An Assessment of Cataract Severity Based on Antioxidant Status and Ascorbic Acid Levels in Aqueous Humor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Sample Collection and Data Acquisition
2.3. Aqueous Humor Antioxidant Capacity
2.4. Statistical Analysis
3. Results
3.1. Study Population
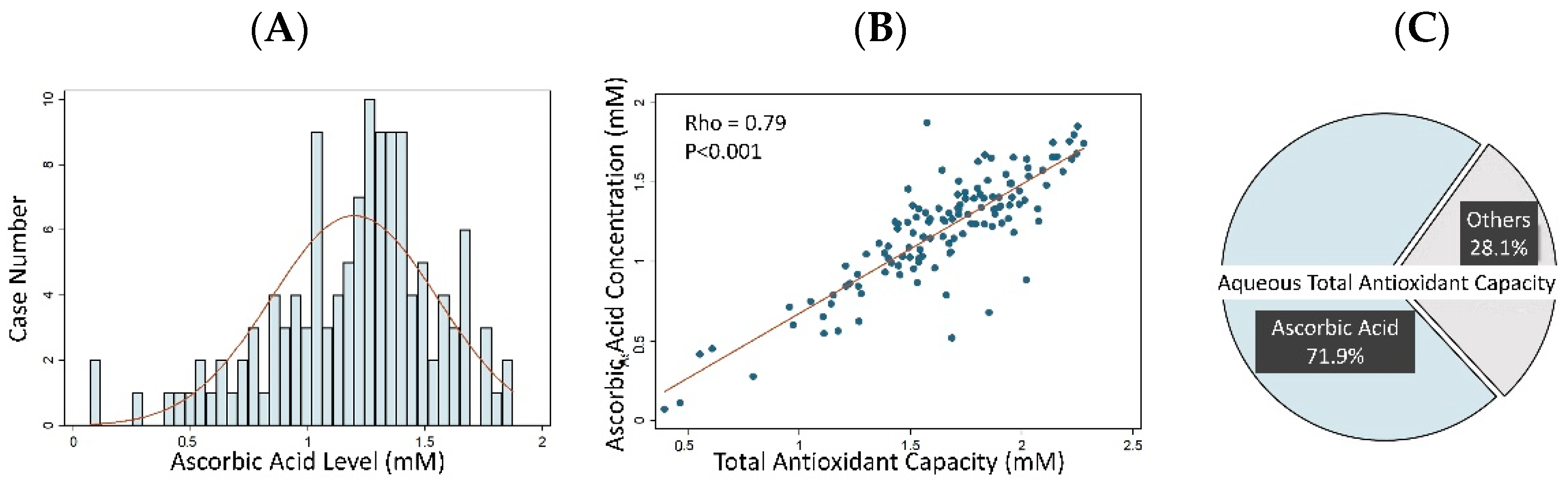
3.2. Aqueous Humor Antioxidant Capacity and Ascorbic Acid Concentration
3.3. Aqueous Humor TAC, Ascorbic Acid, and Cataract Severity
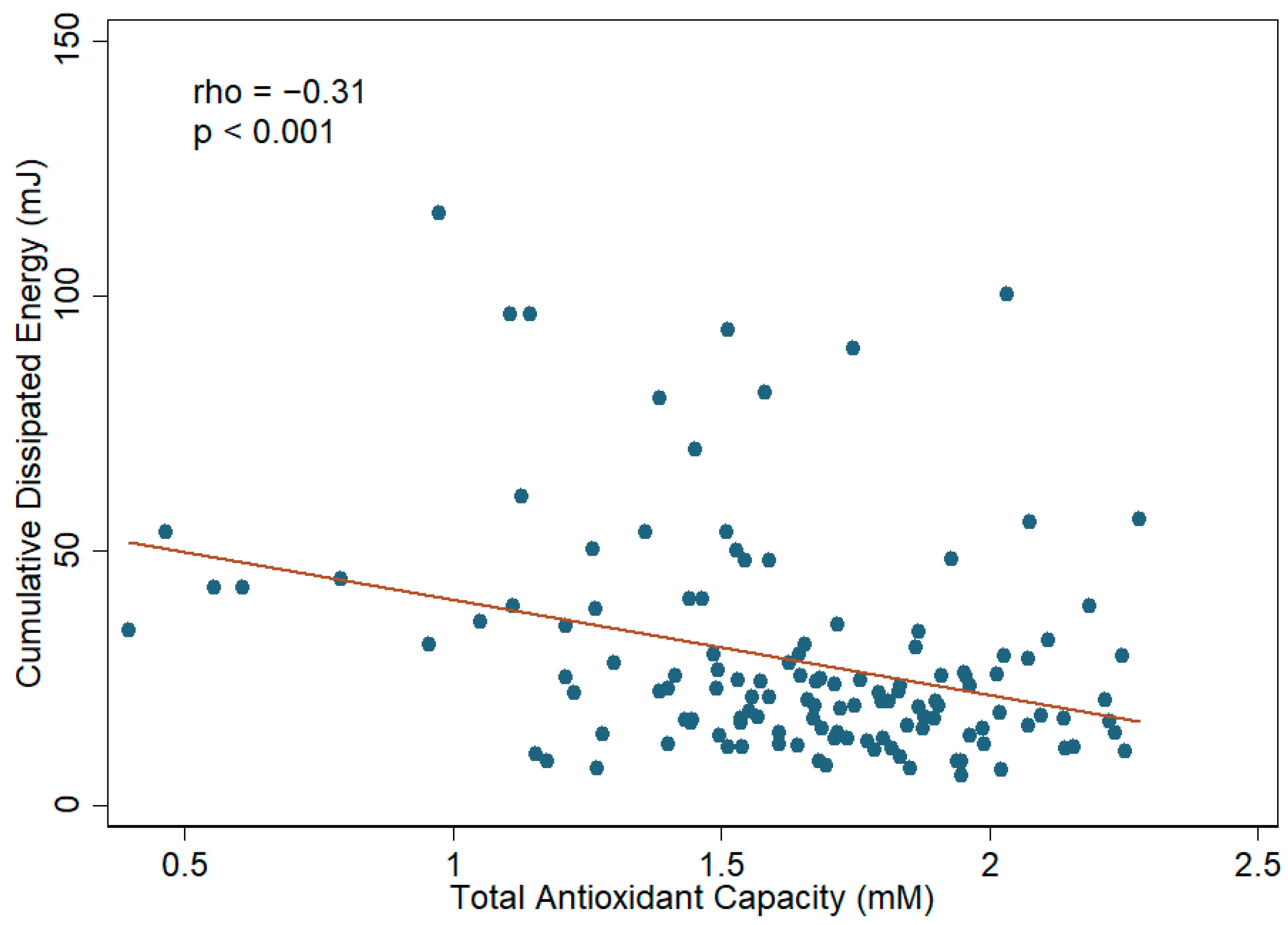
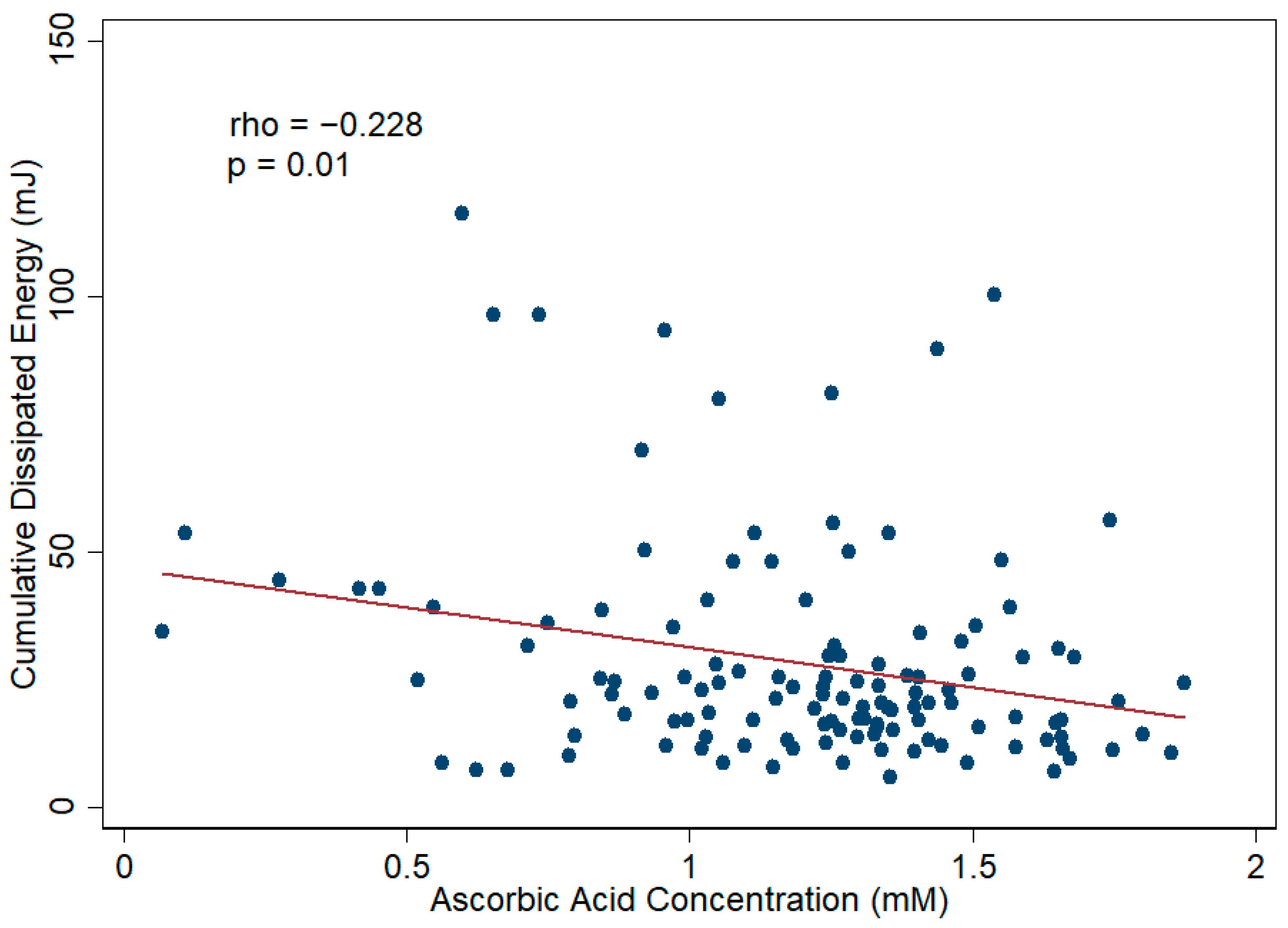
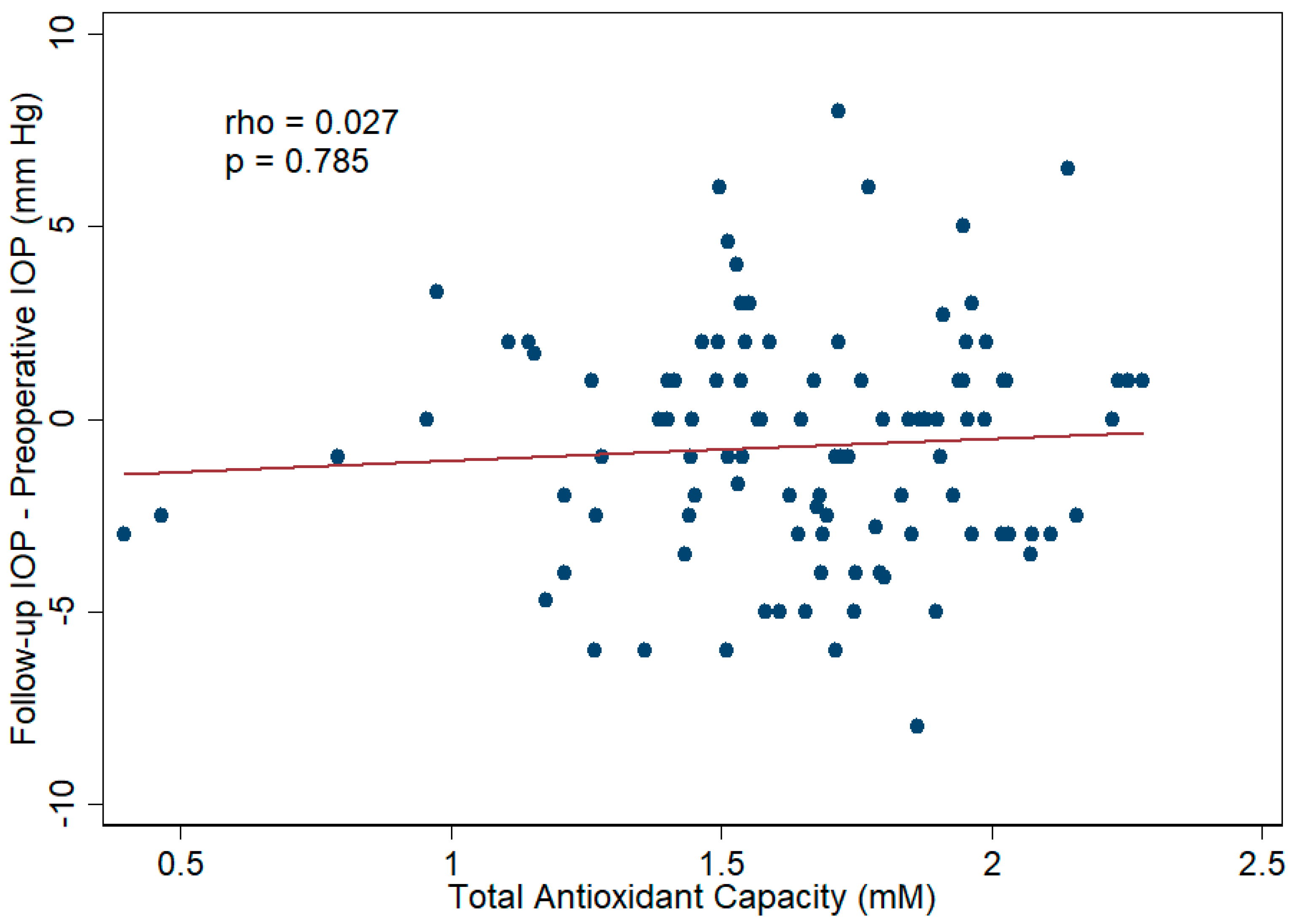
3.4. Aqueous Humor TAC and Cataract Surgical Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hashemi, H.; Pakzad, R.; Yekta, A.; Aghamirsalim, M.; Pakbin, M.; Ramin, S.; Khabazkhoob, M. Global and regional prevalence of age-related cataract: A comprehensive systematic review and meta-analysis. Eye 2020, 34, 1357–1370. [Google Scholar] [CrossRef]
- Pershing, S.; Morrison, D.E.; Hernandez-Boussard, T. Cataract surgery complications and revisit rates among three states. Am. J. Ophthalmol. 2016, 171, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Chylack, L.T., Jr.; Wolfe, J.K.; Singer, D.M.; Leske, M.C.; Bullimore, M.A.; Bailey, I.L.; Friend, J.; McCarthy, D.; Wu, S.-Y. The Lens Opacities Classification System III. Arch. Ophthalmol. 1993, 111, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Schafer, M.E.; Arbisser, B. Quantification of acoustic exposure during cataract surgery. In Proceedings of the IEEE Ultrasonics Symposium, Montreal, QC, Canada, 23–27 August 2004; pp. 1828–1831. [Google Scholar]
- Liu, C.; Liu, Y.; Ye, S.; Liu, L.; Zhang, W.; Wu, M. Effect of topical nonsteroidal anti-inflammatory drugs and nuclear hardness on maintenance of mydriasis during phacoemulsification surgery. J. Ocul. Pharmacol. Ther. 2014, 30, 831–836. [Google Scholar] [CrossRef]
- Sorrentino, F.S.; Matteini, S.; Imburgia, A.; Bonifazzi, C.; Sebastiani, A.; Parmeggiani, F. Torsional phacoemulsification: A pilot study to revise the “harm scale” evaluating the endothelial damage and the visual acuity after cataract surgery. PLoS ONE 2017, 12, e0186975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coco, G.; Cremonesi, P.; Menassa, N.; Pagano, L.; Gadhvi, K.A.; Semeraro, F.; Kaye, S.B.; Romano, V. Changes in pupillometry associated with dissipated energy during phacoemulsification. Eur. J. Ophthalmol. 2020, 31, 2962–2968. [Google Scholar] [CrossRef] [PubMed]
