Prophylactic and Ameliorative Effects of PPAR-γ Agonist Pioglitazone in Improving Oxidative Stress, Germ Cell Apoptosis and Inflammation in Gentamycin-Induced Testicular Damage in Adult Male Albino Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Study Design
2.3. Collection of Samples
2.4. Assessment of Testis Weight and Sperm Parameters (Count and Motility)
2.5. Hormonal Assay
2.6. Measurement of Serum Inflammatory Markers
2.7. Measurement of Oxidative Stress & Antioxidant Markers
2.8. RNA Extraction and Quantitative RT-PCR Detection of Testicular BAX & BCL2 Genes
2.9. Histopathological Examination
2.10. Statistical Analysis
2.11. Ethical Statement
3. Results
3.1. Body Weight, Testis Weight and Relative Testis Weight
3.2. Sperm Parameters
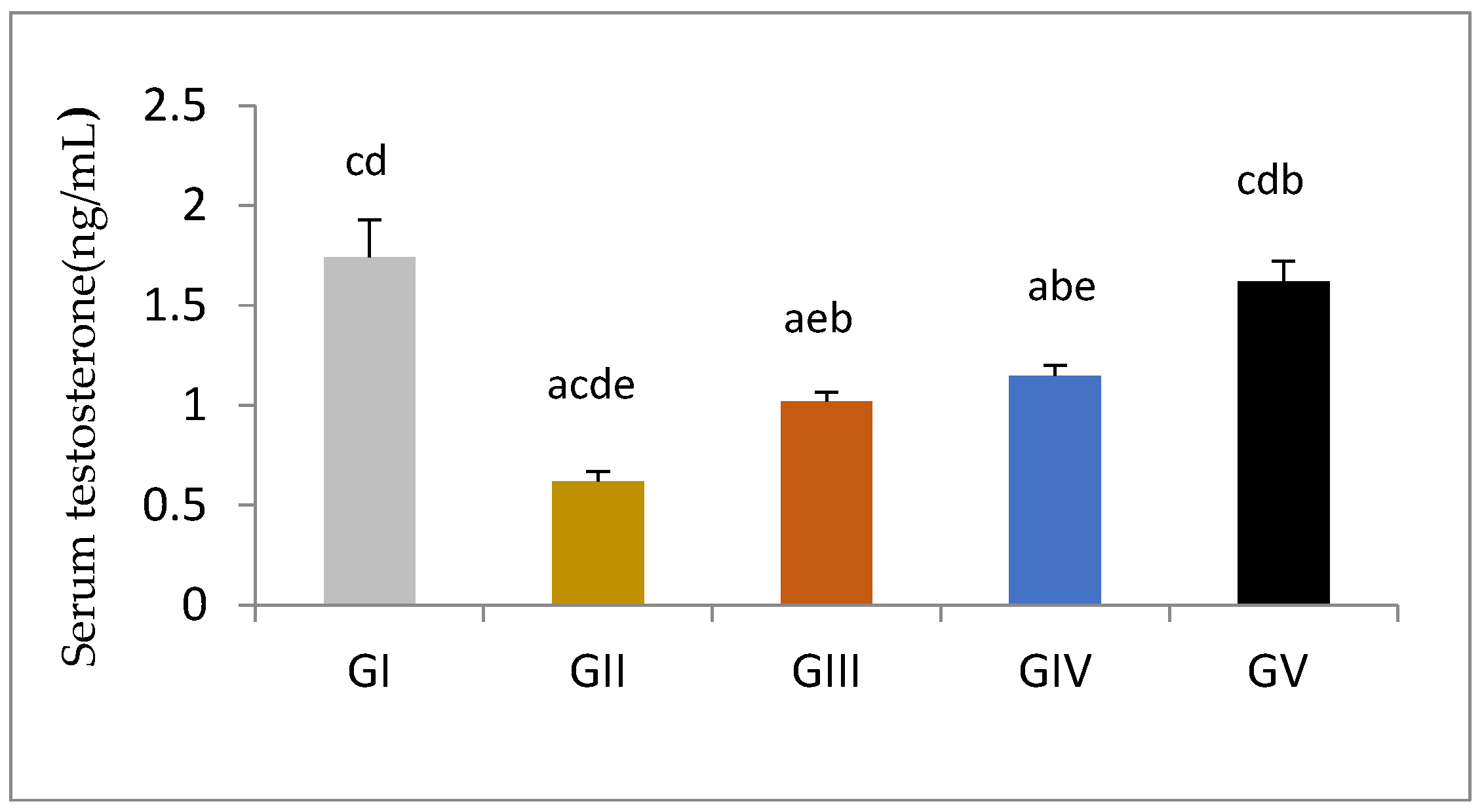
3.3. Pituitary-Testicular Hormonal Axis
3.4. Inflammatory Markers
3.5. Oxidative & Antioxidant State
3.5.1. Oxidative Stress (Total Antioxidant Capacity (TAC) and Total Oxidant Status (TOS) Serum Levels
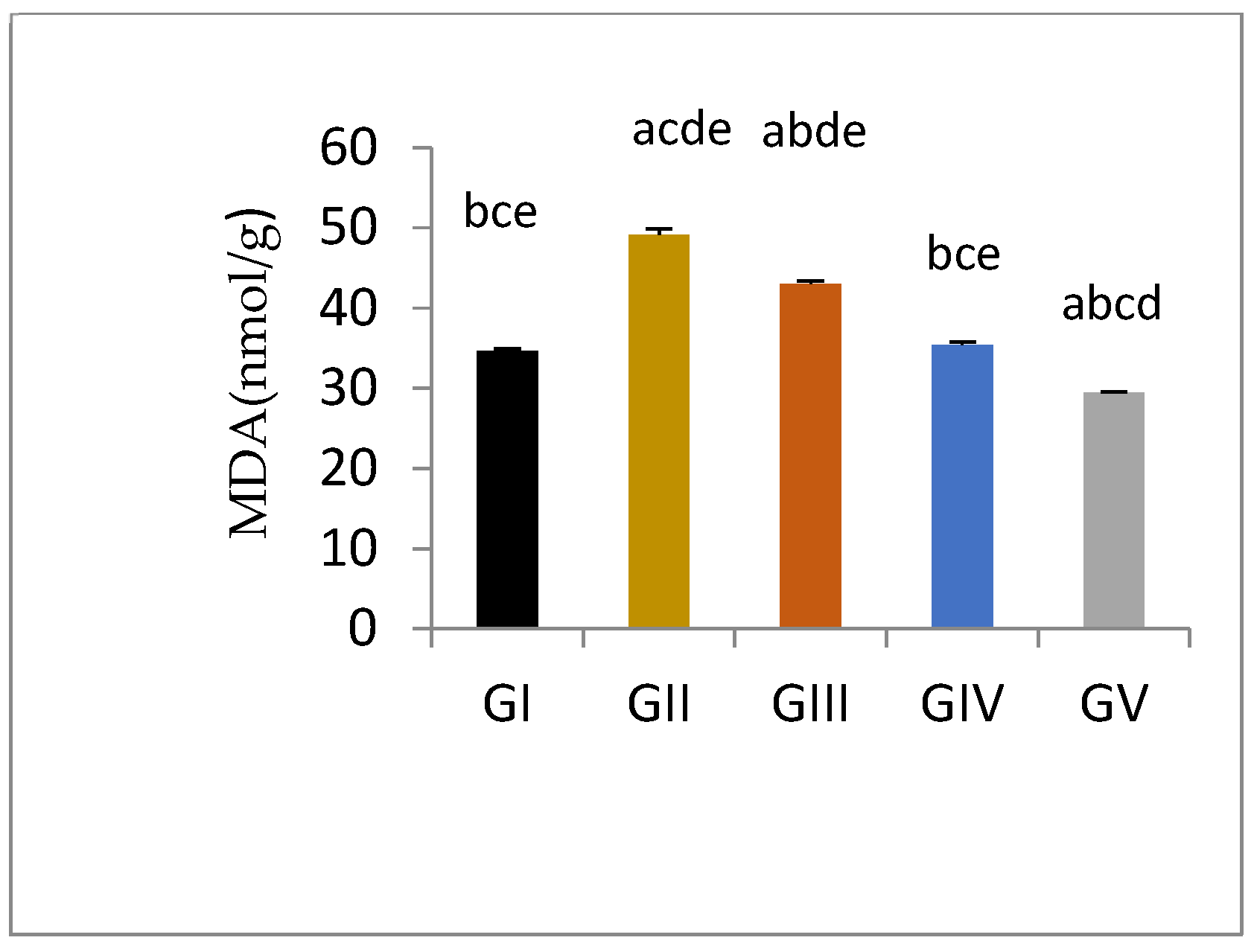
3.5.2. Testicular Tissue Lipid Peroxide Production
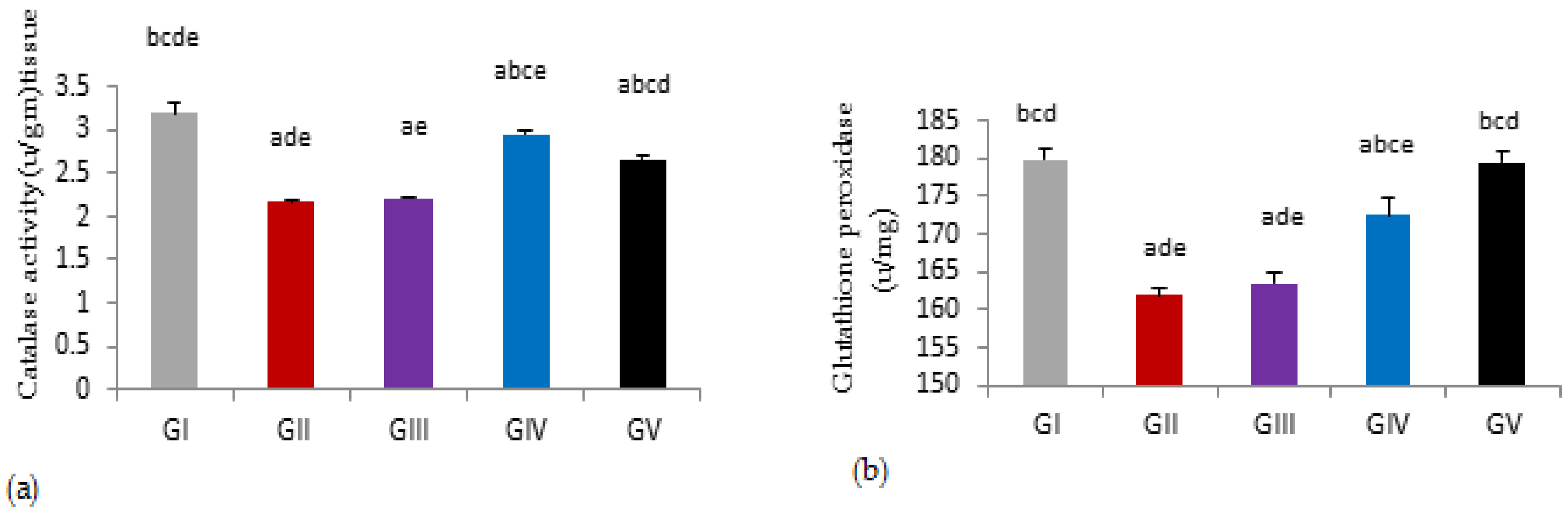
3.5.3. Testicular Antioxidant Activities
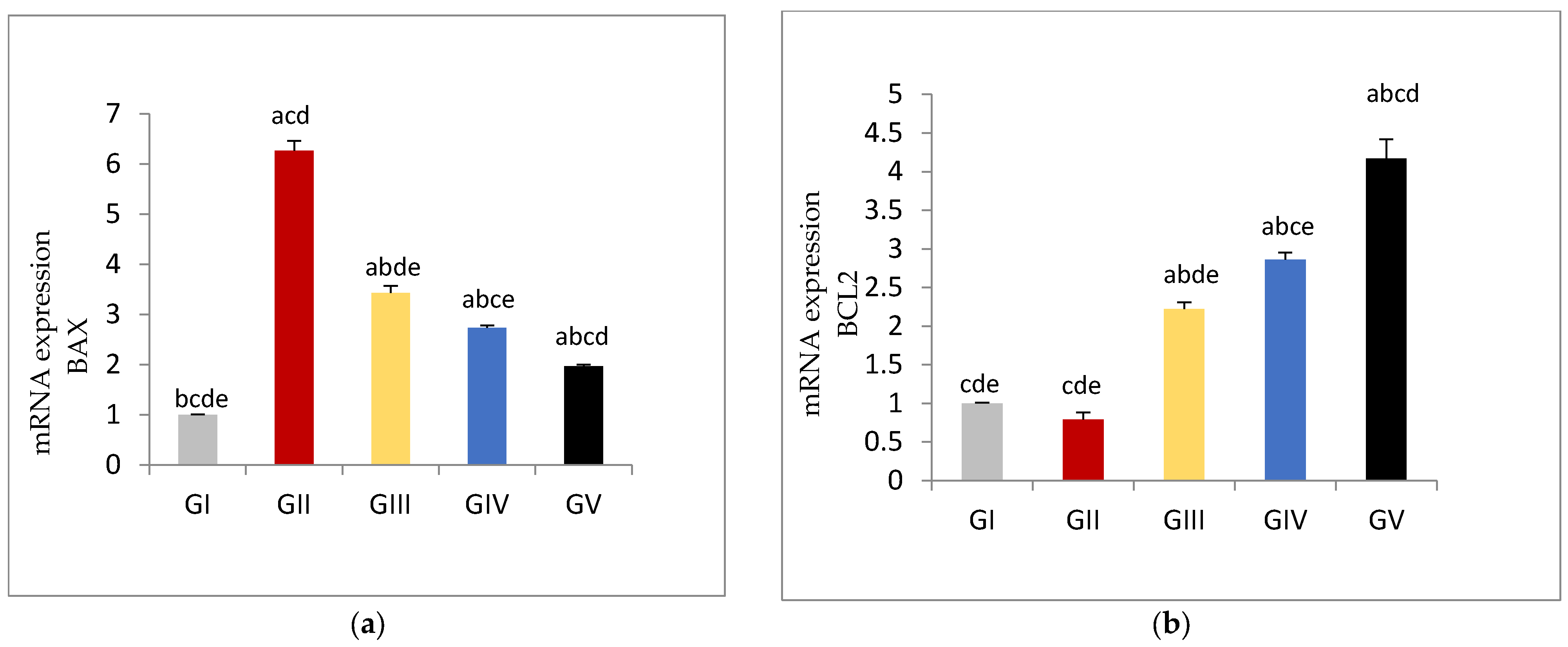
3.6. Apoptotic and Anti-Apoptotic Tissue Expression Levels
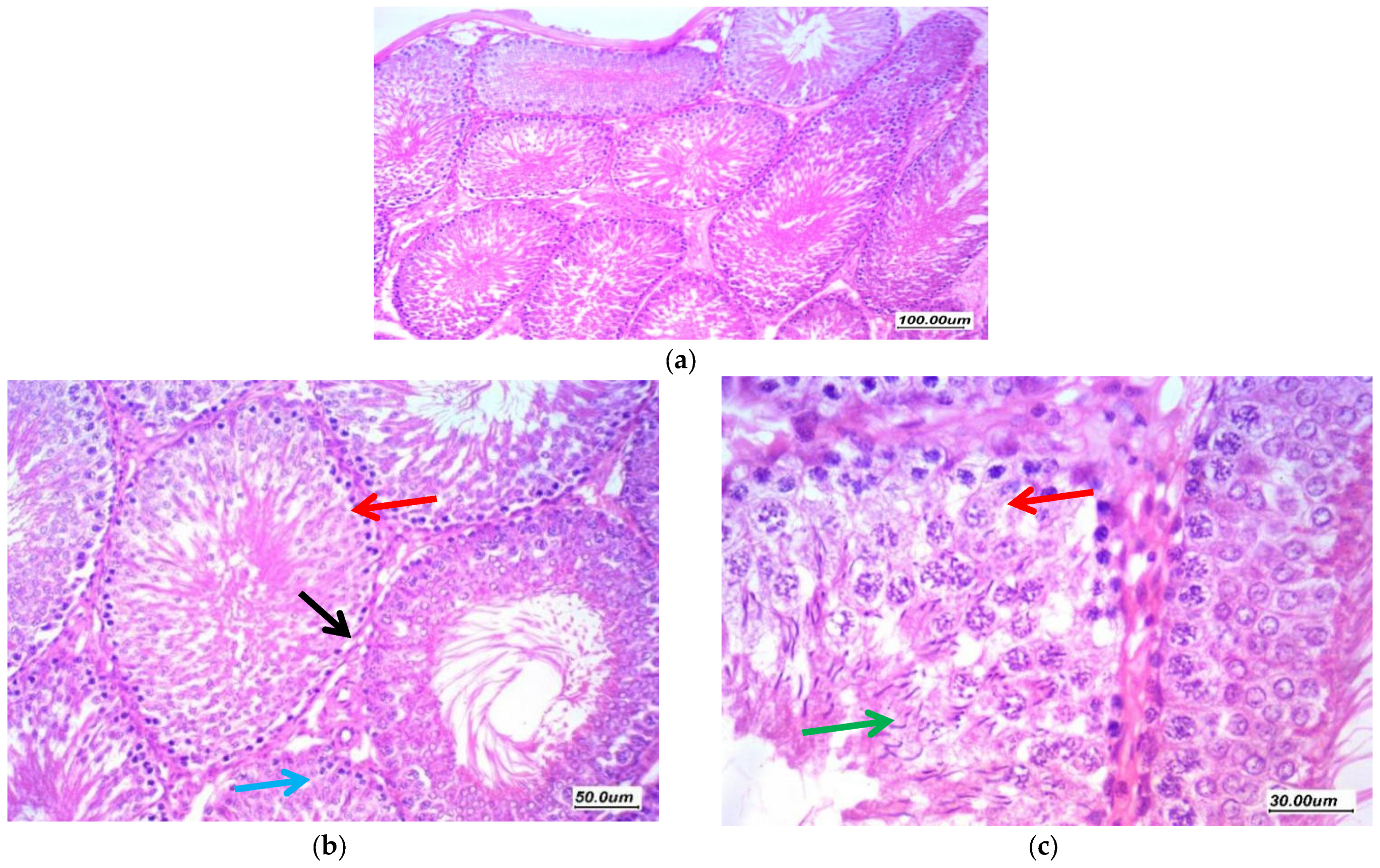
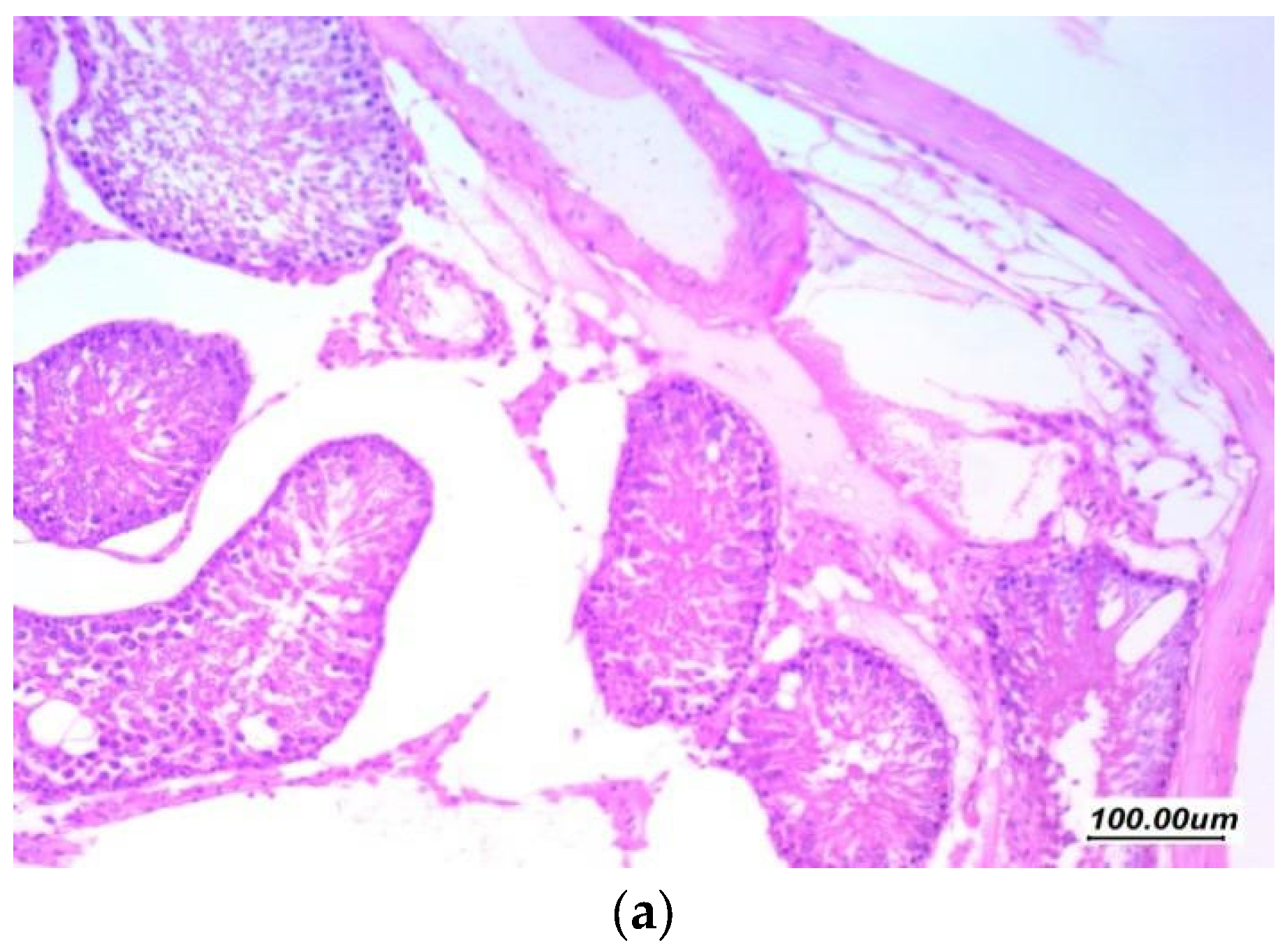
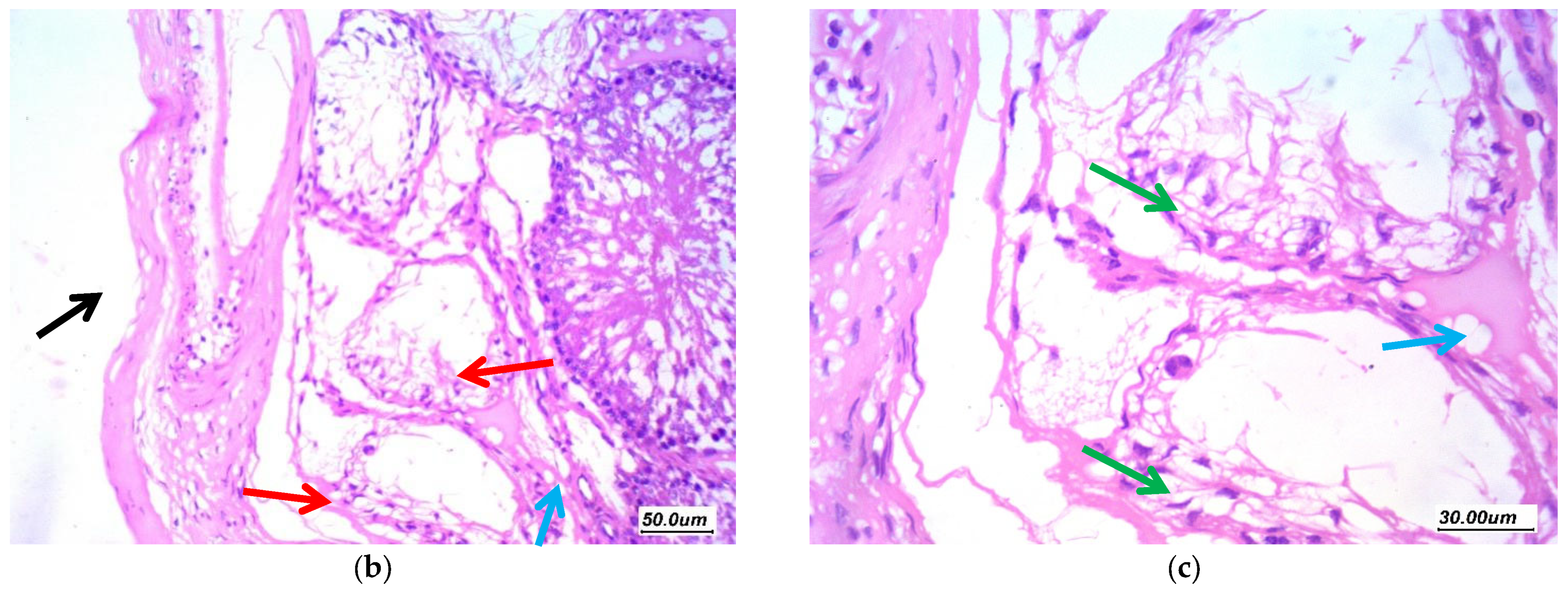
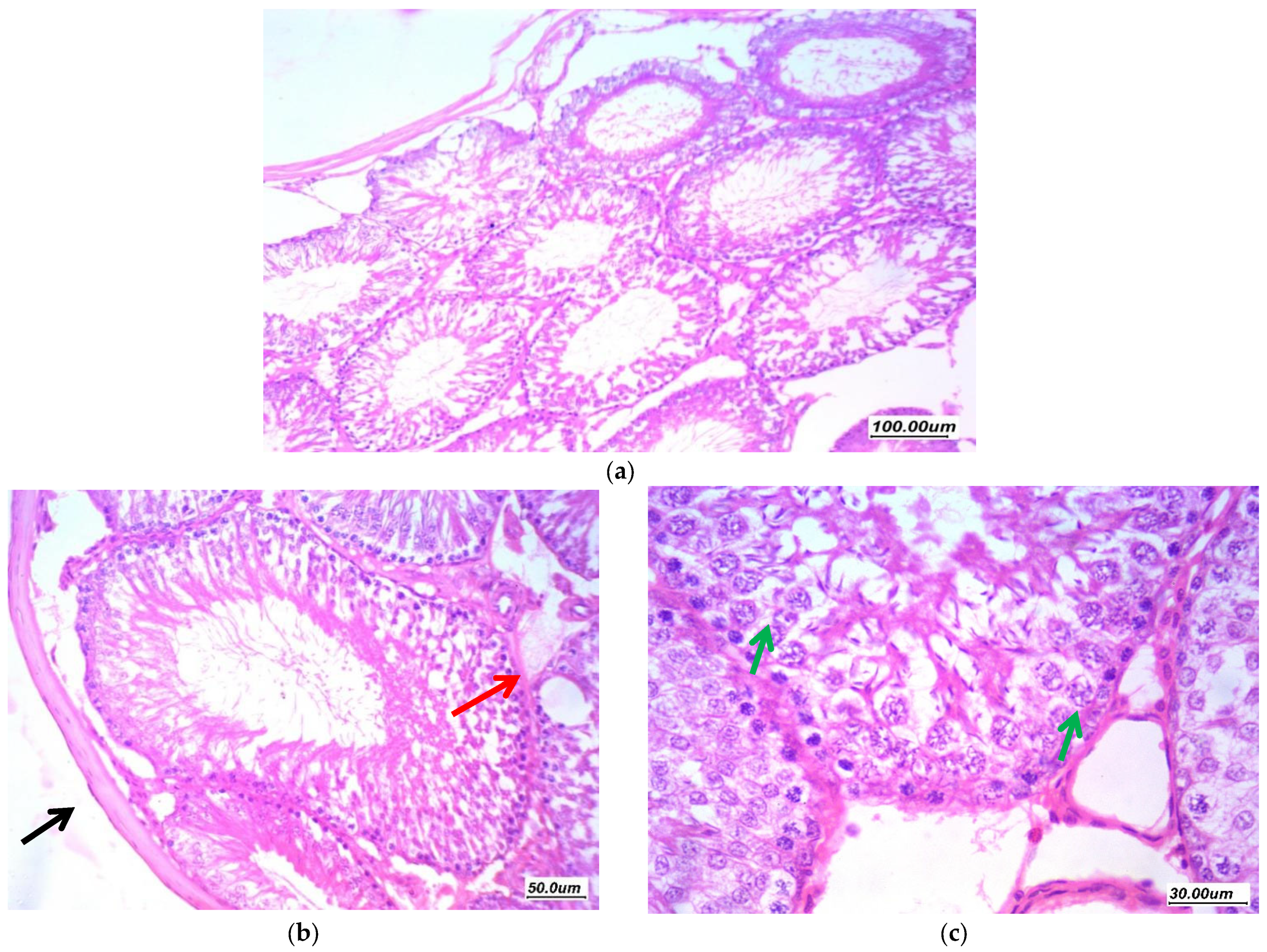
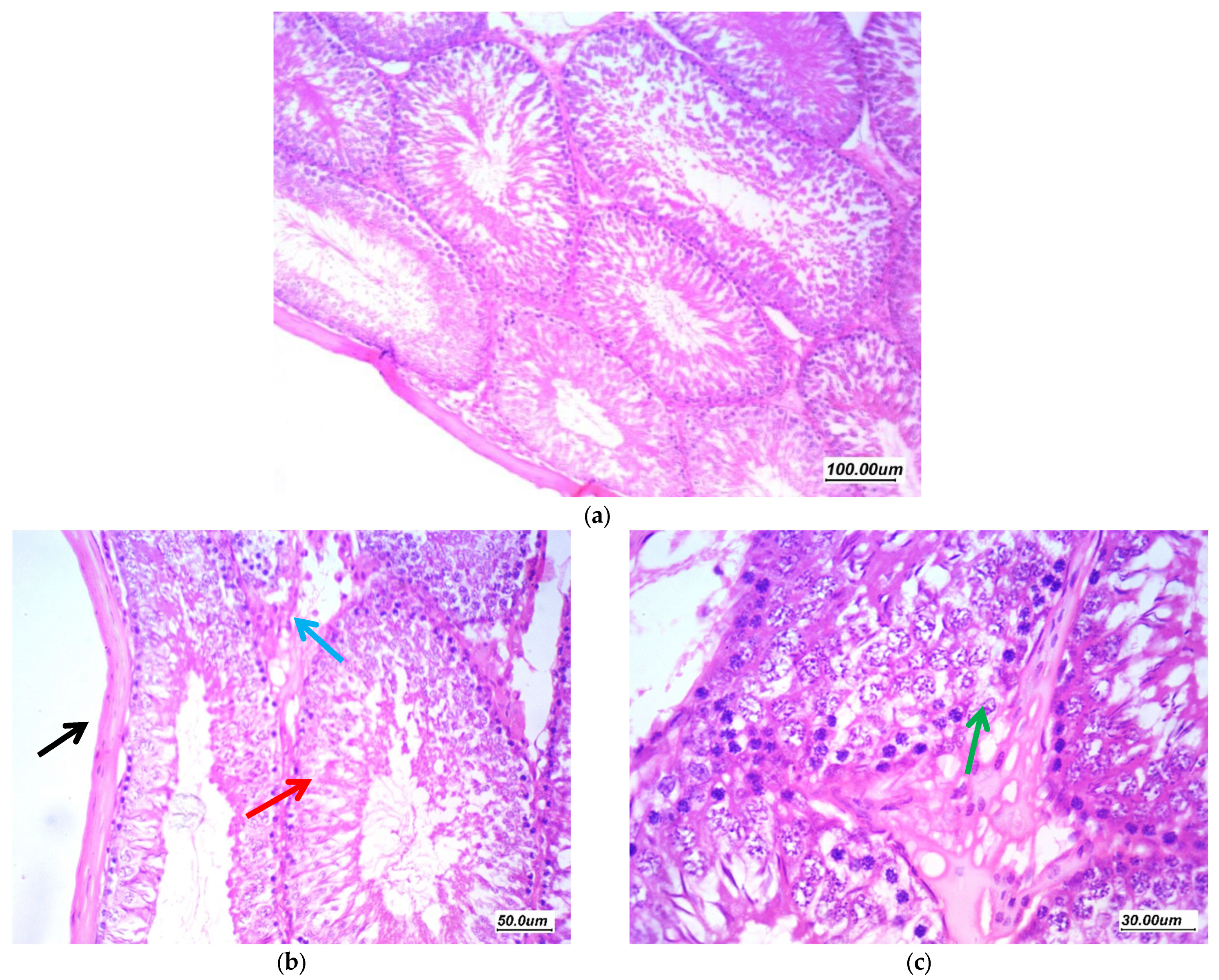
3.7. Histopathological Assessment of the Testicular Tissue
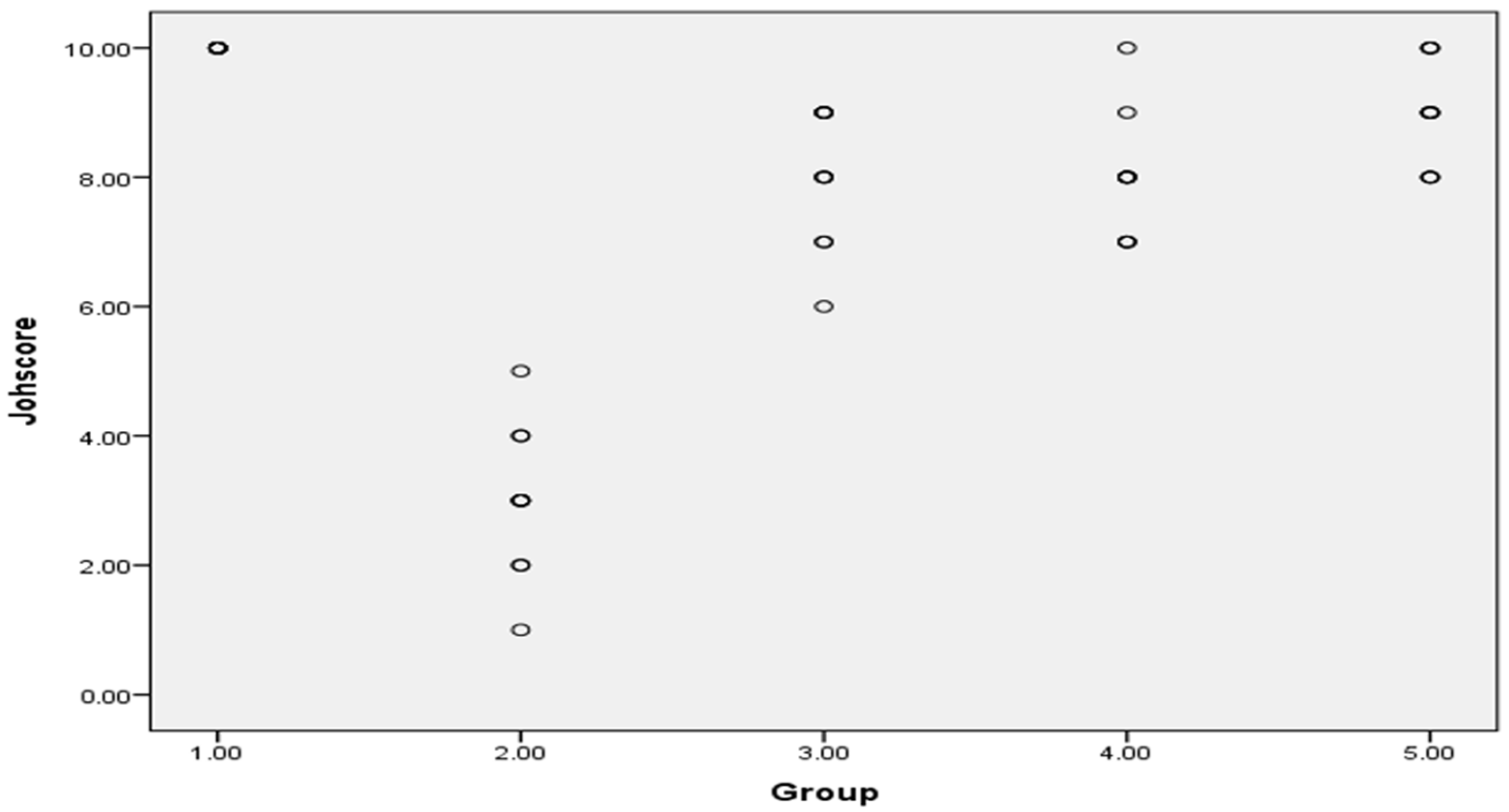
3.7.1. Histopathological Assessment
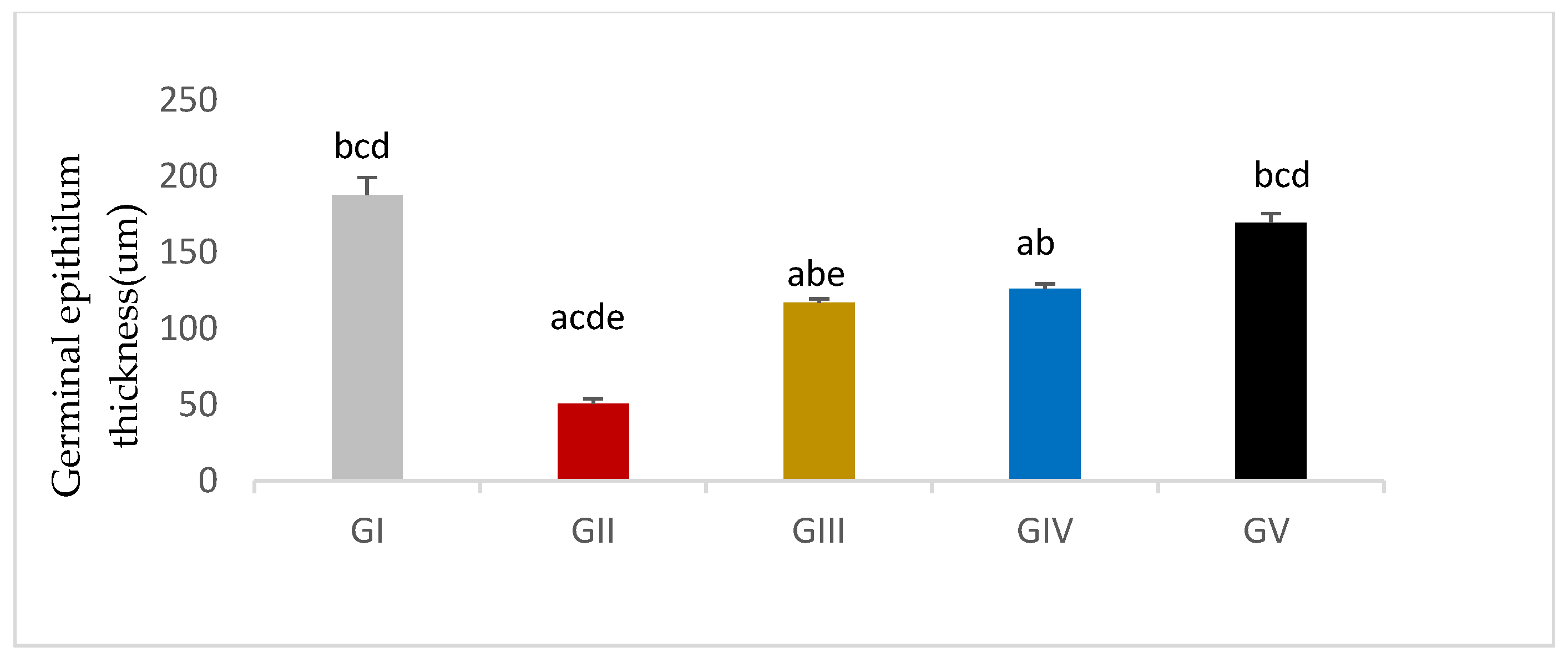
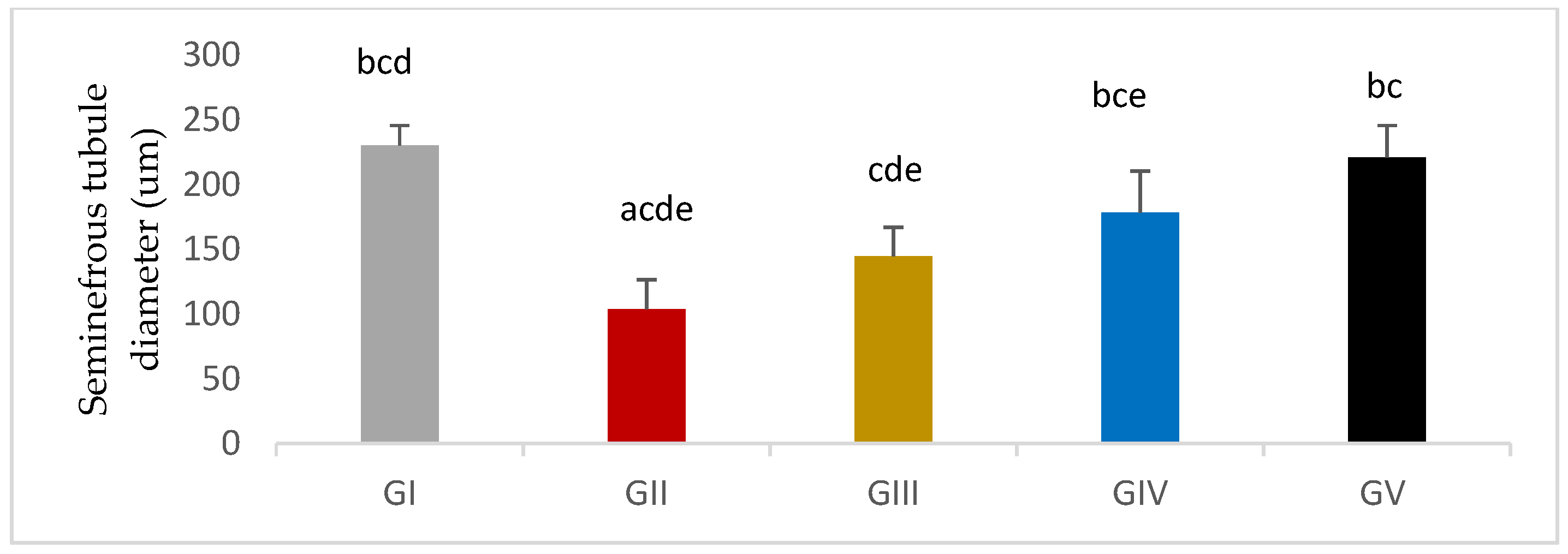
3.7.2. Morphometric Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agarwal, A.; Baskaran, S.; Parekh, N.; Cho, C.-L.; Henkel, R.; Vij, S.; Arafa, M.; Selvam, M.K.P.; Shah, R. Male infertility. Lancet 2021, 397, 319–333. [Google Scholar] [CrossRef]
- Ogundipe, D.J.; Akomolafe, R.O.; Sanusi, A.A.; Imafidon, C.E.; Olukiran, O.S.; Oladele, A.A. Ocimum gratissimum ameliorates gentamicin-induced kidney injury but decreases creatinine clearance following sub-chronic administration in rats. J. Evid. Based Complement. Altern. Med. 2017, 22, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, I.C.; Baek, H.S.; Shin, I.S.; Moon, C.; Kim, S.H.; Yun, W.K.; Nam, K.H.; Kim, H.C.; Kim, J.C. Melatonin prevents gentamicin-induced testicular toxicity and oxidative stress in rats. Andrologia 2014, 46, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Keogh, L.M.; Byrne, P.G.; Silla, A.J. The effect of gentamicin on sperm motility and bacterial abundance during chilled sperm storage in the Booroolong frog. Gen. Comp. Endocrinol. 2017, 243, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, A.; Khaki, A.; Ahmadi, A.H.; Rastgar, H.; Rezazadeh, S. Zingiber officinale protective effects on gentamicin’s toxicity on sperm in rats. J. Med. Plant. 2010, 9, 93–98. [Google Scholar]
