Comparison of the Retention Rates of Synthetic and Natural Astaxanthin in Feeds and Their Effects on Pigmentation, Growth, and Health in Rainbow Trout (Oncorhynchus mykiss)
Abstract
1. Introduction
2. Materials and Methods
2.1. Astaxanthin Sources and Diet Preparation
2.2. Fish and Experimental Conditions
2.3. Sample Collection
2.4. Nutritional Composition, Pigmentation, Astaxanthin Content and Morphological Analysis of Samples
2.5. Plasma and Antioxidant Parameters and qPCR Analysis
2.6. Calculations and Statistical Analysis
3. Results
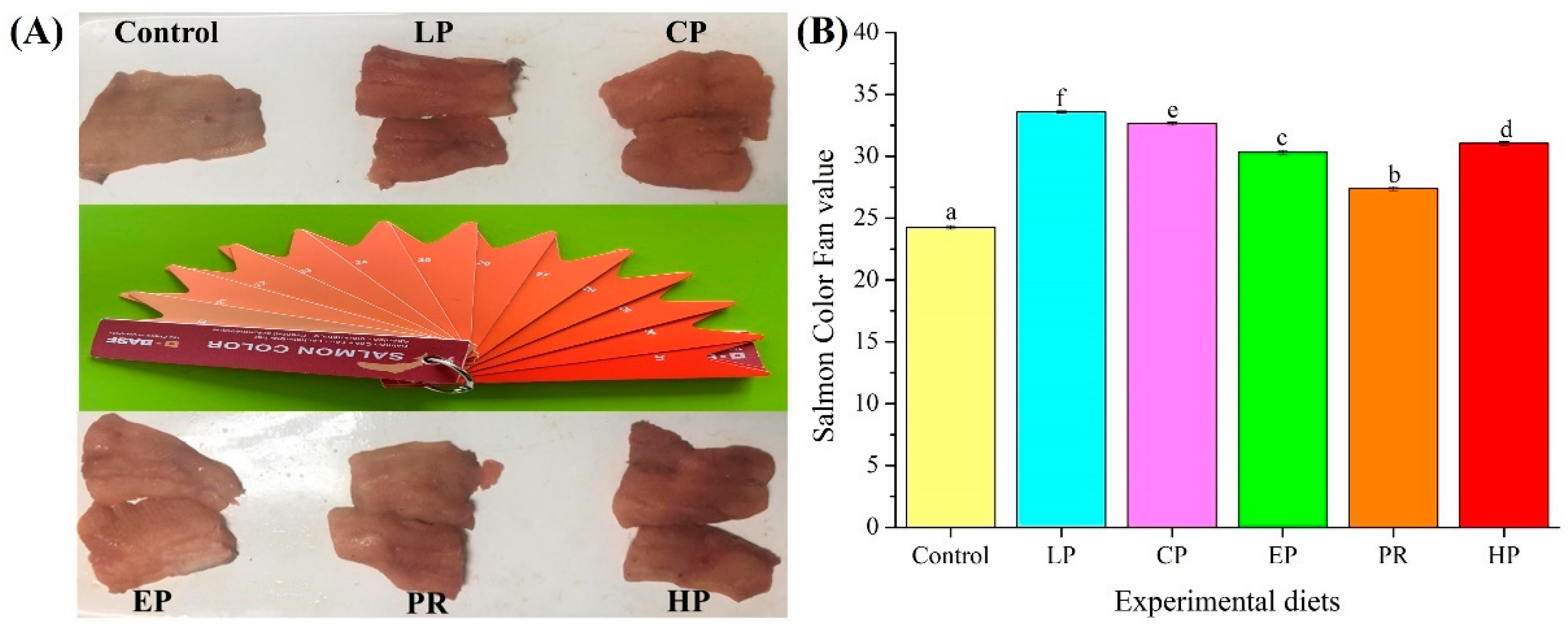
3.1. Pigmentation and Astaxanthin Content
3.2. Retention Rates of Astaxanthin in Feeds
3.3. Biological Parameters
3.4. Whole Body and Muscle Composition Analysis
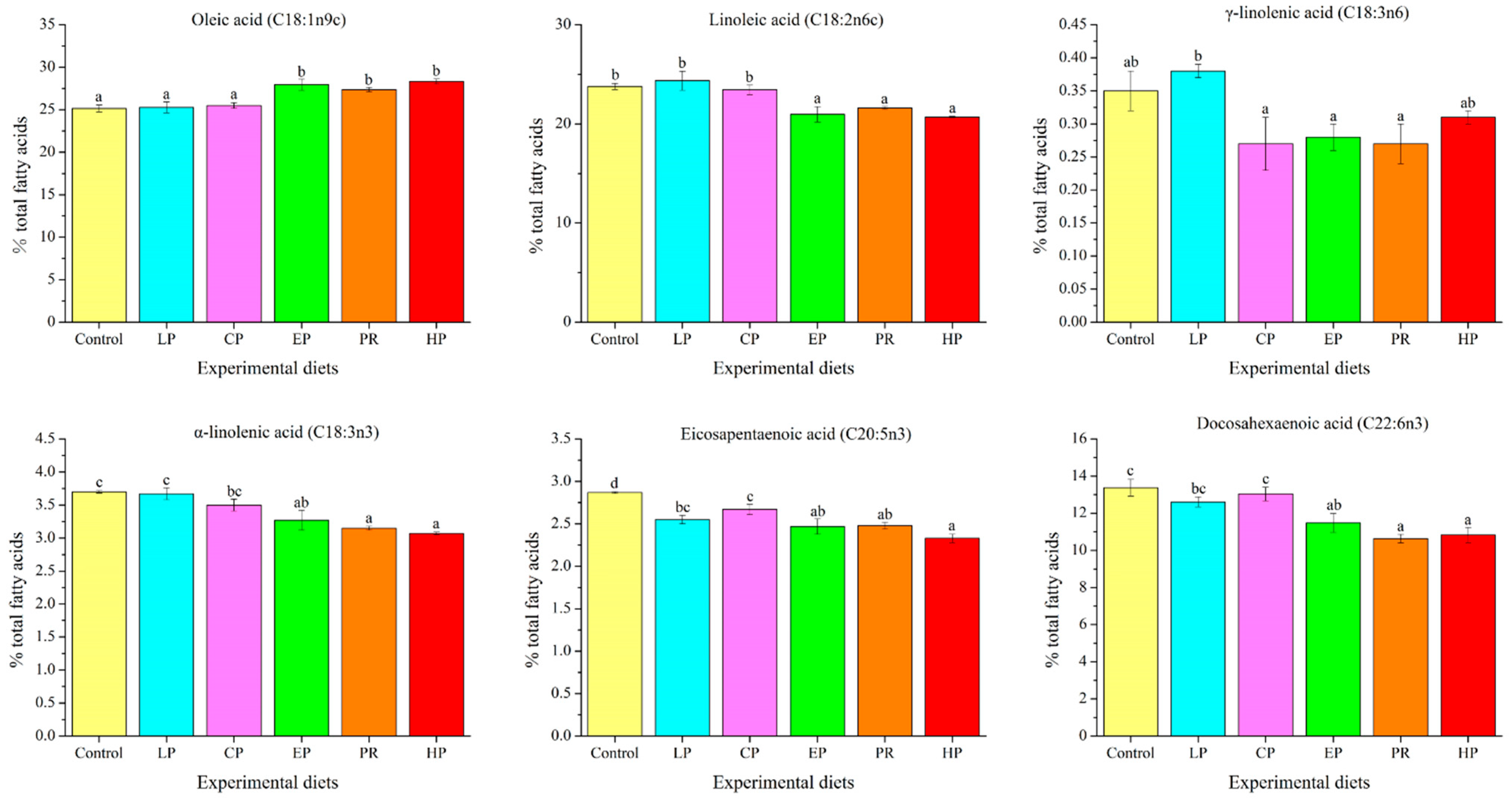
3.5. Amino Acid and Fatty Acid Composition Analysis
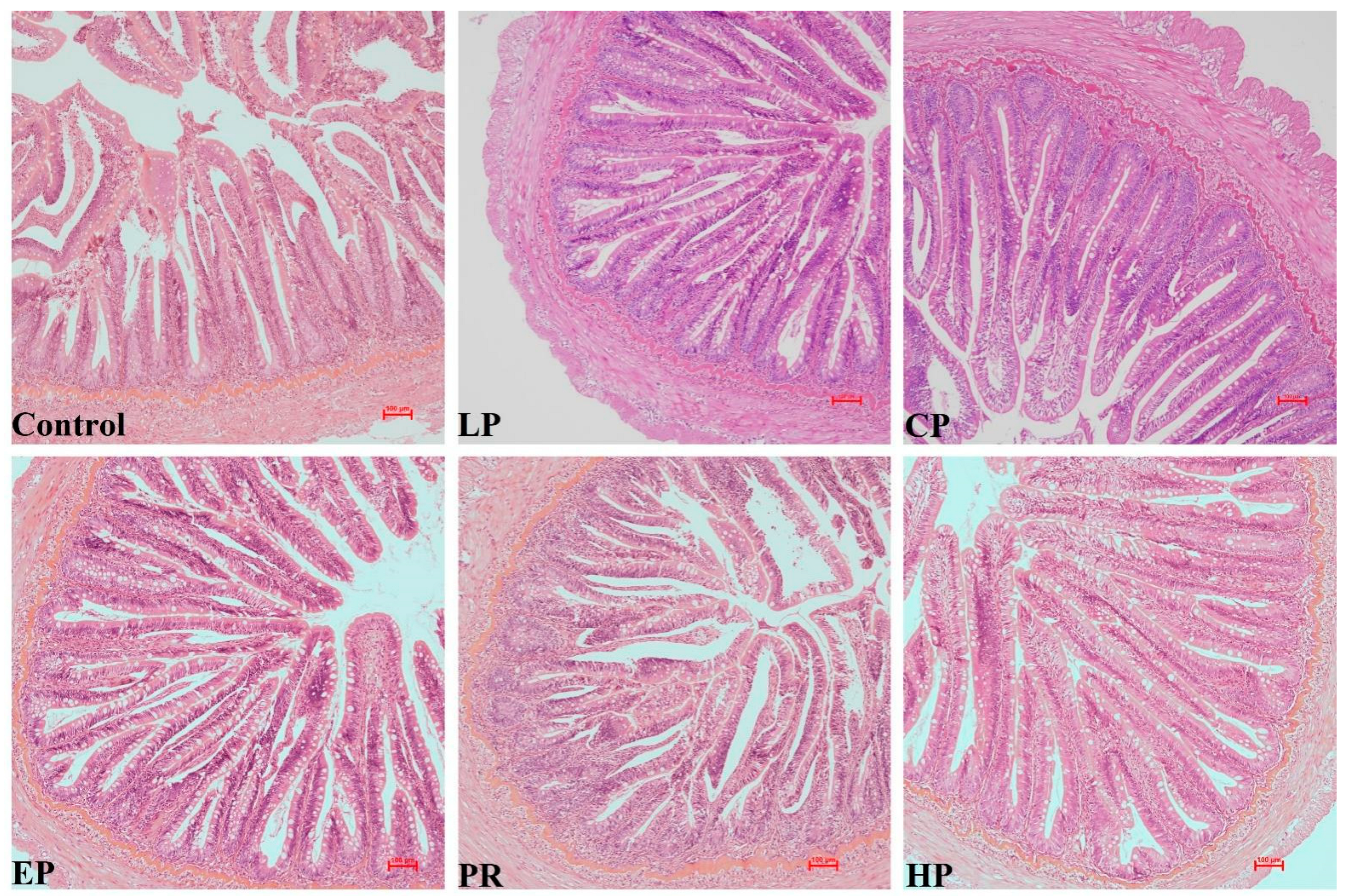
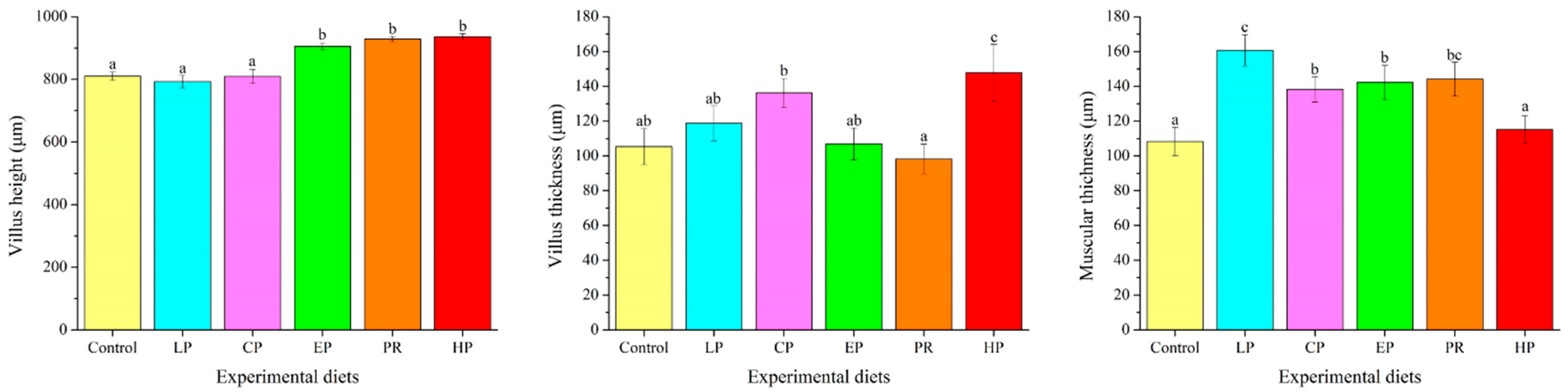
3.6. Morphological Analysis
