Oxidative Stress in the Pathogenesis of Aorta Diseases as a Source of Potential Biomarkers and Therapeutic Targets, with a Particular Focus on Ascending Aorta Aneurysms
Abstract
1. Introduction
2. The Sporadic AsAA and Challenges in Biomarker Identification and Utilization
3. OS in the Aorta Wall: Mechanisms and Pathways Involved in and Significantly Associated with Onset of Aorta Diseases, including AsAA
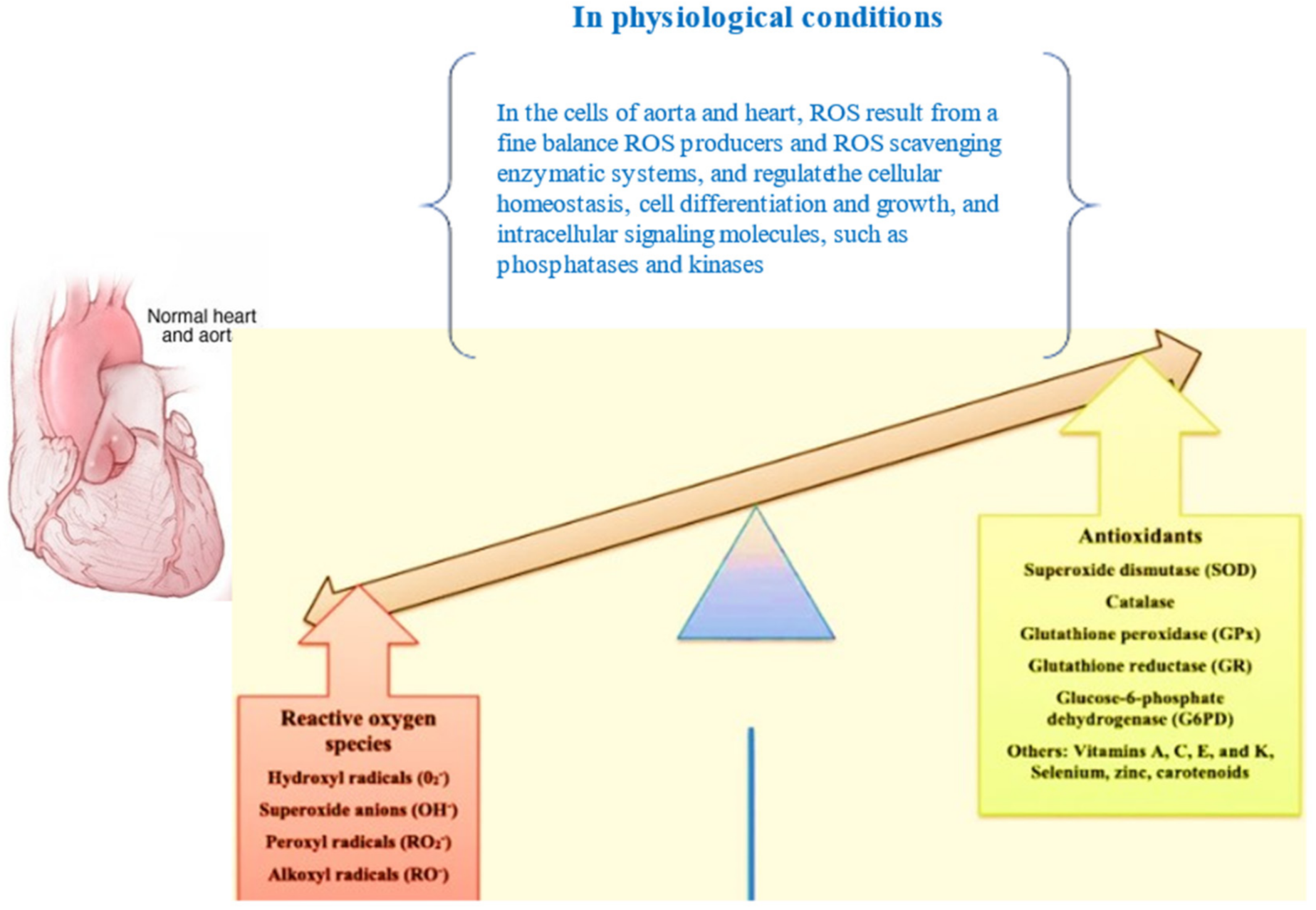
3.1. Physiological Actions of ROS in the Aorta Wall and the Stimulation of OS, with Immediate Effects on Cellular Components
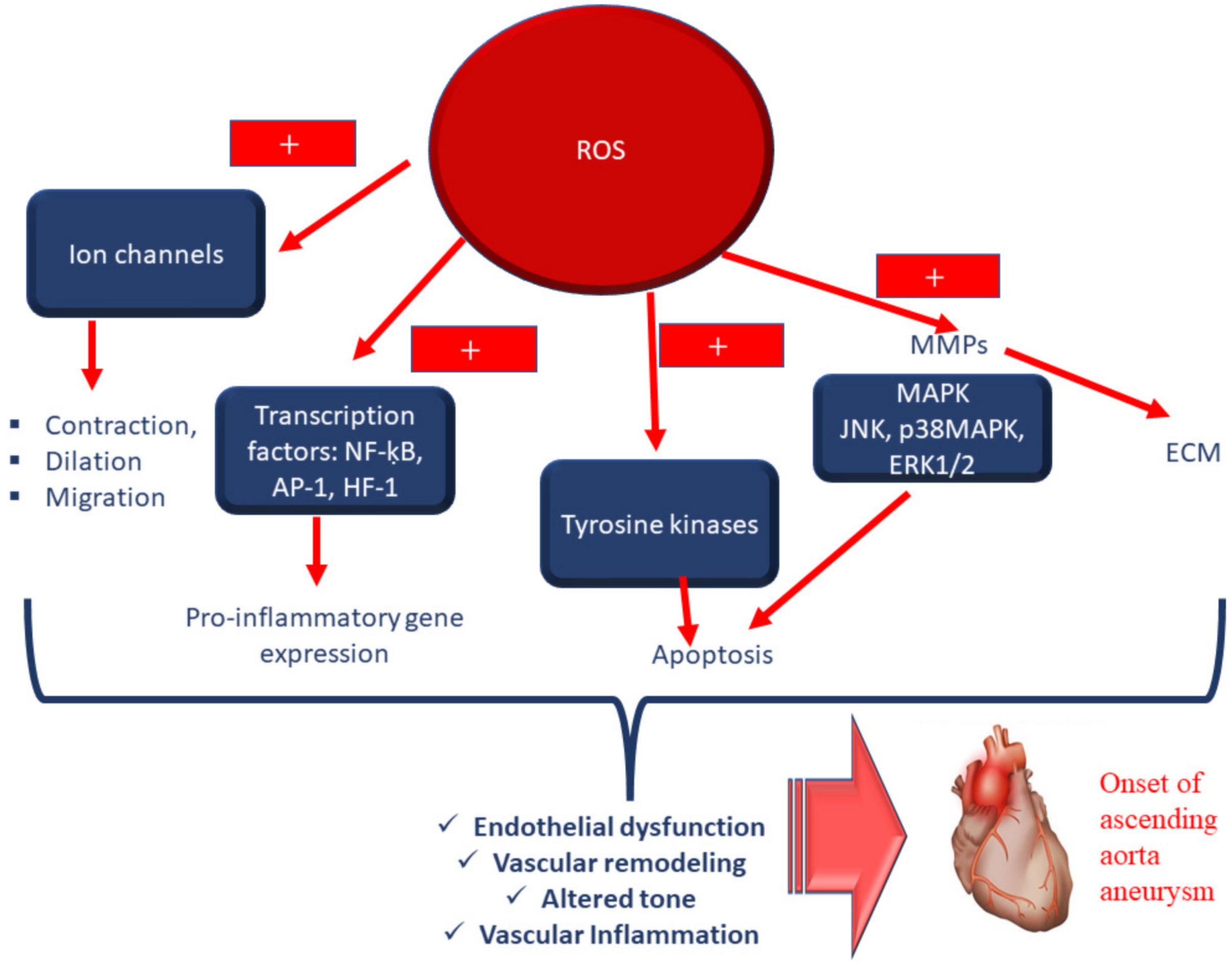
3.2. OS and Aorta Media Degeneration and Remodelling: Associated Pathways
3.3. Molecules and Pathways Related to OS Attenuation in Aorta Wall
4. Suggestions and Recommendations for Further Investigations into the Ideal Scenario for Biomarker Profile Development
5. From the Experimental Aspects to Translational Medicine: Antioxidant Treatments and Targets
5.1. Natural Compounds and the Mediterranean Diet
5.2. Innovative Technologies as Targeted Antioxidant Treatments: Nanomedicine and Its Benefits and Limitations
5.3. New Drugs: Metformin and Melatonin, and the Necessity of Validating Positive Evidence
6. Prebiotics, Probiotics and Synbiotics as Other Therapeutic Possibilities for OS and Aneurysm
7. New Regulators of OS for Developing Targeted Treatments
8. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Heidenreich, P.; Trogdon, J.G.; Khavjou, O.A.; Butler, J.; Dracup, K.; Ezekowitz, M.D.; Finkelstein, E.A.; Hong, Y.; Johnston, S.C.; Khera, A.; et al. Forecasting the future of cardiovascular disease in the United States: A policy statement from the American Heart Association. Circulation 2011, 123, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Livingston, K.A.; Freeman, K.J.; Friedman, S.M.; Stout, R.W.; Lianov, L.S.; Drozek, D.; Shallow, J.; Shurney, D.; Patel, P.M.; Campbell, T.M.; et al. Lifestyle Medicine and Economics: A Proposal for Research Priorities Informed by a Case Series of Disease Reversal. Int. J. Environ. Res. Public Health 2021, 18, 11364. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2020 Update: A Report from the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef]
- Murphy, M.C.; Castner, C.F.; Kouchoukos, N.T. Acute Aortic Syndromes: Diagnosis and Treatment. Mo. Med. 2017, 114, 458–463. [Google Scholar]
- Senser, E.M.; Misra, S.; Henkin, S. Thoracic Aortic Aneurysm: A Clinical Review. Cardiol. Clin. 2021, 39, 505–515. [Google Scholar] [CrossRef]
- Balistreri, C.R.; Pisano, C.; Martorana, A.; Triolo, O.F.; Lio, D.; Candore, G.; Ruvolo, G. Are the leukocyte telomere length attrition and telomerase activity alteration potential predictor biomarkers for sporadic TAA in aged individuals? Age 2014, 36, 9700. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.K.; Tse, H.F. Circulating Biomarkers for Cardiovascular Disease Risk Prediction in Patients with Cardiovascular Disease. Front. Cardiovasc. Med. 2021, 8, 13191. [Google Scholar] [CrossRef]
- Bahbah, E.I.; Noehammer, C.; Pulverer, W.; Jung, M.; Weinhaeusel, A. Salivary biomarkers in cardiovascular disease: An insight into the current evidence. FEBS J. 2021, 288, 6392–6405. [Google Scholar] [CrossRef] [PubMed]
- Ion, A.; Stafie, C.; Mitu, O.; Ciobanu, C.E.; Halitchi, D.I.; Costache, A.D.; Bobric, C.; Troase, R.; Mitu, I.; Huzum, B.; et al. Biomarkers Utility: At the Borderline between Cardiology and Neurology. J. Cardiovasc. Dev. Dis. 2021, 8, 139. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Li, J.; Guo, Y.; Golubnitschaja, O. Mass spectrometry analysis of human tear fluid biomarkers specific for ocular and systemic diseases in the context of 3P medicine. EPMA J. 2021, 12, 449–475. [Google Scholar] [CrossRef] [PubMed]
- Ain, Q.U.; Sarfraz, M.; Prasesti, G.K.; Dewi, T.I.; Kurniati, N.F. Confounders in Identification and Analysis of Inflammatory Biomarkers in Cardiovascular Diseases. Biomolecules 2021, 11, 1464. [Google Scholar] [CrossRef] [PubMed]
