Abstract
Oxidative and nitrosative stress have been linked to thyroid function in both animal and human studies. In the present study, the associations between oxidative and nitrosative stress and thyroid hormones were investigated. Measurements were obtained from 97 Taiwanese pregnant women at the first, second, and third trimesters. Levels of five oxidative and nitrosative stress biomarkers (8-hydroxy-2′-deoxyguanosine [8-OHdG], 8-nitroguanine [8-NO2Gua], 4-hydroxy-2-nonenal-mercapturic acid [HNE-MA], 8-isoprostaglandin F2α [8-isoPGF2α], and malondialdehyde [MDA]) were measured using urine samples, and levels of five thyroid hormones (triiodothyronine [T3], thyroxine [T4], free T4, thyroid-stimulating hormone [TSH], and T4-binding globulin [TBG]) were measured in blood samples. Multiple linear regressions and linear mixed-model regressions were conducted to determine the associations between oxidative or nitrosative stress biomarkers and thyroid hormones in pregnant women. We found that TSH was negatively and significantly associated with 8-NO2Gua (−14%, 95% CI [−26.9% to −1.1%]) and HNE-MA (−23%, 95% CI [−35.9% to −10.0%]) levels. However, T4 (3%, 95% CI [0.2%–5.8%]) and free T4 (4.3%, 95% CI [0.8%–7.8%]) levels were positively and significantly associated with 8-NO2Gua. The T4 to TBG and free T4 to TBG ratios were positively and significantly associated with 8-NO2Gua level (T4/TBG: 3.6%, 95% CI [0.5%–6.7%]; free T4/TBG: 5.6%, 95% CI [0.2%–11.1%]). However, the TSH to T4 ratio was negatively and significantly associated with 8-NO2Gua level (−17.3%, 95% CI [−30.4% to −4.3%]). The T3 to TSH ratio was positively and significantly associated with HNE-MA level (25.2%, 95% CI [11.2%–39.2%]). However, the TSH to T4 and TSH to free T4 ratios were negatively and significantly associated with HNE-MA level (TSH/T4: −21.2%, 95% CI [−34.5% to −7.8%] and TSH/free T4: −24.0%, 95% CI [−38.3% to −9.6%]). Our findings suggest that an imbalance of oxidative and nitrosative stress may alter thyroid hormone homeostasis during pregnancy. Disruption of the maternal thyroid homeostasis during pregnancy would affect embryonic and fetal development.
1. Introduction
Oxidative and nitrosative stress are defined as an imbalance between the synthesis of prooxidant substances and antioxidant defenses. The most important prooxidants are reactive oxygen species (ROS) and reactive nitrogen species (RNS). ROS and RNS are key pathogenetic factors that play roles in a wide range of illnesses. ROS and RNS are highly chemically reactive molecules due to their single unpaired electrons in their outer orbitals. Therefore, ROS free radicals, such as superoxide anions and hydroxyls, play a unique pathogenetic role [1]. Oxidative stress is associated with a variety of chronic diseases, including cardiovascular disorders [2].
The thyroid hormones, namely triiodothyronine (T3) and thyroxine (T4), are key regulators of growth, development, and metabolism that are produced and released by the thyroid gland. Thyroid hormones control cell proliferation and the basal metabolic rate by altering metabolic and energy homeostasis, thermogenesis, and the transcription of genes that regulate cell proliferation [3,4]. Thus, thyroid hormones play a role in a variety of physiological activities, including energy metabolism, central nervous system growth and formation, tissue differentiation, and reproduction [5].
Thyroid hormones exert a major influence on oxidative stress because of their function in cellular metabolism and oxygen consumption. The negative feedback mechanisms in the endocrine system regulate thyroid mechanisms to maintain them at appropriate levels. If any changes were to occur in thyroid hormone levels, such changes may alter the redox environment by altering the amount and activity of mitochondrial respiratory chain components, leading to an increase in ROS generation, which are generally limited by antioxidants. An overproduction of ROS induces thyroid hormones to consume more oxygen, causing oxidative stress and damage to cellular structures, lipids, and proteins [6].
Some studies have suggested that maternal or fetal oxidative stress plays a key role in the pathophysiology of low birth weight [7,8,9]. For example, Matsubasa et al. reported that as birth weights at sampling increased, the average levels of urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG) decreased (infants under 1000 g with 8-OHdG levels 29.5 ± 16.4 μmol/mol creatinine, 1000–1500 g with 23.8 ± 14.9, over 1500 g with 16.1 ± 8.5, and control with 10.9 ± 7.2) [9]. Kim et al. reported that the concentrations of maternal urinary 8-OHdG and malondialdehyde (MDA) were inversely associated with the birth weight of full-term deliveries after controlling for potential confounders [10]. Therefore, monitoring oxidative stress in pregnant women is key in uncovering the link between oxidative stress and pregnancy outcomes [10]. Furthermore, maintaining maternal thyroid homeostasis is essential for fetal growth and development during pregnancy [11]. Hyperthyroidism and hypothyroidism in pregnant women have been associated with adverse birth outcomes, such as preterm births and low birth weights [12,13]. Minor changes in thyroid function in pregnant women might negatively affect fetal health [14,15]. However, a few studies have assessed the impact of oxidative and nitrosative stress.
In the present study, we aimed to explore the relationships between oxidative or nitrosative stress and thyroid hormone in pregnant Taiwanese women. The hypothesis is that an imbalance of oxidative and nitrosative stress would alter thyroid hormone homeostasis at different trimesters during pregnancy. We investigate the associations that oxidative or nitrosative stress has on thyroid hormone levels through an exploration of the effects of lipid peroxidation (4-hydroxy-2-nonenal-mercapturic acid [HNE-MA], 8-isoprostaglandin F2α [8-isoPGF2α], and MDA) and oxidative (8-OHdG) and nitrosative (8-nitroguanine [8-NO2Gua]) DNA damage on the thyroid functions of Taiwanese pregnant women at their first, second, and third trimester visits through repeated measurements analysis [1,16]. The chosen biomarkers were based on relevant studies [1,10,16,17,18,19,20], in which some chosen oxidative and nitrosative stress biomarkers were linked with birth outcomes and different endocrine-disrupting chemicals (EDCs), such as phenols and phthalates. Meanwhile, exposure window of different trimesters may vary and induce different levels of oxidative and nitrosative stress.
2. Materials and Methods
2.1. Participants and Study Design
The data for this study were obtained from the Tainan Birth Cohort Study (TBCS 2013–14) project. Our data collection process has been detailed in our prior publications [20,21,22]. In brief, our study included pregnant women whose blood biochemical examination (alpha fetal protein and free-hCG) were abnormal, or whose advanced maternal age (>35 years) indicated the suggestion for amniocentesis, as recommended by gynecologists at the National Cheng Kung University Hospital. Pregnant women with preeclampsia, abnormal chromosomal disease, gestational diabetes mellitus, intrauterine growth restriction, or other complications were excluded. A total of 97 pregnant women with normal-DNA infant were included for analysis. At the initial study visit (median: 18 gestational weeks), the participants provided sociodemographic information (age, education, occupational history, and social economic status.), pregnancy history (gestational age, menarche age, and parity), lifestyle habits (tobacco use, passive smoking, and alcohol consumption), and exposure history (exposure to di-(2-ethylhexyl) phthalate [DEHP]-contaminated products before the DEHP episode and nutritional supplement consumption). Urine and blood samples of pregnant women were collected at three visits at median gestation times of 18, 26, and 39 weeks.
The study protocol was approved by the Research Ethics Committee of the National Health Research Institutes (No. EC1020302) at 23 September 2013 and the Institutional Review Board of National Cheng Kung University Hospital (No. A-ER-102-104) on 27 June 2013 in Taiwan. Informed consent was obtained from each participant before study enrollment.
2.2. Analytical Method for Oxidative and Nitrosative Stress Biomarkers
Using an isotope dilution liquid chromatography–tandem mass spectrometry (LC–MS/MS), we evaluated the levels of four oxidative and nitrosative stress biomarkers, 8-OHdG, 8-NO2Gua, 8-IsoPGF2α, and HNE-MA per a method in the literature [16,20]. The levels of MDA in urine samples were assessed by measuring the level of thiobarbituric acid (TBA) reactive substances, which result from the reaction that occurs between MDA and TBA at high temperatures ranging from 90 to 100 °C. A commercial kit (Cayman Chemicals No.10009055, Ann Arbor, MI, USA) was used to analyze urine samples according to the manufacturer’s instructions. At 530–540 nm, the formed MDA–TBA product was colorimetrically measured. The intra-assay and inter-assay coefficients of variation were 7.6% and 5.1%, respectively [23].