- Shajari, M.; Rusev, V.; Mayer, W.; Diakonis, V.; Petermann, K.; Kohnen, T. Impact of lens density and lens thickness on cumulative dissipated energy in femtosecond laser–assisted cataract surgery. Lasers Med. Sci. 2019, 34, 1229–1234. [Google Scholar] [CrossRef]
- Al-Osaily, A.M.; Al-Jindan, M.Y. Intra-correlations between cataract density based on Scheimpflug image, phacodynamics, surgery duration, and endothelial cell loss after phacoemulsification. Saudi J. Ophthalmol. 2018, 32, 188–193. [Google Scholar] [CrossRef]
- Kaur, J.; Kukreja, S.; Kaur, A.; Malhotra, N.; Kaur, R. The oxidative stress in cataract patients. J. Clin. Diagn. Res. 2012, 6, 1629–1632. [Google Scholar] [CrossRef]
- Tsao, Y.T.; Wu, W.C.; Chen, K.J.; Yeh, L.K.; Hwang, Y.S.; Hsueh, Y.J.; Chen, H.C.; Cheng, C.M. Analysis of aqueous humor total antioxidant capacity and its correlation with corneal endothelial health. Bioeng. Transl. Med. 2020, 6, e10199. [Google Scholar] [CrossRef]
- Sorkhabi, R.; Ghorbanihaghjo, A.; Javadzadeh, A.; Rashtchizadeh, N.; Moharrery, M. Oxidative DNA damage and total antioxidant status in glaucoma patients. Mol. Vis. 2011, 17, 41–46. [Google Scholar] [PubMed]
- Gawali, K.S.; Patil, N.B. Catalase activity and total antioxidant capacity in lens epithelial cells of different morphological types of senile cataract patients. Int. J. Res. Med. 2016, 5, 92–96. [Google Scholar]
- Mathew, M.C.; Ervin, A.M.; Tao, J.; Davis, R.M. Antioxidant vitamin supplementation for preventing and slowing the progression of age-related cataract. Cochrane Database Syst. Rev. 2012, 6, CD004567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacques, P.F.; Chylack, L.T., Jr. Epidemiologic evidence of a role for the antioxidant vitamins and carotenoids in cataract prevention. Am. J. Clin. Nutr. 1991, 53, 352S–355S. [Google Scholar] [CrossRef]
- Mares, J. Food antioxidants to prevent cataract. JAMA 2015, 313, 1048–1049. [Google Scholar] [CrossRef]