- Revuelta-López, E.; Barallat, J.; Cserkóová, A.; Gálvez-Montón, C.; Jaffe, A.S.; Januzzi, J.L.; Bayes-Genis, A. Pre-analytical considerations in biomarker research: Focus on cardiovascular disease. Clin. Chem. Lab. Med. 2021, 59, 1747–1760. [Google Scholar] [CrossRef]
- Scioli, M.G.; Storti, G.; D’amico, F.; Guzmán, R.R.; Centofanti, F.; Doldo, E.; Miranda, E.M.C.; Orlandi, A. Oxidative Stress and New Pathogenetic Mechanisms in Endothelial Dysfunction: Potential Diagnostic Biomarkers and Therapeutic Targets. J. Clin. Med. 2020, 9, 1995. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Q.; Zhu, J.; Xiao, Q.; Zhang, L. Reactive oxygen species: Key regulators in vascular health and diseases. Br. J. Pharmacol. 2018, 175, 1279–1292. [Google Scholar] [CrossRef]
- Magenta, A.; Greco, S.; Gaetano, C.; Martelli, F. Oxidative stress and microRNAs in vascular diseases. Int. J. Mol. Sci. 2013, 14, 17319–17346. [Google Scholar] [CrossRef]
- Daiber, A.; Di Lisa, F.; Oelze, M.; Kröller-Schön, S.; Steven, S.; Schulz, E.; Münzel, T. Crosstalk of mitochondria with NADPH oxidase via reactive oxygen and nitrogen species signalling and its role for vascular function. Br. J. Pharmacol. 2017, 174, 1670–1689. [Google Scholar] [CrossRef]
- Wortmann, M.; Peters, A.S.; Erhart, P.; Körfer, D.; Böckler, D.; Dihlmann, S. Inflammasomes in the Pathophysiology of Aortic Disease. Cells 2021, 10, 2433. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Li, T.; Zhu, S.; Zhong, W.; Li, F.; Wang, Y. Induction of SPARC on Oxidative Stress, Inflammatory Phenotype Transformation, and Apoptosis of Human Brain Smooth Muscle Cells Via TGF-β1-NOX4 Pathway. J. Mol. Neurosci. 2020, 70, 1728–1741. [Google Scholar] [CrossRef]
- Daiber, A. Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochim Biophys Acta. 2010, 1797, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Liao, M.; Yang, J.; Huang, T.; Green, M.; Wu, J.; Qu, L. Heat shock protein 27 plays a protective role in thoracic aortic dissection by promoting cell proliferation and inhibiting apoptosis. Cell. Mol. Biol. Lett. 2017, 22, 24. [Google Scholar] [CrossRef] [PubMed]
- Afanas’ev, I. ROS and RNS signaling in heart disorders: Could antioxidant treatment be successful? Oxid. Med. Cell. Longev. 2011, 2011, 293769. [Google Scholar] [CrossRef]
- Jha, J.C.; Watson, A.M.D.; Mathew, G.; de Vos, L.C.; Jandeleit-Dahm, K. The emerging role of NADPH oxidase NOX5 in vascular disease. Clin. Sci. 2017, 131, 981–990. [Google Scholar] [CrossRef]
- Konior, A.; Schramm, A.; Czesnikiewicz-Guzik, M.; Guzik, T.J. NADPH oxidases in vascular pathology. Antioxid. Redox Signal. 2014, 20, 2794–2814. [Google Scholar] [CrossRef]
- D’Oria, R.; Schipani, R.; Leonardini, A.; Natalicchio, A.; Perrini, S.; Cignarelli, A.; Laviola, L.; Giorgino, F. The Role of Oxidative Stress in Cardiac Disease: From Physiological Response to Injury Factor. Oxid. Med. Cell. Longev. 2020, 2020, 5732956. [Google Scholar] [CrossRef] [PubMed]
- Mandal, D.; Fu, P.; Levine, A.D. Redox regulation of il-13 signaling in intestinal epithelial cells: Usage of alternate pathways mediates distinct gene expression patterns. Cell. Signal. 2010, 22, 1485–1494. [Google Scholar] [CrossRef]
- Abimannan, T.; Peroumal, D.; Parida, J.R.; Barik, P.K.; Padhan, P.; Devadas, S. Oxidative stress modulates the cytokine response of differentiated th17 and th1 cells. Free Radic. Biol. Med. 2016, 99, 352–363. [Google Scholar] [CrossRef]
- Choi, M.H.; Lee, I.K.; Kim, G.W.; Kim, B.U.; Han, Y.H.; Yu, D.Y.; Park, H.S.; Kim, K.Y.; Lee, J.S.; Choi, C.; et al. Regulation of pdgf signalling and vascular remodelling by peroxiredoxin ii. Nature 2005, 435, 347–353. [Google Scholar] [CrossRef]
- Fujino, G.; Noguchi, T.; Matsuzawa, A.; Yamauchi, S.; Saitoh, M.; Takeda, K.; Ichijo, H. Thioredoxin and traf family proteins regulate reactive oxygen species-dependent activation of ask1 through reciprocal modulation of the n-terminal homophilic interaction of ask1. Mol. Cell. Biol. 2007, 27, 8152–8163. [Google Scholar] [CrossRef]
