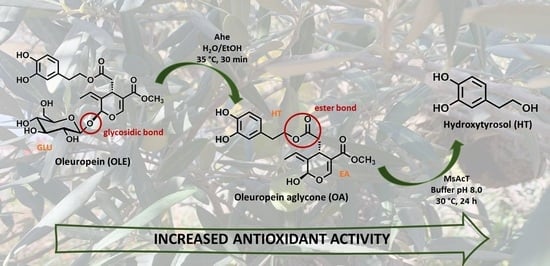
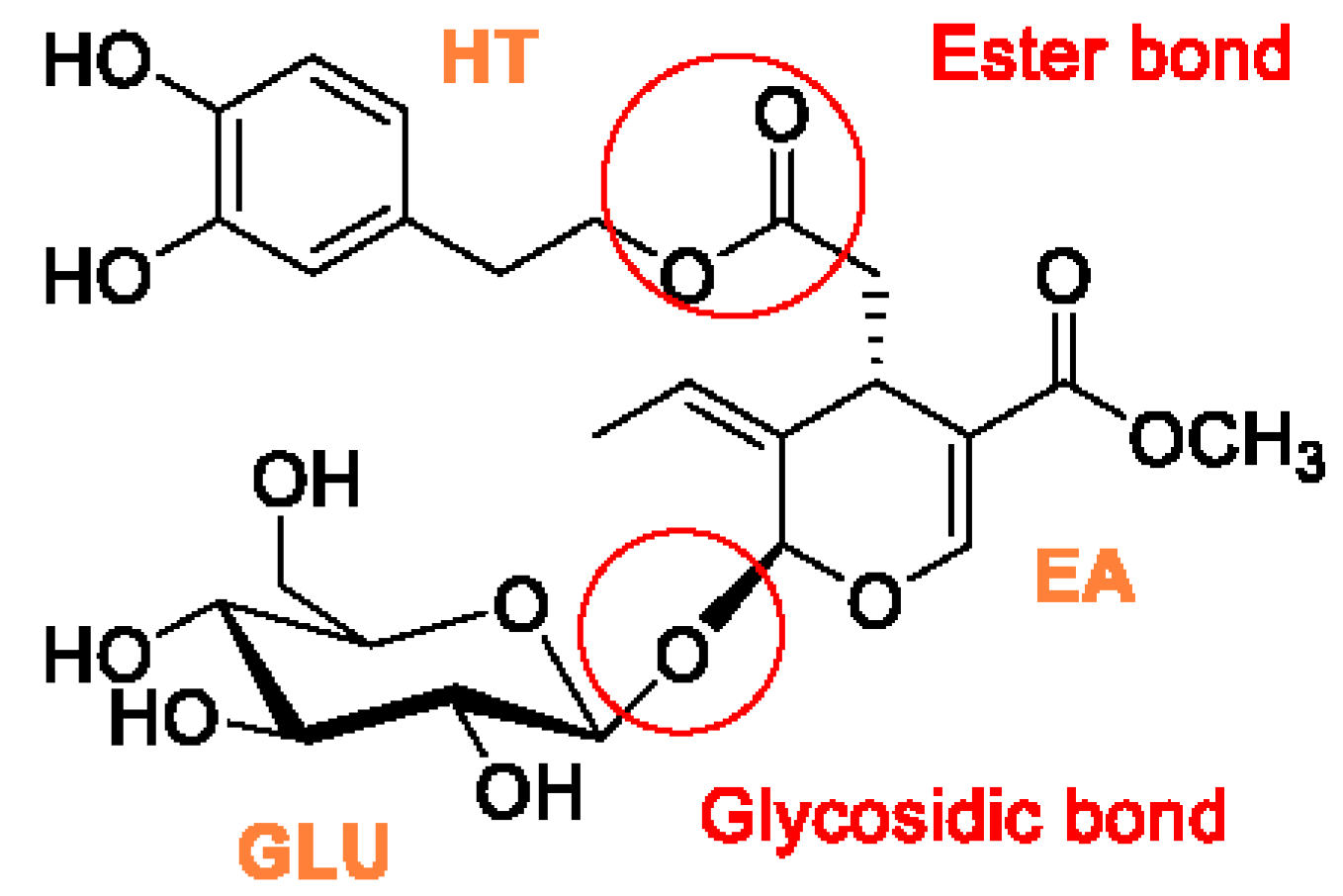
Efficient 2-Step Enzymatic Cascade for the Bioconversion of Oleuropein into Hydroxytyrosol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Expression and Purification of Ahe
2.3. Ahe Activity Assay
2.4. Biotransformation of OLE to the Corresponding Aglycone
2.5. Expression and Purification of MsAcT
2.6. MsAcT Activity Assay
2.7. Biotransformation of Oleuropein Aglycone to Hydroxytyrosol
2.8. HPLC Analysis
2.9. DPPH Radical Scavenging Assay
3. Results and Discussion
3.1. Preparation of Ole Aglycone through Ahe β-Glucosidase
3.2. Preparation of Hydroxytyrosol
3.3. Determination of the Free Radical Scavenging Capacity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chatzikonstantinou, A.V.; Gkantzou, E.; Thomou, E.; Chalmpes, N.; Lyra, K.M.; Kontogianni, V.G.; Spyrou, K.; Patila, M.; Gournis, D.; Stamatis, H. Enzymatic conversion of oleuropein to hydroxytyrosol using immobilized β-glucosidase on porous carbon cuboids. Nanomaterials 2019, 9, 1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Bock, M.; Thorstensen, E.B.; Derraik, J.G.B.; Henderson, H.V.; Hofman, P.L.; Cutfield, W.S. Human absorption and metabolism of oleuropein and hydroxytyrosol ingested as olive (Olea europaea L.) leaf extract. Mol. Nutr. Food Res. 2013, 57, 2079–2085. [Google Scholar] [CrossRef] [PubMed]
- Nediani, C.; Ruzzolini, J.; Romani, A.; Calorini, L. Oleuropein, a bioactive compound from olea europaea l., as a potential preventive and therapeutic agent in non-communicable diseases. Antioxidants 2019, 8, 578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, J.J.; Wang, C.Z.; Ye, J.Z.; Tao, R.; Zhang, Y.S. Enzymatic hydrolysis of oleuropein from Olea europea (olive) leaf extract and antioxidant activities. Molecules 2015, 20, 2903–2921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalampaliki, A.D.; Giannouli, V.; Skaltsounis, A.L.; Kostakis, I.K. A three-step, gram-scale synthesis of hydroxytyrosol, hydroxytyrosol acetate, and 3,4-dihydroxyphenylglycol. Molecules 2019, 24, 3239. [Google Scholar] [CrossRef] [Green Version]
- Contente, M.L.; Annunziata, F.; Cannazza, P.; Donzella, S.; Pinna, C.; Romano, D.; Tamborini, L.; Barbosa, F.G.; Molinari, F.; Pinto, A. Biocatalytic Approaches for an Efficient and Sustainable Preparation of Polyphenols and Their Derivatives. J. Agric. Food Chem. 2021, 69, 13669–13681. [Google Scholar] [CrossRef]
- Liu, M.; Yong, Q.; Yu, S. Efficient bioconversion of oleuropein from olive leaf extract to antioxidant hydroxytyrosol by enzymatic hydrolysis and high-temperature degradation. Biotechnol. Appl. Biochem. 2018, 65, 680–689. [Google Scholar] [CrossRef]
