The Importance of SGLT-2 Inhibitors as Both the Prevention and the Treatment of Diabetic Cardiomyopathy
Abstract
1. Introduction
2. Type 2 Diabetes Mellitus and Diabetic Cardiomyopathy
2.1. Epidemiology
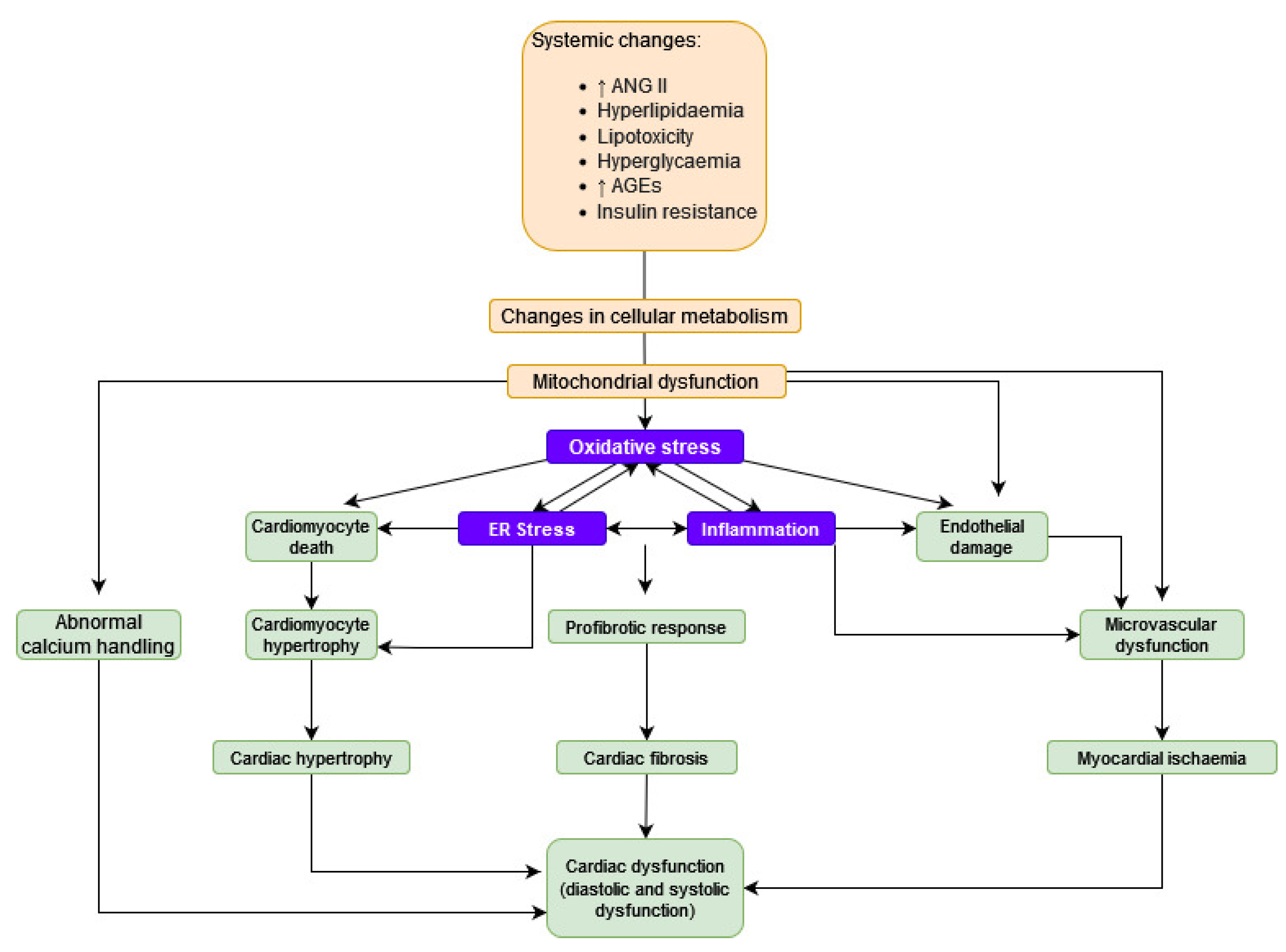
2.2. Pathophysiology
2.2.1. Lipotoxicity
- Diacylglycerol, which leads to exacerbation of insulin resistance and oxidative stress by activation of protein kinase C (PKC);
- Ceramide, which leads to oxidative stress and the dysfunction of mitochondria.
