Mini-Review on the Enzymatic Lipophilization of Phenolics Present in Plant Extracts with the Special Emphasis on Anthocyanins
Abstract
:1. Introduction
2. Lipophilization of Extracts from Fruits
3. Enzyme-Assisted Derivatization of Phenolics Present in Flower Extracts
4. Lipophilization of Extracts from Plant Leaves
5. Acylation of Other Plant Derived Matrices Rich in Phenolic Compounds
6. Other Unconventional Uses of Polyphenols Lipophilization
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, Isolation and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Barberi, R.; Coppo, E.; Marchese, A.; Daglia, M.; Sabarzo-Sanchez, E.; Nabavi, S.F.; Nabavi, S.M. Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. Microbiol. Res. 2017, 196, 44–68. [Google Scholar] [CrossRef] [PubMed]
- Strumiłło, J.; Gerszon, J.; Rodacka, A. Charakterystyka związków fenolowych pochodzenia naturalnego ze szczególnym uwzględnieniem ich roli w prewencji chorób neurodegeneracyjnych. In Bory Tucholskie i Inne Obszary Leśne. Ochrona i Monitoring; Gwoździński, K., Ed.; Wydawnictwo Uniwersytetu Łódzkiego: Łódź, Poland, 2015; pp. 231–245. [Google Scholar]
- Figueroa-Espinoza, M.C.; Bourlieu, C.; Durand, E.; Lecomte, J.; Villeneuve, P. Lipophilized Antioxidants. In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Academic Press: Cambridge, MA, USA, 2019; Volume 2, pp. 193–201. [Google Scholar] [CrossRef]
- Ramadan, M.F. Pheno-Phospholipids and Lipo-Phenolics. Novel Structured Antioxidants; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Farooq, S.; Abdullah; Zhang, H.; Weiss, J. A comprehensive review on polarity, partitioning, and interactions of phenolic antioxidants at oil-water interface of food emulsions. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4250–4277. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, M.E.M.B.; Franco, Y.E.M.; Messias, M.C.F.; Longato, G.B.; Pamphile, J.A.; Carvalho, P.D.O. Biocatalytic Synthesis of Flavonoid Esters by Lipase and Their Biological Benefits. Planta Med. 2017, 83, 7–22. [Google Scholar] [CrossRef]
- Szajdek, A.; Borowska, J. Właściwości przeciwutleniające żywności pochodzenia roślinnego. Żywn. Nauka Technol. Jakość 2004, 11, 5–28. [Google Scholar]
- Cosmulescu, S.; Trandafir, I.; Nour, V. Phenolic acids and flavonoids profiles of extracts from edible wild fruits and their antioxidant properties. Int. J. Food Prop. 2017, 20, 3124–3134. [Google Scholar] [CrossRef]
- Aladedunye, F.; Niehaus, K.; Bednarz, H.; Thiyam-Hollander, U.; Fehling, E.; Matthaus, B. Enzymatic lipophilization of phenolic extract from rowanberry (Sorbus aucuparia) and evaluation of antioxidative activity in edible oil. LWT 2015, 60, 52–62. [Google Scholar] [CrossRef]
- Yang, W.; Kortesniemi, M.; Yang, B.; Zheng, J. Enzymatic Acylation of Anthocyanins Isolated from Alpine Bearberry (Arctostaphylos alpina) and Lipophilic Properties, Thermostability, and Antioxidant Capacity of the Derivatives. J. Agric. Food Chem. 2018, 66, 2909–2916. [Google Scholar] [CrossRef]