- Monteiro, M.; Silva, A.F.R.; Resende, D.; Braga, S.S.; Coimbra, M.A.; Silva, A.M.S.; Cardoso, S.M. Strategies to broaden the applications of olive biophenols oleuropein and hydroxytyrosol in food products. Antioxidants 2021, 10, 444. [Google Scholar] [CrossRef]
- De Leonardis, A.; Aretini, A.; Alfano, G.; MacCiola, V.; Ranalli, G. Isolation of a hydroxytyrosol-rich extract from olive leaves (Olea europaea L.) and evaluation of its antioxidant properties and bioactivity. Eur. Food Res. Technol. 2008, 226, 653–659. [Google Scholar] [CrossRef]
- Vilaplana-Pérez, C.; Auñón, D.; García-Flores, L.A.; Gil-Izquierdo, A. Hydroxytyrosol and Potential Uses in Cardiovascular Diseases, Cancer, and AIDS. Front. Nutr. 2014, 1, 18. [Google Scholar] [CrossRef] [Green Version]
- Goldsmith, C.D.; Bond, D.R.; Jankowski, H.; Weidenhofer, J.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C.J. The olive biophenols oleuropein and hydroxytyrosol selectively reduce proliferation, influence the cell cycle, and induce apoptosis in pancreatic cancer cells. Int. J. Mol. Sci. 2018, 19, 1937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez, L.; Ros, G.; Nieto, G. Hydroxytyrosol: Health Benefits and Use as Functional Ingredient in Meat. Medicines 2018, 5, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Britton, J.; Davis, R.; O’Connor, K.E. Chemical, physical and biotechnological approaches to the production of the potent antioxidant hydroxytyrosol. Appl. Microbiol. Biotechnol. 2019, 103, 5957–5974. [Google Scholar] [CrossRef] [PubMed]
- Angelino, D.; Gennari, L.; Blasa, M.; Selvaggini, R.; Urbani, S.; Esposto, S.; Servili, M.; Ninfali, P. Chemical and cellular antioxidant activity of phytochemicals purified from olive mill waste waters. J. Agric. Food Chem. 2011, 59, 2011–2018. [Google Scholar] [CrossRef] [PubMed]
- Zulueta, A.; Esteve, M.J.; Frígola, A. ORAC and TEAC assays comparison to measure the antioxidant capacity of food products. Food Chem. 2009, 114, 310–316. [Google Scholar] [CrossRef]
- Turck, D.; Bresson, J.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of hydroxytyrosol as a novel food pursuant to Regulation (EC) No 258/97. EFSA J. 2017, 15, e04728. [Google Scholar] [CrossRef]
- Xu, F.; Li, Y.; Zheng, M.; Xi, X.; Zhang, X.; Han, C. Structure properties, acquisition protocols, and biological activities of oleuropein aglycone. Front. Chem. 2018, 6, 239. [Google Scholar] [CrossRef] [Green Version]
- Wichers, H.J.; Soler-Rivas, C.; Espın, J.C. Review Oleuropein and related compounds. J. Sci. Food Agric. 2000, 80, 1013–1023. [Google Scholar]
- Contente, M.L.; Paradisi, F. Self-sustaining closed-loop multienzyme-mediated conversion of amines into alcohols in continuous reactions. Nat. Catal. 2018, 1, 452–459. [Google Scholar] [CrossRef]
