Supplementation with a Cocoa–Carob Blend, Alone or in Combination with Metformin, Attenuates Diabetic Cardiomyopathy, Cardiac Oxidative Stress and Inflammation in Zucker Diabetic Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Cocoa–Carob Blend Diet
2.3. Animals and Experimental Design
2.4. Biochemical Determination
2.5. Glucose Tolerance Test
2.6. Echocardiographic Measurements
2.7. Histological and Immunohistochemical Analyses
2.8. Determination of ROS
2.9. Determination of Protein Carbonyl Content
2.10. GSH Levels and GPx and GR Activities
2.11. Statistical Analysis
3. Results
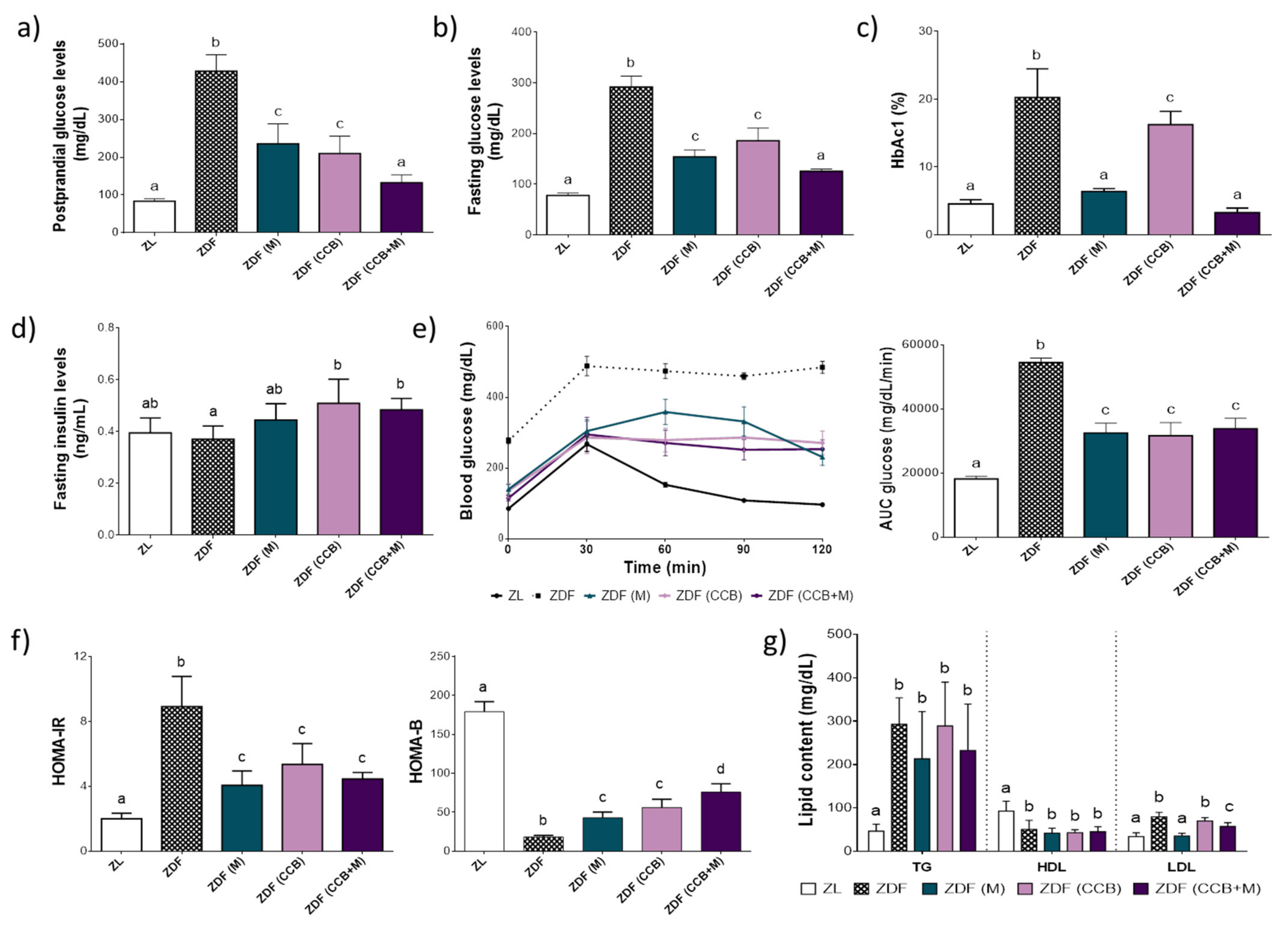
3.1. Effect of CCB Alone or in Combination with Metformin on Glucose Homeostasis and Lipid Profile in Zucker Diabetic Rats
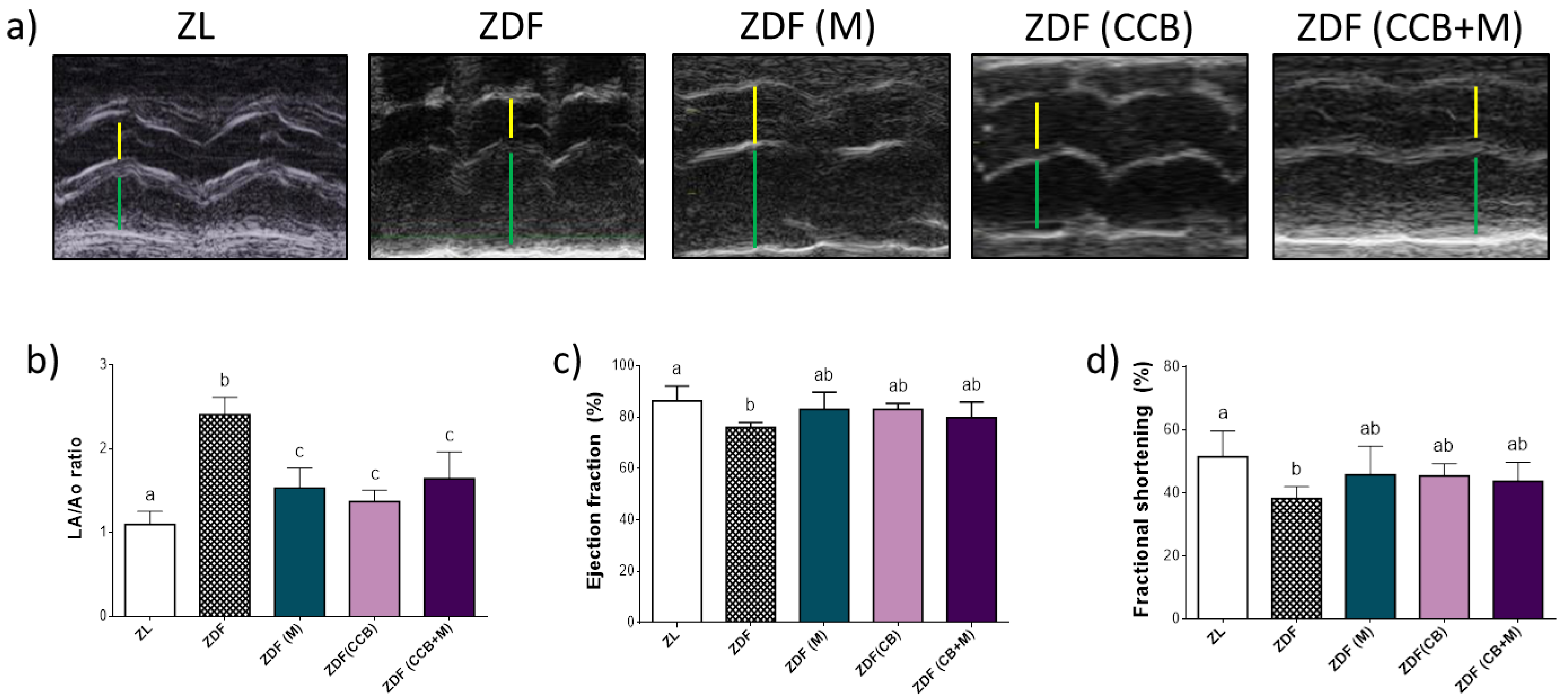
