Total Polyphenol Content in Food Samples and Nutraceuticals: Antioxidant Indices versus High Performance Liquid Chromatography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Instruments and Apparatus
2.3. Samples
2.4. Sample Treatment
2.5. Spectrophotometric Indices
2.6. Chromatographic Method
2.7. Data Analysis
3. Results and Discussion
3.1. HPLC and MS Analysis
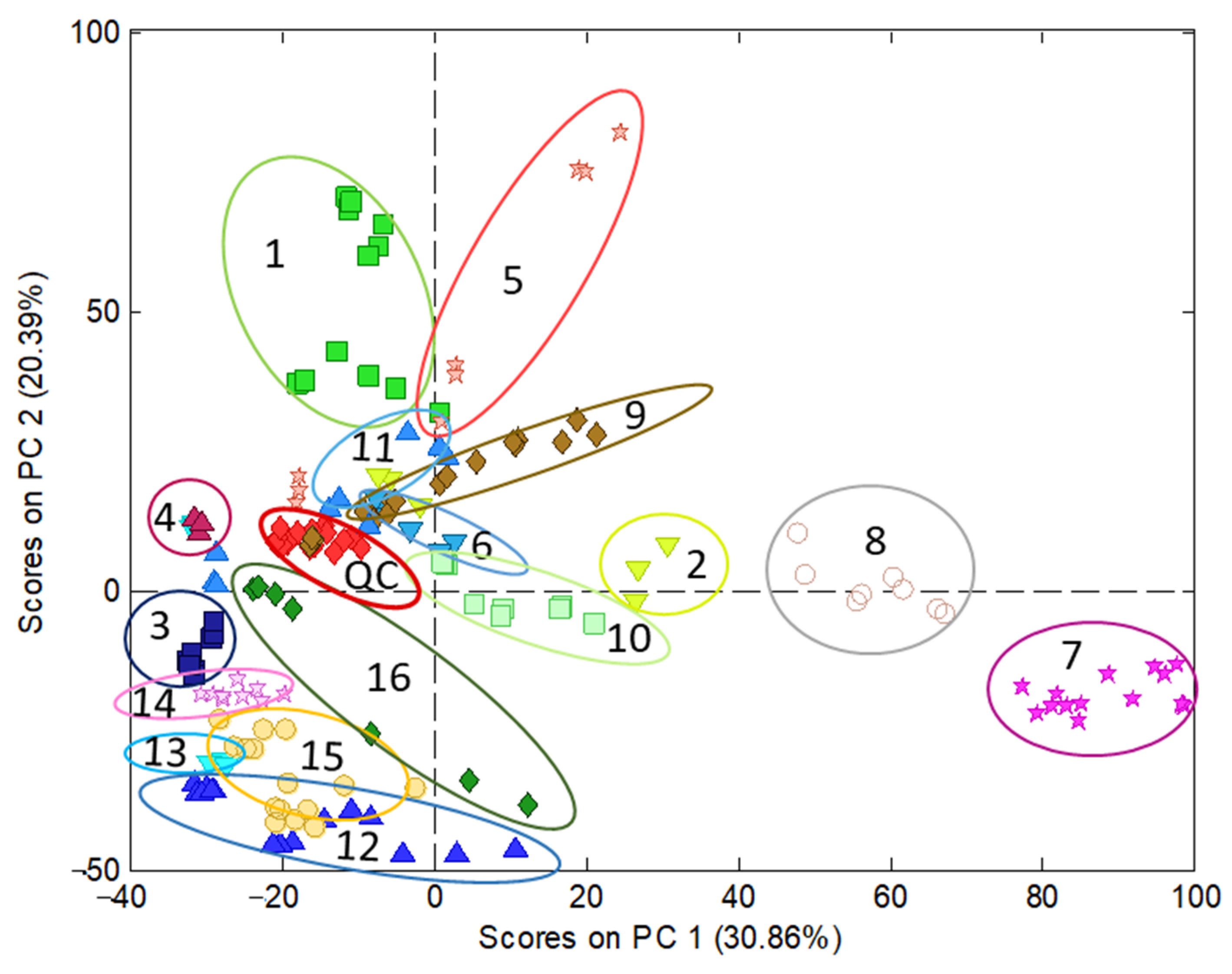
3.2. Sample Characterization by PCA
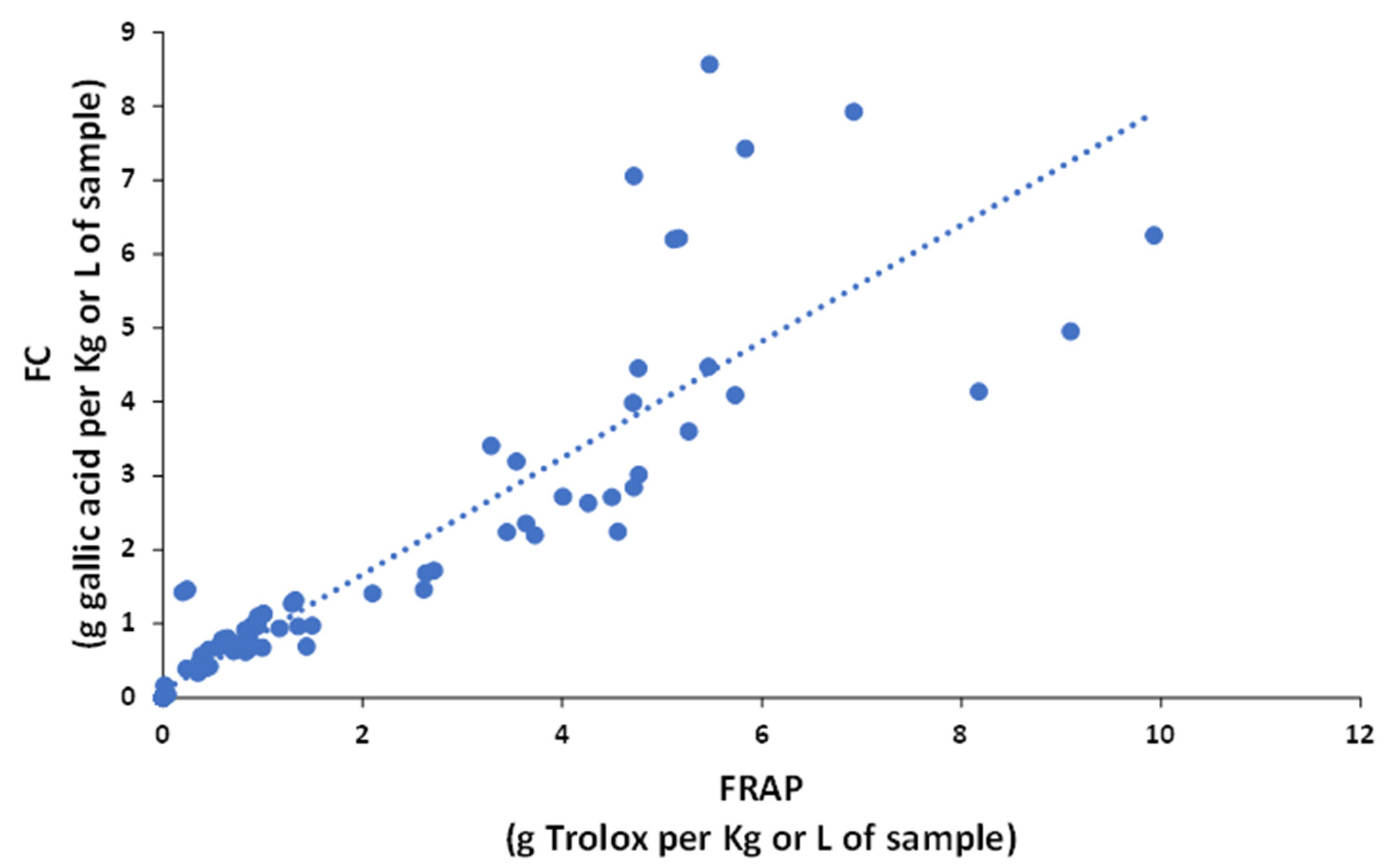
3.3. Determination of the Antioxidant Capacity of Different Sample Classes by Spectrophotometric Indices
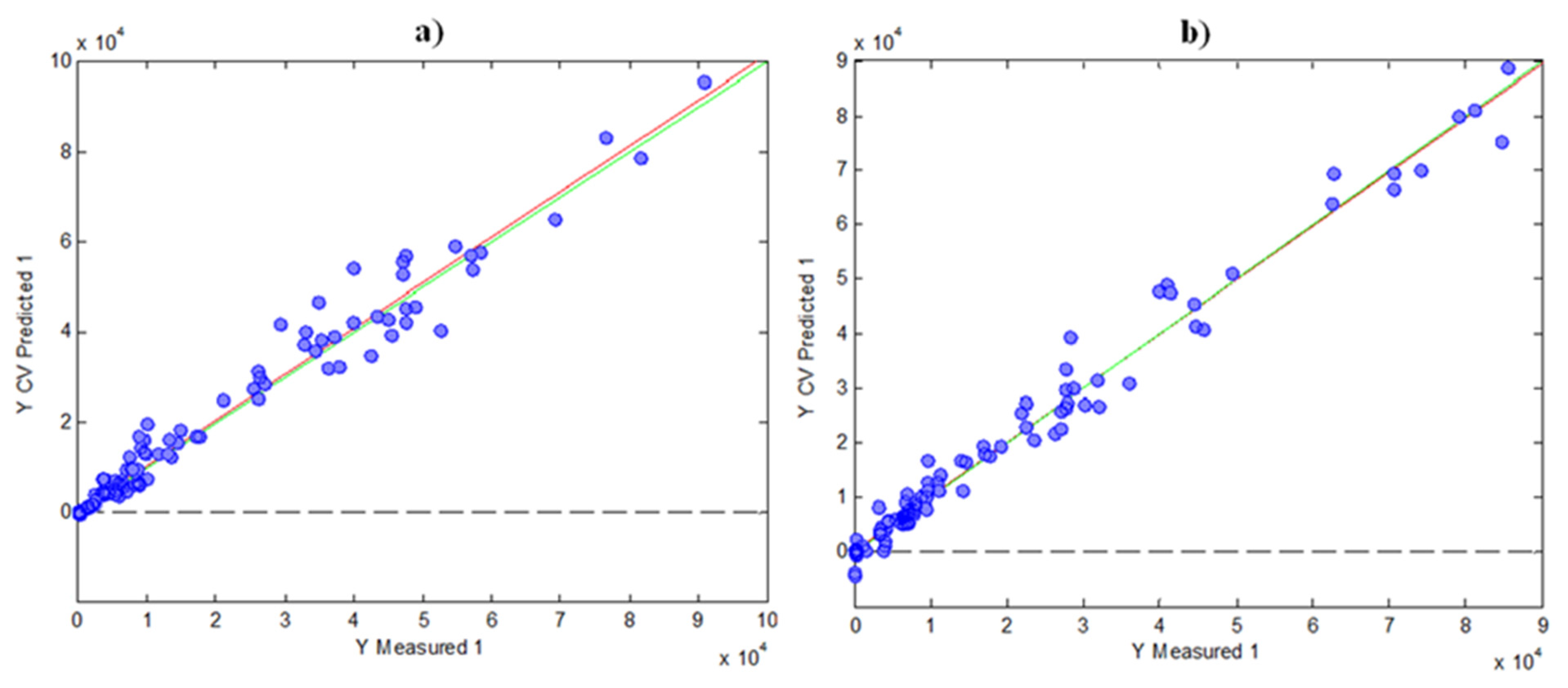
3.4. Correlation between HPLC Fingerprints and Spectrophotometric Indices by PLS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alcalde, B.; Granados, M.; Saurina, J. Exploring the Antioxidant Features of Polyphenols by Spectroscopic and Electrochemical Methods. Antioxidants 2019, 8, 523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arts, I.C.W.; Hollman, P.C.H. Polyphenols and Disease Risk in Epidemiologic Studies. Am. J. Clin. Nutr. 2005, 81 (Suppl. S1), 317S–325S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef] [PubMed]
- Šeruga, M.; Novak, I.; Jakobek, L. Determination of Polyphenols Content and Antioxidant Activity of Some Red Wines by Differential Pulse Voltammetry, HPLC and Spectrophotometric Methods. Food Chem. 2011, 124, 1208–1216. [Google Scholar] [CrossRef]
- Vidal-Casanella, O.; Nunez, O.; Granados, M.; Saurina, J.; Sentellas, S. Analytical Methods for Exploring Nutraceuticals Based on Phenolic Acids and Polyphenols. App. Sci. 2021, 11, 8276. [Google Scholar] [CrossRef]
