A Potential Probiotic Lactobacillus plantarum JBC5 Improves Longevity and Healthy Aging by Modulating Antioxidative, Innate Immunity and Serotonin-Signaling Pathways in Caenorhabditis elegans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bacterial Strains and Growth Conditions
2.3. Identification for the Presence of Probiotic Marker Genes in LPJBC5
2.4. Phylogenetic Tree
2.5. Survival to the Gastrointestinal (GIT) Transit
2.6. Assay for Bile Acid Tolerance
2.7. Adhesion to Intestinal Cells
2.8. Longevity Assay
2.9. Determination of Pharynx Pumping and Locomotor Activity
2.10. Developmental Rate Assay
2.11. Measurement of Body Size
2.12. Colonization Efficiency
2.13. Aging Pigment Accumulation
2.14. Brood Size
2.15. Determination of Fat Accumulation
2.16. Food Preference and Learning Memory
2.17. Thermotolerance and Oxidative Stress-Resistance Assay
2.18. Determination of Resistance against Pathogenic Bacterial Infections
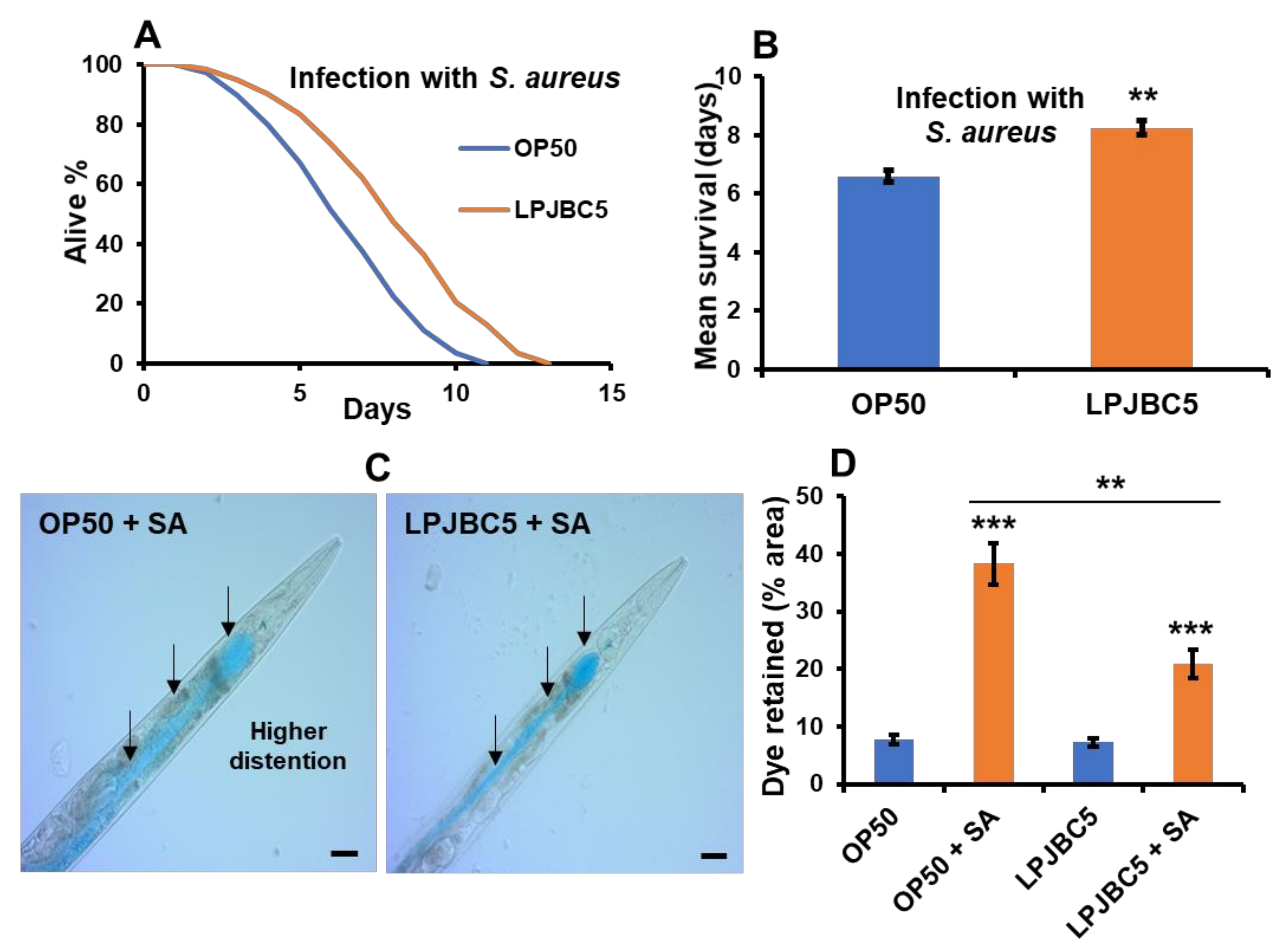
2.19. Measurement of Intestinal Integrity against Pathogenic Infection (Smurf Assay)
2.20. RNA Isolation, cDNA Synthesis, and Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
2.21. GSH/GSSG Assay
2.22. SOD Activity Assay
2.23. Measurement of Intracellular ROS Generation
2.24. Determination of Reactive Oxygen Production and Change in Transmembrane Potential of Mitochondria
2.25. Measurement of Intracellular Adenosine Triphosphate (ATP) Concertation
2.26. Quantification of Apoptosis by Tunnel-Assay
2.27. Statistical Analysis
3. Results
3.1. Molecular Taxonomic Characterisation of LPJBC5 and Persistence in In Vitro Gastrointestinal Conditions
3.2. LPJBC5 Increases Longevity and Slows the Development of Worms
3.3. LPJBC5 Is Efficiently Colonized into the Gut of Worms
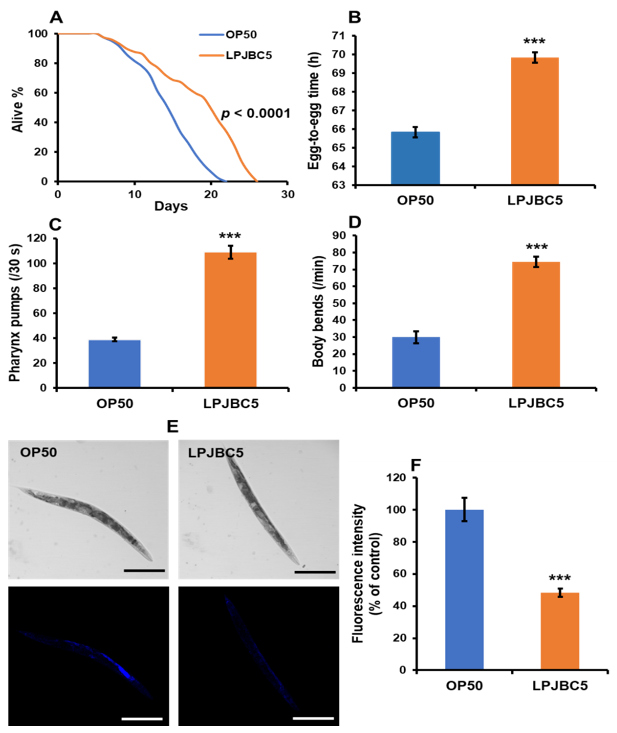
3.4. Feeding of LPJBC5 Delayed Aging in Worms
3.5. Feeding of LPJBC5 Reduced the Accumulation of Fat in Worms
3.6. LPJBC5 Improved Learning and Memory in Worms
3.7. Feeding of LPJBC5 Conferred Resistance against Abiotic and Biotic Stress Conditions
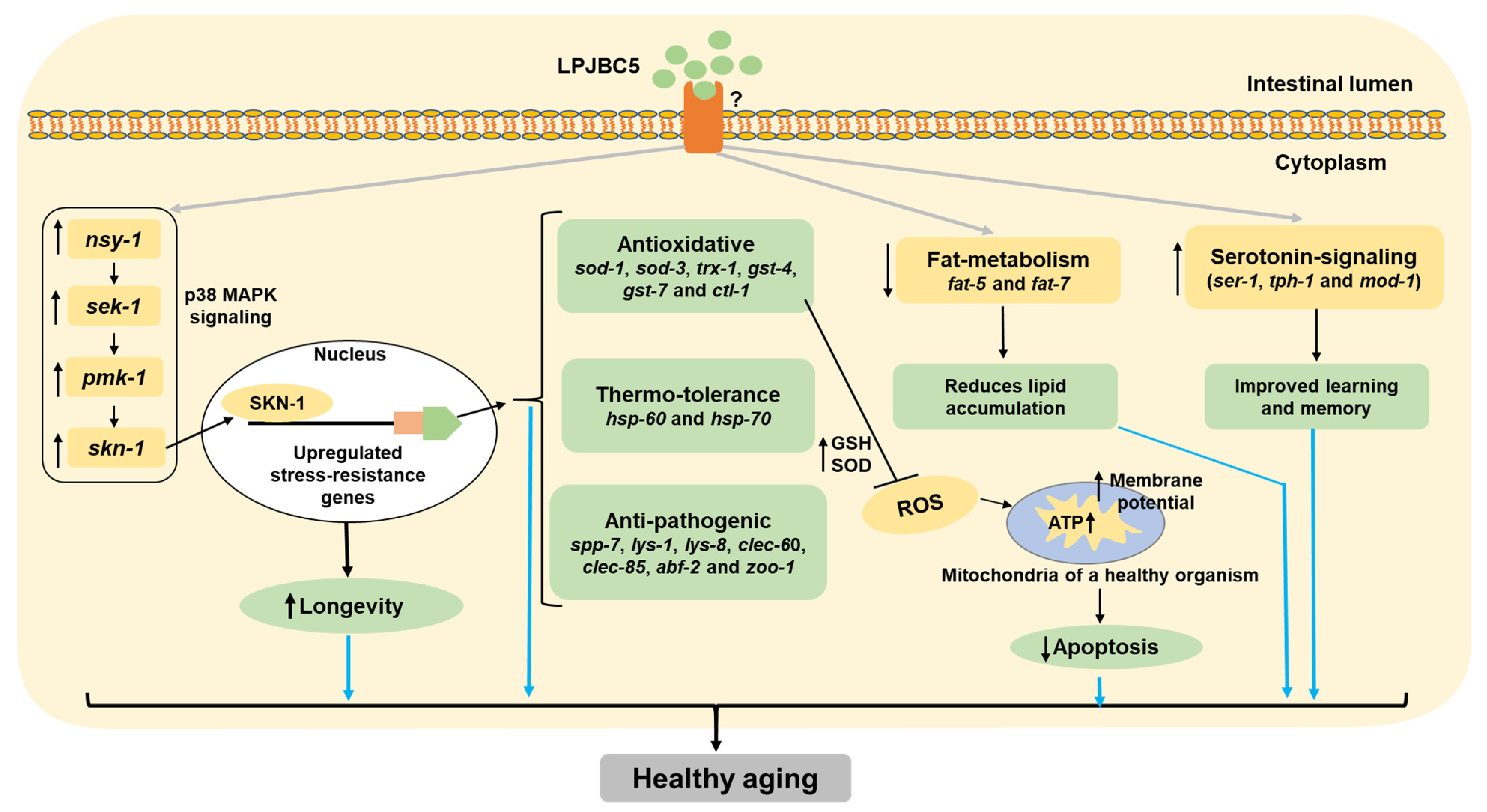
3.8. Understanding the Pathway Involved in Pro-Longevity Effect of LPJBC5-Fed Worms
3.9. Elucidating the Molecular Mechanisms of LPJBC5-Induced Healthy Aging in Worms
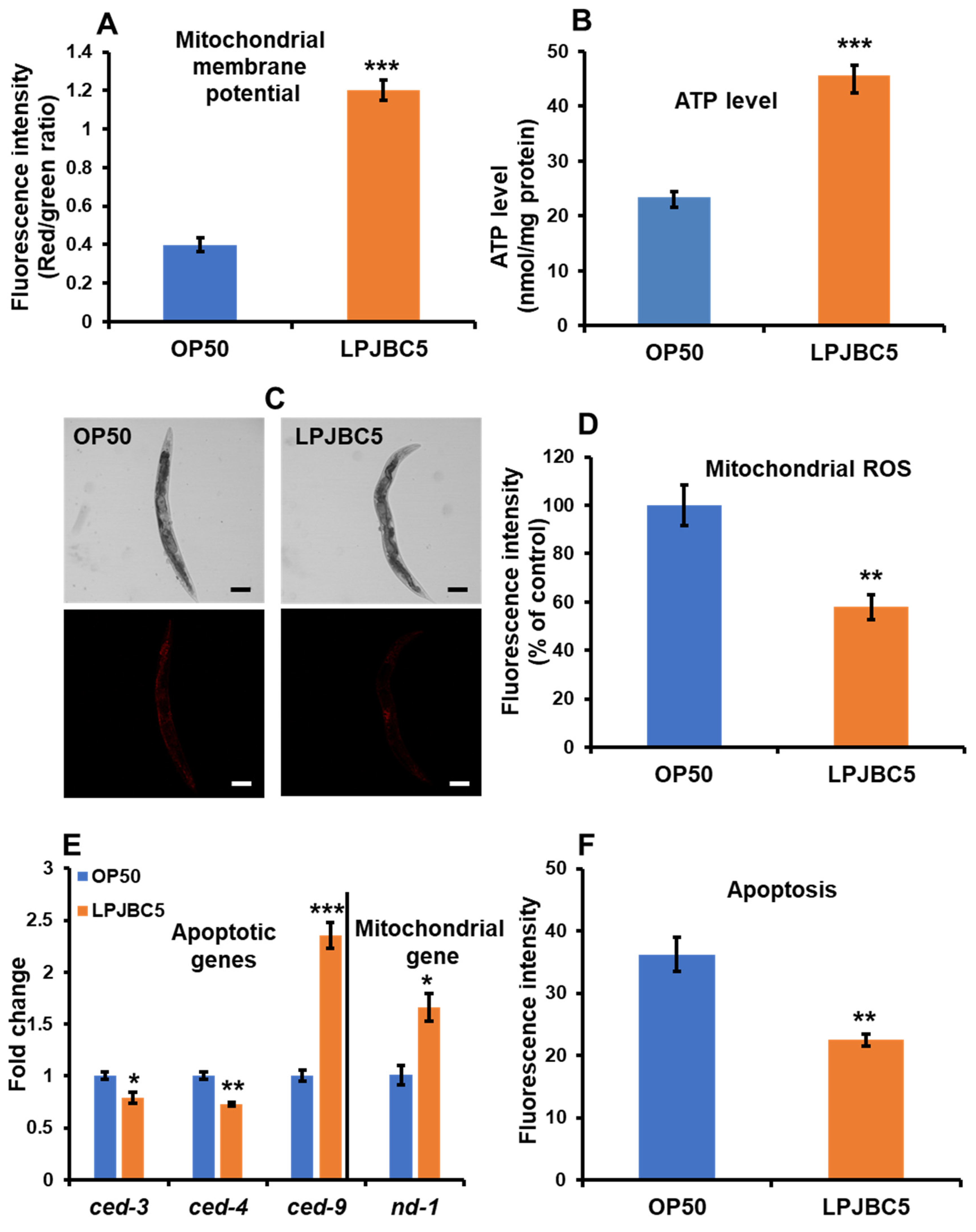
3.10. Feeding of LPJBC5 Reduced the Production of Reactive Oxygen Species
3.11. LPJBC5 Improved Mitochondrial Function in Worms
3.12. LPJBC5 Retards Programmed Cell Death in Worms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bixby, R.L. Impacts of aging on the federal budget and economy: A cross-cutting challenge. Public Policy Aging Rep. 2020, 30, 46–51. [Google Scholar] [CrossRef]
- Michel, J.-P.; Sadana, R. “Healthy aging” concepts and measures. J. Am. Med. Dir. Assoc. 2017, 18, 460–464. [Google Scholar] [CrossRef] [PubMed]
- United Nations. World Population Ageing 2019: Highlights (ST/ESA/SER. A/430); Department of Economic and Social Affairs, Population Division: New York, NY, USA, 2019. [Google Scholar]