- Taylor, A.; Jacques, P.F.; Nadler, D.; Morrow, F.; Sulsky, S.I.; Shepard, D. Relationship in humans between ascorbic acid consumption and levels of total and reduced ascorbic acid in lens, aqueous humor, and plasma. Curr. Eye Res. 1991, 10, 751–759. [Google Scholar] [CrossRef]
- Huang, W.; Koralewska-Makár, A.; Bauer, B.; Åkesson, B. Extracellular glutathione peroxidase and ascorbic acid in aqueous humor and serum of patients operated on for cataract. Clin. Chim. Acta 1997, 261, 117–130. [Google Scholar] [CrossRef]
- Cox, J.T.; Subburaman, G.-B.B.; Munoz, B.; Friedman, D.S.; Ravindran, R.D. Visual acuity outcomes after cataract surgery: High-volume versus low-volume surgeons. Ophthalmology 2019, 126, 1480–1489. [Google Scholar] [CrossRef] [Green Version]
- Schulze-Bonsel, K.; Feltgen, N.; Burau, H.; Hansen, L.; Bach, M. Visual Acuities “Hand Motion” and “Counting Fingers” Can Be Quantified with the Freiburg Visual Acuity Test. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1236–1240. [Google Scholar] [CrossRef]
- Shichi, H.; Page, T.; Sahouri, M.J.; Shin, D.H. Microplate assay of ascorbic acid in aqueous humor with bicinchoninic acid. J. Ocul. Pharmacol. Ther. 1997, 13, 201–206. [Google Scholar] [CrossRef]
- Rubio, C.P.; Hernández-Ruiz, J.; Martinez-Subiela, S.; Tvarijonaviciute, A.; Ceron, J.J. Spectrophotometric assays for total antioxidant capacity (TAC) in dog serum: An update. BMC Vet. Res. 2016, 12, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choy, C.K.M.; Benzie, I.F.F.; Cho, P. Ascorbic acid concentration and total antioxidant activity of human tear fluid measured using the FRASC assay. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3293–3298. [Google Scholar]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Gale, C.R.; Hall, N.F.; Phillips, D.I.; Martyn, C.N. Plasma antioxidant vitamins and carotenoids and age-related cataract. Ophthalmology 2001, 108, 1992–1998. [Google Scholar] [CrossRef]
- Li, L.; Duker, J.S.; Yoshida, Y.; Niki, E.; Rasmussen, H.; Russell, R.M.; Yeum, K.-J. Oxidative stress and antioxidant status in older adults with early cataract. Eye 2009, 23, 1464–1468. [Google Scholar] [CrossRef] [Green Version]
- Cao, G.; Alessio, H.M.; Cutler, R.G. Oxygen-radical absorbance capacity assay for antioxidants. Free Radic. Biol. Med. 1993, 14, 303–311. [Google Scholar] [CrossRef] [Green Version]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef] [Green Version]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef]
