Chemical Composition and Antioxidant Activity of Ammi visnaga L. Essential Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Characterization of A. visnaga Essential Oil
2.1.1. Plant Material
2.1.2. Isolation of Essential Oil
2.1.3. Chemicals and Reagents
2.1.4. Gas Chromatography–Mass Spectrometry of Essential Oil
2.2. The In Vitro Antioxidant Assay
2.2.1. Total Polyphenol Determination
2.2.2. Total Flavonoid Determination
2.2.3. Condensed Tannins Determination
2.2.4. 2,2′-Diphenyl-1-picrylhydrazyl (DPPH) Assay
2.2.5. Reducing Power Assay
2.3. The In Vivo Antioxidant Assay
2.3.1. Animal Models and Induction of Oxidative Stress
2.3.2. Study Design
- Group I was designated as vehicle and was treated with 0.1% CMC;
- Group II (negative control) received no treatment but had free access to water and food;
- Groups III (toxic control), IV, V, and VI received a single intraperitoneal injection of APAP (400 mg/kg, ip) before the start of the experiment to induce hepato-renal oxidative injury. Group IV served as the standard and received AA, 200 mg/kg body weight. Groups V and VI received A. visnaga L. essential oil at doses of 600 and 1200 mg/kg body weight.
2.3.3. Body and Organ Weight
2.3.4. Preparation of Tissue Homogenates
2.3.5. Assessment of Oxidative Stress Biomarkers in Tissue Homogenate
Evaluation of the Enzymatic Activity of Catalase
Evaluation of the Enzymatic Activity of Superoxide Dismutase
Evaluation of the Enzymatic Activity of Reduced Glutathione
Estimation of Lipid Peroxidation
2.4. Statistical Analysis
3. Results
3.1. Chemical Characterization of A. visnaga L. Essential Oil
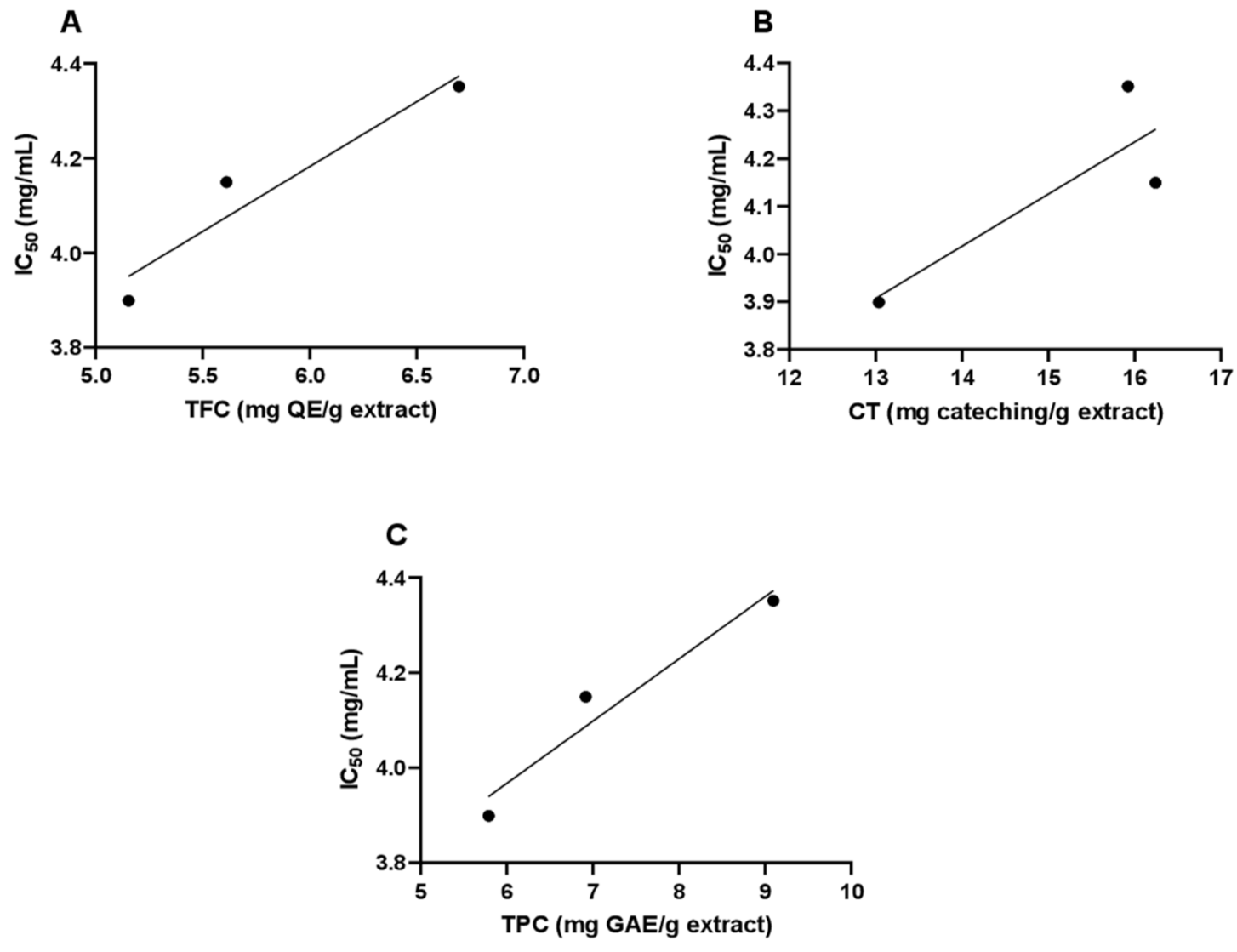
3.2. Total Phenol, Total Flavonoid, and Total Condensed Tannins Contents of A. visnaga L. Essential Oil
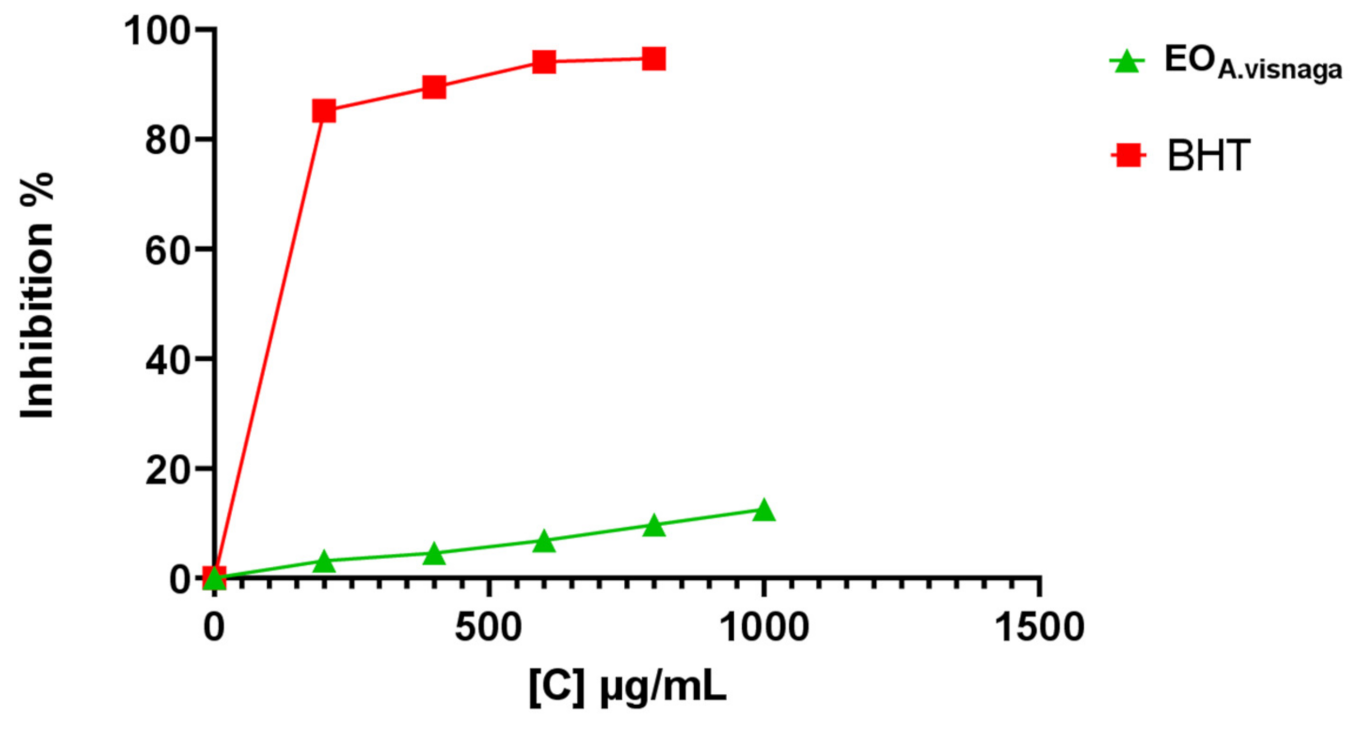
3.3. Determination of the In Vitro Antioxidant Activities
3.4. Determination of In Vivo Antioxidant Potential
3.4.1. The Effects of the Essential Oil of A. visnaga L. on the Body Weight of the Treated Mice
3.4.2. Antioxidant Activity Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zahra, K.F.; Lefter, R.; Ali, A.; Abdellah, E.-C.; Trus, C.; Ciobica, A.; Timofte, D. The Involvement of the Oxidative Stress Status in Cancer Pathology: A Double View on the Role of the Antioxidants. Oxid. Med. Cell. Longev. 2021, 2021, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanciu, G.D.; Luca, A.; Rusu, R.N.; Bild, V.; Chiriac, S.I.B.; Solcan, C.; Bild, W.; Ababei, D.C. Alzheimer’s Disease Pharmacotherapy in Relation to Cholinergic System Involvement. Biomolecules 2020, 10, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanciu, G.; Rusu, R.; Bild, V.; Filipiuc, L.; Tamba, B.-I.; Ababei, D. Systemic Actions of SGLT2 Inhibition on Chronic mTOR Activation as a Shared Pathogenic Mechanism between Alzheimer’s Disease and Diabetes. Biomedicines 2021, 9, 576. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Free radicals and antioxidants–quo vadis? Trends Pharmacol. Sci. 2011, 32, 125–130. [Google Scholar] [CrossRef]