- Marathe, S.J.; Shah, N.N.; Bajaj, S.R.; Singhal, R.S. Esterification of anthocyanins isolated from floral waste: Characterization of the esters and their application in various food systems. Food Biosci. 2021, 40, 100852. [Google Scholar] [CrossRef]
- Marquez-Rodriguez, A.S.; Guimaraes, M.; Mateus, N.; de Freitas, V.; Ballinas-Casarrubias, M.L.; Fuentes-Montero, M.E.; Salas, E.; Cruz, L. Disaccharide anthocyanin delphinidin 3-O-sambubioside from Hibiscus sabdariffa L.: Candida antarctica lipase B-catalyzed fatty acid acylation and study of its color properties. Food Chem. 2021, 344, 128603. [Google Scholar] [CrossRef]
- Fernandez-Aulis, F.; Torres, A.; Sanchez-Mendoza, E.; Cruz, L.; Navarro-Ocana, A. New acylated cyanidin glycosides extracted from underutilized potential sources: Enzymatic synthesis, antioxidant activity and thermostability. Food Chem. 2020, 309, 125796. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Jin, X.; Song, Y.; Zhang, Y.; Li, X.; Wang, F. Enzymatic Acylation of Proanthocyanidin Dimers from Acacia Mearnsii Bark: Effect on Lipophilic and Antioxidant Properties. J. Bioresour. Bioprod. 2021, 6, 359–366. [Google Scholar] [CrossRef]
- Cruz, L.; Benhoud, M.; Rayner, C.M.; Mateus, N.; de Freitas, V.; Blackburn, R.S. Selective enzymatic lipohilization of anthocyanin glucosides from blackcurrant (Ribes nigrum L.) skin extract and characterization of estrified anthocyanins. Food Chem. 2018, 266, 415–419. [Google Scholar] [CrossRef]
- Yang, W.; Kortesniemi, M.; Ma, X.; Zheng, J.; Yang, B. Enzymatic acylation of blaccurrant (Ribes nigrum) anthocyanins and evaluation of lipohilic properties and antioxidant capacity of derivatives. Food Chem. 2019, 281, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Mi, Y.; Cao, H.; Chen, L. Enzymatic acylation of raspberry anthocyanin: Evaluations on its stability and oxidative stress prevention. Food Chem. 2022, 372, 130766. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, D.E.; Wibisono, R.; Jensen, D.J.; Stanley, R.A.; Cooney, J.M. Direct acylation of flavonoid glycosides with phenolic acids catalysed by Candida antarcitca lipase B (Novozym 435). Enzyme Microb. Technol. 2006, 39, 1236–1241. [Google Scholar] [CrossRef]
- Aladedunye, F.; Matthäus, B. Effective lipophilic antioxidant enzymatically derived from Canadian crabapple. Eur. J. Lipid Sci. Technol. 2016, 118, 919–927. [Google Scholar] [CrossRef]
- Chen, M.; Yu, S.-J. Lipophilized Grape Seed Proanthocyanidin Derivatives as Novel Antioxidants. J. Agric. Food Chem. 2017, 65, 1598–1605. [Google Scholar] [CrossRef]
- Do Nascimento, M.A.; Vargas, J.P.C.; Rodrigues, J.G.A.; Leao, R.A.C.; de Moura, P.H.B.; Leal, I.C.R.; Bassut, J.; de Souza, R.O.M.A.; Wojcieszak, R.; Itabaiana, I., Jr. Lipase-catalyzed acylation of levoglucosan in continuous flow: Antibacterial and biosurfactant studies. RSC Adv. 2022, 12, 3027–3035. [Google Scholar] [CrossRef]
- de Castro, V.C.; da Silva, P.H.A.; de Oliveira, E.B.; Desobry, S.; Humeau, C. Extraction, identification and enzymatic synthesis of acylated derivatives of anthocyanins from jaboticaba (Myrciaria cauliflowa) fruits. Int. J. Food Sci. Technol. 2014, 49, 196–204. [Google Scholar] [CrossRef]
