Health Benefits and Applications of Goji Berries in Functional Food Products Development: A Review
Abstract
1. Introduction
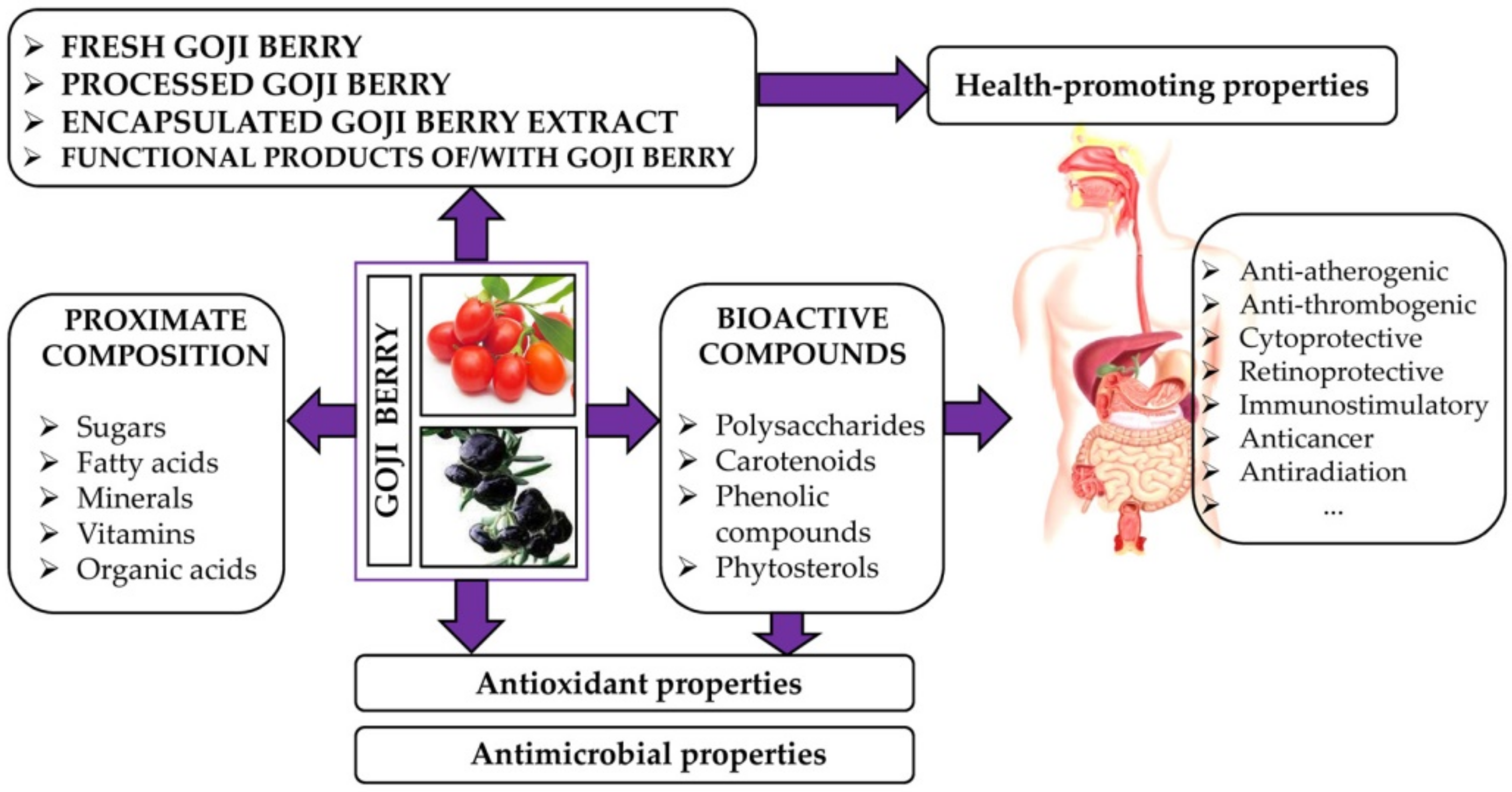
2. Nutritional Value and Bioactive Compounds of Goji Berries
3. Antioxidant Properties of Goji Berries
4. Health Benefits and Side Effects of Goji Berry Consumption
4.1. Health Benefits of Goji Berry Consumption
4.2. Side Effects of Goji Berry Consumption
5. Processing of Goji Berries
5.1. Oven and Freeze-Drying Dehydration Techniques
5.2. Advanced Techniques
5.3. Encapsulation of Bioactive Compounds from Goji Berries
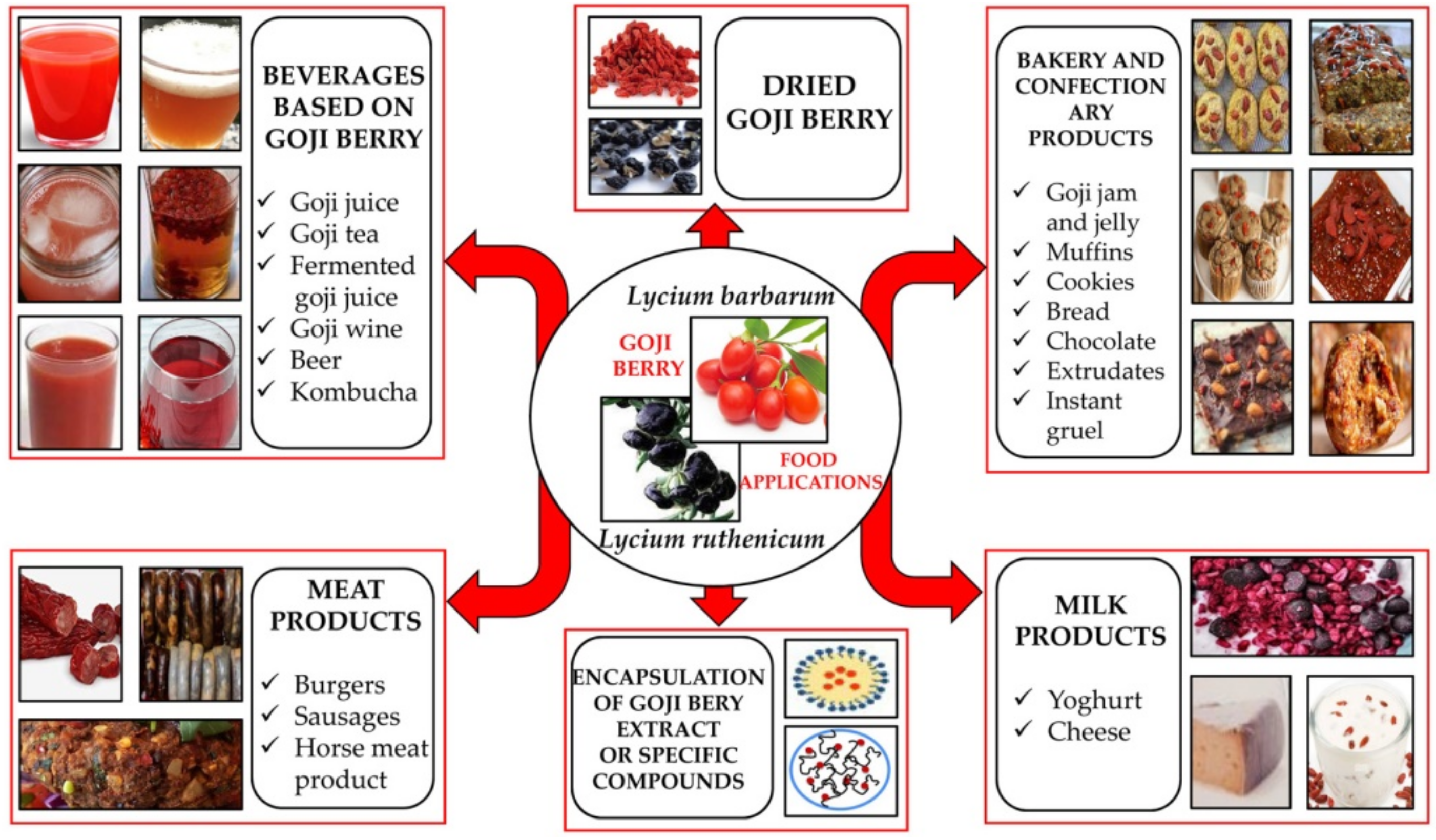
6. Goji Berry as Source of Functional Ingredients in Different Food Products
6.1. Goji Berry as a Raw Material or a Functional Ingredient in the Production of Beverages
6.2. Goji Berries as Functional Ingredients in Meat Products
6.3. Goji Berries as Functional Ingredients in Confectionery and Bakery Products
6.4. Goji Berries as Functional Ingredients in Milk Products
| Products | Goji Berries | Microbial Species Involved in Fermentation | Main Observations | Specific Note | Reference | |
|---|---|---|---|---|---|---|
| Functional Properties | Sensory and Texture Properties | |||||
| BEVERAGES | ||||||
| Kombucha beverages | Dried goji berries: 1. Red goji berry (Lycium barbarum L.) 2. Black goji berry (Lycium ruthenicum Murr.) | Kombucha culture: Symbiosis of acetic acid bacteria and yeast species | 1. High TPC 2. High antioxidant properties (DPPH•, FRAP and CUPRAC) | 1. Decrease colour intensity due to microbial transformation of phenolics (high score) 2. Odour highly acceptable(smell from fruity to acetic acid) 3. Taste (fruity, sour and sparkling flavour) | Increased TPC and antioxidant properties (except DPPH•) after in vitro digestion | [161] |
| Amber ale beer | Dry goji berries | Saccharomyces cerevisiae yeast | 1. High TPC 2. High rutin, p-coumaric and ferulic acid content 3. High content of AA-2βG 4. High antioxidant activity (TEAC and ORAC) | Hedonic score: Appreciation 1. Lower intensity 2. High colour intensity 3. Odour (red fruit, grainy, honey, caramel, coffee, hay-like and smoky) 4. Taste (bitterness, sourness, red fruit-, caramel-, coffee- and grainy-like) | Goji berries were added to ale type beer at different stages of the production process | [162] |
| Fermented goji juice | Goji berries extract | Lactobacillus plantarum RV21797 | 1. High TPC, TFC and TAcy 2. Expressed in vitro antioxidant properties (DPPH•, ABTS•+, FRAP and ORAC) 3. High cellular antioxidant activity (HepG2 Cells) | / | / | [155] |
| Fermented goji juice | Dried goji berries (soaked goji berries) | Bacillus velezensis, Bacillus licheniformis, Lactobacillus reuteri, L. rhamnosus and L. plantarum | 1. High TPC and TFC 2. High p-hydroxybenzoic acid, p-coumaric acid and rutin content 3. High individually volatile compounds content 4. High antioxidant activity (DPPH• and hydroxyl radical) | 1. Colour is moderate, very good 2. Flavour (aroma of goji juice is pure and has no odour) 3. Taste (slightly acidic or sweet) 4. Acceptability (very like) | / | [157] |
| Goji berry tea | Dried goji berries: 1. Red goji berry (Lycium barbarum L.) 2. Black goji berry (Lycium ruthenicum) | / | 1. High TPC 2. High LBP content 3. High antioxidant activity (DPPH•, ABTS•+ and FRAP) | 1. Colour of red and black goji berry tea were light yellow and purple, respectively, and then colour gradually changed to darker with the increase of time and temperature of soaking | This study monitored the effects of various temperatures and times of soak on antioxidant properties of specific goji berry tea. | [70] |
| Fermented goji juice by probiotics | Dried goji berries (soaked goji berries) | Lactobacillus plantarum, L. reuteri and Streptococcus thermophilus | 1. Decreases the levels of pro-inflammatory cytokines and total superoxide dismutase in serum and colon 2. Increased the levels of anti-inflammatory cytokines, myeloperoxidase and glutathione peroxidase 3. Decreases intestinal permeability 4. Modulate gut microbiota | / | Probiotics fermentation of goji berry juice contributing to enhanced the anti-ulcerative colitis function. | [157] |
| Fermented goji juice | Fully ripe and frozen goji berries (goji purre crashed with pectinase) | Lactiplantibacillus plantarum, Lactobacillus acidophilus, L. helveticus, Fructobacillus fructosus, Weissella cibaria | 1. Highly individually volatile compounds content (93 volatile compounds and seven non-volatile organic acids) | 1. Juices fermented with L. plantarum or L. acidophilus were described with ‘honey’, ‘wild jujube’ odours and ‘sour’ taste 2. Juices with L. helveticus were described with ‘goji berry’, ‘floral’ and sweetness 3. Juices with F. fructosus or W. cibaria were described with ‘vinegar’ and sweetness | L. helveticus 6024 is the most active strain able to retain or liberated the key compounds positively associated with ‘goji berry’ note. | [158] |
| Goji juice and goji capsules | Goji berries | / | 1. Source of nutritional and mineral elements | / | Goji capsules contained higher concentration of all individually minerals compared to goji juice samples. | [154] |
| Goji wine | Dried goji berries (goji puree) | Saccharomyces cerevisiae | 1. High TPC and TFC 2. High LBPs content 3. High individually volatile compounds content 4. High antioxidant activity (DPPH• and ABTS•+) | 1. Flavour (woody, vanillia and clove aroma), aroma related with compounds such as cis- and trans-whisky lactone, vanillin, eugenol, isoeugenol, and 4-vinylguaiacol 2. The highest score in olfactory and gustative attributes | This study monitored the effects of various oak matrices (medium toast barrel, medium toast shavings, non-toast chips, light toast chips, medium toast chips, and heavy toast chips) on the volatiles and antioxidant activity in Goji wine. Thus, Goji wine treated with oak shavings had the highest antioxidant activity, phenolics and flavonoids content | [159] |
| Goji berry juice | Dehydrated goji berries | / | 1. High protocatehuic acid, vanillic acid, p-coumaric acid, catechin and rutin content 2. Goji juice caused toxicity and reduced the lifespan of Caenorhabditis elegans 3. Goji juice increased lipofuscin, glucose levels, number of apoptotic bodies and lipase activity | / | High concentration of goji juice showed toxic effects and promoted premature aging in C. elegans. Thus, goji juice should be carefully consumed until further studies are conducted. | [102] |
| Black goji extract as source of natural colour | Dried black goji berries | / | 1. High content of petunidin derivatives, primarily cis and trans isomers of petunidin-3-p-coumaroyl-rutinoside-5-O-glucoside | Acylated petunidin anthocyanins are responsible for colour retention and improvement of colour intensity and stability. | Black goji anthocyanins produced various colour shades in broad ranges of pH. | [163] |
| Goji wine | Dried goji berries (mixed with water and decomposed with pectolase) | Saccharomyces cerevisiae | / | / | Ethyl carbamate was formed during the fermentation and storage processes of goji wine. | [160] |
| MEAT PRODUCTS | ||||||
| Minced catfish | Goji berry extract | / | / | High score for odour, texture, colour and overall quality of catfish minced blended with chitosan/goji berry extract, immediately after mixing and after 14 days of storage. | Chitosan/goji berry extract can be used as a biopreservative and anti-listerial agents (prevents the growth of Listeria monocytogenes), and also enhanced sensory properties and storage stability, when is mixed with catfish minced. | [164] |
| Beef burgers | Goji puree (0%; 2.5%; and 5%) | / | Burgers with goji: 1. Increased TPC 2. Improved antioxidant properties (DPPH•, ABTS•+ and ORAC) 3. Decreased lipid peroxidation | Burgers had acceptable appearance, odour, taste, flavour and texture for all groups of consumers (young, adult and elderly) | Burgers with goji had significantly higher TPC and antioxidant properties after in vitro digestion | [169] |
| Cooked sausages | Dried goji berries (0.5% and 1%) | / | / | The addition of 0.5% goji berries had the highest contribution to the preservation of bright red colour, fresh aroma and taste of functional cooked sausages | The addition of 0.5% and 1% of goji berries effectively inhibited protein oxidation, lipolysis, and lipid oxidation in functional cooked sausages | [167] |
