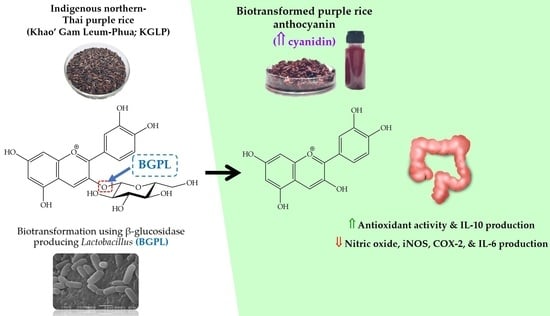
Enhancement of the Colorectal Chemopreventive and Immunization Potential of Northern Thai Purple Rice Anthocyanin Using the Biotransformation by β-Glucosidase-Producing Lactobacillus
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Selection of Indigenous Northern Thai Purple Rice
2.3. Selection of β-Glucosidase-Producing Lactobacillus Strain
2.4. Stability of β-Glucosidase Enzyme Activity at Various pH and Temperature
2.5. Purple Rice Anthocyanin Biotransformation Study
2.6. Effect of Biotransformed Anthocyanin on In Vitro Antioxidant Activity
2.6.1. 2,2′-Azino-bis-3-ethylbenzothiazoline-6-sulfonic Acid (ABTS) Radical-Scavenging Assay
2.6.2. Superoxide Anion Radical-Scavenging Assay
2.6.3. Nitric Oxide Radical-Scavenging Assay
2.7. Effect of Biotransformed Anthocyanin on Colorectal Chemopreventive and Immunization Potential in HT-29 Cells
3. Results
3.1. Selection of Thai Purple Rice
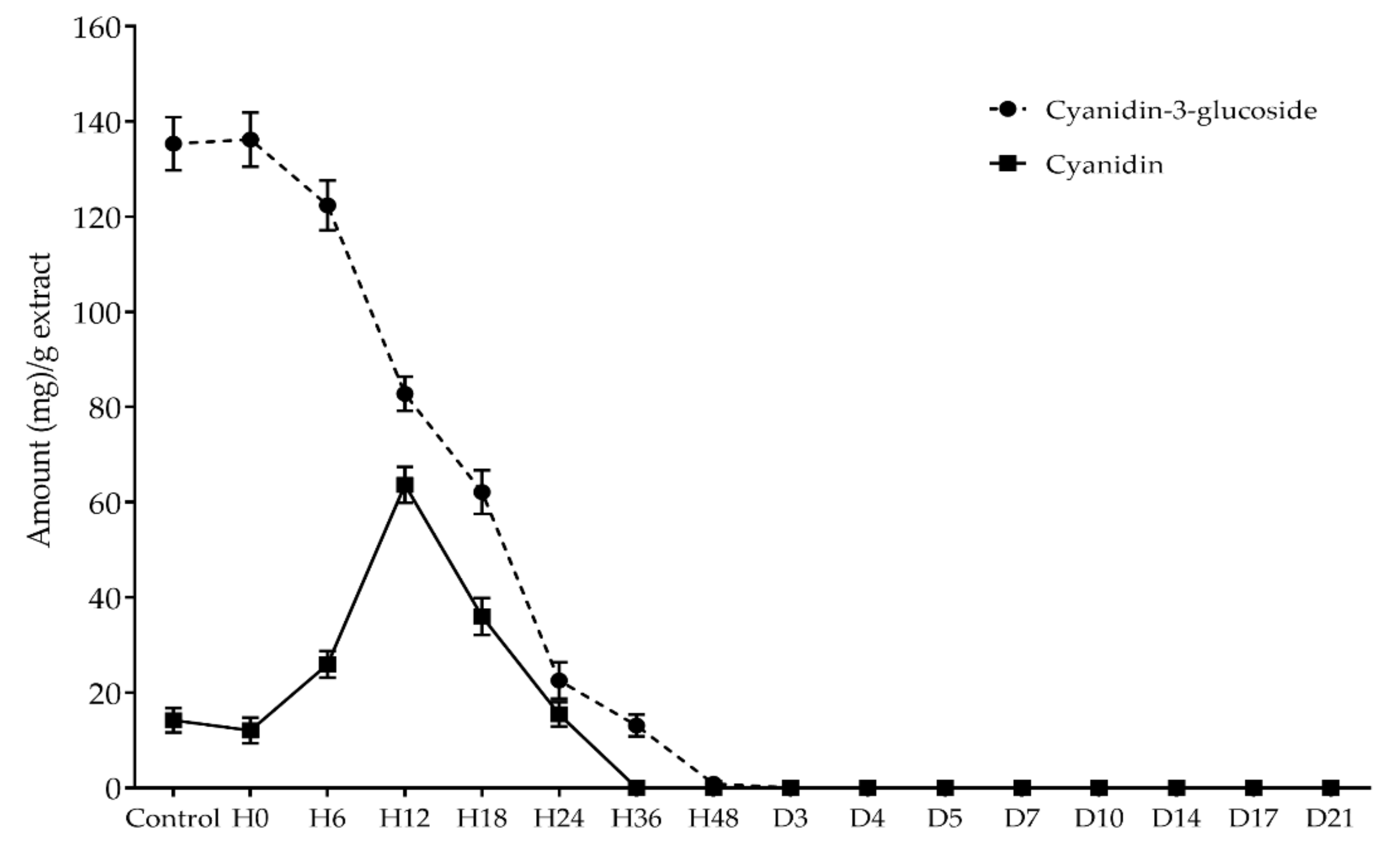
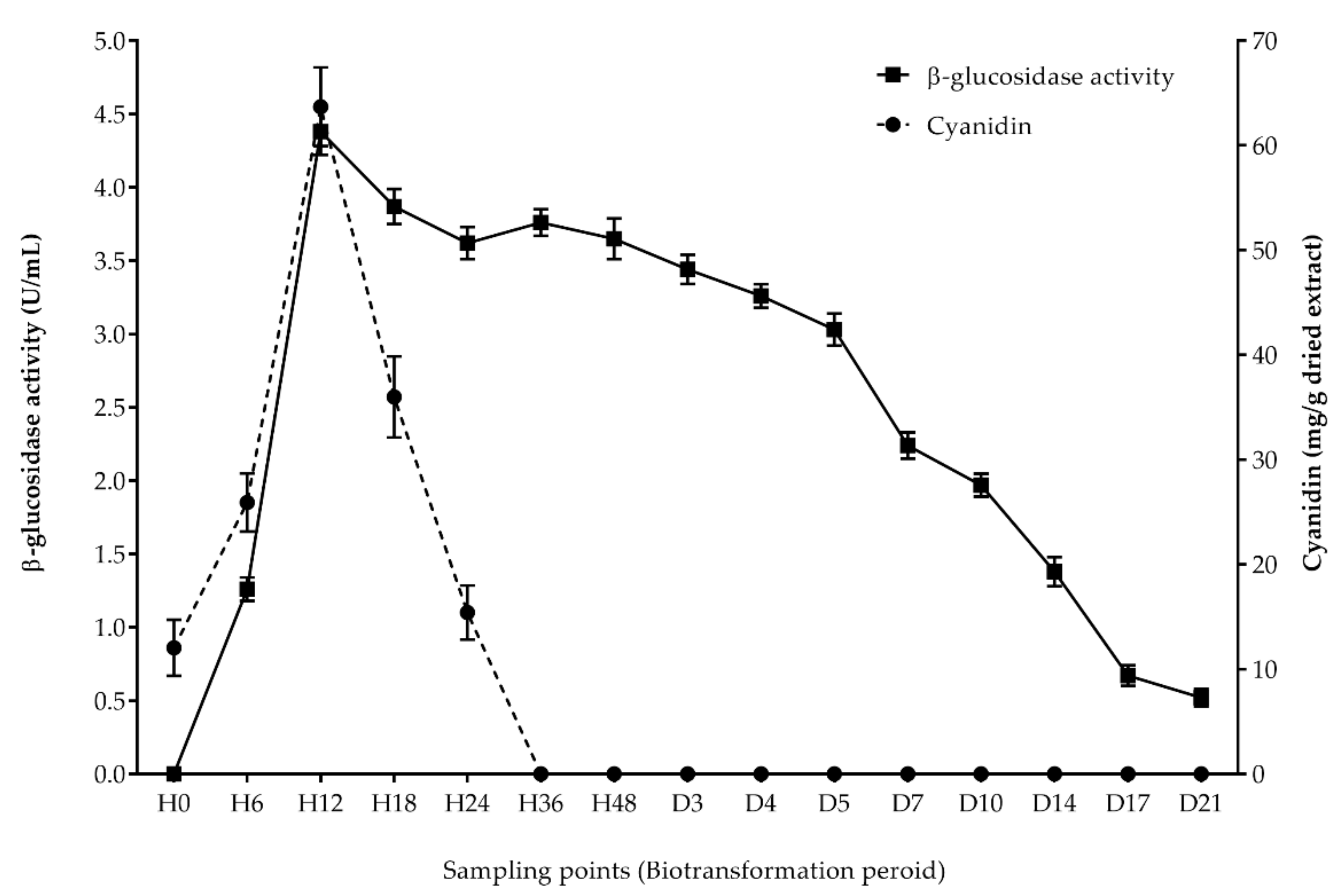
3.2. Kinetics of Anthocyanin Content during Biotransformation Process
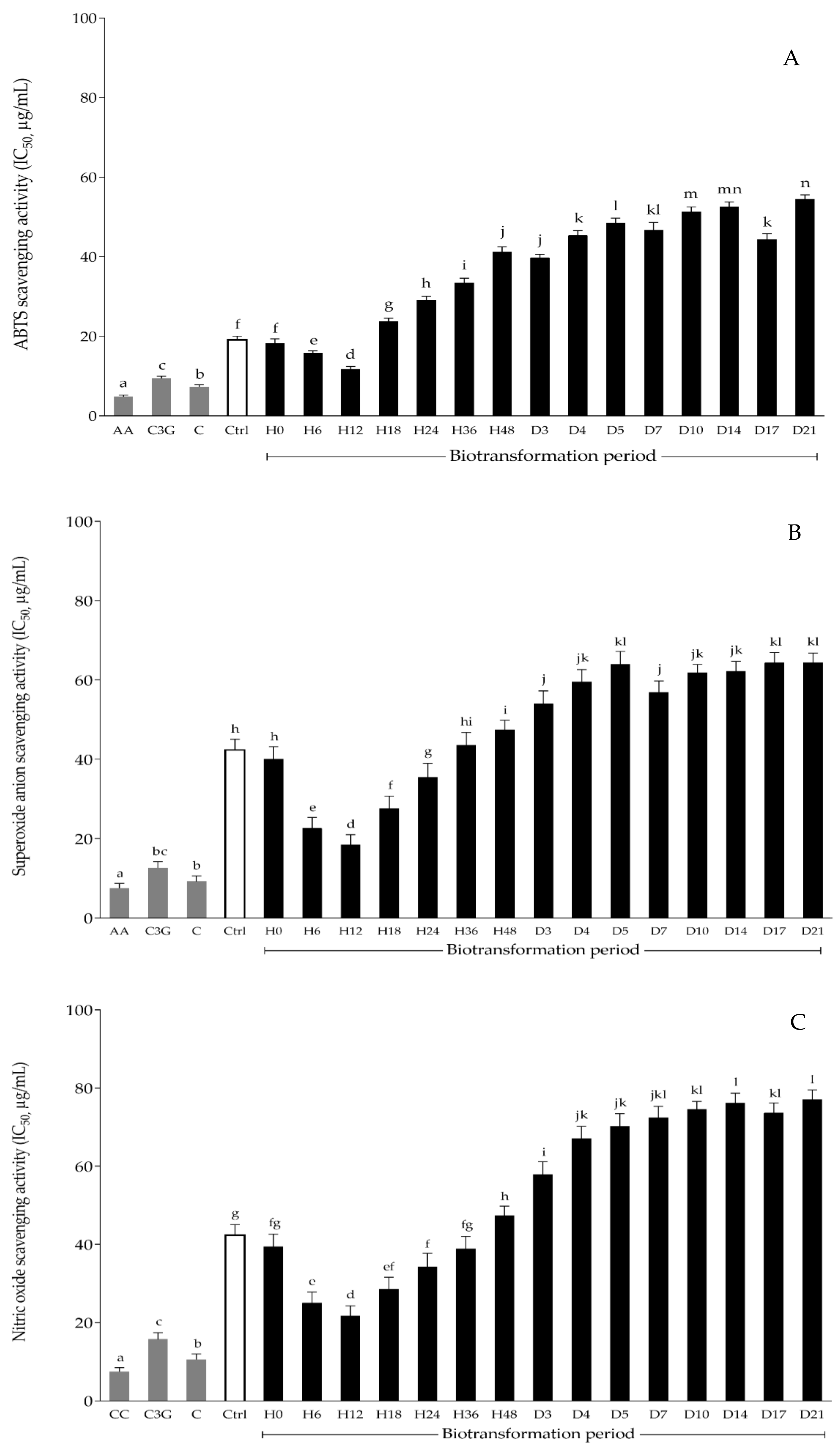
3.3. Effect of Biotransformed Anthocyanin on In Vitro Antioxidant Activities
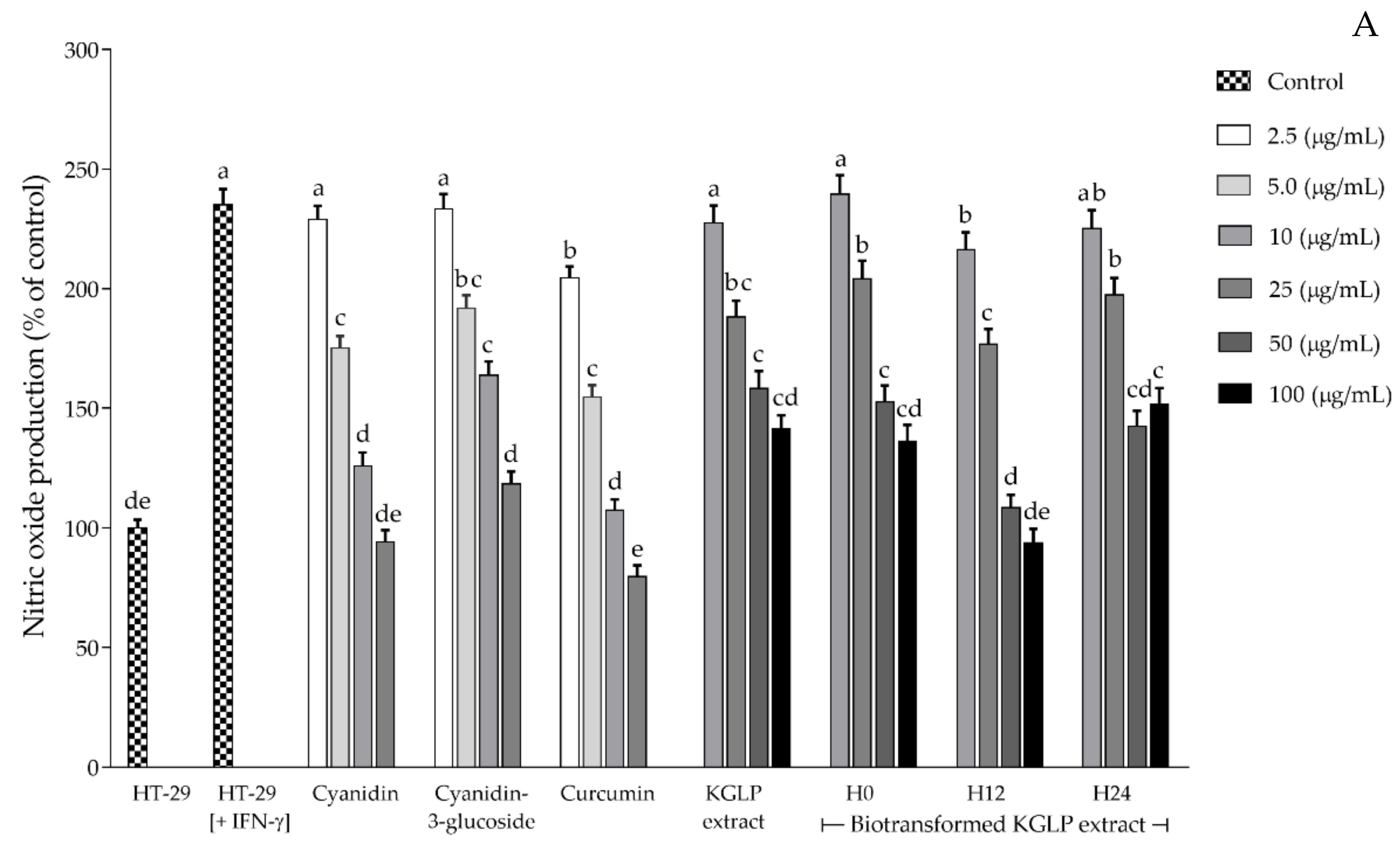
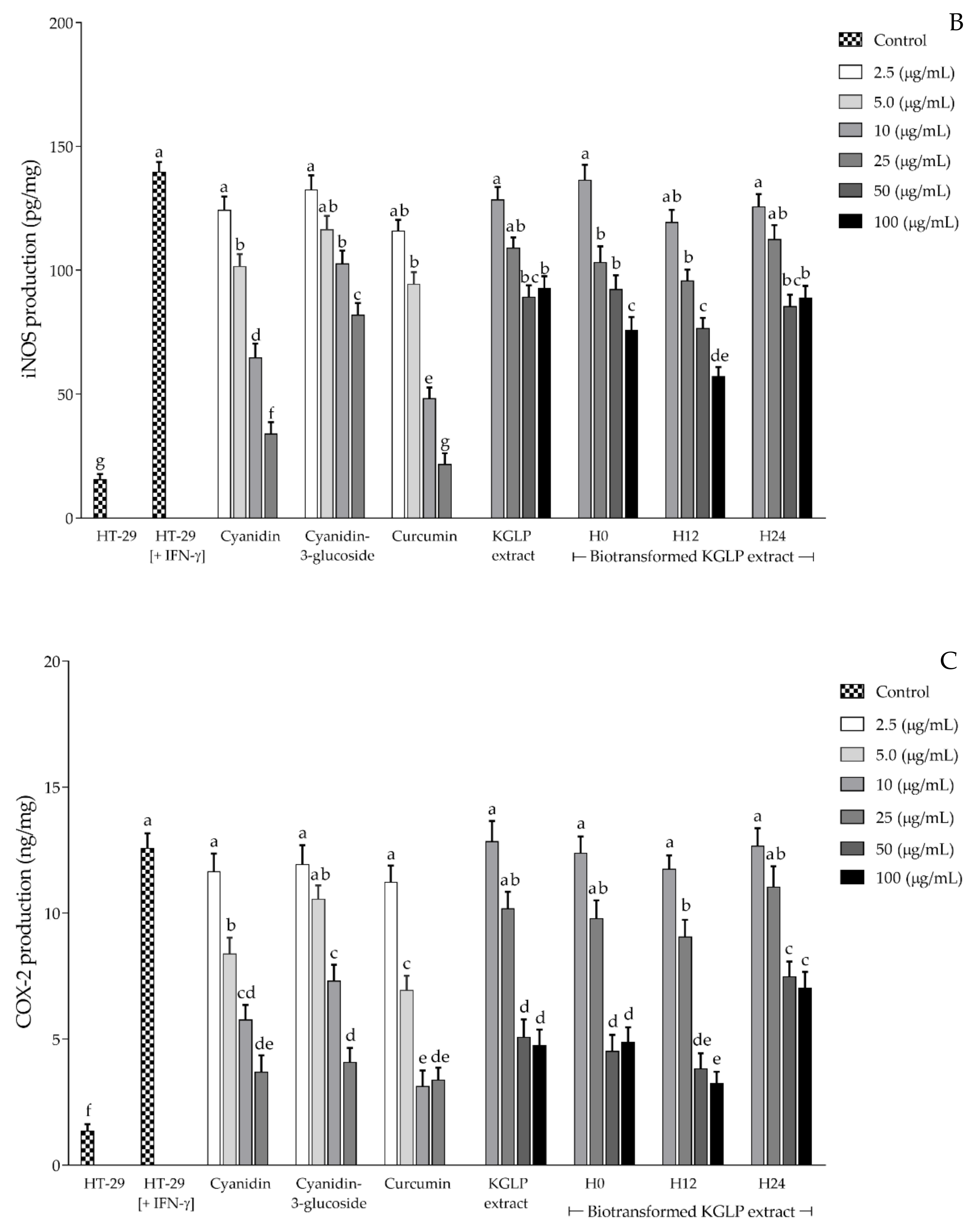
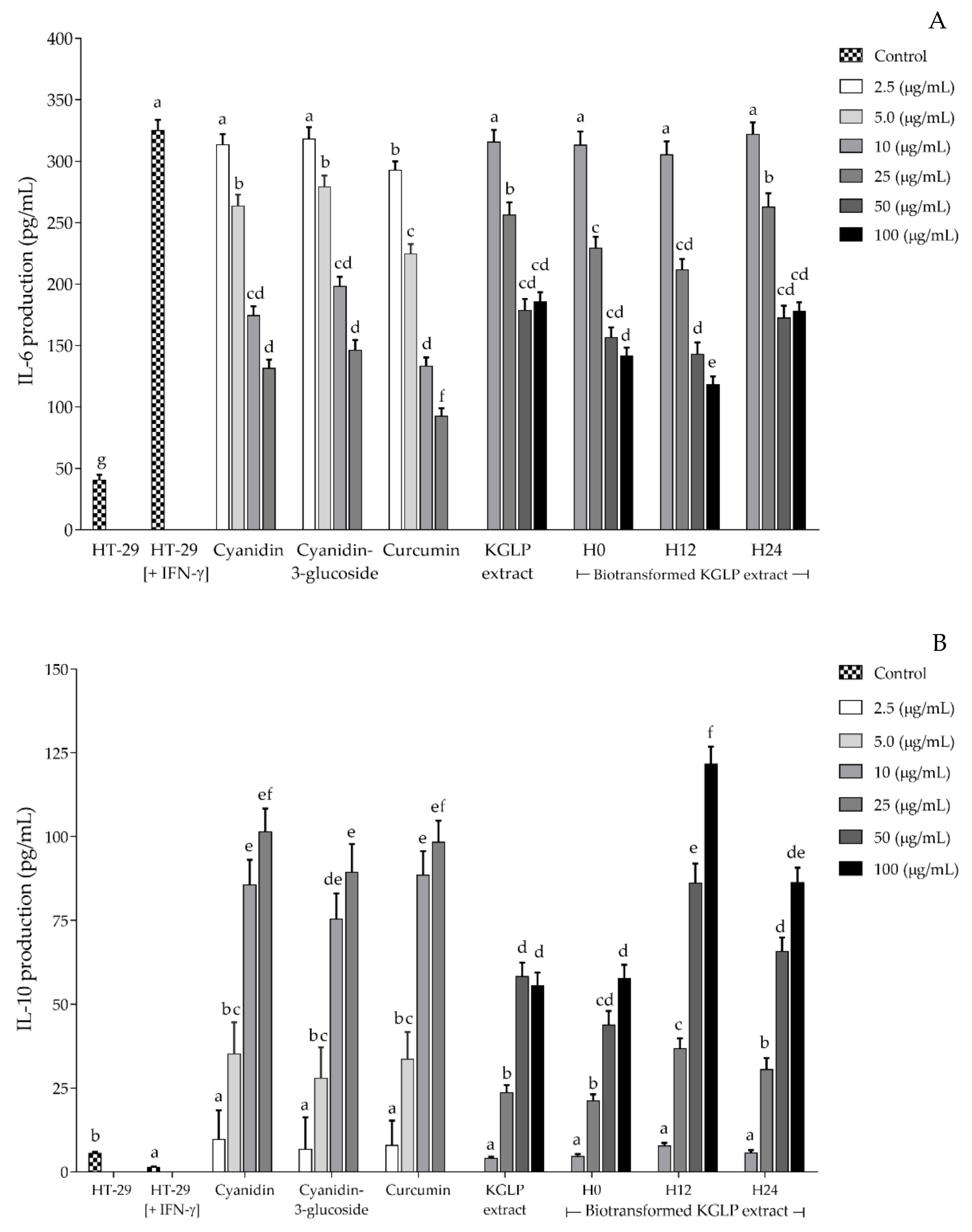
3.4. Effect of Biotransformed Anthocyanin on Colorectal Chemoprevention and Immunization Potential in HT-29 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kelloff, G.J.; Boone, C.W.; Steele, V.E.; Fay, J.R.; Lubet, R.A.; Crowell, J.A.; Sigman, C.C. Mechanistic considerations in chemopreventive drug development. J. Cell. Biochem. Suppl. 1994, 20, 1–24. [Google Scholar] [CrossRef]
- Hoeijmakers, J.H.J. DNA damage, aging, and cancer. N. Engl. J. Med. 2009, 361, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y. Molecular mechanisms of chemopreventive effects of selected dietary and medicinal phenolic substances. Mutat. Res. 1999, 428, 305–327. [Google Scholar] [CrossRef]
