Phenylpropanoid Glycoside and Phenolic Acid Profiles and Biological Activities of Biomass Extracts from Different Types of Verbena officinalis Microshoot Cultures and Soil-Grown Plant
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Initiation of In Vitro Cultures
2.3. Experimental In Vitro Cultures
2.4. Extraction
2.4.1. For HPLC–DAD Analyses
2.4.2. For UHPLC-HR-QTOF-MS Qualitative Analyses
2.4.3. For Biological Investigations
2.5. Chromatographic Analyses—HPLC–DAD
2.5.1. Phenylpropanoid Glycosides
2.5.2. Phenolic Acids
2.5.3. Chromatographic Analyses—UHPLC-HR-QTOF-MS
2.5.4. Determination of Total Phenolic Content
2.6. Antioxidant Activity
2.6.1. Free Radical Scavenging Activity
2.6.2. Reducing Power Assay
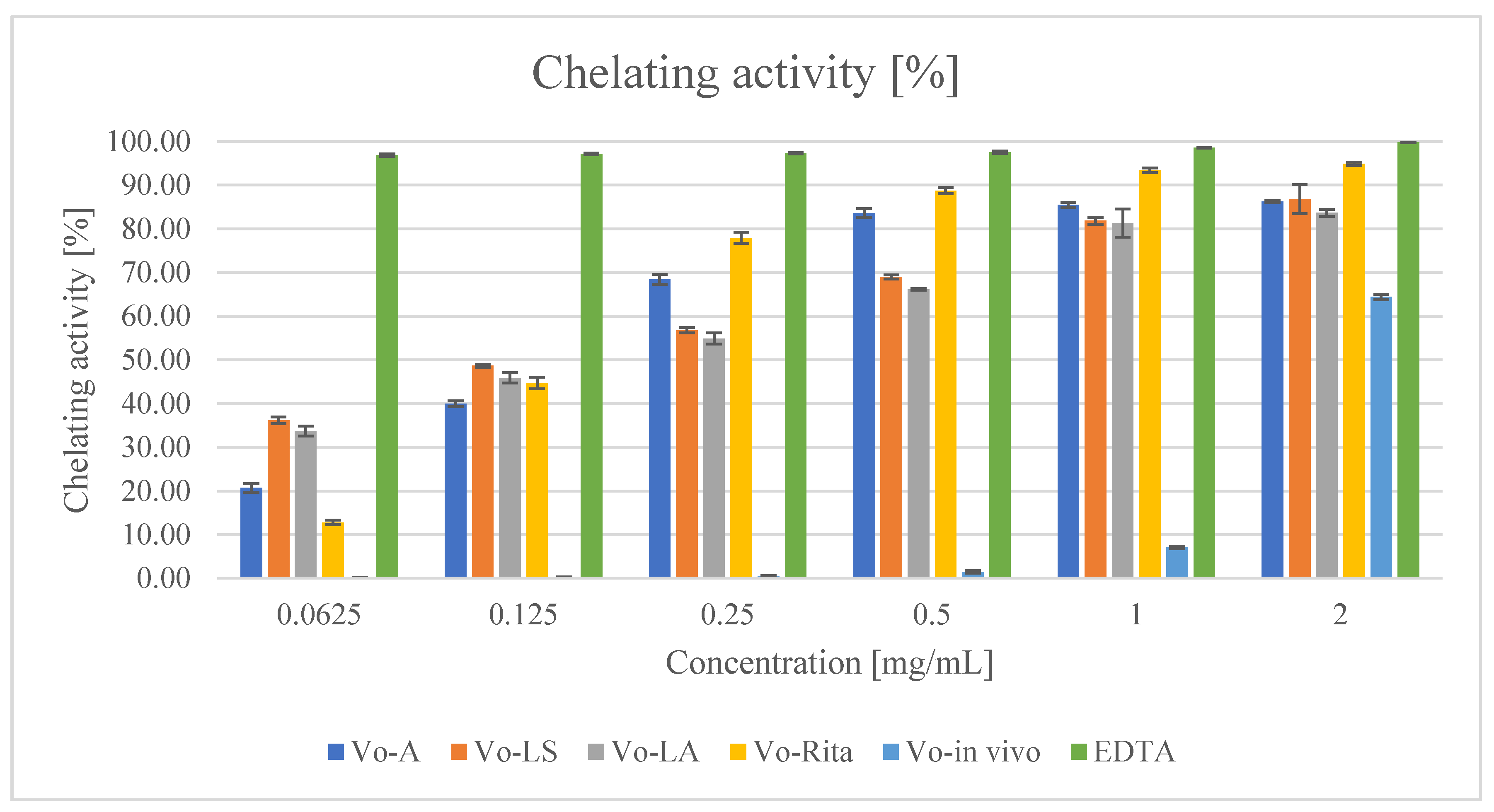
2.6.3. Ferrous Ions (Fe2+) Chelating Activity
2.7. Antibacterial Activity
