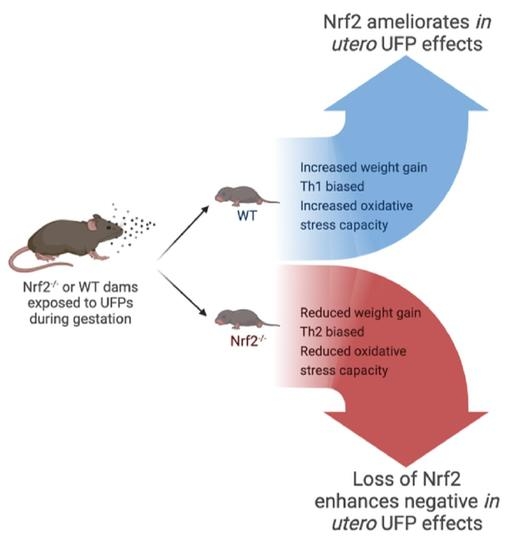
NRF2 Protects against Altered Pulmonary T Cell Differentiation in Neonates Following In Utero Ultrafine Particulate Matter Exposure
Abstract
:1. Introduction
2. Methods and Materials
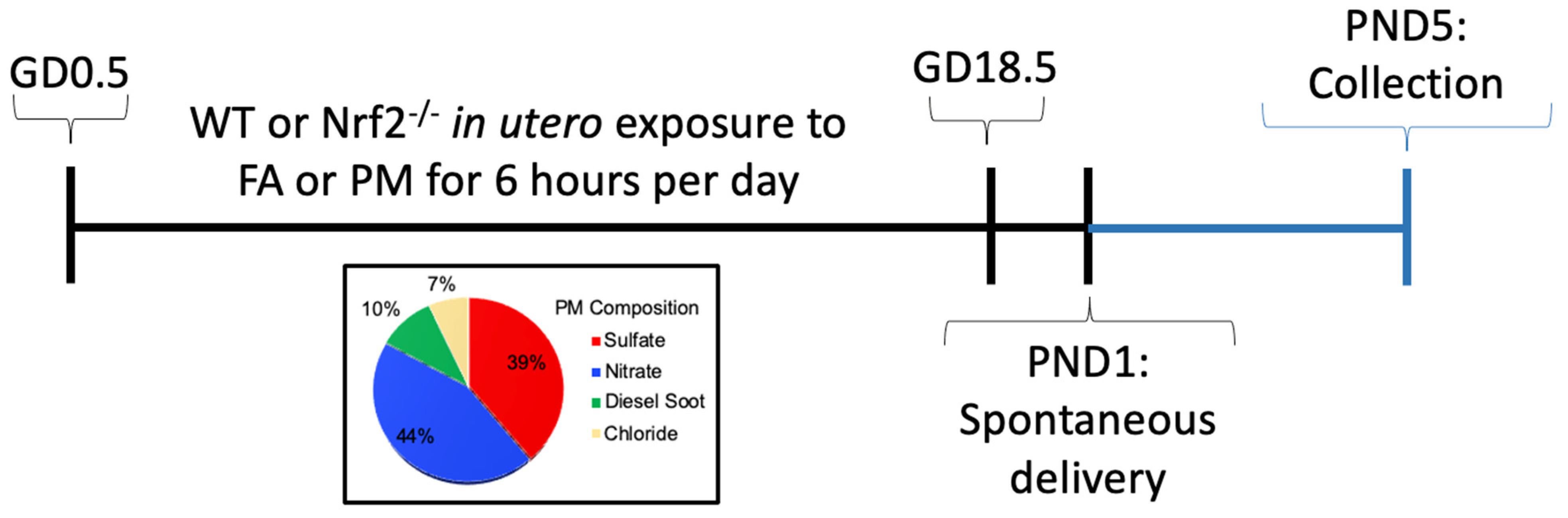
2.1. Ultrafine Particle Exposure