- Carta, L.; Smaldone, S.; Zilberberg, L.; Loch, D.; Dietz, H.C.; Rifkin, D.B.; Ramirez, F. P38 MAPK is an early determinant of promiscuous Smad2/3 signaling in the aortas of fibrillin-1 (Fbn1)-null mice. J. Biol. Chem. 2009, 284, 5630–5636. [Google Scholar] [CrossRef]
- Guido, M.; Debbas, V.; Salemi, V.; Tavares, E.R.; Meirelles, T.; Araujo, T.L.S.; Nolasco, P.; Ferreira-Filho, J.C.A.; Takimura, C.K.; Pereira, L.V.; et al. Effect of the antioxidant lipoic acid in aortic phenotype in a Marfan syndrome mouse model. Oxid. Med. Cell. Longev. 2018, 2018, 3967213. [Google Scholar] [CrossRef]
- Soto, M.E.; Soria-Castro, E.; Lans, V.G.; Ontiveros, E.M.; Mejia, B.I.H.; Hernandez, J.H.M.; Garcia, R.B.; Herrera, V.; Perez-Torres, I. Analysis of oxidative stress enzymes and structural and functional proteins on human aortic tissue from different aortopathies. Oxid. Med. Cell. Longev. 2014, 2014, 760694. [Google Scholar] [CrossRef] [PubMed]
- Billaud, M.; Phillippi, J.A.; Kotlarczyk, M.P.; Hill, J.C.; Ellis, B.W.; St Croix, C.M.; Cantu-Medèllin, N.; Kelley, E.E.; Gleason, T.G. Elevated oxidative stress in the aortic media of patients with bicuspid aortic valve. J. Thorac. Cardiovasc. Surg. 2017, 154, 1756–1762. [Google Scholar] [CrossRef]
- Phillippi, J.A.; Hill, J.C.; Billaud, M.; Green, B.R.; Kotlarczyk, M.P.; Gleason, T.G. Bicuspid Aortic Valve morphotype correlates with regional antioxidant gene expression profiles in the proximal ascending aorta. Ann. Thorac. Surg. 2017, 104, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Phillippi, J.A.; Billaud, M.; Hill, J.C.; Buchawald, J.E.; Kotlarczyk, M.P.; Cantu-Medellin, N.; Valayutham, M.; Cardounel, A.J.; Kelley, E.E.; Gleason, T.G. Elevated oxidative stress induces smooth muscle cell death via superoxide anion in Bicuspid Aortic valve-associated aortopathy [abstract]. Circulation 2015, 132, A19655. [Google Scholar] [CrossRef]
- Soto, M.E.; Manzano-Pech, L.G.; Guarner-Lans, V.; Diaz-Galindo, J.A.; Vàsquez, X.; Castrejòn-Tellez, V.; Gamboa, R.; Huesca, C.; Fuentevilla-Alvarez, G.; Pèrez-Torres, I. Oxidant/antioxidant profile in the thoracic aneurysm of patients with the Loeys- Dietz syndrome. Oxid. Med. Cell. Longev. 2020, 2020, 5392454. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Jiang, W.; Wang, Z.; Li, L.; Hu, Z. NOX1 Negatively Modulates Fibulin-5 in Vascular Smooth Muscle Cells to Affect Aortic Dissection. Biol. Pharm. Bull. 2019, 42, 1464–1470. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.H.; van Breemen, C.; Chung, A.W. Vasomotor dysfunction in the thoracic aorta of Marfan syndrome is associated with accumulation of oxidative stress. Vasc. Pharmacol. 2010, 52, 37–45. [Google Scholar] [CrossRef]
- Liu, W.; Wang, B.; Wang, T.; Liu, X.; He, X.; Liu, Y.; Li, Z.; Zeng, H. Ursodeoxycholic acid attenuates acute aortic dissection formation in angiotensin II-Infused apolipoprotein E-Deficient mice associated with reduced ros and increased Nrf2 levels. Cell. Physiol. Biochem. 2016, 38, 1391–1405. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Tan, X.; Zhu, S.; Zhong, W.; Huang, B.; Sun, J.; Li, F.; Wang, Y. SPARC induces phenotypic modulation of human brain vascular smooth muscle cells via AMPK/mTOR-mediated autophagy. Neurosci Lett. 2019, 712, 134485. [Google Scholar] [CrossRef]
- Pisano, C.; Balistreri, C.R.; Ricasoli, A.; Ruvolo, G. Cardiovascular Disease in Ageing: An Overview on Thoracic Aortic Aneurysm as an Emerging Inflammatory Disease. Mediat. Inflamm. 2017, 2017, 1274034. [Google Scholar] [CrossRef]
- Balistreri, C.R.; Ruvolo, G.; Lio, D.; Madonna, R. Toll-like receptor-4 signaling pathway in aorta aging and diseases: “Its double nature”. J. Mol. Cell. Cardiol. 2017, 110, 38–53. [Google Scholar] [CrossRef]
- You, W.; Hong, Y.; He, H.; Huang, X.; Tao, W.; Liang, X.; Zhang, Y.; Li, X. TGF-β mediates aortic smooth muscle cell senescence in Marfan syndrome. Aging 2019, 11, 3574–3584. [Google Scholar] [CrossRef] [PubMed]