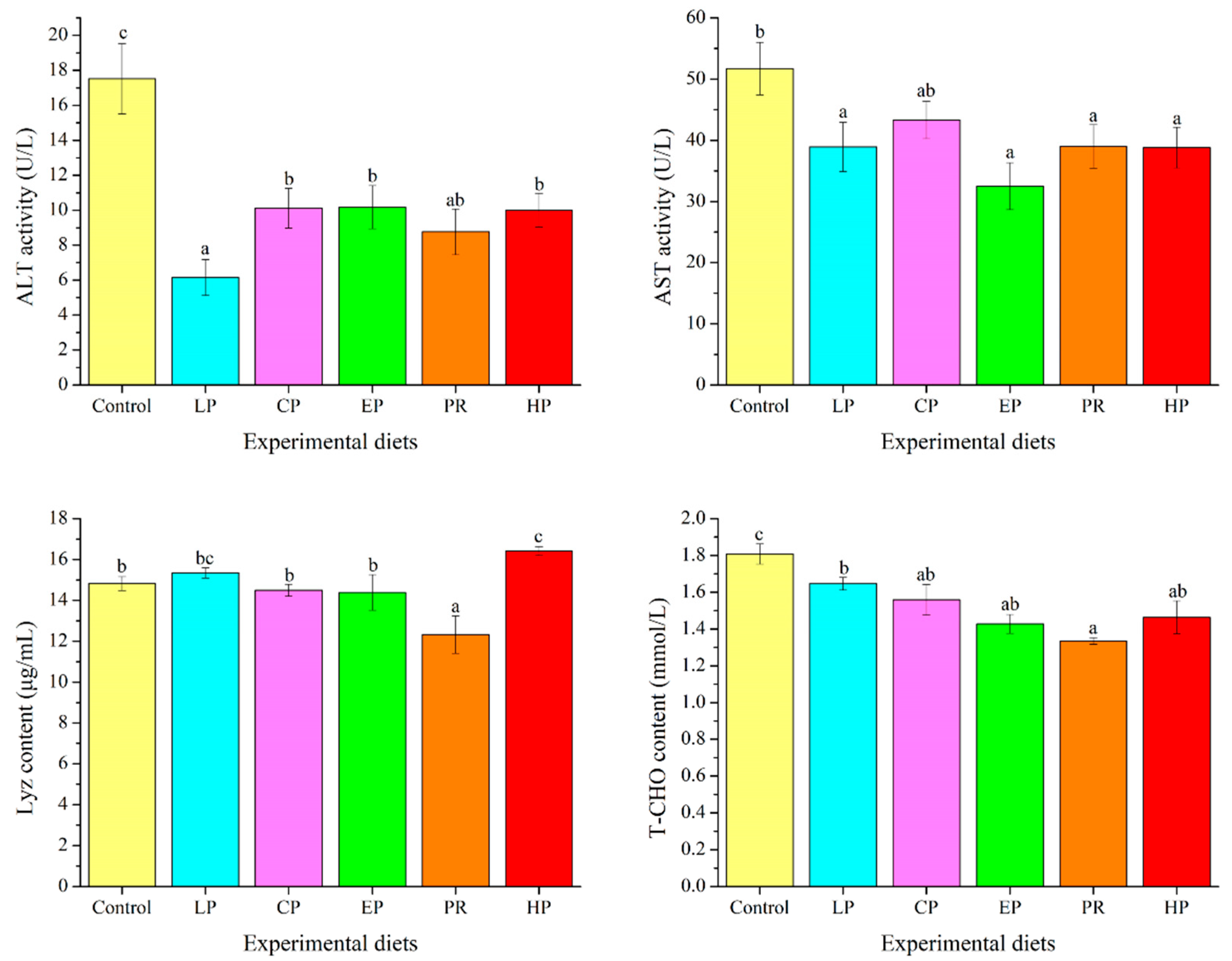
3.7. Plasma Biochemical Parameters
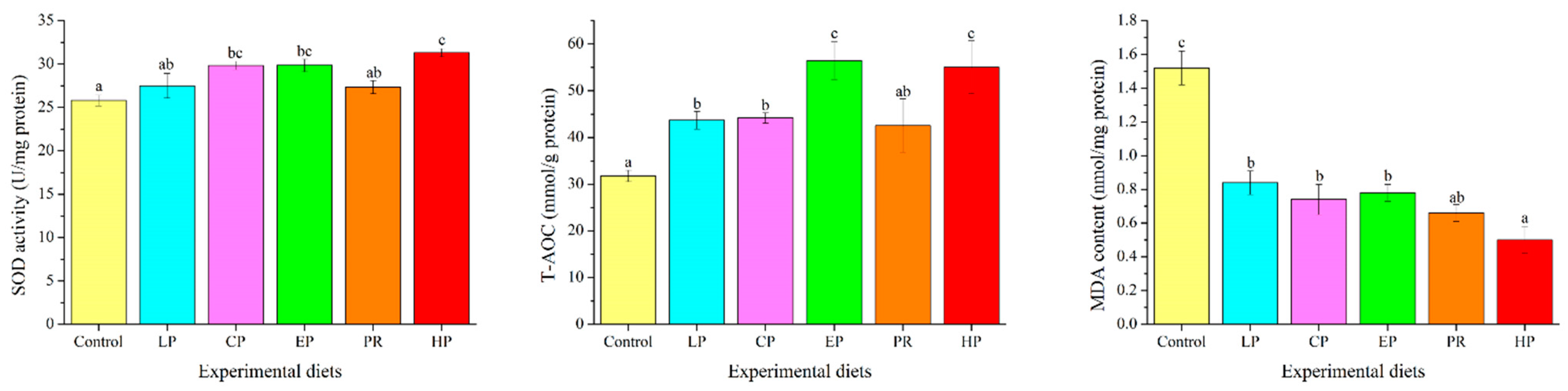
3.8. Antioxidant Parameters in the Liver
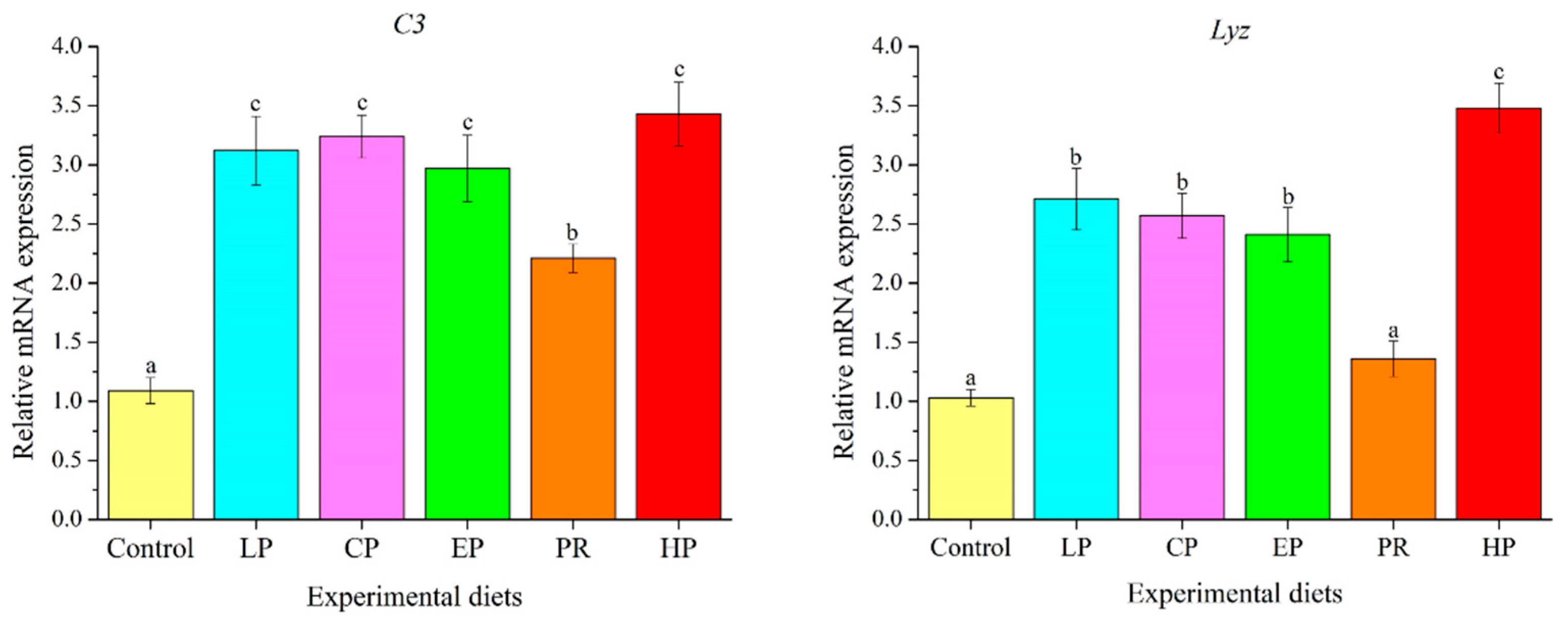
3.9. Expression Analysis of Immune-Related Genes in Liver
4. Discussion
4.1. Pigmentation and Astaxanthin Accumulation
4.2. Retention Rate of Astaxanthin in Feed
4.3. Growth Parameters and Intestinal Morphology
4.4. Flesh Quality
4.5. Plasma Biochemical Indices
4.6. Antioxidation Property
4.7. Immunological Characteristics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO—Food and Agriculture Organization of the United Nations. The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Teimouri, M.; Amirkolaie, A.K.; Yeganeh, S. The effects of Spirulina platensis meal as a feed supplement on growth performance and pigmentation of rainbow trout (Oncorhynchus mykiss). Aquaculture 2013, 396–399, 14–19. [Google Scholar] [CrossRef]
