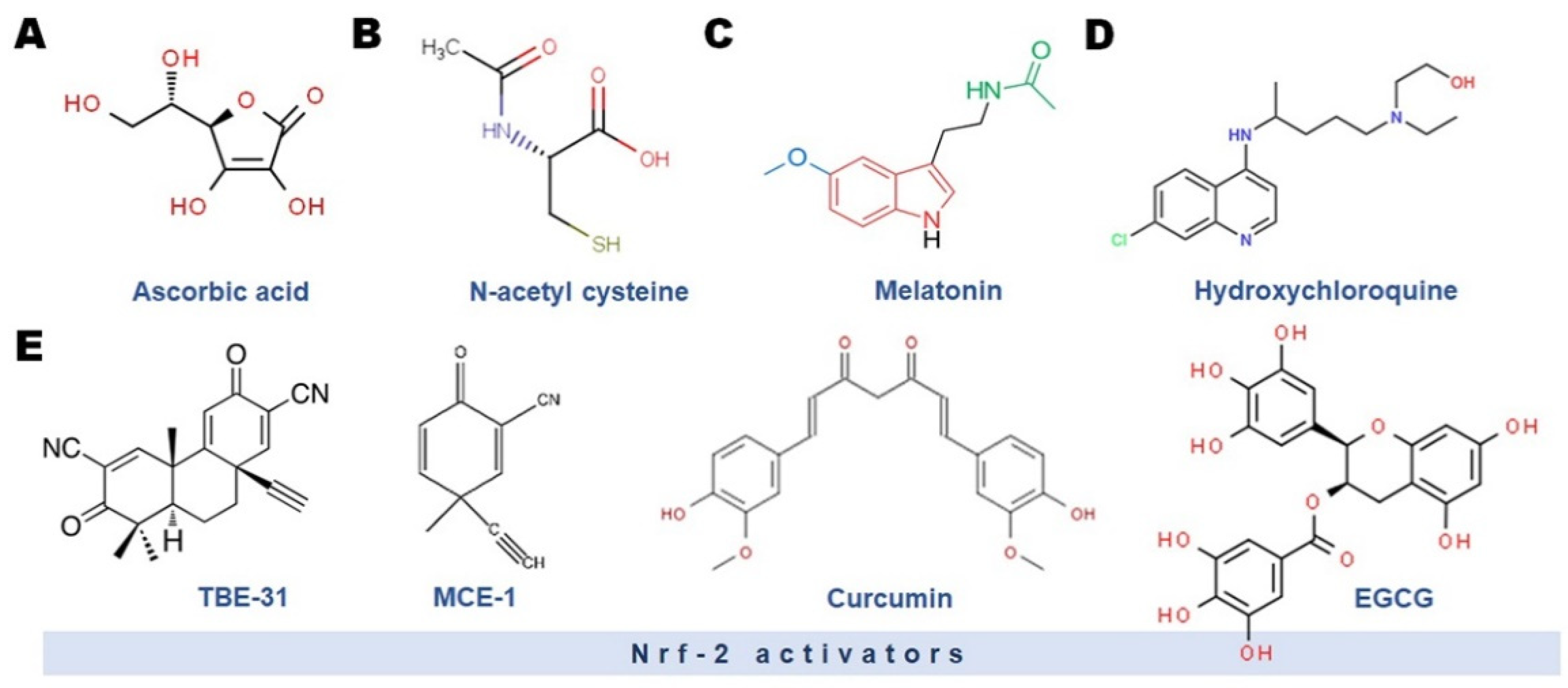
3. COVID-19, Oxidative Stress (OS) and Male Infertility: Role of Antioxidants
3.1. Vitamin C
3.2. N-Acetyl Cysteine (NAC)
3.3. Melatonin
3.4. Selenium (Se)
3.5. Nrf-2 Activators and Flavonoids
3.6. Drugs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.l.; Hui, D.S. Clinical characteristics of coronavirus disease 2019 in China. N. Eng. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Naming the Coronavirus Disease (COVID-19) and the Virus that Causes It. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(COVID-2019)-and-the-virus-that-causes-it (accessed on 17 February 2022).