- Annunziata, F.; Contente, M.L.; Pinna, C.; Tamborini, L.; Pinto, A. Biocatalyzed Flow Oxidation of Tyrosol to Hydroxytyrosol and Efficient Production of Their Acetate Esters. Antioxidants 2021, 10, 1142. [Google Scholar] [CrossRef]
- Delgado, L.; Heckmann, C.M.; Di Pisa, F.; Gourlay, L.; Paradisi, F. Release of Soybean Isoflavones by Using a β-Glucosidase from Alicyclobacillus herbarius. ChemBioChem 2021, 22, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, M.; Sheng, X.; Kroutil, W.; Himo, F. Computational Study of Mycobacterium smegmatis Acyl Transferase Reaction Mechanism and Specificity. ACS Catal. 2018, 8, 10698–10706. [Google Scholar] [CrossRef]
- Mathews, I.; Soltis, M.; Saldajeno, M.; Ganshaw, G.; Sala, R.; Weyler, W.; Cervin, M.A.; Whited, G.; Bott, R. Structure of a novel enzyme that catalyzes acyl transfer to alcohols in aqueous conditions. Biochemistry 2007, 46, 8969–8979. [Google Scholar] [CrossRef] [PubMed]
- Contente, M.L.; Pinto, A.; Molinari, F.; Paradisi, F. Biocatalytic N-Acylation of Amines in Water Using an Acyltransferase from Mycobacterium smegmatis. Adv. Synth. Catal. 2018, 360, 4814–4819. [Google Scholar] [CrossRef] [Green Version]
- Perdomo, I.C.; Gianolio, S.; Pinto, A.; Romano, D.; Contente, M.L.; Paradisi, F.; Molinari, F. Efficient Enzymatic Preparation of Flavor Esters in Water. J. Agric. Food Chem. 2019, 67, 6517–6522. [Google Scholar] [CrossRef]
- Contente, M.L.; Roura Padrosa, D.; Molinari, F.; Paradisi, F. A strategic Ser/Cys exchange in the catalytic triad unlocks an acyltransferase-mediated synthesis of thioesters and tertiary amides. Nat. Catal. 2020, 3, 1020–1026. [Google Scholar] [CrossRef]
- Benitez-Mateos, A.I.; Contente, M.L. Agarose vs. Methacrylate as Material Supports for Enzyme Immobilization and Continuous Processing. Catalysts 2021, 11, 814. [Google Scholar] [CrossRef]
- Pérez-Trujillo, M.; Gómez-Caravaca, A.M.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Parella, T. Separation and Identification of Phenolic Compounds of Extra Virgin Olive Oil from Olea europaea L. by HPLC-DAD-SPE-NMR/MS. Identification of a New Diastereoisomer of the Aldehydic Form of Oleuropein Aglycone. J. Agric. Food Chem. 2010, 58, 9129–9136. [Google Scholar] [CrossRef]
- Bianco, A.; Uccella, N. Biophenolic components of olives. Food Res. Int. 2000, 33, 475–485. [Google Scholar] [CrossRef]
- Vasella, A.; Davies, G.J.; Böhm, M. Glycosidase mechanisms. Curr. Opin. Chem. Biol. 2002, 6, 619–629. [Google Scholar] [CrossRef]