- Ebner, H.; Dienstbach, F.; Sandritter, W. Hormonelle Beeinflussung des experimentellen Portiocarcinoms. Natl. Libr. Med. 1967, 73, 366–369. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough Study of Reactivity of Various Compound Classes toward the Folin-Ciocalteu Reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef] [Green Version]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoʇlu, E. Antioxidant Activity/Capacity Measurement. 1. Classification, Physicochemical Principles, Mechanisms, and Electron Transfer (ET)-Based Assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef]
- Ou, B.; Huang, D.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Analysis of Antioxidant Activities of Common Vegetables Employing Oxygen Radical Absorbance Capacity (ORAC) and Ferric Reducing Antioxidant Power (FRAP) Assays: A Comparative Study. J. Agric. Food Chem. 2002, 50, 3122–3128. [Google Scholar] [CrossRef] [PubMed]
- Alén-Ruiz, F.; García-Falcón, M.S.; Pérez-Lamela, M.C.; Martínez-Carballo, E.; Simal-Gándara, J. Influence of Major Polyphenols on Antioxidant Activity in Mencía and Brancellao Red Wines. Food Chem. 2009, 113, 53–60. [Google Scholar] [CrossRef]
- Alonso, Á.M.; Guillén, D.A.; Barroso, C.G.; Puertas, B.; García, A. Determination of Antioxidant Activity of Wine Byproducts and Its Correlation with Polyphenolic Content. J. Agric. Food Chem. 2002, 50, 5832–5836. [Google Scholar] [CrossRef] [PubMed]