2.7.1. Bacterial Strains and Preparation of Inoculum
2.7.2. MIC and MBC Determination
3. Results and Discussion
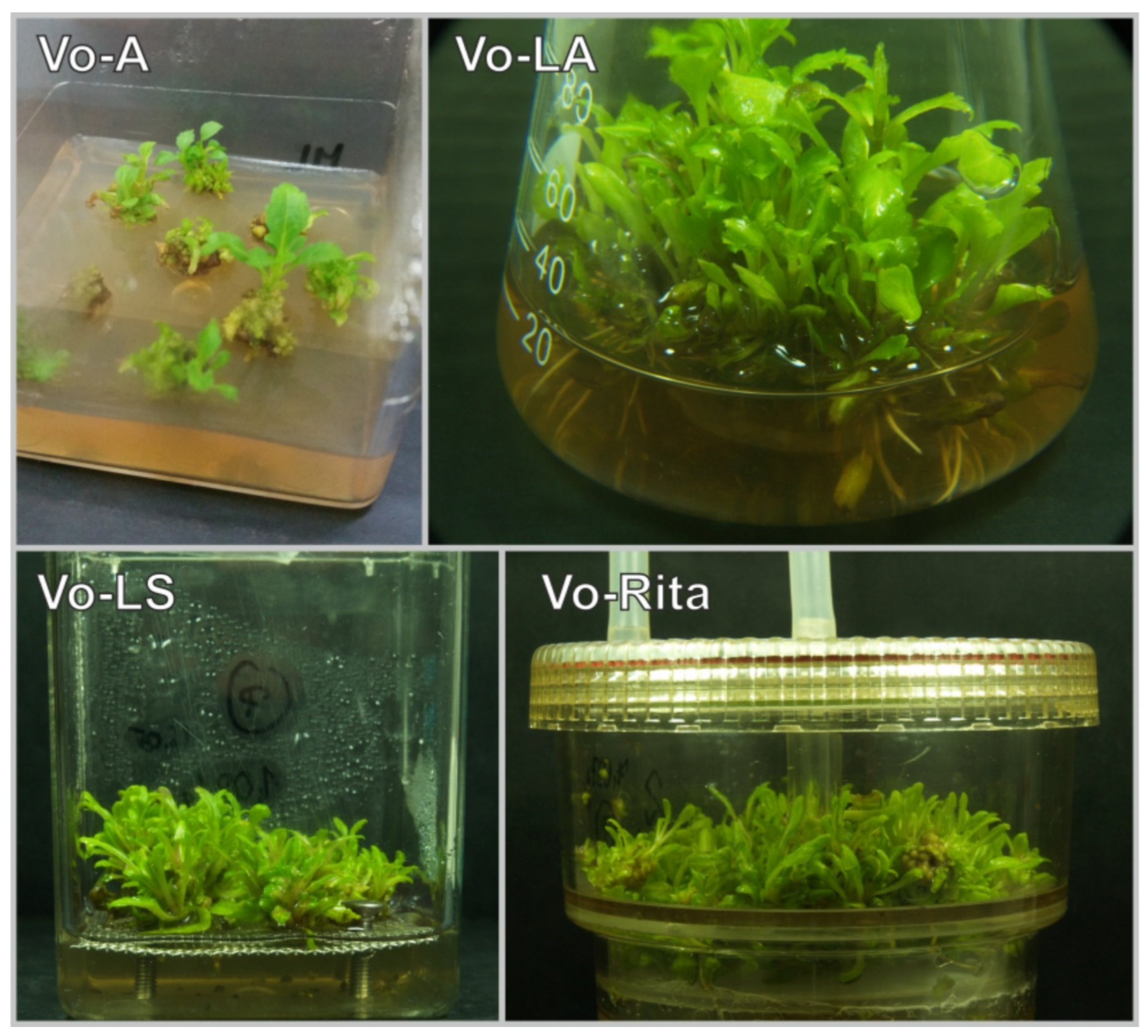
3.1. Influence of Cultivation Mode on Microshoot Appearance and Biomass Increments
3.2. Target Metabolic Profiles
3.3. Biological Activities of the Studied In Vitro Cultures and Soil-Grown Plants
3.3.1. Antioxidant Activity
3.3.2. Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chevallier, A. The Encyclopedia of Medicinal Plants; Dorling Kindersley Limited: London, UK, 1996. [Google Scholar]
- Bradley, P.R. British Herbal Compendium; British Herbal Medicine Association: Bournemouth, UK, 2006; Volume 2. [Google Scholar]
- Verbena Herb. In European Pharmacopoeia, 10th ed.; European Directorate for the Quality of Medicines: Strasburg, France, 2020; pp. 1665–1667.