- Ding, M.; Feng, R.; Wang, S.Y.; Bowman, L.; Lu, Y.; Qian, Y.; Castranova, V.; Jiang, B.H.; Shi, X. Cyanidin-3-glucoside, a natural product derived from blackberry, exhibits chemopreventive and chemotherapeutic activity. J. Biol. Chem. 2006, 281, 17359–17368. [Google Scholar] [CrossRef] [PubMed]
- Emilsson, L.; Holme, Ø.; Bretthauer, M.; Cook, N.R.; Buring, J.E.; Løberg, M.; Adami, H.O.; Sesso, H.D.; Gaziano, M.J.; Kalager, M. Systematic review with meta-analysis: The comparative effectiveness of aspirin vs. screening for colorectal cancer prevention. Aliment. Pharmacol. Ther. 2017, 45, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Cooper, K.; Squires, H.; Carroll, C.; Papaioannou, D.; Booth, A.; Logan, R.F.; Maguire, C.; Hind, D.; Tappenden, P. Chemoprevention of colorectal cancer: Systematic review and economic evaluation. Health Technol. Assess. 2010, 14, 1–206. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Ávila, M.; Hidalgo, M.; Sánchez-Moreno, C.; Pelaez, C.; Requena, T.; Pascual-Teresa, S.d. Bioconversion of anthocyanin glycosides by Bifidobacteria and Lactobacillus. Food Res. Int. 2009, 42, 1453–1461. [Google Scholar] [CrossRef]
- Eker, M.E.; Aaby, K.; Budic-Leto, I.; Rimac Brnčić, S.; El, S.N.; Karakaya, S.; Simsek, S.; Manach, C.; Wiczkowski, W.; de Pascual-Teresa, S. A review of factors affecting anthocyanin bioavailability: Possible implications for the inter-individual variability. Foods 2020, 9, 2. [Google Scholar] [CrossRef]
- Matsumoto, H.; Inaba, H.; Kishi, M.; Tominaga, S.; Hirayama, M.; Tsuda, T. Orally administered delphinidin 3-rutinoside and cyanidin 3-rutinoside are directly absorbed in rats and humans and appear in the blood as the intact forms. J. Agric. Food Chem. 2001, 49, 1546–1551. [Google Scholar] [CrossRef]
- McGhie, T.K.; Ainge, G.D.; Barnett, L.E.; Cooney, J.M.; Jensen, D.J. Anthocyanin glycosides from berry fruit are absorbed and excreted unmetabolized by both humans and rats. J. Agric. Food Chem. 2003, 51, 4539–4548. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, I.L.; Dragsted, L.O.; Ravn-Haren, G.; Freese, R.; Rasmussen, S.E. Absorption and excretion of black currant anthocyanins in humans and watanabe heritable hyperlipidemic rabbits. J. Agric. Food Chem. 2003, 51, 2813–2820. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Fang, J. Bioavailability of anthocyanins. Drug Metab. Rev. 2014, 46, 508–520. [Google Scholar] [CrossRef]