- Chang, S.K.; Alasalvar, C.; Shahidi, F. Superfruits: Phytochemicals, antioxidant efficacies, and health effects—A comprehensive review. Cri. Rev. Food Sci. Nutr. 2018, 59, 1580–1604. [Google Scholar] [CrossRef] [PubMed]
- Sari, P.; Setiawan, A.; Siswoyo, T.A. Stability and antioxidant activity of acylated jambolan (Syzygium cumini) anthocyanins synthesized by lipase-catalyzed transesterification. Int. Food Res. J. 2015, 22, 671–676. [Google Scholar]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R.; SantosBuelga, C.; Ferreira, I.C.F.R. Edible flowers as sources of phenolic compounds with bioactive potential. Food Res. Int. 2018, 105, 580–588. [Google Scholar] [CrossRef]
- Pires, E.d.O., Jr.; Di Gioia, F.; Rouphael, Y.; Ferreira, I.C.F.R.; Caleja, C.; Barros, L.; Petropoulos, S.A. The Compositional Aspects of Edible Flowers as an Emerging Horticultural Product. Molecules 2021, 26, 6940. [Google Scholar] [CrossRef]
- Ma, X.; Yan, R.; Yu, S.; Lu, Y.; Li, Z.; Lu, H.H. Enzymatic acylation of isoorientin and isovitexin from bamboo-leaf extracts with fatty acids and antiradical activity of the acylated derivatives. J. Agric. Food Chem. 2012, 60, 10844–10849. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Qian, J.; Li, S. Enzymatic acylation of isoorientin isolated from antioxidant of bamboo leaves with palmitic acid and antiradical activity of the acylated derivatives. Eur. Food Res. Technol. 2014, 239, 661–667. [Google Scholar] [CrossRef]
- Ma, X.; Wang, E.; Lu, Y.; Wang, Y.; Ou, S.; Yan, R. Acylation of antioxidant of bamboo leaves with fatty acids by lipase and the acylated derivatives’ efficiency in the inhibition of acrylamide formation in fried potato crisps. PLoS ONE 2015, 10, e0130680. [Google Scholar] [CrossRef]
- Porter, W.L.; Black, E.D.; Drolet, A.M. Use of polyamide oxidative fluorescence test on lipid emulsions—contrast in relative effectiveness of antioxidants in bulk versus dispersed systems. J. Agric. Food Chem. 1989, 373, 615–624. [Google Scholar] [CrossRef]
- Mellou, F.; Lazari, D.; Skaltsa, H.; Tselepis, A.D.; Kolisis, F.N.; Stamatis, H. Biocatalytic preparation of acylated derivatives of flavonoid glycosides enhances their antioxidant and antimicrobial activity. J. Biotechnol. 2005, 116, 295–304. [Google Scholar] [CrossRef]
- Hernandez, C.E.; Chen, H.-H.; Chang, C.I.; Huang, T.-C. Direct lipase-catalyzed lipophilization of chlorogenic acid from coffee pulp in supercritical carbon dioxide. Ind. Crops Prod. 2009, 30, 359–365. [Google Scholar] [CrossRef]
- Figueroa-Espinoza, M.C.; Villeneuve, P. Phenolic acids enzymatic lipophilization. J. Agric. Food Chem. 2005, 53, 2779–2787. [Google Scholar] [CrossRef]
- Lopez Giraldo, L.J.; Laguerre, M.; Lecomte, J.; Figueroa-Espinoza, M.C.; Barouh, N.; Barea, B.; Villeneuve, P. Lipase-catalyzed synthesis of chlorogenate fatty esters in solvent-free medium. Enzyme Microb. Technol. 2007, 41, 721–726. [Google Scholar] [CrossRef]
