Pre-Exposure to Stress-Inducing Agents Increase the Anticancer Efficacy of Focused Ultrasound against Aggressive Prostate Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Focused Ultrasound
2.3. Experimental Procedure
2.4. Cell Proliferation Assay
2.5. Flow Cytometry
2.6. Hanging Drop Culture
2.7. Scratch-Wound Assay
2.8. Colony-Forming Unit Assay
2.9. ROS Measurement
2.10. Immunoblot Assay
2.11. Statistical Analysis
3. Results
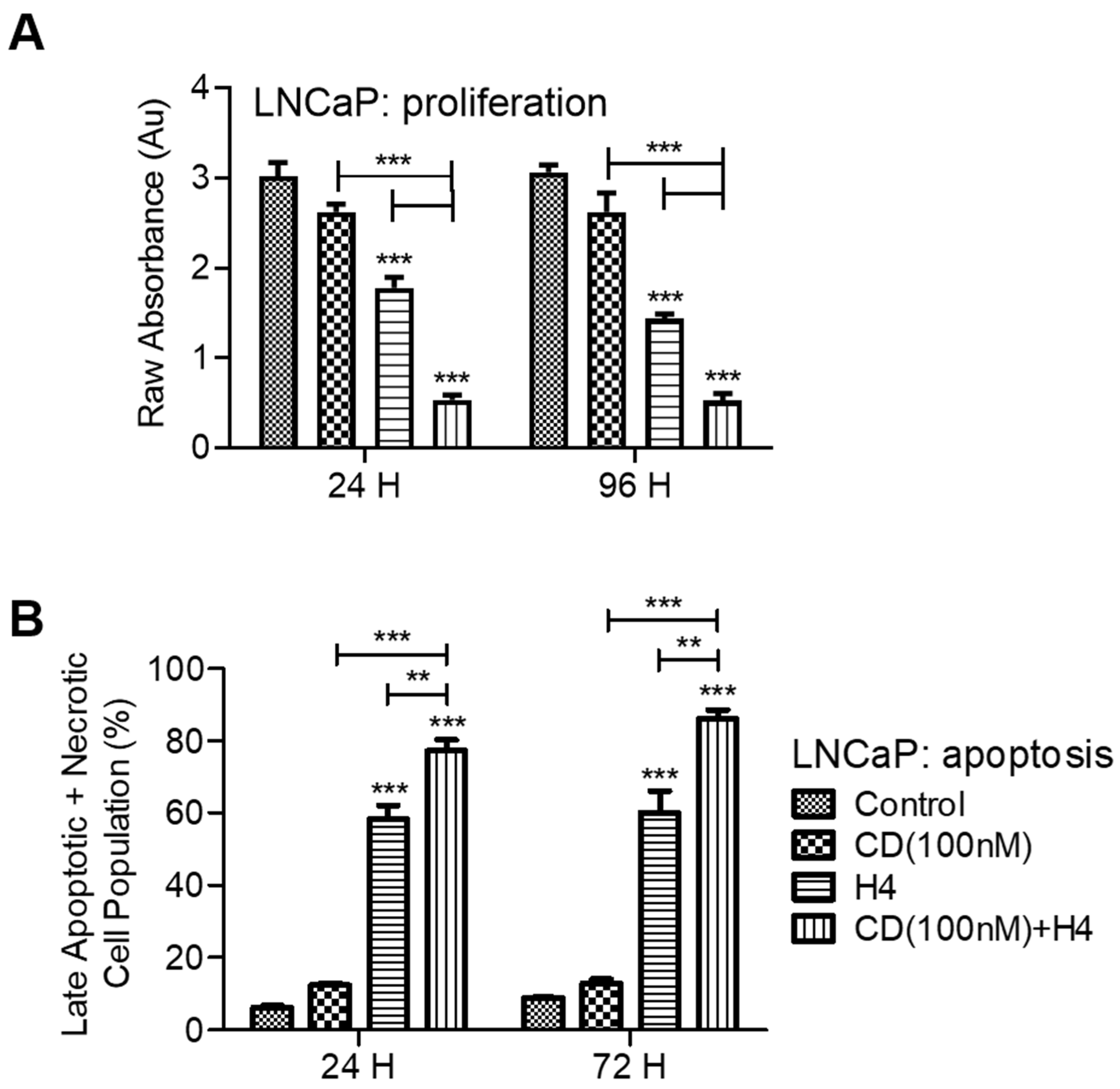
3.1. Pre-Sensitization of LNCaP Cells with CDDO-Me Increases the Cytotoxic Efficacy of FUS
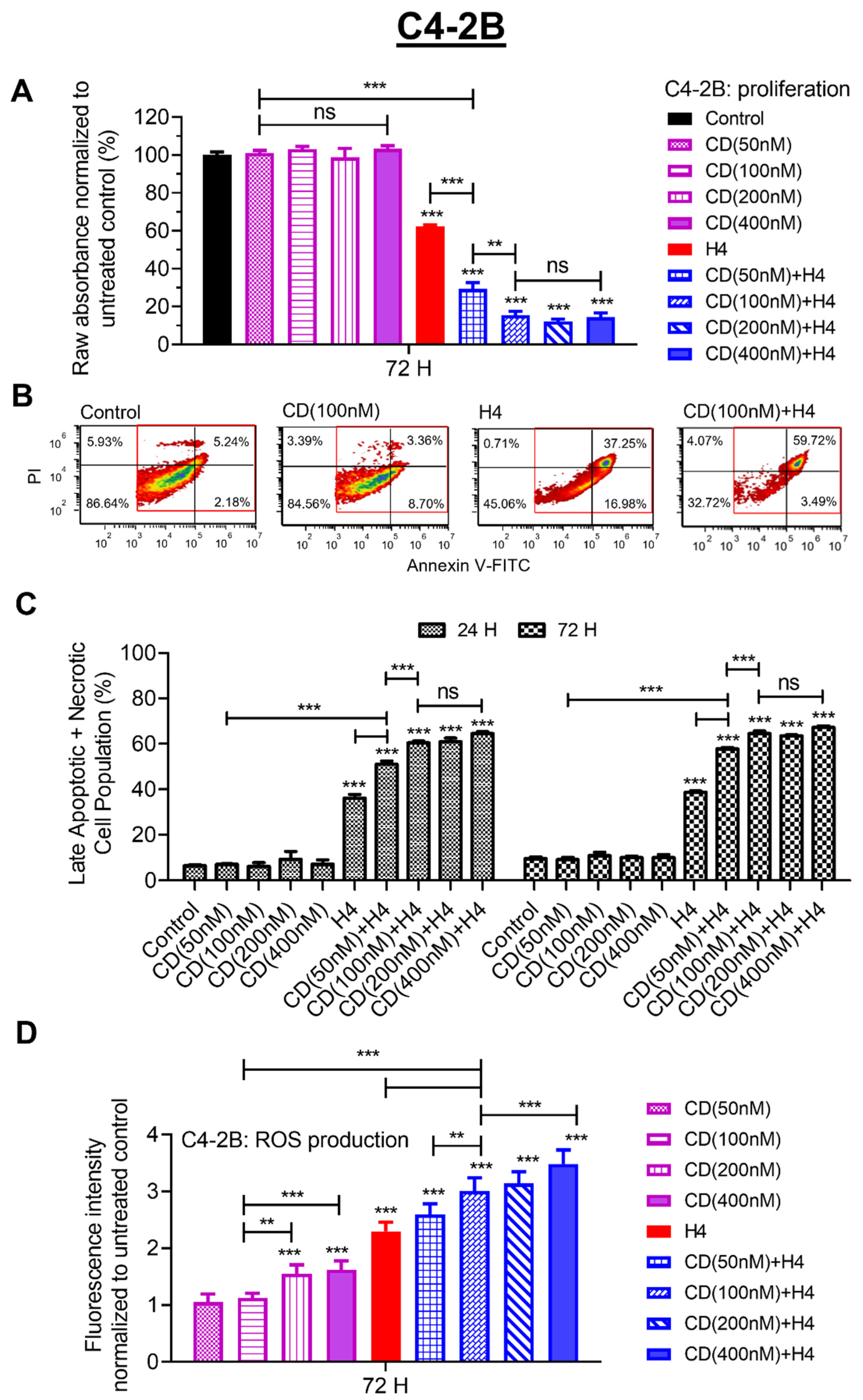
3.2. Pre-Sensitization with CDDO-Me Increases FUS-Induced Oxidative Stress and Enhances Cytotoxicity in C4-2B Cells
3.3. Pre-Treatment with CDDO-Me Enhances FUS-Mediated Suppression of Aggressive Phenotype and Decreases NF-κB Induction in the Surviving C4-2B Cells
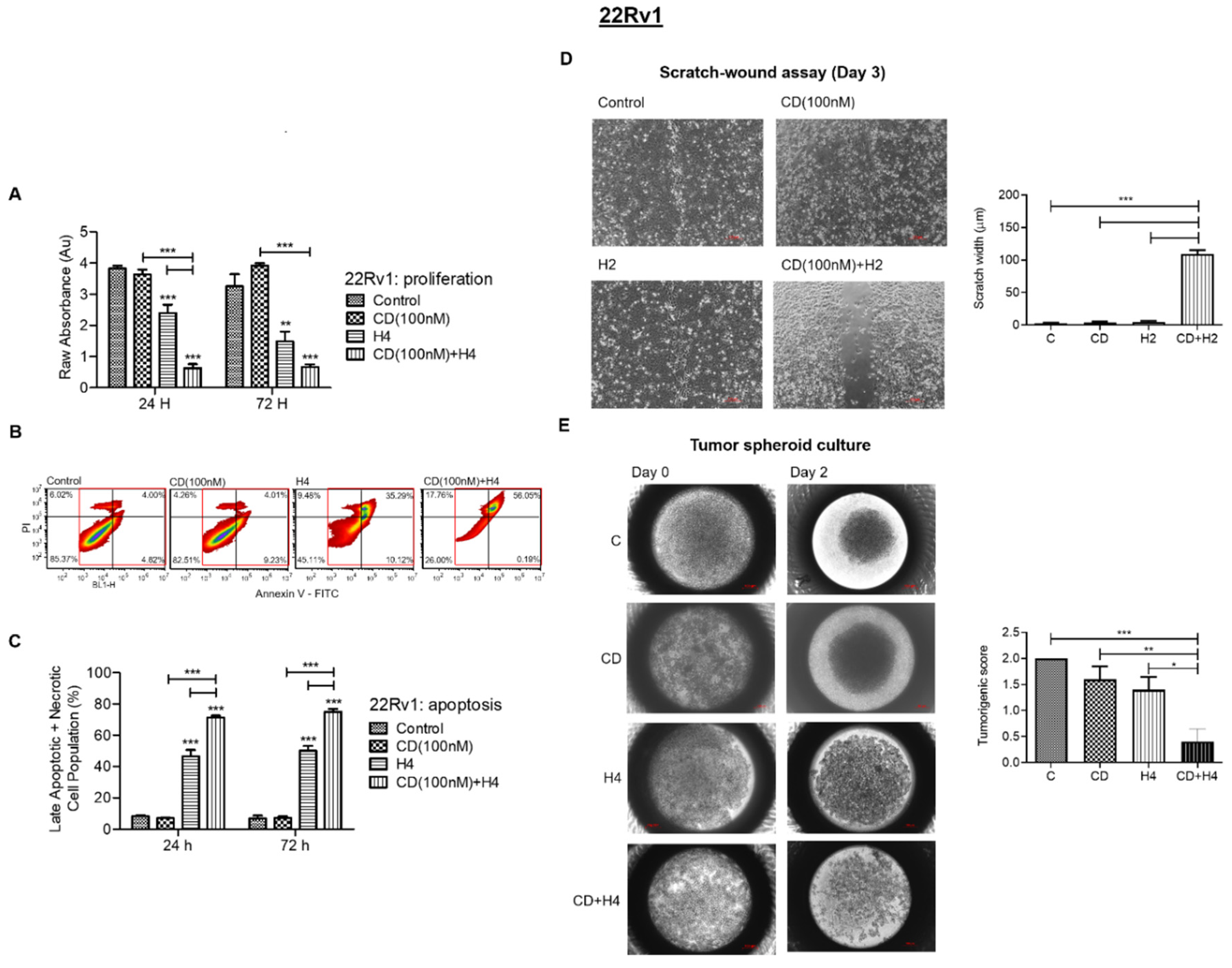
3.4. CDDO-Me Increases FUS-Mediated Suppression in Proliferation, Migration and Colonizing Ability and Enhances FUS-Induced Apoptosis and Necrosis in 22Rv1 Cells
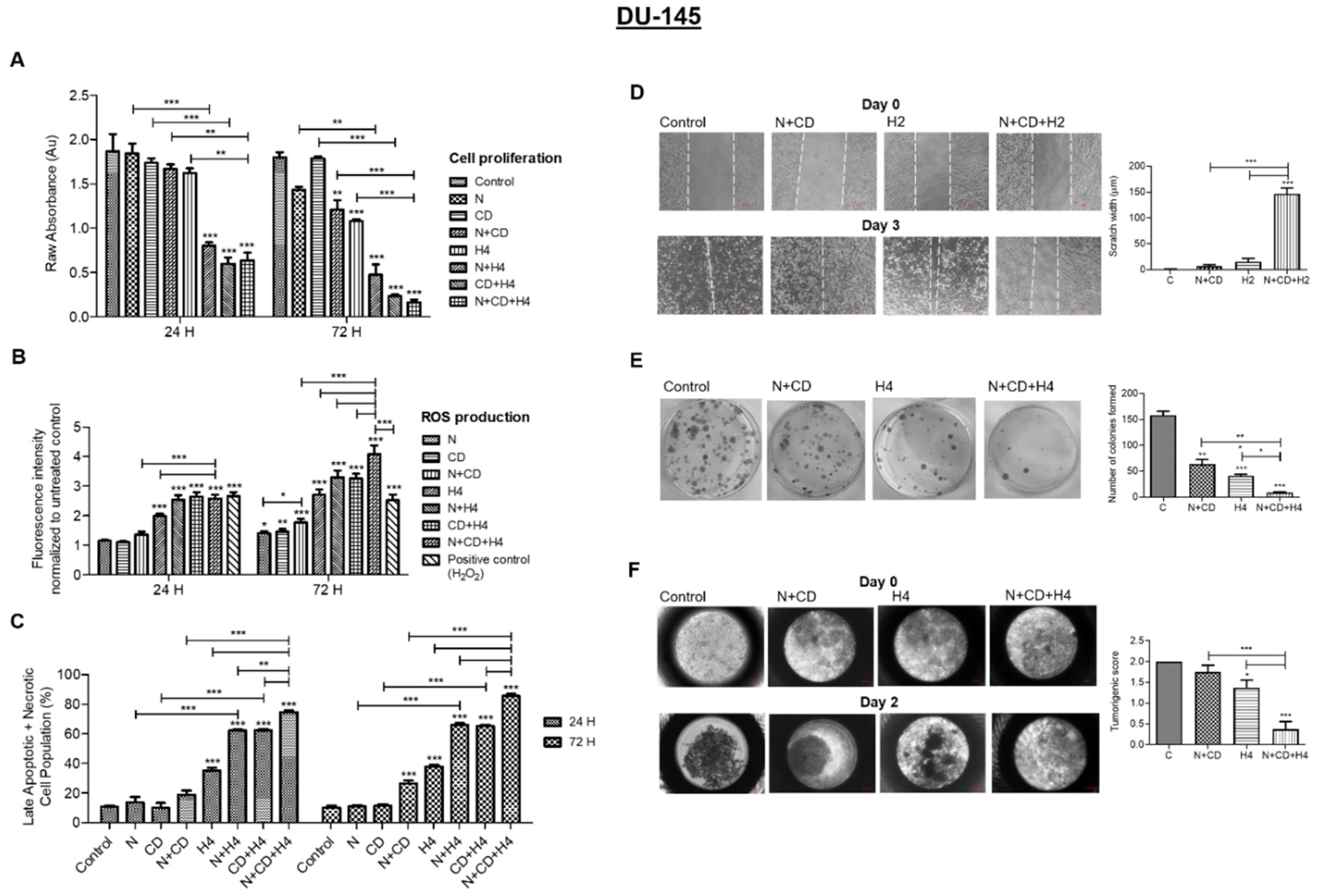
3.5. In DU145 Cells, CDDO-Me and Nelfinavir Co-Exposure Further Increases FUS-Induced Oxidative Stress and Suppresses Their Aggressive Phenotype
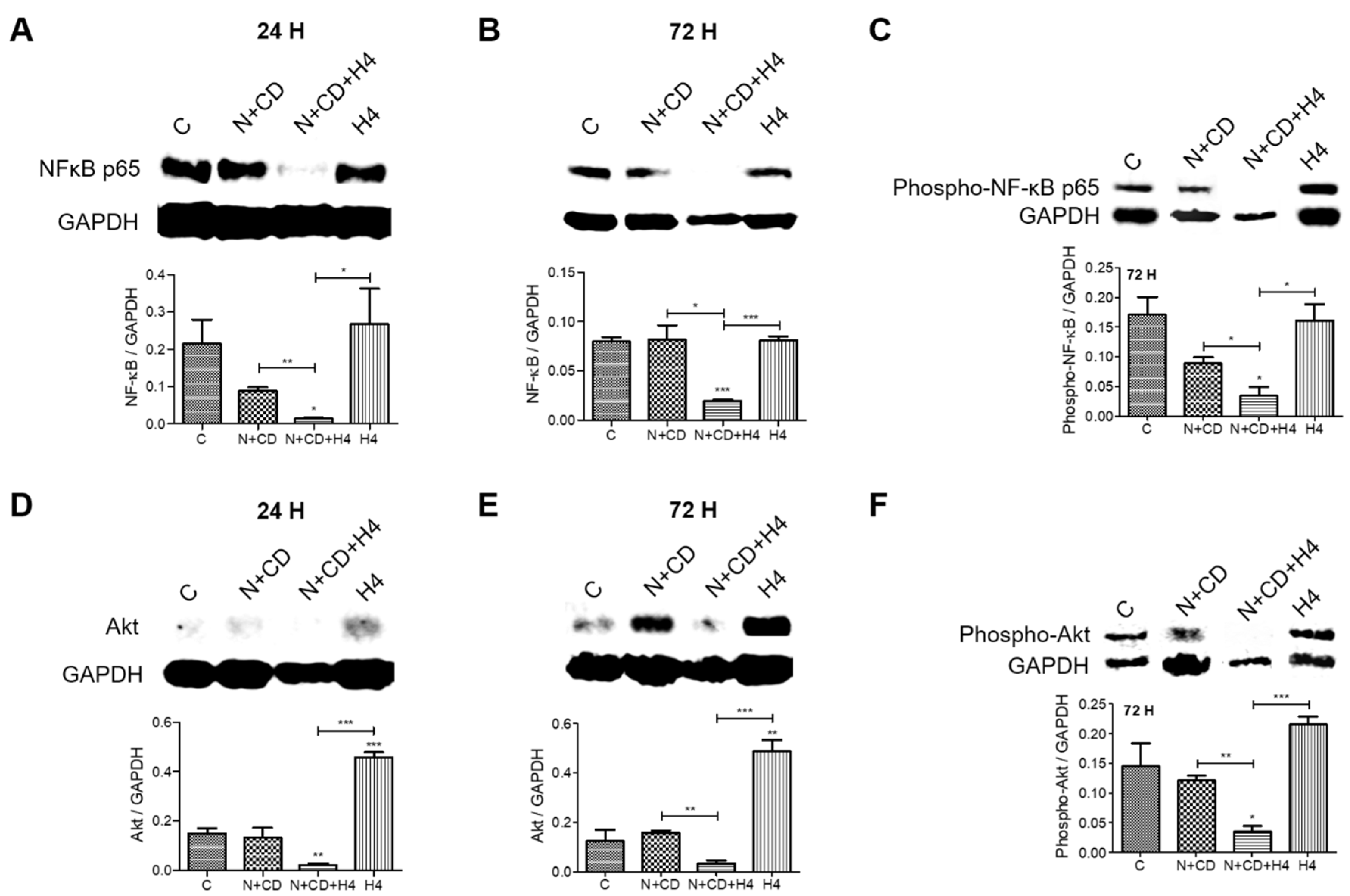
3.6. Pre-Treatment with CDDO-Me and Nelfinavir Decreases Both NF-κB and Akt Transcription Factor Levels in DU145 Cells
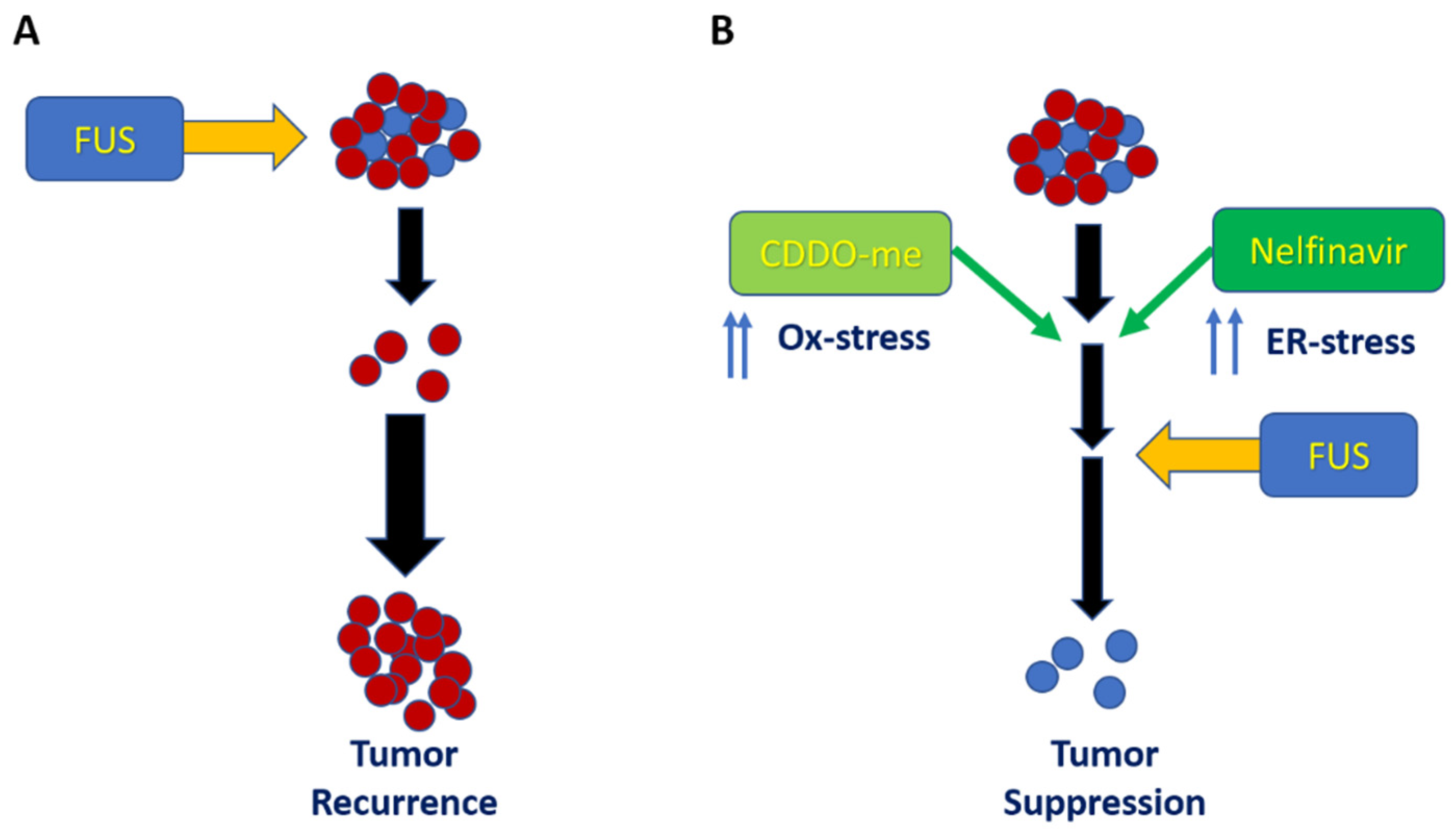
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef] [PubMed]
- Stangelberger, A.; Waldert, M.; Djavan, B. Prostate cancer in elderly men. Rev. Urol. 2008, 10, 111–119. [Google Scholar] [PubMed]
- Falci, C.; Morello, E.; Droz, J.P. Treatment of prostate cancer in unfit senior adult patients. Cancer Treat Rev. 2009, 35, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Roach, M. Current trends for the use of androgen deprivation therapy in conjunction with radiotherapy for patients with unfavorable intermediate-risk, high-risk, localized, and locally advanced prostate cancer. Cancer 2014, 120, 1620–1629. [Google Scholar] [CrossRef]
- Yap, T.A.; Zivi, A.; Omlin, A.; de Bono, J.S. The changing therapeutic landscape of castration-resistant prostate cancer. Nat. Rev. Clin. Oncol. 2011, 8, 597–610. [Google Scholar] [CrossRef]
