Bioavailability Study of Isothiocyanates and Other Bioactive Compounds of Brassica oleracea L. var. Italica Boiled or Steamed: Functional Food or Dietary Supplement?
Abstract
1. Introduction
2. Experimental Section
2.1. Chemicals and Reagents
2.2. Broccoli Sampling and Cooking Treatments
2.3. Study Design
2.4. Dietary Intervention and Biological Sample Collection
2.5. Total GLS in Broccoli as ITCs Equivalents and ITCs Plasma Level
2.6. Carotenoids in Broccoli and Plasma
2.7. Phylloquinone in Broccoli and Plasma
2.8. Statistical Analysis
3. Results
3.1. ITCs Equivalent, Phylloquinone, Lutein and β-carotene in Broccoli
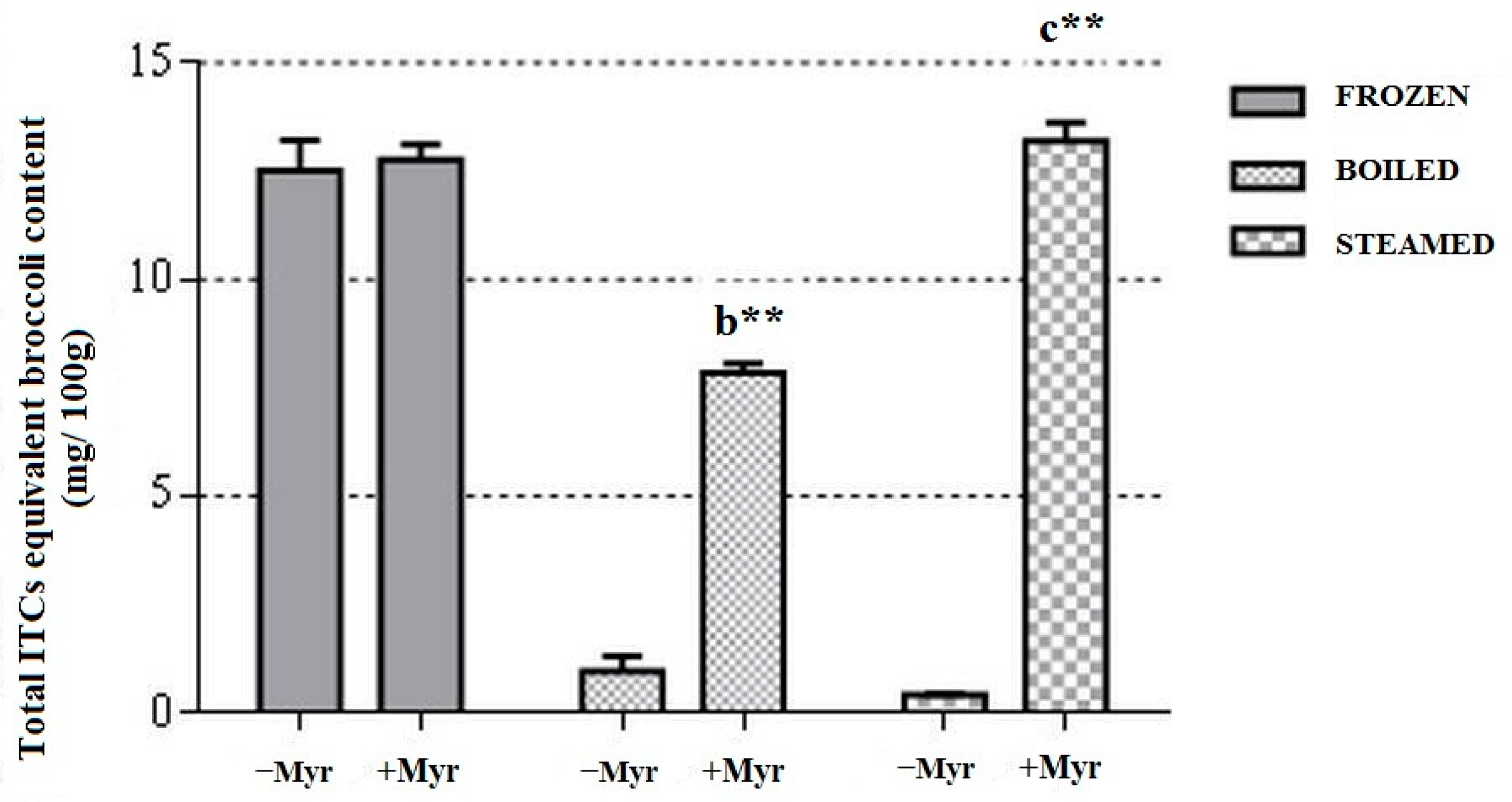
3.1.1. ITCs Equivalent
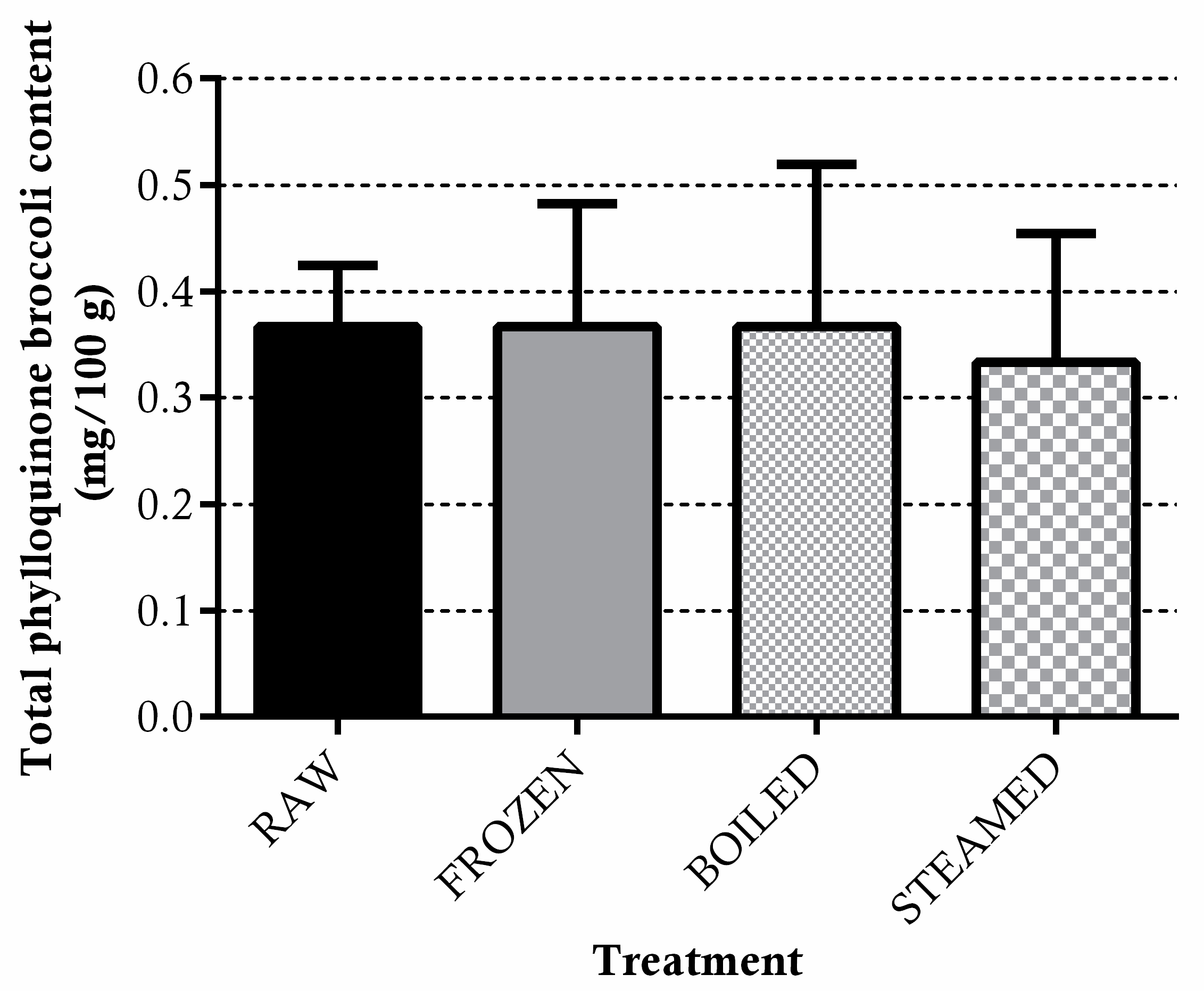
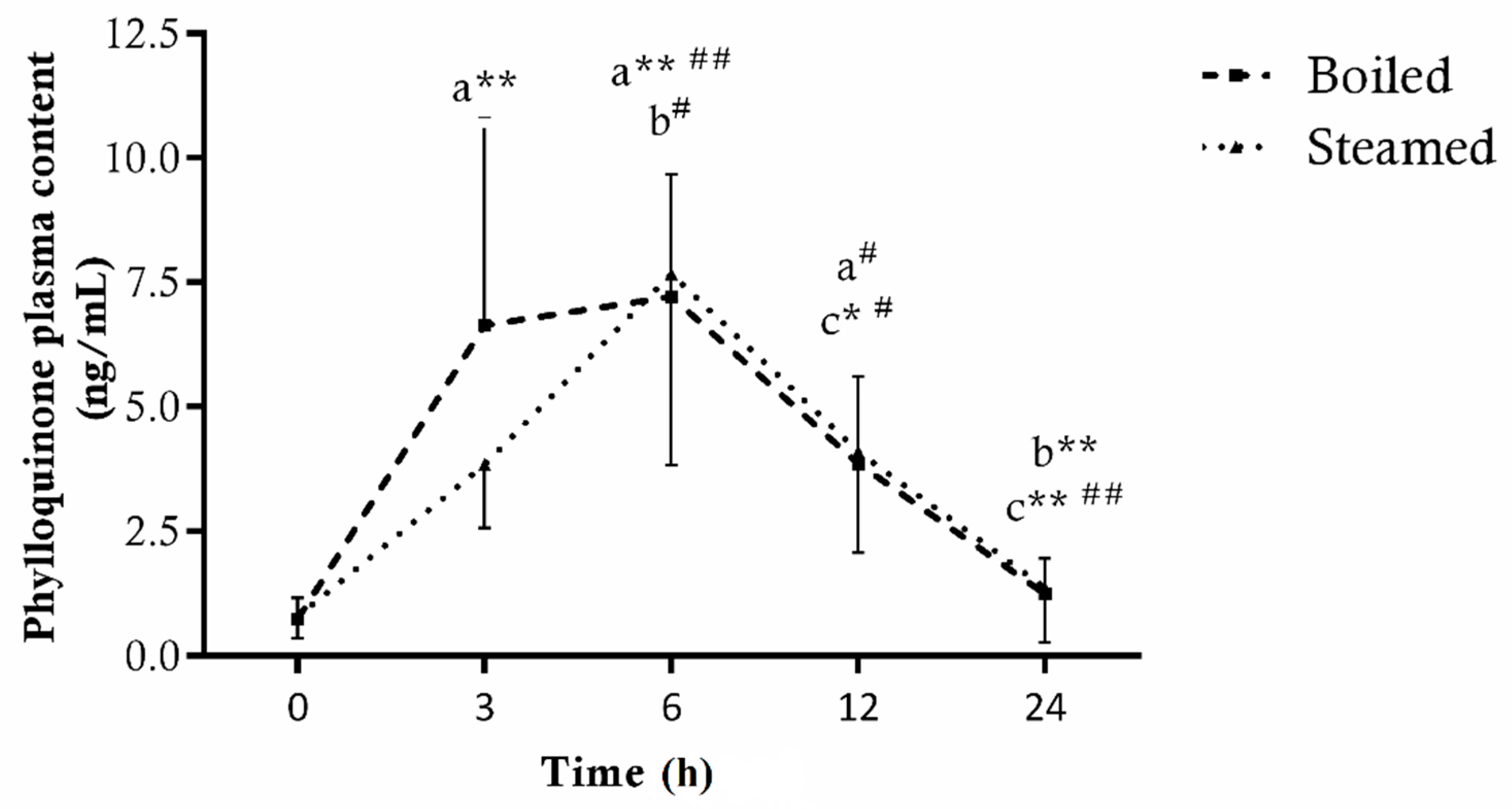
3.1.2. Phylloquinone
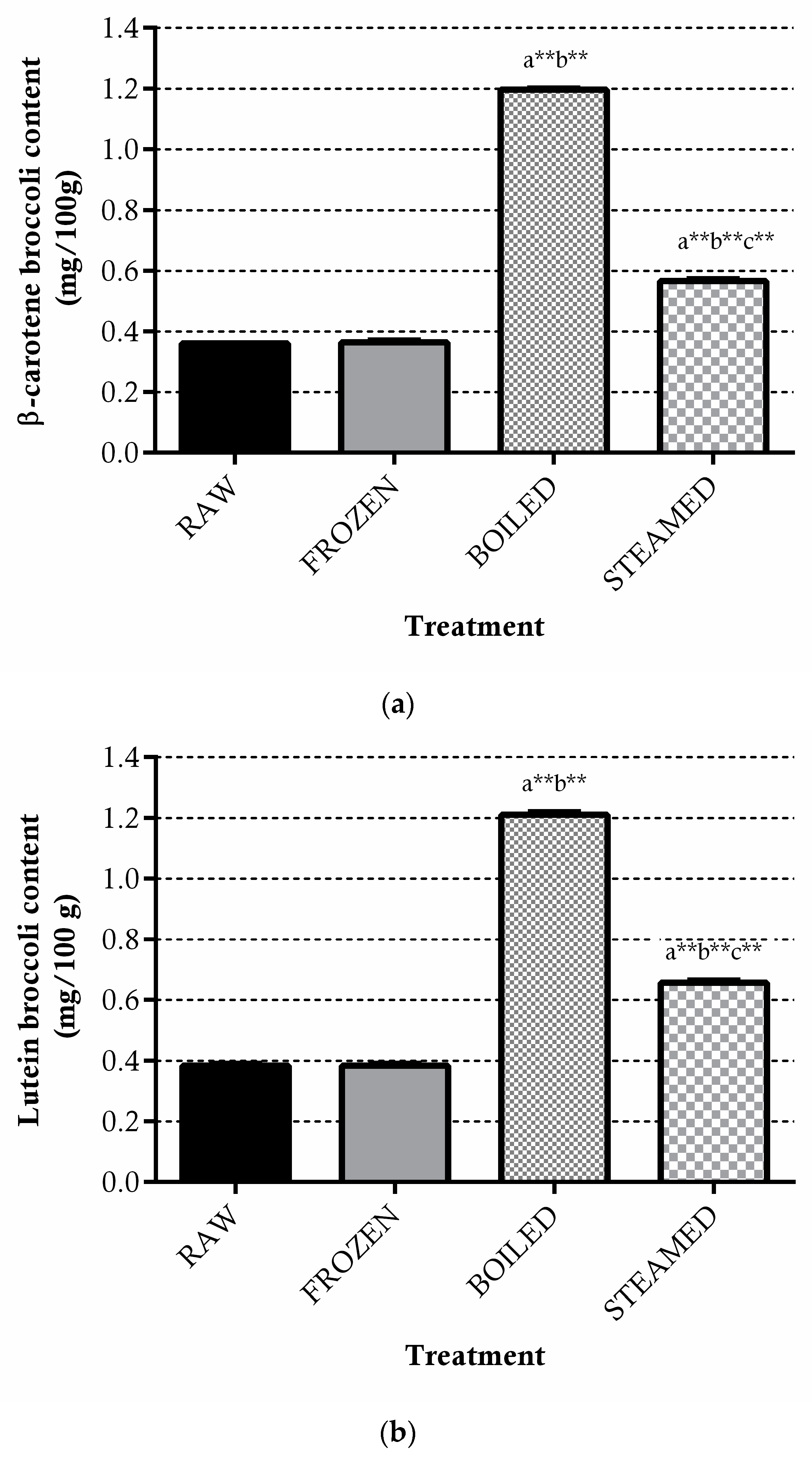
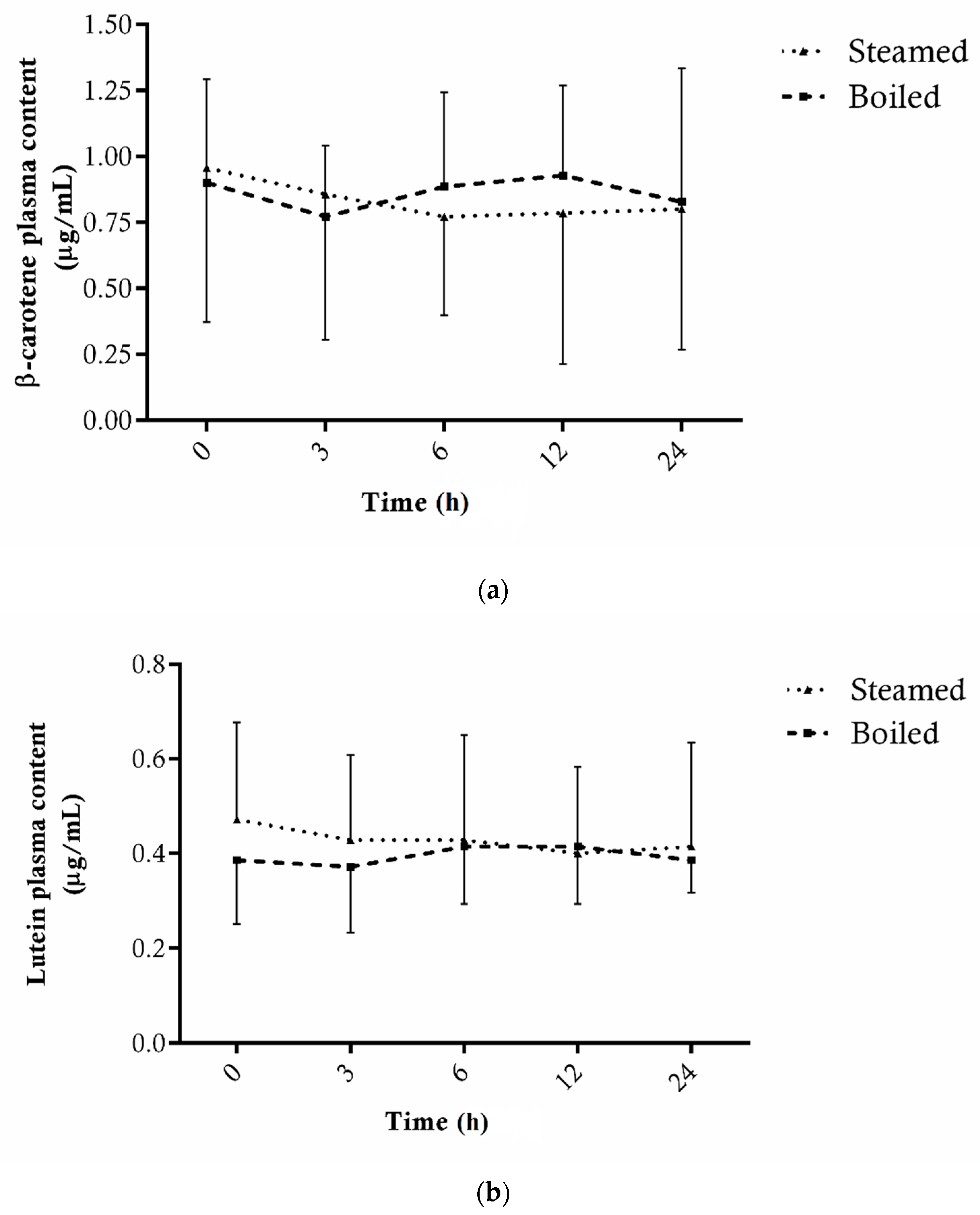
3.1.3. Carotenoids
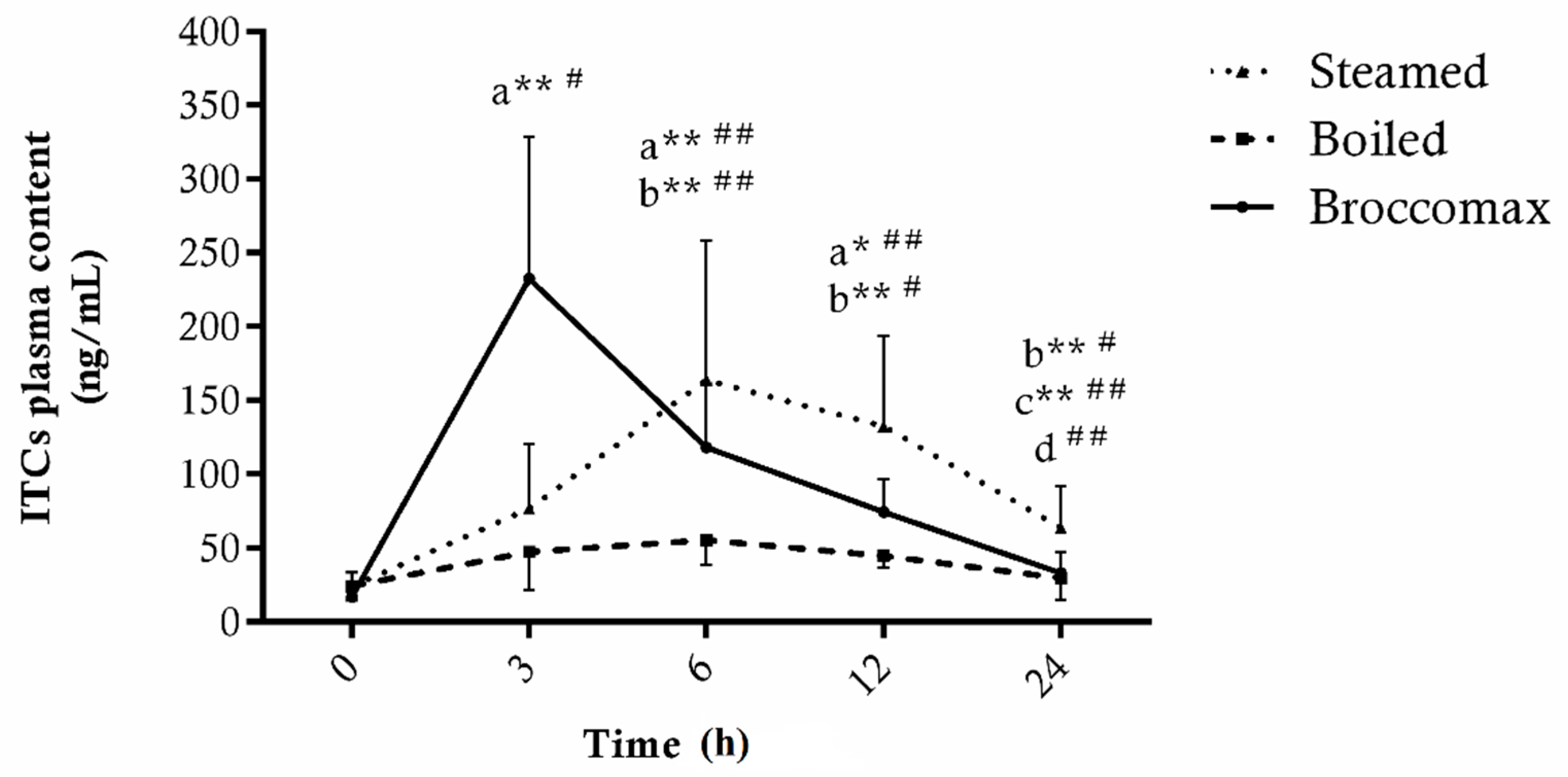
3.2. Plasma Bioavailability of Bioactive Compounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Björkman, M.; Klingen, I.; Birch, A.N.E.; Bones, A.M.; Bruce, T.J.A.; Johansen, T.J.; Meadow, R.; Molmann, J.; Seljasen, R.; Smart, L.E.; et al. Phytochemicals of Brassicaceae in plant protection and human health—Influences of climate, environment and agronomic practice. Phytochemistry 2011, 72, 538–556. [Google Scholar] [CrossRef] [PubMed]
- Granado, F.; Olmedilla, B.; Herrero, C.; Pérez-Sacristán, B.; Blanco, I.; Blázquez, S. Bioavailability of carotenoids and tocopherols from broccoli: In vivo and in vitro assessment. Exp. Biol. Med. 2006, 231, 1733–1738. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, K.E.; Juvik, J.A. Feasibility for improving phytonutrient content in vegetable crops using conventional breeding strategies; case study with carotenoids and tocopherols in sweet corn and broccoli. J. Agric. Food Chem. 2009, 57, 4636–4644. [Google Scholar] [CrossRef]
- Kulczyński, B.; Gramza-Michałowska, A.; Kobus-Cisowska, J.; Kmiecik, D. The role of carotenoids in the prevention and treatment of cardiovascular disease—Current state of knowledge. J. Funct. Foods 2017, 38, 45–65. [Google Scholar] [CrossRef]
- Vasanthi, H.R.; Mukherjee, S.; Das, D.K. Potential Health Benefits of Broccoli—A Chemico-Biological Overview. Med. Chem. 2009, 9, 749–759. [Google Scholar] [CrossRef]