- Tobyn, G.; Denham, A.; Whitelegg, M. Verbena officinalis, vervain. In Medical Herbs; Elsevier: Amsterdam, The Netherlands, 2011; pp. 327–336. ISBN 9780443103445. [Google Scholar]
- Kubica, P.; Szopa, A.; Dominiak, J.; Luczkiewicz, M.; Ekiert, H. Verbena officinalis (common vervain)—A review on the investigations of this medicinally important plant species. Planta Med. 2020, 86, 1241–1257. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.W.; Khan, A.U.; Ahmed, T. Anticonvulsant, anxiolytic, and sedative activities of Verbena officinalis. Front. Pharmacol. 2016, 7, 499. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van Wyk, B.-E.; Wink, M. Medicinal Plants of the World: An Illustrated Scientific Guide to Important Medicinal Plants and Their Uses; Timber Press: Portland, OR, USA, 2004; ISBN 9812329331. [Google Scholar]
- Rehecho, S.; Hidalgo, O.; García-Iñiguez de Ciriano, M.; Navarro-Blasco, I.; Astiasarán, I.; Ansorena-Artieda, D.; Cavero, R.Y.; Calvo, M.I. Chemical composition, mineral content and antioxidant activity of Verbena officinalis L. LWT-Food Sci. Technol. 2011, 44, 875–882. [Google Scholar] [CrossRef]
- Casanova, E.; García-Mina, J.M.; Calvo, M.I. Antioxidant and antifungal activity of Verbena officinalis L. leaves. Plant Foods Hum. Nutr. 2008, 63, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Jornet, P.; Camacho-Alonso, F.; Gómez-Garcia, F.; Molina Miñano, F.; Cañas, X.; Serafín, A.; Castillo, J.; Vicente-Ortega, V. Effects of potassium apigenin and verbena extract on the wound healing process of SKH-1 mouse skin. Int. Wound J. 2014, 11, 489–495. [Google Scholar] [CrossRef]
- Speroni, E.; Cervellati, R.; Costa, S.; Guerra, M.C.; Utan, A.; Govoni, P.; Berger, A.; Müller, A.; Stuppner, H. Effects of differential extraction of Verbena officinalis on rat models of inflammation, cicatrization and gastric damage. Planta Med. 2007, 73, 227–235. [Google Scholar] [CrossRef]
- EFSA. EFSA Panel on Dietetic Products, Nutrition and Allergies. 2010, p. 1489. Available online: https://efsa.onlinelibrary.wiley.com/doi/pdfdirect/10.2903/j.efsa.2010.1489 (accessed on 14 January 2022).
- De Martino, L.; D’Arena, G.; Minervini, M.M.; Deaglio, S.; Fusco, B.M.; Cascavilla, N.; De Feo, V. Verbena officinalis essential oil and its component citral as apoptotic-inducing agent in chronic lymphocytic leukemia. Int. J. Immunopathol. Pharmacol. 2009, 22, 1097–1104. [Google Scholar] [CrossRef]
- Shu, J.; Chou, G.; Wang, Z. Two new iridoids from Verbena officinalis L. Molecules 2014, 19, 10473–10479. [Google Scholar] [CrossRef]
- Verma, V.K.; Siddiqui, N.U. Bioactive chemical constituents from the plant Verbena officinalis Linn. Int. J. Pharm. Pharm. Sci. 2011, 3, 108–109. [Google Scholar]
- Duan, K.; Yuan, Z.; Guo, W.; Meng, Y.; Cui, Y.; Kong, D.; Zhang, L.; Wang, N. LC-MS/MS determination and pharmacokinetic study of five flavone components after solvent extraction/acid hydrolysis in rat plasma after oral administration of Verbena officinalis L. extract. J. Ethnopharmacol. 2011, 135, 201–208. [Google Scholar] [CrossRef]
- Shu, J.-C.; Liu, J.-Q.; Chou, G.-X. A new triterpenoid from Verbena officinalis L. Nat. Prod. Res. 2013, 27, 1293–1297. [Google Scholar] [CrossRef] [PubMed]
- Deepak, M.; Handa, S.S. Antiinflammatory activity and chemical composition of extracts of Verbena officinalis. Phyther. Res. 2000, 14, 463–465. [Google Scholar] [CrossRef]