- Karantanos, T.; Corn, P.G.; Thompson, T.C. Prostate cancer progression after androgen deprivation therapy: Mechanisms of castrate resistance and novel therapeutic approaches. Oncogene 2013, 32, 5501–5511. [Google Scholar] [CrossRef]
- Lin, T.T.; Chen, Y.H.; Wu, Y.P.; Chen, S.Z.; Li, X.D.; Lin, Y.Z.; Chen, S.H.; Zheng, Q.S.; Wei, Y.; Xu, N.; et al. Risk factors for progression to castration-resistant prostate cancer in metastatic prostate cancer patients. J. Cancer. 2019, 10, 5608–5613. [Google Scholar] [CrossRef]
- Mauri, G.; Nicosia, L.; Xu, Z.; Di Pietro, S.; Monfardini, L.; Bonomo, G.; Varano, G.M.; Prada, F.; Della Vigna, P.; Orsi, F. Focused ultrasound: Tumour ablation and its potential to enhance immunological therapy to cancer. Br. J. Radiol. 2018, 91, 20170641. [Google Scholar] [CrossRef]
- Arora, J.S.; Murad, H.Y.; Ashe, S.; Halliburton, G.; Yu, H.; He, J.; John, V.T.; Khismatullin, D.B. Ablative Focused Ultrasound Synergistically Enhances Thermally Triggered Chemotherapy for Prostate Cancer in Vitro. Mol. Pharm 2016, 13, 3080–3090. [Google Scholar] [CrossRef]
- Ashida, R.; Kawabata, K.I.; Maruoka, T.; Yamanaka, K.; Yoshikawa, H.; Ioka, T.; Katayama, K.; Tanaka, S. Transluminal Approach with Bubble-Seeded Histotripsy for Cancer Treatment with Ultrasonic Mechanical Effects. Ultrasound Med. Biol 2018, 44, 1031–1043. [Google Scholar] [CrossRef]
- van den Bijgaart, R.J.; Eikelenboom, D.C.; Hoogenboom, M.; Futterer, J.J.; den Brok, M.H.; Adema, G.J. Thermal and mechanical high-intensity focused ultrasound: Perspectives on tumor ablation, immune effects and combination strategies. Cancer Immunol. Immunother 2017, 66, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Haar, G.T.; Coussios, C. High intensity focused ultrasound: Physical principles and devices. Int. J. Hyperth. 2007, 23, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Iberti, C.T.; Mohamed, N.; Palese, M.A. A review of focal therapy techniques in prostate cancer: Clinical results for high-intensity focused ultrasound and focal cryoablation. Rev. Urol. 2011, 13, e196–e202. [Google Scholar] [PubMed]
- Chaussy, C.G.; Thuroff, S. High-Intensity Focused Ultrasound for the Treatment of Prostate Cancer: A Review. J. Endourol. 2017, 31, S30–S37. [Google Scholar] [CrossRef]