2.3. Analytical Method for Measuring Serum Thyroid Hormones and Urinary Creatinine
Serum thyroid function and urinary creatinine concentrations were measured by a Taiwan Accreditation Foundation–certified laboratory (Nos. 1447 and 1673), which has been recognized by the International Laboratory Accreditation Cooperation Mutual Recognition Arrangement [24]. For creatinine, combined clinical chemistry and immunoassay tests (Modular Analytics Serum Work Area; Roche Diagnostics) were employed to analyze urine samples (which had been stored at −20 °C within one week before analysis). Thyroid function was assessed using an electrochemiluminescence immunoassay (Elecsys 2010 and Modular Analytics E170; Roche Diagnostics) to measure the serum concentrations of T4, free T4, T3, thyroid-stimulating hormone (TSH), and thyroxine-binding globulin (TBG). All analyses were conducted by a blinded technician and in a random order.
2.4. Statistical Analysis
The characteristics of the participants were described in terms of the mean and standard deviation for the continuous variables of age and gestational week at enrollment and in terms of percentage for education level, household income, primipara, folic acid consumption, alcohol consumption, and tobacco exposure. For each visit, detection rates, geometrical means (GMs), and medians were used to describe the distributions of oxidative or nitrosative stress biomarkers and thyroid hormones. To analyze the trend across each visit, linear mixed models with random intercepts for study participants and study visit as an independent variable were used to compare GMs between the three visits [18]. Spearman’s correlation coefficients were used to assess the correlations between oxidative and nitrosative stress biomarkers and thyroid hormones. To investigate intraindividual variability in measurements across study visits, intraclass correlation coefficients (ICCs) for oxidative and nitrosative stress biomarkers and thyroid hormones were calculated. The natural logarithm (ln) of the levels of oxidative and nitrosative stress biomarkers and thyroid hormones were used to meet the normality assumption. Multiple linear regressions were fitted to investigate the associations between oxidative and nitrosative stress biomarkers and thyroid hormones. The associations between the intertertile increase in oxidative or nitrosative stress biomarkers and the percentage of change in thyroid hormones across visits were then assessed using linear mixed models with first-order autoregressive covariance structures and random intercepts for participants, in which concentration variables (i.e., oxidative and nitrosative stress biomarkers) were classified into tertiles (i.e., 0–2) and enter as continuous variables into regression. Selection of covariates was based on the literature review, availability, and their statistical significance in the models. In order to separate any impact of creatinine on the associations, urinary creatinine as a covariate has been suggested as an alternative to creatinine-corrected analytes [25]. Finally, we chose the continuous covariates of age and urinary creatinine level for adjusting. All statistical analyses were conducted in R version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Demographic Characteristics of Participants
At the time of enrollment, the 97 participating pregnant women had a mean gestation time of 18.3 ± 1.3 weeks and an average age of 35.1 ± 3.5 years. Primiparas accounted for approximately half of the participants (43.3%). The majority (95.9%) had a university-level education, and 82.1% were from households with an annual income more than NTD 500,000 (approximately USD 15,625). Over half of the participants (55.4%) had consumed folic acid throughout the month preceding the start of the study. Few reported having drunk alcohol (1.0%) or smoked (2.1%); however, 16.5% reported secondhand smoke exposure (Table 1).

Table 1.
Demographic characteristics of participated Taiwan’s pregnant women (N = 97).
3.2. Distributions of Oxidative and Nitrosative Stress Biomarkers and Thyroid Hormones
The distributions of oxidative and nitrosative stress biomarkers and serum thyroid hormone levels are summarized in Table 2. With the exception of 8-isoPGF2α, which had a detection rate ranging from 61% to 77% across the three study visits, nearly all the oxidative and nitrosative stress biomarkers and thyroid hormones had detection rates of 100%.

Table 2.
Distribution of oxidative/nitrosative stress biomarkers, thyroid hormones, and creatinine in pregnant women by study visits (N = 218) a.
No significant differences were noted between each of these oxidative and nitrosative stress biomarkers and thyroid hormones across the three visits. However, a higher TSH level was noted at the third visit (GM = 2.4 μIU/mL) than those noted at the first and second visits (GM = 1.2 and 1.1 μIU/mL, respectively), and a lower HNE-MA level was noted at the third visit (GM = 26.9 ng/mL) than those noted at the first and second visits (GM = 52.9 and 54.3 ng/mL, respectively). The GM ranges across the three study visits were observed as follows 105.6–122.3 ng/dL for T3, 8.7–8.9 μg/dL for T4, 0.6–0.8 ng/dL for free T4, 34.7–38.3 μg/mL for TBG, 8.2–10.5 μmole/L for MDA, 2.6–2.9 ng/mL for 8-OHdG, 1.5–1.6 ng/mL for 8-NO2Gua, and 0.6–0.9 ng/mL for 8-isoPGF2α. The ICCs for thyroid hormones ranged from 8.4% (free T4) to 63.1% (TBG), and the ICCs for oxidative and nitrosative stress biomarkers ranged from −2.8% (8-isoPGF2α) to 18.8% (8-OHdG). Table 3 presents distribution of thyroid hormone ratios in pregnant women by study visits.

Table 3.
Distribution of thyroid hormone ratios in pregnant women by study visits (N =218) a.
We ran Mann–Whitney U test and Kruskal–Wallis tests to compare oxidative/nitrosative stress and thyroid hormone levels in classified groups based on potential confounding factors listed in Table 1, but the results were not significant (Supplementary Table S1). We also compared the thyroid hormone levels of participants against a reference value for pregnant women in each trimester. For TSH, 26% of our participants had higher TSH levels than the reference value for pregnant women in the third trimester. For T3, 52% and 74% of our participants had lower T3 levels than the reference values for pregnant women in the second and third trimesters, respectively. For T4, 29% and 28% of our participants had higher T4 levels than the reference values for pregnant women in the first and third trimesters, respectively. For free T4, 54% and 40% of our participants had lower free T4 levels than the reference values for pregnant women in the first and second trimesters, respectively. For TBG, 67%, 41% and 40% of our participants had higher TBG levels than the reference values for pregnant women in the first, second, and third trimesters, respectively (Supplementary Table S2).