- Jeon, A.; Suk, K.; Ji, G.E.; Hwang, I.K. Assay of β-glucosidase activity of Bifidobacteria and the hydrolysis of isoflavone glycosides by Bifidobacterium sp. Int-57 in soymilk fermentation. J. Microbiol. Biotechnol. 2002, 12, 8–13. [Google Scholar]
- Matsuda, S.; Norimoto, F.; Matsumoto, Y.; Ohba, R.; Teramoto, Y.; Ohta, N.; Ueda, S. Solubilization of a novel isoflavone glycoside-hydrolyzing β-glucosidase from Lactobacillus casei subsp. rhamnosus. J. Ferment. Bioeng. 1994, 77, 439–441. [Google Scholar] [CrossRef]
- Otieno, D.O.; Ashton, J.F.; Shah, N.E. Stability of β-glucosidase activity produced by Bifidobacterium and Lactobacillus spp. in fermented soymilk during processing and storage. J. Food Sci. 2005, 70, M236–M241. [Google Scholar] [CrossRef]
- Pyo, Y.-H.; Lee, T.-C.; Lee, Y.-C. Enrichment of bioactive isoflavones in soymilk fermented with β-glucosidase-producing lactic acid bacteria. Food Res. Int. 2005, 38, 551–559. [Google Scholar] [CrossRef]
- Yin, L.-j.; Li, L.-t.; Liu, H.; Saito, M.; Tatsumi, E. Effects of fermentation temperature on the content and composition of isoflavones and β-glucosidase activity in Sufu. Biosci. Biotechnol. Biochem. 2005, 69, 267–272. [Google Scholar] [CrossRef][Green Version]
- Leal-Sánchez, M.V.; Ruiz-Barba, J.L.; Sánchez, A.H.; Rejano, L.; Jiménez-Díaz, R.; Garrido, A. Fermentation profile and optimization of green olive fermentation using Lactobacillus plantarum LPCO10 as a starter culture. Food Microbiol. 2003, 20, 421–430. [Google Scholar] [CrossRef]
- Hu, S.; Hong, K.; Song, Y.-C.; Liu, J.-Y.; Tan, R. Biotransformation of soybean isoflavones by a marine Streptomyces sp 060524 and cytotoxicity of the products. World J. Microbiol. Biotechnol. 2009, 25, 115–121. [Google Scholar] [CrossRef]
- Chotimarkorn, C.; Benjakul, S.; Silalai, N. Antioxidant components and properties of five long-grained rice bran extracts from commercial available cultivars in Thailand. Food Chem. 2008, 111, 636–641. [Google Scholar] [CrossRef]