- Ambati, R.R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.C.; Yusoff, F.M.; Shariff, M.; Kamarudin, M.S. Astaxanthin as feed supplement in aquatic animals. Rev. Aquac. 2018, 10, 738–773. [Google Scholar] [CrossRef]
- Lu, Q.; Li, H.; Zou, Y.; Liu, H.; Yang, L. Astaxanthin as a microalgal metabolite for aquaculture: A review on the synthetic mechanisms, production techniques, and practical application. Algal Res. 2021, 54, 102178. [Google Scholar] [CrossRef]
- Nogueira, N.; Canada, P.; Caboz, J.; Andrade, C.; Cordeiro, N. Effect of different levels of synthetic astaxanthin on growth, skin color and lipid metabolism of commercial sized red porgy (Pagrus pagrus). Anim. Feed Sci. Technol. 2021, 276, 114916. [Google Scholar] [CrossRef]
- Angell, A.; Nys, R.D.; Mangott, A.; Vucko, M.J. The effects of concentration and supplementation time of natural and synthetic sources of astaxanthin on the colouration of the prawn Penaeus monodon. Algal Res. 2018, 35, 577–585. [Google Scholar] [CrossRef]
- Sommer, T.R.; Potts, W.T.; Morrissy, N.M. Utilization of microalgal astaxanthin by rainbow trout (Oncorhynchus mykiss). Aquaculture 1991, 94, 79–88. [Google Scholar] [CrossRef]
- Choubert, G.; Heinrich, O. Carotenoid pigments of the green alga Haematococcus pluvialis: Assay on rainbow trout, Oncorhynchus mykiss, pigmentation in comparison with synthetic astaxanthin and canthaxanthin. Aquaculture 1993, 112, 217–226. [Google Scholar] [CrossRef]
- Zhao, W.; Cui, X.; Wang, Z.; Yao, R.; Chen, M.; Gao, B.; Zhang, C.; Niu, J. Effects of Barranca yajiagengensis powder in the diet of Trachinotus ovatus on the growth performance, antioxidant capacity, immunity and morphology of the liver and intestine. Antioxidants 2022, 11, 1220. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 1995. [Google Scholar]
- Mastoraki, M.; Ferrándiz, P.M.; Vardali, S.C.; Kontodimas, D.C.; Kotzamanis, Y.P.; Gsaco, L.; Chatzifotis, S.; Antonopoulou, E. A comparative study