- Kubica, P.; Szopa, A.; Ekiert, H. Production of verbascoside and phenolic acids in biomass of Verbena officinalis L. (vervain) cultured under different in vitro conditions. Nat. Prod. Res. 2017, 31, 1663–1668. [Google Scholar] [CrossRef] [PubMed]
- Kubica, P.; Szopa, A.; Kokotkiewicz, A.; Miceli, N.; Taviano, M.F.; Maugeri, A.; Cirmi, S.; Synowiec, A.; Gniewosz, M.; Elansary, H.O.; et al. Production of verbascoside, isoverbascoside and phenolic acids in callus, suspension, and bioreactor cultures of Verbena officinalis and biological properties of biomass extracts. Molecules 2020, 25, 5609. [Google Scholar] [CrossRef] [PubMed]
- Kubica, P.; Szopa, A.; Prokopiuk, B.; Komsta, Ł.; Pawłowska, B.; Ekiert, H. The influence of light quality on the production of bioactive metabolites–verbascoside, isoverbascoside and phenolic acids and the content of photosynthetic pigments in biomass of Verbena officinalis L. cultured in vitro. J. Photochem. Photobiol. B Biol. 2020, 203, 111768. [Google Scholar] [CrossRef] [PubMed]
- Alipieva, K.; Korkina, L.; Orhan, I.E.; Georgiev, M.I. Verbascoside—A review of its occurrence, (bio)synthesis and pharmacological significance. Biotechnol. Adv. 2014, 32, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Yang, B. Phenylethanoid glycosides: Research advances in their phytochemistry, pharmacological activity and pharmacokinetics. Molecules 2016, 21, 991. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Pang, H.; Wong, Y. Naturally Occurring Phenylethanoid Glycosides: Potential Leads for New Therapeutics. Curr. Med. Chem. 2008, 15, 2592–2613. [Google Scholar] [CrossRef]
- Szopa, A.; Starzec, A.; Ekiert, H. The importance of monochromatic lights in the production of phenolic acids and flavonoids in shoot cultures of Aronia melanocarpa, Aronia arbutifolia and Aronia × prunifolia. J. Photochem. Photobiol. B Biol. 2018, 179, 91–97. [Google Scholar] [CrossRef]
- Szopa, A.; Kokotkiewicz, A.; Bednarz, M.; Luczkiewicz, M.; Ekiert, H. Studies on the accumulation of phenolic acids and flavonoids in different in vitro culture systems of Schisandra chinensis (Turcz.) Baill. using a DAD-HPLC method. Phytochem. Lett. 2017, 20, 462–469. [Google Scholar] [CrossRef]
- Ohlsson, A.B.; Berglund, T. Gibberellic acid-induced changes in glutathione metabolism and anthocyanin content in plant tissue. Plant Cell Tissue Organ Cult. 2001, 64, 77–80. [Google Scholar] [CrossRef]
- Schenk, R.U.; Hildebrandt, A.C. Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can. J. Bot. 1972, 50, 199–204. [Google Scholar] [CrossRef]
- Schönbichler, S.A.; Bittner, L.K.H.; Pallua, J.D.; Popp, M.; Abel, G.; Bonn, G.K.; Huck, C.W. Simultaneous quantification of verbenalin and verbascoside in Verbena officinalis by ATR-IR and NIR spectroscopy. J. Pharm. Biomed. Anal. 2013, 84, 97–102. [Google Scholar] [CrossRef]
- Ellnain-Wojtaszek, M.; Zgórka, G. High-performance liquid chromatography and thin-layer chromatography of phenolic acids from Ginkgo biloba L. leaves collected within vegetative period. J. Liq. Chromatogr. Relat. Technol. 1999, 22, 1457–1471. [Google Scholar] [CrossRef]
- Sułkowska-Ziaja, K.; Maślanka, A.; Szewczyk, A.; Muszyńska, B. Determination of Physiologically Active Compounds in Four Species of Genus Phellinus. Nat. Prod. Commun. 2017, 12, 363–366. [Google Scholar] [PubMed]