- Yan, Z.; Li, C.; Zhang, L.; Liu, Q.; Ou, S.; Zeng, X. Enzymatic acylation of anthocyanin isolated from black rice with methyl aromatic acid ester as donor: Stability of the acylated derivatives. J. Agric. Food Chem. 2016, 64, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Pinto, G.; Illiano, A.; Carpentieri, A.; Spinelli, M.; Melchiorre, C.; Fontanarosa, C.; di Serio, M.; Amoresano, A. Quantification of polyphenols and metals in Chinese tea infusions by mass spectrometry. Foods 2020, 9, 835. [Google Scholar] [CrossRef] [PubMed]
- Klepacka, J.; Tońska, E.; Rafałowski, R.; Czarnowska-Kujawska, M.; Opara, B. Tea as a Source of Biologically Active Compounds in the Human Diet. Molecules 2021, 26, 1487. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-S.; Luo, S.-Z.; Zheng, Z.; Zhao, Y.-Y.; Pang, M.; Jiang, S.-T. Enzymatic lipophilization of epicatechin with free fatty acids and its effect on antioxidative capacity in crude camellia seed oil. J. Sci. Food Agric. 2017, 97, 868–874. [Google Scholar] [CrossRef]
- Luo, S.-Z.; Chen, S.-S.; Pan, L.-H.; Qin, X.-S.; Zheng, Z.; Zhao, Y.-Y.; Pang, M.; Jiang, S.-T. Antioxidative capacity of crude camellia seed oil: Impact of lipohilization products of blueberry anthocyanin. Int. J. Food Prop. 2017, 20, 1627–1636. [Google Scholar] [CrossRef]
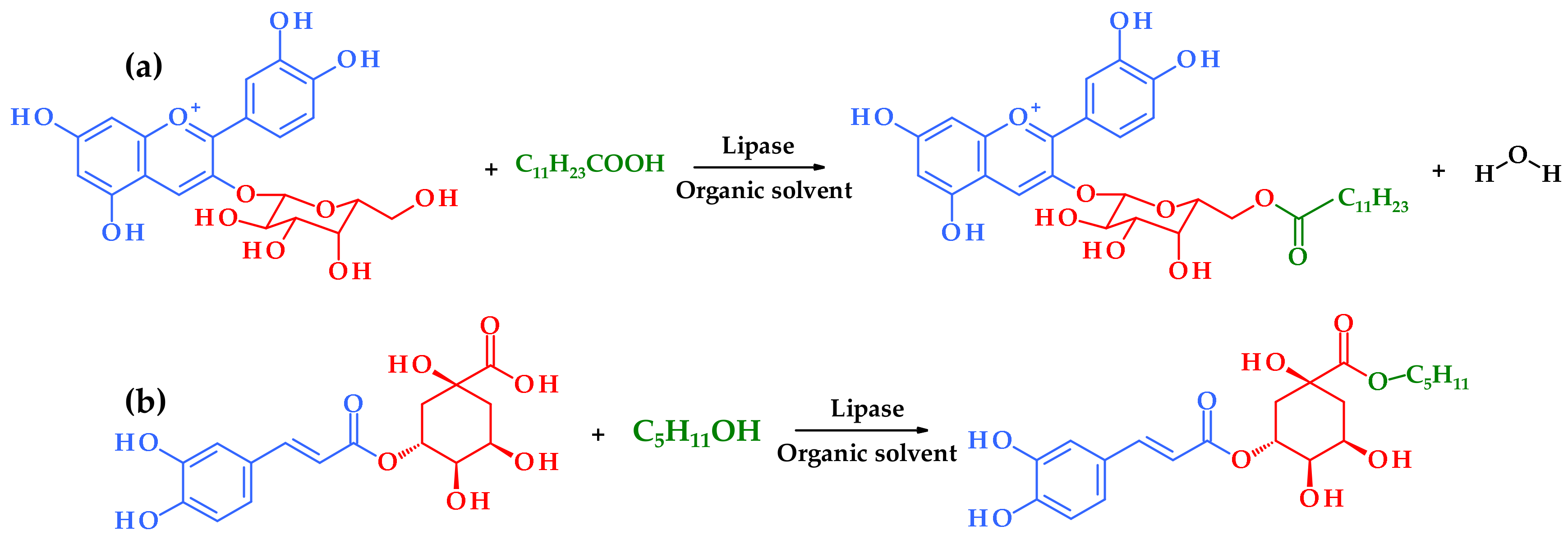

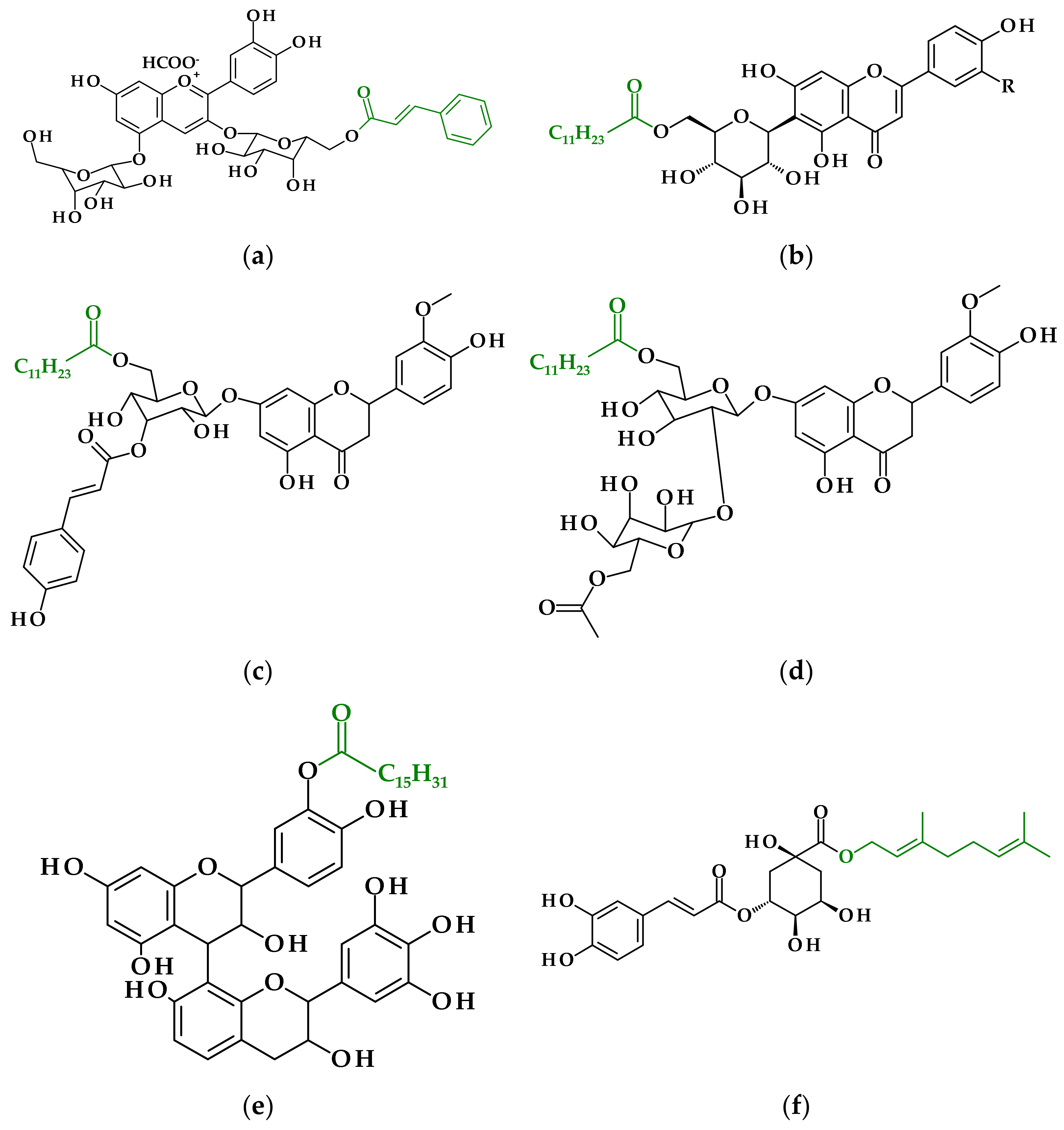
| The Origin of the Plant Extract | Main Components of the Extract | Used Enzyme | Reaction Conditions | The Obtained Ester(s) | Research Highlights | Reference |
|---|---|---|---|---|---|---|
| Rowan (Sorbus aucuparia L.) | Chlorogenic acid | Candida antarctica lipase B | 55 °C, 120 h, 2-methyl-2-butanol as a solvent, molar ratio of 1:20 (acid:alcohol) | Octadecyl chlorogenate | (a) A 43% decrease in peroxide value of rapeseed oil after fortifying with octadecyl chlorogenate. (b) Inhibition of triacylglycerols polymerization and polar compound formation during potato chip frying due to the addition of lipophilized extract. | [10] |
| Alpine bearberry (Arctostaphylos alpina) | Cyanidin-3-O-galactoside | Candida antarctica lipase B | 60 °C, 72 h, tert-butanol as a solvent and the molar ratio of anthocyanin to lauric acid was 1:10 | Cyanidin-3-O-(6″-dodecanoyl)galactoside | (a) Improved thermostability of the obtained ester. (b) Improved lipophilicity (higher logP value). | [11] |
| Blackcurrant (Ribes nigrum L.) | Delphinidin-3-O-rutinoside, Cyanidin-3-O-rutinoside, Delphinidin-3-O-glucoside, Cyanidin-3-O-glucoside | Candida antarctica lipase B | 60 °C, 9 h, acetonitrile:DMSO 10:1 (v/v), 20 g/L of enzyme, molecular sieves (100 g/L), a anthocyanins:octanoic acid ratio of 1:100 | Cyanidin-3-O-(6″-octanoyl)glucoside, Delphinidin-3-O-(6″-octanoyl)glucoside | (a) Lipophilization as a new anthocyanin separation technique with different glycosidic moieties. | [18] |
| Blackcurrant (Ribes nigrum L.) | Delphinidin-3-O-rutinoside, Cyanidin-3-O-rutinoside, Delphinidin-3-O-glucoside, Cyanidin-3-O-glucoside | Candida antarctica lipase B | 60 °C, 72 h, tert-butanol as a solvent and the molar ratio of anthocyanin to lauric acid was 1:10, 10 g/L of enzyme, molecular sieves (100 g/L) | Cyanidin-3-O-(6″-dodecanoyl)glucoside, Delphinidin-3-O-(6″-dodecanoyl)glucoside, Cyanidin-3-O-(6″-dodecanoyl)rutinoside, Delphinidin-3-O-(6″-dodecanoyl)rutinoside, | (a) Improved thermostability due to the enzymatic acylation. (b) Acylation significantly improved inbitition of lipid peroxidation in the β-carotene bleaching method. | [19] |