2.2.2. Oxidative Stress
- Abnormal regulation of the electron transport chain in mitochondria;
- Increased nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity;
- Influence of advanced glycation end products (AGEs)
2.2.3. Abnormal Calcium Handling
2.3. Clinical Picture
2.4. Prognosis
2.5. Therapeutical Grip Points
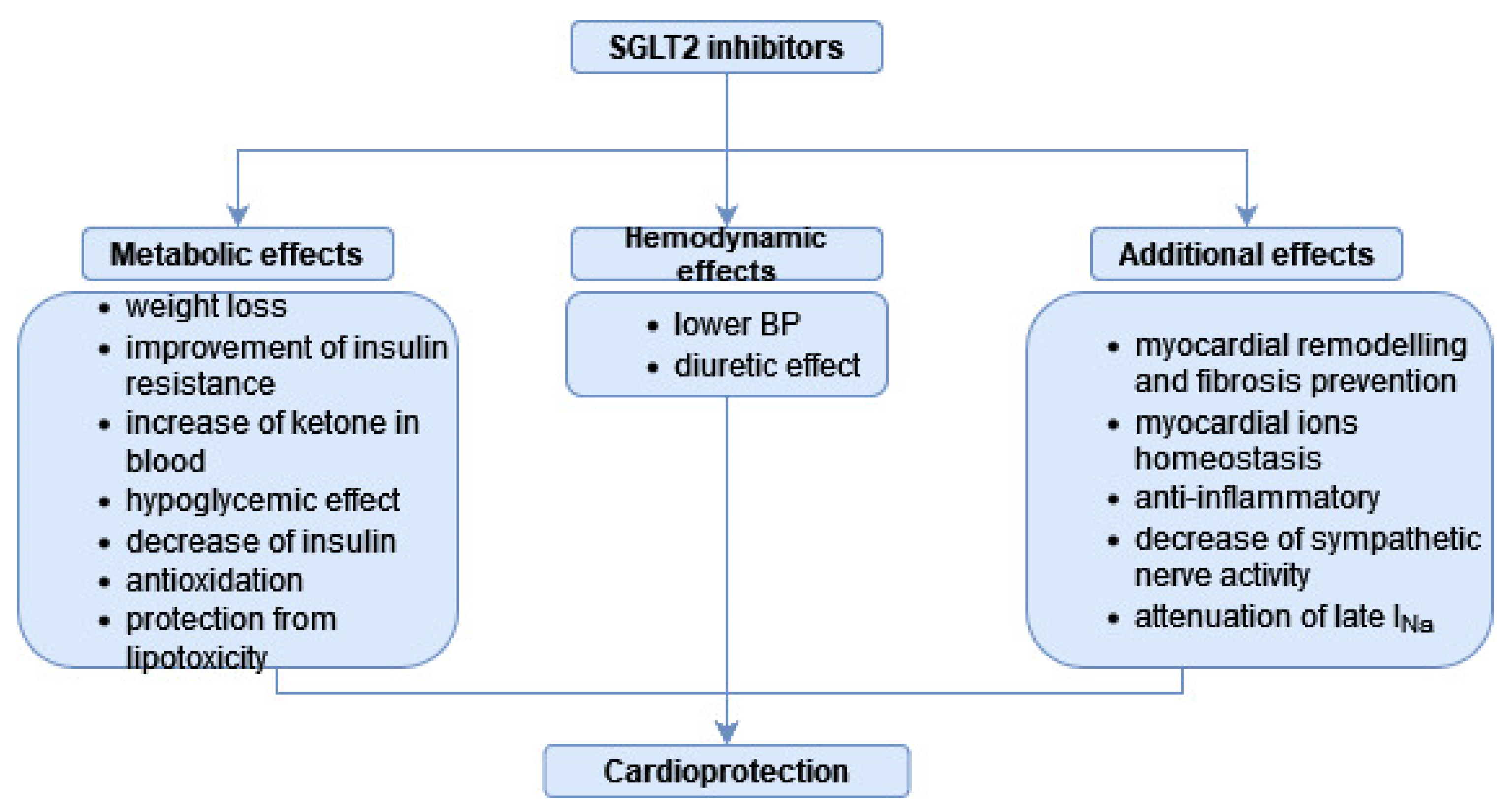
3. SGLT-2is as a Drug Class
4. SGLT-2is in DC—The Overview of Research Results
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kopp, W. How Western Diet and Lifestyle Drive the Pandemic of Obesity and Civilization Diseases. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 2221. [Google Scholar] [CrossRef] [PubMed]
- Cordain, L.; Eades, M.R.; Eades, M.D. Hyperinsulinemic Diseases of Civilization: More than Just Syndrome X. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2003, 136, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The Metabolic Syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.L. A Comprehensive Definition for Metabolic Syndrome. Dis. Model. Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Stegbauer, C.; Falivena, C.; Moreno, A.; Hentschel, A.; Rosenmöller, M.; Heise, T.; Szecsenyi, J.; Schliess, F. Costs and Its Drivers for Diabetes Mellitus Type 2 Patients in France and Germany: A Systematic Review of Economic Studies. BMC Health Serv. Res. 2020, 20, 1043. [Google Scholar] [CrossRef]
- Seuring, T.; Archangelidi, O.; Suhrcke, M. The Economic Costs of Type 2 Diabetes: A Global Systematic Review. Pharmacoeconomics 2015, 33, 811–831. [Google Scholar] [CrossRef]
- National Institute of Public Health. National Institute of Hygiene, Commissions for the Assessment of the Epidemiology of Diabetes in Poland and the Costs of Diabetes and Their Determinants in Poland, Committee for Public Health of the Polish Academy of Sciences, P.P. Summary of the Project: The Prevalence of Diabetes and its Costs to the National Health Fund and to the Patients—A.D. 2017; National Institute of Public Health: Warsaw, Poland, 2019. [Google Scholar]
- Dillmann, W.H. Diabetic Cardiomyopathy. Circ. Res. 2019, 124, 1160–1162. [Google Scholar] [CrossRef]
- Jia, G.; DeMarco, V.G.; Sowers, J.R. Insulin Resistance and Hyperinsulinaemia in Diabetic Cardiomyopathy. Nat. Rev. Endocrinol. 2016, 12, 144–153. [Google Scholar] [CrossRef]
- Penpargkul, S.; Fein, F.; Sonnenblick, E.H.; Scheuer, J. Depressed Cardiac Sarcoplasmic Reticular Function from Diabetic Rats. J. Mol. Cell. Cardiol. 1981, 13, 303–309. [Google Scholar] [CrossRef]
- Dia, M.; Gomez, L.; Thibault, H.; Tessier, N.; Leon, C.; Chouabe, C.; Ducreux, S.; Gallo-Bona, N.; Tubbs, E.; Bendridi, N.; et al. Reduced Reticulum-Mitochondria Ca2+ Transfer Is an Early and Reversible Trigger of Mitochondrial Dysfunctions in Diabetic Cardiomyopathy. Basic Res. Cardiol. 2020, 115, 74. [Google Scholar] [CrossRef]
- Giugliano, D.; Longo, M.; Scappaticcio, L.; Caruso, P.; Esposito, K. Sodium-Glucose Transporter-2 Inhibitors for Prevention and Treatment of Cardiorenal Complications of Type 2 Diabetes. Cardiovasc. Diabetol. 2021, 20, 17. [Google Scholar] [CrossRef] [PubMed]
- Henning, R.J. Type-2 Diabetes Mellitus and Cardiovascular Disease. Future Cardiol. 2018, 14, 491–509. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I.; et al. Type 2 Diabetes Mellitus. Nat. Rev. Dis. Prim. 2015, 1, 15019. [Google Scholar] [CrossRef] [PubMed]
- Tinajero, M.G.; Malik, V.S. An Update on the Epidemiology of Type 2 Diabetes: A Global Perspective. Endocrinol. Metab. Clin. N. Am. 2021, 50, 337–355. [Google Scholar] [CrossRef]
- Martín-Timón, I.; Sevillano-Collantes, C.; Segura-Galindo, A.; Cañizo-Gómez, F.J. del Type 2 Diabetes and Cardiovascular Disease: Have All Risk Factors the Same Strength? World J. Diabetes 2014, 5, 444. [Google Scholar] [CrossRef]
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Prevalence of Cardiovascular Disease in Type 2 Diabetes: A Systematic Literature Review of Scientific Evidence from across the World in 2007–2017. Cardiovasc. Diabetol. 2018, 17, 83. [Google Scholar] [CrossRef]
- Raghavan, S.; Vassy, J.L.; Ho, Y.L.; Song, R.J.; Gagnon, D.R.; Cho, K.; Wilson, P.W.F.; Phillips, L.S. Diabetes Mellitus-Related All-Cause and Cardiovascular Mortality in a National Cohort of Adults. J. Am. Heart Assoc. 2019, 8, e011295. [Google Scholar] [CrossRef]
- Nakamura, K.; Miyoshi, T.; Yoshida, M.; Akagi, S.; Saito, Y.; Ejiri, K.; Matsuo, N.; Ichikawa, K.; Iwasaki, K.; Naito, T.; et al. Pathophysiology and Treatment of Diabetic Cardiomyopathy and Heart Failure in Patients with Diabetes Mellitus. Int. J. Mol. Sci. 2022, 23, 3587. [Google Scholar] [CrossRef]