- Miceli, N.; Filocamo, A.; Ragusa, S.; Cacciola, F.; Dugo, P.; Mondello, L.; Celano, M.; Maggisano, V.; Taviano, M.F. Chemical characterization and biological activities of phenolic-rich fraction from cauline leaves of Isatis tinctoria L. (Brassicaceae) growing in Sicily, Italy. Chem. Biodivers. 2017, 14, e1700073. [Google Scholar] [CrossRef] [PubMed]
- National Committee For Clinical Laboratory Standards. Methods for Dilution Antimicrobial a Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard–Eighth Edition CLSI Document M0-A8; National Committee For Clinical Laboratory Standards: Wayne, PA, USA, 2009. [Google Scholar]
- National Committee For Clinical Laboratory Standards. Method for Determining Bactericidal Activity of Antimicrobial Agents; Approved Guide Standard-Eight Edition CLSI Document M07-A8; National Committee For Clinical Laboratory Standards: Wayne, PA, USA, 2009. [Google Scholar]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef]
- Rangasamy, O.; Raoelison, G.; Rakotoniriana, F.E.; Cheuk, K.; Urverg-Ratsimamanga, S.; Quetin-Leclercq, J.; Gurib-Fakim, A.; Subratty, A.H. Screening for anti-infective properties of several medicinal plants of the Mauritians flora. J. Ethnopharmacol. 2007, 109, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Luczkiewicz, M.; Kokotkiewicz, A.; Glod, D. Plant growth regulators affect biosynthesis and accumulation profile of isoflavone phytoestrogens in high-productive in vitro cultures of Genista tinctoria. Plant Cell Tissue Organ Cult. 2014, 118, 419–429. [Google Scholar] [CrossRef]
- Szopa, A.; Kokotkiewicz, A.; Marzec-Wróblewska, U.; Bucinski, A.; Luczkiewicz, M.; Ekiert, H. Accumulation of dibenzocyclooctadiene lignans in agar cultures and in stationary and agitated liquid cultures of Schisandra chinensis (Turcz.) Baill. Appl. Microbiol. Biotechnol. 2016, 100, 3965–3977. [Google Scholar] [CrossRef]
- Szopa, A.; Kokotkiewicz, A.; Luczkiewicz, M.; Ekiert, H. Schisandra lignans production regulated by different bioreactor type. J. Biotechnol. 2017, 247, 11–17. [Google Scholar] [CrossRef]
- Klimek-Szczykutowicz, M.; Szopa, A.; Dziurka, M.; Komsta, Ł.; Tomczyk, M.; Ekiert, H. The Influence of Nasturtium officinale R. Br. Agar and Agitated Microshoot Culture Media on Glucosinolate and Phenolic Acid Production, and Antioxidant Activity. Biomolecules 2020, 10, 1216. [Google Scholar] [CrossRef] [PubMed]
- Jesionek, A.; Kokotkiewicz, A.; Wlodarska, P.; Zabiegala, B.; Bucinski, A.; Luczkiewicz, M. Bioreactor shoot cultures of Rhododendron tomentosum (Ledum palustre) for a large-scale production of bioactive volatile compounds. Plant Cell Tissue Organ Cult. 2017, 131, 51–64. [Google Scholar] [CrossRef]
- Piątczak, E.; Talar, A.; Kuźma, Ł.; Wysokińska, H. Iridoid and phenylethanoid glycoside production in multiple shoots and regenerated Rehmannia elata N.E. Brown ex Prain plants following micropropagation. Acta Physiol. Plant. 2015, 37, 255. [Google Scholar] [CrossRef]
- Grabkowska, R.; Mielicki, W.; Wielanek, M.; Wysokińska, H. Changes of phenylethanoid and iridoid glycoside distribution in various tissues of shoot cultures and regenerated plants of Harpagophytum procumbens (Burch.) DC. ex Meisn. S. Afr. J. Bot. 2014, 95, 159–164. [Google Scholar] [CrossRef]