3.3. Correlations between Oxidative and Nitrosative Stress Biomarkers and Thyroid Hormones
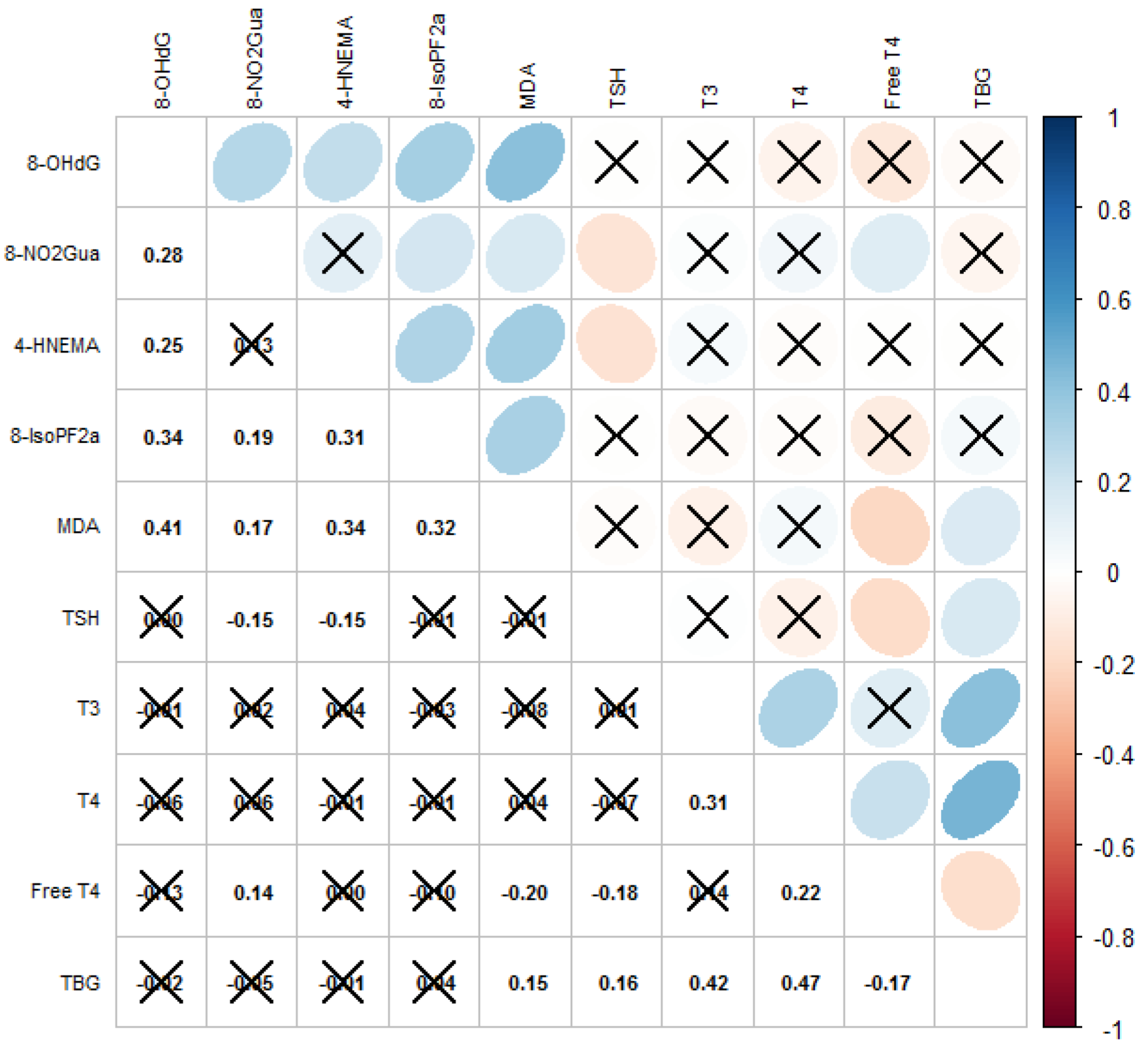
The correlation matrix with the Spearman’s coefficients of the oxidative and nitrosative stress biomarkers and thyroid hormones is displayed in Figure 1. Levels of most biomarkers of oxidative or nitrosative stress as well as thyroid hormones were moderately correlated (Spearman’s ρ = −0.18–0.47). We observed that the level of TSH was significantly correlated with levels of 8-NO2Gua (Spearman’s ρ = −0.15) and HNE-MA (Spearman’s ρ = −0.16), that the level of free T4 was significantly correlated with the levels of 8-NO2Gua (Spearman’s ρ = 0.14) and MDA (Spearman’s ρ = −0.20), and that the level of TBG was significantly correlated with the level of MDA (Spearman’s ρ = 0.15).
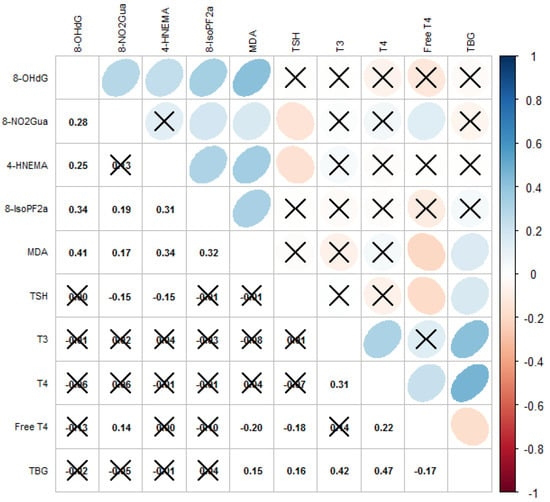
Figure 1.
Correlations among oxidative/nitrosative stress biomarkers and thyroid hormones. Cross mark means correlation is not significant (p-value ≥ 0.05); blue means positively correlated; red means negatively correlated.
3.4. Associations between Oxidative and Nitrosative Stress Biomarkers and Thyroid Hormones in Multiple Linear Regression and Linear Mixed Models
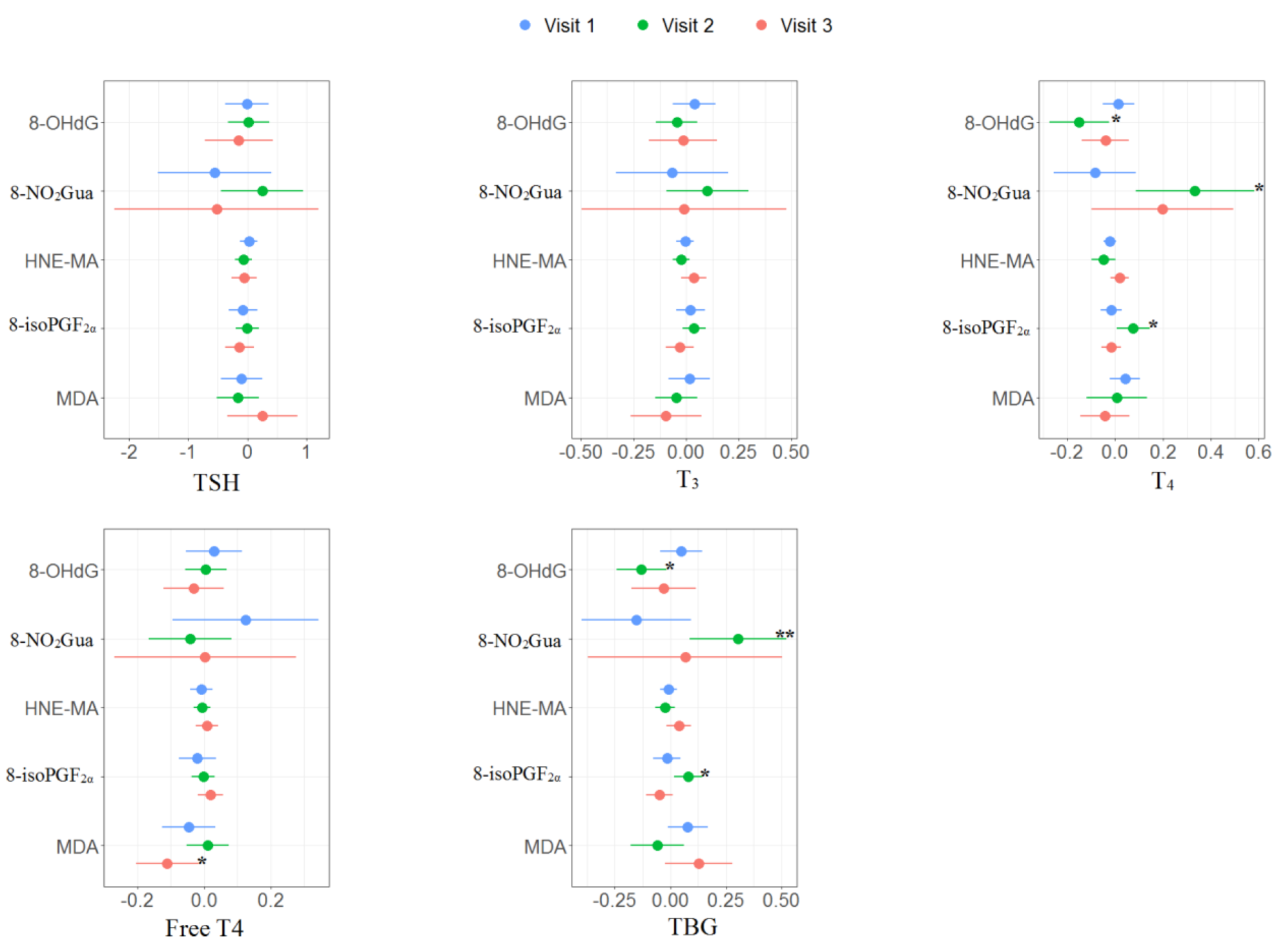
The multiple linear regression results of the association between oxidative or nitrosative stress biomarkers and thyroid hormones for each trimester are presented in Supplementary Tables S3–S5 and Figure 2. No significant association was found in the first trimester. For the second trimester, 8-OHdG was negatively associated with T4 (β: −0.15, 95% CI [−0.28 to −0.02]) and TBG (β: −0.13, 95% CI [−0.25 to −0.02]); 8-NO2Gua was positively associated with T4 (β: 0.33, 95% CI [0.08 to 0.59]) and TBG (β: 0.30, 95% CI [0.08 to 0.53]); 8-isoPGF2α was positively associated with T4 (β: 0.08, 95% CI [0.01 to 0.15]) and TBG (β: 0.08, 95% CI [0.01 to 0.15]). For the third trimester, MDA was negatively associated with free T4 (β: −0.11, 95% CI [−0.21 to −0.01]).