2.2. Flow Cytometry
2.3. Gene Expression
2.4. Thiol Redox Analysis
2.4.1. Sample Preparation
2.4.2. HPLC Analysis
2.5. Statistical Analysis
3. Results
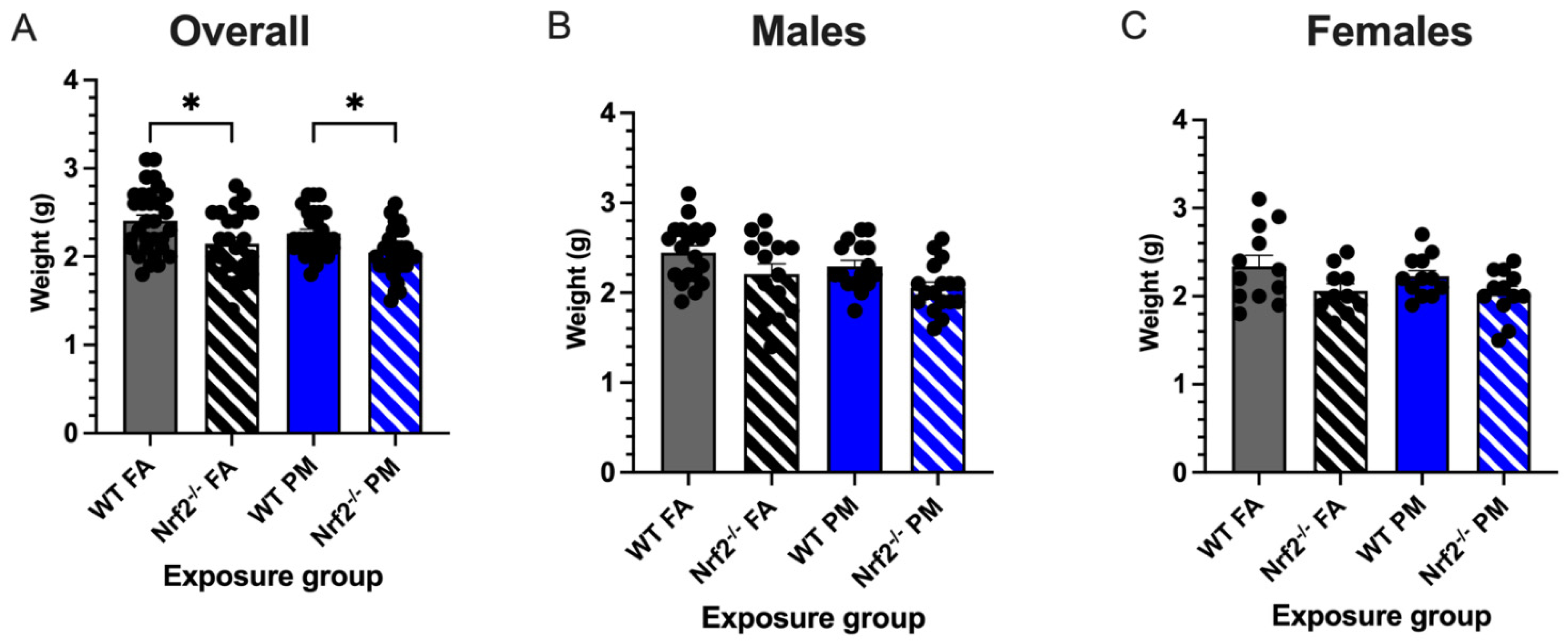
3.1. In Utero UFP Exposure Alters Neonatal Weights in Nrf2−/− Mice
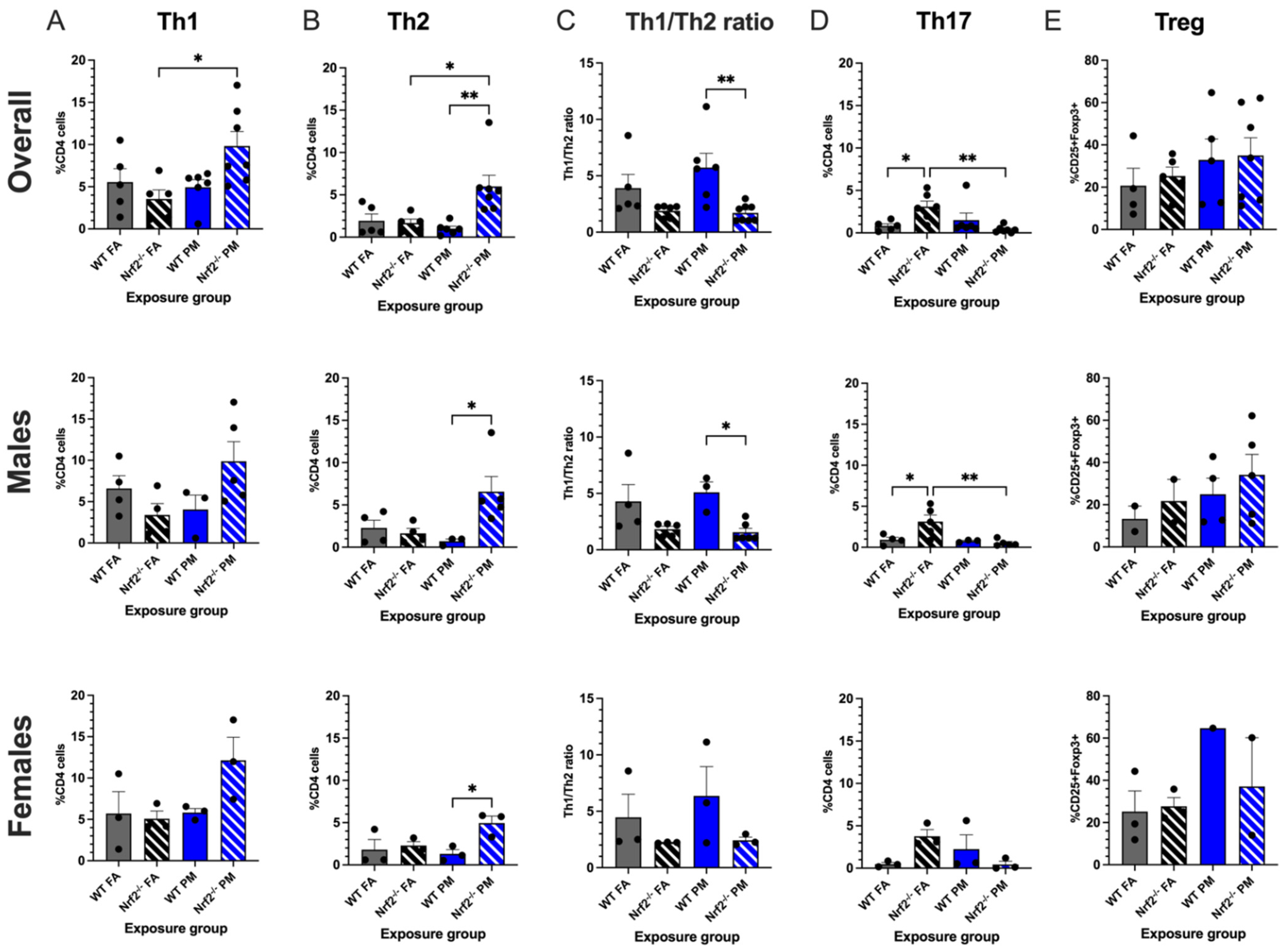