- Heinonen, S.; Nurmi, T.; Liukkonen, K.; Poutanen, K.; Wähälä, K.; Deyama, T.; Nishibe, S.; Adlercreutz, H. In vitro metabolism of plant lignans: New precursors of mammalian lignans enterolactone and enterodiol. J. Agric. Food Chem. 2001, 49, 3178–3186. [Google Scholar] [CrossRef]
- Young, J.E.; Zhao, X.; Carey, E.E.; Welti, R.; Yang, S.S.; Wang, W. Phytochemical phenolics in organically grown vegetables. Mol. Nutr. Food Res. 2005, 49, 1136–1142. [Google Scholar] [CrossRef]
- Podsȩdek, A.; Sosnowska, D.; Redzynia, M.; Anders, B. Antioxidant capacity and content of Brassica oleracea dietary antioxidants. Intern. J. Food Sci. Technol. 2006, 41 (Suppl. 1), 49–58. [Google Scholar] [CrossRef]
- Lin, L.Z.; Harnly, J.M. Identification of the phenolic components of collard greens, kale, and chinese Broccoli. J. Agric. Food Chem. 2009, 57, 7401–7408. [Google Scholar] [CrossRef]
- Ishida, M.; Hara, M.; Fukino, N.; Kakizaki, T.; Morimitsu, Y. Glucosinolate metabolism, functionality and breeding for the improvement of brassicaceae vegetables. Breed. Sci. 2014, 64, 48–59. [Google Scholar] [CrossRef]
- Pérez-Balibrea, S.; Moreno, D.A.; García-Viguera, C. Glucosinolates in broccoli sprouts (Brassica oleracea var. italica) as Conditioned by sulphate supply during germination. J. Food Sci. 2010, 75, C673–C677. [Google Scholar] [CrossRef] [PubMed]
- Narbad, A.; Rossiter, J.T. Gut Glucosinolate Metabolism and Isothiocyanate Production. Mol. Nutr. Food Res. 2018, 62, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Liu, X.; Lei, P.; Zhang, X.; Shan, Y. Microbiota: A mediator to transform glucosinolate precursors in cruciferous vegetables to the active isothiocyanates. J. Sci. Food Agric. 2018, 98, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Louda, S.; Mole, S. Glucosinolates: Chemistry and ecology. In Herbivores, Their Interactions with Secondary Plant Metabolites, 2nd ed.; Rosenthal, G.A., Berenbaum, M.R., Eds.; Academic Press: San Diego, CA, USA, 1991; pp. 123–164. [Google Scholar] [CrossRef]
- Sadasivam, S.; Thayumanavan, B. Molecular Host Plant Resistance to Pests; Marcel Dekker Inc.: New York, NY, USA, 2003; pp. 164–195. [Google Scholar]