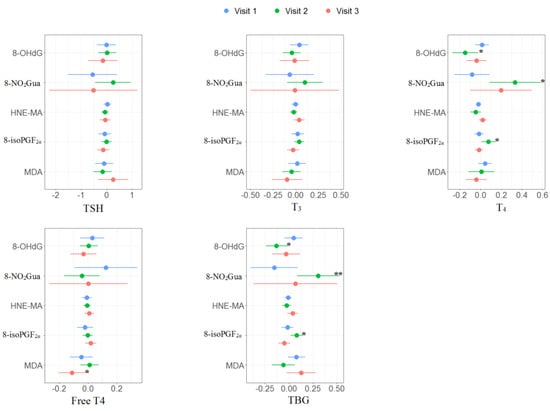
Figure 2.
Unit change in oxidative/nitrosative stress biomarkers concentrations in association with concentrations of thyroid hormones across three visits. Multiple linear regressions are adjusted for age and creatinine; * p < 0.05, ** p < 0.01, *** p < 0.001.
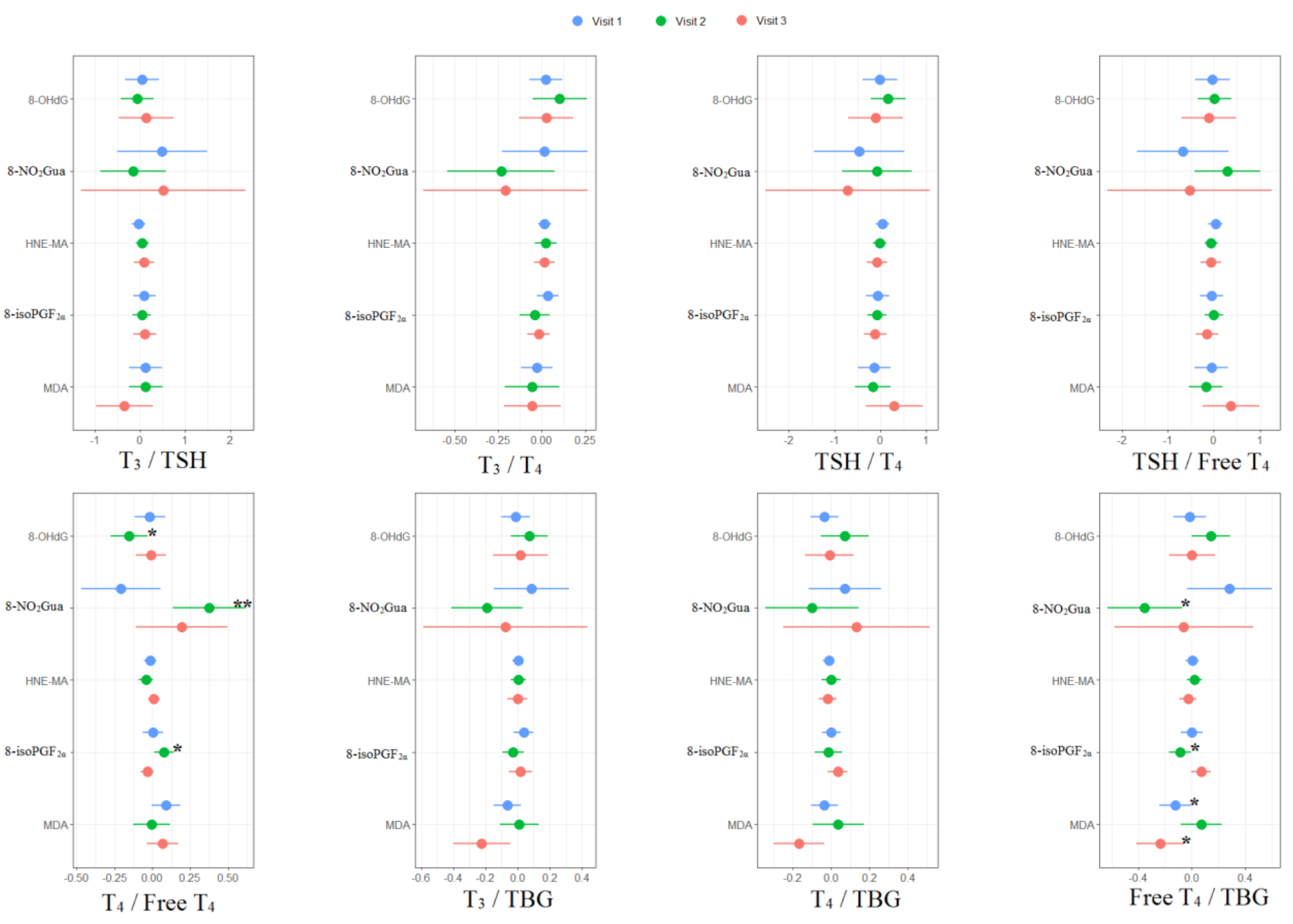
For the associations between thyroid hormone ratios and oxidative or nitrosative stress biomarkers, MDA was negatively associated with free T4 to TBG ratio (β: −0.12, 95% CI [−0.24 to −0.01]) in the first trimester. For the second trimester, 8-OHdG was negatively associated with T4 to free T4 ratio (β: −0.15, 95% CI [−0.28 to −0.03]); 8-NO2Gua was positively associated with T4 to free T4 ratio (β: 0.37, 95% CI [0.13 to 0.62]) but negatively associated with free T4 to TBG ratio (β: −0.35, 95% CI [−0.64 to −0.07]); 8-isoPGF2α was positively associated with T4 to free T4 ratio (β: 0.08, 95% CI [0.01 to 0.15]) but negatively associated with free T4 to TBG ratio (β: −0.09, 95% CI [−0.18 to −0.01]). For the third trimester, MDA was negatively associated with free T4 to TBG ratio (β: −0.24, 95% CI [−0.42 to −0.05]) (Supplementary Tables S3–S5 and Figure 3).
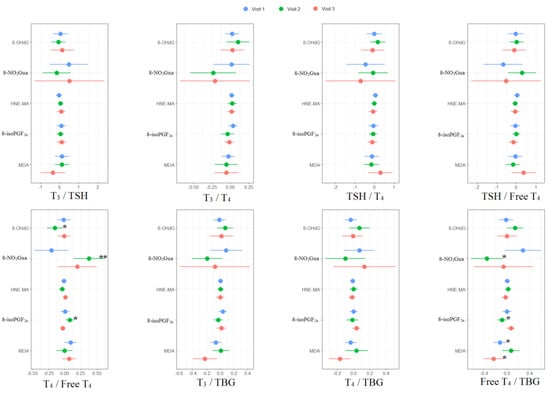
Figure 3.
Unit change in oxidative/nitrosative stress biomarker concentrations in association with concentrations of thyroid hormone ratios across three visits. Multiple linear regressions are adjusted for age and creatinine; * p < 0.05, ** p < 0.01, *** p < 0.001.