3.2. Nrf2−/− Neonates Exhibit Th2 Pulmonary Immune Bias
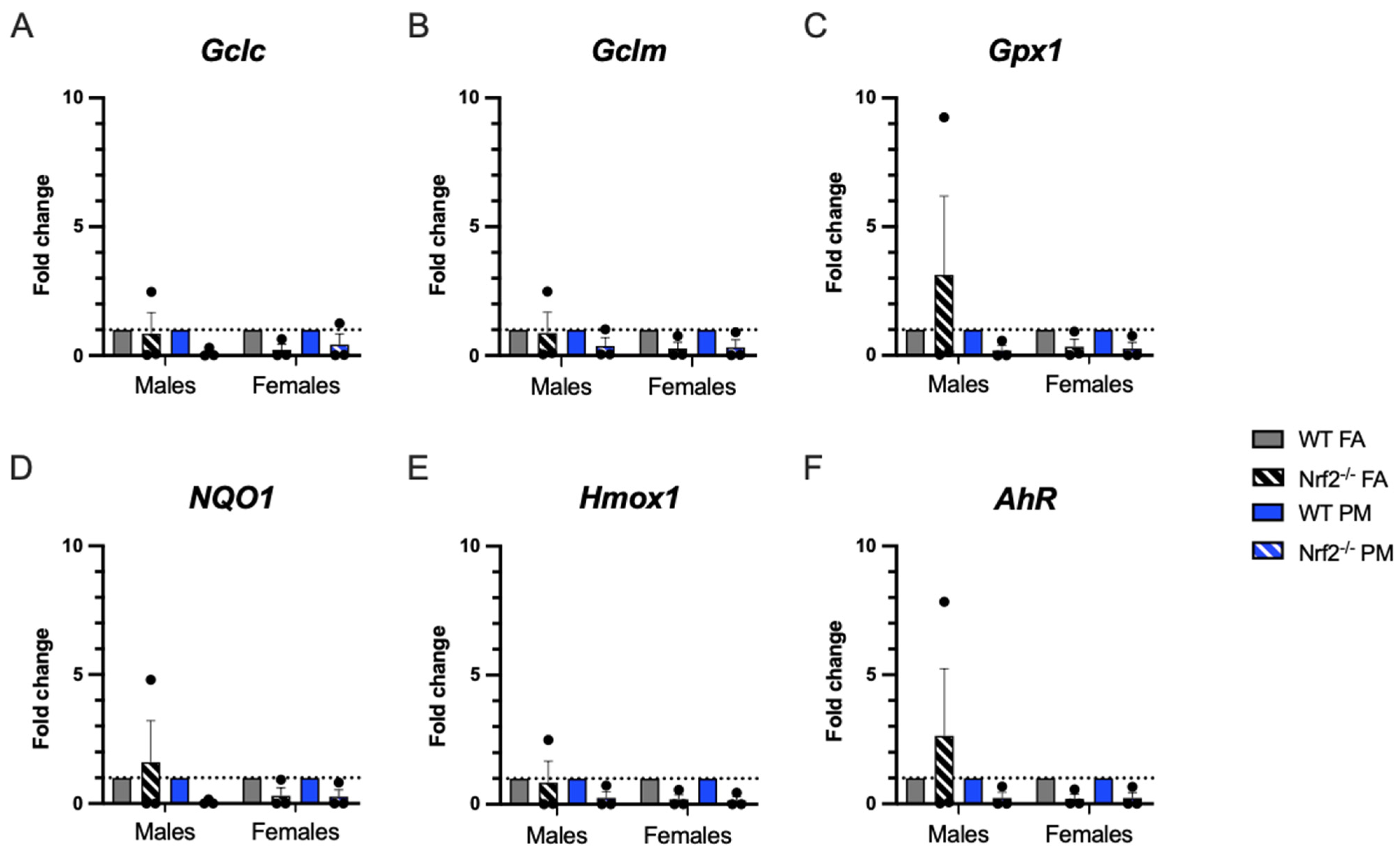
3.3. Expression of Oxidative Stress-Related Genes in Pulmonary Tissue
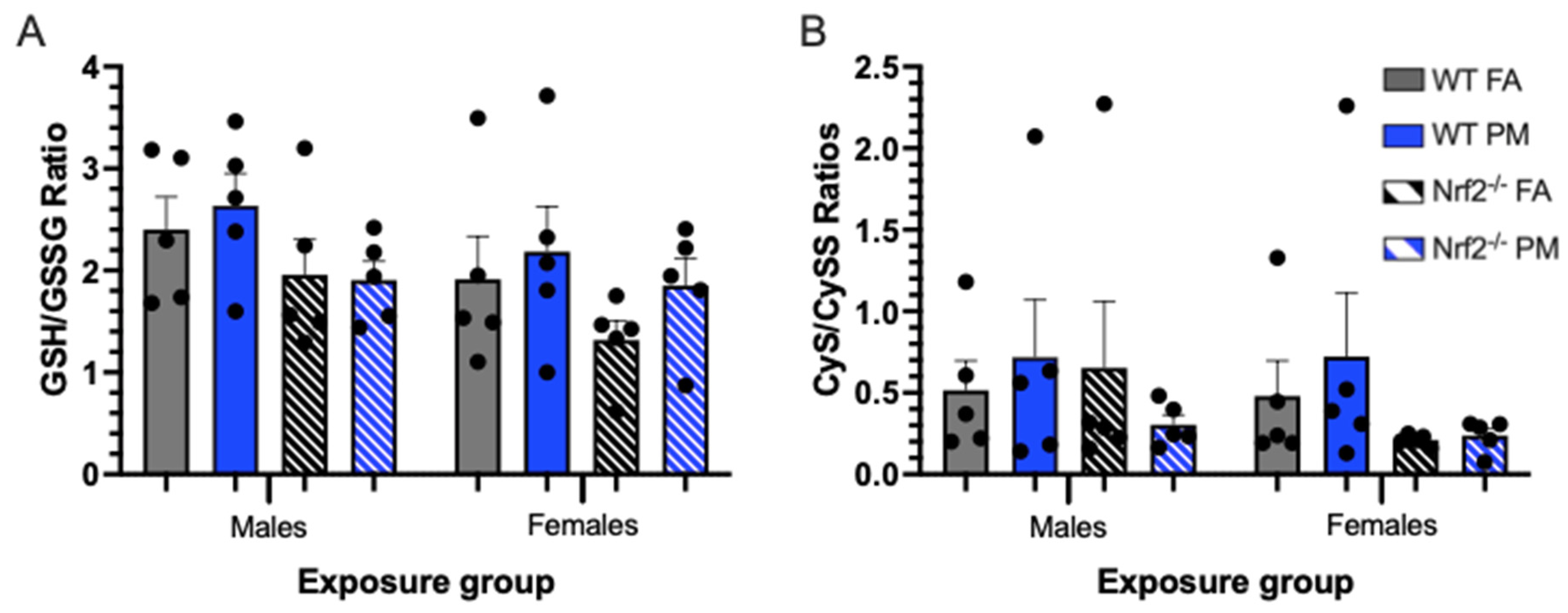
3.4. Thiol Ratios in Hepatic Tissue
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, N.M.; Hoffmann, A.R.; Behlen, J.C.; Lau, C.; Pendleton, D.; Harvey, N.; Shore, R.; Li, Y.; Chen, J.; Tian, Y.; et al. Air pollution and children’s health—A review of adverse effects associated with prenatal exposure from fine to ultrafine particulate matter. Environ. Health Prev. Med. 2021, 26, 72. [Google Scholar] [CrossRef]
- Health Effects Institute. Health Effects Institute State of Global Air 2019; Health Effects Institute: Boston, MA, USA, 2019; Volume 24. [Google Scholar]
- WHO. WHO’s Global Air-Quality Guidelines; WHO: Geneva, Switzerland, 2021; ISBN 9789240034228.