- Esteve, M. Mechanisms Underlying Biological Effects of Cruciferous Glucosinolate-Derived Isothiocyanates/Indoles: A Focus on Metabolic Syndrome. Front. Nutr. 2020, 7, 111. [Google Scholar] [CrossRef]
- Bricker, G.V.; Riedl, K.M.; Ralston, R.A.; Tober, K.L.; Oberyszyn, T.M.; Schwartz, S.J. Isothiocyanate metabolism, distribution, and interconversion in mice following consumption of thermally processed broccoli sprouts or purified sulforaphane. Mol. Nutr. Food Res. 2014, 58, 1991–2000. [Google Scholar] [CrossRef]
- Moon, J.K.; Kim, J.R.; Ahn, Y.J.; Shibamoto, T. Analysis and anti- helicobacter activity of sulforaphane and related compounds present in Broccoli (Brassica oleracea L.) sprouts. J. Agric. Food Chem. 2010, 58, 6672–6677. [Google Scholar] [CrossRef]
- Yang, M.; Wang, H.; Zhou, M.; Liu, W.; Kuang, P.; Liang, H.; Yuan, Q. The natural compound sulforaphene, as a novel anticancer reagent, targeting PI3K-AKT signaling pathway in lung cancer. Oncotarget 2016, 7, 76656–76666. [Google Scholar] [CrossRef]
- Subedi, L.; Cho, K.; Park, Y.U.; Choi, H.J.; Kim, S.Y. Sulforaphane-Enriched Broccoli Sprouts Pretreated by Pulsed Electric Fields Reduces Neuroinflammation and Ameliorates Scopolamine-Induced Amnesia in Mouse Brain through Its Antioxidant Ability via Nrf2-HO-1 Activation. Oxidative Med. Cell. Longev. 2019, 2019, 3549274. [Google Scholar] [CrossRef]
- Ninh Le, T.; Luong, H.Q.; Li, H.P.; Chiu, C.H.; Hsieh, P.C. Broccoli (Brassica oleracea L. var. Italica) Sprouts as the Potential Food Source for Bioactive Properties: A comprehensive study on in vitro disease models. Foods 2019, 8, 532. [Google Scholar]
- Conaway, C.C.; Getahun, S.M.; Liebes, L.L.; Pusateri, D.J.; Topham, D.K.W.; Botero-Omary, M.; Chung, F.L. Disposition of glucosinolates and sulforaphane in humans after ingestion of steamed and fresh broccoli. Nutr. Cancer 2000, 38, 168–178. [Google Scholar] [CrossRef]
- Wang, Z.; Kwan, M.L.; Pratt, R.; Roh, J.M.; Kushi, L.H.; Danforth, K.N.; Zhang, Y.; Ambrosone, C.B.; Tang, L. Effects of cooking methods on total isothiocyanate yield from cruciferous vegetables. Food Sci. Nutr. 2020, 8, 5673–5682. [Google Scholar] [CrossRef]
- Mirmiran, P.; Bahadoran, Z.; Hosseinpanah, F.; Keyzad, A.; Azizi, F. Effects of broccoli sprout with high sulforaphane concentration on inflammatory markers in type 2 diabetic patients: A randomized double-blind placebo-controlled clinical trial. J. Funct. Foods 2012, 4, 837–841. [Google Scholar] [CrossRef]