- Jin, K.; Simpkins, J.W.; Ji, X.; Leis, M.; Stambler, I. The critical need to promote research of aging and aging-related diseases to improve health and longevity of the elderly population. Aging Dis. 2015, 6, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontana, L.; Partridge, L. Promoting health and longevity through diet: From model organisms to humans. Cell 2015, 161, 106–118. [Google Scholar] [CrossRef] [Green Version]
- Brooks-Wilson, A.R. Genetics of healthy aging and longevity. Hum. Genet. 2013, 132, 1323–1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ros, M.; Carrascosa, J.M. Current nutritional and pharmacological anti-aging interventions. Biochim. Biophys. Acta-Mol. Basis Dis. 2020, 1866, 165612. [Google Scholar] [CrossRef]
- Han, B.; Sivaramakrishnan, P.; Lin, C.-C.J.; Neve, I.A.; He, J.; Tay, L.W.R.; Sowa, J.N.; Sizovs, A.; Du, G.; Wang, J. Microbial genetic composition tunes host longevity. Cell 2017, 169, 1249–1262.e1213. [Google Scholar] [CrossRef] [Green Version]
- Van Der Lugt, B.; Van Beek, A.A.; Aalvink, S.; Meijer, B.; Sovran, B.; Vermeij, W.P.; Brandt, R.M.; De Vos, W.M.; Savelkoul, H.F.; Steegenga, W.T. Akkermansia muciniphila ameliorates the age-related decline in colonic mucus thickness and attenuates immune activation in accelerated aging Ercc1−/Δ7 mice. Immun. Ageing 2019, 16, 6. [Google Scholar] [CrossRef]
- Xiao, R.; Chun, L.; Ronan, E.A.; Friedman, D.I.; Liu, J.; Xu, X.S. RNAi interrogation of dietary modulation of development, metabolism, behavior, and aging in C. elegans. Cell Rep. 2015, 11, 1123–1133. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Baruah, A.; Tomioka, M.; Iino, Y.; Kalita, M.C.; Khan, M. Caenorhabditis elegans: A model to understand host–microbe interactions. Cell. Mol. Life Sci. 2020, 77, 1229–1249. [Google Scholar] [CrossRef]
- Nakagawa, H.; Shiozaki, T.; Kobatake, E.; Hosoya, T.; Moriya, T.; Sakai, F.; Taru, H.; Miyazaki, T. Effects and mechanisms of prolongevity induced by Lactobacillus gasseri SBT2055 in Caenorhabditis elegans. Aging Cell 2016, 15, 227–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Yun, H.S.; Cho, K.W.; Oh, S.; Kim, S.H.; Chun, T.; Kim, B.; Whang, K.Y. Evaluation of probiotic characteristics of newly isolated Lactobacillus spp.: Immune modulation and longevity. Int. J. Food Microbiol. 2011, 148, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Yasui, C.; Hoshino, K.; Arikawa, K.; Nishikawa, Y. Influence of lactic acid bacteria on longevity of Caenorhabditis elegans and host defense against Salmonella enterica serovar enteritidis. Appl. Environ. Microbiol. 2007, 73, 6404–6409. [Google Scholar] [CrossRef] [Green Version]
- Fontana, L.; Partridge, L.; Longo, V.D. Extending healthy life span—From yeast to humans. Science 2010, 328, 321–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez, B.; Delgado, S.; Blanco-Míguez, A.; Lourenço, A.; Gueimonde, M.; Margolles, A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 2017, 61, 1600240. [Google Scholar] [CrossRef] [Green Version]
- Metchnikoff, E. The Prolongation of Life: Optimistic Studies; Mitchell, P.C., Ed.; GP Putnam’s Sons: New York, NY, USA, 1907. [Google Scholar]