- Balistreri, C.R.; Marullo, A.G.M.; Madonna, M.; Cavarretta, E.; Allegra, A.; Cesarini, V.; Iaccarino, A.; Schiavon, S.; Peruzzi, M.; Greco, E.; et al. Deregulation of TLR4 signaling pathway characterizes Bicuspid Aortic valve syndrome. Sci. Rep. 2019, 9, 11028. [Google Scholar] [CrossRef]
- Buffa, S.; Borzì, D.; Chiarelli, R.; Crapanzano, F.; Lena, A.M.; Nania, M.; Candi, E.; Triolo, F.; Ruvolo, G.; Melino, G.; et al. Biomarkers for vascular ageing in aorta tissues and blood samples. Exp. Gerontol. 2019, 128, 110741. [Google Scholar] [CrossRef] [PubMed]
- Ruvolo, G.; Pisano, C.; Candore, G.; Lio, D.; Palmeri, C.; Maresi, E.; Balistreri, C.R. Can the TLR-4-mediated signaling pathway be “a key inflammatory promoter for sporadic TAA”? Mediat. Inflamm. 2014, 2014, 349476. [Google Scholar] [CrossRef]
- Branchetti, E.; Poggio, P.; Sainger, R.; Shang, E.; Grau, J.B.; Jackson, B.M.; Lai, E.K.; Parmacek, M.S.; Gorman, R.C.; Gorman, J.H.; et al. Oxidative stress modulates vascular smooth muscle cell phenotype via CTGF in thoracic aortic aneurysm. Cardiovasc. Res. 2013, 100, 316–324. [Google Scholar] [CrossRef]
- Portelli, S.S.; Hambly, B.D.; Jeremy, R.W.; Robertson, E.N. Oxidative stress in genetically triggered thoracic aortic aneurysm: Role in pathogenesis and therapeutic opportunities. Redox Rep. 2021, 26, 45–52. [Google Scholar] [CrossRef]
- Qiu, L.; Yi, S.; Yu, T.; Hao, Y. Sirt3 Protects Against Thoracic Aortic Dissection Formation by Reducing Reactive Oxygen Species, Vascular Inflammation, and Apoptosis of Smooth Muscle Cells. Front. Cardiovasc. Med. 2021, 8, 675647. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.J.; Park, J.G.; Oh, G.T. Peroxiredoxins as Potential Targets for Cardiovascular Disease. Antioxidants 2021, 10, 1244. [Google Scholar] [CrossRef]
- Scola, L.; Giarratana, R.M.; Torre, S.; Argano, V.; Lio, D.; Balistreri, C.R. On the Road to Accurate Biomarkers for Cardiometabolic Diseases by Integrating Precision and Gender Medicine Approaches. Int. J. Mol. Sci. 2019, 20, 6015. [Google Scholar] [CrossRef]
- Balistreri, C.R. To the research of treatments for the typical calcific disease of old aortic valve in the omics era: Is the miR-195 a therapeutic signature via targetable p38-MAPK/VWF axis in bicuspid aortic valve? Int. J. Cardiol. 2020, 309, 108–109. [Google Scholar] [CrossRef]
- Olivier, M.; Asmis, R.; Hawkins, G.A.; Howard, T.D.; Cox, L.A. The Need for Multi-Omics Biomarker Signatures in Precision Medicine. Int. J. Mol. Sci. 2019, 20, 4781. [Google Scholar] [CrossRef] [PubMed]
- Griendling, K.K.; Touyz, R.M.; Zweier, J.L.; Dikalov, S.; Chiallian, W.; Chen, Y.R.; Harrison, D.G.; Bhatnagar, A. Measurement of reactive oxygen species, reactive nitrogen species, and redox-dependent signaling in the cardiovascular system. Circ. Res. 2016, 119, e39–e75. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- George, B.P.; Chandran, R.; Abrahamse, H. Role of Phytochemicals in Cancer Chemoprevention: Insights. Antioxidants 2021, 10, 1455. [Google Scholar] [CrossRef]
- Kiokias, S.; Oreopoulou, V. A Review of the Health Protective Effects of Phenolic Acids against a Range of Severe Pathologic Conditions (Including Coronavirus-Based Infections). Molecules 2021, 26, 5405. [Google Scholar] [CrossRef]
- Bandiwadekar, A.; Jose, J.; Khayatkashani, M.; Habtemariam, S.; Khayat Kashani, H.R.; Nabavi, S.M. Emerging Novel Approaches for the Enhanced Delivery of Natural Products for the Management of Neurodegenerative Diseases. J. Mol. Neurosci. 2021; online ahead of print. [Google Scholar] [CrossRef]
- Mehta, J.; Rayalam, S.; Wang, X. Cytoprotective Effects of Natural Compounds against Oxidative Stress. Antioxidants 2018, 7, 147. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sun, K.; Hu, F.; Chen, L.; Zhang, X.; Wang, F.; Yan, B. Protective effects of natural compounds against oxidative stress in ischemic diseases and cancers via activating the Nrf2 signaling pathway: A mini review. J. Biochem. Mol. Toxicol. 2021, 35, e22658. [Google Scholar] [CrossRef]