- Aly, H.A.; Hassan, M.H. Potential testicular toxicity of gentamicin in adult rats. Biochem. Biophys. Res. Commun. 2018, 497, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Lee, I.-C.; Lim, J.-H.; Moon, C.; Bae, C.-S.; Kim, S.-H.; Shin, D.-H.; Kim, H.-C.; Kim, J.-C. Spermatotoxic effects of α-chlorohydrin in rats. Lab. Anim. Res. 2012, 28, 11–16. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Matsushita, T.; Kusakabe, Y.; Kitamura, A.; Okada, S.; Murase, K. Protective effect of hydrogen-rich water against gentamicin-induced nephrotoxicity in rats using blood oxygenation level-dependent MR imaging. Magn. Reson. Med. Sci. 2011, 10, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.-L.; Xian, H.; Cao, J.-C.; Zhang, C.; Zhang, Y.-H.; Chen, M.-M.; Qian, Y.; Jiang, M. Peroxisome proliferator-activated receptor gamma signaling in human sperm physiology. Asian J. Androl. 2015, 17, 942. [Google Scholar]
- Akinola, O.; Dosumu, O.; Sanusi, S.; Ajayi, T.; Olajide, T. PPAR-γ agonist pioglitazone improves semen quality and testicular histomorphometrics with partial reversal of hyperglycaemia in alloxan-induced diabetic rats. MEFS J. 2015, 20, 271–279. [Google Scholar] [CrossRef]
- Koppula, S.; Kumar, H.; Kim, I.S.; Choi, D.-K. Reactive oxygen species and inhibitors of inflammatory enzymes, NADPH oxidase, and iNOS in experimental models of Parkinson’s disease. Mediat. Inflamm. 2012, 2012, 823902. [Google Scholar] [CrossRef] [PubMed]
- Chiarelli, F.; Di Marzio, D. Peroxisome proliferator-activated receptor-γ agonists and diabetes: Current evidence and future perspectives. Vasc. Health Risk Manag. 2008, 4, 297. [Google Scholar]
- Ishibashi, M.; Egashira, K.; Hiasa, K.-i.; Inoue, S.; Ni, W.; Zhao, Q.; Usui, M.; Kitamoto, S.; Ichiki, T.; Takeshita, A. Antiinflammatory and antiarteriosclerotic effects of pioglitazone. Hypertension 2002, 40, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y.; Shimoda, M.; Hamamoto, S.; Tawaramoto, K.; Kawasaki, F.; Hashiramoto, M.; Nakashima, K.; Matsuki, M.; Kaku, K. Molecular mechanism by which pioglitazone preserves pancreatic β-cells in obese diabetic mice: Evidence for acute and chronic actions as a PPAR-γ agonist. Am. J. Physiol.-Endocrinol. Metab. 2010, 298, E278–E286. [Google Scholar] [CrossRef]
- Mayoral, R.; Osborn, O.; McNelis, J.; Johnson, A.M.; Izquierdo, C.L.; Chung, H.; Li, P.; Traves, P.G.; Bandyopadhyay, G.; Pessentheiner, A.R. Adipocyte SIRT1 knockout promotes PPAR-γ activity, adipogenesis and insulin sensitivity in chronic-HFD and obesity. Mol. Metab. 2015, 4, 378–391. [Google Scholar] [CrossRef]
- Fürnsinn, C.; Waldhäusl, W. Thiazolidinediones: Metabolic actions in vitro C. Fürnsinn, W. Waldhäusl: Thiazolidinediones: Metabolic action in vitro. Diabetologia 2002, 45, 1211–1223. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S.; Gupta, P.; Saini, A.S.; Kaushal, C.; Sharma, S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J. Adv. Pharm. Technol. Res. 2011, 2, 236. [Google Scholar] [CrossRef]
- Dehmer, T.; Heneka, M.T.; Sastre, M.; Dichgans, J.; Schulz, J.B. Protection by pioglitazone in the MPTP model of Parkinson’s disease correlates with IκBα induction and block of NFκB and iNOS activation. J. Neurochem. 2004, 88, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Khodeer, D.M.; Zaitone, S.A.; Farag, N.E.; Moustafa, Y.M. Cardioprotective effect of pioglitazone in diabetic and non-diabetic rats subjected to acute myocardial infarction involves suppression of AGE-RAGE axis and inhibition of apoptosis. Can. J. Physiol. Pharmacol. 2016, 94, 463–476. [Google Scholar] [CrossRef]