- da Costa e Oliveira, J.R.; Base, L.H.; de Abreu, L.C.; Filho, C.F.; Ferreira, C.; Morawska, L. Ultrafine particles and children’s health: Literature review. Paediatr. Respir. Rev. 2019, 32, 73–81. [Google Scholar] [CrossRef]
- Ohlwein, S.; Kappeler, R.; Kutlar Joss, M.; Künzli, N.; Hoffmann, B. Health effects of ultrafine particles: A systematic literature review update of epidemiological evidence. Int. J. Public Health 2019, 64, 547–559. [Google Scholar] [CrossRef] [PubMed]
- Bové, H.; Bongaerts, E.; Slenders, E.; Bijnens, E.M.; Saenen, N.D.; Gyselaers, W.; Van Eyken, P.; Plusquin, M.; Roeffaers, M.B.J.; Ameloot, M.; et al. Ambient black carbon particles reach the fetal side of human placenta. Nat. Commun. 2019, 10, 3866. [Google Scholar] [CrossRef] [PubMed]
- Uwak, I.; Olson, N.; Fuentes, A.; Moriarty, M.; Pulczinski, J.; Lam, J.; Xu, X.; Taylor, B.D.; Taiwo, S.; Koehler, K.; et al. Application of the navigation guide systematic review methodology to evaluate prenatal exposure to particulate matter air pollution and infant birth weight. Environ. Int. 2021, 148, 106378. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tang, Y.; Song, X.; Lazar, L.; Li, Z.; Zhao, J. Impact of ambient PM2.5 on adverse birth outcome and potential molecular mechanism. Ecotoxicol. Environ. Saf. 2019, 169, 248–254. [Google Scholar] [CrossRef]
- Percy, Z.; Defranco, E.; Xu, F.; Hall, E.S.; Haynes, E.N.; Jones, D.; Muglia, L.J.; Chen, A. Trimester Specific PM 2.5 Exposure and Fetal Growth in Ohio, HHS Public Access. Environ. Res. 2019, 171, 111–118. [Google Scholar] [CrossRef]
- Jedrychowski, W.A.; Perera, F.P.; Spengler, J.D.; Mroz, E.; Stigter, L.; Zbieta Flak, E.; Majewska, R.; Klimaszewska-Rembiasz, M.; Jacek, R. Intrauterine exposure to fine particulate matter as a risk factor for increased susceptibility to acute broncho-pulmonary infections in early childhood. Int. J. Hyg. Environ. Health 2013, 216, 395–401. [Google Scholar] [CrossRef] [Green Version]
- Jedrychowski, W.; Perera, F.P.; Maugeri, U.; Mrozek-Budzyn, D.; Mroz, E.; Flak, E.; Edwards, S.; Spengler, J.D.; Jacek, R.; Sowa, A.; et al. Early wheezing phenotypes and severity of respiratory illness in very early childhood. Study on intrauterine exposure to fine particle matter. Environ. Int. 2009, 35, 877–884. [Google Scholar] [CrossRef] [Green Version]
- Bernstein, J.A.; Alexis, N.; Barnes, C.; Bernstein, I.L.; Bernstein, J.A.; Nel, A.; Peden, D.; Diaz-Sanchez, D.; Tarlo, S.M.; Williams, P.B. Health effects of air pollution. J. Allergy Clin. Immunol. 2004, 114, 1116–1123. [Google Scholar] [CrossRef]
- Lavigne, E.; Donelle, J.; Hatzopoulou, M.; Van Ryswyk, K.; Van Donkelaar, A.; Martin, R.V.; Chen, H.; Stieb, D.M.; Gasparrini, A.; Crighton, E.; et al. Spatiotemporal variations in ambient ultrafine particles and the incidence of childhood asthma. Am. J. Respir. Crit. Care Med. 2019, 199, 1487–1495. [Google Scholar] [CrossRef] [Green Version]
- Wright, R.J.; Coull, B.A. Small but mighty: Prenatal ultrafine particle exposure linked to childhood asthma incidence. Am. J. Respir. Crit. Care Med. 2019, 199, 1448–1450. [Google Scholar] [CrossRef]