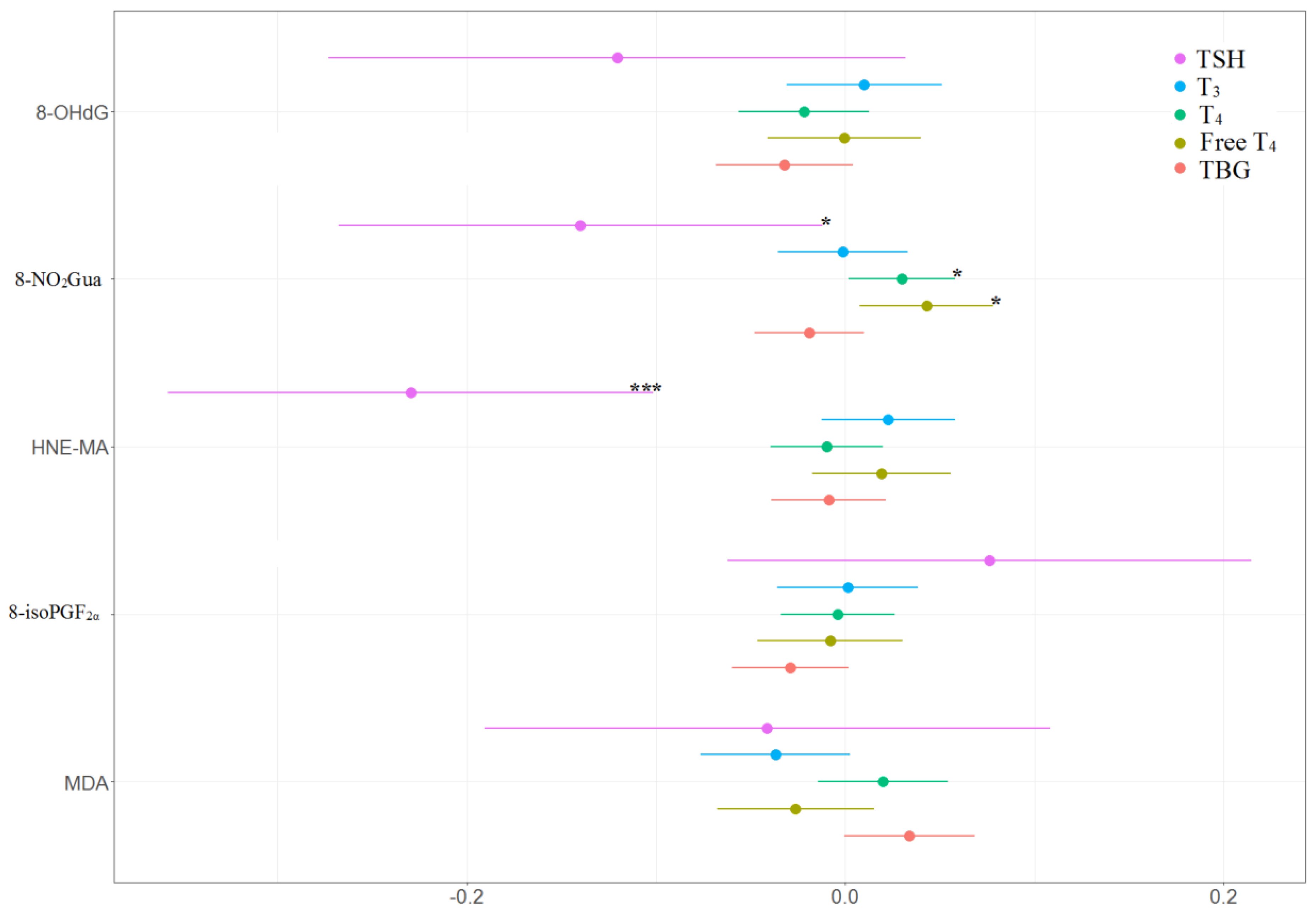
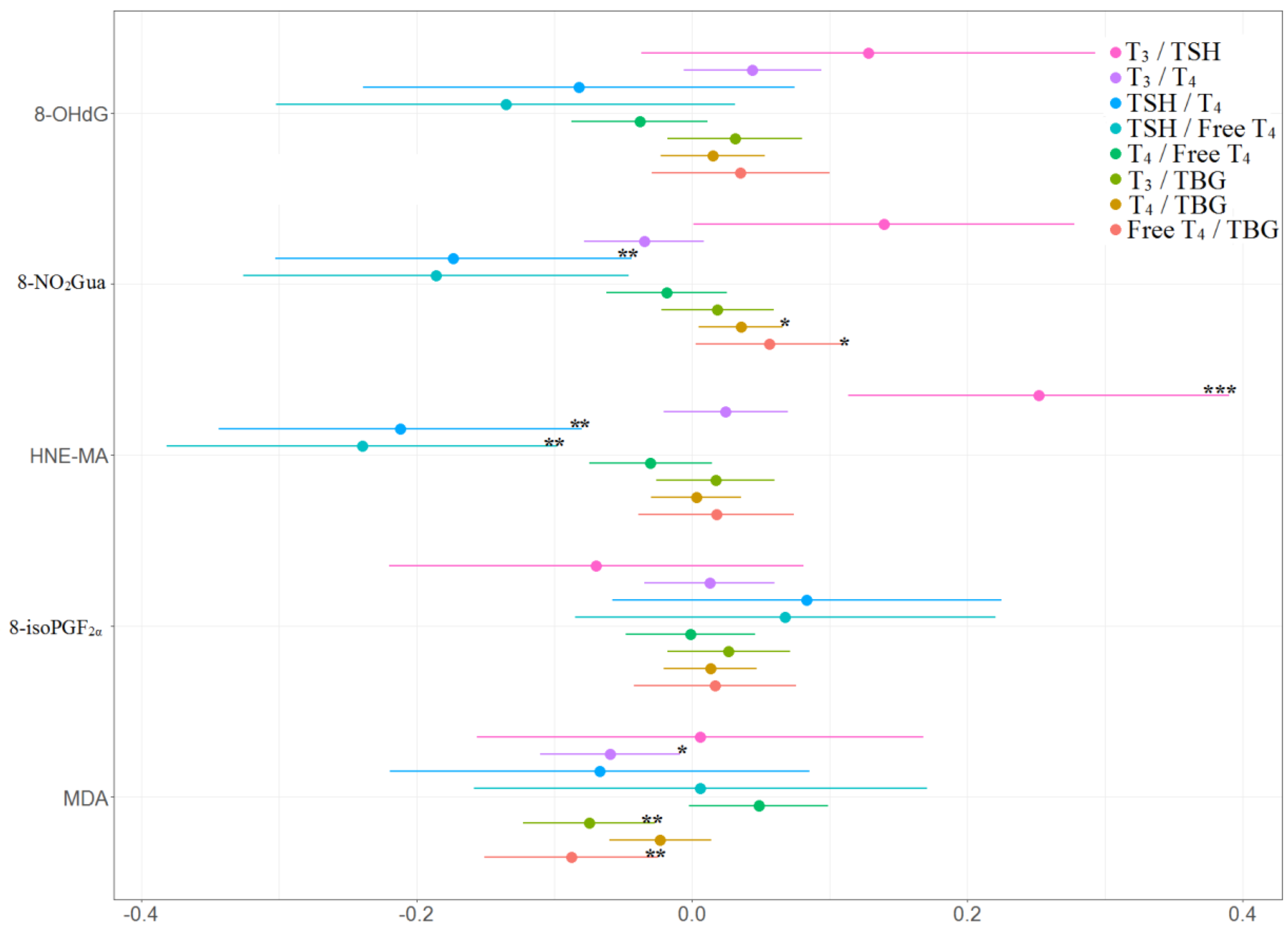
The associations between the percentage of change in concentrations of thyroid hormones and the tertile increases in oxidative or nitrosative stress biomarker concentrations across three visits are presented in Table 4 and Figure 4 and Figure 5. TSH level was negatively and significantly associated with 8-NO2Gua level (−14%, 95% CI [−26.9% to −1.1%]) and HNE-MA level (−23%, 95% CI [−35.9% to −10.0%]). T4 level was only positively and significantly associated with 8-NO2Gua level (3%, 95% CI [0.2%–5.8%]), and free T4 was only positively and significantly associated with 8-NO2Gua (4.3%, 95% CI [0.8%–7.8%]). The T4 to TBG and free T4 to TBG ratios were positively and significantly associated with 8-NO2Gua level (T4/TBG: 3.6%, 95% CI [0.5%–6.7%]; free T4/TBG: 5.6%, 95% CI [0.2%–11.1%]). However, the TSH to T4 ratio was negatively and significantly associated with 8-NO2Gua level (−17.3%, 95% CI [−30.4% to −4.3%]). The T3 to TSH ratio was positively and significantly associated with HNE-MA level (25.2%, 95% CI [11.2%–39.2%]). However, the TSH to T4 and TSH to free T4 ratios were negatively and significantly associated with HNE-MA level (TSH/T4: −21.2%, 95% CI [−34.5% to −7.8%]; TSH/free T4: −24.0%, 95% CI [−38.3% to −9.6%]). The T3 to T4, T3 to TBG, and free T4 to TBG ratios were negatively and significantly associated with MDA level (T3/T4: −5.9%, 95% CI [−11.0% to −0.8%]; T3/TBG: −7.5%, 95% CI [−12.3% to −2.6%]; free T4/TBG: −8.8%, 95% CI [−15.1% to −2.4%]).