| Rabbit meat | Rabbit feed was supplemented with 3% goji berries | / | / | Consumers gave a higher score for meatballs produced of meat of rabbits which were fed with goji berries dietary supplementation. These samples had more acceptable colour, juiciness, taste and overall liking. | Meat obtained of rabbits fed with goji berries dietary supplementation had reduced TBARS values and significant impact on Lactobacillus spp. prevalence. | [170] |
| Smoked common carp sausages | Goji berry extracts (1% and 2%) | / | / | 1. Sausages with goji berry extract had partial redness colour 2. The highest score of aroma and colour had sausages with 1% goji extract. | Sausages supplemented with goji extracts had decreased TBA values, TVB-N contents and total aerobic mesophilic bacteria during storage, in comparison to control sample (without goji). | [168] |
| Cooked and smoked horse meat product | Goji berry extract and goji berry extract/buckwheat flour | / | / | A high score for appearance, shear, colour, taste, odour and consistency were evaluated for a horsemeat product enriched with goji extract or goji/buckwheat mixture. | / | [165] |
| Rabbit meat | Rabbit feed was supplemented with 1% and 3% goji berries | / | Meat obtained of rabbits fed with goji berries dietary supplementation: 1. Increased TPC 2. Improved antioxidant properties (ORAC) | Goji berries dietary supplementation did not affect the colour, water holding capacity and tenderness of rabbit meat muscle. | Meat obtained of rabbits which were fed with 3% goji berries dietary supplementation showed an increase in oxidative stability. | [171] |
| Kazakh horse-meat product | Goji berry extract (0.5% and 1%) | / | A high score for surface colour, smell and taste were evaluated for a horsemeat product enriched with 0.5% and 1% goji extract. | Horse meat products with 1.0% of goji extract had improved oxidative stability. On the other hand, adding of goji berries had destructive effect on most meat fiber. | [166] | |
| BAKERY AND CONFECTIONERY PRODUCTS | ||||||
| Goji jam and jelly | Dehydrated goji berries | / | 1. High antioxidant activity (DPPH•) | Both goji products had high score for colour, appearance, consistency, flavour and sweet taste, however, for sour taste and aftertaste products had lower scores. | / | [172] |
| Muffins and spritz cookies | Whole goji berries (0% and 10%) Goji powder (0%, 3%, 5% and 10%) | / | / | 1. Pastry products with goji berries had a sour, slightly sweet and specific flavour. 2. Consumer’s preferred muffins with 10% whole goji and cookies with 5% goji powder. | / | [176] |
| Muffins and cookies | Goji berry by-products (0%, 10%, 20%, 30% and 40%) | / | Bakery products enriched with goji by-products: 1. Increased TPC 2. Increased insoluble and soluble fibre | 1. Increased goji by-products level decreased muffin firmness, that is, hardness and fracturability of cookies. 2. Muffins with 20% of goji by-products and cookies with 10% of goji by-products had the best sensory properties. | / | [175] |
| Prebiotic white chocolate | Dried goji berries (9% w/w) | / | / | 1. According to quantitative descriptive analysis, adding goji berries in chocolate reduced the perception of most aroma and flavour attributes, and improved the bitter taste, bitter aftertaste, astringency, and most of the texture attributes 2. Increased adherence, grittiness, hardness, astringency and goji berry aroma in comparison with control sample. 3. Chocolates enriched with goji berries had acceptance scores above 6 on a 9-point scale. | / | [178] |
| Rice flour based extrudates | Dry goji berries (0%, 13%, 23% and 28.5%) | / | Increasing goji berry level in rice flour based extrudates resulted in: 1. Increased TPC 2. Increased antioxidant activity (DPPH• and ABTS•+) 3. Increased rutin, zeaxanthin dipalmitate and AA-2βG content (content of listed compounds were higher in samples before extrusion) | / | / | [173] |
| Instant gruels | Dry goji berries (1%, 3% and 5%) | / | Increasing goji berry level in instant corn gruels resulted in: 1. Increased TPC 2. Increased antioxidant activity (TEAC and TLC-DPPH•) 3. Increased protocatehuic, 4-OH-benzoic, p-coumaric, ferulic, isoferulic and salicylic acid content | / | This study also monitored time (10 and 15 min) and rotation speed of the extruder screw (80 rpm, 100 rpm and 120 rpm) | [174] |
| Gluten-free bread | Dried goji berries (0%, 3%, 6%, 9%, 12% and 15%) | / | / | 1. Goji addition in bread, range 3–12% had no significant influence on bread volume, while addition of 15% caused reduction in volume 2. Goji addition in bread reduced lightness and increased redness of bread crumb 3. Increasing of goji in bread influenced on decreased hardness of bread crumb and increased elasticity 4. Increasing goji content from 3% to 6% in bread influenced on increased cohesion of the crumb | / | [177] |
| MILK PRODUCTS | ||||||
| Yoghurt | Dried goji berries (aqueous/ethanolic extract) (0.05%, 0.1% and 0.15% w/v) | Commercial yoghurt culture (yo-FAST-88), Hansen, Denmark | Increasing goji extract level in yoghurt resulted in: 1. Increased TPC 2. Increased antioxidant activity (DPPH•) | Increasing goji extract level in yogurt decreased consumer acceptability, with the same trend at the 1st day and after 20 days. | / | [179] |
| Yoghurt | Goji berries with/without honey (0%, 3%, 5% and 7%) | Starter mezophylic culture Lyofast Y450B (Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus (ratio 1:1)) | / | Consumers preferred yoghurt with 7% goji berries (8.21 points on hedonic scale) | Goji berries maintained viability of lactic acid bacteria in yoghurt during storage | [180] |
| Yoghurt | Dry and ground goji berries (2%, 4% and 6%) | Lactobacillus delbreukii ssp. bulgaricus, Streptococcus thermophilus | Increasing goji extract level in yoghurt resulted in: 1. Increased TPC 2. Increased antioxidant activity (DPPH•) | / | Total phenolic content and antioxidant activity of yogurt enriched with goji berries is continuously reduced after 3th, 7th and 14th days of storage. | [181] |
| Cheese | 1. Dried goji berries (3% water extract) 2. Goji extract/fish collagen | Lactic acid bacteria | 1. Cheese with goji extract showed decreased ACE inhibitory activity 2. Cheese enriched with goji extract and fish collagen had the most enhanced peptides production after 14th and 28th days of storage, and potential anti-ACE activity. | / | / | [182] |
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Souza, V.R.; Pereira, P.A.P.; da Silva, T.L.T.; de Oliveira Lima, L.C.; Pio, R.; Queiroz, F. Determination of the bioactive compounds, antioxidant activity and chemical composition of Brazilian blackberry, red raspberry, strawberry, blueberry and sweet cherry fruits. Food Chem. 2014, 156, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. Composition of sugars, organic acids, and total Phenolics in 25 wild or cultivated berry species. J. Food Sci. 2012, 77, C1064–C1070. [Google Scholar] [CrossRef] [PubMed]
- Golovinskaia, O.; Wang, C.K. Review of functional and pharmacological activities of berries. Molecules 2021, 26, 3904. [Google Scholar] [CrossRef] [PubMed]
- Salo, H.M.; Nguyen, N.; Alakärppä, E.; Klavins, L.; Hykkerud, A.L.; Karppinen, K.; Jaakola, L.; Klavins, M.; Häggman, H. Authentication of berries and berry-based food products. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5197–5225. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.K.; Alasalvar, C.; Shahidi, F. Superfruits: Phytochemicals, antioxidant efficacies, and health effects—A comprehensive review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1580–1604. [Google Scholar] [CrossRef] [PubMed]
- Lasekan, O. Exotic berries as a functional food. Curr. Opin. Clin. Nutr. Metab. Care. 2014, 17, 589–595. [Google Scholar] [CrossRef]
- Yao, R.; Heinrich, M.; Weckerle, C.S. The genus Lycium as food and medicine: A botanical, ethnobotanical and historical review. J. Ethnopharmacol. 2018, 212, 50–66. [Google Scholar] [CrossRef]
- Donno, D.; Beccaro, G.L.; Mellano, M.G.; Cerutti, A.K.; Bounous, G. Goji berry fruit (Lycium spp.): Antioxidant compound fingerprint and bioactivity evaluation. J. Funct. Foods 2015, 18, 1070–1085. [Google Scholar] [CrossRef]
- Potterat, O. Goji (Lycium barbarum and L. chinense): Phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity. Planta Med. 2010, 76, 7–19. [Google Scholar] [CrossRef]