- Jomehzadeh, N.; Javaherizadeh, H.; Amin, M.; Saki, M.; Al-Ouqaili, M.T.; Hamidi, H.; Seyedmahmoudi, M.; Gorjian, Z. Isolation and identification of potential probiotic Lactobacillus species from feces of infants in southwest Iran. Int. J. Infect. Dis. 2020, 96, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.J.; Singh, S.K.; Panaich, S.; Cardozo, L. The aging gut and the role of prebiotics, probiotics, and synbiotics: A review. J. Clin. Gerontol. Geriatr. 2014, 5, 3–6. [Google Scholar] [CrossRef] [Green Version]
- Lew, L.; Hor, Y.; Jaafar, M.; Lau, A.; Ong, J.; Chuah, L.; Yap, K.; Azzam, G.; Azlan, A.; Liong, M. Lactobacilli modulated AMPK activity and prevented telomere shortening in ageing rats. Benef. Microbes 2019, 10, 883–892. [Google Scholar] [CrossRef]
- Nam, B.; Kim, S.A.; Nam, W.; Jeung, W.H.; Park, S.-D.; Lee, J.-L.; Sim, J.-H.; Jang, S.S. Lactobacillus plantarum HY7714 restores TNF-α induced defects on tight junctions. Prev. Nutr. Food Sci. 2019, 24, 64. [Google Scholar] [CrossRef]
- Poupet, C.; Saraoui, T.; Veisseire, P.; Bonnet, M.; Dausset, C.; Gachinat, M.; Camarès, O.; Chassard, C.; Nivoliez, A.; Bornes, S. Lactobacillus rhamnosus Lcr35 as an effective treatment for preventing Candida albicans infection in the invertebrate model Caenorhabditis elegans: First mechanistic insights. PLoS ONE 2019, 14, e0216184. [Google Scholar] [CrossRef]
- Dinić, M.; Herholz, M.; Kačarević, U.; Radojević, D.; Novović, K.; Đokić, J.; Trifunović, A.; Golić, N. Host–commensal interaction promotes health and lifespan in Caenorhabditis elegans through the activation of HLH-30/TFEB-mediated autophagy. Aging 2021, 13, 8040. [Google Scholar] [CrossRef]
- Yu, X.; Li, S.; Yang, D.; Qiu, L.; Wu, Y.; Wang, D.; Shah, N.P.; Xu, F.; Wei, H. A novel strain of Lactobacillus mucosae isolated from a Gaotian villager improves in vitro and in vivo antioxidant as well as biological properties in D-galactose-induced aging mice. J. Dairy Sci. 2016, 99, 903–914. [Google Scholar] [CrossRef] [Green Version]
- Park, M.R.; Shin, M.; Mun, D.; Jeong, S.-Y.; Jeong, D.-Y.; Song, M.; Ko, G.; Unno, T.; Kim, Y.; Oh, S. Probiotic Lactobacillus fermentum strain JDFM216 improves cognitive behavior and modulates immune response with gut microbiota. Sci. Rep. 2020, 10, 21701. [Google Scholar] [CrossRef] [PubMed]
- Schifano, E.; Zinno, P.; Guantario, B.; Roselli, M.; Marcoccia, S.; Devirgiliis, C.; Uccelletti, D. The foodborne strain Lactobacillus fermentum MBC2 triggers pept-1-dependent pro-longevity effects in Caenorhabditis elegans. Microorganisms 2019, 7, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmadi, S.; Wang, S.; Nagpal, R.; Wang, B.; Jain, S.; Razazan, A.; Mishra, S.P.; Zhu, X.; Wang, Z.; Kavanagh, K. A human-origin probiotic cocktail ameliorates aging-related leaky gut and inflammation via modulating the microbiota/taurine/tight junction axis. JCI Insight 2020, 5, e132055. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Park, E.-J.; Kim, S.-H.; Lee, H.-J. Lactobacillus plantarum ATG-K2 and ATG-K6 Ameliorates High-Fat with High-Fructose Induced Intestinal Inflammation. Int. J. Mol. Sci. 2021, 22, 4444. [Google Scholar] [CrossRef]