Table 4.
Percent changes a in concentrations of thyroid hormones in association with tertile increase in oxidative/nitrosative stress biomarker concentrations across three visits (N = 218).
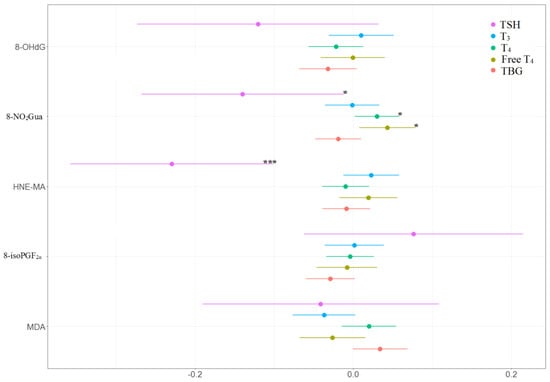
Figure 4.
Percent changes in concentrations of thyroid hormones in association with tertile increase in oxidative/nitrosative stress biomarkers concentrations across three visits. Linear mixed models with random intercept were adjusted for age and creatinine; * p < 0.05, ** p < 0.01, *** p < 0.001.
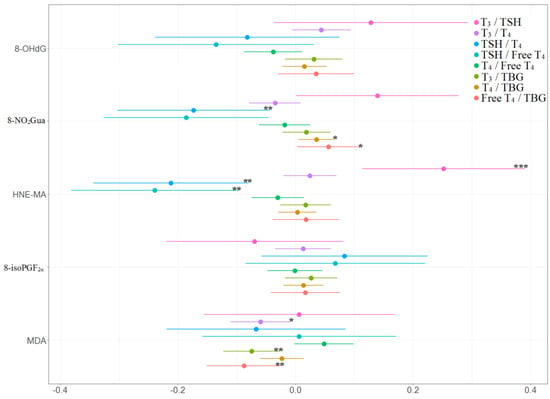
Figure 5.
Percent changes in concentrations of thyroid hormone ratios in association with tertile increase in oxidative/nitrosative stress biomarkers concentrations across three visits. Linear mixed models with random intercept were adjusted for age and creatinine; * p < 0.05, ** p < 0.01, *** p < 0.001.
4. Discussion
To our knowledge, this is the first study of pregnant Taiwanese women to investigate the relationships between oxidative and nitrosative stress and thyroid hormones by using repeated measurements analysis. In our study, urinary levels of 8-isoPGF2α, 8-OHdG, HNE-MA, and 8-NO2Gua remained mostly stable across three visits (Table 2), falling within the ranges reported for Taiwanese pregnant women in the third trimester [28] and those reported for the general healthy population [16]. The levels of 8-isoPGF2α and MDA reported for pregnant women in the United States [29] and Korea [10] were similar to our measurements. Nitrosative stress, measured using 8-NO2Gua, was positively associated with T4 and free T4 levels but negatively associated with TSH level. Lipid peroxidation, measured using HNE-MA, was negatively associated with TSH level. These indicated that the nitrosative stress and lipid peroxidation may alter thyroid hormone homeostasis during pregnancy. In general Taiwanese adults, Huang et al. observed negative associations between 8-NO2Gua and T4 levels and between MDA and T4 levels; they also observed positive associations between 8-NO2Gua and free T4 levels, and between MDA/8-OHdG and free T4 levels [23]. Zhang et al. reported negative associations between 8-isoPGF2α and free T3/free T4 levels for patients with benign thyroid nodules, and they reported a positive association between 8-isoPGF2α and free T4 levels among patients with malignant thyroid nodules [30]. However, we did not observe any significant association between 8-OHdG or 8-isoPGF2α and thyroid hormones in our study. Variations of the associations between oxidative or nitrosative stress and thyroid hormone levels could be due to differences in study design and participants.
In this study, we investigated the impacts of oxidative or nitrosative stress on thyroid hormones; however, thyroid hormones could also cause oxidative stress in target tissues. Thyroid hormones have long been known to induce lipid oxidative damage. Studies have demonstrated that mice are less susceptible to thyroid hormone-induced lipid oxidative damage. In addition, hyperthyroidism was demonstrated to increase protein oxidation in rat livers and testes, as indicated by higher protein-bound carbonyl concentration [31]. Enhanced oxidative stress involving enzymatic and nonenzymatic antioxidants is associated with both hyperthyroidism and hypothyroidism [32,33]. Furthermore, some complications from hyperthyroidism are specifically related to oxidative stress in target tissues [33,34]. The interactions between oxidative stress and thyroid hormones are complicated because oxidative stress could also be induced by thyroid hormones.
An excess of TSH has been linked to the development of oxidative stress in patients with thyroid carcinoma [1,35]. Other studies have observed lipid peroxidation in both overt and subclinical hypothyroidism, as indicated by an increase in MDA level [1,36]. However, in the present study, the increase in nitrosative stress (measured using 8-NO2Gua) was related to a decrease in TSH and increase in T4/free T4 levels; the increase in lipid peroxidation (measured using HNE-MA) was related to a decrease in TSH. Such opposite findings could result from pregnancy, in which raised oxidative stress with consequent enhanced lipid peroxidation as the TSH levels decreased would lower the risk of miscarriage. In addition, the increase in nitrosative stress (measured using 8-NO2Gua) was related to a decrease in the TSH to T4 ratio and increases in the T4 to TBG and in the free T4 to TBG ratios; an increase in lipid peroxidation (measured using HNE-MA) was related to an increase in the T3 to TSH ratio and decreases in the TSH to T4 as well as the TSH to free T4 ratios; the increase in lipid peroxidation (measured using MDA) was related to decreases in the T3 to T4, T3 to TBG, and free T4 to TBG ratios. These indicated that nitrosative stress and lipid peroxidation would alter the thyroid hormone homeostasis during pregnancy.
Because higher metabolic turnover and tissue oxygen consumption occur during pregnancy, it is physiologically linked to increased oxidative stress and reduced antioxidant capacity [37,38]. Antioxidant upregulation may be generated by environmental exposure-induced lipid peroxidation [1], which may contribute to the counteracting effects of oxidative stress on thyroid hormone transfer and function in the placenta. Placental oxidative stress plays a key role in the pathophysiology of placental-related disorders that put fetal development at risk [39]. However, the implication and relevance of oxidative stress in thyroid function and thyroid hormone balance require further clarification [40].
Normal fetal growth and development, particularly early fetal neurodevelopment, require thyroid homeostasis during pregnancy [11]. The mother is the main source of thyroid hormones for the fetus in early pregnancy (before 20 gestational weeks), while fetal thyroid function begins later in pregnancy (after 20 gestational weeks), although maternal thyroid hormones are still relatively essential [41]. Higher maternal free T4 levels in the first trimester of pregnancy have been linked to lower birth weight and an increased risk of small for gestational age [42]. In the present study, the increase in nitrosative stress (measured using 8-NO2Gua) was related to increase in T4 and free T4 levels, which indicated that an imbalance of oxidative and nitrosative stress could alter thyroid hormone homeostasis during pregnancy and affect fetal health. The treatment of hyper or hypothyroidism during pregnancy was debated. Thus, to avoid potential consequences, mitigation of possible risk factors are crucial, i.e., consumption of natural antioxidants would be recommended.
Huang et al. [23] and Zhang et al. [30] found that nitrosative DNA damage and lipid peroxidation may play mediating roles in the effects of phthalate exposure on thyroid hormones in humans. Yao et al. [40] reported a mediating role of oxidative stress biomarkers in the associations between organophosphate esters exposure and thyroid hormones in a population of pregnant women. Waits et al. [20] demonstrated that exposure to phthalates in pregnant women, particularly later in pregnancy, may lead to oxidative and nitrosative stress through DNA damage and lipid peroxidation. Thyroid homeostasis disruption caused by phthalate exposure may contribute to oxidative stress because thyroid hormones may also function as oxidants and cause DNA damage, most likely through phenolic groups [1,20,43]. Furthermore, environmental exposure may influence thyroid hormone levels through induced oxidative and nitrosative stress.