- Ziglioli, F.; Baciarello, M.; Maspero, G.; Bellini, V.; Bocchialini, T.; Cavalieri, D.; Bignami, E.G.; Maestroni, U. Oncologic outcome, side effects and comorbidity of high-intensity focused ultrasound (HIFU) for localized prostate cancer. A review. Ann. Med. Surg. 2020, 56, 110–115. [Google Scholar] [CrossRef]
- Guo, X.; Zhu, H.; Zhou, K.; Jin, C.; Yang, Y.; Zhang, J.; Yang, W.; Ran, L.; Dimitrov, D.D. Effects of high-intensity focused ultrasound treatment on peripancreatic arterial and venous blood vessels in pancreatic cancer. Oncol. Lett. 2020, 19, 3839–3850. [Google Scholar] [CrossRef]
- Murad, H.Y.; Yu, H.; Luo, D.; Bortz, E.P.; Halliburton, G.M.; Sholl, A.B.; Khismatullin, D.B. Mechanochemical disruption suppresses metastatic phenotype and pushes prostate cancer cells toward apoptosis. Mol. Cancer Res. 2019, 17, 1087–1101. [Google Scholar] [CrossRef]
- Chang, C.J.; Hsu, S.H.; Lin, F.T.; Chang, H.; Chang, C.S. Low-intensity-ultrasound-accelerated nerve regeneration using cell-seeded poly(D,L-lactic acid-co-glycolic acid) conduits: An in vivo and in vitro study. J. Biomed Mater Res. B Appl. Biomater 2005, 75, 99–107. [Google Scholar] [CrossRef]
- Claes, L.; Willie, B. The enhancement of bone regeneration by ultrasound. Prog. Biophys Mol. Biol. 2007, 93, 384–398. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, Y.S.; Ye, M.L.; Shen, F.; Liu, W.; Hu, H.S.; Li, S.W.; Wu, H.W.; Chen, Q.H.; Zhou, W.B. Overexpression and correlation of HIF-2alpha, VEGFA and EphA2 in residual hepatocellular carcinoma following high-intensity focused ultrasound treatment: Implications for tumor recurrence and progression. Exp. Ther. Med. 2017, 13, 3529–3534. [Google Scholar] [CrossRef][Green Version]
- Pfeiffer, D.; Berger, J.; Gross, A.J. Single application of high-intensity focused ultrasound as a first-line therapy for clinically localized prostate cancer: 5-year outcomes. BJU Int. 2012, 110, 1702–1707. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.M.; Hong, J.H.; Choi, H.Y. High-intensity focused ultrasound therapy for clinically localized prostate cancer. Prostate Cancer Prostatic Dis. 2006, 9, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Murad, H.Y.; Bortz, E.P.; Yu, H.; Luo, D.; Halliburton, G.M.; Sholl, A.B.; Khismatullin, D.B. Phenotypic alterations in liver cancer cells induced by mechanochemical disruption. Sci. Rep. 2018, 9, 19538. [Google Scholar] [CrossRef] [PubMed]