- Dubois-Deruy, E.; Peugnet, V.; Turkieh, A.; Pinet, F. Oxidative Stress in Cardiovascular Diseases. Antioxidants 2020, 9, 864. [Google Scholar] [CrossRef]
- Bjelakovic, G.; Nikolova, D.; Gluud, L.L.; Simonetti, R.G.; Gluud, C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention. Systematic review and meta-analysis. JAMA 2007, 297, 842–857. [Google Scholar] [CrossRef]
- Davies, A.M.; Holt, A.G. Why antioxidant therapies have failed in clinical trials. J. Theor. Biol. 2018, 457, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural Products for Drug Discovery in the 21st Century: Innovations for Novel Drug Discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef]
- Meephat, S.; Prasatthong, P.; Potue, P.; Bunbupha, S.; Pakdeechote, P.; Maneesai, P. Diosmetin Ameliorates Vascular Dysfunction and Remodeling by Modulation of Nrf2/HO-1 and p-JNK/p-NF-κB Expression in Hypertensive Rats. Antioxidants 2021, 10, 1487. [Google Scholar] [CrossRef]
- Guo, Y.; Pizzol, R.; Gabbanini, S.; Baschieri, A.; Amorati, R.; Valgimigli, L. Absolute Antioxidant Activity of Five Phenol-Rich Essential Oils. Molecules 2021, 26, 5237. [Google Scholar] [CrossRef]
- do Nascimento, L.D.; Moraes, A.A.B.; Costa, K.S.D.; Galúcio, J.M.P.; Taube, P.S.; Costa, C.M.L.; Cruz, J.N.; Andrade, E.H.d.A.; Faria, L.J.G. Bioactive Natural Compounds and Antioxidant Activity of Essential Oils from Spice Plants: New Findings and Potential Applications. Biomolecules 2020, 10, 988. [Google Scholar] [CrossRef] [PubMed]
- Achiri, R.; Fouzia, M.; Benomari, F.Z.; Djabou, N.; Boufeldja, T.; Muselli, A.; Dib, M.E.A. Chemical composition/pharmacophore modelling- based, virtual screening, molecular docking and dynamic simulation studies for the discovery of novel superoxide dismutase (SODs) of bioactive molecules from aerial parts of Inula Montana as antioxydant’s agents. J. Biomol. Struct. Dyn. 2021, 1–22. [Google Scholar] [CrossRef]
- Sirangelo, I.; Borriello, M.; Liccardo, M.; Scafuro, M.; Russo, P.; Iannuzzi, C. Hydroxytyrosol Selectively Affects Non-Enzymatic Glycation in Human Insulin and Protects by AGEs Cytotoxicity. Antioxidants 2021, 10, 1127. [Google Scholar] [CrossRef]
- Dinu, M.; Pagliai, G.; Casini, A.; Sofi, F. Mediterranean diet and multiple health outcomes: An umbrella review of meta-analyses of observational studies and randomised trials. Eur. J. Clin. Nutr. 2018, 72, 30–43. [Google Scholar] [CrossRef]
- Kaluza, J.; Stackelberg, O.; Harris, H.R.; Akesson, A.; Björck, M.; Wolk, A. Mediterranean Diet is Associated with Reduced Risk of Abdominal Aortic Aneurysm in Smokers: Results of Two Prospective Cohort Studies. Eur. J. Vasc. Endovasc. Surg. 2021, 62, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Czekajło, A. Role of diet-related factors in cerebral aneurysm formation and rupture. Rocz. Panstw. Zakl. Hig. 2019, 70, 119–126. [Google Scholar] [CrossRef]
- Bucciantini, M.; Leri, M.; Nardiello, P.; Casamenti, F.; Stefani, M. Olive Polyphenols: Antioxidant and Anti-Inflammatory Properties. Antioxidants 2021, 10, 1044. [Google Scholar] [CrossRef] [PubMed]
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; Stefani, M. Healthy Effects of Plant Polyphenols: Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 1250. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Ye, H.; Kamaraj, R.; Zhang, T.; Zhang, J.; Pavek, P. A review on pharmacological activities and synergistic effect of quercetin with small molecule agents. Phytomedicine 2021, 92, 153736. [Google Scholar] [CrossRef]
- Tzara, A.; Lambrinidis, G.; Kourounakis, A. Design of Multifaceted Antioxidants: Shifting towards Anti-Inflammatory and Antihyperlipidemic Activity. Molecules 2021, 26, 4928. [Google Scholar] [CrossRef]
- Chauvierre, C.; Letourneur, D. The European project NanoAthero to fight CVDusing nanotechnologies. Nanomedicine 2015, 10, 3391–3400. [Google Scholar] [CrossRef]