3.2. Effect of CCB, Metformin and Their Combination on Cardiac Function in Zucker Diabetic Rats
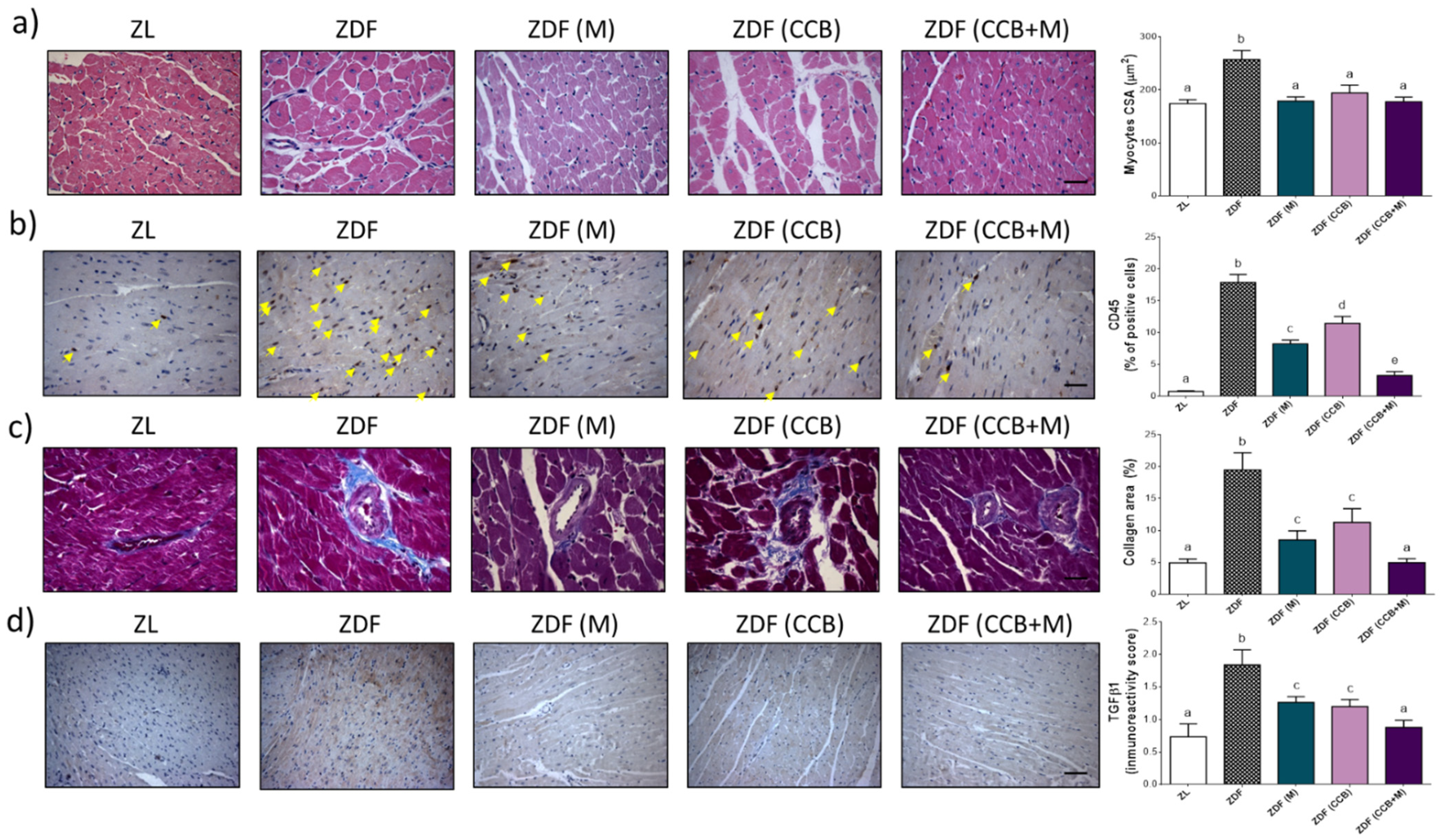
3.3. Effect of CCB, Metformin and Their Combination on LV Remodelling in Zucker Diabetic Rats
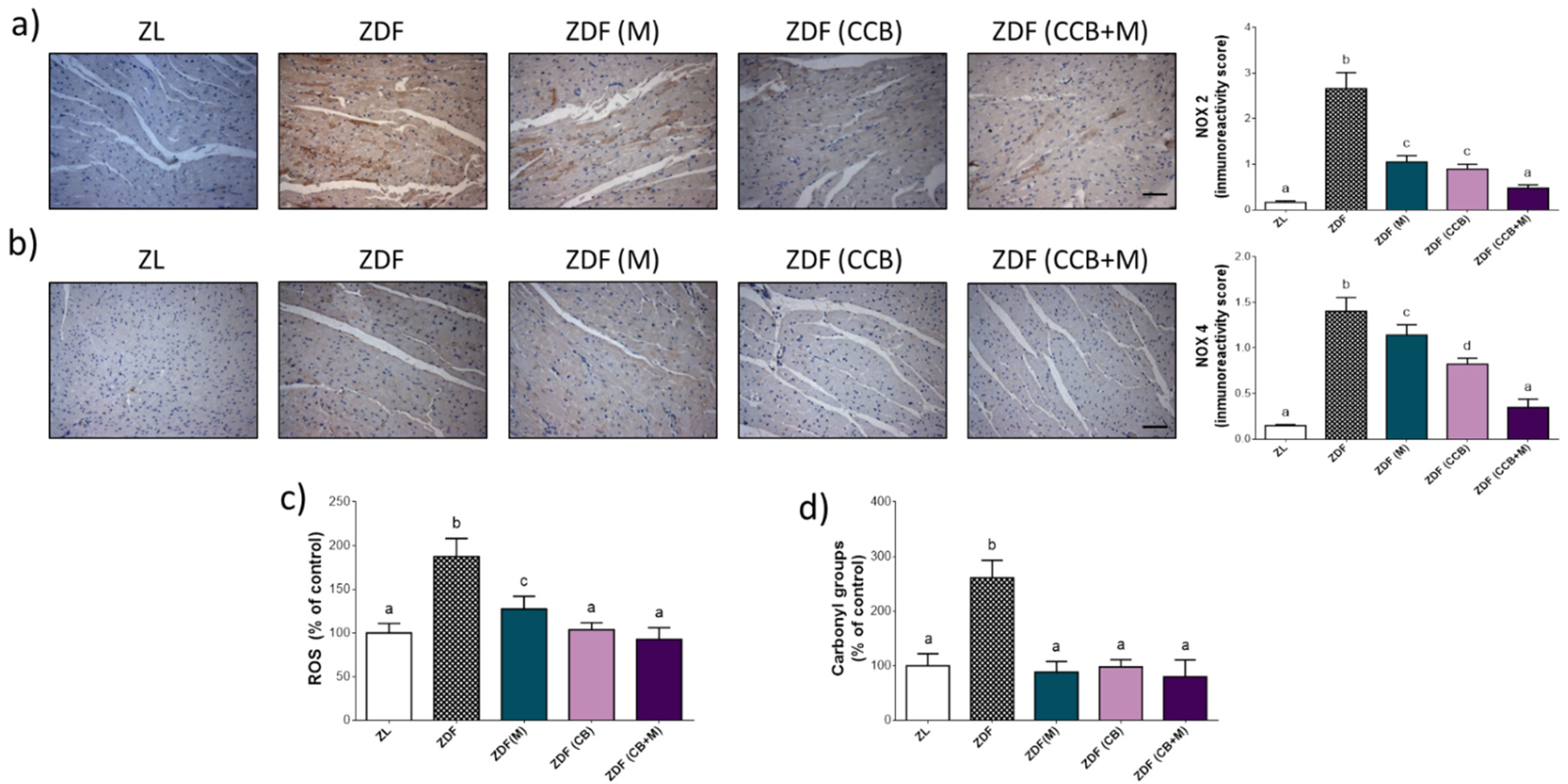
3.4. Effect of CCB, Alone or in Combination with Metformin on Cardiac Oxidative Stress in Zucker Diabetic Rats
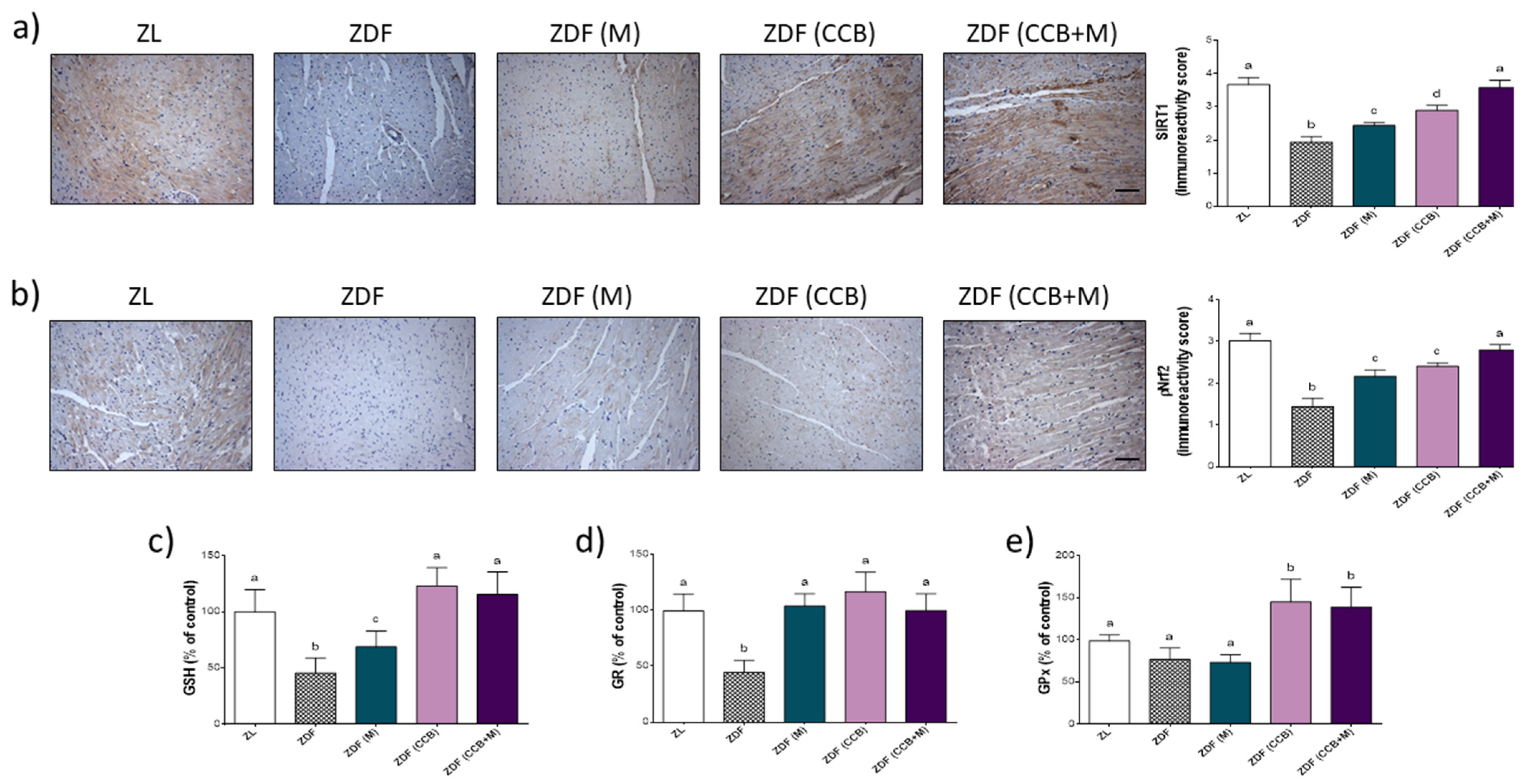