- Devaraj, S.; Leonard, S.; Traber, M.G.; Jialal, I. Gamma-tocopherol supplementation alone and in combination with al-pha-tocopherol alters biomarkers of oxidative stress and inflammation in subjects with metabolic syndrome. Free Radic. Biol. Med. 2008, 44, 1203–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouayed, J.; Rammal, H.; Soulimani, R. Oxidative Stress and Anxiety: Relationship and Cellular Pathways. Oxidative Med. Cell. Longev. 2009, 2, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, K.R.C.; dos Santos, C.P.; de Medeiros, L.A.; Mendes, J.A.; Cunha, T.M.; De Angelis, K.; Penha-Silva, N.; de Oliveira, E.P.; Crispim, C.A. Night workers have lower levels of antioxidant defenses and higher levels of oxidative stress damage when compared to day workers. Sci. Rep. 2019, 9, 4455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ávila-Escalante, M.L.; Coop-Gamas, F.; Cervantes-Rodríguez, M.; Méndez-Iturbide, D.; Aranda-González, I.I. The effect of diet on oxidative stress and metabolic diseases—Clinically controlled trials. J. Food Biochem. 2020, 44, e13191. [Google Scholar] [CrossRef] [PubMed]
- Guemouri, L.; Artur, Y.; Herbeth, B.; Jeandel, C.; Cuny, G.; Siest, G. Biological variability of superoxide dismutase, glutathione peroxidase, and catalase in blood. Clin. Chem. 1991, 37, 1932–1937. [Google Scholar] [CrossRef]
- Gorni, D.; Finco, A. Oxidative stress in elderly population: A prevention screening study. Aging Med. 2020, 3, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Hassan, W.; Noreen, H.; Rehman, S.; Gul, S.; Amjad Kamal, M.; Paul Kamdem, J.; Zaman, B.; da Rocha, J.B.T. Oxidative Stress and Antioxidant Potential of One Hundred Medicinal Plants. Curr. Top. Med. Chem. 2017, 17, 1336–1370. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.-P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.-J.; Li, H.-B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Jeyabalan, J.; Aqil, F.; Soper, L.; Schultz, D.J.; Gupta, R.C. Potent Chemopreventive/Antioxidant Activity Detected in Common Spices of the Apiaceae Family. Nutr. Cancer 2015, 67, 1201–1207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Önder, A.; Çinar, A.S.; Sarialtin, S.Y.; Izgi, M.N.; Çoban, T. Evaluation of the Antioxidant Potency of Seseli L. Species (Apiaceae). Turk. J. Pharm. Sci. 2020, 17, 197–202. [Google Scholar] [CrossRef]
- Thiviya, P.; Gamage, A.; Piumali, D.; Merah, O.; Madhujith, T. Apiaceae as an Important Source of Antioxidants and Their Applications. Cosmetics 2021, 8, 111. [Google Scholar] [CrossRef]
- Khalil, N.; Bishr, M.; Desouky, S.; Salama, O. Ammi Visnaga L., a Potential Medicinal Plant: A Review. Molecules 2020, 25, 301. [Google Scholar] [CrossRef] [Green Version]
- Bhagavathula, A.S.; Al-Khatib, A.J.M.; Elnour, A.A.; Al Kalbani, N.M.; Shehab, A. Ammi Visnaga in treatment of urolithiasis and hypertriglyceridemia. Pharm. Res. 2014, 7, 397–400. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Kim, D.-O.; Chun, O.K.; Kim, Y.J.; Moon, H.-Y.; Lee, C.Y. Quantification of Polyphenolics and Their Antioxidant Capacity in Fresh Plums. J. Agric. Food Chem. 2003, 51, 6509–6515. [Google Scholar] [CrossRef]