- Taneja, G.; Sud, A.; Pendse, N.; Panigrahi, B.; Kumar, A.; Sharma, A.K. Nano-medicine and vascular endothelial dysfunction: Options and delivery strategies. Cardiovasc. Toxicol. 2019, 19, 1–12. [Google Scholar] [CrossRef]
- Quispe, C.; Cruz-Martins, N.; Manca, M.L.; Manconi, M.; Sytar, O.; Hudz, N.; Shanaida, M.; Kumar, M.; Taheri, Y.; Martorell, M.; et al. Nano-Derived Therapeutic Formulations with Curcumin in Inflammation-Related Diseases. Oxid. Med. Cell. Longev. 2021, 2021, 3149223. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, R.; Liu, C.; Pan, L.; Qi, Y.; Cheng, J.; Guo, J.; Jia, Y.; Ding, J.; Zhang, J.; et al. A pH/ROS dual-responsive and targeting nanotherapy for vascular inflammatory diseases. Biomaterials 2020, 230, 119605. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P.; Sobarzo-Sánchez, E.; Uddin, M.S.; Bru, R.; Clément, C.; Jacquard, C.; Nabavi, S.F.; Khayatkashani, M.; Batiha, G.E.; Khan, H.; et al. Resveratrol and cyclodextrins, an easy alliance: Applications in nanomedicine, green chemistry, and biotechnology. Biotechnol. Adv. 2021, 53, 107844. [Google Scholar] [CrossRef]
- Younis, M.A.; Tawfeek, H.M.; Abdellatif, A.A.H.; Abdel-Aleem, J.A.; Harashima, H. Clinical translation of nanomedicines: Challenges, opportunities, and keys. Adv Drug Deliv Rev. 2021, 181, 114083. [Google Scholar] [CrossRef]
- King, R.W.; Bonaca, M.P. Acute aortic syndromes: A review of what we know and future considerations. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 1197–1203. [Google Scholar] [CrossRef]
- Harky, A.; Sokal, P.A.; Hasan, K.; Papaleontiou, A. The Aortic Pathologies: How Far We Understand It and Its Implications on Thoracic Aortic Surgery. Braz. J. Cardiovasc. Surg. 2021, 36, 535–549. [Google Scholar] [CrossRef]
- Wang, Y.W.; He, S.J.; Feng, X.; Cheng, J.; Luo, Y.T.; Tian, L.; Huang, Q. Metformin: A review of its potential indications. Drug Des. Dev. Ther. 2017, 11, 2421–2429. [Google Scholar] [CrossRef]
- Li, S.; Shi, Y.; Liu, P.; Song, Y.; Liu, Y.; Ying, L.; Quan, K.; Yu, G.; Fan, Z.; Zhu, W. Metformin inhibits intracranial aneurysm formation and progression by regulating vascular smooth muscle cell phenotype switching via the AMPK/ACC pathway. J. Neuroinflamm. 2020, 17, 191. [Google Scholar] [CrossRef]
- Yu, X.; Jiang, D.; Wang, J.; Wang, R.; Chen, T.; Wang, K.; Durgahee, M.S.A.; Wei, X.; Cao, S. Metformin prescription and aortic aneurysm: Systematic review and meta-analysis. Heart 2019, 105, 1351–1357. [Google Scholar] [CrossRef]
- Hinchliffe, R.J. Metformin and Abdominal Aortic Aneurysm. Eur. J. Vasc. Endovasc. Surg. 2017, 54, 679–680. [Google Scholar] [CrossRef][Green Version]
- El-Arabey, A.A.; Abdalla, M.; Eltayb, W.A. Metformin: Ongoing journey with superdrug revolution. Adv. Pharm. Bull. 2019, 9, 1–4. [Google Scholar] [CrossRef]
- Balistreri, C.R. Anti-Inflamm-Ageing and/or Anti-Age-Related Disease Emerging Treatments: A Historical Alchemy or Revolutionary Effective Procedures? Mediat. Inflamm. 2018, 2018, 3705389. [Google Scholar] [CrossRef]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef]
- Kumar, J.; Haldar, C.; Verma, R. Melatonin Ameliorates LPS-Induced Testicular Nitro-oxidative Stress (iNOS/TNFα) and Inflammation (NF-kB/COX-2) via Modulation of SIRT-1. Reprod. Sci. 2021, 28, 3417–3430. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Sun, C.; Zhu, H.; Zhai, M.; Zhang, L.; Jiang, L.; Hou, P.; Li, J.; Li, K.; Liu, Z.; et al. Melatonin protects against thoracic aortic aneurysm and dissection through SIRT1-dependent regulation of oxidative stress and vascular smooth muscle cell loss. J. Pineal Res. 2020, 69, e12661. [Google Scholar] [CrossRef] [PubMed]
- Ballard, J.W.O.; Towarnicki, S.G. Mitochondria, the gut microbiome and ROS. Cell. Signal. 2020, 75, 109737. [Google Scholar] [CrossRef] [PubMed]