- Jordan, R.E.; Adab, P.; Cheng, K. COVID-19: Risk factors for severe disease and death. BMJ 2020, 26, 368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poma, A.M.; Bonuccelli, D.; Giannini, R.; Macerola, E.; Vignali, P.; Ugolini, C.; Torregrossa, L.; Proietti, A.; Pistello, M.; Basolo, A.; et al. COVID-19 autopsy cases: Detection of virus in endocrine tissues. J. Endocrinol. Investig. 2022, 45, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Jin, M.; Bao, P.; Zhao, W.; Zhang, S. Clinical characteristics and results of semen tests among men with coronavirus disease 2019. JAMA Net Open 2020, 3, 08292. [Google Scholar] [CrossRef]
- Song, C.; Wang, Y.; Li, W.; Hu, B.; Chen, G.; Xia, P.; Wang, W.; Li, C.; Hu, Z.; Yang, X. Detection of 2019 novel coronavirus in semen and testicular biopsy specimen of COVID-19 patients. MedRxiv 2020, 103, 4–6. [Google Scholar] [CrossRef]
- Gong, J.; Zeng, Q.; Yu, D.; Duan, Y.G. T lymphocytes and testicular immunity: A new insight into immune regulation in testes. Int. J. Mol. Sci. 2020, 22, 57. [Google Scholar] [CrossRef]
- Li, H.; Xiao, X.; Zhang, J.; Zafar, M.I.; Wu, C.; Long, Y.; Lu, W.; Pan, F.; Meng, T.; Zhao, K.; et al. Impaired spermatogenesis in COVID-19 patients. EClinicalMedicine 2020, 28, 100604. [Google Scholar] [CrossRef]
- Ma, L.; Xie, W.; Li, D.; Shi, L.; Ye, G.; Mao, Y.; Xiong, Y.; Sun, H.; Zheng, F.; Chen, Z.; et al. Evaluation of sex-related hormones and semen characteristics in reproductive-aged male COVID-19 patients. J. Med. Virol. 2021, 93, 456–462. [Google Scholar] [CrossRef]
- Koç, E.; Keseroğlu, B.B. Does COVID-19 worsen the semen parameters? Early results of a tertiary healthcare center. Urol. Int. 2021, 105, 743–748. [Google Scholar] [CrossRef]
- Cinislioglu, A.E.; Cinislioglu, N.; Demirdogen, S.O.; Sam, E.; Akkas, F.; Altay, M.S.; Utlu, M.; Sen, I.A.; Yildirim, F.; Kartal, S.; et al. The relationship of serum testosterone levels with the clinical course and prognosis of COVID-19 disease in male patients: A prospective study. Andrology 2022, 10, 24–33. [Google Scholar] [CrossRef]
- Temiz, M.Z.; Dincer, M.M.; Hacibey, I.; Yazar, R.O.; Celik, C.; Kucuk, S.H.; Alkurt, G.; Doganay, L.; Yuruk, E.; Muslumanoglu, A.Y. Investigation of SARS-CoV-2 in semen samples and the effects of COVID-19 on male sexual health by using semen analysis and serum male hormone profile: A cross-sectional, pilot study. Andrologia 2021, 53, 13912. [Google Scholar] [CrossRef] [PubMed]
- Gacci, M.; Coppi, M.; Baldi, E.; Sebastianelli, A.; Zaccaro, C.; Morselli, S.; Pecoraro, A.; Manera, A.; Nicoletti, R.; Liaci, A.; et al. Semen impairment and occurrence of SARS-CoV-2 virus in semen after recovery from COVID-19. Hum. Reprod. 2021, 36, 1520–1529. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Roche, L.; Mesta, F. Oxidative stress as key player in severe acute respiratory syndrome coronavirus (SARS-CoV) infection. Arch. Med. Res. 2020, 51, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Bisht, S.; Faiq, M.; Tolahunase, M.; Dada, R. Oxidative stress and male infertility. Nat. Rev. Urol. 2017, 14, 470. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X. Clinical features of patients infected with 2019 novel coronavirus in wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Dutta, S.; Sandhu, N.; Sengupta, P.; Alves, M.G.; Henkel, R.; Agarwal, A. Somatic-Immune Cells Crosstalk in-the-Making of Testicular Immune Privilege. 2021. Available online: https://link.springer.com/article/10.1007/s43032-021-00721-0 (accessed on 17 February 2022).