- Price, M.L.; Van Scoyoc, S.; Butler, L.G. A critical evaluation of the vanillin reaction as an assay for tannin in sorghum grain. J. Agric. Food Chem. 1978, 26, 1214–1218. [Google Scholar] [CrossRef]
- Bougandoura, N.; Bendimerad, N. Evaluation de l’activité antioxydante des extraits aqueux et méthanolique de Satureja calamintha ssp.Nepeta (L.) Briq. Rev. Nat. Technol. 2013, 5, 14–19. [Google Scholar]
- Samarth, R.M.; Panwar, M.; Kumar, M.; Soni, A.; Kumar, M.; Kumar, A. Evaluation of antioxidant and radical-scavenging activities of certain radioprotective plant extracts. Food Chem. 2008, 106, 868–873. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction. Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Huang, B.; He, J.; Han, L.; Zhan, Y.; Wang, Y. In vitro and in vivo antioxidant effects of the ethanolic extract of Swertia chirayita. J. Ethnopharmacol. 2011, 136, 309–315. [Google Scholar] [CrossRef]
- Merghem, M.; Dahamna, S. In-Vitro Antioxidant Activity and Total Phenolic Content of Ruta montana L. Extracts. J. Drug Deliv. Ther. 2020, 10, 69–75. [Google Scholar] [CrossRef]
- Aebi, H. Catalase. Methods of enzymatic analysis. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Verlag Chemie, Academic Press Inc.: Cambridge, MA, USA, 1974; pp. 673–684. [Google Scholar]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; pp. 121–126. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Kakkar, P.; Das, B.; Viswanathan, P.N. A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys. 1984, 21, 130–132. [Google Scholar]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Sastre, J.; Pallardó, F.V.; de la Asunción, J.G.; Viña, J. Mitochondria, oxidative stress and aging. Free Radic. Res. 2000, 32, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Keddad, A.; Baaliouamer, A.; Hazzit, M. Chemical Composition and Antioxidant Activity of Essential Oils from Umbels of Algerian Ammi visnaga (L.). J. Essent. Oil Bear. Plants 2016, 19, 1243–1250. [Google Scholar] [CrossRef]
- Khadhri, A.; El Mokni, R.; Mguis, K.; Araujo, M.E.M. Variability of two essential oils of Ammi visnaga (L.) Lam. a traditional Tunisian medicinal plant. J. Med. Plant Res. 2011, 5, 5079–5082. [Google Scholar]
- Khalfallah, A.; Labed, A.; Semra, Z.; Kaki, B.; Kabouche, A.; Touzani, R.; Kabouche, Z. Antibacterial activity and chemical composition of the essential oil of Ammi visnaga L. (Apiaceae) from Constantine, Algeria. Int. J. Med. Aromat. Plants 2011, 1, 302–305. [Google Scholar]
- Satrani, B.; Farah, A.; Talbi, M. Composition chimique et activité antibactérienne et antifongique de l’huile essentielle extraite du bois de Tetraclinis articulata du Maroc. Ann. Falsif. Expert. Chim. Toxicol. 2004, 97, 75–84. [Google Scholar]
- Günaydin, K.; Beyazit, N. The chemical investigations on the ripe fruits of Ammi visnaga (Lam.) Lamarck growing in Turkey. Nat. Prod. Res. 2004, 18, 169–175. [Google Scholar] [CrossRef]