- Todorovic, V.; Redovnikovic, I.R.; Todorovic, Z.; Jankovic, G.; Dodevska, M.; Sobajic, S. Polyphenols, Methylxanthines, and Antioxidant Capacity of Chocolates Produced in Serbia. J. Food Compos. Anal. 2015, 41, 137–143. [Google Scholar] [CrossRef]
- Hawrył, A.; Hawrył, M. Chromatographic Fingerprinting of Some Basils and the Evaluation of Their Antioxidant Properties with Chemometric Calculations. J. Liq. Chromatogr. Relat. Technol. 2020, 43, 750–760. [Google Scholar] [CrossRef]
- Vidal-Casanella, O.; Arias-Alpizar, K.; Nuñez, O.; Saurina, J. Hydrophilic Interaction Liquid Chromatography to Characterize Nutraceuticals and Food Supplements Based on Flavanols and Related Compounds. Separations 2021, 8, 17. [Google Scholar] [CrossRef]
- Jolliffe, I.T.; Cadima, J. Principal Component Analysis: A Review and Recent Developments. Philos. Trans. Ser. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef]
- Kozak, M.; Scaman, C.H. Unsupervised classification methods in food sciences: Discussion and outlook. J. Sci. Food Agric. 2008, 88, 1115–1127. [Google Scholar] [CrossRef]
- Granato, D.; Putnik, P.; Kovacevic, D.B.; Santos, J.S.; Calado, V.; Rocha, R.S.; Da Cruz, A.G.; Jarvis, B.; Rodionova, O.Y.; Pomerantsev, A. Trends in Chemometrics: Food Authentication, Microbiology, and Effects of Processing. Compr. Rev. Food Sci. Food Saf. 2018, 17, 663–677. [Google Scholar] [CrossRef] [Green Version]
- Tapia-Quiros, P.; Montenegro-Landivar, M.F.; Reig, M.; Vecino, X.; Alvarino, T.; Cortina, J.L.; Saurina, J.; Granados, M. Olive Mill and Winery Wastes as Viable Sources of Bioactive Compounds: A Study on Polyphenols Recovery. Antioxidants 2020, 9, 1074. [Google Scholar] [CrossRef]