- Schuppe, H.-C.; Meinhardt, A. Immune privilege and inflammation of the testis. In Immunology of Gametes and Embryo Implantation; Karger Publishers: Basel, Switzerland, 2005; Volume 88, pp. 1–14. [Google Scholar]
- Dutta, S.; Sengupta, P.; Chhikara, B.S. Reproductive inflammatory mediators and male infertility. Chem. Biol. Lett. 2020, 7, 73–74. [Google Scholar]
- Fan, C.; Li, K.; Ding, Y.; Lu, W.L.; Wang, J. Ace2 expression in kidney and testis may cause kidney and testis damage after 2019-ncov infection. Front. Med. 2020, 7, 563893. [Google Scholar] [CrossRef]
- Hedger, M.P. Immunophysiology and pathology of inflammation in the testis and epididymis. J. Androl. 2011, 32, 625–640. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P.; Hassan, M.F.; Biswas, A. Role of toll-like receptors in the reproductive tract inflammation and male infertility. Chem. Biol. Lett. 2020, 7, 113–123. [Google Scholar]
- Ren, L.L.; Wang, Y.M.; Wu, Z.Q.; Xiang, Z.C.; Guo, L.; Xu, T.; Jiang, Y.Z.; Xiong, Y.; Li, Y.J.; Li, X.W. Identification of a novel coronavirus causing severe pneumonia in human: A descriptive study. Chin. Med. J. 2020, 133, 1015–1024. [Google Scholar] [CrossRef]
- Merad, M.; Martin, J.C. Pathological inflammation in patients with COVID-19: A key role for monocytes and macrophages. Nat. Rev. Immunol. 2020, 20, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Majzoub, A.; Agarwal, A. Oxidative stress and sperm function: A systematic review on evaluation and management. Arab. J. Urol. 2019, 17, 87–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sengupta, P.; Dutta, S.; Alahmar, A.T.; D’souza, U.J.A. Reproductive tract infection, inflammation and male infertility. Chem. Biol. Lett. 2020, 7, 75–84. [Google Scholar]
- Ma, L.; Xie, W.; Li, D.; Shi, L.; Mao, Y.; Xiong, Y.; Zhang, Y.; Zhang, M. Effect of SARS-CoV-2 infection upon male gonadal function: A single center-based study. MedRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Darbandi, M.; Darbandi, S.; Agarwal, A.; Sengupta, P.; Durairajanayagam, D.; Henkel, R.; Sadeghi, M.R. Reactive oxygen species and male reproductive hormones. Reprod. Biol. Endocrinol. 2018, 16, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Moghimi, N.; Farsani, B.E.; Ghadipasha, M.; Mahmoudiasl, G.-R.; Piryaei, A.; Aliaghaei, A.; Abdi, S.; Abbaszadeh, H.-A.; Abdollahifar, M.-A.; Forozesh, M. COVID-19 disrupts spermatogenesis through the oxidative stress pathway following induction of apoptosis. Apoptosis 2021, 26, 415–430. [Google Scholar] [CrossRef]
- Izuka, E.; Menuba, I.; Sengupta, P.; Dutta, S.; Nwagha, U. Antioxidants, anti-inflammatory drugs and antibiotics in the treatment of reproductive tract infections and their association with male infertility. Chem. Biol. Lett. 2020, 7, 156–165. [Google Scholar]
- Liu, Q.; Gao, Y.; Ci, X. Role of nrf2 and its activators in respiratory diseases. Oxidat. Med. Cell. Longev. 2019, 2019, 7090534. [Google Scholar] [CrossRef] [Green Version]
- Nabzdyk, C.S.; Bittner, E.A. Vitamin C in the critically ill-indications and controversies. World J. Crit. Care Med. 2018, 7, 52. [Google Scholar] [CrossRef]
- Li, J. Evidence is stronger than you think: A meta-analysis of vitamin c use in patients with sepsis. Crit. Care 2018, 22, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Patel, V.; Dial, K.; Wu, J.; Gauthier, A.G.; Wu, W.; Lin, M.; Espey, M.G.; Thomas, D.D.; Ashby, C.R.; Mantell, L.L. Dietary antioxidants significantly attenuate hyperoxia-induced acute inflammatory lung injury by enhancing macrophage function via reducing the accumulation of airway hmgb1. Int. J. Mol. Sci. 2020, 21, 977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angulo, C.; Maldonado, R.; Pulgar, E.; Mancilla, H.; Córdova, A.; Villarroel, F.; Castro, M.A.; Concha, I.I. Vitamin C and oxidative stress in the seminiferous epithelium. Biol. Res. 2011, 44, 169–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sönmez, M.; Türk, G.; Yüce, A. The effect of ascorbic acid supplementation on sperm quality, lipid peroxidation and testosterone levels of male wistar rats. Theriogenology 2005, 63, 2063–2072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayinde, O.C.; Ogunnowo, S.; Ogedegbe, R.A. Influence of vitamin c and vitamin e on testicular zinc content and testicular toxicity in lead exposed albino rats. BMC Pharm. Toxicol 2012, 13, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behairy, A.; El-Sharkawy, N.I.; Saber, T.M.; Soliman, M.M.; Metwally, M.M.M.; Abd El-Rahman, G.I.; Abd-Elhakim, Y.M.; El Deib, M.M. The modulatory role of vitamin C in boldenone undecylenate induced testicular oxidative damage and androgen receptor dysregulation in adult male rats. Antioxidants 2020, 9, 1053. [Google Scholar] [CrossRef]
- National Cancer Institute. High-Dose Vitamin C (pdq®)–Health Professional Version. Available online: https://www.cancer.gov/about-cancer/treatment/cam/hp/vitamin-c-pdq (accessed on 17 February 2022).