- Prior, R.L.; Cao, G. In vivo total antioxidant capacity: Comparison of different analytical methods1. Free Radic. Biol. Med. 1999, 27, 1173–1181. [Google Scholar] [CrossRef]
- Cao, G.; Prior, R.L. Comparison of different analytical methods for assessing total antioxidant capacity of human serum. Clin. Chem. 1998, 44, 1309–1315. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, H.; Keles, S.; Koçer, I.; Akçay, F. Diabetic cataract and the total antioxidant status in aqueous humor. De Gruyter 2001, 39, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Mancino, R.; Di Pierro, D.; Varesi, C.; Cerulli, A.; Feraco, A.; Cedrone, C.; Pinazo-Duran, M.D.; Coletta, M.; Nucci, C. Lipid peroxidation and total antioxidant capacity in vitreous, aqueous humor, and blood samples from patients with diabetic retinopathy. Mol. Vis. 2011, 17, 1298. [Google Scholar] [PubMed]
- Beyazyıldız, E.; Çankaya, A.B.; Ergan, E.; Anayol, M.A.; Özdamar, Y.; Sezer, S.; Tırhış, M.H.; Yılmazbaş, P.; Öztürk, F. Changes of total antioxidant capacity and total oxidant status of aqueous humor in diabetes patients and correlations with diabetic retinopathy. Int. J. Ophthalmol. 2013, 6, 531. [Google Scholar] [PubMed] [Green Version]
- Nucci, C.; Di Pierro, D.; Varesi, C.; Ciuffoletti, E.; Russo, R.; Gentile, R.; Cedrone, C.; Duran, M.D.P.; Coletta, M.; Mancino, R. Increased malondialdehyde concentration and reduced total antioxidant capacity in aqueous humor and blood samples from patients with glaucoma. Mol. Vis. 2013, 19, 1841. [Google Scholar]
- Beyazyıldız, E.; Çankaya, A.B.; Beyazyıldız, Ö.; Ergan, E.; Çelik, H.T.; Yılmazbaş, P.; Öztürk, F. Disturbed oxidant/antioxidant balance in aqueous humour of patients with exfoliation syndrome. Jpn. J. Ophthalmol. 2014, 58, 353–358. [Google Scholar] [CrossRef]
- Kirboga, K.; Ozec, A.V.; Kosker, M.; Dursun, A.; Toker, M.I.; Aydin, H.; Erdogan, H.; Topalkara, A.; Arici, M.K. The association between diabetic retinopathy and levels of ischemia-modified albumin, total thiol, total antioxidant capacity, and total oxidative stress in serum and aqueous humor. J. Ophthalmol. 2014, 2014, 820853. [Google Scholar] [CrossRef] [Green Version]