- Abshire, C.; Murad, H.Y.; Arora, J.S.; Liu, J.; Mandava, S.H.; John, V.T.; Khismatullin, D.B.; Lee, B.R. Focused Ultrasound-Triggered Release of Tyrosine Kinase Inhibitor from Thermosensitive Liposomes for Treatment of Renal Cell Carcinoma. J. Pharm Sci. 2017, 106, 1355–1362. [Google Scholar] [CrossRef]
- Yue, P.; Zhou, Z.; Khuri, F.R.; Sun, S.Y. Depletion of intracellular glutathione contributes to JNK-mediated death receptor 5 upregulation and apoptosis induction by the novel synthetic triterpenoid methyl-2-cyano-3, 12-dioxooleana-1, 9-dien-28-oate (CDDO-Me). Cancer Biol. Ther. 2006, 5, 492–497. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zhang, X.H.; Peng, L.; Liu, Z.; Yang, Y.X.; He, Z.X.; Dang, H.W.; Zhou, S.F. Bardoxolone methyl (CDDO-Me or RTA402) induces cell cycle arrest, apoptosis and autophagy via PI3K/Akt/mTOR and p38 MAPK/Erk1/2 signaling pathways in K562 cells. Am. J. Transl Res. 2017, 9, 4652–4672. [Google Scholar] [PubMed]
- Gills, J.J.; Lopiccolo, J.; Dennis, P.A. Nelfinavir, a new anti-cancer drug with pleiotropic effects and many paths to autophagy. Autophagy. 2008, 4, 107–109. [Google Scholar] [CrossRef]
- Okubo, K.; Sato, A.; Isono, M.; Asano, T. Nelfinavir Induces Endoplasmic Reticulum Stress and Sensitizes Renal Cancer Cells to TRAIL. Anticancer Res. 2018, 38, 4505–4514. [Google Scholar] [CrossRef]
- Hong, D.S.; Kurzrock, R.; Supko, J.G.; He, X.; Naing, A.; Wheler, J.; Lawrence, D.; Eder, J.P.; Meyer, C.J.; Ferguson, D.A.; et al. A phase I first-in-human trial of bardoxolone methyl in patients with advanced solid tumors and lymphomas. Clin. Cancer Res. 2012, 18, 3396–3406. [Google Scholar] [CrossRef]
- Lin, C.; Verma, V.; Lazenby, A.; Ly, Q.P.; Berim, L.D.; Schwarz, J.K.; Madiyalakan, M.; Nicodemus, C.F.; Hollingsworth, M.A.; Meza, J.L.; et al. Phase I/II Trial of Neoadjuvant Oregovomab-based Chemoimmunotherapy Followed by Stereotactic Body Radiotherapy and Nelfinavir for Locally Advanced Pancreatic Adenocarcinoma. Am. J. Clin. Oncol. 2019, 42, 755–760. [Google Scholar] [CrossRef]