3.5. Effect of CCB and Their Combination with Metformin on SIRT/Nrf2 Pathway and Antioxidant Response in Zucker Diabetic Rats
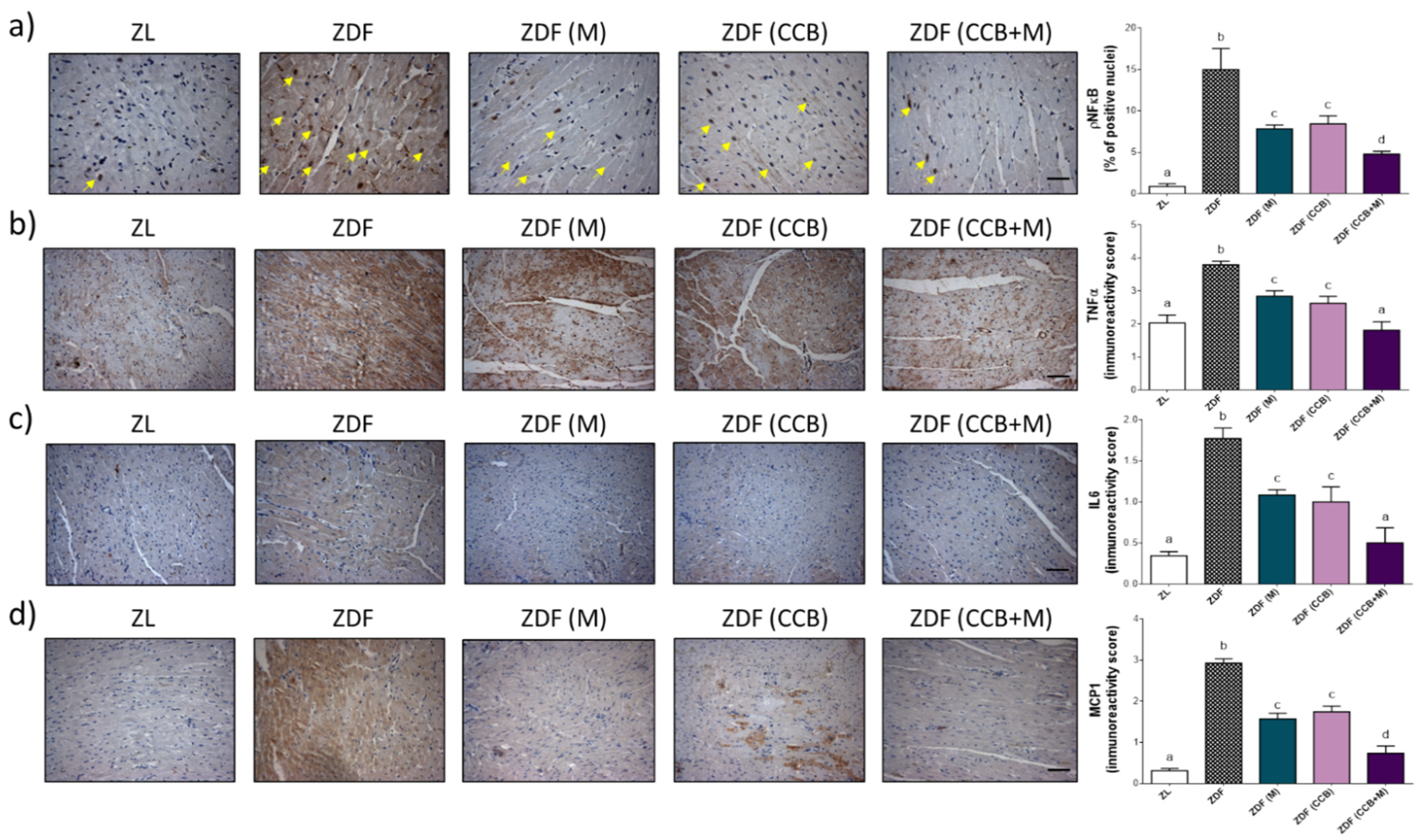
3.6. Effect of CCB, Metformin and Their Combination on Myocardial Inflammation in Zucker Diabetic Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; Available online: https://www.diabetesatlas.org (accessed on 16 February 2022).