- Butsat, S.; Siriamornpun, S. Antioxidant capacities and phenolic compounds of the husk, bran and endosperm of Thai rice. Food Chem. 2010, 119, 606–613. [Google Scholar] [CrossRef]
- Pattananandecha, T.; Sirithunyalug, J.; Sirithunyalug, B.; Thiankhanithikun, K.; Khanongnuch, C.; Saenjum, C. Bioactive compounds constituent and anti-inflammatory activity of natural rice bran oil produced from colored and non- pigmented rice in northern Thailand. J. Pharm. Nutr. Sci. 2019, 9, 205–212. [Google Scholar]
- Pengkumsri, N.; Chaiyasut, C.; Saenjum, C.; Sirilun, S.; Peerajan, S.; Suwannalert, P.; Sirisattha, S.; Sivamaruthi, B.S. Physicochemical and antioxidative properties of black, brown and red rice varieties of northern Thailand. Food Sci. Technol. 2015, 35, 331–338. [Google Scholar] [CrossRef]
- Otieno, D.O.; Ashton, J.F.; Shah, N.P. Evaluation of enzymic potential for biotransformation of isoflavone phytoestrogen in soymilk by Bifidobacterium animalis, Lactobacillus acidophilus and Lactobacillus casei. Food Res. Int. 2006, 39, 394–407. [Google Scholar] [CrossRef]
- Michlmayr, H.; Kneifel, W. β-Glucosidase activities of lactic acid bacteria: Mechanisms, impact on fermented food and human health. FEMS Microbiol. Lett. 2014, 352, 1–10. [Google Scholar] [CrossRef]
- Sirilun, S.; Chaiyasut, C.; Kantachote, D.; Luxananil, P. Functional properties of β-glucosidase-producing Lactobacillus plantarum SC 359 isolated from Thai fermented soybean food. Acta Aliment. 2012, 41, 451–464. [Google Scholar] [CrossRef]
- Massi, M.; Vitali, B.; Federici, F.; Matteuzzi, D.; Brigidi, P. Identification method based on PCR combined with automated ribotyping for tracking probiotic Lactobacillus strains colonizing the human gut and vagina. J. Appl. Microbiol. 2004, 96, 777–786. [Google Scholar] [CrossRef]
- Phromnoi, K.; Suttajit, M.; Saenjum, C. Polyphenols and rosmarinic acid contents, antioxidant and anti-inflammatory activities of different solvent fractions from Nga-Mon (Perilla frutescens) leaf. J. Pharm. Nutr. Sci. 2019, 9, 239–246. [Google Scholar] [CrossRef]