- Yao, R.; Heinrich, M.; Zou, Y.; Reich, E.; Zhang, X.; Chen, Y.; Weckerle, C.S. Quality variation of goji (fruits of Lycium spp.) in China: A comparative morphological and metabolomic analysis. Front. Pharmacol. 2018, 9, 151. [Google Scholar] [CrossRef]
- Yao, R.; Heinrich, M.; Zhao, X.; Wang, Q.; Wei, J.; Xiao, P.G. What’s the choice for goji: Lycium barbarum L. or L. chinense Mill? J. Ethnopharmacol. 2021, 276, 114185. [Google Scholar] [CrossRef] [PubMed]
- Amagase, H.; Farnsworth, N.R. A review of botanical characteristics, phytochemistry, clinical relevance in efficacy and safety of Lycium barbarum fruit (Goji). Food Res. Int. 2011, 44, 1702–1717. [Google Scholar] [CrossRef]
- Pedro, A.C.; Sánchez-Mata, M.-C.; Pérez-Rodríguez, M.L.; Cámara, M.; López-Colón, J.L.; Bach, F.; Bellettini, M.; Haminiuk, C.W.I. Qualitative and nutritional comparison of goji berry fruits produced in organic and conventional systems. Sci. Hortic. 2019, 257, 108660. [Google Scholar] [CrossRef]
- Niro, S.; Fratianni, A.; Panfili, G.; Falasca, L.; Cinquanta, L.; Alam, M.R. Nutritional evaluation of fresh and dried goji berries cultivated in Italy. Ital. J. Food Sci. 2017, 29, 398–408. [Google Scholar]
- Benchennouf, A.; Grigorakis, S.; Loupassaki, S.; Kokkalou, E. Phytochemical analysis and antioxidant activity of Lycium barbarum (Goji) cultivated in Greece. Pharm. Biol. 2017, 55, 596–602. [Google Scholar] [CrossRef]
- Mocan, A.; Moldovan, C.; Zengin, G.; Bender, O.; Locatelli, M.; Simirgiotis, M.; Atalay, A.; Vodnar, D.C.; Rohn, S.; Crișan, G. UHPLC-QTOF-MS analysis of bioactive constituents from two Romanian Goji (Lycium barbarum L.) berries cultivars and their antioxidant, enzyme inhibitory, and real-time cytotoxicological evaluation. Food Chem. Toxicol. 2018, 115, 414–424. [Google Scholar] [CrossRef]
- Ruffo, M.; Parisi, O.I.; Amone, F.; Malivindi, R.; Gorgoglione, D.; De Biasio, F.; Scrivano, L.; Pezzi, V.; Puoci, F. Calabrian Goji vs. Chinese Goji: A comparative study on biological properties. Foods 2017, 6, 30. [Google Scholar] [CrossRef]
- Jiang, Y.; Fang, Z.; Leonard, W.; Zhang, P. Phenolic compounds in Lycium berry: Composition, health benefits and industrial applications. J. Funct. Foods 2021, 77, 104340. [Google Scholar] [CrossRef]
- Povolo, C.; Foschini, A.; Ribaudo, G. Optimization of the extraction of bioactive molecules from Lycium barbarum fruits and evaluation of the antioxidant activity: A combined study. Nat. Prod. Res. 2019, 33, 2694–2698. [Google Scholar] [CrossRef]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Santos-Buelga, C.; Ferreira, I.C.F.R. Phenolic compounds profile, nutritional compounds and bioactive properties of Lycium barbarum L.: A comparative study with stems and fruits. Ind. Crops Prod. 2018, 122, 574–581. [Google Scholar] [CrossRef]
- Kafkaletou, M.; Christopoulos, M.V.; Tsaniklidis, G.; Papadakis, I.; Ioannou, D.; Tzoutzoukou, C.; Tsantili, E. Nutritional value and consumer-perceived quality of fresh goji berries (Lycium barbarum L. and L. chinense L.) from plants cultivated in Southern Europe. Fruits 2018, 73, 5–12. [Google Scholar] [CrossRef]
- Skenderidis, P.; Kerasioti, E.; Karkanta, E.; Stagos, D.; Kouretas, D.; Petrotos, K.; Hadjichristodoulou, C.; Tsakalof, A. Assessment of the antioxidant and antimutagenic activity of extracts from goji berry of Greek cultivation. Toxicol. Rep. 2018, 5, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Dzhugalov, H.; Lichev, V.; Yordanov, A.; Kaymakanov, P.; Dimitrova, V.; Kutoranov, G. First results of testing Goji berry (Lycium barbarum L.) in Plovdiv region, Bulgaria. Sci. Pap. Ser. B Hortic. 2015, LIX, 47–50. [Google Scholar]
- Vulić, J.J.; Čanadanović-Brunet, J.M.; Ćetković, G.S.; Djilas, S.M.; Tumbas Šaponjac, V.T.; Stajčić, S.S. Bioactive compounds and antioxidant properties of goji fruits (Lycium barbarum L.) cultivated in Serbia. J. Am. Coll. Nutr. 2016, 35, 692–698. [Google Scholar] [CrossRef]
- Ilić, T.; Dodevska, M.; Marčetić, M.; Božić, D.; Kodranov, I.; Vidović, B. Chemical characterization, antioxidant and antimicrobial properties of goji berries cultivated in Serbia. Foods 2020, 9, 1614. [Google Scholar] [CrossRef] [PubMed]
- Covaci, E.; Senilă, M.; Leopold, L.F.; Olah, N.K.; Cobzac, C.; Petropulos, V.I.; Balabanova, B.; Cadar, O.; Becze, A.; Ponta, M.; et al. Characterization of Lycium barbarum L. berry cultivated in North Macedonia: A chemometric approach. J. Berry Res. 2020, 10, 223–241. [Google Scholar] [CrossRef]
- Kosinska-Cagnazzo, A.; Weber, B.; Chablais, R.; Vouillamoz, J.F.; Molnár, B.; Crovadore, J.; Lefort, F.; Andlauer, W. Bioactive compound profile and antioxidant activity of fruits from six goji cultivars cultivated in Switzerland. J. Berry Res. 2017, 7, 43–59. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Bąbelewski, P. Phenolic and carotenoid profile of new goji cultivars and their anti-hyperglycemic, anti-aging and antioxidant properties. J. Funct. Foods 2018, 48, 632–642. [Google Scholar] [CrossRef]
- Kulaitienė, J.; Vaitkevičienė, N.; Jarienė, E.; Černiauskienė, J.; Jeznach, M.; Paulauskienė, A. Concentrations of minerals, soluble solids, vitamin C, carotenoids and toxigenic elements in organic goji berries (Lycium barbarum L.) cultivated in Lithuania. Biol. Agric. Hortic. 2020, 36, 130–140. [Google Scholar] [CrossRef]
- Lopatriello, A.; Previtera, R.; Pace, S.; Werner, M.; Rubino, L.; Werz, O.; Taglialatela-Scafati, O.; Forino, M. NMR-based identification of the major bioactive molecules from an Italian cultivar of Lycium barbarum. Phytochemistry 2017, 144, 52–57. [Google Scholar] [CrossRef]
- Carnés, J.; de Larramendi, C.H.; López-Matas, M.A.; Ferrer, A.; Huertas, J. Allergenic sensitisation mediated by Wolfberry. In Lycium barbarum and Human Health, 1st ed.; Chang, R.C.-C., So, K.-F., Eds.; Springer: Dordrecht, The Netherlands; Berlin/Heidelberg, Germany; New York, NY, USA, 2015; pp. 179–198. [Google Scholar]
- Uasuf, C.G.; De Angelis, E.; Guagnano, R.; Pilolli, R.; D’Anna, C.; Villalta, D.; Brusca, I.; Monaci, L. Emerging allergens in goji berry superfruit: The identification of new IgE binding proteins towards allergic patients’ sera. Biomolecules 2020, 10, 689. [Google Scholar] [CrossRef] [PubMed]
- Montesano, D.; Rocchetti, G.; Cossignani, L.; Lucini, L.; Simonetti, M.S.; Blasi, F. Italian Lycium barbarum L. berry: Chemical characterization and nutraceutical value. Nat. Prod. Commun. 2018, 13, 1151–1156. [Google Scholar] [CrossRef]
- Zhao, D.; Li, S.; Han, X.; Li, C.; Ni, Y.; Hao, J. Physico-chemical properties and free amino acids profiles of six wolfberry cultivars in Zhongning. J. Food Compos. Anal. 2020, 88, 103460. [Google Scholar] [CrossRef]
- Bertoldi, D.; Cossignani, L.; Blasi, F.; Perini, M.; Barbero, A.; Pianezze, S.; Montesano, D. Characterisation and geographical traceability of Italian goji berries. Food Chem. 2019, 275, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Wojcieszek, J.; Kwiatkowski, P.; Ruzik, L. Speciation analysis and bioaccessibility evaluation of trace elements in goji berries (Lycium barbarum L.). J. Chromatogr. A 2017, 1492, 70–78. [Google Scholar] [CrossRef]
- Cossignani, L.; Blasi, F.; Simonetti, M.S.; Montesano, D. Fatty acids and phytosterols to discriminate geographic origin of Lycium barbarum berry. Food. Anal. Methods 2017, 11, 1180–1188. [Google Scholar] [CrossRef]
- Lu, Y.; Guo, S.; Zhang, F.; Yan, H.; Qian, D.W.; Shang, E.X.; Wang, H.Q.; Duan, J.A. Nutritional components characterization of goji berries from different regions in China. J. Pharm. Biomed. Anal. 2021, 195, 113859. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, W.; Li, X.; Xu, Y.; Cao, J.; Jiang, W. The anti-obesogenic effects of dietary berry fruits: A review. Food Res. Int. 2021, 147, 110539. [Google Scholar] [CrossRef]
- Zhu, W.; Zhou, S.; Liu, J.; McLean, R.J.C.; Chu, W. Prebiotic, immuno-stimulating and gut microbiota-modulating effects of Lycium barbarum polysaccharide. Biomed. Pharmacother. 2020, 121, 109591. [Google Scholar] [CrossRef]
- Ma, Z.F.; Zhang, H.; Teh, S.S.; Wang, C.W.; Zhang, Y.; Hayford, F.; Wang, L.; Ma, T.; Dong, Z.; Zhang, Y.; et al. Goji berries as a potential natural antioxidant medicine: An insight into their molecular mechanisms of action. Oxid. Med. Cell. Longev. 2019, 2019, 2437397. [Google Scholar] [CrossRef]