- Murtaza, G.; Virk, H.U.H.; Khalid, M.; Lavie, C.J.; Ventura, H.; Mukherjee, D.; Bhogal, S.; Kumar, G.; Shanmugasundaram, M.; Paul, T.K. Diabetic Cardiomyopathy-A Comprehensive Updated Review. Prog. Cardiovasc. Dis. 2019, 62, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Paolillo, S.; Marsico, F.; Prastaro, M.; Renga, F.; Esposito, L.; De Martino, F.; Di Napoli, P.; Esposito, I.; Ambrosio, A.; Ianniruberto, M.; et al. Diabetic Cardiomyopathy: Definition, Diagnosis, and Therapeutic Implications. Heart Fail. Clin. 2019, 15, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.J.; Gill, E.K.; Abudalo, R.A.; Edgar, K.S.; Watson, C.J.; Grieve, D.J. Reactive oxygen species signalling in the diabetic heart: Emerging prospect for therapeutic targeting. Heart 2018, 104, 293–299. [Google Scholar] [CrossRef]
- Faria, A.; Persaud, S.J. Cardiac oxidative stress in diabetes: Mechanisms and therapeutic potential. Pharmacol. Ther. 2017, 172, 50–62. [Google Scholar] [CrossRef] [Green Version]
- Teshima, Y.; Takahashi, N.; Nishio, S.; Saito, S.; Kondo, H.; Fukui, A.; Aoki, K.; Yufu, K.; Nakagawa, M.; Saikawa, T. Production of reactive oxygen species in the diabetic heart: Roles of mitochondria and NADPH oxidase. Circ. J. 2014, 78, 300–306. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Guo, W.; Shang, F.; Li, Y.; Li, W.; Liu, J.; Ma, C.; Teng, J. Bakuchiol Alleviates Hyperglycemia-Induced Diabetic Cardiomyopathy by Reducing Myocardial Oxidative Stress via Activating the SIRT1/Nrf2 Signaling Pathway. Oxid. Med. Cell. Longev. 2020, 2020, 3732718. [Google Scholar] [CrossRef]
- Jin, Q.; Zhu, Q.; Wang, K.; Chen, M.; Li, X. Allisartan isoproxil attenuates oxidative stress and inflammation through the SIRT1/Nrf2/NF-κB signalling pathway in diabetic cardiomyopathy rats. Mol. Med. Rep. 2021, 23, 205. [Google Scholar] [CrossRef]
- Li, L.; Luo, W.; Qian, Y.; Zhu, W.; Qian, J.; Li, J.; Jin, Y.; Xu, X.; Liang, G. Luteolin protects against diabetic cardiomyopathy by inhibiting NF-kappaB mediated inflammation and activating the Nrf2-mediated antioxidant responses. Phytomedicine 2019, 59, 152774. [Google Scholar] [CrossRef]
- Sheida, A.; Taghavi, T.; Shafabakhsh, R.; Ostadian, A.; Razaghi Bahabadi, Z.; Khaksary Mahabady, M.; Hamblin, M.R.; Mirzaei, H. Potential of natural products in the treatment of myocardial infarction: Focus on molecular mechanisms. Crit. Rev. Food Sci. Nutr. 2022, 3, 1. [Google Scholar] [CrossRef]
- Yao, Y.; Goh, H.M.; Kim, J.E. The Roles of Carotenoid Consumption and Bioavailability in Cardiovascular Health. Antioxidants 2021, 11, 10. [Google Scholar] [CrossRef]
- Grassi, D.; Necozione, S.; Desideri, G.; Abballe, S.; Mai, F.; De Feo, M.; Carducci, A.; Ferri, C. Acute and Long Term Effects of a Nutraceutical Combination on Lipid Profile, Glucose Metabolism and Vascular Function in Patients with Dyslipidaemia with and Without Cigarette Smoking. High Blood Press. Cardiovasc. Prev. 2021, 28, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Blahova, J.; Martiniakova, M.; Babikova, M.; Kovacova, V.; Mondockova, V.; Omelka, R. Pharmaceutical Drugs and Natural Therapeutic Products for the Treatment of Type 2 Diabetes Mellitus. Pharmaceuticals 2021, 14, 806. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Deb, P.K.; Priya, S.; Medina, K.D.; Devi, R.; Walode, S.G.; Rudrapal, M. Dietary Flavonoids: Cardioprotective Potential with Antioxidant Effects and Their Pharmacokinetic, Toxicological and Therapeutic Concerns. Molecules 2021, 26, 4021. [Google Scholar] [CrossRef]
- Jubaidi, F.F.; Zainalabidin, S.; Taib, I.S.; Hamid, Z.A.; Budin, S.B. The Potential Role of Flavonoids in Ameliorating Diabetic Cardiomyopathy via Alleviation of Cardiac Oxidative Stress, Inflammation and Apoptosis. Int. J. Mol. Sci. 2021, 22, 5094. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S.; Martín, M.A.; Goya, L. Effects of Cocoa Antioxidants in Type 2 Diabetes Mellitus. Antioxidants 2017, 31, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, M.A.; Goya, L.; Ramos, S. Antidiabetic actions of cocoa flavanols. Mol. Nutr. Food Res. 2016, 60, 1756–1769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferri, C.; Desideri, G.; Ferri, L.; Proietti, I.; Di Agostino, S.; Martella, L.; Mai, F.; Di Giosia, P.; Grassi, D. Cocoa, blood pressure, and cardiovascular health. J. Agric. Food Chem. 2015, 63, 9901. [Google Scholar] [CrossRef]
- Álvarez-Cilleros, D.; López-Oliva, M.E.; Morales-Cano, D.; Barreira, B.; Pérez-Vizcaíno, F.; Goya, L.; Ramos, S.; Martín, M.A. Dietary Cocoa Prevents Aortic Remodeling and Vascular Oxidative Stress in Diabetic Rats. Mol. Nutr. Food Res. 2019, 63, 1900044. [Google Scholar] [CrossRef]