- Saenjum, C.; Chaiyasut, C.; Kadchumsang, S.; Chansakaow, S.; Suttajit, M. Antioxidant activity and protective effects on DNA damage of Caesalpinia sappan L. extract. J. Med. Plants Res. 2010, 4, 1594–1600. [Google Scholar]
- Pengkumsri, N.; Chaiyasut, C.; Sivamaruthi, B.S.; Saenjum, C.; Sirilun, S.; Peerajan, S.; Suwannalert, P.; Sirisattha, S.; Chaiyasut, K.; Kesika, P. The influence of extraction methods on composition and antioxidant properties of rice bran oil. Food Sci. Technol. 2015, 35, 493–501. [Google Scholar] [CrossRef]
- Hong, C.H.; Hur, S.K.; Oh, O.J.; Kim, S.S.; Nam, K.A.; Lee, S.K. Evaluation of natural products on inhibition of inducible cyclooxygenase (COX-2) and nitric oxide synthase (iNOS) in cultured mouse macrophage cells. J. Ethnopharmacol. 2002, 83, 153–159. [Google Scholar] [CrossRef]
- Sirithunyalug, B.; Saenjum, C.; Charumanee, S.; Sivamaruthi, B.S.; Chaiyasut, C.; Sirithunyalug, J.; Tipduangta, P. Development of colorectal-targeted dietary supplement tablets containing natural purple rice bran oil as a colorectal chemopreventive. Nutrients 2018, 10, 444. [Google Scholar] [CrossRef] [PubMed]
- Phromnoi, K.; Suttajit, M.; Saenjum, C.; Limtrakul Dejkriengkraikul, P. Inhibitory effect of a rosmarinic acid-enriched fraction prepared from Nga-Mon (Perilla frutescens) seed meal on osteoclastogenesis through the RANK signaling pathway. Antioxidants 2021, 10, 307. [Google Scholar] [CrossRef]
- Rao, M.N.A. Nitric oxide scavenging by curcuminoids. J. Pharm. Pharmacol. 1997, 49, 105–107. [Google Scholar]
- Pitija, K.; Nakornriab, M.; Sriseadka, T.; Vanavichit, A.; Wongpornchai, S. Anthocyanin content and antioxidant capacity in bran extracts of some Thai black rice varieties. Int. J. Food Sci. Technol. 2013, 48, 300–308. [Google Scholar] [CrossRef]
- Tan, J.; Li, Y.; Hou, D.-X.; Wu, S. The effects and mechanisms of Cyanidin-3-glucoside and its phenolic metabolites in maintaining intestinal integrity. Antioxidants 2019, 8, 479. [Google Scholar] [CrossRef]
- Wang, D.; Xia, M.; Yan, X.; Li, D.; Wang, L.; Xu, Y.; Jin, T.; Ling, W. Gut microbiota metabolism of anthocyanin promotes reverse cholesterol transport in mice via repressing miRNA-10b. Circ. Res. 2012, 111, 967–981. [Google Scholar] [CrossRef]