- Qian, D.; Zhao, Y.; Yang, G.; Huang, L. Systematic review of chemical constituents in the genus Lycium (Solanaceae). Molecules 2017, 22, 911. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Huang, Q.; Zhao, K.; Shang, P. Biological activities and potential health benefit effects of polysaccharides isolated from Lycium barbarum L. Int. J. Biol. Macromol. 2013, 54, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Redgwell, R.J.; Curti, D.; Wang, J.; Dobruchowska, J.M.; Gerwig, G.J.; Kamerling, J.P.; Bucheli, P. Cell wall polysaccharides of Chinese Wolfberry (Lycium barbarum): Part 1. Characterisation of soluble and insoluble polymer fractions. Carbohydr. Polym. 2011, 84, 1344–1349. [Google Scholar] [CrossRef]
- Wu, D.; Guo, H.; Lin, S.; Lam, S.C.; Zhao, L.; Lin, D.; Qin, W. Review of the structural characterization, quality evaluation, and industrial application of Lycium barbarum polysaccharides. Trends Food Sci. Technol. 2018, 79, 171–183. [Google Scholar] [CrossRef]
- Xie, J.; Wu, D.T.; Li, W.Z.; Ning, C.G.; Tang, Y.P.; Zhao, J.; Li, S.P. Effects of polysaccharides in Lycium barbarum berries from different regions of China on macrophages function and their correlation to the glycosidic linkages. J. Food Sci. 2017, 82, 2411–2420. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, H.; Dong, X.; Yang, S.; Ma, S.; Ni, J. Quality evaluation of Lycium barbarum (wolfberry) from different regions in China based on polysaccharide structure, yield and bioactivities. Chin. Med. 2019, 14, 49. [Google Scholar] [CrossRef]
- Masci, A.; Carradori, S.; Casadei, M.A.; Paolicelli, P.; Petralito, S.; Ragno, R.; Cesa, S. Lycium barbarum polysaccharides: Extraction, purification, structural characterisation and evidence about hypoglycaemic and hypolipidaemic effects. A review. Food Chem. 2018, 254, 377–389. [Google Scholar] [CrossRef]
- Wu, D.T.; Cheong, K.L.; Deng, Y.; Lin, P.C.; Wei, F.; Lv, X.J.; Long, Z.R.; Zhao, J.; Ma, S.C.; Li, S.P. Characterization and comparison of polysaccharides from Lycium barbarum in China using saccharide mapping based on PACE and HPTLC. Carbohydr. Polym. 2015, 134, 12–19. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, J.; Zhang, S. Study on the composition of Lycium barbarum polysaccharides and its effects on the growth of weanling mice. Wei Sheng Yan Jiu 2002, 31, 118–119. [Google Scholar]
- Zhou, Z.Q.; Xiao, J.; Fan, H.X.; Yu, Y.; He, R.R.; Feng, X.L.; Kurihara, H.; So, K.F.; Yao, X.S.; Gao, H. Polyphenols from wolfberry and their bioactivities. Food Chem. 2017, 214, 644–654. [Google Scholar] [CrossRef]
- Shah, T.; Bule, M.; Niaz, K. Goji Berry (Lycium barbarum)—A superfood. In Nonvitamin and Nonmineral Nutritional Supplements; Nabavi, S.M., Silva, A.S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 257–264. [Google Scholar]
- Chen, S.; Zhou, H.; Zhang, G.; Meng, J.; Deng, K.; Zhou, W.; Wang, H.; Wang, Z.; Hu, N.; Suo, Y. Anthocyanins from Lycium ruthenicum Murr. ameliorated D-galactose-induced memory impairment, oxidative stress, and neuroinflammation in adult rats. J. Agric. Food Chem. 2019, 67, 3140–3149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Chen, S.; Zhou, W.; Meng, J.; Deng, K.; Zhou, H.; Hu, N.; Suo, Y. Anthocyanin composition of fruit extracts from Lycium ruthenicum and their protective effect for gouty arthritis. Ind. Crop. Prod. 2019, 129, 414–423. [Google Scholar] [CrossRef]
- Luo, Y.; Fang, J.L.; Yuan, K.; Jin, S.H.; Guo, Y. Ameliorative effect of purified anthocyanin from Lycium ruthenicum on atherosclerosis in rats through synergistic modulation of the gut microbiota and NF-κB/SREBP-2 pathways. J. Funct. Foods 2019, 59, 223–233. [Google Scholar] [CrossRef]
- Yin, J.J.; Wu, T. Anthocyanins from black wolfberry (Lycium ruthenicum Murr.) prevent inflammation and increase fecal fatty acid in diet-induced obese rats. RSC Adv. 2017, 7, 47848–47853. [Google Scholar] [CrossRef]
- Gamage, G.C.V.; Lim, Y.Y.; Choo, W.S. Black goji berry anthocyanins: Extraction, stability, health benefits, and applications. ACS Agric. Sci. Technol. 2021, 8, 1360–1370. [Google Scholar]
- Nimalaratne, C.; Lopes-Lutz, D.; Schieber, A.; Wu, J. Effect of domestic cooking methods on egg yolk xanthophylls. J. Agric. Food Chem. 2012, 60, 12547–12552. [Google Scholar] [CrossRef]
- Toyoda-Ono, Y.; Maeda, M.; Nakao, M.; Yoshimura, M.; Sugiura-Tomimori, N.; Fukami, H. 2-O-(beta-D-glucopyranosyl) ascorbic acid, a novel ascorbic acid analogue isolated from Lycium fruit. J. Agric. Food Chem. 2004, 52, 2092–2096. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, W.; Zhao, J.; Xi, W. Functional constituents and antioxidant activities of eight Chinese native goji genotypes. Food Chem. 2016, 200, 230–236. [Google Scholar] [CrossRef]
- Skenderidis, P.; Lampakis, D.; Giavasis, I.; Leontopoulos, S.; Petrotos, K.; Hadjichristodoulou, C.; Tsakalof, A. Chemical properties, fatty-acid composition, and antioxidant activity of goji berry (Lycium barbarum L. and Lycium chinense Mill.) fruits. Antioxidants 2019, 8, 60. [Google Scholar] [CrossRef]
- Zhou, Y.; Qu, H.; Zhu, H.; Wang, Y.; Jiang, Y. Postharvest Handling of Fresh Goji Berries in Phytochemicals in Goji Berries, 1st ed.; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Islam, T.; Yu, X.; Singh Badwai, T.; Xu, B. Comparative studies on phenolic profiles, antioxidant capacities and carotenoid contents of red goji berry (Lycium barbarum) and black goji berry (Lycium ruthenicum). Chem. Cent. J. 2017, 11, 59. [Google Scholar] [CrossRef]
- Yossa Nzeuwa, I.B.; Guo, B.; Zhang, T.; Wang, L.; Ji, Q.; Xia, H.; Sun, G. Comparative metabolic profiling of Lycium fruits (Lycium barbarum and Lycium chinense) from different areas in China and from Nepal. J. Food Qual. 2019, 2019, 4396027. [Google Scholar] [CrossRef]
- Ozkan, E.E.; Ozden, T.Y.; Toplan, G.G.; Mat, A. Phenolic content and biological activities of Lycium barbarum L (Solanaceae) fruits (Goji berries) cultivated in Konya, Turkey. Trop. J. Pharm. Res. 2018, 17, 2047–2053. [Google Scholar] [CrossRef]
- Xin, G.; Zhu, F.; Du, B.; Xu, B. Antioxidants distribution in pulp and seeds of black and red goji berries as affected by boiling processing. J. Food Qual. 2017, 2017, 3145946. [Google Scholar] [CrossRef]
- Zhao, W.; Shi, Y. Comprehensive analysis of phenolic compounds in four varieties berries at different ripening stages by UPLC-MS/MS. J. Food Compost. Anal. 2021, 106, 104279. [Google Scholar] [CrossRef]
- Lu, Y.; Kong, X.; Zhang, J.; Guo, C.; Qu, Z.; Jin, L.; Wang, H. Composition changes in Lycium ruthenicum fruit dried by different methods. Front. Nutr. 2021, 8, 737521. [Google Scholar] [CrossRef]
- Wu, T.; Lv, H.; Wang, F.; Wang, Y. Characterization of polyphenols from Lycium ruthenicum fruit by UPLC-Q-TOF/MSE and their antioxidant activity in Caco 2 cells. J. Agric. Food Chem. 2016, 64, 2280–2288. [Google Scholar] [CrossRef]
- Liu, B.; Xu, Q.; Sun, Y. Black goji berry (Lycium ruthenicum) tea has higher phytochemical contents and in vitro antioxidant properties than red goji berry (Lycium barbarum) tea. Food Qual. Saf. 2020, 4, 193–201. [Google Scholar] [CrossRef]
- Kulczyński, B.; Gramza-Michałowska, A. Goji berry (Lycium barbarum): Composition and health effects—A review. Pol. J. Food Nutr. Sci. 2016, 66, 67–75. [Google Scholar] [CrossRef]
- Niki, E.; Noguchi, N. Evaluation of antioxidant capacity. What capacity is being measured by which method? IUBMB Life 2000, 50, 323–329. [Google Scholar] [CrossRef]
- Lin, C.L.; Wang, C.C.; Chang, S.C.; Inbaraj, B.S.; Chen, B.H. Antioxidative activity of polysaccharide fractions isolated from Lycium barbarum Linnaeus. Int. J. Biol. Macromol. 2009, 45, 146–151. [Google Scholar] [CrossRef]
- Wang, C.C.; Chang, S.C.; Inbaraj, B.S.; Chen, B.H. Isolation of carotenoids, flavonoids and polysaccharides from Lycium barbarum L. and evaluation of antioxidant activity. Food Chem. 2010, 120, 184–192. [Google Scholar] [CrossRef]
- Asker, M.M.S.; Mahmoud, M.G.; Ibrahim, G.S. Structural characterization and biological activity of acidic polysaccharide fractions isolated from Bacillus polymyxa NRC-A. Res. J. Appl. Sci. 2007, 3, 1170–1177. [Google Scholar]
- Bucheli, P.; Gao, Q.; Redgwell, R.; Karine, V.; Wang, J.; Zhang, W.; Nong, S.; Cao, B. Biomolecular and Clinical Aspects of Chinese Wolfberry. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Benzie, I., Wachtel, G.S., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 289–314. [Google Scholar]
- Zhang, Z.; Liu, X.; Zhang, X.; Liu, J.; Hao, Y.; Yang, X.; Wang, Y. Comparative evaluation of the antioxidant effects of the natural vitamin C analog 2-O-beta-D-glucopyranosyl-L-ascorbic acid isolated from goji berry fruit. Arch. Pharm Res. 2011, 34, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, B.; Wen, H.; Tao, Y.; Shao, Y. Phytochemical profiles, nutritional constituents and activity of black wolfberry (Lycium ruthenicum Murr.). Ind. Crops Prod. 2020, 154, 112692. [Google Scholar] [CrossRef]