Ours is the first study on oxidative and nitrosative stress biomarkers and thyroid hormones in pregnant Taiwanese women; in addition, we included five different biomarkers for lipid peroxidation and oxidative and nitrosative DNA damage, some of which have not been previously assessed in relation to thyroid hormones. Another strength of our study was the use of repeated measurements analysis, which resulted in more robust estimations of the associations between oxidative and nitrosative stress and thyroid hormones during pregnancy. However, the small sample size of our study limits the generalizability of our findings. Because we considered only a few covariates, unmeasured confounding likely occurred. Furthermore, because intraindividual variability in oxidative and nitrosative stress biomarkers remains undetermined [44], the single spot urine sampling of our investigation is an additional limitation.
5. Conclusions
Our findings suggest that an imbalance of oxidative and nitrosative stress may alter thyroid hormone homeostasis during pregnancy. Disruption of the maternal thyroid homeostasis during pregnancy would affect embryonic and fetal development. To verify these associations, further mechanistic, prospective, and large-scale epidemiological studies are required.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox11020334/s1, Table S1: Potential confounding factors check, Table S2: Proportions (%) of thyroid hormones outside reference ranges for pregnant women, Table S3: Multiple linear regression in concentrations of thyroid hormones in association with unit change in oxidative/ nitrosative stress biomarkers concentrations in visit 1 (N = 97), Table S4: Multiple linear regression in concentrations of thyroid hormones in association with unit change in oxidative/ nitrosative stress biomarkers concentrations in visit 2 (N = 63), Table S5: Multiple linear regression in concentrations of thyroid hormones in association with unit change in oxidative/ nitrosative stress biomarkers concentrations in visit 3 (N = 58).
Author Contributions
Conceptualization, P.-C.H.; methodology, P.-K.C., H.-C.C. and P.-C.H.; software, P.-C.H.; formal analysis, P.-K.C.; investigation, P.-K.C., W.-T.C. and P.-C.H.; resources, P.-C.H.; writing—original draft preparation, P.-K.C. and P.-C.H.; writing—review and editing, H.-C.C., P.-L.K., J.-W.C. and P.-C.H.; supervision, P.-C.H.; project administration, P.-C.H.; funding acquisition, P.-C.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Health Research Institutes (EM-110-PP-11, EM-111-PP-11) and Ministry of Science of Technology (MOST 109-2314-B-400-022-MY3). This work was supported partially by the Research Center for Environmental Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan and by Kaohsiung Medical University Research Center Grant (KMU-TC109A01-1).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Research Ethics Committee of the National Health Research Institutes (No. EC1020302) and the Institutional Review Board of National Cheng Kung University Hospital (No. A-ER-102-104) in Taiwan.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available in this manuscript and supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mancini, A.; Di Segni, C.; Raimondo, S.; Olivieri, G.; Silvestrini, A.; Meucci, E.; Currò, D. Thyroid hormones, oxidative stress, and inflammation. Mediat. Inflamm. 2016, 2016, 6757154. [Google Scholar] [CrossRef] [Green Version]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Silvestri, E.; Schiavo, L.; Lombardi, A.; Goglia, F. Thyroid hormones as molecular determinants of thermogenesis. Acta Physiol. Scand. 2005, 184, 265–283. [Google Scholar] [CrossRef]
- Schriks, M.; Roessig, J.M.; Murk, A.J.; Furlow, J.D. Thyroid hormone receptor isoform selectivity of thyroid hormone disrupting compounds quantified with an in vitro reporter gene assay. Environ. Toxicol. Pharmacol. 2007, 23, 302–307. [Google Scholar] [CrossRef]
- Wu, Y.; Koenig, R.J. Gene regulation by thyroid hormone. Trends Endocrinol. Metab. 2000, 11, 207–211. [Google Scholar] [CrossRef]
- Cheserek, M.J.; Wu, G.R.; Ntazinda, A.; Shi, Y.H.; Shen, L.Y.; Le, G.W. Association between thyroid hormones, lipids and oxidative stress markers in subclinical hypothyroidism. J. Med. Biochem. 2015, 34, 323–331. [Google Scholar] [CrossRef]
- Scholl, T.O.; Stein, T.P. Oxidant damage to DNA and pregnancy outcome. J. Matern. Fetal Neonatal Med. 2001, 10, 182–185. [Google Scholar] [CrossRef]
- Wang, Y.; Walsh, S.W. Increased superoxide generation is associated with decreased superoxide dismutase activity and mRNA expression in placental trophoblast cells in pre-eclampsia. Placenta 2001, 22, 206–212. [Google Scholar] [CrossRef]
- Matsubasa, T.; Uchino, T.; Karashima, S.; Tanimura, M.; Endo, F. Oxidative stress in very low birth weight infants as measured by urinary 8-OHdG. Free Radic. Res. 2002, 36, 189–193. [Google Scholar] [CrossRef]
- Kim, Y.J.; Hong, Y.C.; Lee, K.H.; Park, H.J.; Park, E.A.; Moon, H.S.; Ha, E.H. Oxidative stress in pregnant women and birth weight reduction. Reprod. Toxicol. 2005, 19, 487–492. [Google Scholar] [CrossRef]
- Hartoft-Nielsen, M.L.; Boas, M.; Bliddal, S.; Rasmussen, Å.K.; Main, K.; Feldt-Rasmussen, U. Do thyroid disrupting chemicals influence foetal development during pregnancy? J. Thyroid. Res. 2011, 2011, 342189. [Google Scholar] [CrossRef] [Green Version]
- Aggarawal, N.; Suri, V.; Singla, R.; Chopra, S.; Sikka, P.; Shah, V.N.; Bhansali, A. Pregnancy outcome in hyperthyroidism: A case control study. Gynecol. Obstet. Investig. 2014, 77, 94–99. [Google Scholar] [CrossRef]
- Chen, L.M.; Du, W.J.; Dai, J.; Zhang, Q.; Si, G.X.; Yang, H.; Ye, E.L.; Chen, Q.S.; Yu, L.C.; Zhang, C.; et al. Effects of subclinical hypothyroidism on maternal and perinatal outcomes during pregnancy: A single-center cohort study of a Chinese population. PLoS ONE 2014, 9, e109364. [Google Scholar] [CrossRef] [Green Version]
- Henrichs, J.; Bongers-Schokking, J.J.; Schenk, J.J.; Ghassabian, A.; Schmidt, H.G.; Visser, T.J.; Hooijkaas, H.; de Muinck Keizer-Schrama, S.M.P.F.; Hofman, A.; Jaddoe, V.V.W.; et al. Maternal thyroid function during early pregnancy and cognitive functioning in early childhood: The generation R study. J. Clin. Endocrinol. Metab. 2010, 95, 4227–4234. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Shan, Z.; Teng, W.; Yu, X.; Li, Y.; Fan, C.; Teng, X.; Guo, R.; Wang, H.; Li, J.; et al. Abnormalities of maternal thyroid function during pregnancy affect neuropsychological development of their children at 25–30 months. Clin. Endocrinol. 2010, 72, 825–829. [Google Scholar] [CrossRef]
- Wu, C.; Chen, S.T.; Peng, K.H.; Cheng, T.J.; Wu, K.Y. Concurrent quantification of multiple biomarkers indicative of oxidative stress status using liquid chromatography—Tandem mass spectrometry. Anal. Biochem. 2016, 512, 26–35. [Google Scholar] [CrossRef]
- Peter Stein, T.; Scholl, T.O.; Schluter, M.D.; Leskiw, M.J.; Chen, X.; Spur, B.W.; Rodriguez, A. Oxidative stress early in pregnancy and pregnancy outcome. Free Radic. Res. 2008, 42, 841–848. [Google Scholar] [CrossRef]