| Trueno (Ligustrum japonicum) fruits | Cyanidin-3-O-rutinoside | Candida antarctica lipase B | 60 °C, 48 h, 300 rpm, 20 g/L of enzyme, tert-butanol as a solvent, a cyanidin:vinyl cinnamate ratio of 1:250, molecular sieves (100 g/L) | Cyanidin-3-O-(4‴-cinnamoyl)rutinoside | (a) Optimized synthesis methodology with 45.5% conversion yield. | [14] |
| Raspberry (Rubus L.) | Cyanidin-3-O-glucoside | Novozym 435 (Candida antarctica lipase B) | 40 °C, 24 h, pyridine (10 mL), 0.9 KPa of pressure (rotary evaporator), 10 mg of purified anthocyanins, 500 mg of methyl salicylate, 200 mg of Novozym 435 | Cyanidin-3-(6-salicyloyl)glucoside | (a) Acylation improved thermostability and stability in the light and oxidation environments. | [12] |
| Penglai apple polyphenolic extract | Phloridzin | Novozym 435 (Candida antarctica lipase B) | 60 °C, 168 h, 20 mg of fruit extract, acyl donor substrate (2 molar equivalents), 100 mg of enzyme, 100 mg of molecular sieves, tert-butanol as a solvent (1 mL, containing 0.2% of BHT) | Phloridzyl palmitate, Phloridzyl 4-hydroxyphenylpropionate, Phloridzyl 2-hydroxyphenylpropionate, Phloridzyl 3,4-dihydroxyphenylpropionate, phloridzyl cinnamate, Phloridzyl 3-phenylpropionate | (a) Acylation improved the solubility of the obtained derivatives in the reaction solvent. | [20] |
| Blueberry extract | Chlorogenic acid, Quercetin-3-glycosides, Delphinidin, cyanidin, petunidin and malvidin glycosides | Novozym 435 (Candida antarctica lipase B) | 60 °C, 168 h, 20 mg of fruit extract, acyl donor substrate (2 molar equivalents), 100 mg of enzyme, 100 mg of molecular sieves, tert-butanol as a solvent (1 mL, containing 0.2% of BHT) | 3-phenylpropionate esters of quercetin, isoquercetin, and delphinidin, cyanidin, petunidin and malvidin glycosides | (a) Novozym 435 showed selectivity for a primary aliphatic hydroxyl group of the sugar moiety in the acylation of different groups of polyphenols. | [20] |
| Canadian crabapple | Phloridzin | Candida antarctica lipase B | 55 °C, 120 h, 250 rpm, 2-methyl-2-butanol (10 mL) as a solvent, 1000 mg of enzyme, molecular sieves (1000 mg), phenolic extract (750 mg), octadecanoic acid (acyl donor, 1500 mg) | Phloridzyl octadecanoate | (a) Improved stability of the rapeseed oil during potato chip frying (inhibition of polymerization of triacylglycerols and polar components formation). (b) Higher amount of tocopherols presented in deep fried potato chips in the oil with the addition of phloridzyl octadecanoate. | [13] |
| Grape seeds (GSP) | Epicatechin, Procyanidin B1, and other 9 phenolic compounds | Lipozyme TL IM (immobilized lipase from Thermomyces lanuginosus) | 45 °C, 22 h, a ratio of lauric acid:grape seeds extract of 1:1, enzyme (2%), ethanol as a solvent | Lauroyl epicatechin, Tri-lauroyl epicatechin gallate, Lauroyl catechin | (a) GSP derivatives had the highest DPPH· scavenging activity compared to GSP, BHT (butylated hydroxytoluene) and TBHQ (tert-butylhydroquinone) | [21] |