- Hempel, J.; Schädle, C.N.; Sprenger, J.; Heller, A.; Carle, R.; Schweiggert, R.M. Ultrastructural deposition forms and bioaccessibility of carotenoids and carotenoid esters from goji berries (Lycium barbarum L.). Food Chem. 2017, 218, 525–533. [Google Scholar] [CrossRef]
- Trevithick-Sutton, C.C.; Foote, C.S.; Collins, M.; Trevithick, J.R. The retinal carotenoids zeaxanthin and lutein scavenge superoxide and hydroxyl radicals: A chemiluminescence and ESR study. Mol. Vis. 2006, 12, 1127–1135. [Google Scholar] [PubMed]
- Cheng, C.Y.; Chung, W.Y.; Szeto, Y.T.; Benzie, I.F. Fasting plasma zeaxanthin response to Fructus barbarum L. (wolfberry; Kei Tze) in a food-based human supplementation trial. Br. J. Nutr. 2005, 93, 123–130. [Google Scholar] [CrossRef]
- Amagase, H.; Nance, D.M. A randomized, double-blind, placebo-controlled, clinical study of the general effects of a standardized Lycium barbarum (Goji) juice, GoChi. J. Altern Complement. Med. 2008, 14, 403–412. [Google Scholar] [CrossRef]
- Amagase, H.; Sun, B.; Borek, C. Lycium barbarum (goji) juice improves in vivo antioxidant biomarkers in serum of healthy adults. Nutr. Res. 2009, 29, 19–25. [Google Scholar] [CrossRef]
- Yu, D.H.; Wu, J.M.; Niu, A.J. Health-promoting effect of LBP and healthy Qigong exercise on physiological functions in old subjects. Carbohydr. Polym. 2009, 75, 312–316. [Google Scholar] [CrossRef]
- Amagase, H.; Sun, B.; Nance, D.M. Immunomodulatory effects of a standardized Lycium barbarum fruit juice in Chinese older healthy human subjects. J. Med. Food. 2009, 12, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Amagase, H.; Nance, D.M. Lycium barbarum increases caloric expenditure and decreases waist circumference in healthy overweight men and women: Pilot study. J. Am. Coll. Nutr. 2011, 30, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Bucheli, P.; Vidal, K.; Shen, L.; Gu, Z.; Zhang, C.; Miller, L.E.; Wang, J. Goji berry effects on macular characteristics and plasma antioxidant levels. Optom. Vis. Sci. 2011, 88, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Vidal, K.; Bucheli, P.; Gao, Q.; Moulin, J.; Shen, L.S.; Wang, J.; Blum, S.; Benyacoub, J. Immunomodulatory effects of dietary supplementation with a milk-based wolfberry formulation in healthy elderly: A randomized, double-blind, placebo-controlled trial. Rejuvenation Res. 2012, 15, 89–97. [Google Scholar] [CrossRef]
- Cai, H.; Liu, F.; Zuo, P.; Huang, G.; Song, Z.; Wang, T.; Lu, H.; Guo, F.; Han, C.; Sun, G. Practical application of antidiabetic efficacy of Lycium barbarum polysaccharide in patients with type 2 diabetes. Med. Chem. 2015, 11, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Ahn, Y.; Kwon, O.; Lee, M.Y.; Lee, C.H.; Lee, S.; Park, T.; Kwon, S.W.; Kim, J.Y. Dietary wolfberry extract modifies oxidative stress by controlling the expression of inflammatory mRNAs in overweight and hypercholesterolemic subjects: A randomized, double-blind, placebo-controlled trial. J. Agric. Food Chem. 2017, 65, 309–316. [Google Scholar] [CrossRef]
- De Souza Zanchet, M.Z.; Nardi, G.M.; de Oliveira Souza Bratti, L.; Filippin-Monteiro, F.B.; Locatelli, C. Lycium barbarum reduces abdominal fat and improves lipid profile and antioxidant status in patients with metabolic syndrome. Oxid. Med. Cell Longev. 2017, 2017, 9763210. [Google Scholar] [CrossRef]
- Chan, H.H.; Lam, H.I.; Choi, K.Y.; Li, S.Z.; Lakshmanan, Y.; Yu, W.Y.; Chang, R.C.; Lai, J.S.; So, K.F. Delay of cone degeneration in retinitis pigmentosa using a 12-month treatment with Lycium barbarum supplement. J. Ethnopharmacol. 2019, 236, 336–344. [Google Scholar] [CrossRef]
- Van den Driessche, J.J.; Plat, J.; Plasqui, G.; Mensink, R.P. A single dose of goji berries does not affect postprandial energy expenditure and substrate oxidation in healthy, overweight men. J. Nutr. Metab. 2019, 2019, 4057143. [Google Scholar] [CrossRef]
- Toh, D.W.K.; Xia, X.; Sutanto, C.N.; Low, J.H.M.; Poh, K.K.; Wang, J.W.; Foo, R.S.; Kim, J.E. Enhancing the cardiovascular protective effects of a healthy dietary pattern with wolfberry (Lycium barbarum): A randomized controlled trial. Am. J. Clin. Nutr. 2021, 114, 80–89. [Google Scholar] [CrossRef]
- Toh, D.; Lee, W.Y.; Zhou, H.; Sutanto, C.N.; Lee, D.; Tan, D.; Kim, J.E. Wolfberry (Lycium barbarum) consumption with a healthy dietary pattern lowers oxidative stress in middle-aged and older adults: A randomized controlled trial. Antioxidants 2021, 10, 567. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Holt, R.R.; Keen, C.L.; Morse, L.S.; Yiu, G.; Hackman, R.M. Goji berry intake increases macular pigment optical density in healthy adults: A randomized pilot trial. Nutrients 2021, 13, 4409. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.F.; Li, Z.H.; Cai, H.; Li, D. The effects of Lycium barbarum L. (L. barbarum) on cardiometabolic risk factors: A meta-analysis of randomized controlled trials. Food Funct. 2017, 8, 1741–1748. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Chung, W.Y.; Wang, J.; Richelle, M.; Bucheli, P. Enhanced bioavailability of zeaxanthin in a milk-based formulation of wolfberry (Gou Qi Zi; Fructus barbarum L.). Br. J. Nutr. 2006, 96, 154–160. [Google Scholar] [CrossRef]
- Neelam, K.; Dey, S.; Sim, R.; Lee, J.; Au Eong, K.G. Fructus lycii: A natural dietary supplement for amelioration of retinal diseases. Nutrients 2021, 13, 246. [Google Scholar] [CrossRef]
- Toh, D.W.K.; Low, J.H.M.; Kim, J.E. Cardiovascular disease risk reduction with wolfberry consumption: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Nutr. 2021. [Google Scholar] [CrossRef]
- Amagase, H. General toxicity and histological analysis from acute toxicological study of a standardized Lycium barbarum (Goji) juice (GoChiTM) in rodents. FASEB J. 2008, 22, 722. [Google Scholar] [CrossRef]
- De Freitas Rodrigues, C.; Ramos Boldori, J.; Valandro Soares, M.; Somacal, S.; Emanuelli, T.; Izaguirry, A.; Weber Santos Cibin, F.; Rossini Augusti, P.; Casagrande Denardin, C. Goji berry (Lycium barbarum L.) juice reduces lifespan and premature aging of Caenorhabditis elegans: Is it safe to consume it? Food Res. Int. 2021, 144, 110297. [Google Scholar] [CrossRef]
- Arroyo-Martinez, Q.; Sáenz, M.J.; Argüelles Arias, F.; Acosta, M.S. Lycium barbarum: A new hepatotoxic “natural” agent? Dig. Liver Dis. 2011, 43, 749. [Google Scholar] [CrossRef]
- Karaman, K.; Yılmaz, Y.D.; Özlüer, Y.E.; Çanakçı, S.E.; Deniz, A.T. A new hepatotoxic agent: Goji berry. J. Emerg. Med. Case Rep. 2019, 10, 105–107. [Google Scholar]
- Jeszka-Skowron, M.; Zgoła-Grześkowiak, A.; Stanisz, E.; Waśkiewicz, A. Potential health benefits and quality of dried fruits: Goji fruits, cranberries and raisins. Food Chem. 2017, 221, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qin, J.; Wang, Y.; Zhou, T.; Feng, N.; Ma, C.; Zhu, M. Levels and health risk assessment of pesticides and metals in Lycium barbarum L. from different sources in Ningxia, China. Sci. Rep. 2022, 12, 561. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Luo, X.; Han, J.; Chen, Y.; Zhang, K.; Hu, D. Residual determination of pyrethrins in Lycium barbarum (goji) by GC-MS/MS and a dietary risk assessment of Chinese goji consumption. Food Addit. Contam. Part A 2020, 37, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Bernal, S.; Rodríguez-Pazos, L.; Martínez, F.J.; Ginarte, M.; Rodríguez-Granados, M.T.; Toribio, J. Systemic photosensitivity due to Goji berries. Photodermatol. Photoimmunol. Photomed. 2011, 27, 245–247. [Google Scholar] [CrossRef]
- Larramendi, C.H.; García-Abujeta, J.L.; Vicario, S.; García-Endrino, A.; López-Matas, M.A.; García-Sedeño, M.D.; Carnés, J. Goji berries (Lycium barbarum): Risk of allergic reactions in individuals with food allergy. J. Investig. Allergol. Clin. Immunol. 2012, 22, 345–350. [Google Scholar]
- Monzón Ballarín, S.; López-Matas, M.A.; Sáenz Abad, D.; Pérez-Cinto, N.; Carnés, J. Anaphylaxis associated with the ingestion of Goji berries (Lycium barbarum). J. Investig. Allergol. Clin. Immunol. 2011, 21, 567–570. [Google Scholar]
- Zauli, D.; Mirarchi, M.G. Anaphylaxis induced by Goji berries. Ann. Allergy Asthma Immunol. 2015, 114, 535–536. [Google Scholar] [CrossRef]