- Murtaza, G.; Virk, H.U.H.; Khalid, M.; Lavie, C.J.; Ventura, H.; Mukherjee, D.; Ramu, V.; Bhogal, S.; Kumar, G.; Shanmugasundaram, M.; et al. Diabetic Cardiomyopathy—A Comprehensive Updated Review. Prog. Cardiovasc. Dis. 2019, 62, 315–326. [Google Scholar] [CrossRef]
- Joubert, M.; Manrique, A.; Cariou, B.; Prieur, X. Diabetes-Related Cardiomyopathy: The Sweet Story of Glucose Overload from Epidemiology to Cellular Pathways. Diabetes Metab. 2019, 45, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Zhang, Z.; Zheng, C.; Wintergerst, K.A.; Keller, B.B.; Cai, L. Mechanisms of Diabetic Cardiomyopathy and Potential Therapeutic Strategies: Preclinical and Clinical Evidence. Nat. Rev. Cardiol. 2020, 17, 585–607. [Google Scholar] [CrossRef] [PubMed]
- Bugger, H.; Abel, E.D. Molecular Mechanisms of Diabetic Cardiomyopathy. Diabetologia 2014, 57, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Paolillo, S.; Marsico, F.; Prastaro, M.; Renga, F.; Esposito, L.; De Martino, F.; Di Napoli, P.; Esposito, I.; Ambrosio, A.; Ianniruberto, M.; et al. Diabetic Cardiomyopathy: Definition, Diagnosis, and Therapeutic Implications. Heart Fail. Clin. 2019, 15, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Urbanski, M.; Patel, M.; Zeck, R.E.; Cox, G.G.; Bian, H.; Conway, B.R.; Beavers, M.P.; Rybczynski, P.J.; Demarest, K.T. Heteroaryl-O-Glucosides as Novel Sodium Glucose Co-Transporter 2 Inhibitors. Part 1. Bioorg. Med. Chem. Lett. 2005, 15, 5202–5206. [Google Scholar] [CrossRef]
- Blaschek, W. Natural Products as Lead Compounds for Sodium Glucose Cotransporter (SGLT) Inhibitors. Planta Med. 2017, 83, 985–993. [Google Scholar] [CrossRef]
- Petersen, C. Analyse Des Phloridzins. Ann. Pharm. 1835, 15, 178. [Google Scholar] [CrossRef]
- Chasis, H.; Jolliffe, N.; Smith, H.W. The Action of Phlorizin on the Excretion of Glucose, Xylose, Sucrose, Creatinine and Urea by Man. J. Clin. Investig. 1933, 12, 1083–1090. [Google Scholar] [CrossRef]
- Rieg, T.; Vallon, V. Development of SGLT1 and SGLT2 Inhibitors. Diabetologia 2018, 61, 2079. [Google Scholar] [CrossRef]
- Meng, W.; Ellsworth, B.A.; Nirschl, A.A.; McCann, P.J.; Patel, M.; Girotra, R.N.; Wu, G.; Sher, P.M.; Morrison, E.P.; Biller, S.A.; et al. Discovery of Dapagliflozin: A Potent, Selective Renal Sodium-Dependent Glucose Cotransporter 2 (SGLT2) Inhibitor for the Treatment of Type 2 Diabetes. J. Med. Chem. 2008, 51, 1145–1149. [Google Scholar] [CrossRef]
- Nevola, R.; Alfano, M.; Pafundi, P.C.; Brin, C.; Gragnano, F.; Calabrò, P.; Adinolfi, L.E.; Rinaldi, L.; Sasso, F.C.; Caturano, A. Cardiorenal Impact of SGLT-2 Inhibitors: A Conceptual Revolution in the Management of Type 2 Diabetes, Heart Failure and Chronic Kidney Disease. Rev. Cardiovasc. Med. 2022, 23, 106. [Google Scholar] [CrossRef] [PubMed]
- Al Rifai, M.; Newby, L.K.; Nair, A.P.; Misra, A.; Rogers, J.G.; Fedson, S.; Virani, S.S. SGLT-2 Inhibitors for Patients with Heart Failure: What Have We Learned Recently? Curr. Atheroscler. Rep. 2022, 24, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Zannad, F.; Ferreira, J.P.; Pocock, S.J.; Anker, S.D.; Butler, J.; Filippatos, G.; Brueckmann, M.; Ofstad, A.P.; Pfarr, E.; Jamal, W.; et al. SGLT2 Inhibitors in Patients with Heart Failure with Reduced Ejection Fraction: A Meta-Analysis of the EMPEROR-Reduced and DAPA-HF Trials. Lancet 2020, 396, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, E895–E1032. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Kowalska, K.; Walczak, J.; Femlak, J.; Młynarska, E.; Franczyk, B.; Rysz, J. Empagliflozin—A New Chance for Patients with Chronic Heart Failure. Pharmaceuticals 2021, 15, 47. [Google Scholar] [CrossRef]
- Rossing, P.; Inzucchi, S.E.; Vart, P.; Jongs, N.; Docherty, K.F.; Jhund, P.S.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; et al. Dapagliflozin and New-Onset Type 2 Diabetes in Patients with Chronic Kidney Disease or Heart Failure: Pooled Analysis of the DAPA-CKD and DAPA-HF Trials. Lancet Diabetes Endocrinol. 2022, 10, 24–34. [Google Scholar] [CrossRef]
- Herrington, W.G.; Wanner, C.; Green, J.B.; Hauske, S.J.; Judge, P.; Mayne, K.J.; Ng, S.Y.A.; Sammons, E.; Zhu, D.; Staplin, N.; et al. Design, Recruitment, and Baseline Characteristics of the EMPA-KIDNEY Trial. Nephrol. Dial. Transplant. 2022, 37, 1317–1329. [Google Scholar] [CrossRef]
- Wilding, J.P.H. The Role of the Kidneys in Glucose Homeostasis in Type 2 Diabetes: Clinical Implications and Therapeutic Significance through Sodium Glucose Co-Transporter 2 Inhibitors. Metabolism 2014, 63, 1228–1237. [Google Scholar] [CrossRef]