on the effect of fish meal substitution with three different insect meals on growth, body composition and metabolism of European sea bass (Dicentrarchus labrax L.). Aquaculture 2020, 528, 735511. [Google Scholar] [CrossRef]
- Wang, F.; Gao, B.; Wu, M.; Huang, L.; Zhang, C. A novel strategy for the hyper-production of astaxanthin from the newly isolated microalga Haematococcus pluvialis JNU35. Algal Res. 2019, 39, 101466. [Google Scholar] [CrossRef]
- Zhao, W.; Cui, X.; Wang, Z.; Yao, R.; Xie, S.; Gao, B.; Zhang, C.; Niu, J. Beneficial changes in growth performance, antioxidant capacity, immune response, hepatic health, and flesh quality of Trachinotus ovatus fed with Oedocladium carolinianum. Front. Immunol. 2022, 13, 940929. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Fang, H.H.; Gao, B.Y.; Dai, C.M.; Liu, Z.Z.; Zhang, C.W.; Niu, J. Dietary Tribonema sp. supplementation increased growth performance, antioxidant capacity, immunity and improved hepatic health in golden pompano (Trachinotus ovatus). Aquaculture 2020, 529, 735667. [Google Scholar] [CrossRef]
- Choubert, G.; Cravedi, J.P.; Laurentie, M. Effect of alternate distribution of astaxanthin on rainbow trout (Oncorhynchus mykiss) muscle pigmentation. Aquaculture 2009, 286, 100–104. [Google Scholar] [CrossRef]
- Tejera, N.; Cejas, J.; Rodriguez, C.; Bjerkeng, B.; Jerez, S.; Bolanos, A.; Lorenzo, A. Pigmentation, carotenoids, lipid peroxides and lipid composition of skin of red porgy (Pagrus pagrus) fed diets supplemented with different astaxanthin sources. Aquaculture 2007, 270, 218–230. [Google Scholar] [CrossRef]
- Yi, X.; Xu, W.; Zhou, H.; Zhang, H.Y.; Luo, Y.; Zhang, W.; Mai, K. Effects of dietary astaxanthin and xanthophylls on the growth and skin pigmentation of large yellow croaker Larimichthys croceus. Aquaculture 2014, 433, 377–383. [Google Scholar] [CrossRef]