| Skin of jaboticaba (Myrciaria cauliflora) fruits | Delphinidin-3-O-glucoside and Cyanidin-3-O-glucoside | Novozym 435 (Candida antarctica lipase B) | 50 °C, 48 h, 600 rpm, 200 mbar, 20 g/L of enzyme, 20 mg of jaboticaba extract, palmitic acid as an acyl donor (2 molar equivalents), 2-methyl-2-butanol as a solvent (5 mL) | Delphinidin-3-O-(6″-palmitoyl)glucoside, Cyanidin-3-O-(6″-palmitoyl)glucoside | (a) Acylation increased the hydrophobicity of anthocyanins. | [23] |
| Jambolan (Syzygium cumini) fruits | Anthocyanins | Candida antarctica lipase B | 40 °C, 48 h, acetone with 10% of DMSO as solvents, vinyl cinnamate as an acyl donor | Cinnamate esters of anthocyanins | (a) Higher thermal and light stability in the acylated anthocyanins compared to the native anthocyanins. | [25] |
| The Origin of the Plant Extract | Main Components of the Extract | Used Enzyme | Reaction Conditions | The Obtained Ester(s) | Research Highlights | Reference |
|---|---|---|---|---|---|---|
| Hibiscus sabdariffa flowers | Delphinidin 3-O-sambubioside | Candida antarctica lipase B | 60 °C, 48 h, 20 g/L of enzyme, 2-methyl-2-butanol as a solvent, molecular sieves (100 g/L), a anthocyanin:octanoic acid ratio of 1:250 | Octanoic acid ester of delphinidin-3-O-sambubioside | (a) Stable quinoidal base with blue color at a wide range of pH. | [16] |
| Bottlebrush (Callistemon citrinus) flowers | Cyanidin-3,5-O-diglucoside | Candida antarctica lipase B | 60 °C, 48 h, 300 rpm, 20 g/L of enzyme, tert-butanol as a solvent, a cyanidin:vinyl cinnamate ratio of 1:250, molecular sieves (100 g/L) | Cyanidin-3-O-(6″-cinnamoyl) glucoside-5-O-glucoside | (a) Optimized synthesis methodology with 85.7% conversion yield. | [14] |
| Rose petals | Cyanidin-3,5-O-diglucoside | Fermase CALB™10000 (lipase B from Candida antarctica immobilized on polyacrylate beads) | 40 °C, 24 h, acetonitrile as a solvent, 20 mg/mL of enzyme, a cyanidin:lauric acid molar ratio of 1:100, molecular sieves (100 mg/mL) | Lauryl monoesters of cyanidin-3,5-O-diglucoside | (a) Enhanced color stability in thermal processing of rice extrudates. (b) The use of esterified anthocyanins as a colorant in cupcakes and as a filling in sandwich biscuit cream. | [15] |
| Bamboo leaves | Isoorientin and Isovitexin | Candida antarctica lipase B | 65 °C, 48 h, molecular sieves (100 mg/mL), 10 g/L of enzyme, 2-methyl-2-butanol as a solvent, the acyl donor/flavonoid molar ratio of 5:1 or 10:1 | Isoorientin-6″-laurate, Isovitexin-6″-laurate | (a) The lipophilicity was improved due to the acylation, and simultaneously, reduction in the antioxidant activity was observed. | [28] |
| Bamboo leaves | Isoorientin | Novozym 435 (Candida antarctica lipase B) | 60 °C, 48 h, 210 rpm, molecular sieves (140 mg/mL), 12 g/L of enzyme, 2-methyl-2-butanol as a solvent, the acyl donor/flavonoid molar ratio reached of 12:1 | Isoorientin-6″-palmitate | (a) High yield of the acylation (90%). (b) Acylation improved lipophilicity, but the antioxidant activity of the obtained derivative was lower. | [31] |