- Bakhytkyzy, I.; Nunez, O.; Saurina, J. Determination of flavanols by liquid chromatography with fluorescence detection. Application to the characterization of cranberry-based pharmaceuticals through profiling and fingerprinting approaches. J. Pharm. Biomed. Anal. 2018, 156, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Llopart, A.; Saurina, J. Liquid Chromatographic Approach for the Discrimination and Classification of Cava Samples Based on the Phenolic Composition Using Chemometric Methods. Beverages 2020, 6, 54. [Google Scholar] [CrossRef]
- Vidal-Casanella, O.; Nunez, N.; Sentellas, S.; Nunez, O.; Saurina, J. Characterization of Turmeric and Curry Samples by Liquid Chromatography with Spectroscopic Detection Based on Polyphenolic and Curcuminoid Contents. Separations 2020, 7, 23. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, S.; Campmajo, G.; Saurina, J.; Puignou, L.; Nunez, O. Determination of Phenolic Compounds in Paprika by Ultrahigh Performance Liquid Chromatography-Tandem Mass Spectrometry: Application to Product Designation of Origin Authentication by Chemometrics. J. Agric. Food Chem. 2020, 68, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, S.; Pardo-Mates, N.; Hidalgo-Serrano, M.; Saurina, J.; Puignou, L.; Nunez, O. Detection and Quantitation of Frauds in the Authentication of Cranberry-Based Extracts by UHPLC-HRMS (Orbitrap) Polyphenolic Profiling and Multivariate Calibration Methods. J. Agric. Food Chem. 2018, 66, 9353–9365. [Google Scholar] [CrossRef] [Green Version]