- Braga, A.R.C.; Mesquita, L.M.d.S.; Martins, P.L.G.; Habu, S.; de Rosso, V.V. Lactobacillus fermentation of jussara pulp leads to the enzymatic conversion of anthocyanins increasing antioxidant activity. J. Food Compost. Anal. 2018, 69, 162–170. [Google Scholar] [CrossRef]
- Cheng, J.-R.; Liu, X.-M.; Chen, Z.-Y.; Zhang, Y.-S.; Zhang, Y.-H. Mulberry anthocyanin biotransformation by intestinal probiotics. Food Chem. 2016, 213, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Devi, A.; Konerira Aiyappaa, A.A.; Waterhouse, A.L. Adsorption and biotransformation of anthocyanin glucosides and quercetin glycosides by Oenococcus oeni and Lactobacillus plantarum in model wine solution. J. Sci. Food Agric. 2020, 100, 2110–2120. [Google Scholar] [CrossRef] [PubMed]
- Keppler, K.; Humpf, H.U. Metabolism of anthocyanins and their phenolic degradation products by the intestinal microflora. Bioorg. Med. Chem. 2005, 13, 5195–5205. [Google Scholar] [CrossRef] [PubMed]
- Leong, L.P.; Shui, G. An investigation of antioxidant capacity of fruits in Singapore markets. Food Chem. 2002, 76, 69–75. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharm. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Ahmad, N.; Ammar, A.; Storr, S.J.; Green, A.R.; Rakha, E.; Ellis, I.O.; Martin, S.G. IL-6 and IL-10 are associated with good prognosis in early stage invasive breast cancer patients. Cancer Immunol. Immunother. 2018, 67, 537–549. [Google Scholar] [CrossRef]
- Qu, Z.; Sun, F.; Zhou, J.; Li, L.; Shapiro, S.D.; Xiao, G. Interleukin-6 Prevents the Initiation but Enhances the Progression of Lung Cancer. Cancer Res. 2015, 75, 3209–3215. [Google Scholar] [CrossRef]
- Dennis, K.L.; Blatner, N.R.; Gounari, F.; Khazaie, K. Current status of interleukin-10 and regulatory T-cells in cancer. Curr. Opin. Oncol. 2013, 25, 637–645. [Google Scholar] [CrossRef]
- Sakamoto, T.; Saito, H.; Tatebe, S.; Tsujitani, S.; Ozaki, M.; Ito, H.; Ikeguchi, M. Interleukin-10 expression significantly correlates with minor CD8+ T-cell infiltration and high microvessel density in patients with gastric cancer. Int. J. Cancer 2006, 118, 1909–1914. [Google Scholar] [CrossRef]