- Zięba, K.; Makarewicz-Wujec, M.; Kozłowska-Wojciechowska, M. Cardioprotective Mechanisms of Cocoa. J. Am. Coll. Nutr. 2019, 38, 564–575. [Google Scholar] [CrossRef]
- Akdeniz, E.; Yakışık, E.; Rasouli Pirouzian, H.; Akkın, S.; Turan, B.; Tipigil, E.; Toker, O.S.; Ozcan, O. Carob powder as cocoa substitute in milk and dark compound chocolate formulation. J. Food Sci. Technol. 2021, 58, 4558–4566. [Google Scholar] [CrossRef]
- Qasem, M.A.; Noordin, M.I.; Arya, A.; Alsalahi, A.; Jayash, S.N. Evaluation of the glycemic effect of Ceratonia siliqua pods (Carob) on a streptozotocin-nicotinamide induced diabetic rat model. PeerJ 2018, 6, e4788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brassesco, M.L.; Brandão, T.R.S.; Silva, C.L.M.; Pintado, M. Carob bean (Ceratonia siliqua L.): A new perspective for functional food. Trends Food Sci. Technol. 2021, 114, 310–322. [Google Scholar] [CrossRef]
- De la Fuente-Fernández, M.; González-Hedström, D.; Amor, S.; Tejera-Muñoz, A.; Fernández, N.; Monge, L.; Almodóvar, P.; Andrés-Delgado, L.; Santamaría, L.; Prodanov, M.; et al. Supplementation with a Carob (Ceratonia siliqua L.) Fruit Extract Attenuates the Cardiometabolic Alterations Associated with Metabolic Syndrome in Mice. Antioxidants 2020, 21, 339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1–64. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Millán, E.; Cordero-Herrera, I.; Ramos, S.; Escrivá, F.; Alvarez, C.; Goya, L.; Martın, M.A. Cocoa-rich diet attenuates beta cell mass loss and function in young Zucker diabetic fatty rats by preventing oxidative stress and beta cell apoptosis. Mol. Nutr. Food Res. 2015, 59, 820. [Google Scholar] [CrossRef] [Green Version]
- Kayacan, S.; Yilancioglu, K.; Akdemir, A.S.; Kaya-Dagistanli, F.; Melikoglu, G.; Ozturk, M. Synergistic Effect of Apigenin and Curcumin on Apoptosis, Paraptosis and Autophagy-related Cell Death in HeLa Cells. Anticancer Res. 2021, 41, 1271–1282. [Google Scholar] [CrossRef]
- Liu, H.; Wang, L.; Li, F.; Jiang, Y.; Guan, H.; Wang, D.; Sun-Waterhouse, D.; Wu, M.; Li, D. The synergistic protection of EGCG and quercetin against streptozotocin (STZ)-induced NIT-1 pancreatic β cell damage via upregulation of BCL-2 expression by miR-16-5p. J. Nutr. Biochem. 2021, 96, 108748. [Google Scholar] [CrossRef]
- Nie, T.; Cooper, G.J.S. Mechanisms Underlying the Antidiabetic Activities of Polyphenolic Compounds: A Review. Front. Pharmacol. 2021, 12, 798329. [Google Scholar] [CrossRef]
- Bartella, L.; Di Donna, L.; Napoli, A.; Siciliano, C.; Sindona, G.; Mazzotti, F. A rapid method for the assay of methylxanthines alkaloids: Theobromine, theophylline and caffeine, in cocoa products and drugs by paper spray tandem mass spectrometry. Food Chem. 2019, 278, 261–266. [Google Scholar] [CrossRef]
- Stavrou, I.J.; Christou, A.; Kapnissi-Christodoulou, C.P. Polyphenols in carobs: A review on their composition, antioxidant capacity and cytotoxic effects, and health impact. Food Chem. 2018, 269, 355–374. [Google Scholar] [CrossRef]
- Antonowski, T.; Osowski, A.; Lahuta, L.; Górecki, R.; Rynkiewicz, A.; Wojtkiewicz, J. Health-Promoting Properties of Selected Cyclitols for Metabolic Syndrome and Diabetes. Nutrients 2019, 11, 2314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evangelista, I.; Nuti, R.; Picchioni, T.; Dotta, F.; Palazzuoli, A. Molecular Dysfunction and Phenotypic Derangement in Diabetic Cardiomyopathy. Int. J. Mol. Sci. 2019, 20, 3264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, I.; Frangogiannis, N.G. Diabetes-associated cardiac fibrosis: Cellular effectors, molecular mechanisms and therapeutic opportunities. J. Mol. Cell. Cardiol. 2016, 90, 84–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alex, L.; Russo, I.; Holoborodko, V.; Frangogiannis, N.G. Characterization of a mouse model of obesity-related fibrotic cardiomyopathy that recapitulates features of human heart failure with preserved ejection fraction. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H934–H949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.W.; Wang, X.Y.; Zhang, X.; Gao, L.; Wang, L.F.; Yin, X.H. (–)-Epicatechin protects against myocardial ischemia-induced cardiac injury via activation of the PTEN/PI3K/AKT pathway. Mol. Med. Rep. 2018, 17, 8300–8308. [Google Scholar] [CrossRef] [Green Version]