- Feirouz, B.; Salima, K.-G. Antibacterial Activity and Chemical Composition ofAmmi visnaga, L. Essential Oil Collected from Boumerdes (Algeria) During Three Periods of the Plant Growth. J. Essent. Oil Bear. Plants 2014, 17, 1317–1328. [Google Scholar] [CrossRef]
- Bungau, S.; Behl, T.; Aleya, L.; Bourgeade, P.; Aloui-Sossé, B.; Purza, A.L.; Abid, A.; Samuel, A.D. Expatiating the impact of anthropogenic aspects and climatic factors on long-term soil monitoring and management. Environ. Sci. Pollut. Res. 2021, 28, 30528–30550. [Google Scholar] [CrossRef]
- Gitea, M.A.; Gitea, D.; Tit, D.M.; Purza, L.; Samuel, A.D.; Bungau, S.; Badea, G.E.; Aleya, L. Orchard management under the effects of climate change: Implications for apple, plum, and almond growing. Environ. Sci. Pollut. Res. 2019, 26, 9908–9915. [Google Scholar] [CrossRef]
- Sellami, H.K.; Flamini, G.; Cioni, P.L.; Smiti, S. Composition of the Essential Oils in Various Organs at Different Developmental Stages of Ammi visnaga (L.) Lam. from Tunisia. Chem. Biodivers. 2011, 8, 1990–2004. [Google Scholar] [CrossRef]
- Hashim, S.; Jan, A.; Marwat, K.B.; Khan, M.A. Phytochemistry and medicinal properties of Ammi visnaga (Apiacae). Pak. J. Bot. 2014, 46, 861–867. [Google Scholar]
- Samuel, A.D.; Bungau, S.; Tit, D.M.; Melinte, C.E.; Purza, L.; Badea, G.E. Effects of Long Term Application of Organic and Mineral Fertilizers on Soil Enzymes. Rev. Chim. 2018, 69, 2608–2612. [Google Scholar] [CrossRef]
- Samuel, A.D.; Tit, D.M.; Melinte, C.E.; Iovan, C.; Purza, L.; Gitea, M.; Bungau, S. Enzymological and physico-chemical evaluation of the effects of soil management practices. Rev. Chim. 2017, 68, 2243–2247. [Google Scholar] [CrossRef]
- El Karkouri, J.; Drioiche, A.; Soro, A.; Ailli, A.; Benhlima, N.; Bouzoubaa, A.; El Makhoukhi, F.; Oulhaj, H.; Elombo, F.K.; Zair, T. Identification and antioxidant activity of Ammi visnaga L. polyphenols from the Middle Atlas in Morocco. Mediterr. J. Chem. 2020, 10, 649–658. [Google Scholar] [CrossRef]
- Aourabi, S.; Driouch, M.; Ammor, K.; Sfaira, M.; Touhami, M.E.; Mahjoubi, F. Evaluation of anticorrosion and antioxidant activities of ethanolic extract of Ammi visnaga. Anal. Bioanal. Electrochem. 2018, 10, 912–929. [Google Scholar]
- Imane, B.; Ouafa, R.; Rachid, D. Antimicrobial and antioxidant activity of Ammi visnaga (L) phenolic extracts and their effects on planktonic and biofilm growth of food spoilage Bacillus cereus. Int. J. Biosci. 2016, 9, 32–47. [Google Scholar]
- Darkaoui, N.; Benbrahim, K.F.; Chaibi, M.; Boukhira, S.; Ez-Zriouli, R.; Ouaritini, B. Phytochemical and Antioxidant Activity of the Essential Oil of Ammi visnaga L. from Morocco. Pharma Chem. 2017, 9, 68–72. [Google Scholar]
- Amin, J.N.; Murad, A.; Motasem, A.M.; Ibrahem, S.R.; Ass’ad, J.M.; Ayed, A.M. Phytochemical screening and in-vitro evaluation of antioxidant and antimicrobial activities of the entire Khella plant (Ammi visnaga L.) a member of palestinian flora. Int. J. Pharmacogn. Phytochem. Res. 2015, 7, 137–143. [Google Scholar]