- Olivas-Aguirre, F.J.; Rodrigo-García, J.; Martínez-Ruiz, N.D.; Cárdenas-Robles, A.I.; Mendoza-Díaz, S.O.; Álvarez-Parrilla, E.; González-Aguilar, G.A.; de la Rosa, L.A.; Ramos-Jiménez, A.; Wall-Medrano, A. Cyanidin-3-O-glucoside: Physical-chemistry, foodomics and health effects. Molecules 2016, 21, 1264. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, T.; Li, P.; Ai, J.; Liu, J.; Bai, W.; Tian, L. Dietary fiber modulates the fermentation patterns of Cyanidin-3-O-glucoside in a fiber-type dependent manner. Foods 2021, 10, 1386. [Google Scholar] [CrossRef] [PubMed]
- Serra, D.; Paixão, J.; Nunes, C.; Dinis, T.C.P.; Almeida, L.M. Cyanidin-3-glucoside suppresses cytokine-induced inflammatory response in human intestinal cells: Comparison with 5-aminosalicylic acid. PLoS ONE 2013, 8, e73001. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.R.; Pereira, R.; Figueiredo, I.; Freitas, V.; Dinis, T.C.P.; Almeida, L.M. Comparison of anti-inflammatory activities of an anthocyanin-rich fraction from Portuguese blueberries (Vaccinium corymbosum L.) and 5-aminosalicylic acid in a TNBS-induced colitis rat model. PLoS ONE 2017, 12, e0174116. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, S.; Gascón, S.; Luquin, A.; Laguna, M.; Ancin-Azpilicueta, C.; Rodríguez-Yoldi, M.J. Rosa canina extracts have antiproliferative and antioxidant effects on Caco-2 human colon cancer. PLoS ONE 2016, 11, e0159136. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Fu, Y.; Yang, L.; Chen, J.; Lei, H.; Liu, Q. Cyanidin-3-O-glucoside and cyanidin protect against intestinal barrier damage and 2,4,6-trinitrobenzenesulfonic acid-induced colitis. J. Med. Food 2019, 23, 90–99. [Google Scholar] [CrossRef]
- Roth, S.; Spalinger, M.R.; Gottier, C.; Biedermann, L.; Zeitz, J.; Lang, S.; Weber, A.; Rogler, G.; Scharl, M. Bilberry-derived anthocyanins modulate cytokine expression in the intestine of patients with ulcerative colitis. PLoS ONE 2016, 11, e0154817. [Google Scholar] [CrossRef]