- Ergan, E.; Ozturk, F.; Beyazyildiz, E.; Elgin, U.; Sen, E.; Cankaya, A.B.; Celik, T. Oxidant/antioxidant balance in the aqueous humor of patients with glaucoma. Int. J. Ophthalmol. 2016, 9, 249. [Google Scholar]
- Kulaksızoglu, S.; Karalezli, A. Aqueous humour and serum levels of nitric oxide, malondialdehyde and total antioxidant status in patients with type 2 diabetes with proliferative diabetic retinopathy and nondiabetic senile cataracts. Can. J. Diabetes 2016, 40, 115–119. [Google Scholar] [CrossRef]
- Altinisik, M.; Koytak, A.; Elbay, A.; Toklu, E.; Sezer, T.; Kocyigit, A. Oxidant-antioxidant balance in the aqueous humor of patients with retinal vein occlusion. In The Seminars in Ophthalmology; Taylor & Francis: Abingdon, UK, 2018; pp. 675–682. [Google Scholar]
- Bozkurt, E.; Çakır, B.; Çelik, E.; Doğan, E.; Uçak, T.; Alagöz, G. Correlation of the aqueous humor total antioxidant capacity, total oxidant status, and levels of IL-6 and VEGF with diabetic retinopathy status. Arq. Bras. Oftalmol. 2019, 82, 136–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegfried, C.J.; Shui, Y.-B. Intraocular oxygen and antioxidant status: New insights on the effect of vitrectomy and glaucoma pathogenesis. Am. J. Ophthalmol. 2019, 203, 12–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinson, J.A. Oxidative stress in cataracts. Pathophysiology 2006, 13, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Pardhan, S.; Kulothungan, V.; Swaminathan, G.; Ravichandran, J.S.; Ganesan, S.; Sharma, T.; Raman, R. The prevalence and risk factors for cataract in rural and urban India. Indian J. Ophthalmol. 2019, 67, 477–483. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, X.; Wang, J.; Jin, L.; Huang, W.; Luo, Y.; Lu, Y. Risk factors of age-related cataract in a Chinese adult population: The Taizhou Eye Study. Clin. Exp. Ophthalmol. 2018, 46, 371–379. [Google Scholar] [CrossRef]
- Garner, B.; Davies, M.J.; Truscott, R.J. Formation of hydroxyl radicals in the human lens is related to the severity of nuclear cataract. Exp. Eye Res. 2000, 70, 81–88. [Google Scholar] [CrossRef]