- Benteldjoune, M.; Boudiar, T.; Bakhouche, A.; del Mar Contreras, M.; Lozano-Sánchez, J.; Bensouici, C.; Kabouche, Z.; Segura-Carretero, A. Antioxidant activity and characterization of flavonoids and phenolic acids of Ammoides atlantica by RP–UHPLC–ESI–QTOF–MSn. Nat. Prod. Res. 2021, 35, 1639–1643. [Google Scholar] [CrossRef] [PubMed]
- Bettaieb Rebey, I.; Aidi Wannes, W.; Kaab, S.B.; Bourgou, S.; Tounsi, M.S.; Ksouri, R.; Fauconnier, M.L. Bioactive compounds and antioxidant activity of Pimpinella anisum L. accessions at different ripening stages. Sci. Hortic. 2019, 246, 453–461. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Agrawal, P. Studies on Phytochemicals, Antioxidant, Free Radical Scavenging and Lipid Peroxidation Inhibitory effects of Trachyspermum ammi seeds. Indian J. Pharm. Educ. Res. 2015, 49, 58–65. [Google Scholar] [CrossRef] [Green Version]
- Lim, Y.Y.; Lim, T.T.; Tee, J.J. Antioxidant properties of several tropical fruits: A comparative study. Food Chem. 2007, 103, 1003–1008. [Google Scholar] [CrossRef]
- Rufino, M.D.S.M.; Alves, R.E.; de Brito, E.S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef] [Green Version]
- Zargoosh, Z.; Ghavam, M.; Bacchetta, G.; Tavili, A. Effects of ecological factors on the antioxidant potential and total phenol content of Scrophularia striata Boiss. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knight, T.R.; Fariss, M.W.; Farhood, A.; Jaeschke, H. Role of lipid peroxidation as a mechanism of liver injury after aceta-minophen overdose in mice. Toxicol. Sci. 2003, 76, 229–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, S.J.; Kim, S.-M.; Lee, S.-H.; Moon, J.-Y.; Hwang, H.S.; Kim, J.S.; Park, S.-H.; Jeong, K.H.; Kim, Y.G. Rhabdomyolysis-Induced AKI Was Ameliorated in NLRP3 KO Mice via Alleviation of Mitochondrial Lipid Peroxidation in Renal Tubular Cells. Int. J. Mol. Sci. 2020, 21, 8564. [Google Scholar] [CrossRef]
- Jodynis-Liebert, J.; Matławska, I.; Bylka, W.; Murias, M. Protective effect of Aquilegia vulgaris (L.) on APAP-induced oxidative stress in rats. J. Ethnopharmacol. 2005, 97, 351–358. [Google Scholar] [CrossRef]
- McGill, M.R.; Williams, C.D.; Xie, Y.; Ramachandran, A.; Jaeschke, H. Acetaminophen-induced liver injury in rats and mice: Comparison of protein adducts, mitochondrial dysfunction, and oxidative stress in the mechanism of toxicity. Toxicol. Appl. Pharmacol. 2012, 264, 387–394. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Hao, W.; Hu, J.; Mi, X.; Han, Y.; Ren, S.; Jiang, S.; Wang, Y.; Li, X.; Li, W. Maltol improves APAP-induced hepatotoxicity by inhibiting oxidative stress and inflammation response via NF-κB and PI3K/Akt signal pathways. Antioxidants 2019, 8, 395. [Google Scholar] [CrossRef] [Green Version]
- Takemoto, K.; Hatano, E.; Iwaisako, K.; Takeiri, M.; Noma, N.; Ohmae, S.; Toriguchi, K.; Tanabe, K.; Tanaka, H.; Seo, S.; et al. Necrostatin-1 protects against reactive oxygen species (ROS)-induced hepatotoxicity in acetaminophen-induced acute liver failure. FEBS Open Bio 2014, 4, 777–787. [Google Scholar] [CrossRef] [Green Version]