- Zaydi, A.; Lew, L.-C.; Hor, Y.-Y.; Jaafar, M.; Chuah, L.-O.; Yap, K.-P.; Azlan, A.; Azzam, G.; Liong, M.-T. Lactobacillus plantarum DR7 improved brain health in aging rats via the serotonin, inflammatory and apoptosis pathways. Benef. Microbes 2020, 11, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.S.; Taylor, T.D.; Yong, C.C.; Khoo, B.Y.; Sasidharan, S.; Choi, S.B.; Ohno, H.; Liong, M.T. Lactobacillus plantarum USM8613 aids in wound healing and suppresses Staphylococcus aureus infection at wound sites. Probiotics Antimicrob. Proteins 2020, 12, 125–137. [Google Scholar] [CrossRef]
- Zhao, J.; Tian, F.; Yan, S.; Zhai, Q.; Zhang, H.; Chen, W. Lactobacillus plantarum CCFM10 alleviating oxidative stress and restoring the gut microbiota in d-galactose-induced aging mice. Food Funct. 2018, 9, 917–924. [Google Scholar] [CrossRef]
- Peng, X.; Meng, J.; Chi, T.; Liu, P.; Man, C.; Liu, S.; Guo, Y.; Jiang, Y. Lactobacillus plantarum NDC 75017 alleviates the learning and memory ability in aging rats by reducing mitochondrial dysfunction. Exp. Ther. Med. 2014, 8, 1841–1846. [Google Scholar] [CrossRef] [Green Version]
- Joishy, T.K.; Dehingia, M.; Khan, M.R. Bacterial diversity and metabolite profiles of curd prepared by natural fermentation of raw milk and back sloping of boiled milk. World J. Microbiol. Biotechnol. 2019, 35, 102. [Google Scholar] [CrossRef]
- Kumar, R.; Grover, S.; Batish, V.K. Molecular identification and typing of putative probiotic indigenous Lactobacillus plantarum strain Lp91 of human origin by specific primed-PCR assays. Probiotics Antimicrob. Proteins 2011, 3, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Khemariya, P.; Singh, S.; Jaiswal, N.; Chaurasia, S. Isolation and identification of Lactobacillus plantarum from vegetable samples. Food Biotechnol. 2016, 30, 49–62. [Google Scholar] [CrossRef]
- Kim, E.; Yang, S.-M.; Lim, B.; Park, S.H.; Rackerby, B.; Kim, H.-Y. Design of PCR assays to specifically detect and identify 37 Lactobacillus species in a single 96 well plate. BMC Microbiol. 2020, 20, 96. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Conway, P.; Gorbach, S.; Goldin, B. Survival of lactic acid bacteria in the human stomach and adhesion to intestinal cells. J. Dairy Sci. 1987, 70, 1–12. [Google Scholar] [CrossRef]
- Vinderola, C.G.; Reinheimer, J.A. Lactic acid starter and probiotic bacteria: A comparative “in vitro” study of probiotic characteristics and biological barrier resistance. Food Res. Int. 2003, 36, 895–904. [Google Scholar] [CrossRef]
- Ayeni, F.A.; Sánchez, B.; Adeniyi, B.A.; Clara, G.; Margolles, A.; Ruas-Madiedo, P. Evaluation of the functional potential of Weissella and Lactobacillus isolates obtained from Nigerian traditional fermented foods and cow’s intestine. Int. J. Food Microbiol. 2011, 147, 97–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, G.; Lee, J.; Lim, Y.-H. Dairy Propionibacterium extends the mean lifespan of Caenorhabditis elegans via activation of the innate immune system. Sci. Rep. 2016, 6, 131713. [Google Scholar] [CrossRef] [PubMed]