- McCarty, M.F.; DiNicolantonio, J.J. Nutraceuticals have potential for boosting the type 1 interferon response to rna viruses including influenza and coronavirus. Prog. Card. Dis. 2020, 63, 383. [Google Scholar] [CrossRef]
- Sengupta, P.; Dutta, S.; Slama, P.; Roychoudhury, S. COVID-19, oxidative stress, and male reproductive dysfunctions: Is vitamin c a potential remedy? Physiol. Res. 2022, 71, 19. [Google Scholar]
- Mata, M.; Morcillo, E.; Gimeno, C.; Cortijo, J. N-acetyl-l-cysteine (NAC) inhibit mucin synthesis and pro-inflammatory mediators in alveolar type ii epithelial cells infected with influenza virus a and b and with respiratory syncytial virus (rsv). Biochem. Pharm. 2011, 82, 548–555. [Google Scholar] [CrossRef] [Green Version]
- Polonikov, A. Endogenous deficiency of glutathione as the most likely cause of serious manifestations and death in COVID-19 patients. ACS Infect. Dis. 2020, 6, 1558–1562. [Google Scholar] [CrossRef]
- Sengupta, P.; Dutta, S. COVID-19 and hypogonadism: Secondary immune responses rule-over endocrine mechanisms. Hum. Fertil. 2021, 1–6. [Google Scholar] [CrossRef]
- Wright, J.H.; Caudill, R. Remote treatment delivery in response to the COVID-19 pandemic. Psychother. Psychosom. 2020, 89, 1. [Google Scholar] [CrossRef] [PubMed]
- Jorge-Aarón, R.-M.; Rosa-Ester, M.-P. N-acetylcysteine as a potential treatment for novel coronavirus disease 2019. Fut. Micobiol. 2020, 15, 959–962. [Google Scholar] [CrossRef] [PubMed]
- Memorial Sloan Kettering Cancer Center. A Study of N-Acetylcysteine in Patients with COVID-19 Infection; Clinical Trial Identifier: NCT04374461. Available online: https://clinicaltrials.gov/ct2/show/NCT04374461 (accessed on 17 February 2022).
- Jannatifar, R.; Parivar, K.; Roodbari, N.H.; Nasr-Esfahani, M.H. Effects of n-acetyl-cysteine supplementation on sperm quality, chromatin integrity and level of oxidative stress in infertile men. Reprod. Biol. Endocrinol. 2019, 17, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comhaire, F.H.; Christophe, A.B.; Zalata, A.A.; Dhooge, W.S.; Mahmoud, A.M.; Depuydt, C.E. The effects of combined conventional treatment, oral antioxidants and essential fatty acids on sperm biology in subfertile men. Prostaglandins Leukot. Essent. Fat. Acids 2000, 63, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Schoeman, D.; Fielding, B.C. Coronavirus envelope protein: Current knowledge. Virol. J. 2019, 16, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldini, G.; Altomare, A.; Baron, G.; Vistoli, G.; Carini, M.; Borsani, L.; Sergio, F. N-acetylcysteine as an antioxidant and disulphide breaking agent: The reasons why. Free Rad. Res. 2018, 52, 751–762. [Google Scholar] [CrossRef]
- Semenovich, D.S.; Plotnikov, E.Y.; Titko, O.V.; Lukiyenko, E.P.; Kanunnikova, N.P. Effects of Panthenol and N-Acetylcysteine on Changes in the Redox State of Brain Mitochondria under Oxidative Stress In Vitro. Antioxidants. 2021, 10, 1699. [Google Scholar] [CrossRef]
- Reiter, R.J.; Ma, Q.; Sharma, R. Treatment of ebola and other infectious diseases: Melatonin “goes viral”. Melatonin Res. 2020, 3, 43–57. [Google Scholar] [CrossRef]
- Junaid, A.; Tang, H.; Van Reeuwijk, A.; Abouleila, Y.; Wuelfroth, P.; Van Duinen, V.; Stam, W.; Van Zonneveld, A.J.; Hankemeier, T.; Mashaghi, A. Ebola hemorrhagic shock syndrome-on-a-chip. IScience 2020, 23, 100765. [Google Scholar] [CrossRef] [Green Version]