- Mongi, S.; Riadh, B.; Noura, B.; Fatma, R.; Kamel, J.; Abdelfattah, E.F. Antioxidant and protective effects of Artemisia campestris essential oil against chlorpyrifos-induced kidney and liver injuries in rats. Front. Physiol. 2021, 12, 194. [Google Scholar]
- Martins, M.D.R.; Arantes, S.; Candeias, F.; Tinoco, M.T.; Cruz-Morais, J. Antioxidant, antimicrobial and toxicological properties of Schinus molle L. essential oils. J. Ethnopharmacol. 2014, 151, 485–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, H.; Zhou, H.-Z.; Yang, J.-Y.; Li, R.; Song, H.; Wu, H.-X. In vitro and in vivo antioxidant activities of inulin. PLoS ONE 2018, 13, e0192273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slatnar, A.; Jakopic, J.; Stampar, F.; Veberic, R.; Jamnik, P. The Effect of Bioactive Compounds on In Vitro and In Vivo Antioxidant Activity of Different Berry Juices. PLoS ONE 2012, 7, e47880. [Google Scholar] [CrossRef] [Green Version]
- Power, O.; Jakeman, P.; Fitzgerald, R.J. Antioxidative peptides: Enzymatic production, in vitro and in vivo antioxidant activity and potential applications of milk-derived antioxidative peptides. Amino Acids 2013, 44, 797–820. [Google Scholar] [CrossRef] [PubMed]
- Glevitzky, I.; Dumitrel, G.A.; Glevitzky, M.; Pasca, B.; Otřísal, P.; Bungau, S.; Cioca, G.; Pantis, C.; Popa, M. Statistical Analysis of the Relationship Between Antioxidant Activity and the Structure of Flavonoid Compounds. Rev. Chim. 2019, 70, 3103–3107. [Google Scholar] [CrossRef]
- Pietta, P.; Simonetti, P.; Mauri, P. Antioxidant Activity of Selected Medicinal Plants. J. Agric. Food Chem. 1998, 46, 4487–4490. [Google Scholar] [CrossRef]
- Pietta, P.G.; Rava, A.; Mauri, P.L. Rapid HPLC method for the analysis of terpenes from Ginkgo biloba in extracts, capsules and syrups by reversed phase. J. Pharm. Biomed. Anal. 1992, 10, 1077–1080. [Google Scholar] [CrossRef]
- Ruberto, G.; Baratta, M.T. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000, 69, 167–174. [Google Scholar] [CrossRef]
- Kamal-Eldin, A.; Appelqvist, L.-Å. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 1996, 31, 671–701. [Google Scholar] [CrossRef]
- Edziri, H.; Mastouri, M.; Chéraif, I.; Aouni, M. Chemical composition and antibacterial, antifungal and antioxidant activities of the flower oil ofRetama raetam(Forssk.) Webb from Tunisia. Nat. Prod. Res. 2010, 24, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Dorman, H.J.D.; Surai, P.; Deans, S.G. In Vitro Antioxidant Activity of a Number of Plant Essential Oils and Phytoconstituents. J. Essent. Oil Res. 2000, 12, 241–248. [Google Scholar] [CrossRef]
- Iqbal, T.; Hussain, A.I.; Chatha, S.A.S.; Naqvi, S.A.R.; Bokhari, T.H. Antioxidant Activity and Volatile and Phenolic Profiles of Essential Oil and Different Extracts of Wild Mint (Mentha longifolia) from the Pakistani Flora. J. Anal. Methods Chem. 2013, 2013, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, X.; Huang, H. Protective effect of linalool against lipopolysaccharide/d-galactosamine-induced liver injury in mice. Int. Immunopharmacol. 2014, 23, 523–529. [Google Scholar] [CrossRef]