| Purple Rice Cultivars | Amount of Anthocyanin (mg/100 g Dried Weight) | |||
|---|---|---|---|---|
| Cyanidin-3-glucoside | Peonidine-3-glucoside | Cyanidin | Peonidin | |
| Khao’ Gam Chiang Rai (KGCR) | 37.29 ± 2.43 d | 16.76 ± 2.07 d | ND | ND |
| Khao’ Gam Pha Yao (KGPY) | 35.62 ± 2.28 d | 23.49 ± 1.85 c | ND | ND |
| Khao’ Gam Leum-Phua (KGLP) | 192.19 ± 3.10 a | 84.54 ± 2.78 a | 7.67 ± 0.62 a | ND |
| Khao’ Gam Thor (KGT) | 67.63 ± 2.65 c | 26.35 ± 2.15 c | 3.54 ± 0.48 c | ND |
| Khao’ Gam Boung (KGB) | 108.49 ± 2.78 b | 57.83 ± 2.19 b | 5.31 ± 0.45 b | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirilun, S.; Chaiyasut, C.; Pattananandecha, T.; Apichai, S.; Sirithunyalug, J.; Sirithunyalug, B.; Saenjum, C. Enhancement of the Colorectal Chemopreventive and Immunization Potential of Northern Thai Purple Rice Anthocyanin Using the Biotransformation by β-Glucosidase-Producing Lactobacillus. Antioxidants 2022, 11, 305. https://doi.org/10.3390/antiox11020305
Sirilun S, Chaiyasut C, Pattananandecha T, Apichai S, Sirithunyalug J, Sirithunyalug B, Saenjum C. Enhancement of the Colorectal Chemopreventive and Immunization Potential of Northern Thai Purple Rice Anthocyanin Using the Biotransformation by β-Glucosidase-Producing Lactobacillus. Antioxidants. 2022; 11(2):305. https://doi.org/10.3390/antiox11020305
Chicago/Turabian StyleSirilun, Sasithorn, Chaiyavat Chaiyasut, Thanawat Pattananandecha, Sutasinee Apichai, Jakkapan Sirithunyalug, Busaban Sirithunyalug, and Chalermpong Saenjum. 2022. "Enhancement of the Colorectal Chemopreventive and Immunization Potential of Northern Thai Purple Rice Anthocyanin Using the Biotransformation by β-Glucosidase-Producing Lactobacillus" Antioxidants 11, no. 2: 305. https://doi.org/10.3390/antiox11020305
APA StyleSirilun, S., Chaiyasut, C., Pattananandecha, T., Apichai, S., Sirithunyalug, J., Sirithunyalug, B., & Saenjum, C. (2022). Enhancement of the Colorectal Chemopreventive and Immunization Potential of Northern Thai Purple Rice Anthocyanin Using the Biotransformation by β-Glucosidase-Producing Lactobacillus. Antioxidants, 11(2), 305. https://doi.org/10.3390/antiox11020305