- Patergnani, S.; Bouhamida, E.; Leo, S.; Pinton, P.; Rimessi, A. Mitochondrial Oxidative Stress and “Mito-Inflammation”: Actors in the Diseases. Biomedicines 2021, 9, 216. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Hou, C.Y.; Hsu, W.H.; Tain, Y.L. Cardiovascular diseases of Developmental Origins: Preventive Aspects of Gut Microbiota-Targeted Therapy. Nutrients 2021, 13, 2290. [Google Scholar] [CrossRef]
- Jin, M.; Qian, Z.; Yin, J.; Xu, W.; Zhou, X. The role of intestinal microbiota in cardiovascular disease. J. Cell. Mol. Med. 2019, 23, 2343–2350. [Google Scholar] [CrossRef]
- Xie, J.; Lu, W.; Zhong, L.; Hu, Y.; Li, Q.; Ding, R.; Zhong, Z.; Liu, Z.; Xiao, H.; Xie, D.; et al. Alterations in gut microbiota of abdominal aortic aneurysm mice. BMC Cardiovasc. Disord. 2020, 20, 32. [Google Scholar] [CrossRef] [PubMed]
- Olas, B. Probiotics, Prebiotics and Synbiotics-A Promising Strategy in Prevention and Treatment of Cardiovascular Diseases? Int. J. Mol. Sci. 2020, 21, 9737. [Google Scholar] [CrossRef]
- de LeBlanc, A.A.M.; LeBlanc, J.G. Effect of probiotic administration on the intestinal microbiota, current knowledge, and potential applications. World J. Gastroenterol. 2014, 20, 16518–16528. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Krumbeck, J.A.; Maldonado-Gomez, M.X.; Ramer-Tait, A.E.; Hutkins, R.W. Prebiotics and synbiotics: Dietary strategies for improving gut health. Curr. Opin. Gastroenterol. 2016, 32, 110–119. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef] [PubMed]
- Oniszczuk, A.; Oniszczuk, T.; Gancarz, M.; Szymańska, J. Role of Gut Microbiota, Probiotics and Prebiotics in the Cardiovascular Diseases. Molecules 2021, 26, 1172. [Google Scholar] [CrossRef]
- Liu, H.J.; Li, H.T.; Lin, Y.; Lu, D.L.; Yue, Y.; Xiong, J.; Li, C.Q.; Xu, X.Y.; Feng, Y.G. Identification of 2 Potential Core Genes for Influence of Gut Probiotics on Formation of Intracranial Aneurysms by Bioinformatics Analysis. Med. Sci. Monit. 2020, 26, e920754. [Google Scholar] [CrossRef]
- Sternburg, E.L.; Gruijs da Silva, L.A.; Dormann, D. Post-translational modifications on RNA-binding proteins: Accelerators, brakes, or passengers in neurodegeneration? Trends Biochem. Sci. 2021, 47, 6–22. [Google Scholar] [CrossRef]
- Greene, J.; Baird, A.M.; Brady, L.; Lim, M.; Gray, S.G.; McDermott, R.; Finn, S.P. Circular RNAs: Biogenesis, Function and Role in Human Diseases. Front. Mol. Biosci. 2017, 4, 38. [Google Scholar] [CrossRef]
- Saaoud, F.; Drummer, I.V.C.; Shao, Y.; Sun, Y.; Lu, Y.; Xu, K.; Ni, D.; Jiang, X.; Wang, H.; Yang, X. Circular RNAs are a novel type of non-coding RNAs in ROS regulation, cardiovascular metabolic inflammations and cancers. Pharmacol. Ther. 2021, 220, 107715. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Wan, Y.; Zhao, Y.; Wen, Q.; Tang, X.; Shen, J.; Wu, X.; Li, M.; Li, X.; et al. Circular RNAs in the Regulation of Oxidative Stress. Front. Pharmacol. 2021, 12, 697903. [Google Scholar] [CrossRef]
- Li, L.; Ni, Z.; Si, X.; Jiang, L.; Sang, H.; Xia, W.; Chen, Z.; Huang, J.; Jin, J.; Shao, A.; et al. Emerging Clues of Regulatory Roles of Circular RNAs through Modulating Oxidative Stress: Focus on Neurological and Vascular Diseases. Oxid. Med. Cell. Longev. 2021, 2021, 6659908. [Google Scholar] [CrossRef]

| Enzymes and ROS Molecules | Biological Effects |
|---|---|
| Glutathione (GSH) system | Preventing the initiation of oxidative stress Endothelial and smooth muscle cell dysfunction |
| Metallothionein (MT) | Preventing the initiation of oxidative stress-induced aortic MMP-9 expression |
| Superoxide dismutases (SOD) | Transforming RNS/RNS into less reactive species |
| Glutathione peroxidases (GPx) | Transforming RNS/RNS into less reactive species |
| Peroxiredoxins (PRX) | Transforming RNS/RNS into less reactive species |
| Nox4 | Oxidative damage to multiple cytoskeletal and contractile proteins, and elastic fragmentation |
| Xanthine oxidase (XO) | Alteration of contraction and relaxation, ECM degradation and aortic wall remodelling |