- Stiernagle, T. Maintenance of C. elegans. C. Elegans 1999, 2, 51–67. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.-S.; Nam, H.-J.; Seo, M.; Han, S.K.; Choi, Y.; Nam, H.G.; Lee, S.-J.; Kim, S. OASIS: Online application for the survival analysis of lifespan assays performed in aging research. PLoS ONE 2011, 6, e23525. [Google Scholar] [CrossRef] [Green Version]
- Soukas, A.A.; Kane, E.A.; Carr, C.E.; Melo, J.A.; Ruvkun, G. Rictor/TORC2 regulates fat metabolism, feeding, growth, and life span in Caenorhabditis elegans. Genes Dev. 2009, 23, 496–511. [Google Scholar] [CrossRef] [Green Version]
- Zanni, E.; Laudenzi, C.; Schifano, E.; Palleschi, C.; Perozzi, G.; Uccelletti, D.; Devirgiliis, C. Impact of a complex food microbiota on energy metabolism in the model organism Caenorhabditis elegans. BioMed Res. Int. 2015, 2015, 621709. [Google Scholar] [CrossRef] [Green Version]
- Chelliah, R.; Choi, J.-G.; Hwang, S.-b.; Park, B.-J.; Daliri, E.B.-M.; Kim, S.-H.; Wei, S.; Ramakrishnan, S.R.; Oh, D.-H. In vitro and in vivo defensive effect of probiotic LAB against Pseudomonas aeruginosa using Caenorhabditis elegans model. Virulence 2018, 9, 1489–1507. [Google Scholar] [CrossRef] [Green Version]
- Bendesky, A.; Tsunozaki, M.; Rockman, M.V.; Kruglyak, L.; Bargmann, C.I. Catecholamine receptor polymorphisms affect decision-making in C. elegans. Nature 2011, 472, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Fu, X.; Luo, Y.; Du, F.; Wang, H.; Xing, S.; Li, W.; Ma, J. 2-SeCD treatment extends lifespan, improves healthspan and enhances resistance to stress in Caenorhabditis elegans. RSC Adv. 2017, 7, 48245–48252. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Cho, J.S.; Lambacher, N.; Lee, J.; Lee, S.-J.; Lee, T.H.; Gartner, A.; Koo, H.-S. The Caenorhabditis elegans AMP-activated protein kinase AAK-2 is phosphorylated by LKB1 and is required for resistance to oxidative stress and for normal motility and foraging behavior. J. Biol. Chem. 2008, 283, 14988–14993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Moon, Y. Worm-based alternate assessment of probiotic intervention against gut barrier infection. Nutrients 2019, 11, 2146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Yoon, D.S.; Lee, M.-H.; Cha, D.S. Measurement of Intracellular ROS in Caenorhabditis elegans Using 2′, 7′-Dichlorodihydrofluorescein Diacetate. Bio-Protocol 2018, 8, e2774. [Google Scholar] [CrossRef] [Green Version]
- Dilberger, B.; Baumanns, S.; Schmitt, F.; Schmiedl, T.; Hardt, M.; Wenzel, U.; Eckert, G.P. Mitochondrial oxidative stress impairs energy metabolism and reduces stress resistance and longevity of C. Elegans. Oxidative Med. Cell. Longev. 2019, 2019, 6840540. [Google Scholar] [CrossRef] [Green Version]
- Ganguly, N.; Bhattacharya, S.; Sesikeran, B.; Nair, G.; Ramakrishna, B.; Sachdev, H.; Batish, V.; Kanagasabapathy, A.; Muthuswamy, V.; Kathuria, S. ICMR-DBT guidelines for evaluation of probiotics in food. Indian J. Med. Res. 2011, 134, 22. [Google Scholar]