- Da Silva, P.N.; Da Conceição, R.A.; do Couto Maia, R.; De Castro Barbosa, M.L. Sodium–Glucose Cotransporter 2 (SGLT-2) Inhibitors: A New Antidiabetic Drug Class. MedChemComm 2018, 9, 1273. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Szarek, M.; Pitt, B.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Inzucchi, S.E.; Kosiborod, M.N.; et al. Sotagliflozin in Patients with Diabetes and Chronic Kidney Disease. N. Engl. J. Med. 2021, 384, 129–139. [Google Scholar] [CrossRef]
- Schneider, M.P.; Raff, U.; Kopp, C.; Scheppach, J.B.; Toncar, S.; Wanner, C.; Schlieper, G.; Saritas, T.; Floege, J.; Schmid, M.; et al. Skin Sodium Concentration Correlates with Left Ventricular Hypertrophy in CKD. J. Am. Soc. Nephrol. 2017, 28, 1867–1876. [Google Scholar] [CrossRef]
- Tsapas, A.; Karagiannis, T.; Kakotrichi, P.; Avgerinos, I.; Mantsiou, C.; Tousinas, G.; Manolopoulos, A.; Liakos, A.; Malandris, K.; Matthews, D.R.; et al. Comparative Efficacy of Glucose-Lowering Medications on Body Weight and Blood Pressure in Patients with Type 2 Diabetes: A Systematic Review and Network Meta-Analysis. Diabetes. Obes. Metab. 2021, 23, 2116–2124. [Google Scholar] [CrossRef]
- González-Clemente, J.M.; García-Castillo, M.; Gorgojo-Martínez, J.J.; Jiménez, A.; Llorente, I.; Matute, E.; Tejera, C.; Izarra, A.; Lecube, A. Beyond the Glycaemic Control of Dapagliflozin: Impact on Arterial Stiffness and Macroangiopathy. Diabetes Ther. 2022, 13, 1281–1298. [Google Scholar] [CrossRef]
- Baartscheer, A.; Schumacher, C.A.; Wüst, R.C.I.; Fiolet, J.W.T.; Stienen, G.J.M.; Coronel, R.; Zuurbier, C.J. Empagliflozin Decreases Myocardial Cytoplasmic Na+ through Inhibition of the Cardiac Na+/H+ Exchanger in Rats and Rabbits. Diabetologia 2017, 60, 568–573. [Google Scholar] [CrossRef]
- Uthman, L.; Baartscheer, A.; Bleijlevens, B.; Schumacher, C.A.; Fiolet, J.W.T.; Koeman, A.; Jancev, M.; Hollmann, M.W.; Weber, N.C.; Coronel, R.; et al. Class Effects of SGLT2 Inhibitors in Mouse Cardiomyocytes and Hearts: Inhibition of Na+/H+ Exchanger, Lowering of Cytosolic Na+ and Vasodilation. Diabetologia 2018, 61, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Zannad, F. Effects of Sodium-Glucose Cotransporter 2 Inhibitors for the Treatment of Patients with Heart Failure. JAMA Cardiol. 2017, 2, 1025. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xu, L.; Tian, D.; Xia, P.; Zheng, H.; Wang, L.; Chen, L. Effects of Sodium-Glucose Co-Transporter 2 (SGLT2) Inhibitors on Serum Uric Acid Level: A Meta-Analysis of Randomized Controlled Trials. Diabetes Obes. Metab. 2018, 20, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Calapkulu, M.; Cander, S.; Gul, O.O.; Ersoy, C. Lipid Profile in Type 2 Diabetic Patients with New Dapagliflozin Treatment; Actual Clinical Experience Data of Six Months Retrospective Lipid Profile from Single Center. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1031–1034. [Google Scholar] [CrossRef]
- Ji, W.; Zhao, M.; Wang, M.; Yan, W.; Liu, Y.; Ren, S.; Lu, J.; Wang, B.; Chen, L. Effects of Canagliflozin on Weight Loss in High-Fat Diet-Induced Obese Mice. PLoS ONE 2017, 12, e0179960. [Google Scholar] [CrossRef] [PubMed]
- Basu, D.; Huggins, L.A.; Scerbo, D.; Obunike, J.; Mullick, A.E.; Rothenberg, P.L.; Di Prospero, N.A.; Eckel, R.H.; Goldberg, I.J. Mechanism of Increased LDL (Low-Density Lipoprotein) and Decreased Triglycerides With SGLT2 (Sodium-Glucose Cotransporter 2) Inhibition. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2207–2216. [Google Scholar] [CrossRef]
- Szekeres, Z.; Toth, K.; Szabados, E. The Effects of SGLT2 Inhibitors on Lipid Metabolism. Metabolites 2021, 11, 87. [Google Scholar] [CrossRef]
- Hierro-Bujalance, C.; Infante-Garcia, C.; Del Marco, A.; Herrera, M.; Carranza-Naval, M.J.; Suarez, J.; Alves-Martinez, P.; Lubian-Lopez, S.; Garcia-Alloza, M. Empagliflozin Reduces Vascular Damage and Cognitive Impairment in a Mixed Murine Model of Alzheimer’s Disease and Type 2 Diabetes. Alzheimer’s Res. Ther. 2020, 12, 40. [Google Scholar] [CrossRef]
- Pawlos, A.; Broncel, M.; Woźniak, E.; Gorzelak-Pabiś, P. Neuroprotective Effect of SGLT2 Inhibitors. Molecules 2021, 26, 7213. [Google Scholar] [CrossRef]
- Garg, S.K.; Henry, R.R.; Banks, P.; Buse, J.B.; Davies, M.J.; Fulcher, G.R.; Pozzilli, P.; Gesty-Palmer, D.; Lapuerta, P.; Simó, R.; et al. Effects of Sotagliflozin Added to Insulin in Patients with Type 1 Diabetes. N. Engl. J. Med. 2017, 377, 2337–2348. [Google Scholar] [CrossRef]
- Dandona, P.; Mathieu, C.; Phillip, M.; Hansen, L.; Tschöpe, D.; Thorén, F.; Xu, J.; Langkilde, A.M. Efficacy and Safety of Dapagliflozin in Patients with Inadequately Controlled Type 1 Diabetes: The DEPICT-1 52-Week Study. Diabetes Care 2018, 41, 2552–2559. [Google Scholar] [CrossRef] [PubMed]