| Bamboo leaves | Orientin, Isoorientin, Vitexin, and Isovitexin | Novozym 435 (Candida antarctica lipase B) | 65 °C, 48 h, 2-methyl-2-butanol as a solvent (100 mL), bamboo leaves extract (2.5 g–4.5 mM of falvonoids), 4.51 g of lauric acid (22.5 mM), a ratio of acid:extract of 5:1, 10 g of enzyme, 100 mg/mL of molecular sieves | Orientin-6″-laurate, Vitexin-6″-laurate, Isoorientin-6″-laurate, Isovitexin-6″-laurate | (a) Acylated antioxidants from bamboo leaves in the concentration of 0.05% and 0.1% inhibited the formation of acrylamide during potato chip frying. | [32] |
| The Origin of the Plant Extract | Main Components of the Extract | Used Enzyme | Reaction Conditions | The Obtained Ester(s) | Research Highlights | Reference |
|---|---|---|---|---|---|---|
| Aerials parts of two Greek endemic plants, i.e., Stachys swainsonii ssp. argolica (Boiss.) Phitos and Damboldt and St. swainsonii ssp. swainsonii | Chrysoeriol-7-O-β-D-(3″-E-p-coumaroyl) glucopyranoside and Chrysoeriol-7-[6‴-O-acetyl-β-D-allosyl-(1→2)-β-D-glucopyranoside] | Novozym 435 (Candida antarctica lipase B) | 50 °C, 96 h, 240 rpm, acetone as a solvent (10 mL), flavonoids–0.2 mmol, vinyl laurate as an acyl donor (2 mmol, ratio 1:10), 100 mg of enzyme | Laurate ester of chrysoeriol-7-O-β-D-(3″-E-p-coumaroyl)-glucopyranoside and laurate ester of chrysoeriol-7-[6‴-O-acetyl-β-D-allosyl-(1→2)-β-D-glucopyranoside] | (a) The obtained laurate esters caused higher prolongation of LDL and serum resistance to copper-induced oxidation. | [29] |
| Purple corn (Zea mays) or tiliapo (Sideroxylon palmeri) husks | Cyanidin-3-O-glucoside | Candida antarctica lipase B | 60 °C, 48 h, 300 rpm, 20 g/L of enzyme, tert-butanol as a solvent, a cyanidin:vinyl cinnamate ratio of 1:250, molecular sieves (100 g/L) | Cyanidin-3-O-(6″-cinnamoyl) glucoside, Cyanidin-3-O-(6″-dihydro cinnamoyl)glucoside, Cyanidin-3-O-(6″-dihydro feruloyl)glucoside, Cyanidin-3-O-(6″-dihydro sinapoyl)glucoside | (a) Cyanidin-3-O-(6″-dihydrosinapoyl) glucoside had the highest antioxidant activity in DPPH• assay. | [14] |
| Plum (Prunus domestica) husk | Cyanidin-3-O-rutinoside | Candida antarctica lipase B | 60 °C, 48 h, 300 rpm, 20 g/L of enzyme, tert-butanol as a solvent, a cyanidin:vinyl cinnamate ratio of 1:250, molecular sieves (100 g/L) | Cyanidin-3-O-(4‴-cinnamoyl)rutinoside | (a) Optimized synthesis methodology with a 45.5% conversion yield. | [14] |
| Black rice (Oryza sativa L. subsp. Japonica) | Cyanidin-3-O-glucoside | Novozym 435 (Candida antarctica lipase B) | 40 °C, 48 h, 30 rpm, 900 mbar (vacuum pump), 0.5 g of black rice anthocyanins, 10 mL of acyl donor, pyridine (5 mL) as a solvent, 1 g of enzyme | Cyanidin-3-O-(6″-benzoate) glucoside, Cyanidin-3-O-(6″-salicylate) glucoside, Cyanidin-3-O-(6″-cinnamate) glucoside | (a) Enzymatic acylation improved the half-life times of anthocyanins in the light treatments (dark, UV and fluorescent). (b) Improved thermostability due to the enzymatic acylation was observed. | [36] |