- Medić, B.; Stojanović, M.; Rovčanin, B.; Kekić, D.; Škodrić, S.R.; Jovanović, G.B.; Vujović, K.S.; Divac, N.; Stojanović, R.; Radenković, M. Pioglitazone attenuates kidney injury in an experimental model of gentamicin-induced nephrotoxicity in rats. Sci. Rep. 2019, 9, 13689. [Google Scholar] [CrossRef]
- Al-Azzam, S.; Abdul-Razzak, K.; Jaradat, M. The nephroprotective effects of pioglitazone and glibenclamide against gentamicin-induced nephrotoxicity in rats: A comparative study. J. Chemother. 2010, 22, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Maeshiba, Y.; Kiyota, Y.; Yamashita, K.; Yoshimura, Y.; Motohashi, M.; Tanayama, S.J.A. Disposition of the new antidiabetic agent pioglitazone in rats, dogs, and monkeys. Arzneimittelforschung 1997, 47, 29–34. [Google Scholar]
- Tomita, K.; Azuma, T.; Kitamura, N.; Nishida, J.; Tamiya, G.; Oka, A.; Inokuchi, S.; Nishimura, T.; Suematsu, M.; Ishii, H. Pioglitazone prevents alcohol-induced fatty liver in rats through up-regulation of c-Met. Gastroenterology 2004, 126, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Catanzariti, F.; Cantoro, U.; Lacetera, V.; Muzzonigro, G.; Polito, M. Comparison between WHO (World Health Organization) 2010 and WHO 1999 parameters for semen analysis–interpretation of 529 consecutive samples. Arch. Ital. Urol. Androl. 2013, 85, 125–129. [Google Scholar] [CrossRef]
- Jaâ, M.; Noor, M.M. A Simple Technique for Rapid Assessment of Rat (Rattus norvegicus) Sperm Motility. Biol. Med. Nat. Prod. Chem. 2020, 9, 105–107. [Google Scholar]
- Asl, R.S.; Shariatmadari, F.; Sharafi, M.; Torshizi, M.A.K.; Shahverdi, A. Improvements in semen quality, sperm fatty acids, and reproductive performance in aged Ross breeder roosters fed a diet supplemented with a moderate ratio of n-3: N-6 fatty acids. Poult. Sci. 2018, 97, 4113–4121. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Hamzeh, M.; Hosseinimehr, S.J.; Karimpour, A.; Mohammadi, H.R.; Khalatbary, A.R.; Amiri, F.T. Cerium oxide nanoparticles protect cyclophosphamide-induced testicular toxicity in mice. Int. J. Prev. Med. 2019, 10, 5. [Google Scholar]
- Abdel-Wahab, A.; Hassanin, K.; Mahmoud, A.A.; Abdel-Badeea, W.I.; Abdel-Razik, A.-R.H.; Attia, E.Z.; Abdelmohsen, U.R.; Abdel Aziz, R.L.; Najda, A.; Alanazi, I.S. Physiological Roles of Red Carrot Methanolic Extract and Vitamin E to Abrogate Cadmium-Induced Oxidative Challenge and Apoptosis in Rat Testes: Involvement of the Bax/Bcl-2 Ratio. Antioxidants 2021, 10, 1653. [Google Scholar] [CrossRef]
- Haschek, W.M.; Rousseaux, C.G.; Wallig, M.A.; Bolon, B. Toxicologic pathology: An introduction. In Haschek and Rousseaux’s Handbook of Toxicologic Pathology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–12. [Google Scholar]
- Johnsen, S.G. Testicular biopsy score count—A method for registration of spermatogenesis in human testes: Normal values and results in 335 hypogonadal males. Horm. Res. Paediatr. 1970, 1, 2–25. [Google Scholar] [CrossRef] [PubMed]
- Stumpp, T.; Sasso-Cerri, E.; Freymüller, E.; Miraglia, S.M. Apoptosis and testicular alterations in albino rats treated with etoposide during the prepubertal phase. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. Off. Publ. Am. Assoc. Anat. 2004, 279, 611–622. [Google Scholar] [CrossRef]