- Carnés, J.; de Larramendi, C.H.; Ferrer, A.; Huertas, A.J.; López-Matas, M.A.; Pagán, J.A.; Navarro, L.A.; García-Abujeta, J.L.; Vicario, S.; Peña, M. Recently introduced foods as new allergenic sources: Sensitisation to Goji berries (Lycium barbarum). Food Chem. 2013, 137, 130–135. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, L.; Xie, B. Bleeding due to a probable interaction between warfarin and Gouqizi (Lycium Barbarum L.). Toxicol. Rep. 2015, 2, 1209–1212. [Google Scholar] [CrossRef]
- Guzmán, C.E.; Guzmán-Moreno, C.G.; Assad-Morell, J.L.; Carrizales-Sepúlveda, E.F. Flecainide toxicity associated with the use of goji berries: A case report. Eur. Heart J. Case Rep. 2021, 5, ytab204. [Google Scholar] [CrossRef]
- Adiletta, G.; Alam, M.; Cinquanta, L.; Russo, P.; Albanese, D.; Matteo, M. Effect of abrasive pretreatment on hot dried goji berry. Chem. Eng. Trans. 2015, 44, 127–132. [Google Scholar]
- Deng, L.Z.; Mujumdar, A.S.; Zhang, Q.; Yang, X.H.; Wang, J.; Zheng, Z.A.; Gao, Z.J.; Xiao, H.W. Chemical and physical pretreatments of fruits and vegetables: Effects on drying characteristics and quality attributes—A comprehensive review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1408–1432. [Google Scholar] [CrossRef] [PubMed]
- Fratianni, A.; Niro, S.; Alam, M.; Cinquanta, L.; Matteo, M.; Adiletta, G.; Panfili, G. Effect of a physical pre-treatment and drying on carotenoids of goji berries (Lycium barbarum L.). LWT 2018, 92, 318–323. [Google Scholar] [CrossRef]
- Ni, J.; Ding, C.; Zhang, Y.; Song, Z. Impact of different pretreatment methods on drying characteristics and microstructure of goji berry under electrohydrodynamic (EHD) drying process. Innov. Food Sci. Emerg. Technol. 2020, 61, 102318. [Google Scholar] [CrossRef]
- Russo, P.; Adiletta, G.; Matteo, M.; Senadeera, W.; Cinquanta, L. The effect of abrasive pre-treatment on the drying kinetics and phenolic compounds in goji berries (Lycium barbarum L.). J. Food Process. Preserv. 2020, 44, e14933. [Google Scholar] [CrossRef]
- Zhao, D.; Wei, J.; Hao, J.; Han, X.; Ding, S.; Yang, L.; Zhang, Z. Effect of sodium carbonate solution pretreatment on drying kinetics, antioxidant capacity changes, and final quality of wolfberry (Lycium barbarum) during drying. LWT 2018, 99, 254–261. [Google Scholar] [CrossRef]
- Song, H.; Bi, J.; Chen, Q.; Zhou, M.; Wu, X.; Song, J. Structural and health functionality of dried goji berries as affected by coupled dewaxing pre-treatment and hybrid drying methods. Int. J. Food Prop. 2018, 21, 2527–2538. [Google Scholar] [CrossRef]
- Dermesonlouoglou, E.; Chalkia, A.; Dimopoulos, G.; Taoukis, P. Combined effect of pulsed electric field and osmotic dehydration pre-treatments on mass transfer and quality of air dried goji berry. Innov. Food Sci. Emerg. Technol. 2018, 49, 106–115. [Google Scholar] [CrossRef]
- Dermesonlouoglou, E.; Chalkia, A.; Taoukis, P. Application of osmotic dehydration to improve the quality of dried goji berry. J. Food Eng. 2018, 232, 36–43. [Google Scholar] [CrossRef]
- Donno, D.; Mellano, M.G.; Raimondo, E.; Cerutti, A.K.; Prgomet, Z.; Beccaro, G.L. Influence of applied drying methods on phytochemical composition in fresh and dried goji fruits by HPLC fingerprint. Eur. Food Res. Technol. 2016, 242, 1961–1974. [Google Scholar] [CrossRef]
- Rodrigues Sá, R.; da Cruz Caldas, J.; de Andrade Santana, D.; Vieira Lopes, M.; dos Santos, W.N.L.; Graças Andrade Korn, M.; de Freitas Santos Júnior, A. Multielementar/centesimal composition and determination of bioactive phenolics in dried fruits and capsules containing Goji berries (Lycium barbarum L.). Food Chem. 2019, 273, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Luo, W.; Sun, D.W. Effects of liquid nitrogen quick freezing on polyphenol oxidase and peroxide activities, cell water states and epidermal microstructure of wolfberry. LWT 2020, 120, 108923. [Google Scholar] [CrossRef]
- Donno, D.; Mellano, M.G.; Riondato, I.; Biaggi, M.; Andriamaniraka, H.; Gamba, G.; Beccaro, G. Traditional and unconventional dried fruit snacks as a source of health-promoting compounds. Antioxidants 2019, 8, 396. [Google Scholar] [CrossRef] [PubMed]
- Rybicka, I.; Kiewlicz, J.; Kowalczewski, P.Ł.; Gliszczyńska-Świgło, A. Selected dried fruits as a source of nutrients. Eur. Food Res. Technol. 2021, 247, 2409–2419. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Vidyarthi, S.K.; Zhong, C.S.; Zheng, Z.A.; An, Y.; Wang, J.; Wei, Q.; Xiao, H.-W. Cold plasma enhances drying and colour, rehydration ratio and polyphenols of wolfberry via microstructure and ultrastructure alteration. LWT 2020, 134, 110173. [Google Scholar] [CrossRef]
- Huang, D.; Yang, P.; Tang, X.; Luo, L.; Sunden, B. Application of infrared radiation in the drying of food products. Trends Food Sci. Technol. 2021, 110, 765–777. [Google Scholar] [CrossRef]
- Xie, L.; Mujumdar, A.S.; Fang, X.-M.; Wang, J.; Dai, J.-W.; Du, Z.-L.; Xiao, H.-W.; Liu, Y.; Gao, Z.-J. Far-infrared radiation heating assisted pulsed vacuum drying (FIR-PVD) of wolfberry (Lycium barbarum L.): Effects on drying kinetics and quality attributes. Food Bioprod. Process. 2017, 102, 320–331. [Google Scholar] [CrossRef]
- Zhang, W.-P.; Chen, C.; Pan, Z.; Xiao, H.-W.; Xie, L.; Gao, Z.-J.; Zheng, Z.-A. Design and performance evaluation of a pilot-scale pulsed vacuum infrared drying (PVID) system for drying of berries. Dry. Technol. 2020, 38, 1340–1355. [Google Scholar] [CrossRef]
- Yu, F.; Li, Y.; Wu, Z.; Wan, N.; Yang, M. Dehydration of wolfberry fruit using pulsed vacuum drying combined with carboxymethyl cellulose coating pretreatment. LWT 2020, 134, 110159. [Google Scholar] [CrossRef]
- Qi, Y.; Yu, F.; Wang, X.; Wan, N.; Yang, M.; Wu, Z.; Li, Y. Drying of wolfberry fruit juice using low-intensity pulsed ultrasound. LWT 2021, 141, 110953. [Google Scholar] [CrossRef]
- Skenderidis, P.; Petrotos, K.; Giavasis, I.; Hadjichristodoulou, C.; Tsakalof, A. Optimization of ultrasound assisted extraction of of goji berry (Lycium barbarum) fruits and evaluation of extracts’ bioactivity. J. Food Process. Eng. 2017, 40, 12522. [Google Scholar] [CrossRef]
- Vilkhu, K.; Mawson, R.; Simons, L.; Bates, D. Applications and opportunities for ultrasound assisted extraction in the food industry—A review. Innov. Food Sci. Emerg. Technol. 2008, 9, 161–169. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Zhang, H.; Duan, Y.; Ma, H. Recent advances in the extraction of bioactive compounds with subcritical water: A review. Trends Food Sci. Technol. 2020, 95, 183–195. [Google Scholar] [CrossRef]
- De Moura, C.; dos Reis, A.S.; da Silva, L.D.; de Lima, V.A.; Oldoni, T.L.C.; Pereira, C.; Carpes, S.T. Optimization of phenolic compounds extraction with antioxidant activity from açaí, blueberry and goji berry using response surface methodology. Emir. J. Food Agric. 2018, 30, 180–189. [Google Scholar]
- Pedro, A.C.; Maurer, J.B.B.; Zawadzki-Baggio, S.F.; Ávila, S.; Maciel, G.M.; Haminiuk, C.W.I. Bioactive compounds of organic goji berry (Lycium barbarum L.) prevent oxidative deterioration of soybean oil. Ind. Crops Prod. 2018, 112, 90–97. [Google Scholar] [CrossRef]
- Zhou, S.; Rahman, A.; Li, J.; Wei, C.; Chen, J.; Linhardt, R.J.; Ye, X.; Chen, S. Extraction methods affect the structure of Goji (Lycium barbarum) polysaccharides. Molecules 2020, 25, 936. [Google Scholar] [CrossRef]
- Ahmadi, S.; Yu, C.; Zaeim, D.; Wu, D.; Hu, X.; Ye, X.; Chen, S. Increasing RG-I content and lipase inhibitory activity of pectic polysaccharides extracted from goji berry and raspberry by high-pressure processing. Food Hydrocolloid. 2021, 126, 107477. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, X.; Ji, T.; Wen, C.; Ye, Z.; Liu, X.; Liang, L.; Liu, G.; Xu, X. Digestion and absorption properties of Lycium barbarum polysaccharides stabilized selenium nanoparticles. Food Chem. 2022, 373, 131637. [Google Scholar] [CrossRef]
- He, Z.; Ma, T.; Zhang, W.; Su, E.; Cao, F.; Huang, M.; Wang, Y. Heat-induced gel formation by whey protein isolate-Lycium barbarum polysaccharides at varying pHs. Food Hydrocoll. 2021, 115, 106607. [Google Scholar] [CrossRef]
- Wang, H.; Ke, L.; Ding, Y.; Rao, P.; Xu, T.; Han, H.; Zhou, J.; Ding, W.; Shang, X. Effect of calcium ions on rheological properties and structure of Lycium barbarum L. polysaccharide and its gelation mechanism. Food Hydrocoll. 2022, 122, 107079. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.; Cui, B.; Wang, M.; Fu, H.; Wang, Y. Carotenoid-enriched oil preparation and stability analysis during storage: Influence of oils chain length and fatty acid saturation. LWT 2021, 151, 112163. [Google Scholar] [CrossRef]