- Wang, P.W.; Chen, M.L.; Huang, L.W.; Yang, W.; Wu, K.Y.; Huang, Y.F. Prenatal nonylphenol exposure, oxidative and nitrative stress, and birth outcomes: A cohort study in Taiwan. Environ. Pollut. 2015, 207, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Eick, S.M.; Ferguson, K.K.; Milne, G.L.; Rios-McConnell, R.; Vélez-Vega, C.; Rosario, Z.; Alshawabkeh, A.; Cordero, J.F.; Meeker, J.D. Repeated measures of urinary oxidative stress biomarkers and preterm birth in Puerto Rico. Free. Radic. Biol. Med. 2020, 146, 299–305. [Google Scholar] [CrossRef]
- Waits, A.; Chen, H.C.; Kuo, P.L.; Wang, C.W.; Huang, H.B.; Chang, W.H.; Shih, S.F.; Huang, P.C. Urinary phthalate metabolites are associated with biomarkers of DNA damage and lipid peroxidation in pregnant women—Tainan Birth Cohort Study (TBCS). Environ. Res. 2020, 188, 109863. [Google Scholar] [CrossRef]
- Huang, P.C.; Tsai, C.H.; Liang, W.Y.; Li, S.S.; Huang, H.B.; Kuo, P.L. Early phthalates exposure in pregnant women is associated with alteration of thyroid hormones. PLoS ONE 2016, 11, e0159398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.B.; Kuo, P.L.; Chang, J.W.; Jaakkola, J.J.K.; Liao, K.W.; Huang, P.C. Longitudinal assessment of prenatal phthalate exposure on serum and cord thyroid hormones homeostasis during pregnancy—Tainan birth cohort study (TBCS). Sci. Total Environ. 2018, 619–620, 1058–1065. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.C.; Waits, A.; Chen, H.C.; Chang, W.T.; Jaakkola, J.J.; Huang, H.B. Mediating role of oxidative/nitrosative stress biomarkers in the associations between phthalate exposure and thyroid function in Taiwanese adults. Environ. Int. 2020, 140, 105751. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.B.; Pan, W.H.; Chang, J.W.; Chiang, H.C.; Guo, Y.L.; Jaakkola, J.J.; Huang, P.C. Does exposure to phthalates influence thyroid function and growth hormone homeostasis? The Taiwan Environmental Survey for Toxicants (TEST) 2013. Environ. Res. 2017, 153, 63–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barr, D.B.; Wilder, L.C.; Caudill, S.P.; Gonzalez, A.J.; Needham, L.L.; Pirkle, J.L. Urinary creatinine concentrations in the US population: Implications for urinary biologic monitoring measurements. Environ. Health Perspect. 2005, 113, 192–200. [Google Scholar] [CrossRef] [Green Version]
- Carney, L.A.; Quinlan, J.D.; West, J.M. Thyroid disease in pregnancy. Am. Fam. Physician 2014, 89, 273–278. [Google Scholar]
- Alexander, E.K.; Pearce, E.N.; Brent, G.A.; Brown, R.S.; Chen, H.; Dosiou, C.; Grobman, W.A.; Laurberg, P.; Lazarus, J.H.; Mandel, S.J.; et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid 2017, 27, 315–389. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.F.; Wang, P.W.; Huang, L.W.; Lai, C.H.; Yang, W.; Wu, K.Y.; Lu, C.A.; Chen, H.C.; Chen, M.L. Prenatal nonylphenol and bisphenol A exposures and inflammation are determinants of oxidative/nitrative stress: A Taiwanese cohort study. Environ. Sci. Technol. 2017, 51, 6422–6429. [Google Scholar] [CrossRef]
- van’t Erve, T.J.; Rosen, E.M.; Barrett, E.S.; Nguyen, R.H.; Sathyanarayana, S.; Milne, G.L.; Calafat, A.M.; Swan, S.H.; Ferguson, K.K. Phthalates and phthalate alternatives have diverse associations with oxidative stress and inflammation in pregnant women. Environ. Sci. Technol. 2019, 53, 3258–3267. [Google Scholar] [CrossRef]
- Zhang, M.; Deng, Y.L.; Liu, C.; Chen, P.P.; Luo, Q.; Miao, Y.; Cui, F.P.; Wang, L.Q.; Jiang, M.; Zeng, Q. Urinary phthalate metabolite concentrations, oxidative stress and thyroid function biomarkers among patients with thyroid nodules. Environ. Pollut. 2021, 272, 116416. [Google Scholar] [CrossRef]
- Venditti, P.; Di Meo, S. Thyroid hormone-induced oxidative stress. Cell. Mol. Life Sci. CMLS 2006, 63, 414–434. [Google Scholar] [CrossRef] [PubMed]
- Resch, U.; Helsel, G.; Tatzber, F.; Sinzinger, H. Antioxidant Status in Thyroid Dysfunction. Clin. Chem. Lab. Med. 2002, 40, 1132–1134. [Google Scholar] [CrossRef] [PubMed]
- Mancini, A.; Raimondo, S.; Di Segni, C.; Persano, M.; Gadotti, G.; Silvestrini, A.; Festa, R.; Tiano, L.; Pontecorvi, A.; Meucci, E. Thyroid hormones and antioxidant systems: Focus on oxidative stress in cardiovascular and pulmonary diseases. Int. J. Mol. Sci. 2013, 14, 23893–23909. [Google Scholar] [CrossRef]
- Asayama, K.; Kato, K. Oxidative muscular injury and its relevance to hyperthyroidism. Free Radic. Biol. Med. 1990, 8, 293–303. [Google Scholar] [CrossRef]
- Dardano, A.; Ghiadoni, L.; Plantinga, Y.; Caraccio, N.; Bemi, A.; Duranti, E.; Taddei, S.; Ferrannini, E.; Salvetti, A.; Monzani, F. Recombinant human thyrotropin reduces endothelium-dependent vasodilation in patients monitored for differentiated thyroid carcinoma. J. Clin. Endocrinol. Metab. 2006, 91, 4175–4178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haribabu, A.; Reddy, V.S.; Pallavi, C.H.; Bitla, A.R.; Sachan, A.; Pullaiah, P.; Suresh, V.; Rao, P.V.L.N.S.; Suchitra, M.M. Evaluation of protein oxidation and its association with lipid peroxidation and thyrotropin levels in overt and subclinical hypothyroidism. Endocrine 2013, 44, 152–157. [Google Scholar] [CrossRef]
- Manti, S.; Marseglia, L.; D’Angelo, G.; Cuppari, C.; Cusumano, E.; Arrigo, T.; Gitto, E.; Salpietro, C. “Cumulative Stress”: The effects of maternal and neonatal oxidative stress and oxidative stress-inducible genes on programming of atopy. Oxid. Med. Cell. Longev. 2016, 2016, 8651820. [Google Scholar] [CrossRef] [Green Version]
- Derouiche, S.; DoudiDalal, A.N. Study of oxidative stress during pregnancy. Glob. J. Pharm. Pharm. Sci. 2018, 4, 555646. [Google Scholar]
- Domínguez-Perles, R.; Gil-Izquierdo, A.; Ferreres, F.; Medina, S. Update on oxidative stress and inflammation in pregnant women, unborn children (nasciturus), and newborns—Nutritional and dietary effects. Free Radic. Biol. Med. 2019, 142, 38–51. [Google Scholar] [CrossRef]
- Yao, Y.; Li, M.; Pan, L.; Duan, Y.; Duan, X.; Li, Y.; Sun, H. Exposure to organophosphate ester flame retardants and plasticizers during pregnancy: Thyroid endocrine disruption and mediation role of oxidative stress. Environ. Int. 2021, 146, 106215. [Google Scholar] [CrossRef]
- Obregón, M.J.; Calvo, R.M.; Del Rey, F.E.; De Escobar, G.M. Ontogenesis of thyroid function and interactions with maternal function. Thyroid Gland Dev. Funct. 2007, 10, 86–98. [Google Scholar]
- Medici, M.; de Rijke, Y.B.; Peeters, R.P.; Visser, W.; de Muinck Keizer-Schrama, S.M.; Jaddoe, V.V.; Hofman, A.; Hooijkaas, H.; Steegers, E.A.; Tiemeier, H.; et al. Maternal early pregnancy and newborn thyroid hormone parameters: The Generation R study. J. Clin. Endocrinol. Metab. 2012, 97, 646–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobrzyńska, M.M.; Baumgartner, A.; Anderson, D. Antioxidants modulate thyroid hormone-and noradrenaline-induced DNA damage in human sperm. Mutagenesis 2004, 19, 325–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Il’yasova, D.; Scarbrough, P.; Spasojevic, I. Urinary biomarkers of oxidative status. Clin. Chim. Acta 2012, 413, 1446–1453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).