| SmgGDS (Small GTP-Binding Protein GDP Dissociation Stimulator) | Maintaining the contractile phenotype of VSMCs. A deficiency induces severe aortic dilatation and severe elastic fragmentation, higher levels of ROS and MMPs, and inflammatory cell migration |
| Myeloperoxidase (MPO) | Increased MMP-2 and MMP-9 expression; increased ECM fragmentation and apoptosis |
| ERK1/2 signalling pathway | The increased activation of non-canonical ERK1/2 signalling due to inflammatory cells is known to be associated with TAA pathogenesis, predominately through the downstream effect of increased MMP expression |
| Myeloperoxidase (MPO)/hypochlorous acid (HOCl) system | The MPO/HOCl system produced in neutrophils has been demonstrated to be a local mediator of tissue damage, particularly in ascending aorta aneurysms |
| Nox 2 and Nox 4 | A specific increase in endothelial ROS production in Nox2 transgenic mice was sufficient to cause Ang II–mediated aortic dissection |
| Enzymes | Biological Effects |
|---|---|
| NADPH oxidases (NOX1, NOX2) | Generation of ROS Major sources of ROS in the artery wall The NOX2 phagocyte plays a role in catalysing the respiratory burst NOX2 facilitates oxygen reduction, resulting in the formation of superoxide |
| MPO (enzyme myeloperoxidase) | MPO is a peroxidase; excessive levels of these toxic molecules cause tissue damage. MPO-producing macrophages can infiltrate TAA and are likely involved in the progression of TAA disease. MPO can exacerbate mechanisms of damage to the ECM and DNA, activation of inflammatory signalling and an increase in endothelial dysfunction |
| ERK1/2 | Increased MMP expression Increased oxidative stress due to inflammation Can disrupt aortic wall homeostasis by altering the characteristics of VSMCs Promotion of human aortic VSMC migration |
| 3-Nitrotyrosine | Markers of MPO-mediated oxidative damage Promotion of human aortic VSMC migration Contributing to VSMC dysfunction |
| 3-Chlorothyrosine | Markers of MPO-mediated oxidative damage Promotion of human aortic VSMC migration |
| MAPK signalling pathway | Expression of relevant target genes, including the upregulation of MMPs |
| MPO-derived oxidants | DNA modification, causing DNA damage Affecting VSMC DNA |
| MPO-derived HOCl | Damage to specific DNA bases Inhibition of DNA repair enzymes |
| Nitric oxide (NO) | Important regulator of vascular tone Reduced NO production causes endothelial dysfunction |
| NOX4 | Reduced elastin fragmentation, less endothelial dysfunction and an increase in contractile markers |
| Glutathione peroxidase (GPx) and glutathione reductase (GR) | Higher lipid peroxide fluorochromes, expressed as U/g aorta, than in controls in both abdominal aortic aneurysms (AAA) and atherosclerotic occlusive disease (AOD) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pisano, C.; Benedetto, U.; Ruvolo, G.; Balistreri, C.R. Oxidative Stress in the Pathogenesis of Aorta Diseases as a Source of Potential Biomarkers and Therapeutic Targets, with a Particular Focus on Ascending Aorta Aneurysms. Antioxidants 2022, 11, 182. https://doi.org/10.3390/antiox11020182
Pisano C, Benedetto U, Ruvolo G, Balistreri CR. Oxidative Stress in the Pathogenesis of Aorta Diseases as a Source of Potential Biomarkers and Therapeutic Targets, with a Particular Focus on Ascending Aorta Aneurysms. Antioxidants. 2022; 11(2):182. https://doi.org/10.3390/antiox11020182
Chicago/Turabian StylePisano, Calogera, Umberto Benedetto, Giovanni Ruvolo, and Carmela Rita Balistreri. 2022. "Oxidative Stress in the Pathogenesis of Aorta Diseases as a Source of Potential Biomarkers and Therapeutic Targets, with a Particular Focus on Ascending Aorta Aneurysms" Antioxidants 11, no. 2: 182. https://doi.org/10.3390/antiox11020182
APA StylePisano, C., Benedetto, U., Ruvolo, G., & Balistreri, C. R. (2022). Oxidative Stress in the Pathogenesis of Aorta Diseases as a Source of Potential Biomarkers and Therapeutic Targets, with a Particular Focus on Ascending Aorta Aneurysms. Antioxidants, 11(2), 182. https://doi.org/10.3390/antiox11020182