- Sanchez, P.M.; Villarreal, M.L.; Herrera-Ruiz, M.; Zamilpa, A.; Jiménez-Ferrer, E.; Trejo-Tapia, G. In vivo anti-inflammatory and anti-ulcerogenic activities of extracts from wild growing and in vitro plants of Castilleja tenuiflora Benth. (Orobanchaceae). J. Ethnopharmacol. 2013, 150, 1032–1037. [Google Scholar] [CrossRef] [PubMed]
- Budzianowska, A.; Skrzypczak, L.; Budzianowski, J. Phenylethanoid glucosides from in vitro propagated plants and callus cultures of Plantago lanceolata. Planta Med. 2004, 70, 834–840. [Google Scholar] [CrossRef]
- Ellis, B.E. Production of hydroxyphenylethanol glycosides in suspension cultures of Syringa vulgaris. Phytochemistry 1983, 22, 1941–1943. [Google Scholar] [CrossRef]
- Estrada-Zúñiga, M.E.; Cruz-Sosa, F.; Rodríguez-Monroy, M.; Verde-Calvo, J.R.; Vernon-Carter, E.J. Phenylpropanoid production in callus and cell suspension cultures of Buddleja cordata Kunth. Plant Cell Tissue Organ Cult. 2009, 97, 39–47. [Google Scholar] [CrossRef]
- Budzianowska, A.; Kikowska, M.; Małkiewicz, M.; Karolak, I.; Budzianowski, J. Phenylethanoid glycosides in Plantago media L. organs obtained in in vitro cultures. Acta Biol. Crac. Ser. Bot. 2019, 61, 7–18. [Google Scholar] [CrossRef]
- Liu, J.Y.; Guo, Z.G.; Zeng, Z.L. Improved accumulation of phenylethanoid glycosides by precursor feeding to suspension culture of Cistanche salsa. Biochem. Eng. J. 2007, 33, 88–93. [Google Scholar] [CrossRef]
- Georgiev, M.; Ludwig-Müller, J.; Weber, J.; Stancheva, N.; Bley, T. Bioactive metabolite production and stress-related hormones in Devil’s claw cell suspension cultures grown in bioreactors. Appl. Microbiol. Biotechnol. 2011, 89, 1683–1691. [Google Scholar] [CrossRef]
- Piątczak, E.; Kuźma, Ł.; Wysokińska, H. The influence of methyl jasmonate and salicylic acid on secondary metabolite production in Rehmannia glutinosa Libosch. hairy root culture. Acta Biol. Crac. Ser. Bot. 2016, 58, 57–65. [Google Scholar] [CrossRef]
- Piątczak, E.; Kuźma, Ł.; Skała, E.; Żebrowska, M.; Balcerczak, E.; Wysokińska, H. Iridoid and phenylethanoid glycoside production and phenotypical changes in plants regenerated from hairy roots of Rehmannia glutinosa Libosch. Plant Cell Tissue Organ Cult. 2015, 122, 259–266. [Google Scholar] [CrossRef]
- Grąbkowska, R.; Królicka, A.; Mielicki, W.; Wielanek, M.; Wysokińska, H. Genetic transformation of Harpagophytum procumbens by Agrobacterium rhizogenes: Iridoid and phenylethanoid glycoside accumulation in hairy root cultures. Acta Physiol. Plant. 2010, 32, 665–673. [Google Scholar] [CrossRef]
- Kawka, B.; Kwiecień, I.; Ekiert, H. Production of specific flavonoids and verbascoside in shoot cultures of Scutellaria baicalensis. In Plant Cell and Tissue Differentiation and Secondary Metabolites; Ramawat, K., Ekiert, H., Goyal, S., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2021; pp. 249–272. ISBN 9783030112530. [Google Scholar]
- Grzegorczyk-Karolak, I.; Kuźma, Ł.; Wysokińska, H. Study on the chemical composition and antioxidant activity of extracts from shoot culture and regenerated plants of Scutellaria altissima L. Acta Physiol. Plant. 2015, 37, 1736. [Google Scholar] [CrossRef]