- Fang, J.; Song, X.; Xu, H.; Wu, R.; Song, J.; Xie, Y.; Xu, X.; Zeng, Y.; Wang, T.; Zhu, Y.; et al. Associations of ultrafine and fine particles with childhood emergency room visits for respiratory diseases in a megacity. Thorax 2021, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Darrow, L.A.; Klein, M.; Flanders, W.D.; Mulholland, J.A.; Tolbert, P.E.; Strickland, M.J. Air pollution and acute respiratory infections among children 0-4 years of age: An 18-year time-series study. Am. J. Epidemiol. 2014, 180, 968–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kensler, T.W.; Wakabayashi, N. Nrf2: Friend or foe for chemoprevention? Carcinogenesis 2010, 31, 90–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva-Islas, C.A.; Maldonado, P.D. Canonical and non-canonical mechanisms of Nrf2 activation. Pharmacol. Res. 2018, 134, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Jang, J.H.; Chen, C.Y.; Na, H.K.; Surh, Y.J. A formulated red ginseng extract rescues PC12 cells from PCB-induced oxidative cell death through Nrf2-mediated upregulation of heme oxygenase-1 and glutamate cysteine ligase. Toxicology 2010, 278, 131–139. [Google Scholar] [CrossRef]
- Li, Y.; Kawada, T.; Azuma, A. Nrf2 is a protective factor against oxidative stresses induced by diesel exhaust particle in allergic asthma. Oxid. Med. Cell. Longev. 2013, 2013, 323607. [Google Scholar] [CrossRef] [Green Version]
- Rychlik, K.A.; Secrest, J.R.; Lau, C.; Pulczinski, J.; Zamora, M.L.; Leal, J.; Langley, R.; Myatt, L.G.; Raju, M.; Chang, R.C.A.; et al. In utero ultrafine particulate matter exposure causes offspring pulmonary immunosuppression. Proc. Natl. Acad. Sci. USA 2019, 116, 3443–3448. [Google Scholar] [CrossRef] [Green Version]
- Behlen, J.C.; Lau, C.H.; Li, Y.; Dhagat, P.; Stanley, J.A.; Hoffman, A.R.; Golding, M.C.; Zhang, R.; Johnson, N.M. Gestational exposure to ultrafine particles reveals sex- and dose-specific changes in offspring birth outcomes, placental morphology, and gene networks. Toxicol. Sci. 2021, 184, 204–213. [Google Scholar] [CrossRef]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I.; et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.P.; Liang, Y. Measuring the poise of thiol/disulfide couples in vivo. Free Radic. Biol. Med. 2009, 47, 1329–1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castañeda, A.R.; Bein, K.J.; Smiley-Jewell, S.; Pinkerton, K.E. Fine particulate matter (PM 2.5) enhances allergic sensitization in BALB/c mice. J. Toxicol. Environ. Health Part A 2017, 80, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Kweider, N.; Huppertz, B.; Rath, W.; Lambertz, J.; Caspers, R.; ElMoursi, M.; Pecks, U.; Kadyrov, M.; Fragoulis, A.; Pufe, T.; et al. The effects of Nrf2 deletion on placental morphology and exchange capacity in the mouse. J. Matern. Neonatal. Med. 2017, 30, 2068–2073. [Google Scholar] [CrossRef]
- Nezu, M.; Souma, T.; Yu, L.; Sekine, H.; Takahashi, N.; Zu-Sern Wei, A.; Ito, S.; Fukamizu, A.; Zsengeller, Z.K.; Nakamura, T.; et al. Nrf2 inactivation enhances placental angiogenesis in a preeclampsia mouse model and improves maternal and fetal outcomes. Sci. Signal. 2017, 10, eaam5711. [Google Scholar] [CrossRef]
