Sweeteners from Different Lingonberry Jams Influence on Bioaccessibility of Vitamin C, Anthocyanins and Antioxidant Capacity under In Vitro Gastrointestinal Digestion
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Jam Preparation
2.3. In Vitro Simulated GI Digestion
2.4. High Performance Liquid Chromatography (HPLC) Analysis of Anthocyanins
2.5. Spectrophotometric Measurements
2.5.1. Vitamin C Determination
2.5.2. Total Anthocyanin Content (TAC) Determination
2.5.3. Antioxidant Capacity (AC) Determination
2.6. Statistics
3. Results and Discussion
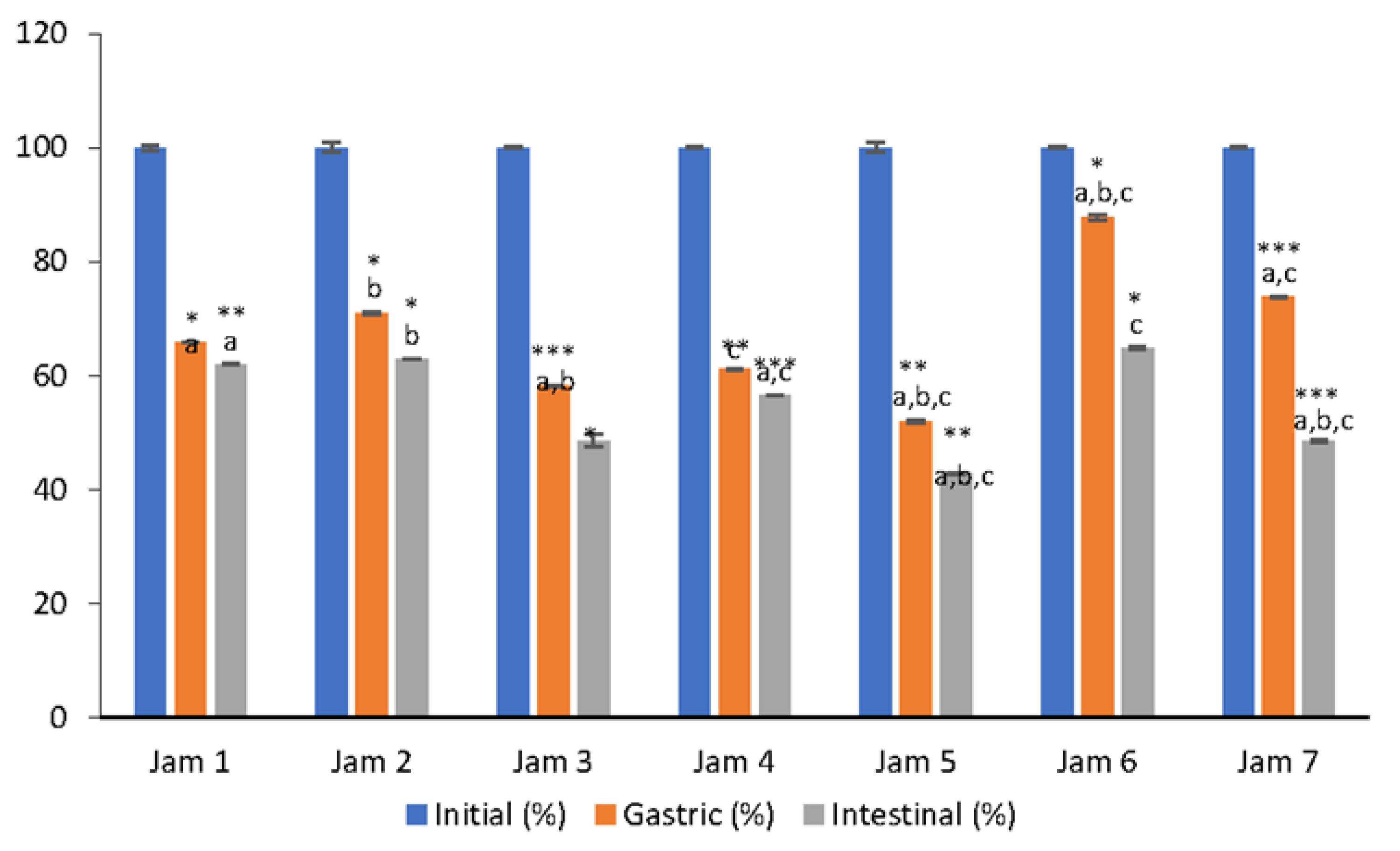
3.1. Bioaccessibility of Vitamin C
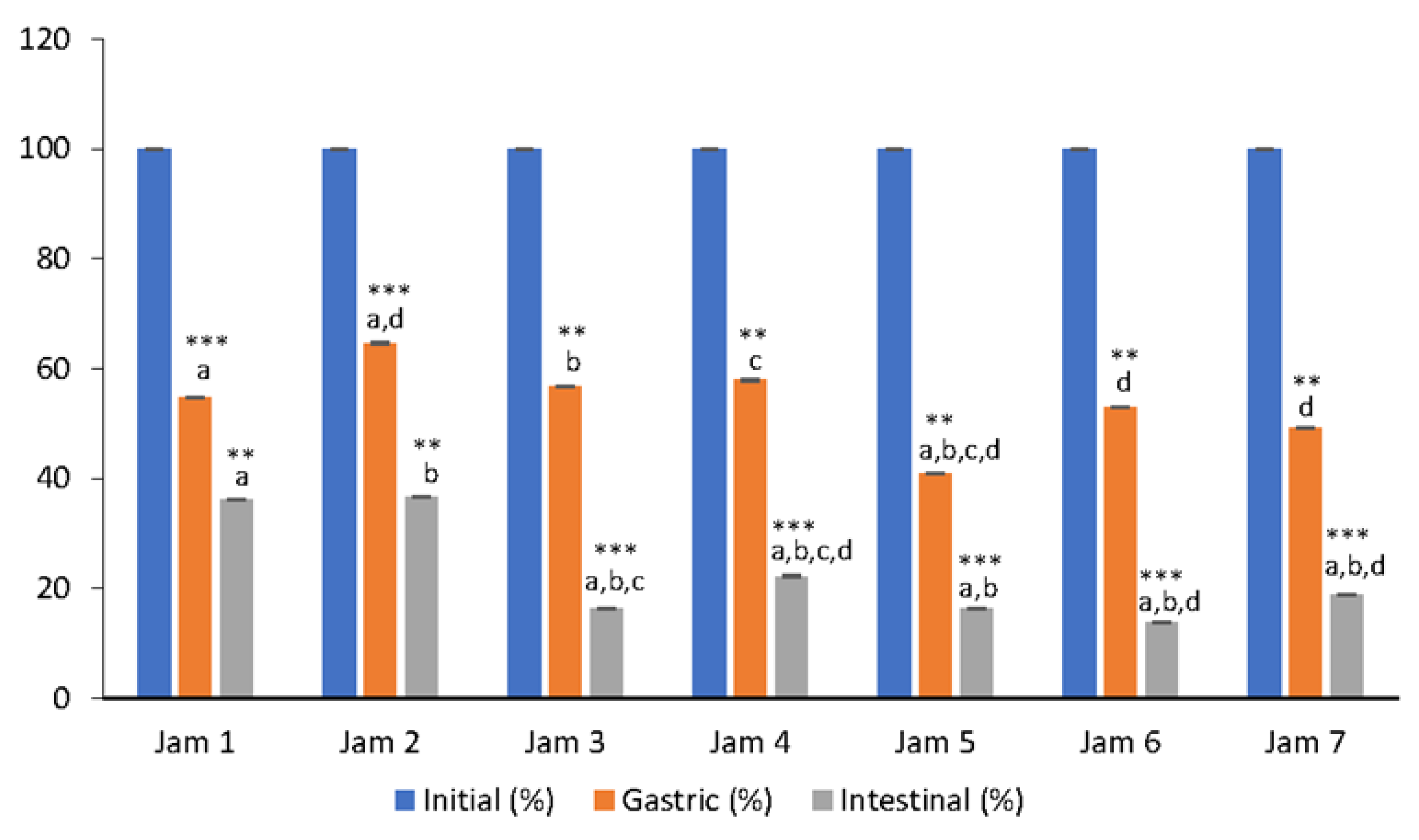
3.2. Bioaccessibility of Anthocyanins
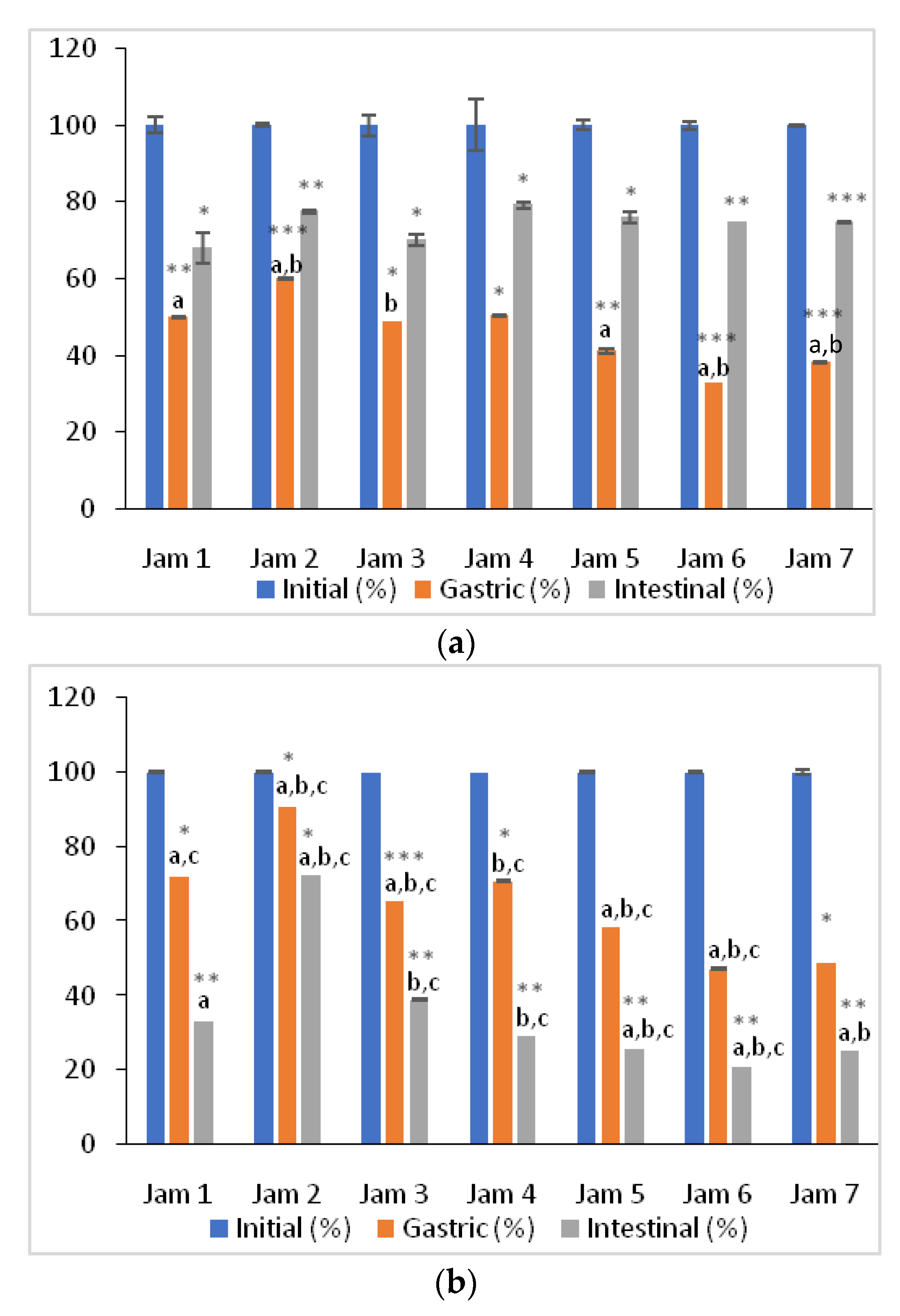
3.3. Changes in AC of Lingonberry Jams during In Vitro Digestion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mane, C.; Loonis, M.; Juhel, C.; Dufour, C.; Malien-Aubert, C. Food Grade Lingonberry Extract: Polyphenolic Composition and In Vivo Protective Effect against Oxidative Stress. J. Agric. Food Chem. 2011, 59, 3330–3339. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, K. Lingonberry (Vaccinium vitis-idaea L.) Fruit as a Source of Bioactive Compounds with Health-Promoting Effects—A Review. Int. J. Mol. Sci. 2021, 22, 5126. [Google Scholar] [CrossRef] [PubMed]
- Drózdz, P.; Šežiene, V.; Wójcik, J.; Pyrzynska, K. Evaluation of Bioactive Compounds, Minerals and Antioxidant Activity of Lingonberry (Vaccinium vitis-idaea L.) Fruits. Molecules 2018, 23, 53. [Google Scholar] [CrossRef] [PubMed]
- Kivimäki, A.S.; Siltari, A.; Ehlers, P.I.; Korpel, R.; Vapaatalo, H. Lingonberry juice negates the effects of a high salt diet on vascular function and low-grade inflammation. J. Funct. Foods 2014, 7, 238–245. [Google Scholar] [CrossRef]