- Hamid, Z.A.; Budin, S.B.; Jie, N.W.; Hamid, A.; Husain, K.; Mohamed, J. Nephroprotective effects of Zingiber zerumbet Smith ethyl acetate extract against paracetamol-induced nephrotoxicity and oxidative stress in rats. J. Zhejiang Univ. Sci. B 2012, 13, 176–185. [Google Scholar] [CrossRef] [Green Version]
- Jaeschke, H.; Xie, Y.; McGill, M. Acetaminophen-induced Liver Injury: From Animal Models to Humans. J. Clin. Transl. Hepatol. 2014, 2, 153–161. [Google Scholar] [CrossRef]
- Dargan, P.; Kalsi, S.S.; Wood, D.; Waring, W.S. Does cytochrome P450 liver isoenzyme induction increase the risk of liver toxicity after paracetamol overdose? Open Access Emerg. Med. 2011, 3, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Emam, H.T.; Madboly, A.G. Ameliorative effects of hesperidin and melatonin against acetaminophen-induced nephrotoxicity in adult albino rats. Egypt. J. Forensic Sci. Appl. Toxicol. 2021, 21, 31–46. [Google Scholar] [CrossRef]
- Ez-zahir, A.; Naya, A.; Seddik, N.; Marnissi, F.; Belghmi, K.; Oudghiri, M. In vivo anti-psoriasis, anti-inflammatory activities and oral toxicity studies of aqueous extract from seeds of Ammi visnaga L. Int. J. Res. Pharm. Sci. 2020, 11, 1025–1037. [Google Scholar] [CrossRef]
- Koriem, K.M.M.; Arbid, M.S.; El-Attar, M.A. Acute and subacute toxicity of Ammi visnaga on rats. Interdiscip. Toxicol. 2019, 12, 26–35. [Google Scholar] [CrossRef] [Green Version]
- FDA. Food and Drug Administration Guidance for Industry: Estimating the Maximum Safe Starting dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. 2005. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/estimating-maximum-safe-starting-dose-initial-clinical-trials-therapeutics-adult-healthy-volunteers (accessed on 20 December 2021).
- Guba, R. Toxicity myths: The actual risks of essential oil use. Perfum. Flavorist 2000, 25, 10–28. [Google Scholar]
- Kohlert, C.; Van Rensen, I.; März, R.; Schindler, G.; Graefe, E.U.; Veit, M. Bioavailability and Pharmacokinetics of Natural Volatile Terpenes in Animals and Humans. Planta Med. 2000, 66, 495–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schäfer, R.; Schäfer, W. Die perkutane Resorption verschiedener Terpene-Menthol, Campher, Limo-nen, Isobornylacetat, α-Pinen-aus Badezusätzen. Drug Res. 1982, 32, 56–58. [Google Scholar]
- Schuster, O.; Haag, F.; Priester, H. Transdermale Absorption von Terpenen aus den etherischen Ölen der Pinimenthol-S-Salbe. Die Med. Welt 1986, 37, 100–102. [Google Scholar]
- Bogdan, M.A.; Bungau, S.; Tit, D.M.; Zaha, D.C.; Nechifor, A.C.; Behl, T.; Chambre, D.; Lupitu, A.I.; Copolovici, L.; Copolovici, D.M. Chemical Profile, Antioxidant Capacity, and Antimicrobial Activity of Essential Oils Extracted from Three Different Varieties (Moldoveanca 4, Vis Magic 10, and Alba 7) of Lavandula angustifolia. Molecules 2021, 26, 4381. [Google Scholar] [CrossRef]