- Boga, J.A.; Coto-Montes, A.; Rosales-Corral, S.A.; Tan, D.X.; Reiter, R.J. Beneficial actions of melatonin in the management of viral infections: A new use for this ”molecular handyman”? Rev. Med. Virol. 2012, 22, 323–338. [Google Scholar] [CrossRef]
- Feitosa, E.L.; Júnior, F.; Nery Neto, J.A.O.; Matos, L.F.L.; Moura, M.H.S.; Rosales, T.O.; De Freitas, G.B.L. COVID-19: Rational discovery of the therapeutic potential of melatonin as a SARS-CoV-2 main protease inhibitor. Int. J. Med. Sci. 2020, 17, 2133–2146. [Google Scholar] [CrossRef] [PubMed]
- Ben-Nathan, D.; Maestroni, G.; Lustig, S.; Conti, A. Protective effects of melatonin in mice infected with encephalitis viruses. Arch. Virol. 1995, 140, 223–230. [Google Scholar] [CrossRef] [PubMed]
- El-Missiry, M.A.; El-Missiry, Z.M.A.; Othman, A.I. Melatonin is a potential adjuvant to improve clinical outcomes in individuals with obesity and diabetes with coexistence of COVID-19. Eur. J. Pharmacol. 2020, 882, 173329. [Google Scholar] [CrossRef]
- Shneider, A.; Kudriavtsev, A.; Vakhrusheva, A. Can melatonin reduce the severity of COVID-19 pandemic? Int. Rev. Immunol. 2020, 39, 153–162. [Google Scholar] [CrossRef]
- Li, C.; Zhou, X. Melatonin and male reproduction. Clin. Chim. Acta. 2015, 446, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Riviere, E.; Rossi, S.P.; Tavalieri, Y.E.; Muñoz de Toro, M.M.; Ponzio, R.; Puigdomenech, E.; Levalle, O.; Martinez, G.; Terradas, C.; Calandra, R.S.; et al. Melatonin daily oral supplementation attenuates inflammation and oxidative stress in testes of men with altered spermatogenesis of unknown aetiology. Mol. Cell. Endocrinol. 2020, 515, 110889. [Google Scholar] [CrossRef]
- Deng, S.L.; Zhang, B.L.; Reiter, R.J.; Liu, Y.X. Melatonin ameliorates inflammation and oxidative stress by suppressing the p38mapk signaling pathway in lps-induced sheep orchitis. Antioxidants 2020, 9, 1277. [Google Scholar] [CrossRef]
- Zhang, J.; Taylor, E.W.; Bennett, K.; Saad, R.; Rayman, M.P. Association between regional selenium status and reported outcome of COVID-19 cases in china. Am. J. Clin. Nutr. 2020, 111, 1297–1299. [Google Scholar] [CrossRef]
- Alexander, J.; Tinkov, A.; Strand, T.A.; Alehagen, U.; Skalny, A.; Aaseth, J. Early nutritional interventions with zinc, selenium and vitamin d for raising anti-viral resistance against progressive COVID-19. Nutrients 2020, 12, 2358. [Google Scholar] [CrossRef]
- Sies, H.; Parnham, M.J. Potential therapeutic use of ebselen for COVID-19 and other respiratory viral infections. Free Rad. Biol. Med. 2020, 156, 107–112. [Google Scholar] [CrossRef]
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C.; et al. Structure of mpro from SARS-CoV-2 and discovery of its inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moslemi, M.K.; Tavanbakhsh, S. Selenium-vitamin E supplementation in infertile men: Effects on semen parameters and pregnancy rate. Int. J. Gen. Med. 2011, 4, 99–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazjin, M.A.; Salehi, Z.; Mashayekhi, F.; Bahadori, M. Evaluation of gpx1 pro198leu polymorphism in idiopathic male infertility. Molekul. Biol. 2016, 50, 89–93. [Google Scholar] [CrossRef]
- Seale, L.A.; Torres, D.J.; Berry, M.J.; Pitts, M.W. A role for selenium-dependent gpx1 in SARS-CoV-2 virulence. Am. J. Clin. Nutr. 2020, 112, 447–448. [Google Scholar] [CrossRef]