- Fan, Z.L.; Wang, Z.Y.; Liu, J.R. Cold-field fruit extracts exert different antioxidant and antiproliferative activities in vitro. Food Chem. 2011, 129, 402–407. [Google Scholar] [CrossRef]
- Brown, E.; Gill, C.; Stewarta, D.; McDougall, G. Lingonberries (Vaccinium vitis-idaea L.) and blueberries (Vaccinium corymbosum) contain discrete epicatechin anthocyanin derivatives linked by ethyl bridges. J. Berry Res. 2016, 6, 13–23. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Pasli, A.A.; Ozcelik, B.; Van Camp, J.; Capanoglu, E. Colour retention, anthocyanin stability and antioxidant capacity in black carrot (Daucus carota) jams and marmalades: Effect of processing, storage conditions and in vitro gastrointestinal digestion. J. Funct. Foods 2015, 13, 1–10. [Google Scholar] [CrossRef]
- David, L.; Danciu, V.; Moldovan, B.; Filip, A. Effects of In Vitro Gastrointestinal Digestion on the Antioxidant Capacity and Anthocyanin Content of Cornelian Cherry Fruit Extract. Antioxidants 2019, 8, 114. [Google Scholar] [CrossRef]
- Lee, J.; Finn, C.E. Lingonberry (Vaccinium vitis-idaea L.) grown in the Pacific Northwest of North America: Anthocyanin and free amino acid composition. J. Funct. Foods 2012, 4, 213–218. [Google Scholar] [CrossRef]
- Zanini, D.J.; Silva, M.H.; Aguiar-Oliveira, E.; Mazalli, M.R.; Kamimura, E.S.; Maldonado, R.R. Spectrophotometric analysis of Vitamin C in different matrices utilizing potassium permanganate. EIJST 2018, 7, 70–84. [Google Scholar]
- Scrob, T.; Varodi, S.M.; Vintilă, G.A. Effect of sweeteners and storage on the acidity, soluble solids and sensorial profile of lingonberry jams. Studia Univ. Babes-Bolyai Chem. 2021, 66, 97–106. [Google Scholar]
- Cilla, A.; González-Sarrías, A.; Tomás-Barberán, F.A.; Espín, J.C.; Barberá, R. Availability of polyphenols in fruit beverages subjected to in vitro gastrointestinal digestion and their effects on proliferation, cell-cycle and apoptosis in human colon cancer Caco-2 cells. Food Chem. 2009, 114, 813–820. [Google Scholar] [CrossRef]