- Lirdi, L.C.; Stumpp, T.; Sasso-Cerri, E.; Miraglia, S.M. Amifostine protective effect on cisplatin-treated rat testis. Anat. Rec. Adv. Integr. Anat. Evol. Biol. Adv. Integr. Anat. Evol. Biol. 2008, 291, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.; Berndtson, W.E.; Cardoso, F. Plasma and testicular testosterone levels, volume density and number of Leydig cells and spermatogenic efficiency of rabbits. Braz. J. Med. Biol. Res. 2002, 35, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Babaeenezhad, E.; Nouryazdan, N.; Nasri, M.; Ahmadvand, H.; Sarabi, M.M. Cinnamic acid ameliorate gentamicin-induced liver dysfunctions and nephrotoxicity in rats through induction of antioxidant activities. Heliyon 2021, 7, e07465. [Google Scholar] [CrossRef]
- Sekulic-Jablanovic, M.; Wright, M.B.; Petkovic, V.; Bodmer, D. Pioglitazone Ameliorates Gentamicin Ototoxicity by Affecting the TLR and STAT Pathways in the Early Postnatal Organ of Corti. Front. Cell. Neurosci. 2020, 14, 566148. [Google Scholar] [CrossRef] [PubMed]
- Papich, M. Kanamycin Sulfatein: Saunders Handbook of Veterinary Drugs; Elsevier: London, UK, 2016. [Google Scholar]
- Elsawah, H.K.; Kandiel, M.M.; Amin, A.A.; Mokhimar, H.M.; El Mahmoudy, A.M. Gentamicin and amikacin adversely affect male infertility indicated by pharmacological, andrological and pathological evidence. J. Basic Clin. Physiol. Pharmacol. 2020, 9, 218. [Google Scholar] [CrossRef]
- Aly, H. Testicular toxicity of gentamicin in adult rats: Ameliorative effect of lycopene. Hum. Exp. Toxicol. 2019, 38, 1302–1313. [Google Scholar] [CrossRef] [PubMed]
- Karaman, M.; Budak, H.; Çiftci, M. Amoxicillin and gentamicin antibiotics treatment adversely influence the fertility and morphology through decreasing the Dazl gene expression level and increasing the oxidative stress. Arch. Physiol. Biochem. 2019, 125, 447–455. [Google Scholar] [CrossRef]
- Kerr, J. Spontaneous degeneration of germ cells in normal rat testis: Assessment of cell types and frequency during the spermatogenic cycle. Reproduction 1992, 95, 825–830. [Google Scholar] [CrossRef][Green Version]
- Khaki, A.; Novin, M.G.; Khaki, A.; Fathiazad, F.; Khaberi, M.; Hossinchi, J.; Sehizadeh, R. Ultra structural study of gentamicin and ofloxacin effect on testis tissue in rats: Light and transmission electron microscopy. Afr. J. Pharm. Pharmacol. 2009, 3, 105–109. [Google Scholar]
- Narayana, K. An aminoglycoside antibiotic gentamycin induces oxidative stress, reduces antioxidant reserve and impairs spermatogenesis in rats. J. Toxicol. Sci. 2008, 33, 85–96. [Google Scholar] [CrossRef]
- Jalilvand, N.; Hosseini, M.; Beheshti, F.; Ebrahimzadeh-Bideskan, A. Protective effect of PPAR-γ agonist pioglitazone, on testicular tissue and sperm parameters in hypothyroid rats. Toxin Rev. 2019, 40, 267–276. [Google Scholar] [CrossRef]
- Mahmoud, N.M.; Kabil, S.L. Pioglitazone abrogates testicular damage induced by testicular torsion/detorsion in rats. Iran. J. Basic Med. Sci. 2019, 22, 884. [Google Scholar] [PubMed]
- Surapaneni, K.; Jainu, M. Comparative effect of pioglitazone, quercetin and hydroxy citric acid on the status of lipid peroxidation and antioxidants in experimental non-alcoholic steatohepatitis. J. Physiol. Pharmacol. 2014, 65, 67–74. [Google Scholar] [PubMed]