- Kawka, B.; Kwiecień, I.; Ekiert, H. Endogenous production of specific flavonoids and verbascoside in agar and agitated microshoot cultures of Scutellaria lateriflora L. and biotransformation potential. Plant Cell Tissue Organ Cult. 2020, 142, 471–482. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Kuźma, Ł.; Wysokińska, H. In vitro cultures of Scutellaria alpina as a source of pharmacologically active metabolites. Acta Physiol. Plant. 2016, 38, 1–9. [Google Scholar] [CrossRef]
- Sreeramulu, D.; Reddy, C.V.K.; Chauhan, A.; Balakrishna, N.; Raghunath, M. Natural antioxidant activity of commonly consumed plant foods in India: Effect of domestic processing. Oxid. Med. Cell. Longev. 2013, 2013, 369479. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of natural plant origins: From sources to food industry applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef]
- Ebrahimzadeh, M.A.; Pourmorad, F.; Bekhradnia, A.R. Iron chelating activity, phenol and flavonoid content of some medicinal plants from Iran. Afr. J. Biotechnol. 2008, 7, 3188–3192. [Google Scholar] [CrossRef]
- De Andrade Lima, C.S.; Cavalcanti de Amorim, E.L.; Ribeiro de Sena, K.X.d.F.; De Andrade Chiappeta, A.; Pereira Nunes, X.; Agra, M.d.F.; Leitão da-Cunha, E.V.; Da Silva, M.S.; Barbosa-Fílho, J.M. Antimicrobial activity of a mixture of two isomeric phenylpropanoid glycosides from Arrabidaea harleyi A.H. Gentry (Bignoniaceae). Rev. Bras. Cienc. Farm. J. Pharm. Sci. 2003, 39, 77–81. [Google Scholar] [CrossRef]
- Shikanga, E.A.; Combrinck, S.; Regnier, T. South African Lippia herbal infusions: Total phenolic content, antioxidant and antibacterial activities. S. Afr. J. Bot. 2010, 76, 567–571. [Google Scholar] [CrossRef]
- Funes, L.; Laporta, O.; Cerdán-Calero, M.; Micol, V. Effects of verbascoside, a phenylpropanoid glycoside from lemon verbena, on phospholipid model membranes. Chem. Phys. Lipids 2010, 163, 190–199. [Google Scholar] [CrossRef]
- Avila, J.G.; De Liverant, J.G.; Martínez, A.; Martínez, G.; Muñoz, J.L.; Arciniegas, A.; Romo De Vivar, A. Mode of action of Buddleja cordata verbascoside against Staphylococcus aureus. J. Ethnopharmacol. 1999, 66, 75–78. [Google Scholar] [CrossRef]



| Rt (min) | Analyte |
|---|---|
| 4.3 | Verbascoside M+ Na+: 647.1932, 471.1488, 325.0914, 163.0381 |
| 4.5 | Isoverbascoside M + Na+: 647.1932, 471.1488, 325.0914, 163.0381 |
| 4.7, 4.9, 5.1 | Leucoseptoside A/isomers M+ Na+: 661.2083, 485.1635, 339.1034, 177.0533 |
| 5.6, 5.8 | Cistanoside D/isomer M+ Na+: 675.2227, 485.1636, 339.1056, 177.0532 |
| Extract | Verbascoside | Isoverbascoside |
|---|---|---|
| Vo-A | 4818.22 ± 79.89 | 330.56 ± 0.17 |
| Vo-LS | 4777.11 ± 5.23 | 264.90 ± 1.33 |
| Vo-LA | 4881.61 ± 99.7 | 451.80 ± 2.85 |
| Vo-RITA | 4722.20 ± 142.74 | 266.50 ± 2.32 |
| Vo-in vivo | 1728.97 ± 29.78 | 78.34 ± 3.28 |
| Extract | Ferulic Acid | Protocatechuic Acid | Rosmarinic Acid | Total Phenolics [mg GAE/g DW] |
|---|---|---|---|---|
| Vo-A | 8.35 ± 0.54 | 3.76 ± 0.07 | nd | 163.58 ± 1.28 |
| Vo-LS | 17.19 ± 0.48 | 5.30 ± 0.19 | nd | 137.96 ± 4.25 |
| Vo-LA | 23.69 ± 0.21 | 7.59 ± 0.48 | nd | 122.93 ± 2.08 |
| Vo-RITA | 16.66 ± 0.75 | 3.51 ± 0.08 | nd | 129.25 ± 1.63 |
| Vo-in vivo | 29.76 ± 2.51 | 25.75 ± 1.65 | 2.53 ± 0.11 | 137.51 ± 3.60 |
| Extract | DPPH Test, IC50 [mg/mL] | Reducing Power Assay [ASE/mL] | Fe2+ Chelating Activity, IC50 [mg/mL] |
|---|---|---|---|
| Vo-A | 0.081 ± 0.036 | 4.215 ± 0.006 | 0.110 ± 0.037 |
| Vo-LS | 0.184 ± 0.051 | 3.048 ± 0.006 | 0.085 ± 0.020 |
| Vo-LA | 0.313 ± 0.022 | 2.452 ± 0.003 | 0.182 ± 0.036 |