- Rosenstock, J.; Marquard, J.; Laffel, L.M.; Neubacher, D.; Kaspers, S.; Cherney, D.Z.; Zinman, B.; Skyler, J.S.; George, J.; Soleymanlou, N.; et al. Empagliflozin as Adjunctive to Insulin Therapy in Type 1 Diabetes: The EASE Trials. Diabetes Care 2018, 41, 2560–2569. [Google Scholar] [CrossRef]
- Liu, H.; Sridhar, V.S.; Perkins, B.A.; Rosenstock, J.; Cherney, D.Z.I. SGLT2 Inhibition in Type 1 Diabetes with Diabetic Kidney Disease: Potential Cardiorenal Benefits Can Outweigh Preventable Risk of Diabetic Ketoacidosis. Curr. Diabetes Rep. 2022, 22, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Kamoshima, H.; Nomoto, H.; Yamashita, K.; Takahashi, Y.; Tsuchida, K.; Kuwabara, S.; Miya, A.; Cho, K.Y.; Kameda, H.; Nakamura, A.; et al. Do the Benefits of Sodium-Glucose Cotransporter 2 Inhibitors Exceed the Risks in Patients with Type 1 Diabetes? Endocr. J. 2022, 69, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.M.; Lau, Y.M.; Dhandhania, V.; Cai, Z.J.; Lee, Y.K.; Lai, W.H.; Tse, H.F.; Siu, C.W. Empagliflozin Ammeliorates High Glucose Induced-Cardiac Dysfuntion in Human IPSC-Derived Cardiomyocytes. Sci. Rep. 2018, 8, 14872. [Google Scholar] [CrossRef]
- Kohsaka, S.; Kumamaru, H.; Nishimura, S.; Shoji, S.; Nakatani, E.; Ichihara, N.; Yamamoto, H.; Miyachi, Y.; Miyata, H. Incidence of Adverse Cardiovascular Events in Type 2 Diabetes Mellitus Patients after Initiation of Glucose-Lowering Agents: A Population-Based Community Study from the Shizuoka Kokuho Database. J. Diabetes Investig. 2021, 12, 1452–1461. [Google Scholar] [CrossRef]
- Berg, D.D.; Wiviott, S.D.; Scirica, B.M.; Gurmu, Y.; Mosenzon, O.; Murphy, S.A.; Bhatt, D.L.; Leiter, L.A.; McGuire, D.K.; Wilding, J.P.H.; et al. Heart Failure Risk Stratification and Efficacy of Sodium-Glucose Cotransporter-2 Inhibitors in Patients with Type 2 Diabetes Mellitus. Circulation 2019, 140, 1569–1577. [Google Scholar] [CrossRef]
- Kosiborod, M.; Birkeland, K.I.; Cavender, M.A.; Fu, A.Z.; Wilding, J.P.; Khunti, K.; Holl, R.W.; Norhammar, A.; Jørgensen, M.E.; Wittbrodt, E.T.; et al. Rates of Myocardial Infarction and Stroke in Patients Initiating Treatment with SGLT2-Inhibitors versus Other Glucose-Lowering Agents in Real-World Clinical Practice: Results from the CVD-REAL Study. Diabetes Obes. Metab. 2018, 20, 1983–1987. [Google Scholar] [CrossRef]
- Persson, F.; Nyström, T.; Jørgensen, M.E.; Carstensen, B.; Gulseth, H.L.; Thuresson, M.; Fenici, P.; Nathanson, D.; Eriksson, J.W.; Norhammar, A.; et al. Dapagliflozin Is Associated with Lower Risk of Cardiovascular Events and All-Cause Mortality in People with Type 2 Diabetes (CVD-REAL Nordic) When Compared with Dipeptidyl Peptidase-4 Inhibitor Therapy: A Multinational Observational Study. Diabetes Obes. Metab. 2018, 20, 344–351. [Google Scholar] [CrossRef]
- Kosiborod, M.; Gause-Nilsson, I.; Xu, J.; Sonesson, C.; Johnsson, E. Efficacy and Safety of Dapagliflozin in Patients with Type 2 Diabetes and Concomitant Heart Failure. J. Diabetes Complicat. 2017, 31, 1215–1221. [Google Scholar] [CrossRef]
- Matsutani, D.; Sakamoto, M.; Kayama, Y.; Takeda, N.; Horiuchi, R.; Utsunomiya, K. Effect of Canagliflozin on Left Ventricular Diastolic Function in Patients with Type 2 Diabetes. Cardiovasc. Diabetol. 2018, 17, 73. [Google Scholar] [CrossRef] [PubMed]
- Shimohata, H.; Iwaki, Y.; Yamashita, M.; Ohgi, K.; Maruyama, H.; Takayasu, M.; Hirayama, K.; Kobayashi, M. The Effect of Sodium-Glucose Cotransporter 2 Inhibitor (Tofogliflozin) on Renal Tubular Damage in Diabetic Patients without Albuminuria. Int. Urol. Nephrol. 2021, 54, 1907–1914. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tran, D.; Yang, H.C.; Nylander, S.; Birnbaum, Y.; Ye, Y. Dapagliflozin and Ticagrelor Have Additive Effects on the Attenuation of the Activation of the NLRP3 Inflammasome and the Progression of Diabetic Cardiomyopathy: An AMPK–MTOR Interplay. Cardiovasc. Drugs Ther. 2020, 34, 443–461. [Google Scholar] [CrossRef]
- Ye, Y.; Bajaj, M.; Yang, H.C.; Perez-Polo, J.R.; Birnbaum, Y. SGLT-2 Inhibition with Dapagliflozin Reduces the Activation of the Nlrp3/ASC Inflammasome and Attenuates the Development of Diabetic Cardiomyopathy in Mice with Type 2 Diabetes. Further Augmentation of the Effects with Saxagliptin, a DPP4 Inhibitor. Cardiovasc. Drugs Ther. 2017, 31, 119–132. [Google Scholar] [CrossRef]