- Hasan, M.M.; El-Shal, A.S.; Mackawy, A.M.; Ibrahim, E.M.; Abdelghany, E.M.; Saeed, A.A.; El-Gendy, J. Ameliorative effect of combined low dose of Pioglitazone and omega-3 on spermatogenesis and steroidogenesis in diabetic rats. J. Cell Biochem. 2020, 121, 1524–1540. [Google Scholar] [CrossRef] [PubMed]
- Fetouh, F.A.; Azab, A.E.S. Ameliorating effects of curcumin and propolis against the reproductive toxicity of gentamicin in adult male guinea pigs: Quantitative analysis and morphological study. Am. J. Life Sci. 2014, 2, 138–149. [Google Scholar] [CrossRef]
- Sharpe, R.; Maddocks, S.; Millar, M.; Kerr, J.; Saunders, P.; McKinnell, C. Testosterone and Spermatogenesis Identification of Stage-Specific, Androgen-Regulated Proteins Secreted by Adult Rat Seminiferous Tubules. J. Androl. 1992, 13, 172–184. [Google Scholar] [PubMed]
- Wang, R.-S.; Yeh, S.; Tzeng, C.-R.; Chang, C. Androgen receptor roles in spermatogenesis and fertility: Lessons from testicular cell-specific androgen receptor knockout mice. Endocr. Rev. 2009, 30, 119–132. [Google Scholar] [CrossRef]
- Aly, H.A.; Alahdal, A.M.; Nagy, A.A.; Abdallah, H.M.; Abdel-Sattar, E.A.; Azhar, A.S. Lipoic acid and Calligonum comosumon attenuate aroclor 1260-induced testicular toxicity in adult rats. Environ. Toxicol. 2017, 32, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Chen, Y.-T.; Yeh, S.-D.; Xu, Q.; Wang, R.-S.; Guillou, F.; Lardy, H.; Yeh, S. Infertility with defective spermatogenesis and hypotestosteronemia in male mice lacking the androgen receptor in Sertoli cells. Proc. Natl. Acad. Sci. USA 2004, 101, 6876–6881. [Google Scholar] [CrossRef]
- Holdcraft, R.W.; Braun, R.E. Androgen receptor function is required in Sertoli cells for the terminal differentiation of haploid spermatids. Development 2004, 131, 459–467. [Google Scholar] [CrossRef]
- Blanco-Rodriguez, J.; Martinez-Garcia, C. Apoptosis precedes detachment of germ cells from the seminiferous epithelium after hormone suppression by short-term oestradiol treatment of rats. Int. J. Androl. 1998, 21, 109–115. [Google Scholar] [CrossRef]
- Badri, S.; Vanithakumari, G.; Malini, T. Studies on methotrexate effects on testicular steroidogenesis in rats. Endocr. Res. 2000, 26, 247–262. [Google Scholar] [CrossRef]
- Chen, H.; Pechenino, A.S.; Liu, J.; Beattie, M.C.; Brown, T.R.; Zirkin, B.R. Effect of glutathione depletion on Leydig cell steroidogenesis in young and old brown Norway rats. J. Endocrinol. 2008, 149, 2612–2619. [Google Scholar] [CrossRef] [PubMed]
- Carageorgiou, H.K.; Stratakis, C.A.; Damoulis, P.D.; Varonos, D.D.; Messari, I.D.; Sideris, A.C.; Sfikakis, A.P. Reversible plasma testosterone levels reduction after gentamicin administration and freund’s adjuvant arthritis in rats. Indian J. Physiol. Pharmacol. 2005, 49, 443. [Google Scholar]
- Nouri, M.; Khaki, A.; Fathiazar, F.; Rashidi, M.R. The protective effects of carrot seed extract on spermatogenesis and cauda epididymal sperm reserves in gentamicin treated rats. Cell J. 2009, 11, 327–333. [Google Scholar]