- Kan, X.; Yan, Y.; Ran, L.; Lu, L.; Mi, J.; Zhang, Z.; Li, X.; Zeng, X.; Cao, Y. Evaluation of bioaccessibility of zeaxanthin dipalmitate from the fruits of Lycium barbarum in oil-in-water emulsions. Food Hydrocoll. 2020, 105, 105781. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, Y.; Guo, H.; Fu, H. Evaluation of the bioaccessibility of carotenoid esters from Lycium barbarum L. in nano-emulsions: A kinetic approach. Food Res. Int. 2020, 136, 109611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jia, G.; Wanbin, Z.; Minghao, J.; Wei, Y.; Hao, J.; Liu, X.; Gan, Z.; Sun, A. Nanoencapsulation of zeaxanthin extracted from Lycium barbarum L. by complex coacervation with gelatin and CMC. Food Hydrocoll. 2021, 112, 106280. [Google Scholar] [CrossRef]
- De Campo, C.; Dick, M.; dos Santos, P.P.; Costa, T.M.H.; Paese, K.; Guterres, S.S.; de Oliveira Rios, A.; Flores, S.H. Zeaxanthin nanoencapsulation with Opuntia monacantha mucilage as structuring material: Characterization and stability evaluation under different temperatures. Coll. Surf. A Physicochem. Eng. 2018, 558, 410–421. [Google Scholar] [CrossRef]
- Zu, M.; Song, H.; Zhang, J.; Chen, Q.; Deng, S.; Canup, B.S.; Yuan, Y.; Xiao, B. Lycium barbarum lipid-based edible nanoparticles protect against experimental colitis. Coll. Surf. B 2020, 187, 110747. [Google Scholar] [CrossRef] [PubMed]
- Păvăloiu, R.D.; Sha’at, F.; Neagu, G.; Deaconu, M.; Bubueanu, C.; Albulescu, A.; Sha’at, M.; Hlevca, C. Encapsulation of polyphenols from Lycium barbarum leaves into liposomes as a strategy to improve their delivery. Nanomaterials 2021, 11, 1938. [Google Scholar] [CrossRef] [PubMed]
- Skenderidis, P.; Mitsagga, C.; Lampakis, D.; Petrotos, K.; Giavasis, I. The Effect of Encapsulated Powder of Goji Berry (Lycium barbarum) on Growth and Survival of Probiotic Bacteria. Microorganisms 2019, 8, 57. [Google Scholar] [CrossRef]
- Skenderidis, P.; Mitsagga, C.; Giavasis, I.; Petrotos, K.; Lampakis, D.; Leontopoulos, S.; Hadjichristodoulou, C.; Tsakalof, A. The in vitro antimicrobial activity assessment of ultrasound assisted Lycium barbarum fruit extracts and pomegranate fruit peels. J. Food Meas. Charact. 2019, 13, 2017–2031. [Google Scholar] [CrossRef]
- Llorent-Martínez, E.J.; Fernández-de Córdova, M.L.; Ortega-Barrales, P.; Ruiz-Medina, A. Characterization and comparison of the chemical composition of exotic superfoods. Microchem. J. 2013, 110, 444–451. [Google Scholar] [CrossRef]
- Feng, L.; Tang, N.; Liu, R.; Nie, R.; Guo, Y.; Liu, R.; Chang, M. Effects of different processing methods on bioactive substances and antioxidation properties of Lycium barbarum (goji berry) from China. Food Biosci. 2021, 42, 101048. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, H.; Liu, H.; Ma, R.; Ma, J.; Fang, H. Fermentation by multiple bacterial strains improves the production of bioactive compounds and antioxidant activity of goji juice. Molecules 2019, 24, 3519. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fang, H.; Liu, H.; Cheng, H.; Pan, L.; Hu, M.; Li, X. Goji berry juice fermented by probiotics attenuates dextran sodium sulfate-induced ulcerative colitis in mice. J. Funct. Foods 2021, 83, 104491. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, P.; Laaksonen, O.; Wei, B.; Zhu, Y.; Zhang, B.; Zhi, B.; Li, H. Lactic acid bacteria incubation and aging drives flavor enhancement of goji berry juice. J. Food Compost. Anal. 2022, 105, 104202. [Google Scholar] [CrossRef]
- Niu, M.; Huang, J.; Jin, Y.; Wu, C.; Zhou, R. Volatiles and antioxidant activity of fermented Goji (Lycium chinese) wine: Effect of different oak matrix (barrel, shavings and chips). Int. J. Food Prop. 2017, 20, 1–13. [Google Scholar] [CrossRef]
- Xia, Q.; Niu, M.; Wu, C.; Zhou, R. Formation of ethyl carbamate in goji wines: Effect of goji fruit composition. Food Sci. Biotechnol. 2016, 25, 921–927. [Google Scholar] [CrossRef]
- Abuduaibifu, A.; Tamer, C.E. Evaluation of physicochemical and bioaccessibility properties of goji berry kombucha. J. Food Process Preserv. 2019, 43, e14077. [Google Scholar] [CrossRef]
- Ducruet, J.; Rébénaque, P.; Diserens, S.; Kosińska-Cagnazzo, A.; Héritier, I.; Andlauer, W. Amber ale beer enriched with goji berries—The effect on bioactive compound content and sensorial properties. Food Chem. 2017, 226, 109–118. [Google Scholar] [CrossRef]
- Tang, P.; Giusti, M. Black goji as a potential source of natural colour in a wide pH range. Food Chem. 2018, 269, 419–426. [Google Scholar] [CrossRef]
- Alsaggaf, M.S.; Moussa, S.H.; Elguindy, N.M.; Tayel, A.A. Fungal chitosan and Lycium barbarum extract as anti-listeria and quality preservatives in minced catfish. Int. J. Biol. Macromol. 2017, 104, 854–861. [Google Scholar] [CrossRef]
- Kaldarbekova, M.; Uzakov, Y.; Chernukha, I.; Kurmanbekova, A.; Jetpisbayeva, B. Dtudying the effect of multicomponent pickle on the quality of cooked and smoked horse meat product. Period. Tche. Quim. 2019, 16, 259–265. [Google Scholar]
- Uzakov, Y.; Kaldarbekova, M.; Kuznetsova, O. Improved technology for new-generationKazak h national meat products. Foods Raw Mater. 2020, 8, 76–83. [Google Scholar] [CrossRef]
- Bulambaeva, A.A.; Vlahova-Vangelova, D.; Dragoev, S.; Balev, D.; Uzakov, Y.M. Development of new functional cooked sausages by addition of goji berry and pumpkin powder. Am. J. Food Technol. 2014, 9, 180–189. [Google Scholar] [CrossRef][Green Version]
- Fadıloglu, E.; Çoban, M. The effects of goji berry (Lycium barbarum L.) extract on some chemical, microbiological and sensory characteristics of liquid smoked common carp (Cyprinus carpio L., 1758) sausages. Yuz. Yil Univ. J. Agric. Sci. 2019, 29, 702–710. [Google Scholar]
- Antonini, E.; Torri, L.; Piochi, M.; Cabrino, G.; Meli, M.A.; De Bellis, R. Nutritional, antioxidant and sensory properties of functional beef burgers formulated with chia seeds and goji puree, before and after in vitro digestion. Meat Sci. 2020, 161, 108021. [Google Scholar] [CrossRef] [PubMed]
- Castrica, M.; Menchetti, L.; Balzaretti, C.M.; Branciari, R.; Ranucci, D.; Cotozzolo, E.; Vigo, D.; Curone, G.; Brecchia, G.; Miraglia, D. Impact of dietary supplementation with goji berries (Lycium barbarum) on microbiological quality, physico-chemical, and sensory characteristics of rabbit meat. Foods 2020, 9, 1480. [Google Scholar] [CrossRef]
- Menchetti, L.; Brecchia, G.; Branciari, R.; Barbato, O.; Fioretti, B.; Codini, M.; Bellezza, E.; Trabalza-Marinucci, M.; Miraglia, D. The effect of Goji berries (Lycium barbarum) dietary supplementation on rabbit meat quality. Meat Sci. 2020, 161, 108018. [Google Scholar] [CrossRef]
- Istrati, D.; Vizireanu, C.; Iordachescu, G.; Dima, F.; Garnai, M. Physico-chemical characteristics and antioxidant activity of goji fruits jam and jelly during storage. Ann. Univ. Dunarea Jos Galati Fascicle VI—Food Technol. 2013, 37, 100–110. [Google Scholar]
- Kosińska-Cagnazzo, A.; Bocquel, D.; Marmillod, I.; Andlauer, W. Stability of goji bioactives during extrusion cooking process. Food Chem. 2017, 230, 250–256. [Google Scholar] [CrossRef]
- Olech, M.; Kasprzak, K.; Wójtowicz, A.; Oniszczuk, T.; Nowak, R.; Waksmundzka-Hajnos, M.; Combrzyński, M.; Gancarz, M.; Kowalska, I.; Krajewska, A.; et al. Polyphenol composition and antioxidant potential of instant gruels enriched with Lycium barbarum L. fruit. Molecules 2020, 25, 4538. [Google Scholar] [CrossRef]
- Bora, P.; Ragaee, S.; Abdel-Aal, E.S.M. Effect of incorporation of goji berry by-product on biochemical, physical and sensory properties of selected bakery products. LWT 2019, 112, 108225. [Google Scholar] [CrossRef]
- Pop, A.; Muste, S.; Man, S.; Crina, M. Study on the valorification of Lycium barbarum fruit (goji) in pastry products. Bull. Univ. Agric. Sci. Vet. Med. 2013, 70, 93–98. [Google Scholar] [CrossRef][Green Version]
- Ziemichód, A.; Różyło, R. Effect of the addition of goji berries on the physical properties of gluten-free bread. Acta Agroph. 2018, 25, 117–127. [Google Scholar] [CrossRef]
- Morais Ferreira, J.M.; Azevedo, B.M.; Luccas, V.; Bolini, H.M. Sensory profile and consumer acceptability of prebiotic white chocolate with sucrose substitutes and the addition of goji berry (Lycium barbarum). J. Food Sci. 2017, 82, 818–824. [Google Scholar] [CrossRef]