- Nakazawa, Y.; Nagai, N.; Ishimori, N.; Oguchi, J.; Tamura, H. Administration of antioxidant compounds affects the lens chaperone activity and prevents the onset of cataracts. Biomed. Pharmacother. 2017, 95, 137–143. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, J.; Li, J.; Jiang, H.; Kong, J. Klotho ameliorates the onset and progression of cataract via suppressing oxidative stress and inflammation in the lens in streptozotocin-induced diabetic rats. Int. Immunopharmacol. 2020, 85, 106582. [Google Scholar] [CrossRef]
- Davison, J.A.; Chylack, L.T., Jr. Clinical application of the lens opacities classification system III in the performance of phacoemulsification. J. Cataract Refract. Surg. 2003, 29, 138–145. [Google Scholar] [CrossRef]
- Lee, R.Y.; Chen, R.I.; Kasuga, T.; Cui, Q.N.; Porco, T.C.; Lin, S.C. The Effect of Cumulative Dissipated Energy on Changes in Intraocular Pressure Following Uncomplicated Cataract Surgery by Phacoemulsification. J. Glaucoma 2016, 25, 565. [Google Scholar] [CrossRef] [Green Version]
- Sahu, P.; Das, G.; Malik, A.; Biakthangi, L. Dry eye following phacoemulsification surgery and its relation to associated intraoperative risk factors. Middle East Afr. J. Ophthalmol. 2015, 22, 472. [Google Scholar] [CrossRef]
- Charles Crozafon, P.; Bouchet, C.; Zignani, M.; Griner, R.; Foster, S.D.; Zou, M.; Dhariwal, M. Comparison of real-world treatment outcomes of femtosecond laser-assisted cataract surgery and phacoemulsification cataract surgery: A retrospective, observational study from an outpatient clinic in France. Eur. J. Ophthalmol. 2021, 31, 1809–1816. [Google Scholar] [CrossRef] [PubMed]
- Anastasilakis, K.; Mourgela, A.; Symeonidis, C.; Dimitrakos, S.A.; Ekonomidis, P.; Tsinopoulos, I. Macular edema after uncomplicated cataract surgery: A role for phacoemulsification energy and vitreoretinal interface status? Eur. J. Ophthalmol. 2015, 25, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, M.; Wang, Y.; Ben, S.; Gao, M.; Zhang, S.; Liu, H.; Sun, X. Short-term changes in retinal vasculature and layer thickness after phacoemulsification surgery. Curr. Eye Res. 2020, 45, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Mahdy, M.A.S.; Eid, M.Z.; Mohammed, M.A.-B.; Hafez, A.; Bhatia, J. Relationship between endothelial cell loss and microcoaxial phacoemulsification parameters in noncomplicated cataract surgery. Clin. Ophthalmol. 