- Tahara, A.; Kurosaki, E.; Yokono, M.; Yamajuku, D.; Kihara, R.; Hayashizaki, Y.; Takasu, T.; Imamura, M.; Li, Q.; Tomiyama, H.; et al. Effects of Sodium-Glucose Cotransporter 2 Selective Inhibitor Ipragliflozin on Hyperglycaemia, Oxidative Stress, Inflammation and Liver Injury in Streptozotocin-Induced Type 1 Diabetic Rats. J. Pharm. Pharmacol. 2014, 66, 975–987. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Mazer, C.D.; Yan, A.T.; Mason, T.; Garg, V.; Teoh, H.; Zuo, F.; Quan, A.; Farkouh, M.E.; Fitchett, D.H.; et al. Effect of Empagliflozin on Left Ventricular Mass in Patients with Type 2 Diabetes Mellitus and Coronary Artery Disease: The EMPA-HEART CardioLink-6 Randomized Clinical Trial. Circulation 2019, 140, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- Carbone, S.; Canada, J.M.; Billingsley, H.E.; Kadariya, D.; Dixon, D.L.; Trankle, C.R.; Buckley, L.F.; Markley, R.; Vo, C.; Medina de Chazal, H.; et al. Effects of Empagliflozin on Cardiorespiratory Fitness and Significant Interaction of Loop Diuretics. Diabetes Obes. Metab. 2018, 20, 2014–2018. [Google Scholar] [CrossRef]
- Oka, S.; Kai, T.; Hoshino, K.; Watanabe, K.; Nakamura, J.; Abe, M.; Watanabe, A. Effects of Empagliflozin in Different Phases of Diabetes Mellitus-Related Cardiomyopathy: A Prospective Observational Study. BMC Cardiovasc. Disord. 2021, 21, 217. [Google Scholar] [CrossRef]
- Moellmann, J.; Klinkhammer, B.M.; Droste, P.; Kappel, B.; Haj-Yehia, E.; Maxeiner, S.; Artati, A.; Adamski, J.; Boor, P.; Schütt, K.; et al. Empagliflozin Improves Left Ventricular Diastolic Function of Db/Db Mice. Biochim. Biophys. Acta—Mol. Basis Dis. 2020, 1866, 165807. [Google Scholar] [CrossRef]
- Adingupu, D.D.; Göpel, S.O.; Grönros, J.; Behrendt, M.; Sotak, M.; Miliotis, T.; Dahlqvist, U.; Gan, L.M.; Jönsson-Rylander, A.C. SGLT2 Inhibition with Empagliflozin Improves Coronary Microvascular Function and Cardiac Contractility in Prediabetic Ob/Ob-/- Mice. Cardiovasc. Diabetol. 2019, 18, 16. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, W. The Sodium-Glucose Co-Transporter 2 Inhibitor, Empagliflozin, Protects against Diabetic Cardiomyopathy by Inhibition of the Endoplasmic Reticulum Stress Pathway. Cell. Physiol. Biochem. 2017, 41, 2503–2512. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, J.; Xue, M.; Li, X.; Han, F.; Liu, X.; Xu, L.; Lu, Y.; Cheng, Y.; Li, T.; et al. SGLT2 Inhibition with Empagliflozin Attenuates Myocardial Oxidative Stress and Fibrosis in Diabetic Mice Heart. Cardiovasc. Diabetol. 2019, 18, 15. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.I.; Chen, Y.C.; Lin, Y.K.; Chung, C.C.; Lu, Y.Y.; Kao, Y.H.; Chen, Y.J. Empagliflozin Attenuates Myocardial Sodium and Calcium Dysregulation and Reverses Cardiac Remodeling in Streptozotocin-Induced Diabetic Rats. Int. J. Mol. Sci. 2019, 20, 1680. [Google Scholar] [CrossRef] [PubMed]
- Moellmann, J.; Mann, P.A.; Kappel, B.A.; Kahles, F.; Klinkhammer, B.M.; Boor, P.; Kramann, R.; Ghesquiere, B.; Lebherz, C.; Marx, N.; et al. The SGLT2 Inhibitor Ertugliflozin Modifies the Signature of Cardiac Substrate Metabolism and Reduces Cardiac mTOR signaling, Endoplasmic Reticulum Stress and Apoptosis. Diabetes Obes. Metab. 2022, 24, 2263–2272. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, N.; Mostafa, Y.M.; AboGresha, N.M.; Ahmed, A.A.M.; Mahmoud, I.Z.; El-Sayed, N.M. Dapagliflozin Attenuates Diabetic Cardiomyopathy through Erythropoietin Up-Regulation of AKT/JAK/MAPK Pathways in Streptozotocin-Induced Diabetic Rats. Chem. Biol. Interact. 2021, 347, 109617. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.; Li, L.; Fu, H.; Yin, Y.; Du, B.; Wang, F.; Ding, Y.; Liu, Y.; Zhao, R.; Zhang, Z.; et al. Effect of Dapagliflozin on Diabetic Patients with Cardiovascular Disease via MAPK Signalling Pathway. J. Cell. Mol. Med. 2021, 25, 7500–7512. [Google Scholar] [CrossRef] [PubMed]
- Trang, N.N.; Chung, C.C.; Lee, T.W.; Cheng, W.L.; Kao, Y.H.; Huang, S.Y.; Lee, T.I.; Chen, Y.J. Empagliflozin and Liraglutide Differentially Modulate Cardiac Metabolism in Diabetic Cardiomyopathy in Rats. Int. J. Mol. Sci. 2021, 22, 1177. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Li, T.; Wang, Y.; Chang, Y.; Cheng, Y.; Lu, Y.; Liu, X.; Xu, L.; Li, X.; Yu, X.; et al. Empagliflozin Prevents Cardiomyopathy via SGC-CGMP-PKG Pathway in Type 2 Diabetes Mice. Clin. Sci. 2019, 133, 1705–1720. [Google Scholar] [CrossRef]
- Marfella, R.; D’Onofrio, N.; Trotta, M.C.; Sardu, C.; Scisciola, L.; Amarelli, C.; Balestrieri, M.L.; Grimaldi, V.; Mansueto, G.; Esposito, S.; et al. Sodium/Glucose Cotransporter 2 (SGLT2) Inhibitors Improve Cardiac Function by Reducing JunD Expression in Human Diabetic Hearts. Metabolism 2022, 127, 154936. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Li, C.; Tang, D.; Dai, Y. Metabolomics Study Reveals the Alteration of Fatty Acid Oxidation in the Hearts of Diabetic Mice by Empagliflozin. Mol. Omics 2022, 18, 643–651. [Google Scholar] [CrossRef]