- Park, M.R.; Oh, S.; Son, S.J.; Park, D.-J.; Oh, S.; Kim, S.H.; Jeong, D.-Y.; Oh, N.S.; Lee, Y.; Song, M. Bacillus licheniformis isolated from traditional Korean food resources enhances the longevity of Caenorhabditis elegans through serotonin signaling. J. Agric. Food Chem. 2015, 63, 10227–10233. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Ren, Y.; Wang, Y.; Fang, J.; Yue, H.; Ma, S.; Guan, F. Aging and age-related diseases: From mechanisms to therapeutic strategies. Biogerontology 2021, 22, 165–187. [Google Scholar] [CrossRef]
- World Health Organization. World Report on Ageing and Health; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Jena, P.K.; Trivedi, D.; Thakore, K.; Chaudhary, H.; Giri, S.S.; Seshadri, S. Isolation and characterization of probiotic properties of lactobacilli isolated from rat fecal microbiota. Microbiol. Immunol. 2013, 57, 407–416. [Google Scholar] [CrossRef]
- Roselli, M.; Schifano, E.; Guantario, B.; Zinno, P.; Uccelletti, D.; Devirgiliis, C. Caenorhabditis elegans and probiotics interactions from a prolongevity perspective. Int. J. Mol. Sci. 2019, 20, 5020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, M.; Liu, X.; Yu, H.; Gong, J. Lactobacillus Regulates Caenorhabditis elegans Cell Signaling to Combat Salmonella Infection. Front. Immunol. 2021, 12, 569. [Google Scholar] [CrossRef] [PubMed]
- Marchionni, S.; Sell, C.; Lorenzini, A. Development and Longevity: Cellular and Molecular Determinants—A Mini-Review. Gerontology 2020, 66, 223–230. [Google Scholar] [CrossRef]
- Lee, W.-S.; Monaghan, P.; Metcalfe, N.B. Experimental demonstration of the growth rate–lifespan trade-off. Proc. R. Soc. B Biol. Sci. 2013, 280, 20122370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saul, N.; Möller, S.; Cirulli, F.; Berry, A.; Luyten, W.; Fuellen, G. Health and longevity studies in C. elegans: The “healthy worm database” reveals strengths, weaknesses and gaps of test compound-based studies. Biogerontology 2021, 22, 215–236. [Google Scholar] [CrossRef]
- Johnson, A.A.; Stolzing, A. The role of lipid metabolism in aging, lifespan regulation, and age-related disease. Aging Cell 2019, 18, e13048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Gilst, M.R.; Hadjivassiliou, H.; Jolly, A.; Yamamoto, K.R. Nuclear hormone receptor NHR-49 controls fat consumption and fatty acid composition in C. elegans. PLoS Biol. 2005, 3, e53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brock, T.J.; Browse, J.; Watts, J.L. Fatty acid desaturation and the regulation of adiposity in Caenorhabditis elegans. Genetics 2007, 176, 865–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, S.; Schroeder, E.A.; Silva-García, C.G.; Hebestreit, K.; Mair, W.B.; Brunet, A. Mono-unsaturated fatty acids link H3K4me3 modifiers to C. elegans lifespan. Nature 2017, 544, 185–190. [Google Scholar] [CrossRef]