- Sari, N.; Katanasaka, Y.; Honda, H.; Miyazaki, Y.; Sunagawa, Y.; Funamoto, M.; Shimizu, K.; Shimizu, S.; Wada, H.; Hasegawa, K.; et al. Cacao Bean Polyphenols Inhibit Cardiac Hypertrophy and Systolic Dysfunction in Pressure Overload-induced Heart Failure Model Mice. Planta Med. 2020, 86, 1304–1312. [Google Scholar] [CrossRef]
- Valero-Muñoz, M.; Martín-Fernández, B.; Ballesteros, S.; Lahera, V.; De las Heras, N. Carob pod insoluble fiber exerts anti-atherosclerotic effects in rabbits through sirtuin-1 and peroxisome proliferator-activated receptor-γ coactivator-1α. J. Nutr. 2014, 144, 1378–1384. [Google Scholar] [CrossRef] [Green Version]
- El Hayek, M.S.; Ernande, L.; Benitah, J.P.; Gomez, A.M.; Pereira, L. The role of hyperglycaemia in the development of diabetic cardiomyopathy. Arch. Cardiovasc. Dis. 2021, 114, 748–760. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, Y.; Liu, H.; Li, P.; Zhang, H.; Cheng, G. Fortunellin protects against high fructose-induced diabetic heart injury in mice by suppressing inflammation and oxidative stress via AMPK/Nrf-2 pathway regulation. Biochem. Biophys. Res. Commun. 2017, 490, 552–559. [Google Scholar] [CrossRef]
- Althunibat, O.Y.; Al Hroob, A.M.; Abukhalil, M.H.; Germoush, M.O.; Bin-Jumah, M.; Mahmoud, A.M. Fisetin ameliorates oxidative stress, inflammation and apoptosis in diabetic cardiomyopathy. Life Sci. 2019, 221, 83–92. [Google Scholar] [CrossRef]
- Jia, Q.; Wang, Y.; Liu, X.; Ma, S.; Yang, R. Effects of genistein on Nrf2/HO-1 pathway in myocardial tissues of diabetic rats. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2019, 44, 850–856. [Google Scholar] [PubMed]
- Liao, H.H.; Zhu, J.X.; Feng, H.; Ni, J.; Zhang, N.; Chen, S.; Liu, H.J.; Yang, Z.; Deng, W.; Tang, Q.Z. Myricetin Possesses Potential Protective Effects on Diabetic Cardiomyopathy through Inhibiting IkB/NFkB and Enhancing Nrf2/HO-1. Oxid. Med. Cell. Longev. 2017, 2017, 8370593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, Z.-D.; Lian, Q.; Mao, X.; Xia, Z. Current Status and Challenges of NRF2 as a Potential Therapeutic Target for Diabetic Cardiomyopathy. Int. Heart J. 2019, 60, 512–520. [Google Scholar] [CrossRef] [Green Version]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Crosstalk between oxidative stress and SIRT1: Impact on the aging process. Int. J. Mol. Sci. 2013, 14, 3834–3859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laddha, A.P.; Kulkarni, Y.A. Daidzein mitigates myocardial injury in streptozotocin-induced diabetes in rats. Life Sci. 2021, 284, 119664. [Google Scholar] [CrossRef]
- Ren, B.C.; Zhang, Y.F.; Liu, S.S.; Cheng, X.J.; Yang, X.; Cui, X.G.; Zhao, X.R.; Zhao, H.; Hao, M.F.; Li, M.D.; et al. Curcumin alleviates oxidative stress and inhibits apoptosis in diabetic cardiomyopathy via Sirt1-Foxo1 and PI3K-Akt signalling pathways. J. Cell. Mol. Med. 2020, 24, 12355–12367. [Google Scholar] [CrossRef]
- Testai, L.; Piragine, E.; Piano, I.; Flori, L.; Da Pozzo, E.; Miragliotta, V.; Pirone, A.; Citi, V.; Di Cesare Mannelli, L.; Brogi, S.; et al. The citrus flavonoid naringenin protects the myocardium from ageing-dependent dysfunction: Potential role of SIRT1. Oxid. Med. Cell Longev. 2020, 2020, 4650207. [Google Scholar] [CrossRef] [Green Version]
- Dong, Z.; Wan, L.; Wang, R.; Shi, Y.; Liu, G.; Zheng, S.; Hou, H.; Han, W.; Hai, X. (–)-Epicatechin Suppresses Angiotensin II-induced Cardiac Hypertrophy via the Activation of the SP1/SIRT1 Signaling Pathway. Cell. Physiol. Biochem. 2017, 41, 2004–2015. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-J.; Paneni, F.; Stein, S.; Matter, C.M. Modulating Sirtuin Biology and Nicotinamide Adenine Diphosphate Metabolism in Cardiovascular Disease—From Bench to Bedside. Front. Physiol. 2021, 12, 755060. [Google Scholar] [CrossRef]
- Hong, Y.; Wu, W.; Song, F.; Wu, C.; Li, G.; Wang, Y. Cardiac senescence is alleviated by the natural flavone acacetin via enhancing mitophagy. Aging 2021, 13, 16381–16403. [Google Scholar] [CrossRef]
- Han, W.-M.; Chen, X.-C.; Li, G.-R.; Wang, Y. Acacetin Protects Against High Glucose-Induced Endothelial Cells Injury by Preserving Mitochondrial Function via Activating Sirt1/Sirt3/AMPK Signals. Front. Pharmacol. 2020, 11, 607796. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, A.; Pillai, V.B.; Samant, S.; Gupta, M.; Gupta, M.P. The nuclear and mitochondrial sirtuins, Sirt6 and Sirt3, regulate each other’s activity and protect the heart from developing obesity-mediated diabetic cardiomyopathy. FASEB J. 2019, 33, 10872–10888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishida, K.; Otsu, K. Inflammation and metabolic cardiomyopathy. Cardiovasc. Res. 2017, 113, 389–398. [Google Scholar] [CrossRef] [Green Version]