- Fernandes, I.; Faria, A.; Calhaub, C.; de Freitas, V.; Mateus, N. Bioavailability of anthocyanins and derivatives. J. Funct. Foods 2014, 7, 54–66. [Google Scholar] [CrossRef]
- Rodríguez-Roque, M.J.; Rojas-Grau, M.A.; Elez-Martínez, P.; Belloso, O.M. Changes in Vitamin C, Phenolic, and Carotenoid Profiles Throughout In Vitro Gastrointestinal Digestion of a Blended Fruit Juice. J. Agric. Food Chem. 2013, 61, 1859–1867. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Alminger, M.; Aura, A.M.; Bohn, T.; Dufour, C.; El, S.N.; Gomes, A.; Karakaya, S.; Martínez-Cuesta, M.C.; McDougall, G.J.; Requena, T.; et al. In vitro models for studying secondary plant metabolite digestion and bioaccessibility. Compr. Rev. Food Sci. Food Saf. 2014, 13, 413–436. [Google Scholar] [CrossRef]
- Sutwal, R.; Dhankhar, J.; Kind, P.; Mehla, R. Development of low calorie jam by replacement of sugar with natural sweetener stevia. Int. J. Curr. Res. 2019, 11, 9–16. [Google Scholar] [CrossRef]
- Seraglio, S.K.T.; Valese, A.C.; Daguerb, H.; Bergamo, G.; Azevedo, M.S.; Nehring, P.; Gonzaga, V.; Fett, R.; Costa, A.C.O. Effect of in vitro gastrointestinal digestion of phenolic compounds, minerals, and antioxidant capacity of Mimosa scabrella Bentham honeydew honeys. Food Res. Int. 2017, 99, 670–678. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Oxygen Radical Absorbing Capacity of Phenolics in Blueberries, Cranberries, Chokeberries, and Lingonberries. J. Agric. Food Chem. 2003, 51, 502–509. [Google Scholar] [CrossRef]