- Khurana, N.; Chandra, P.K.; Kim, H.; Abdel-Mageed, A.B.; Mondal, D.; Sikka, S.C. Bardoxolone-Methyl (CDDO-Me) Suppresses Androgen Receptor and Its Splice-Variant AR-V7 and Enhances Efficacy of Enzalutamide in Prostate Cancer Cells. Antioxidants 2020, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Mathur, A.; Abd Elmageed, Z.Y.; Liu, X.; Kostochka, M.L.; Zhang, H.; Abdel-Mageed, A.B.; Mondal, D. Subverting ER-stress towards apoptosis by nelfinavir and curcumin coexposure augments docetaxel efficacy in castration resistant prostate cancer cells. PLoS ONE. 2014, 9, e103109. [Google Scholar] [CrossRef] [PubMed]
- Deeb, D.; Gao, X.; Dulchavsky, S.A.; Gautam, S.C. CDDO-me induces apoptosis and inhibits Akt, mTOR and NF-kappaB signaling proteins in prostate cancer cells. Anticancer Res. 2007, 27, 3035–3044. [Google Scholar] [PubMed]
- Subeha, M.R.; Telleria, C.M. The Anti-Cancer Properties of the HIV Protease Inhibitor Nelfinavir. Cancers 2020, 12, 3437. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Goldstein, A.S. Adaptation or selection--mechanisms of castration-resistant prostate cancer. Nat. Rev. Urol. 2013, 10, 90–98. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, X.; Liang, X.; Jiang, G. Molecular and cellular mechanisms of castration resistant prostate cancer. Oncol. Lett. 2018, 15, 6063–6076. [Google Scholar] [CrossRef]
- Ferrari, N.; Granata, I.; Capaia, M.; Piccirillo, M.; Guarracino, M.R.; Vene, R.; Brizzolara, A.; Petretto, A.; Inglese, E.; Morini, M.; et al. Adaptive phenotype drives resistance to androgen deprivation therapy in prostate cancer. Cell Commun. Signal 2017, 15, 51. [Google Scholar] [CrossRef]
- Uchida, T.; Ohkusa, H.; Nagata, Y.; Hyodo, T.; Satoh, T.; Irie, A. Treatment of localized prostate cancer using high-intensity focused ultrasound. BJU Int. 2006, 97, 56–61. [Google Scholar] [CrossRef]
- Deeb, D.; Gao, X.; Jiang, H.; Janic, B.; Arbab, A.S.; Rojanasakul, Y.; Dulchavsky, S.A.; Gautam, S.C. Oleanane triterpenoid CDDO-Me inhibits growth and induces apoptosis in prostate cancer cells through a ROS-dependent mechanism. Biochem. Pharm. 2010, 79, 350–360. [Google Scholar] [CrossRef]
- Ishiguro, T.; Ohata, H.; Sato, A.; Yamawaki, K.; Enomoto, T.; Okamoto, K. Tumor-derived spheroids: Relevance to cancer stem cells and clinical applications. Cancer Sci. 2017, 108, 283–289. [Google Scholar] [CrossRef]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct Target Ther. 2020, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Pires, B.R.; Mencalha, A.L.; Ferreira, G.M.; de Souza, W.F.; Morgado-Díaz, J.A.; Maia, A.M.; Corrêa, S.; Abdelhay, E.S. NF-kappaB Is Involved in the Regulation of EMT Genes in Breast Cancer Cells. PLoS ONE. 2017, 12, e0169622. [Google Scholar] [CrossRef] [PubMed]