- Tsui, D.; van der Kooy, D. Serotonin mediates a learned increase in attraction to high concentrations of benzaldehyde in aged C. elegans. Learn. Mem. 2008, 15, 844–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouse, J.; Cohen, P.; Trigon, S.; Morange, M.; Alonso-Llamazares, A.; Zamanillo, D.; Hunt, T.; Nebreda, A.R. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell 1994, 78, 1027–1037. [Google Scholar] [CrossRef]
- Freshney, N.W.; Rawlinson, L.; Guesdon, F.; Jones, E.; Cowley, S.; Hsuan, J.; Saklatvala, J. Interleukin-1 activates a novel protein kinase cascade that results in the phosphorylation of Hsp27. Cell 1994, 78, 1039–1049. [Google Scholar] [CrossRef]
- Han, J.; Lee, J.; Bibbs, L.; Ulevitch, R. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 1994, 265, 808–811. [Google Scholar] [CrossRef]
- Kim, D.H.; Feinbaum, R.; Alloing, G.; Emerson, F.E.; Garsin, D.A.; Inoue, H.; Tanaka-Hino, M.; Hisamoto, N.; Matsumoto, K.; Tan, M.-W. A conserved p38 MAP kinase pathway in Caenorhabditis elegans innate immunity. Science 2002, 297, 623–626. [Google Scholar] [CrossRef] [Green Version]
- Sifri, C.D.; Begun, J.; Ausubel, F.M.; Calderwood, S.B. Caenorhabditis elegans as a model host for Staphylococcus aureus pathogenesis. Infect. Immun. 2003, 71, 2208–2217. [Google Scholar] [CrossRef] [Green Version]
- Mallo, G.V.; Kurz, C.L.; Couillault, C.; Pujol, N.; Granjeaud, S.; Kohara, Y.; Ewbank, J.J. Inducible antibacterial defense system in C. elegans. Curr. Biol. 2002, 12, 1209–1214. [Google Scholar] [CrossRef] [Green Version]
- Roeder, T.; Stanisak, M.; Gelhaus, C.; Bruchhaus, I.; Grötzinger, J.; Leippe, M. Caenopores are antimicrobial peptides in the nematode Caenorhabditis elegans instrumental in nutrition and immunity. Dev. Comp. Immunol. 2010, 34, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Miltsch, S.M.; Seeberger, P.H.; Lepenies, B. The C-type lectin-like domain containing proteins Clec-39 and Clec-49 are crucial for Caenorhabditis elegans immunity against Serratia marcescens infection. Dev. Comp. Immunol. 2014, 45, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Aizawa, T.; Hoshino, H.; Kawano, K.; Nitta, K.; Zhang, H. abf-1 and abf-2, ASABF-type antimicrobial peptide genes in Caenorhabditis elegans. Biochem. J. 2002, 361, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-Y.; Wang, M.; Zhang, J.; Barve, S.S.; McClain, C.J.; Joshi-Barve, S. Acrolein disrupts tight junction proteins and causes endoplasmic reticulum stress-mediated epithelial cell death leading to intestinal barrier dysfunction and permeability. Am. J. Pathol. 2017, 187, 2686–2697. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, C.Y.; Osaka, T.; Moriyama, E.; Date, Y.; Kikuchi, J.; Tsuneda, S. Strengthening of the intestinal epithelial tight junction by Bifidobacterium bifidum. Physiol. Rep. 2015, 3, e12327. [Google Scholar] [CrossRef] [Green Version]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757. [Google Scholar] [CrossRef] [Green Version]
- Weydert, C.J.; Cullen, J.J. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat. Protoc. 2010, 5, 51–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lithgow, G.J.; Walker, G.A. Stress resistance as a determinate of C. elegans lifespan. Mech. Ageing Dev. 2002, 123, 765–771. [Google Scholar] [CrossRef]