- Moldovan, B.; Hosu, A.; David, L.; Cimpoiu, C. Total Phenolics, Total Anthocyanins, Antioxidant and Pro-oxidant Activity of Some Red Fruits Teas. Acta Chim. Slov. 2015, 63, 213–219. [Google Scholar] [CrossRef][Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Hosu, A.; Cimpoiu, C.; David, L.; Moldovan, B. Study of the antioxidant property variation of cornelian cherry fruits during storage using HPTLC and spectrophotometric assays. J. Anal. Methods Chem. 2016, 2016, 2345375. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Vicente, A.; Gil-Izquierdo, A.; García-Viguera, C. In vitro gastrointestinal digestion study of pomegranate juice phenolic compounds, anthocyanins, and vitamin C. J. Agric. Food Chem. 2002, 50, 2308–2312. [Google Scholar] [CrossRef]
- Kroyer, G. Stevioside and Stevia-sweetener in food: Application, stability and interaction with food ingredients. J. Verbr. Lebensm. 2010, 5, 225–229. [Google Scholar] [CrossRef]
- Rodríguez-Roque, M.J.; de Ancos, B.; Sánchez-Moreno, C.; Cano, M.P.; Elez-Martínez, P.; Martín-Belloso, O. Impact of food matrix and processing on the in vitro bioaccessibility of vitamin C, phenolic compounds, and hydrophilic antioxidant activity from fruit juice-based beverages. J. Funct. Foods 2015, 14, 33–43. [Google Scholar] [CrossRef]
- Cilla, A.; Perales, S.; Lagarda, M.J.; Barberá, R.; Clemente, G.; Farré, R. Influence of storage and in vitro gastrointestinal digestion on total antioxidant capacity of fruit beverages. J. Food Compos. Anal. 2011, 24, 87–94. [Google Scholar] [CrossRef]
- Hanske, L.; Engst, W.; Loh, G.; Sczesny, S.; Blaut, M.; Braune, A. Contribution of gut bacteria to the metabolism of cyanidin 3-glucoside in human microbiota-associated rats. Br. J. Nutr. 2013, 109, 1433–1441. [Google Scholar] [CrossRef]
- Hana, F.; Yanga, P.; Wang, H.; Fernandes, I.; Mateus, N.; Liu, Y. Digestion and absorption of red grape and wine anthocyanins through the gastrointestinal tract. Trends Food Sci. Technol. 2019, 83, 211–224. [Google Scholar] [CrossRef]