- Kallio, H.M.L.; Hieta, R.; Latonen, L.; Brofeldt, A.; Annala, M.; Kivinummi, K.; Tammela, T.L.; Nykter, M.; Isaacs, W.B.; Lilja, H.G.; et al. Constitutively active androgen receptor splice variants AR-V3, AR-V7 and AR-V9 are co-expressed in castration-resistant prostate cancer metastases. Br. J. Cancer. 2018, 119, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Sharp, A.; Coleman, I.; Yuan, W.; Sprenger, C.; Dolling, D.; Rodrigues, D.N.; Russo, J.W.; Figueiredo, I.; Bertan, C.; Seed, G.; et al. Androgen receptor splice variant-7 expression emerges with castration resistance in prostate cancer. J. Clin. Investig. 2019, 129, 192–208. [Google Scholar] [CrossRef]
- Orsi, F.; Zhang, L.; Arnone, P.; Orgera, G.; Bonomo, G.; Vigna, P.D.; Monfardini, L.; Zhou, K.; Chen, W.; Wang, Z.; et al. High-intensity focused ultrasound ablation: Effective and safe therapy for solid tumors in difficult locations. AJR Am. J. Roentgenol. 2010, 195, W245–W252. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Wang, R.; Xu, J.; Chen, Q.; Liang, C.; Chen, J.; Zhao, J.; Chu, J.; Fan, Q.; Archibong, E.; et al. In situ thermal ablation of tumors in combination with nano-adjuvant and immune checkpoint blockade to inhibit cancer metastasis and recurrence. Biomaterials 2019, 224, 119490. [Google Scholar] [CrossRef]
- Sheybani, N.D.; Witter, A.R.; Thim, E.A.; Yagita, H.; Bullock, T.N.J.; Price, R.J. Combination of thermally ablative focused ultrasound with gemcitabine controls breast cancer via adaptive immunity. J. Immunother Cancer. 2020, 8, e001008. [Google Scholar] [CrossRef]
- Abdollahi, A.; Domhan, S.; Jenne, J.W.; Hallaj, M.; Dell’Aqua, G.; Mueckenthaler, M.; Richter, A.; Martin, H.; Debus, J.; Ansorge, W.; et al. Apoptosis signals in lymphoblasts induced by focused ultrasound. FASEB J. 2004, 18, 1413–1414. [Google Scholar] [CrossRef]
- Wood, A.K.; Sehgal, C.M. A review of low-intensity ultrasound for cancer therapy. Ultrasound Med. Biol. 2015, 41, 905–928. [Google Scholar] [CrossRef]