- Tower, J. Heat shock proteins and Drosophila aging. Exp. Gerontol. 2011, 46, 355–362. [Google Scholar] [CrossRef] [Green Version]
- Barja, G. The mitochondrial free radical theory of aging. In Progress in Molecular Biology and Translational Science; RG Landes: Austin, TX, USA, 2014; Volume 127, pp. 1–27. [Google Scholar]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta-Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Youle, R.J. The role of mitochondria in apoptosis. Annu. Rev. Genet. 2009, 43, 95–118. [Google Scholar] [CrossRef] [Green Version]




| Strain | Bacterial Source | Mean Life Span ± SEM (Days) | Total Worms (150) = Dead/Censored | p-Value (L. plantarum JBC5 versus E. coli OP50) |
|---|---|---|---|---|
| N2 (wild-type) | OP50 | 14.56 ± 0.34 | 138/12 | p < 0.0001 (***) |
| LPJBC5 | 18.61 ± 0.48 | 143/7 | ||
| daf-2 (e1368) | OP50 | 19.67 ± 0.45 | 141/9 | p < 0.0001 (***) |
| LPJBC5 | 23.48 ± 0.51 | 142/8 | ||
| daf-16 (mgDf50) | OP50 | 12.54 ± 0.24 | 139/11 | p < 0.0001 (***) |
| LPJBC5 | 15.22 ± 0.32 | 141/9 | ||
| nsy-1 (ag3) | OP50 | 13.04 ± 0.28 | 134/16 | p > 0.05 (N.S.) |
| LPJBC5 | 13.50 ± 0.26 | 137/13 | ||
| sek-1 (ag1) | OP50 | 12.72 ± 0.27 | 138/12 | p > 0.05 (N.S.) |
| LPJBC5 | 13.13 ± 0.22 | 140/10 | ||
| pmk-1 (km25) | OP50 | 13.51 ± 0.25 | 142/8 | p > 0.05 (N.S.) |
| LPJBC5 | 13.94 ± 0.30 | 143/7 | ||
| skn-1 (zu67) | OP50 | 13.03 ± 0.23 | 140/10 | p > 0.05 (N.S.) |
| LPJBC5 | 13.49 ± 0.27 | 142/8 | ||
| skn-1 (zu135) | OP50 | 13.83 ± 0.19 | 139/11 | p > 0.05 (N.S.) |
| LPJBC5 | 14.37 ± 0.28 | 136/14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, A.; Joishy, T.; Das, S.; Kalita, M.C.; Mukherjee, A.K.; Khan, M.R. A Potential Probiotic Lactobacillus plantarum JBC5 Improves Longevity and Healthy Aging by Modulating Antioxidative, Innate Immunity and Serotonin-Signaling Pathways in Caenorhabditis elegans. Antioxidants 2022, 11, 268. https://doi.org/10.3390/antiox11020268
Kumar A, Joishy T, Das S, Kalita MC, Mukherjee AK, Khan MR. A Potential Probiotic Lactobacillus plantarum JBC5 Improves Longevity and Healthy Aging by Modulating Antioxidative, Innate Immunity and Serotonin-Signaling Pathways in Caenorhabditis elegans. Antioxidants. 2022; 11(2):268. https://doi.org/10.3390/antiox11020268
Chicago/Turabian StyleKumar, Arun, Tulsi Joishy, Santanu Das, Mohan C. Kalita, Ashis K. Mukherjee, and Mojibur R. Khan. 2022. "A Potential Probiotic Lactobacillus plantarum JBC5 Improves Longevity and Healthy Aging by Modulating Antioxidative, Innate Immunity and Serotonin-Signaling Pathways in Caenorhabditis elegans" Antioxidants 11, no. 2: 268. https://doi.org/10.3390/antiox11020268
APA StyleKumar, A., Joishy, T., Das, S., Kalita, M. C., Mukherjee, A. K., & Khan, M. R. (2022). A Potential Probiotic Lactobacillus plantarum JBC5 Improves Longevity and Healthy Aging by Modulating Antioxidative, Innate Immunity and Serotonin-Signaling Pathways in Caenorhabditis elegans. Antioxidants, 11(2), 268. https://doi.org/10.3390/antiox11020268