- Liang, L.; Wu, X.; Zhao, T.; Zhao, J.; Li, F.; Zou, Y.; Mao, G.; Yang, L. In vitro bioaccessibility and antioxidant activity of anthocyanins from mulberry (Morus atropurpurea Roxb.) following simulated gastro-intestinal digestion. Food Res. Int. 2012, 46, 76–82. [Google Scholar] [CrossRef]
- Correa-Betanzo, J.; Allen-Vercoe, E.; McDonald, J.; Schroeter, K.; Corredig, N.; Palliyath, G. Stability and biological activity of wild blueberry (Vaccinium angustifolium) polyphenols during simulated in vitro gastrointestinal digestion. Food Chem. 2014, 165, 522–531. [Google Scholar] [CrossRef]
- Liang, Z.; Liang, H.; Guo, Y.; Yang, D. Cyanidin 3-O-galactoside: A Natural Compound with Multiple Health Benefits. Int. J. Mol. Sci. 2021, 22, 2261. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Moon, J.K.; Hur, S.J.; Lee, J. Structural changes in mulberry (Morus Microphylla. Buckl) and chokeberry (Aronia melanocarpa) anthocyanins during simulated in vitro human digestion. Food Chem. 2020, 318, 126449–126458. [Google Scholar] [CrossRef] [PubMed]
- Danciu, V.; Hosu, A.; Cimpoiu, C. Comparative evaluation of antioxidant activity using 1,1-diphenyl-2-picrylhydrazyl and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) methods. JPC Mod. TLC 2016, 29, 306–309. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Verzelloni, E.; Bertolini, D.; Conte, A. In vitro bioaccessibility and antioxidant activity of grape polyphenols. Food Chem. 2010, 120, 599–606. [Google Scholar] [CrossRef]
- Fawole, O.A.; Opara, U.L. Stability of total phenolic concentration and antioxidant capacity of extracts from pomegranate co-products subjected to in vitro digestion. BMC 2016, 16, 358–368. [Google Scholar] [CrossRef]
- Chen, G.L.; Chen, S.G.; Zhao, Y.Y.; Luo, C.X.; Li, J.; Gao, Y.Q. Total phenolic contents of 33 fruits and their antioxidant capacities before and after in vitro digestion. Ind. Crops Prod. 2014, 57, 150–157. [Google Scholar] [CrossRef]