- Armah, C.N.; Traka, M.H.; Dainty, J.R.; Defernez, M.; Janssens, A.; Leung, W.; Doleman, J.F.; Potter, J.F.; Mithen, R.F. A diet rich in high-glucoraphanin broccoli interacts with genotype to reduce discordance in plasma metabolite profiles by modulating mitochondrial function. Am. J. Clin. Nutr. 2013, 98, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Armah, C.N.; Derdemezis, C.; Traka, M.H.; Dainty, J.R.; Doleman, J.F.; Saha, S.; Leung, W.; Potter, J.F.; Lovegrove, J.A.; Mithen, R.F. Diet rich in high glucoraphanin broccoli reduces plasma LDL cholesterol: Evidence from randomised controlled trials. Mol. Nutr. Food Res. 2015, 59, 918–926. [Google Scholar] [CrossRef]
- Axelsson, A.S.; Tubbs, E.; Mecham, B.; Chacko, S.; Nenonen, H.A.; Tang, Y.; Fahey, J.W.; Derry, M.J.J.; Wollheim, C.B.; Wierup, N.; et al. Sulforaphane reduces hepatic glucose production and improves glucose control in patients with type 2 diabetes. Sci. Transl. Med. 2017, 9, 394. [Google Scholar] [CrossRef] [PubMed]
- Sedlak, T.W.; Nucifora, L.G.; Koga, M.; Shaffer, L.S.; Higgs, C.; Tanaka, T.; Wang, A.M.; Coughlin, J.M.; Barker, P.B.; Fahey, J.W.; et al. Sulforaphane Augments Glutathione and Influences Brain Metabolites in Human Subjects: A Clinical Pilot Study. Mol. Neuropsychiatry 2017, 3, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Alanís-Garza, P.A.; Becerra-Moreno, A.; Mora-Nieves, J.L.; Mora-Mora, J.P.; Jacobo-Velázquez, D.A. Effect of industrial freezing on the stability of chemopreventive compounds in broccoli. Int. J. Food Sci. Nutr. 2015, 66, 282–288. [Google Scholar] [CrossRef]
- González-Hidalgo, I.; Moreno, D.A.; García-Viguera, C.; Ros-García, J.M. Effect of industrial freezing on the physical and nutritional quality traits in broccoli. Food Sci. Techol. Int. 2019, 25, 56–65. [Google Scholar] [CrossRef]
- Orlando, P.; Giardinieri, A.; Lucci, P.; Nartea, A.; Balzano, M.; Pacetti, D.; Frega, N.G.; Silvestri, S.; Tiano, L. Impact of traditional and mild oven cooking treatments on antioxidant compounds levels and oxidative status of Atlantic salmon (Salmo salar) fillets. LWT 2020, 134, 110011. [Google Scholar] [CrossRef]