- Xie, B.; Ramirez, W.; Mills, A.M.; Huckestein, B.R.; Anderson, M.; Pangburn, M.M.; Lang, E.Y.; Mullet, S.J.; Chuan, B.W.; Guo, L.; et al. Empagliflozin Restores Cardiac Metabolic Flexibility in Diet-Induced Obese C57BL6/J Mice. Curr. Res. Physiol. 2022, 5, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Rawat, S.; Ho, K.L.; Wagg, C.S.; Zhang, L.; Teoh, H.; Dyck, J.E.; Uddin, G.M.; Oudit, G.Y.; Mayoux, E.; et al. Empagliflozin Increases Cardiac Energy Production in Diabetes: Novel Translational Insights into the Heart Failure Benefits of SGLT2 Inhibitors. JACC Basic Transl. Sci. 2018, 3, 575–587. [Google Scholar] [CrossRef]
- Thirunavukarasu, S.; Jex, N.; Chowdhary, A.; Hassan, I.U.; Straw, S.; Craven, T.P.; Gorecka, M.; Broadbent, D.; Swoboda, P.; Witte, K.K.; et al. Empagliflozin Treatment Is Associated with Improvements in Cardiac Energetics and Function and Reductions in Myocardial Cellular Volume in Patients with Type 2 Diabetes. Diabetes 2021, 70, 2810–2822. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Deshpande, M.; Pang, H.; Palaniyandi, S.S. Precision Medicine Approach: Empagliflozin for Diabetic Cardiomyopathy in Mice with Aldehyde Dehydrogenase (ALDH) 2*2 Mutation, a Specific Genetic Mutation in Millions of East Asians. Eur. J. Pharmacol. 2018, 839, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Steven, S.; Oelze, M.; Hanf, A.; Kröller-Schön, S.; Kashani, F.; Roohani, S.; Welschof, P.; Kopp, M.; Gödtel-Armbrust, U.; Xia, N.; et al. The SGLT2 Inhibitor Empagliflozin Improves the Primary Diabetic Complications in ZDF Rats. Redox Biol. 2017, 13, 370–385. [Google Scholar] [CrossRef] [PubMed]
- Alshnbari, A.S.; Millar, S.A.; O’Sullivan, S.E.; Idris, I. Effect of Sodium-Glucose Cotransporter-2 Inhibitors on Endothelial Function: A Systematic Review of Preclinical Studies. Diabetes Ther. 2020, 11, 1947–1963. [Google Scholar] [CrossRef] [PubMed]
- Salim, H.M.; Fukuda, D.; Yagi, S.; Soeki, T.; Shimabukuro, M.; Sata, M. Glycemic Control with Ipragliflozin, a Novel Selective SGLT2 Inhibitor, Ameliorated Endothelial Dysfunction in Streptozotocin-Induced Diabetic Mouse. Front. Cardiovasc. Med. 2016, 3, 43. [Google Scholar] [CrossRef]
- Mone, P.; Varzideh, F.; Jankauskas, S.S.; Pansini, A.; Lombardi, A.; Frullone, S.; Santulli, G. SGLT2 Inhibition via Empagliflozin Improves Endothelial Function and Reduces Mitochondrial Oxidative Stress: Insights From Frail Hypertensive and Diabetic Patients. Hypertension 2022, 79, 1633–1643. [Google Scholar] [CrossRef]
- Zhang, P. CaMKII: The Molecular Villain That Aggravates Cardiovascular Disease (Review). Exp. Ther. Med. 2017, 13, 815–820. [Google Scholar] [CrossRef]
- Arow, M.; Waldman, M.; Yadin, D.; Nudelman, V.; Shainberg, A.; Abraham, N.G.; Freimark, D.; Kornowski, R.; Aravot, D.; Hochhauser, E.; et al. Sodium-Glucose Cotransporter 2 Inhibitor Dapagliflozin Attenuates Diabetic Cardiomyopathy. Cardiovasc. Diabetol. 2020, 19, 7. [Google Scholar] [CrossRef]
- Osaka, N.; Mori, Y.; Terasaki, M.; Hiromura, M.; Saito, T.; Yashima, H.; Shiraga, Y.; Kawakami, R.; Ohara, M.; Fukui, T.; et al. Luseogliflozin Inhibits High Glucose-Induced TGF-Β2 Expression in Mouse Cardiomyocytes by Suppressing NHE-1 Activity. J. Int. Med. Res. 2022, 50, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Belen, E.; Canbolat, I.P.; Yigittürk, G.; Cetinarslan, Ö.; Akdeniz, C.S.; Karaca, M.; Sönmez, M.; Erbas, O. Cardio-Protective Effect of Dapagliflozin against Doxorubicin Induced Cardiomyopathy in Rats. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 4403–4408. [Google Scholar] [CrossRef]
- Hussein, A.M.; Eid, E.A.; Taha, M.; Elshazli, R.M.; Bedir, R.F.; Lashin, L.S. Comparative Study of the Effects of GLP1 Analog and SGLT2 Inhibitor against Diabetic Cardiomyopathy in Type 2 Diabetic Rats: Possible Underlying Mechanisms. Biomedicines 2020, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Giannattasio, S.; Citarella, A.; Trocchianesi, S.; Filardi, T.; Morano, S.; Lenzi, A.; Ferretti, E.; Crescioli, C. Cell-Target-Specific Anti-Inflammatory Effect of Empagliflozin: In Vitro Evidence in Human Cardiomyocytes. Front. Mol. Biosci. 2022, 9, 879522. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Yan, T.; Liang, Y. Targeting TRAF3IP2 Alleviates High Glucose-Induced Cardiomyocyte Inflammation and Apoptosis. Drug Dev. Res. 2022, 83, 167–175. [Google Scholar] [CrossRef]