- Wootton-Beard, P.C.; Moran, A.; Ryan, L. Stability of the total antioxidant capacity and total polyphenol content of 23 commercially available vegetable juices before and after in vitro digestion measured by FRAP, DPPH, ABTS+ and Folin–Ciocalteu methods. Food Res. Int. 2011, 44, 217–224. [Google Scholar] [CrossRef]
- Bender, C.; Killermann, K.V.; Rehmann, D.; Weidlich, H.H. Effect of Stevia rebaudiana Bert. Addition on the Antioxidant Activity of Red Raspberry (Rubusidaeus, L.) Juices. Beverages 2018, 4, 52. [Google Scholar] [CrossRef]
- Phillips, M.K.; Carlsen, M.H.; Blomhoff, R. Total Antioxidant Content of Alternatives to Refined Sugar. J. Acad. Nutr. Diet. 2009, 109, 64–71. [Google Scholar] [CrossRef]


| Lingonberry Jam | Vitamin C (mg/g jam) | TAC (mg Cy-3-gly/g Jam) | AC (μmols Trolox/g Jam) | ACFRAP (mg Vitamin C/g Jam) |
|---|---|---|---|---|
| Jam 1 (white sugar) | 12.85 ± 0.49 a | 0.212 ± 0.001 a | 56.2 ± 2.0 a | 5.39 ± 0.10 a |
| Jam 2 (fructose) | 13.37 ± 0.85 b | 0.254 ± 0.002 a,b | 51.4 ± 0.4 b | 5.64 ± 0.08 b |
| Jam 3 (erythritol) | 9.92 ± 0.15 a,b | 0.164 ± 0.003 a,b,c | 45.3 ± 2.7 a | 4.64 ± 0.02 a,b,c |
| Jam 4 (brown sugar) | 12.50 ± 0.15 b,c | 0.214 ± 0.009 b,c,d | 57.3 ± 6.6 c | 5.36 ± 0.06 c |
| Jam 5 (coconut sugar) | 10.31 ± 0.89 d | 0.204 ± 0.007 b,c | 72.4 ± 1.3 a,b | 7.82 ± 0.10 a,b,c |
| Jam 6 (stevia) | 15.51 ± 0.15 a,b,c,d | 0.261 ± 0.027 c | 93.8 ± 1.1 a,b,c | 9.37 ± 0.23 a,b,c |
| Jam 7 (saccharine) | 13.57 ± 0.03 b,c,d | 0.263 ± 0.005 a,c,d | 72.1 ± 0.2 a,b | 8.85 ± 0.68 a,b,c |
| Gastric Digestion | GI Digestion | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Anthocyanins | TAC | Cy-3-gal | Cy-3-glu | Cy-3-ara | TAC | Cy-3-gal | Cy-3-glu | Cy-3-ara | |
| Jam | |||||||||