- Lee, V.; De Kretser, D.; Hudson, B.; Wang, C.J.R. Variations in serum FSH, LH and testosterone levels in male rats from birth to sexual maturity. Reproduction 1975, 42, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Makary, S.; Abdo, M.; Fekry, E. Oxidative stress burden inhibits spermatogenesis in adult male rats: Testosterone protective effect. Can. J. Physiol. Pharmacol. 2018, 96, 372–381. [Google Scholar] [CrossRef] [PubMed]
- El-Sahar, A.E.; Safar, M.M.; Zaki, H.F.; Attia, A.S.; Ain-Shoka, A.A.J.P.R. Neuroprotective effects of pioglitazone against transient cerebral ischemic reperfusion injury in diabetic rats: Modulation of antioxidant, anti-inflammatory, and anti-apoptotic biomarkers. Pharmacol. Rep. 2015, 67, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wang, D.; Ye, L.; Li, P.; Hao, W.; Chen, X.; Ma, J.; Wang, B.; Shang, J.; Li, D. Rosmarinic acid protects against inflammation and cardiomyocyte apoptosis during myocardial ischemia/reperfusion injury by activating peroxisome proliferator-activated receptor gamma. Front. Pharmacol. 2017, 8, 456. [Google Scholar] [CrossRef]
- Lenzi, A.; Gandini, L.; Lombardo, F.; Picardo, M.; Maresca, V.; Panfili, E.; Tramer, F.; Boitani, C.; Dondero, F. Polyunsaturated fatty acids of germ cell membranes, glutathione and blutathione-dependent enzyme-PHGPx: From basic to clinic. Contraception 2002, 65, 301–304. [Google Scholar] [CrossRef]
- Ichikawa, T.; Oeda, T.; Ohmori, H.; Schill, W. Reactive oxygen species influence the acrosome reaction but not acrosin activity in human spermatozoa. Int. J. Androl. 1999, 22, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Griveau, J.; Lannou, D.L. Reactive oxygen species and human spermatozoa: Physiology and pathology. Int. J. Androl. 1997, 20, 61–69. [Google Scholar] [CrossRef]
- Somi, M.; Hajipour, B.; Asl, N.; Estakhri, R.; Azar, A.; Zade, M.; Haghjou, A.; Vatankhah, A. Pioglitazone Attenuates Ischemia/Reperfusion–Induced Liver Injury in Rats. In Transplantation Proceedings; Elsevier: Amsterdam, The Netherlands, 2009; pp. 4105–4109. [Google Scholar]
- Kuru Karabas, M.; Ayhan, M.; Guney, E.; Serter, M.; Meteoglu, I. The effect of pioglitazone on antioxidant levels and renal histopathology in streptozotocin-induced diabetic rats. ISRN Endocrinol. 2013, 2013, 858690. [Google Scholar] [CrossRef]
- Abd El-Twab, S.M.; Mohamed, H.M.; Mahmoud, A.M. Taurine and pioglitazone attenuate diabetes-induced testicular damage by abrogation of oxidative stress and up-regulation of the pituitary–gonadal axis. Can. J. Physiol. Pharmacol. 2016, 94, 651–661. [Google Scholar] [CrossRef]
- Erdamar, H.; Demirci, H.; Yaman, H.; Erbil, M.K.; Yakar, T.; Sancak, B.; Elbeg, S.; Biberoğlu, G.; Yetkin, I. The effect of hypothyroidism, hyperthyroidism, and their treatment on parameters of oxidative stress and antioxidant status. Clin. Chem. Lab. Med. 2008, 46, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Shaha, C.; Tripathi, R.; Mishra, D.P. Male germ cell apoptosis: Regulation and biology. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 1501–1515. [Google Scholar] [CrossRef] [PubMed]
- Kitazumi, I.; Tsukahara, M. Regulation of DNA fragmentation: The role of caspases and phosphorylation. FEBS J. 2011, 278, 427–441. [Google Scholar] [CrossRef]
- Turner, T.T.; Bang, H.J.; Lysiak, J.L. The molecular pathology of experimental testicular torsion suggests adjunct therapy to surgical repair. J. Urol. 2004, 172, 2574–2578. [Google Scholar] [CrossRef] [PubMed]