- Singh, G.; Vinod, A.K.V. Catalytic properties, functional attributes and industrial applications of β-glucosidases. 3 Biotech 2016, 6, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sørensen, A.; Lübeck, M.; Lübeck, P.S.; Ahring, B.K. Fungal beta-glucosidases: A bottleneck in industrial use of lignocellulosic materials. Biomolecules 2013, 3, 612–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Zhang, X.; Yin, Q.; Fang, W.; Fang, Z.; Wang, X.; Zhang, X.; Xiao, Y. A mechanism of glucose tolerance and stimulation of GH1 β-glucosidases. Sci. Rep. 2015, 5, 1–12. [Google Scholar] [CrossRef]
- Chen, K.; Arnold, F.H. Engineering new catalytic activities in enzymes. Nat. Catal. 2020, 3, 203–213. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Basso, A.; Brady, D. New frontiers in enzyme immobilisation: Robust biocatalysts for a circular bio-based economy. Chem. Soc. Rev. 2021, 50, 5850–5862. [Google Scholar] [CrossRef]
- Elleuche, S.; Schröder, C.; Sahm, K.; Antranikian, G. Extremozymes-biocatalysts with unique properties from extremophilic microorganisms. Curr. Opin. Biotechnol. 2014, 29, 116–123. [Google Scholar] [CrossRef]
- De Leeuw, N.; Torrelo, G.; Bisterfeld, C.; Resch, V.; Mestrom, L.; Straulino, E.; Van Der Weel, L.; Hanefeld, U. Ester Synthesis in Water: Mycobacterium smegmatis Acyl Transferase for Kinetic Resolutions. Adv. Synth. Catal. 2018, 360, 242–249. [Google Scholar] [CrossRef]
- Mestrom, L.; Claessen, J.G.R.; Hanefeld, U. Enzyme-Catalyzed Synthesis of Esters in Water. ChemCatChem 2019, 11, 2004–2010. [Google Scholar] [CrossRef] [Green Version]
- Contente, M.L.; Tamborini, L.; Molinari, F.; Paradisi, F. Aromas flow: Eco-friendly, continuous, and scalable preparation of flavour esters. J. Flow Chem. 2020, 10, 235–240. [Google Scholar] [CrossRef]
- Contente, M.L.; Farris, S.; Tamborini, L.; Molinari, F.; Paradisi, F. Flow-based enzymatic synthesis of melatonin and other high value tryptamine derivatives: A five-minute intensified process. Green Chem. 2019, 21, 3263–3266. [Google Scholar] [CrossRef] [Green Version]
- Annunziata, F.; Contente, M.L.; Betti, D.; Pinna, C.; Molinari, F.; Tamborini, L.; Pinto, A. Efficient chemo-enzymatic flow synthesis of high value amides and esters. Catalysts 2020, 10, 939. [Google Scholar] [CrossRef]
- Roura Padrosa, D.; Contente, M.L. Multi-gram preparation of cinnamoyl tryptamines as skin whitening agents through a chemo-enzymatic flow process. Tetrahedron Lett. 2021, 86, 153453–153459. [Google Scholar] [CrossRef]
- Dinu, C.Z.; Zhu, G.; Bale, S.S.; Anand, G.; Reeder, P.J.; Sanford, K.; Whited, G.; Kane, R.S.; Dordick, J.S. Enzyme-based nanoscale composites for use as active decontamination surfaces. Adv. Funct. Mater. 2010, 20, 392–398. [Google Scholar] [CrossRef]