| Jam 1 | 54.8 a | 69.2 a | 72.9 a | 65.9 a | 36.1 a | 45.1 a | 46.1 a | 41.8 a | |
| Jam 2 | 64.6 a,d | 61.3 a,d | 65.9 a,d | 66.6 a,d | 36.5 b | 47.5 b | 43.8 b | 56.4 b | |
| Jam 3 | 56.7 b | 68.9 b | 74.5 b | 66.9 b | 16.4 a,b,c | 27.7 a,b,c | 30.1 a,b,c | 25.9 | |
| Jam 4 | 57.9 c | 60.2 c | 63.7 c | 57.2 c | 22.2 a,b,c,d | 26.2 a,b,c,d | 29.1 a,b,c,d | 24.2 a,b,c,d | |
| Jam 5 | 40.9 a,b,c,d | 50.9 a,b,c,d | 45.5 a,b,c,d | 50.9 a,b,c,d | 16.3 a,d | 10.3 a,d | 8.1 a,d | 10.9 a,d | |
| Jam 6 | 52.9 d | 46.7 d | 47.4 d | 42.9 d | 13.7 a,b,d | 8.9 a,b,d | 9.7 a,b,d | 8.7 a,b,d | |
| Jam 7 | 49.2 d | 48.8 d | 50.6 d | 46.4 d | 18.9 a,b,d | 6.4 a,b,d | 6.6 a,b,d | 8.3 a,b,d | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scrob, T.; Hosu, A.; Cimpoiu, C. Sweeteners from Different Lingonberry Jams Influence on Bioaccessibility of Vitamin C, Anthocyanins and Antioxidant Capacity under In Vitro Gastrointestinal Digestion. Antioxidants 2022, 11, 442. https://doi.org/10.3390/antiox11030442
Scrob T, Hosu A, Cimpoiu C. Sweeteners from Different Lingonberry Jams Influence on Bioaccessibility of Vitamin C, Anthocyanins and Antioxidant Capacity under In Vitro Gastrointestinal Digestion. Antioxidants. 2022; 11(3):442. https://doi.org/10.3390/antiox11030442
Chicago/Turabian StyleScrob, Teodora, Anamaria Hosu, and Claudia Cimpoiu. 2022. "Sweeteners from Different Lingonberry Jams Influence on Bioaccessibility of Vitamin C, Anthocyanins and Antioxidant Capacity under In Vitro Gastrointestinal Digestion" Antioxidants 11, no. 3: 442. https://doi.org/10.3390/antiox11030442
APA StyleScrob, T., Hosu, A., & Cimpoiu, C. (2022). Sweeteners from Different Lingonberry Jams Influence on Bioaccessibility of Vitamin C, Anthocyanins and Antioxidant Capacity under In Vitro Gastrointestinal Digestion. Antioxidants, 11(3), 442. https://doi.org/10.3390/antiox11030442