- Gliszczyńska-Świgło, A.; Ciska, E.; Pawlak-Lemańska, K.; Chmielewski, J.; Borkowski, T.; Tyrakowska, B. Changes in the content of health-promoting compounds and antioxidant activity of broccoli after domestic processing. Food Addit. Contam. 2006, 23, 1088–1098. [Google Scholar] [CrossRef]
- Pellegrini, N.; Chiavaro, E.; Gardana, C.; Mazzeo, T.; Contino, D.; Gallo, M.; Riso, P.; Fogliano, V.; Porrini, M. Effect of different cooking methods on color, phytochemical concentration, and antioxidant capacity of raw and frozen brassica vegetables. J. Agric. Food Chem. 2010, 58, 4310–4321. [Google Scholar] [CrossRef]
- Bongoni, R.; Verkerk, R.; Steenbekkers, B.; Dekker, M.; Stieger, M. Evaluation of Different Cooking Conditions on Broccoli (Brassica oleracea var. italica) to Improve the Nutritional Value and Consumer Acceptance. Plant Foods Hum. Nutr. 2014, 69, 228–234. [Google Scholar] [CrossRef]
- Lee, S.; Choi, Y.; Jeong, H.S.; Lee, J.; Sung, J. Effect of different cooking methods on the content of vitamins and true retention in selected vegetables. Food Sci. Biotech. 2018, 27, 333–342. [Google Scholar] [CrossRef]
- Nartea, A.; Fanesi, B.; Falcone, P.M.; Pacetti, D.; Frega, N.G.; Lucci, P. Impact of mild oven cooking treatments on carotenoids and tocopherols of cheddar and depurple cauliflower (Brassica oleracea L. var. Botrytis). Antioxidants 2021, 10, 196. [Google Scholar] [CrossRef]
- Brown, E.D.; Micozzi, M.S.; Craft, N.E.; Bieri, J.G.; Beecher, G.; Edwards, B.K.; Rose, A.; Taylor, P.R.; Smith, J.C. Plasma carotenoids in normal men after a single ingestion of vegetables or purified β-carotene. Am. J. Clin. Nutr. 1989, 49, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Van Het Hof, K.H.; Tijburg, L.B.M.; Pietrzik, K.; Weststrate, J.A. Influence of feeding different vegetables on plasma levels of carotenoids, folate and vitamin C. Effect of disruption of the vegetable matrix. Brit. J. Nutr. 1999, 82, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Riso, P.; Brusamolino, A.; Moro, M.; Porrini, M. Absorption of bioactive compounds from steamed broccoli and their effect on plasma glutathione S-transferase activity. Int. J. Food Sci. Nutr. 2009, 60 (Suppl. 1), 56–71. [Google Scholar] [CrossRef]