- Crawford, E.D.; Heidenreich, A.; Lawrentschuk, N.; Tombal, B.; Pompeo, A.C.L.; Mendoza-Valdes, A.; Miller, K.; Debruyne, F.M.J.; Klotz, L. Androgen-targeted therapy in men with prostate cancer: Evolving practice and future considerations. Prostate Cancer Prostatic Dis. 2019, 22, 24–38. [Google Scholar] [CrossRef]
- Cunningham, D.; You, Z. In vitro and in vivo model systems used in prostate cancer research. J. Biol. Methods 2015, 2, e17. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, Z.; Yi, J.; Shen, H.; Yang, Z.; Yan, L.; Xie, L. Clinicopathological characteristics of androgen receptor splicing variant 7 (AR-V7) expression in patients with castration resistant prostate cancer: A systematic review and meta-analysis. Transl Oncol. 2021, 14, 101145. [Google Scholar] [CrossRef] [PubMed]
- Carver, B.S.; Chapinski, C.; Wongvipat, J.; Hieronymus, H.; Chen, Y.; Chandarlapaty, S.; Arora, V.K.; Le, C.; Koutcher, J.; Scher, H.; et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell 2011, 19, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Chng, W.J.; Zhou, J. Crosstalk between endoplasmic reticulum stress and oxidative stress: A dynamic duo in multiple myeloma. Cell Mol. Life Sci. 2021, 78, 3883–3906. [Google Scholar] [CrossRef]
- Farooqi, A.A.; Li, K.T.; Fayyaz, S.; Chang, Y.T.; Ismail, M.; Liaw, C.C.; Yuan, S.S.; Tang, J.Y.; Chang, H.W. Anticancer drugs for the modulation of endoplasmic reticulum stress and oxidative stress. Tumour Biol. 2015, 36, 5743–5752. [Google Scholar] [CrossRef]
- Cullinan, S.B.; Diehl, J.A. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J. Biol. Chem. 2004, 279, 20108–20117. [Google Scholar] [CrossRef]
- Cullinan, S.B.; Diehl, J.A. Coordination of ER and oxidative stress signaling: The PERK/Nrf2 signaling pathway. Int. J. Biochem Cell Biol. 2006, 38, 317–332. [Google Scholar] [CrossRef]
- Liberman, B.; Gianfelice, D.; Inbar, Y.; Beck, A.; Rabin, T.; Shabshin, N.; Chander, G.; Hengst, S.; Pfeffer, R.; Chechick, A.; et al. Pain palliation in patients with bone metastases using MR-guided focused ultrasound surgery: A multicenter study. Ann. Surg. Oncol. 2009, 16, 140–146. [Google Scholar] [CrossRef]
- Hurwitz, M.D.; Ghanouni, P.; Kanaev, S.V.; Iozeffi, D.; Gianfelice, D.; Fennessy, F.M.; Kuten, A.; Meyer, J.E.; LeBlang, S.D.; Roberts, A.; et al. Magnetic resonance-guided focused ultrasound for patients with painful bone metastases: Phase III trial results. J. Natl. Cancer Inst. 2014, 106, dju082. [Google Scholar] [CrossRef]