| Vo-RITA | 0.125 ± 0.060 | 2.684 ± 0.005 | 0.034 ± 0.017 |
| Vo-in vivo | 0.214 ± 0.011 | 2.800 ± 0.005 | 1.674 ± 0.023 |
| Standard | BHT 0.060 ± 0.003 | BHT 2.776 ± 0.005 | EDTA 0.007 ± 0.002 |
| V. officinalis Extract | Gram-Positive Bacteria | Gram-Negative Bacteria | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. epidermidis | S. aureus | B. cereus | L. monocytogenes | Y. enterocolitica | P. aeruginosa | K. pneumoniae | P. mirabilis | Sh. sonnei | S. enteritidis | E. aerogenes | E. coli | |
| Vo-A | 0.3 (0.6) | 1.1 (2.2) | 2.2 (2.2) | 2.2 (2.2) | 1.1 (1.1) | 1.1 (1.1) | 1.1 (1.1) | 1.1 (1.1) | 2.2 (2.2) | 2.2 (2.2) | 4.5 (4.5) | 4.5 (4.5) |
| Vo-LS | 0.6 (1.1) | 1.1 (1.1) | 1.1 (2.2) | 1.1 (2.2) | 0.6 (0.6) | 0.6 (0.6) | 1.1 (1.1) | 1.1 (1.1) | 1.2 (2.2) | 1.1 (1.1) | 1.1 (2.2) | 2.2 (2.2) |
| Vo-LA | 1.1 (1.1) | 1.1 (1.1) | 2.2 (2.2) | 2.2 (2.2) | 1.1 (1.1) | 1.1 (1.1) | 1.1(1.1) | 2.2 (2.2) | 2.2 (2.2) | 2.2 (2.1) | 4.5 (4.5) | 4.5 (4.5) |
| Vo-RITA | 0.6 (1.1) | 0.6 (1.1) | 1.1 (1.1) | 2.2 (2.2) | 2.2 (2.2) | 1.1 (1.1) | 2.2 (2.2) | 2.2 (2.2) | 2.2 (2.2) | 2.2 (2.2) | 4.5 (4.5) | 4.5 (4.5) |
| Vo-in vivo | 0.6 (9.0) | 1.1 (9.0) | 2.2 (4.5) | 2.2 (9.0) | 1.1 (4.5) | 1.1 (9.0) | 1.1 (4.5) | 2.2 (9.0) | 2.2 (4.5) | 4.5 (9.0) | 4.5 (9.0) | 4.5 (18) |
| MIC [mg/mL] | Vo-A | Vo-LS | Vo-LA | Vo-RITA | Vo-In Vivo |
|---|---|---|---|---|---|
| 0.3 | 8 | 0 | 0 | 0 | 0 |
| 0.6 | 8 | 25 | 0 | 17 | 8 |
| 1.1 | 50 | 92 | 42 | 33 | 42 |
| 2.2 | 83 | 100 | 83 | 83 | 75 |
| 4.5 | 100 | 100 | 100 | 100 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubica, P.; Kokotkiewicz, A.; Malinowska, M.A.; Synowiec, A.; Gniewosz, M.; Hussain, S.; Yaqoob, M.; Bonn, G.K.; Jakschitz, T.; Mahmoud, E.A.; et al. Phenylpropanoid Glycoside and Phenolic Acid Profiles and Biological Activities of Biomass Extracts from Different Types of Verbena officinalis Microshoot Cultures and Soil-Grown Plant. Antioxidants 2022, 11, 409. https://doi.org/10.3390/antiox11020409
Kubica P, Kokotkiewicz A, Malinowska MA, Synowiec A, Gniewosz M, Hussain S, Yaqoob M, Bonn GK, Jakschitz T, Mahmoud EA, et al. Phenylpropanoid Glycoside and Phenolic Acid Profiles and Biological Activities of Biomass Extracts from Different Types of Verbena officinalis Microshoot Cultures and Soil-Grown Plant. Antioxidants. 2022; 11(2):409. https://doi.org/10.3390/antiox11020409
Chicago/Turabian StyleKubica, Paweł, Adam Kokotkiewicz, Magdalena Anna Malinowska, Alicja Synowiec, Małgorzata Gniewosz, Shah Hussain, Muhammad Yaqoob, Günther K. Bonn, Thomas Jakschitz, Eman A. Mahmoud, and et al. 2022. "Phenylpropanoid Glycoside and Phenolic Acid Profiles and Biological Activities of Biomass Extracts from Different Types of Verbena officinalis Microshoot Cultures and Soil-Grown Plant" Antioxidants 11, no. 2: 409. https://doi.org/10.3390/antiox11020409
APA StyleKubica, P., Kokotkiewicz, A., Malinowska, M. A., Synowiec, A., Gniewosz, M., Hussain, S., Yaqoob, M., Bonn, G. K., Jakschitz, T., Mahmoud, E. A., El-Abedin, T. K. Z., Elansary, H. O., Luczkiewicz, M., Ekiert, H., & Szopa, A. (2022). Phenylpropanoid Glycoside and Phenolic Acid Profiles and Biological Activities of Biomass Extracts from Different Types of Verbena officinalis Microshoot Cultures and Soil-Grown Plant. Antioxidants, 11(2), 409. https://doi.org/10.3390/antiox11020409