- Pardo-Mates, N.; Vera, A.; Barbosa, S.; Hidalgo-Serrano, M.; Nunez, O.; Saurina, J.; Hernandez-Cassou, S.; Puignou, L. Characterization, classification and authentication of fruit-based extracts by means of HPLC-UV chromatographic fingerprints, polyphenolic profiles and chemometric methods. Food Chem. 2017, 221, 29–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidal-Casanella, O.; Moreno-Merchan, J.; Granados, M.; Nuñez, O.; Saurina, J.; Sentellas, S. Total Polyphenol Content in Food Samples and Nutraceuticals: Antioxidant Indices versus High Performance Liquid Chromatography. Antioxidants 2022, 11, 324. https://doi.org/10.3390/antiox11020324
Vidal-Casanella O, Moreno-Merchan J, Granados M, Nuñez O, Saurina J, Sentellas S. Total Polyphenol Content in Food Samples and Nutraceuticals: Antioxidant Indices versus High Performance Liquid Chromatography. Antioxidants. 2022; 11(2):324. https://doi.org/10.3390/antiox11020324
Chicago/Turabian StyleVidal-Casanella, Oscar, Javier Moreno-Merchan, Merce Granados, Oscar Nuñez, Javier Saurina, and Sonia Sentellas. 2022. "Total Polyphenol Content in Food Samples and Nutraceuticals: Antioxidant Indices versus High Performance Liquid Chromatography" Antioxidants 11, no. 2: 324. https://doi.org/10.3390/antiox11020324
APA StyleVidal-Casanella, O., Moreno-Merchan, J., Granados, M., Nuñez, O., Saurina, J., & Sentellas, S. (2022). Total Polyphenol Content in Food Samples and Nutraceuticals: Antioxidant Indices versus High Performance Liquid Chromatography. Antioxidants, 11(2), 324. https://doi.org/10.3390/antiox11020324