- Maurya, V.; Aggarwal, M. Impact of aqueous/ethanolic goji berry (Lycium barbarum) fruit extract supplementation on vitamin D stability in yoghurt. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2016–2029. [Google Scholar] [CrossRef]
- Rotar, A.; Vodnar, D.; Bunghez, F.; Catunescu, G.; Pop, C.; Jimborean, M.; Semeniuc, C. Effect of goji berries and honey on lactic acid bacteria viability and shelf life stability of yoghurt. Not. Bot. Horti Agrobot. Cluj Napoca 2015, 43, 196–203. [Google Scholar] [CrossRef]
- Taneva, I.; Zlatev, Z. Total phenolic content and antioxidant activity of yoghurt with goji berries (Lycium barbarum). Sci. Study Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2020, 21, 125–131. [Google Scholar]
- Shori, A.B.; Ling, Y.; Hj Baba, A.S. Effects of Lycium barbarum and fish collagen in cheese on the proteolytic degradation profile with anti-ACE activity. J. Food Process. Preserv. 2021, 45, e15239. [Google Scholar] [CrossRef]
| TPC (mg/g) | TFC (mg/g) | TCC (mg/g) | LBP (mg/g) | Reference |
|---|---|---|---|---|
| L. barbarum | ||||
| 2.56–2.82 | - | 5.7 | - | [8] |
| 11.6–15.7 | - | - | - | [16] |
| 4.0–13.0 | - | 4.0–9.5 | - | [21] |
| 7.17 | 2.37 | 0.43 | - | [24] |
| 1.62 | 2.14 | 0.42 | - | [25] |
| 3.89–8.20 | - | 2.9 | - | [26] |
| 0.71–2.94 | - | - | - | [27] |
| 0.25–1.93 | - | 0.66–4.13 | - | [28] |
| 7.6 | - | - | - | [33] |
| 6.9–8.25 | 3.18–6.14 | 12.93–25.35 | 23.62–42.45 | [38] |
| 16–48 | [47] | |||
| 30.3–73.4 | 38.5–54.7 | 3.64–11.33 | 55.9–62.7 | [60] |
| 6.9–10.9 | - | - | - | [61] |
| 2.17–4.48 | 2.67–3.16 | 0.21–0.23 | - | [63] |
| 8.36–14.13 | - | 0.42–1.01 | - | [64] |
| 8.16–9.04 | 1.78–2.63 | - | - | [65] |
| 3.45–3.47 | 2.20–2.23 | - | - | [66] |
| 0.01–5.47 | - | - | - | [67] |
| L. ruthenicum | ||||
| 2.96 | 0.27 | nd 1 | - | [25] |
| 26.9 | 36.1 | 0.40 | 56.1 2 | [60] |
| 7.26–9.01 | 9.77–12.32 | 0.001–0.003 | - | [63] |
| 3.44–6.45 | 5.66–11.16 | - | - | [66] |
| 21.14–28.52 | 1.23–1.38 | - | - | [68] |
| 49.07 | - | - | - | [69] |
| Sample Origin | Extraction Solvent | DPPH• | ABTS•+ | FRAP | Reference |
|---|---|---|---|---|---|
| L. barbarum | |||||
| China | ethanol (60%, v/v) | 44.63–47.63% | - | 0.15–0.17 µmol Fe+2/g | [66] |
| methanol (80%, v/v) | 35.88–85.46 µmol TE/g fw | 59.3–95.6 µmol TE/g fw | 57.7–92.5 µmol TE/g fw | [60] | |
| Acetone/water/acetic acid (70:29.5:0.5) | 16.07–17.47 µmol TE/g | 53.92–64.38 µmol TE/g | 26.39–46.51 mmol Fe+2/g | [63] | |
| Greece | water | 1.29–3.00 mg/mL (IC50) | 0.42–1.10 mg/mL (IC50) | - | [22] |
| water | 0.83–1.15 mg/mL (IC50) | 0.19–0.4 mg/mL (IC50) | - | [61] | |
| Italy | methanol: water acidified with HCL | - | - | 18.00–20.89 µmol Fe+2/g fw | [8] |
| North Macedonia | water | 1.51–6.25 mg/g dw | 1.94–9.93 mg /g dw | - | [26] |
| Poland | methanol (80%, v/v) + 1% HCl | - | 16.0–68.3 µmol TE/g | 14.4–63.0 µmol TE/g | [28] |
| Portugal | methanol (80%, v/v) | 6.25 mg/mL (EC50) | - | - | [20] |
| Romania | methanol (70%, v/v) | 8.79–9.35 mg TE/g | 24.86–25.12 mg TE/g | 16.91–19.52 mg TE/g | [16] |
| Serbia | methanol (80%, v/v) | 4.52 µmol TE/g fw | 0.12 µmol TE/g fw | 5.32 µmol TE/g fw | [25] |
| Switzerland | methanol | 6.94–13.22 µmol TE/g dw | - | [27] | |
| Turkey | water | 22.64 mg/mL (EC50) | - | 2.93 mM Fe+2 | [65] |
| methanol (80%, v/v) | 18.19 mg/mL (EC50) | - | 2.62 mM Fe+2 | ||
| L. ruthenicum | |||||
| China | ethanol (85%, v/v) | 315.7–460.5 µmol TE/g dw | 327.8–485.6 µmol TE/g dw | 377.0–539.4 µmol TE/g dw | [70] |
| ethanol (60%, v/v) | 63.09–85.15% | - | 0.55–0.62 µmol Fe+2/g | [66] | |
| methanol (80%, v/v) | 49.65 µmol TE/g fw | 47.8 µmol TE/g fw | 56.3 µmol TE/g fw | [60] | |
| acetone/water/ acetic acid (70:29.5:0.5) | 32.29–35.86 µmol TE/g | 147.00–180.03 µmol TE/g | 278.21–363.46 mmol Fe+2/g | [63] | |
| Serbia | methanol (80%, v/v) | 10.22 µmol TE/g fw | 0.28 µmol TE/g fw | 19.43 µmol TE/g fw | [25] |
| Study Design | Study Population | Number | Intervention (Dose) | Main Outcomes | Reference |
|---|---|---|---|---|---|
| Single-blinded, placebo-controlled, parallel design study | Healthy adults | 27 | 28 days (15 g/d wolfberries-estimated to provide ~3 mg/d esterified zeaxanthin) | plasma zeaxanthin increased 2.5-fold | [81] |
| Double-blinded, placebo-controlled RCT | Healthy adults | 34 | 14 days (120 mL/d LBP standardized juice—equivalent at least 150 g of fresh fruit) | ↑ subjective feelings of general well-being, neurologic/psychologic performance and gastrointestinal functions | [82] |
| Double-blinded, placebo-controlled RCT | Healthy adults | 39 | 30 days (120 mL/d LBP-standardized juice) | ↑ SOD, GSH-Px ↓ lipid peroxidation (MDA) | [83] |
| Parallel design intervention study | Healthy elderly subjects | 177 | 3 months (LBPs) | ↓ plasma triglycerides, total cholesterol, and LDL cholesterol ↑ HDL cholesterol | [84] |
| Double-blinded, placebo-controlled RCT | Older healthy adults | 60 | 30 days (120 mL/d LBP standardized juice—equivalent at least 150 g of fresh fruit) | ↑ several immunological responses and subjective feelings of general well-being | [85] |
| Double-blinded, placebo-controlled RCT | Healthy adults | 28 | 14 days (120 mL/d LBP standardized juice—equivalent at least 150 g of fresh fruit) | ↓ waist circumference | [86] |
| Double-blinded, placebo-controlled RCT | Healthy elderly subjects | 150 | 90 days (13.7 g/d milk-based formulation of goji berry, LWB) | ↑ plasma zeaxanthin and antioxidant levels protects from hypopigmentation and soft drusen accumulation in the macula of elderly subjects | [87] |
| Double-blinded, placebo-controlled RCT | Healthy elderly subjects | 150 | 90 days (13.7 g/d milk-based formulation of goji berry, LWB) | ↑ postvaccination serum influenza-specific immunoglobulin G levels and seroconversion rate | [88] |
| Double-blinded, placebo-controlled RCT | Type 2 diabetes patients | 67 | 90 days (300 mg LBPs/d) | ↓ glucose and ↑ insulinogenic index ↑ HDL cholesterol | [89] |
| Double-blinded, placebo-controlled RCT | Healthy overweight and mild hypercholesterolemic subjects | 53 | 8 weaks (80 mL/pouch-contained 13.5 g of WBE) | anti-oxidative and anti-inflammatory effects by modulating mRNA expression | [90] |
| Parallel design RCT | Metabolic syndrome patients | 50 | 45 days (14 g dried goji berry with healthy dietary pattern) | ↓ transaminases and waist circumference ↑ serum antioxidant capacity and GSH ↓ lipid peroxidation | [91] |
| Double-blinded, placebo-controlled RCT | Retinitis pigmentosa (RP) patients | 42 | 12 months (10 g of LB granules/d, estimated to provide 0.175 g LBPs) | LB supplement provides a neuroprotective effect for the retina and could help delay or minimize cone degeneration in RP | [92] |
| Double-blind crossover RCT | Healthy, overweight men | 17 | 25 g of dried LB fruit | ≠ postprandial energy expenditure, substrate oxidation, and markers for lipid and glucose metabolism | [93] |
| Parallel design RCT | Middle-aged and older adults | 40 | 16 weak (15 g/d whole, dried wolfberry with healthy dietary pattern) | improves vascular tone ↓ lipid peroxidation (8-iso-prostaglandin F2α) | [94,95] |
| Parallel design RCT | Healthy, middle-aged subjects | 27 | 3 months (25 g of whole goji berries or supplements of lutein and zeaxhantin) | ↑ macular pigment optical density | [96] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidović, B.B.; Milinčić, D.D.; Marčetić, M.D.; Djuriš, J.D.; Ilić, T.D.; Kostić, A.Ž.; Pešić, M.B. Health Benefits and Applications of Goji Berries in Functional Food Products Development: A Review. Antioxidants 2022, 11, 248. https://doi.org/10.3390/antiox11020248
Vidović BB, Milinčić DD, Marčetić MD, Djuriš JD, Ilić TD, Kostić AŽ, Pešić MB. Health Benefits and Applications of Goji Berries in Functional Food Products Development: A Review. Antioxidants. 2022; 11(2):248. https://doi.org/10.3390/antiox11020248
Chicago/Turabian StyleVidović, Bojana B., Danijel D. Milinčić, Mirjana D. Marčetić, Jelena D. Djuriš, Tijana D. Ilić, Aleksandar Ž. Kostić, and Mirjana B. Pešić. 2022. "Health Benefits and Applications of Goji Berries in Functional Food Products Development: A Review" Antioxidants 11, no. 2: 248. https://doi.org/10.3390/antiox11020248
APA StyleVidović, B. B., Milinčić, D. D., Marčetić, M. D., Djuriš, J. D., Ilić, T. D., Kostić, A. Ž., & Pešić, M. B. (2022). Health Benefits and Applications of Goji Berries in Functional Food Products Development: A Review. Antioxidants, 11(2), 248. https://doi.org/10.3390/antiox11020248