- Falk-Filipsson, A.; Löf, A.; Hagberg, M.; Hjelm, E.W.; Wang, Z. d-Limonene exposure to humans by inhalation: Uptake, distribution, elimination, and effects on the pulmonary function. J. Toxicol. Environ. Health 1993, 38, 77–88. [Google Scholar] [CrossRef]
- Parke, D.V.; Rahman, K.M.Q.; Walker, R. The absorption, distribution and excretion of linalool in the rat. Biochem. Soc. Trans. 1974, 2, 612–615. [Google Scholar] [CrossRef]

| Compounds | a IR | b IR | Area % |
|---|---|---|---|
| Propanoic acid, 2-methyl-, butyl ester | 1011 | 944 | 3.40 |
| Bicyclo[3.1.0]hexane, 4-methylene-1-(1-methylethyl)- | 1018 | 987 | 1.23 |
| β-myrcene | 1022 | 1003 | 1.36 |
| Butanoic acid, 2-methyl-, 2-methyl propyl ester | 1025 | 1015 | 4.39 |
| Butane, 1-(ethenyloxy)-3-methyl- | 1030 | - | 12.28 |
| 4-Carene | 1034 | 1030 | 1.61 |
| (Z)-β-ocimene | 1037 | 1037 | 4.03 |
| Butanoic acid, 2-methyl-, pentyl ester | 1059 | 1121 | 16.13 |
| Linalool | 1064 | 1097 | 22.94 |
| D-Limonene | 1086 | 1030 | 0.69 |
| Pulegone | 1209 | 1209 | 5.45 |
| Lavandulyl isobutyrate | 1275 | 1404 | 0.48 |
| Sample | Sample Concentration (µg/mL) | ||||
|---|---|---|---|---|---|
| 200 | 400 | 600 | 800 | 1000 | |
| Essential oil | 0.13 ± 0.01 * | 0.29 ± 0.02 * | 0.38 ± 0.04 * | 0.57 ± 0.005 * | 0.64 ± 0.005 * |
| Ascorbic acid | 0.43 ± 0.01 | 0.71 ± 0.01 | 0.95 ± 0.03 | 1.21 ± 0.01 | 1.52 ± 0.005 |
| Groups | Mean Body Weight in Gram ± SD | |
|---|---|---|
| Day 0 | Day 14 | |
| C | 29.39 ± 0.29 | 29.58 ± 0.24 |
| CMC | 30.48 ± 0.31 | 30.71 ± 0.30 |
| APAP | 32.54 ± 0.43 | 29.78 ± 0.65 * |
| AA | 27.47 ± 0.28 | 27.92 ± 0.72 |
| AV 1 | 34.53 ± 0.29 | 35.09 ± 0.25 |
| AV 2 | 25.71 ± 0.39 | 26.42 ± 0.88 |
| Organs | Groups | |||||
|---|---|---|---|---|---|---|
| C | CMC | AA | APAP | AV1 | AV2 | |
| Kidneys | 1.37 ± 0.11 | 1.36 ± 0.17 | 1.24 ± 0.08 *;# | 1.04 ± 0.14 ** | 1.15 ± 0.10 * | 1.19 ± 0.07 *;# |
| Liver | 5.26 ± 0.26 | 5.05 ± 0.11 | 4.66 ± 0.16 **;### | 3.88 ± 0.13 *** | 4.04 ± 0.18 *** | 4.83 ± 0.35 *;## |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamal, F.Z.; Stanciu, G.D.; Lefter, R.; Cotea, V.V.; Niculaua, M.; Ababei, D.C.; Ciobica, A.; Ech-Chahad, A. Chemical Composition and Antioxidant Activity of Ammi visnaga L. Essential Oil. Antioxidants 2022, 11, 347. https://doi.org/10.3390/antiox11020347
Kamal FZ, Stanciu GD, Lefter R, Cotea VV, Niculaua M, Ababei DC, Ciobica A, Ech-Chahad A. Chemical Composition and Antioxidant Activity of Ammi visnaga L. Essential Oil. Antioxidants. 2022; 11(2):347. https://doi.org/10.3390/antiox11020347
Chicago/Turabian StyleKamal, Fatima Zahra, Gabriela Dumitrita Stanciu, Radu Lefter, Valeriu V. Cotea, Marius Niculaua, Daniela Carmen Ababei, Alin Ciobica, and Abdellah Ech-Chahad. 2022. "Chemical Composition and Antioxidant Activity of Ammi visnaga L. Essential Oil" Antioxidants 11, no. 2: 347. https://doi.org/10.3390/antiox11020347
APA StyleKamal, F. Z., Stanciu, G. D., Lefter, R., Cotea, V. V., Niculaua, M., Ababei, D. C., Ciobica, A., & Ech-Chahad, A. (2022). Chemical Composition and Antioxidant Activity of Ammi visnaga L. Essential Oil. Antioxidants, 11(2), 347. https://doi.org/10.3390/antiox11020347