- Atwell, L.L.; Hsu, A.; Wong, C.P.; Stevens, J.F.; Bella, D.; Yu, T.W.; Pereira, C.B.; Lohr, C.V.; Christensen, J.M.; Dashwood, R.H.; et al. Absorption and chemopreventive targets of sulforaphane in humans following consumption of broccoli sprouts or a myrosinase-treated broccoli sprout extract. Mol. Nutr. Food Res. 2015, 59, 424–433. [Google Scholar] [CrossRef]
- Ye, L.; Dinkova-Kostova, A.T.; Wade, K.L.; Zhang, Y.; Shapiro, T.A.; Talalay, P. Quantitative determination of dithiocarbamates in human plasma, serum, erythrocytes and urine: Pharmacokinetics of broccoli sprout isothiocyanates in humans. Clin. Chim. Acta 2002, 316, 43–53. [Google Scholar] [CrossRef]
- Chauveau-Duriot, B.; Doreau, M.; Nozière, P.; Graulet, B. Simultaneous quantification of carotenoids, retinol, and tocopherols in forages, bovine plasma, and milk: Validation of a novel UPLC method. Anal. Bioanal. Chem. 2010, 397, 777–790. [Google Scholar] [CrossRef]
- Cirilli, I.; Orlando, P.; Marcheggiani, F.; Dludla, P.V.; Silvestri, S.; Damiani, E.; Tiano, L. The protective role of bioactive quinones in stress-induced senescence phenotype of endothelial cells exposed to cigarette smoke extract. Antioxidants 2020, 9, 1008. [Google Scholar] [CrossRef] [PubMed]
- Biesalski, H.K.; Dragsted, L.O.; Elmadfa, I.; Grossklaus, R.; Müller, M.; Schrenk, D.; Walter, P.; Weber, P. Bioactive compounds: Definition and assessment of activity. Nutrition 2009, 25, 1202–1205. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, F.; Tomás-Barberán, F.A.; Garcia-Viguera, C. Glucosinolates and vitamin C content in edible parts of broccoli florets after domestic cooking. Eur. Food Res. Technol. 2002, 215, 310–316. [Google Scholar]
- Yuan, G.F.; Sun, B.; Yuan, J.; Wang, Q.M. Effects of different cooking methods on health-promoting compounds of broccoli. J. Zhejiang Univ. Sci. B 2009, 10, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Baenas, N.; Marhuenda, J.; García-Viguera, C.; Zafrilla, P.; Moreno, D.A. Influence of cooking methods on glucosinolates and isothiocyanates content in novel cruciferous foods. Foods 2019, 8, 257. [Google Scholar] [CrossRef]
- Oliviero, T.; Verkerk, R.; Dekker, M. Isothiocyanates from Brassica Vegetables—Effects of Processing, Cooking, Mastication, and Digestion. Mol. Nutr. Food Res. 2018, 62, 1–11. [Google Scholar] [CrossRef]