- Frieler, R.A.; Mortensen, R.M. Immune cell and other noncardiomyocyte regulation of cardiac hypertrophy and remodelling. Circulation 2015, 131, 1019–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abukhalil, M.H.; Althunibat, O.Y.; Aladaileh, S.H.; Al-Amarat, W.; Obeidat, H.M.; Al-Khawalde, A.A.A.; Hussein, O.E.; Alfwuaires, M.A.; Algefare, A.I.; Alanazi, K.M.; et al. Galangin attenuates diabetic cardiomyopathy through modulating oxidative stress, inflammation, and apoptosis in rats. Biomed. Pharmacother. 2021, 138, 111410. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmed, N.; Rungatscher, A.; Linardi, D.; Kulsoom, B.; Innamorati, G.; Meo, S.A.; Gebrie, M.A.; Mani, R.; Merigo, F.; et al. Cocoa Flavonoids Reduce Inflammation and Oxidative Stress in a Myocardial Ischemia-Reperfusion Experimental Model. Antioxidants 2020, 9, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagul, P.K.; Deepthi, N.; Sultana, R.; Banerjee, S.K. Resveratrol ameliorates cardiac oxidative stress in diabetes through deacetylation of NFkB-p65 and histone 3. J. Nutr. Biochem. 2015, 26, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
| Component (g/Kg Dry Weight) | Control | Cocoa–Carob Blend |
|---|---|---|
| Casein | 140 | 140 |
| Dextrose | 155 | 155 |
| Sucrose | 100 | 92 |
| Fat | 40 | 40 |
| t-BHQ (tert-butylhydroquinone) | 0.008 | 0.008 |
| Mineral mix. | 35 | 35 |
| Vitamin mix. | 10 | 10 |
| L-Cys | 1.8 | 1.8 |
| Cholin bitartrate | 2.5 | 2.5 |
| Cellulose | 100 | 44.5 |
| Starch | 415.7 | 379 |
| Cocoa–carob powder | - | 100 |
| Energy (KJ/Kg diet) | 15048 | 15048 |
| ZL | ZDF | ZDF (M) | ZDF (CCB) | ZDF (CCB + M) | |
|---|---|---|---|---|---|
| Initial Body weight (g) | 278 ± 9 a | 340 ± 11 b | 341 ± 20 b | 337 ± 26 b | 338 ± 23 b |
| Final Body weight (g) | 377 ± 17 a | 393 ± 13 ab | 423 ± 38 b | 384 ± 26 ab | 411 ± 30 ab |
| Body weight gain (g) | 98 ± 10 a | 55 ± 8 b | 93 ± 17 a | 63 ± 5 b | 74 ± 14 c |
| Total food intake (g in 12 weeks) | 1547 ± 61 a | 2491 ± 38 b | 2390 ± 44 b | 2466 ± 39 b | 2372 ± 48 b |
| Food Efficiency (body weight gain/total food intake) (%) | 6.4 ± 0.6 a | 2.2 ± 0.3 b | 3.9 ± 0.7 c | 2.5 ± 0.2 bd | 3.1 ± 0.6 d |
| ZL | ZDF | ZDF (M) | ZDF (CCB) | ZDF (CCB + M) | |
|---|---|---|---|---|---|
| HW/TL (g/cm) | 0.32 ± 0.02 a | 0.32 ± 0.01 a | 0.35 ± 0.03 a | 0.26 ± 0.01 b | 0.31 ± 0.01 a |
| LVDD (cm) | 0.73 ± 0.05 a | 0.76 ± 0.05 a | 0.75 ± 0.08 a | 0.65 ± 0.05 a | 0.68 ± 0.13 a |
| LVPWD (cm) | 0.22 ± 0.08 a | 0.20 ±0.05 a | 0.20 ± 0.05 a | 0.19 ± 0.04 a | 0.21 ± 0.04 a |
| LVSD (cm) | 0.40 ± 0.06 a | 0.46 ± 0.05 a | 0.38 ± 0.07 a | 0.39 ± 0.06 a | 0.39 ± 0.10 a |
| IVSD (cm) | 0.13 ± 0.05 a | 0.14 ± 0.05 a | 0.15 ± 0.05 a | 0.14 ± 0.05 a | 0.15 ± 0.05 a |
| Ao diameter (cm) | 0.33 ± 0.05 a | 0.23 ± 0.05 b | 0.32 ± 0.04 ab | 0.28 ± 0.08 ab | 0.28 ± 0.04 ab |
| LA diameter (cm) | 0.37 ± 0.08 a | 0.57 ± 0.08 b | 0.50 ± 0.05 b | 0.43 ± 0.07 ab | 0.49 ± 0.04 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Díez, E.; López-Oliva, M.E.; Caro-Vadillo, A.; Pérez-Vizcaíno, F.; Pérez-Jiménez, J.; Ramos, S.; Martín, M.Á. Supplementation with a Cocoa–Carob Blend, Alone or in Combination with Metformin, Attenuates Diabetic Cardiomyopathy, Cardiac Oxidative Stress and Inflammation in Zucker Diabetic Rats. Antioxidants 2022, 11, 432. https://doi.org/10.3390/antiox11020432
García-Díez E, López-Oliva ME, Caro-Vadillo A, Pérez-Vizcaíno F, Pérez-Jiménez J, Ramos S, Martín MÁ. Supplementation with a Cocoa–Carob Blend, Alone or in Combination with Metformin, Attenuates Diabetic Cardiomyopathy, Cardiac Oxidative Stress and Inflammation in Zucker Diabetic Rats. Antioxidants. 2022; 11(2):432. https://doi.org/10.3390/antiox11020432
Chicago/Turabian StyleGarcía-Díez, Esther, María Elvira López-Oliva, Alicia Caro-Vadillo, Francisco Pérez-Vizcaíno, Jara Pérez-Jiménez, Sonia Ramos, and María Ángeles Martín. 2022. "Supplementation with a Cocoa–Carob Blend, Alone or in Combination with Metformin, Attenuates Diabetic Cardiomyopathy, Cardiac Oxidative Stress and Inflammation in Zucker Diabetic Rats" Antioxidants 11, no. 2: 432. https://doi.org/10.3390/antiox11020432
APA StyleGarcía-Díez, E., López-Oliva, M. E., Caro-Vadillo, A., Pérez-Vizcaíno, F., Pérez-Jiménez, J., Ramos, S., & Martín, M. Á. (2022). Supplementation with a Cocoa–Carob Blend, Alone or in Combination with Metformin, Attenuates Diabetic Cardiomyopathy, Cardiac Oxidative Stress and Inflammation in Zucker Diabetic Rats. Antioxidants, 11(2), 432. https://doi.org/10.3390/antiox11020432