- Durante-Mangoni, E.; Grammatikos, A.; Utili, R.; Falagas, M.E. Do we still need the aminoglycosides? Int. J. Antimicrob. Agents 2009, 33, 201–205. [Google Scholar] [CrossRef]
- Hu, H.; Zou, C.; Xi, X.; Shi, Z.; Wang, G.; Huang, X. Protective effects of pioglitazone on renal ischemia-reperfusion injury in mice. J. Surg. Res. 2012, 178, 460–465. [Google Scholar] [CrossRef]

| Group | PIO | GM |
|---|---|---|
| Group I (CTL) | “–” | “–” |
| Group II | – | 100 mg/kg/day for 6 days |
| Group III | 5 mg/kg/day for 21 days | 100 mg/kg/day for 6 days started on day 15 of PIO treatment |
| Group IV | 10 mg/kg/day for 21 days | 100 mg/kg/day for 6 days started on day 15 of PIO treatment |
| Group V | 20 mg/kg/day for 21 days | 100 mg/kg/day for 6 days started on day 15 of PIO treatment |
| Primer | Sequence | Annealing Temperature |
|---|---|---|
| BAX | FOR: 5′-CCAGTTGAAGTTGCCGTCTG-3′ REV: 5′-AAGAAGCTGAGCGAGTGTCT-3′ | 56 |
| BCL2 | FOR: 5′- ATGTGTGTGGAGAGCGTCAA-3′ REV: 5′-GATGCCGGTTCAGGTACTCA-3′ | 57 |
| GAPDH | FOR: 5′-CTCTCTGCTCCTCCCTGTTC-3′ REV: 5′-TACGGCCAAATCCGTTCACA-3′ | 60 |
| Scheme. | Score Description |
|---|---|
| 1 | No cell |
| 2 | Sertoli cells without germ cells |
| 3 | Only spermatogonia |
| 4 | Only a few spermatocytes |
| 5 | Many spermatocytes |
| 6 | Only a few early spermatids |
| 7 | Many early spermatids without differentiation |
| 8 | Few late spermatids |
| 9 | Many late spermatids |
| 10 | Full spermatogenesis |
| Group Variable (Mean ± SEM) | GI (n = 10) | GII (n = 10) | GIII (n = 10) | GIV (n = 10) | GV (n = 10) |
|---|---|---|---|---|---|
| Body weight (gm) | 232.80 ± 8.84 | 229.50 ± 6.89 | 241.60 ± 8.47 | 230.50 ± 5.57 | 236.40 ± 4.39 |
| Absolute testis weight (mg) | 131.50 ± 0.50 b | 106 ± 0.53 acde | 124.50 ± 2.72 be | 133.30 ± 3.47 b | 141.9 ± 1.96 bc |
| Relative testis weight | 0.58 ± 0.04 b | 0.47 ±0.03 ade | 0.52 ± 0.02 | 0.58 ±0.01 b | 0.60 ±0.01 b |
| Group | GI (n = 10) | GII (n = 10) | GIII (n = 10) | GIV (n = 10) | GV (n = 10) |
|---|---|---|---|---|---|
| Sperm count (×106) | 62.23 ± 0.88 bcd | 4.97 ± 0.37 acde | 54.28 ± 0.44 be | 55.85 ± 0.52 abe | 60.18 ± 0.56 bcd |
| Sperm motility (%) | 84.22 ± 0.78 | 65.37 ± 0.91 a | 72.89 ± 0.91 b | 78.57 ± 0.43 b | 82.85 ± 0.89 b |
| Sperm viability (%) | 80.6 ± 1.71 | 59.69 ± 0.32 a | 65.58 ± 0.58 b | 71.6 ± 0.96 b | 76.20 ± 1.67 b |
| Group Variable | GI (n = 10) | GII (n = 10) | GIII (n = 10) | GIV (n = 10) | GV (n = 10) |
|---|---|---|---|---|---|
| Serum Testosterone(ng/mL) | 1.74 ± 0.06 cd | 0.61 ± 0.02 acde | 1.01 ± 0.14 abe | 1.15 ± 0.05 abc | 1.62 ± 0.03 bcd |
| Serum LH (mIU/mL) | 0.56 ± 0.02 bc | 1.69 ± 0.05 acde | 1.30 ± 0.05 abde | 0.68 ± 0.05 bc | 0.62 ± 0.02 bc |
| Serum FSH (mIU/mL) | 1.25 ± 0.03 bcd | 3.32 ± 0.09 acde | 2.49 ± 0.08 abde | 1.64 ± 0.06 abce | 1.3 ± 0.04 bcd |
| Group Variable | GI (n = 10) | GII (n = 10) | GIII (n = 10) | GIV (n = 10) | GV (n = 10) |
|---|---|---|---|---|---|
| TNF-α (pg/mL) | 11.80 ± 0.43 bcde | 79.66 ± 0.89 acde | 36.80 ± 0.49 abde | 32.23 ± 0.39 abde | 20.93 ± 1.03 abcd |
| IL-6 (pg/mL) | 13.68 ± 0.52 bcde | 87.20 ± 1.53 acde | 66.05 ± 0.52 bde | 46.75 ± 3.41 bde | 32.18 ± 0.29 bcd |