2012, 6, 503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonen, T.; Sever, O.; Horozoglu, F.; Yasar, M.; Keskinbora, K.H. Endothelial cell loss: Biaxial small-incision torsional phacoemulsification versus biaxial small-incision longitudinal phacoemulsification. J. Cataract Refract. Surg. 2012, 38, 1918–1924. [Google Scholar] [CrossRef] [PubMed]



| Basic Characteristics | |
|---|---|
| OD/OS, n | 59/71 |
| Age, mean ± SD (Years) | 67.6 ± 8.9 |
| Gender, M/F n (%) | 67 (51.5%)/63 (48.5%) |
| BMI, mean ± SD (Kg/m2) | 24.95 ± 3.49 |
| Disease diagnosis, n (%) | ARC: 116 (89.2%) Juvenile cataract: 14 (10.8%) |
| Underlying Diseases | |
| Hypertension, n (%) | 51 (39.2%) |
| Diabetes mellitus, n (%) | 28 (21.5%) |
| Other underlying systemic diseases, n (%) | 55 (42.3%) |
| Variable | Univariate Regression | Multivariate Regression | ||||
|---|---|---|---|---|---|---|
| B | SE | p Value | B | SE | p Value | |
| Total antioxidant capacity | −18.62 | 4.85 | <0.001 *** | −18.47 | 4.89 | <0.001 *** |
| Basic characteristics | ||||||
| OD/OS | 0.31 | 3.79 | 0.934 | −0.128 | 3.53 | 0.971 |
| Age | 0.45 | 0.21 | 0.034 * | 0.719 | 0.27 | 0.008 ** |
| Gender | −2.58 | 3.77 | 0.496 | 1.130 | 3.65 | 0.758 |
| BMI | −0.01 | 0.54 | 0.99 | −0.426 | 0.55 | 0.444 |
| Disease diagnosis | −0.11 | 6.09 | 0.986 | 13.92 | 7.82 | 0.078 |
| Underlying disease | ||||||
| Hypertension | 0.61 | 3.87 | 0.876 | 0.676 | 4.3 | 0.875 |
| Diabetes mellitus | 11.4 | 4.48 | 0.012 * | 8.623 | 4.44 | 0.055 |
| Other underlying systemic disease | −3.73 | 3.81 | 0.329 | −3.996 | 4.03 | 0.323 |
| Crude Odds Ratio | Adjusted Odds Ratio | |||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Nuclear Opalescence | ||||
| Grade 2 vs. Grade 1 | 0.453 (0.04–5.18) | 0.524 | 0.348 (0.028–4.301) | 0.411 |
| Grade 3 vs. Grade 1 | 0.11 (0.016–0.735) | 0.023 * | 0.078 (0.011–0.571) | 0.012 * |
| Cortical Cataract | ||||
| Grade 2 vs. Grade 1 | 0.027 (0.001–1.055) | 0.053 | 0.001 (0–0.355) | 0.02 * |
| Grade 3 vs. Grade 1 | 0.005 (0–0.147) | 0.002 ** | < 0.001 (0–0.051) | <0.001 *** |
| Reference | Total Antioxidant Capacity (Unit: mmol/L Trolex) | Sample Size | Measurement Method |
|---|---|---|---|
| Aksoy et al. 2001 [34] | 0.41 ± 0.04 | 16 | Spectrophotometric method (elimination of ABTSR+) |