- Hassan, S.M.; Jawad, M.J.; Ahjel, S.W.; Singh, R.B.; Singh, J.; Awad, S.M.; Hadi, N.R. The nrf2 activator (dmf) and COVID-19: Is there a possible role? Med. Arch. 2020, 74, 134–138. [Google Scholar] [CrossRef]
- McCord, J.M.; Hybertson, B.M.; Cota-Gomez, A.; Geraci, K.P.; Gao, B. Nrf2 activator pb125(®) as a potential therapeutic agent against COVID-19. Antioxidants 2020, 9, 518. [Google Scholar] [CrossRef]
- Liu, Z.; Ying, Y. The inhibitory effect of curcumin on virus-induced cytokine storm and its potential use in the associated severe pneumonia. Front. Cell Dev. Biol. 2020, 8, 479. [Google Scholar] [CrossRef]
- Menegazzi, M.; Campagnari, R.; Bertoldi, M.; Crupi, R.; Di Paola, R.; Cuzzocrea, S. Protective effect of epigallocatechin-3-gallate (egcg) in diseases with uncontrolled immune activation: Could such a scenario be helpful to counteract COVID-19? Int. J. Mol. Sci. 2020, 21, 5171. [Google Scholar] [CrossRef]
- Mendonca, P.; Soliman, K.F.A. Flavonoids activation of the transcription factor nrf2 as a hypothesis approach for the prevention and modulation of SARS-CoV-2 infection severity. Antioxidants 2020, 9, 659. [Google Scholar] [CrossRef]
- Jamalan, M.; Ghaffari, M.A.; Hoseinzadeh, P.; Hashemitabar, M.; Zeinali, M. Human sperm quality and metal toxicants: Protective effects of some flavonoids on male reproductive function. Int. J. Fert. Steril. 2016, 10, 215–223. [Google Scholar]
- Pahan, P.; Pahan, K. Smooth or risky revisit of an old malaria drug for COVID-19? J. Neuroimm. Pharmacol. 2020, 15, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.; Perl, A.; Smith, D.; Lewis, T.; Kon, Z.; Goldenberg, R.; Yarta, K.; Staniloae, C.; Williams, M. Therapeutic blockade of inflammation in severe COVID-19 infection with intravenous n-acetylcysteine. Clin. Immunol. 2020, 219, 108544. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.L.D.; Lam, W.C.; Yang, W.; Chan, K.W.; Sze, S.C.W.; Miao, J.; Yung, K.K.L.; Bian, Z.; Wong, V.T. Potential targets for treatment of coronavirus disease 2019 (COVID-19): A review of qing-fei-pai-du-tang and its major herbs. Am. J. Chin. Med. 2020, 48, 1051–1071. [Google Scholar] [CrossRef] [PubMed]
- Sestili, P.; Fimognari, C. Paracetamol use in COVID-19: Friend or enemy? Preprints 2020, 2020, 202080186. [Google Scholar]
- Banihani, S.A. Effect of paracetamol on semen quality. Andrologia 2018, 50, 12874. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sengupta, P.; Dutta, S.; Roychoudhury, S.; D’Souza, U.J.A.; Govindasamy, K.; Kolesarova, A. COVID-19, Oxidative Stress and Male Reproduction: Possible Role of Antioxidants. Antioxidants 2022, 11, 548. https://doi.org/10.3390/antiox11030548
Sengupta P, Dutta S, Roychoudhury S, D’Souza UJA, Govindasamy K, Kolesarova A. COVID-19, Oxidative Stress and Male Reproduction: Possible Role of Antioxidants. Antioxidants. 2022; 11(3):548. https://doi.org/10.3390/antiox11030548
Chicago/Turabian StyleSengupta, Pallav, Sulagna Dutta, Shubhadeep Roychoudhury, Urban John Arnold D’Souza, Kadirvel Govindasamy, and Adriana Kolesarova. 2022. "COVID-19, Oxidative Stress and Male Reproduction: Possible Role of Antioxidants" Antioxidants 11, no. 3: 548. https://doi.org/10.3390/antiox11030548
APA StyleSengupta, P., Dutta, S., Roychoudhury, S., D’Souza, U. J. A., Govindasamy, K., & Kolesarova, A. (2022). COVID-19, Oxidative Stress and Male Reproduction: Possible Role of Antioxidants. Antioxidants, 11(3), 548. https://doi.org/10.3390/antiox11030548