- Borowski, J.; Narwojsz, A.; Borowska, E.J.; Majewska, K. The effect of thermal processing on sensory properties, texture attributes and pectic changes in broccoli. Czech J. Food Sci. 2015, 33, 254–260. [Google Scholar] [CrossRef]
- Rungapamestry, V.; Duncan, A.J.; Fuller, Z.; Ratcliffe, B. Changes in glucosinolate concentrations, myrosinase activity, and production of metabolites of glucosinolates in cabbage (Brassica oleracea var. capitata) cooked for different durations. J. Agric. Food Chem. 2006, 54, 7628–7634. [Google Scholar] [CrossRef]
- Rabot, S.; Nugon-Baudon, L.; Raibaud, P.; Szylit, O. Rape-seed meal toxicity in gnotobiotic rats: Influence of a whole human faecal flora or single human strains of Escherichia coli and Bacteroides vulgatus. Brit. J. Nutr. 1993, 70, 323–331. [Google Scholar] [CrossRef]
- Krul, C.; Humblot, C.; Philippe, C.; Vermeulen, M.; Van Nuenen, M.; Havenaar, R.; Rabot, S. Metabolism of sinigrin (2-propenyl glucosinolate) by the human colonic microflora in a dynamic in vitro large-intestinal model. Carcinogenesis 2002, 23, 1009–1016. [Google Scholar] [CrossRef]
- Rouzaud, G.; Young, S.A.; Duncan, A.J. Hydrolysis of Glucosinolates to Isothiocyanates after Ingestion of Raw or Microwaved Cabbage by Human Volunteers. Cancer Epidem. Biom. Prev. 2004, 13, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Barba, F.J.; Nikmaram, N.; Roohinejad, S.; Khelfa, A.; Zhu, Z.; Koubaa, M. Bioavailability of Glucosinolates and Their Breakdown Products: Impact of Processing. Front. Nutr. 2016, 3, 24. [Google Scholar] [CrossRef]
- Miglio, C.; Chiavaro, E.; Visconti, A.; Fogliano, V.; Pellegrini, N. Effects of different cooking methods on nutritional and physicochemical characteristics of selected vegetables. J. Agric. Food Chem. 2008, 56, 139–147. [Google Scholar] [CrossRef]
- Kurilich, A.C.; Britz, S.J.; Clevidence, B.A.; Novotny, J.A. Isotopic labeling and LC-APCI-MS quantification for investigating absorption of carotenoids and phylloquinone from kale (Brassica oleracea). J. Agric. Food Chem. 2003, 51, 4877–4883. [Google Scholar] [CrossRef] [PubMed]
- Novotny, J.A.; Kurilich, A.C.; Britz, S.J.; Clevidence, B.A. Plasma appearance of labeled β-carotene, lutein, and retinol in humans after consumption of isotopically labeled kale. J. Lip. Res. 2005, 46, 1896–1903. [Google Scholar] [CrossRef]