| Mancino et al. 2011 [35] | 0.94 ± 0.26 | 14 | Fluorescence method (inhibit the peroxidation of β-phycoerythrin) |
| Sorkhabi et al. 2011 [12] | 0.34 ± 0.15 | 27 | Spectrophotometric method (elimination of ABTS+) |
| Beyazyıldız et al. 2013 [36] | 2.54 ± 0.71 | 33 | Colorimetric change in Fe2+ |
| Nucci et al. 2013 [37] | 0.963 ± 0.302 | 26 | Fluorescence method (inhibit the peroxidation of β-phycoerythrin) |
| Beyazyıldız et al. 2014 [38] | 2.5 ± 0.7 | 25 | Colorimetric change in Fe2+ |
| Kirboga et al. 2014 [39] | 0.65 ± 0.09 | 22 | Colorimetric method (reduction of 2,2′-azino-bis radical) |
| Ergan et al. 2016 [40] | 1.80 ± 0.79 | 31 | Colorimetric method (inhibit oxidation of H2O2) |
| Kulaksızoglu et al. 2016 [41] | 0.77 ± 0.07 | 35 | TAS kit (unknown mechanism) |
| Altinisik et al. 2018 [42] | 0.78 ± 0.46 | 28 | Colorimetric change in Fe2+ |
| Bozkurt et al. 2019 [43] | 0.39 [0.39–0.44] | 28 | Colorimetric immunodiagnostic assay (H2O2 elimination) |
| Siegfried et al. 2019 [44] | 0.479 ± 0.146 | 72 | Luminescence method (elimination of alkyl peroxyl radicals) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsao, Y.-T.; Wu, W.-C.; Chen, K.-J.; Liu, C.-F.; Hsueh, Y.-J.; Cheng, C.-M.; Chen, H.-C. An Assessment of Cataract Severity Based on Antioxidant Status and Ascorbic Acid Levels in Aqueous Humor. Antioxidants 2022, 11, 397. https://doi.org/10.3390/antiox11020397
Tsao Y-T, Wu W-C, Chen K-J, Liu C-F, Hsueh Y-J, Cheng C-M, Chen H-C. An Assessment of Cataract Severity Based on Antioxidant Status and Ascorbic Acid Levels in Aqueous Humor. Antioxidants. 2022; 11(2):397. https://doi.org/10.3390/antiox11020397
Chicago/Turabian StyleTsao, Yu-Ting, Wei-Chi Wu, Kuan-Jen Chen, Chun-Fu Liu, Yi-Jen Hsueh, Chao-Min Cheng, and Hung-Chi Chen. 2022. "An Assessment of Cataract Severity Based on Antioxidant Status and Ascorbic Acid Levels in Aqueous Humor" Antioxidants 11, no. 2: 397. https://doi.org/10.3390/antiox11020397
APA StyleTsao, Y.-T., Wu, W.-C., Chen, K.-J., Liu, C.-F., Hsueh, Y.-J., Cheng, C.-M., & Chen, H.-C. (2022). An Assessment of Cataract Severity Based on Antioxidant Status and Ascorbic Acid Levels in Aqueous Humor. Antioxidants, 11(2), 397. https://doi.org/10.3390/antiox11020397