| Acacia mearnsii bark | Heteroduplex composed of procyanidin and prorobinetinidin linked by a single C4–C8 bond (PA dimers) | Novozym 435 (Candida antarctica lipase B) | 60 °C, 12 h, 30 g/L of enzyme, 2-methyl-2-butanol (with 5% of water) as a solvent, a ratio of 10:1 (palmitic acid:PA dimers) | Procyanidin and prorobinetinidin heteroduplex esterified with palmitic acid | (a) The antioxidant activities of PA dimers and obtained palmitate ester were much higher than the activity of vitamin E. | [17] |
| Coffee pulp | Chlorogenic acid | Novozym 435 (Candida antarctica lipase B) | Cell volume of 50 mL, 150 mbar of supercritical carbon dioxide (sCO2), 55 °C, 25 h, tert-butanol as a solvent (10% v/v), 10% (v/v) of acyl donor (1-heptanol or 1-pentanol or geraniol), 20 mg/mL of enzyme, 20 mg/mL of molecular sieves | Heptyl chlorogenate | (a) The supercritical carbon dioxide proved to be useful in the lipophilization of chlorogenic acid from the coffee pulp. | [30] |
| Tea leaves | Epicatechin | Novozym 435 (Candida antarctica lipase B) | 50 °C, 36 h, 14 mg of epicatechin (dissolved in polyoxyethylene stearate, 1:1 (m/m)), 10 g of crude camellia seed oil, 10 mg of enzyme, 2 g of molecular sieves | Epicatechin palmitate, Epicatechin oleate | (a) Reduced content of the free fatty acids as a result of the reaction of blueberry anthocyanins with components of the crude camellia seed oil. (b) The oxidative stability of the oil was improved. | [39] |
| Blueberry anthocyanin extract | Malvidin-3-O-galactoside, Cyanidin-3-O-galactoside, Delphinidin-3-O-galactoside, Malvidin-3-O-glucoside, and Cyanidin-3-O-arabinoside | Novozym 435 (Candida antarctica lipase B) | 50 °C, 36 h, 200 rpm, 36 mg of the blueberry anthocyanin solution (dissolved in polyoxyethylene stearate, 1:2 (m/m)), 10 g of crude camellia seed oil, 10 mg of enzyme, 2 g of molecular sieves | Cyanidin-3-O-(6″-oleoyl) galactoside, Cyanidin-3-O-(6″-palmitoyl) galactoside | (a) Reduced content of the free fatty acids as a result of the reaction of blueberry anthocyanins with components of the crude camellia seed oil. (b) The oxidative stability of the oil was improved. | [40] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jasińska, K.; Fabiszewska, A.; Białecka-Florjańczyk, E.; Zieniuk, B. Mini-Review on the Enzymatic Lipophilization of Phenolics Present in Plant Extracts with the Special Emphasis on Anthocyanins. Antioxidants 2022, 11, 1528. https://doi.org/10.3390/antiox11081528
Jasińska K, Fabiszewska A, Białecka-Florjańczyk E, Zieniuk B. Mini-Review on the Enzymatic Lipophilization of Phenolics Present in Plant Extracts with the Special Emphasis on Anthocyanins. Antioxidants. 2022; 11(8):1528. https://doi.org/10.3390/antiox11081528
Chicago/Turabian StyleJasińska, Karina, Agata Fabiszewska, Ewa Białecka-Florjańczyk, and Bartłomiej Zieniuk. 2022. "Mini-Review on the Enzymatic Lipophilization of Phenolics Present in Plant Extracts with the Special Emphasis on Anthocyanins" Antioxidants 11, no. 8: 1528. https://doi.org/10.3390/antiox11081528
APA StyleJasińska, K., Fabiszewska, A., Białecka-Florjańczyk, E., & Zieniuk, B. (2022). Mini-Review on the Enzymatic Lipophilization of Phenolics Present in Plant Extracts with the Special Emphasis on Anthocyanins. Antioxidants, 11(8), 1528. https://doi.org/10.3390/antiox11081528