- Novotny, J.A.; Kurilich, A.C.; Britz, S.J.; Baer, D.J.; Clevidence, B.A. Vitamin K absorption and kinetics in human subjects after consumption of 13C-labelled phylloquinone from kale. Brit. J. Nutr. 2010, 104, 858–862. [Google Scholar] [CrossRef]
- Garber, A.K.; Binkley, N.C.; Krueger, D.C.; Suttie, J.W. Comparison of phylloquinone bioavailability from food sources or a supplement in human subjects. J. Nutr. 1999, 129, 1201–1203. [Google Scholar] [CrossRef] [PubMed]

| ITCs Equivalent | Phylloquinone | β-Carotene | Lutein | |
| BroccoMax® | 90 | - | - | - |
| Boiled | 38.4 ± 4.4 | 1.36 ± 0.24 | 4.78 ± 0.03 | 4.83 ± 0.04 |
| Steamed | 56.8 ± 2.8 | 1.40 ± 0.72 | 2.26 ± 0.02 | 2.62 ± 0.03 |
| GLS | ||||
| BroccoMax® | 90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlando, P.; Nartea, A.; Silvestri, S.; Marcheggiani, F.; Cirilli, I.; Dludla, P.V.; Fiorini, R.; Pacetti, D.; Loizzo, M.R.; Lucci, P.; et al. Bioavailability Study of Isothiocyanates and Other Bioactive Compounds of Brassica oleracea L. var. Italica Boiled or Steamed: Functional Food or Dietary Supplement? Antioxidants 2022, 11, 209. https://doi.org/10.3390/antiox11020209
Orlando P, Nartea A, Silvestri S, Marcheggiani F, Cirilli I, Dludla PV, Fiorini R, Pacetti D, Loizzo MR, Lucci P, et al. Bioavailability Study of Isothiocyanates and Other Bioactive Compounds of Brassica oleracea L. var. Italica Boiled or Steamed: Functional Food or Dietary Supplement? Antioxidants. 2022; 11(2):209. https://doi.org/10.3390/antiox11020209
Chicago/Turabian StyleOrlando, Patrick, Ancuta Nartea, Sonia Silvestri, Fabio Marcheggiani, Ilenia Cirilli, Phiwayinkosi V. Dludla, Rosamaria Fiorini, Deborah Pacetti, Monica Rosa Loizzo, Paolo Lucci, and et al. 2022. "Bioavailability Study of Isothiocyanates and Other Bioactive Compounds of Brassica oleracea L. var. Italica Boiled or Steamed: Functional Food or Dietary Supplement?" Antioxidants 11, no. 2: 209. https://doi.org/10.3390/antiox11020209
APA StyleOrlando, P., Nartea, A., Silvestri, S., Marcheggiani, F., Cirilli, I., Dludla, P. V., Fiorini, R., Pacetti, D., Loizzo, M. R., Lucci, P., & Tiano, L. (2022). Bioavailability Study of Isothiocyanates and Other Bioactive Compounds of Brassica oleracea L. var. Italica Boiled or Steamed: Functional Food or Dietary Supplement? Antioxidants, 11(2), 209. https://doi.org/10.3390/antiox11020209