- Xing, Y.J.; Liu, B.H.; Wan, S.J.; Cheng, Y.; Zhou, S.M.; Sun, Y.; Yao, X.M.; Hua, Q.; Meng, X.J.; Cheng, J.H.; et al. A SGLT2 Inhibitor Dapagliflozin Alleviates Diabetic Cardiomyopathy by Suppressing High Glucose-Induced Oxidative Stress in Vivo and in Vitro. Front. Pharmacol. 2021, 12, 708177. [Google Scholar] [CrossRef]
- Olgar, Y.; Turan, B. A Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitor Dapagliflozin Comparison with Insulin Shows Important Effects on Zn2+-Transporters in Cardiomyocytes from Insulin-Resistant Metabolic Syndrome Rats through Inhibition of Oxidative Stress. Can. J. Physiol. Pharmacol. 2019, 97, 528–535. [Google Scholar] [CrossRef]
- Joubert, M.; Jagu, B.; Montaigne, D.; Marechal, X.; Tesse, A.; Ayer, A.; Dollet, L.; Le May, C.; Toumaniantz, G.; Manrique, A.; et al. The Sodium-Glucose Cotransporter 2 Inhibitor Dapagliflozin Prevents Cardiomyopathy in a Diabetic Lipodystrophic Mouse Model. Diabetes 2017, 66, 1030–1040. [Google Scholar] [CrossRef]
- Wang, J.; Huang, X.; Liu, H.; Chen, Y.; Li, P.; Liu, L.; Li, J.; Ren, Y.; Huang, J.; Xiong, E.; et al. Empagliflozin Ameliorates Diabetic Cardiomyopathy via Attenuating Oxidative Stress and Improving Mitochondrial Function. Oxid. Med. Cell. Longev. 2022, 2022, 1122494. [Google Scholar] [CrossRef]
- Paolisso, P.; Bergamaschi, L.; Santulli, G.; Gallinoro, E.; Cesaro, A.; Gragnano, F.; Sardu, C.; Mileva, N.; Foà, A.; Armillotta, M.; et al. Infarct size, inflammatory burden, and admission hyperglycemia in diabetic patients with acute myocardial infarction treated with SGLT2-inhibitors: A multicenter international registry. Cardiovasc. Diabetol. 2022, 21, 77. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, J.L.; Yan, X.J.; Sun, L.; Ji, Y.; Wang, F.F. Effect of dapagliflozin on the prognosis of patients with acute myocardial infarction undergoing percutaneous coronary intervention. Cardiovasc. Diabetol. 2022, 21, 186. [Google Scholar] [CrossRef]
- von Lewinski, D.; Kolesnik, E.; Tripolt, N.J.; Pferschy, P.N.; Benedikt, M.; Wallner, M.; Alber, H.; Berger, R.; Lichtenauer, M.; Saely, C.H.; et al. Empagliflozin in acute myocardial infarction: The EMMY trial. Eur. Heart J. 2022, 43, 4421–4432. [Google Scholar] [CrossRef]
- Baker, H.E.; Tune, J.D.; Mather, K.J.; Blaettner, B.S.; Clark, H.E.; Li, F.; Li, X.; Kowala, M.C.; Fliegel, L.; Goodwill, A.G. Acute SGLT-2i treatment improves cardiac efficiency during myocardial ischemia independent of Na+/H+ exchanger-1. Int. J. Cardiol. 2022, 363, 138–148. [Google Scholar] [CrossRef]
| Stage | Pathophysiological Events | Changes in Structure and Morphology | Functional Impairment |
|---|---|---|---|
| Early | Hyperglycaemia, downregulation of GLUT4, insulin resistance, increase in free fatty acids, impairment of Ca2+ homeostasis, sympathetic nervous system activation | Small pathophysiological changes in cardiomyocytes, normal left ventricular mass and LV wall thickness | Little or no left ventricular diastolic dysfunction (LVDD) |
| Advanced | Cardiomyocyte injury and death, fibrosis, activation of RAAs, increased inflammation | Increased left ventricular mass, wall thickness and size | Impairment of left ventricular diastolic function and slightly decreased ejection fraction |
| Late | Myocardial fibrosis, impaired microvascular coronary circulation, severe neurohormonal activation, inflammation | Substantial increase in left ventricular mass, wall thickness and size | Impairment of both diastolic and systolic functions |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalska, K.; Wilczopolski, P.; Buławska, D.; Młynarska, E.; Rysz, J.; Franczyk, B. The Importance of SGLT-2 Inhibitors as Both the Prevention and the Treatment of Diabetic Cardiomyopathy. Antioxidants 2022, 11, 2500. https://doi.org/10.3390/antiox11122500
Kowalska K, Wilczopolski P, Buławska D, Młynarska E, Rysz J, Franczyk B. The Importance of SGLT-2 Inhibitors as Both the Prevention and the Treatment of Diabetic Cardiomyopathy. Antioxidants. 2022; 11(12):2500. https://doi.org/10.3390/antiox11122500
Chicago/Turabian StyleKowalska, Klaudia, Piotr Wilczopolski, Dominika Buławska, Ewelina Młynarska, Jacek Rysz, and Beata Franczyk. 2022. "The Importance of SGLT-2 Inhibitors as Both the Prevention and the Treatment of Diabetic Cardiomyopathy" Antioxidants 11, no. 12: 2500. https://doi.org/10.3390/antiox11122500
APA StyleKowalska, K., Wilczopolski, P., Buławska, D., Młynarska, E., Rysz, J., & Franczyk, B. (2022). The Importance of SGLT-2 Inhibitors as Both the Prevention and the Treatment of Diabetic Cardiomyopathy. Antioxidants, 11(12), 2500. https://doi.org/10.3390/antiox11122500