- Ma, Q.; Battelli, L.; Hubbs, A.F. Multiorgan autoimmune inflammation, enhanced lymphoproliferation, and impaired homeostasis of reactive oxygen species in mice lacking the antioxidant-activated transcription factor Nrf2. Am. J. Pathol. 2006, 168, 1960–1974. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Kim, B.; Lee, S.; Kim, H.; Lee, C.M.; Yu, J.; Kang, M.; Yu, H.; Lee, E.; Jung, Y.; et al. Prenatal particulate matter/tobacco smoke increases infants’ respiratory infections: COCOA study. Allergy Asthma Immunol. Res. 2015, 7, 573–582. [Google Scholar] [CrossRef] [Green Version]
- Perveen, K.; Quach, A.; Stark, M.J.; Prescott, S.L.; Barry, S.C.; Hii, C.S.; Ferrante, A. Characterization of the transient deficiency of pkc isozyme levels in immature cord blood t cells and its connection to anti-allergic cytokine profiles of the matured cells. Int. J. Mol. Sci. 2021, 22, 12650. [Google Scholar] [CrossRef] [PubMed]
- Perveen, K.; Quach, A.; McPhee, A.; Prescott, S.L.; Barry, S.C.; Hii, C.S.; Ferrante, A. Cord blood t cells expressing high and low pkcζ levels develop into cells with a propensity to display th1 and th9 cytokine profiles, respectively. Int. J. Mol. Sci. 2021, 22, 4907. [Google Scholar] [CrossRef]
- Cuadrado, A.; Manda, G.; Hassan, A.; Alcaraz, M.J.; Barbas, C.; Daiber, A.; Ghezzi, P.; León, R.; López, M.G.; Oliva, B.; et al. Transcription factor NRF2 as a therapeutic target for chronic diseases: A systems medicine approach. Pharmacol. Rev. 2018, 70, 348–383. [Google Scholar] [CrossRef] [Green Version]
- Morzadec, C.; Macoch, M.; Sparfel, L.; Kerdine-Römer, S.; Fardel, O.; Vernhet, L. Nrf2 expression and activity in human T lymphocytes: Stimulation by T cell receptor activation and priming by inorganic arsenic and tert-butylhydroquinone. Free Radic. Biol. Med. 2014, 71, 133–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taguchi, K.; Kensler, T.W. Nrf2 in liver toxicology. Arch. Pharm. Res. 2020, 43, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.F.A.; Van Winkle, L.S.; Esser, C.; Haarmann-Stemmann, T. The aryl hydrocarbon receptor as a target of environmental stressors—Implications for pollution mediated stress and inflammatory responses. Redox Biol. 2020, 34, 101530. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lau, C.H.; Pendleton, D.; Drury, N.L.; Zhao, J.; Li, Y.; Zhang, R.; Wright, G.A.; Hoffmann, A.R.; Johnson, N.M. NRF2 Protects against Altered Pulmonary T Cell Differentiation in Neonates Following In Utero Ultrafine Particulate Matter Exposure. Antioxidants 2022, 11, 202. https://doi.org/10.3390/antiox11020202
Lau CH, Pendleton D, Drury NL, Zhao J, Li Y, Zhang R, Wright GA, Hoffmann AR, Johnson NM. NRF2 Protects against Altered Pulmonary T Cell Differentiation in Neonates Following In Utero Ultrafine Particulate Matter Exposure. Antioxidants. 2022; 11(2):202. https://doi.org/10.3390/antiox11020202
Chicago/Turabian StyleLau, Carmen H., Drew Pendleton, Nicholas L. Drury, Jiayun Zhao, Yixin Li, Renyi Zhang, Gus A. Wright, Aline Rodrigues Hoffmann, and Natalie M. Johnson. 2022. "NRF2 Protects against Altered Pulmonary T Cell Differentiation in Neonates Following In Utero Ultrafine Particulate Matter Exposure" Antioxidants 11, no. 2: 202. https://doi.org/10.3390/antiox11020202
APA StyleLau, C. H., Pendleton, D., Drury, N. L., Zhao, J., Li, Y., Zhang, R., Wright, G. A., Hoffmann, A. R., & Johnson, N. M. (2022). NRF2 Protects against Altered Pulmonary T Cell Differentiation in Neonates Following In Utero Ultrafine Particulate Matter Exposure. Antioxidants, 11(2), 202. https://doi.org/10.3390/antiox11020202