| Group Variable | GI (n = 10) | GII (n = 10) | GIII (n = 10) | GIV (n = 10) | GV (n = 10) |
|---|---|---|---|---|---|
| TAC (mM/L) | 1.53 ± 0.10 bc | 0.91 ± 0.05 ade | 1.13 ± 0.03 abe | 1.3 ± 0.04 b | 1.48 ± 0.11 bc |
| TOS (mM/L) | 0.32 ± 0.03 bcd | 2.19 ± 0.10 acde | 1.32 ± 0.05 abde | 1.07 ± 0.02 abe | 0.33 ± 0.03 bcd |
| OSI | 0.22 ± 0.03 bcd | 2.47 ± 0.15 acde | 1.17 ± 0.05 abde | 0.84 ± 0.03 abce | 0.25 ± 0.04 bcd |
| Group Variable (Mean ± SEM) | GI (n = 10) | GII (n = 10) | GIII (n = 10) | GIV (n = 10) | GV (n = 10) |
|---|---|---|---|---|---|
| MDA (nmol/g) | 34.66±0.99 bce | 49.08±2.55 acde | 43.05±0.95 abde | 35.42±1.02 bce | 29.42±0.54 abcd |
| Group Variable (Mean ± SEM) | GI (n = 10) | GII (n = 10) | GIII (n = 10) | GIV (n = 10) | GV (n = 10) |
|---|---|---|---|---|---|
| CAT (u/gm) | 3.24 ± 0.11 bcde | 2.17 ± 0.03 ade | 2.21 ± 0.02 ae | 2.95 ± 0.05 abce | 2.67 ± 0.04 abcd |
| GP (u/mg) | 179.83 ± 1.39 bcd | 161.92 ± 0.88 ade | 163.49 ± 1.27 ade | 172.46 ± 2.29 abce | 179.49 ± 1.65 bcd |
| Group Variable (Mean ± SEM) | GI (n = 10) | GII (n = 10) | GIII (n = 10) | GIV (n = 10) | GV (n = 10) |
|---|---|---|---|---|---|
| BAX | 1.01 ± 0.01 bcde | 6.26 ± 0.21 acd | 3.43 ± 0.14 abde | 2.73 ± 0.05 abce | 2.05 ± 0.03 abcd |
| BCL2 | 0.99 ± 0.08 cde | 0.79 ± 0.07 cde | 2.22 ± 0.09 abde | 2.86 ± 0.08 abce | 4.17 ± 0.25 abcd |
| Group Variable (Mean ± SEM) | GI (n = 10) | GII (n = 10) | GIII (n = 10) | GIV (n = 10) | GV (n = 10) |
|---|---|---|---|---|---|
| Germinal epithelial thickness | 187.93 ± 11.35 bcd | 50.73±3.32 acde | 117.15±2.49 abe | 126.31±3.29 ab | 169.86±5.98 bcd |
| SNT diameter | 230.32 ± 4.84 bcd | 103.89 ± 7.18 acde | 144.75±7.05 cde | 178.65±10.06 bce | 221.11±7.75 bc |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Sayed, K.; Ali, D.A.; Maher, S.A.; Ghareeb, D.; Selim, S.; Albogami, S.; Fayad, E.; Kolieb, E. Prophylactic and Ameliorative Effects of PPAR-γ Agonist Pioglitazone in Improving Oxidative Stress, Germ Cell Apoptosis and Inflammation in Gentamycin-Induced Testicular Damage in Adult Male Albino Rats. Antioxidants 2022, 11, 191. https://doi.org/10.3390/antiox11020191
El-Sayed K, Ali DA, Maher SA, Ghareeb D, Selim S, Albogami S, Fayad E, Kolieb E. Prophylactic and Ameliorative Effects of PPAR-γ Agonist Pioglitazone in Improving Oxidative Stress, Germ Cell Apoptosis and Inflammation in Gentamycin-Induced Testicular Damage in Adult Male Albino Rats. Antioxidants. 2022; 11(2):191. https://doi.org/10.3390/antiox11020191
Chicago/Turabian StyleEl-Sayed, Karima, Dina A. Ali, Shymaa Ahmed Maher, Dalia Ghareeb, Samy Selim, Sarah Albogami, Eman Fayad, and Eman Kolieb. 2022. "Prophylactic and Ameliorative Effects of PPAR-γ Agonist Pioglitazone in Improving Oxidative Stress, Germ Cell Apoptosis and Inflammation in Gentamycin-Induced Testicular Damage in Adult Male Albino Rats" Antioxidants 11, no. 2: 191. https://doi.org/10.3390/antiox11020191
APA StyleEl-Sayed, K., Ali, D. A., Maher, S. A., Ghareeb, D., Selim, S., Albogami, S., Fayad, E., & Kolieb, E. (2022). Prophylactic and Ameliorative Effects of PPAR-γ Agonist Pioglitazone in Improving Oxidative Stress, Germ Cell Apoptosis and Inflammation in Gentamycin-Induced Testicular Damage in Adult Male Albino Rats. Antioxidants, 11(2), 191. https://doi.org/10.3390/antiox11020191