- Szymańska, K.; Odrozek, K.; Zniszczoł, A.; Torrelo, G.; Resch, V.; Hanefeld, U.; Jarzȩbski, A.B. MsAcT in siliceous monolithic microreactors enables quantitative ester synthesis in water. Catal. Sci. Technol. 2016, 6, 4882–4888. [Google Scholar] [CrossRef] [Green Version]
- Pazos, M.; Alonso, A.; Sánchez, I.; Medina, I. Hydroxytyrosol prevents oxidative deterioration in foodstuffs rich in fish lipids. J. Agric. Food Chem. 2008, 56, 3334–3340. [Google Scholar] [CrossRef]
- Fabiani, R.; De Bartolomeo, A.; Rosignoli, P.; Servili, M.; Montedoro, G.F.; Morozzi, G. Cancer chemoprevention by hydroxytyrosol isolated from virgin olive oil through G1 cell cycle arrest and apoptosis. Eur. J. Cancer Prev. 2002, 11, 351–358. [Google Scholar] [CrossRef] [PubMed]
- De Bock, M.; Derraik, J.G.B.; Brennan, C.M.; Biggs, J.B.; Morgan, P.E.; Hodgkinson, S.C.; Hofman, P.L.; Cutfield, W.S. Olive (Olea europaea L.) Leaf Polyphenols Improve Insulin Sensitivity in Middle-Aged Overweight Men: A Randomized, Placebo-Controlled, Crossover Trial. PLoS ONE 2013, 8, e57622. [Google Scholar] [CrossRef]
- Martínez-Zamora, L.; Peñalver, R.; Ros, G.; Nieto, G. Olive tree derivatives and hydroxytyrosol: Their potential effects on human health and its use as functional ingredient in meat. Foods 2021, 10, 2611. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.S.; Jinsmaa, Y.; Sullivan, P.; Holmes, C.; Kopin, I.J.; Sharabi, Y. 3,4-Dihydroxyphenylethanol (Hydroxytyrosol) Mitigates the Increase in Spontaneous Oxidation of Dopamine during Monoamine Oxidase Inhibition in PC12 Cells. Neurochem. Res. 2016, 41, 2173–2178. [Google Scholar] [CrossRef] [Green Version]


| Compound | DPPH Radical Inhibition (%) * |
|---|---|
| OLE | 25 |
| OLE aglycone | 27 |
| HT | 44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catinella, G.; Donzella, S.; Borgonovo, G.; Dallavalle, S.; Contente, M.L.; Pinto, A. Efficient 2-Step Enzymatic Cascade for the Bioconversion of Oleuropein into Hydroxytyrosol. Antioxidants 2022, 11, 260. https://doi.org/10.3390/antiox11020260
Catinella G, Donzella S, Borgonovo G, Dallavalle S, Contente ML, Pinto A. Efficient 2-Step Enzymatic Cascade for the Bioconversion of Oleuropein into Hydroxytyrosol. Antioxidants. 2022; 11(2):260. https://doi.org/10.3390/antiox11020260
Chicago/Turabian StyleCatinella, Giorgia, Silvia Donzella, Gigliola Borgonovo, Sabrina Dallavalle, Martina Letizia Contente, and Andrea Pinto. 2022. "Efficient 2-Step Enzymatic Cascade for the Bioconversion of Oleuropein into Hydroxytyrosol" Antioxidants 11, no. 2: 260. https://doi.org/10.3390/antiox11020260
APA StyleCatinella, G., Donzella, S., Borgonovo, G., Dallavalle, S., Contente, M. L., & Pinto, A. (2022). Efficient 2-Step Enzymatic Cascade for the Bioconversion of Oleuropein into Hydroxytyrosol. Antioxidants, 11(2), 260. https://doi.org/10.3390/antiox11020260