- Jadvar, H.; Desai, B.; Ji, L.; Conti, P.S.; Dorff, T.B.; Groshen, S.G.; Gross, M.E.; Pinski, J.K.; Quinn, D.I. Prospective evaluation of 18F-NaF and 18F-FDG PET/CT in detection of occult metastatic disease in biochemical recurrence of prostate cancer. Clin. Nucl. Med. 2012, 37, 637–643. [Google Scholar] [CrossRef]
- Napoli, A.; Alfieri, G.; Scipione, R.; Leonardi, A.; Fierro, D.; Panebianco, V.; De Nunzio, C.; Leonardo, C.; Catalano, C. High-intensity focused ultrasound for prostate cancer. Expert Rev. Med. Devices. 2020, 17, 427–433. [Google Scholar] [CrossRef] [PubMed]
- El-Ashmawy, M.; Delgado, O.; Cardentey, A.; Wright, W.E.; Shay, J.W. CDDO-Me protects normal lung and breast epithelial cells but not cancer cells from radiation. PLoS ONE. 2014, 23, e115600. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murad, H.Y.; Chandra, P.K.; Kelly, C.A.; Khurana, N.; Yu, H.; Bortz, E.P.; Hong, S.N.; Mondal, D.; Khismatullin, D.B. Pre-Exposure to Stress-Inducing Agents Increase the Anticancer Efficacy of Focused Ultrasound against Aggressive Prostate Cancer Cells. Antioxidants 2022, 11, 341. https://doi.org/10.3390/antiox11020341
Murad HY, Chandra PK, Kelly CA, Khurana N, Yu H, Bortz EP, Hong SN, Mondal D, Khismatullin DB. Pre-Exposure to Stress-Inducing Agents Increase the Anticancer Efficacy of Focused Ultrasound against Aggressive Prostate Cancer Cells. Antioxidants. 2022; 11(2):341. https://doi.org/10.3390/antiox11020341
Chicago/Turabian StyleMurad, Hakm Y., Partha K. Chandra, Charles A. Kelly, Namrata Khurana, Heng Yu, Emma P. Bortz, Shirley N. Hong, Debasis Mondal, and Damir B. Khismatullin. 2022. "Pre-Exposure to Stress-Inducing Agents Increase the Anticancer Efficacy of Focused Ultrasound against Aggressive Prostate Cancer Cells" Antioxidants 11, no. 2: 341. https://doi.org/10.3390/antiox11020341
APA StyleMurad, H. Y., Chandra, P. K., Kelly, C. A., Khurana, N., Yu, H., Bortz, E. P., Hong, S. N., Mondal, D., & Khismatullin, D. B. (2022). Pre-Exposure to Stress-Inducing Agents Increase the Anticancer Efficacy of Focused Ultrasound against Aggressive Prostate Cancer Cells. Antioxidants, 11(2), 341. https://doi.org/10.3390/antiox11020341