- Baker, R.T.; Pfeiffer, A.M.; Schoner, F.J.; Smith-Lemmon, L. Pigmenting efficacy of astaxanthin and canthaxanthin in freshwater-reared Atlantic salmon, Salmo salar. Anim. Feed Sci. Tech. 2002, 99, 97–106. [Google Scholar] [CrossRef]
- White, D.A.; Moody, A.J.; Serwata, R.D.; Bowen, J.; Soutar, C.; Young, A.J.; Davies, S.J. The degree of carotenoid esterification influences the absorption of astaxanthin in rainbow trout, Oncorhynchus mykiss (Walbaum). Aquac. Nutr. 2003, 9, 247–251. [Google Scholar] [CrossRef]
- Burgos-Diaz, C.; Opazo-Navarrete, M.; Soto-Annual, M.; Leal-Calderon, F.; Bustamante, M. Food-grade pickering emulsion as a novel astaxanthin encapsulation system for making powder-based products: Evaluation of astaxanthin stability during processing, storage, and its bioaccessibility. Food Res. Int. 2020, 134, 109244. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, L.; Xu, J.; Qiao, X.; Li, Z.; Wang, Y.; Xue, C. Evaluation of the physicochemical stability and digestibility of microencapsulated esterified astaxanthins using in vitro and in vivo models. Food Chem. 2018, 269, 73–81. [Google Scholar] [CrossRef]
- Amar, E.C.; Kiron, V.; Satoh, S.; Watanabe, T. Influence of various dietary synthetic carotenoids on bio-defence mechanisms in rainbow trout, Oncorhynchus mykiss (Walbaum). Aquac. Res. 2001, 32, 162–173. [Google Scholar] [CrossRef]
- Bazyar, L.A.A.; Ahamadi, M.R.; Safi, S.; Ytrestoyl, T.; Bjerkeng, B. Growth performance, mortality and carotenoid pigmentation of fry offspring as affected by dietary supplementation of astaxanthin to female rainbow trout (Oncorhynchus mykiss) broodstock. J. Appl. Icthyol. 2010, 26, 35–39. [Google Scholar] [CrossRef]
- Talebi, M.; Khara, H.; Zorriehzahra, M.E.J.; Ghobadi, S. Efficacy of astaxanthin fed to Rainbow Trout (Oncorhynchus mykiss): Effect on growth, pigmentation and blood indices. Int. J. Oceanogr. Aquac. 2020, 4, 000197. [Google Scholar] [CrossRef]
- Li, M.; Wu, W.; Zhou, P.; Xie, F.; Zhou, Q.; Mai, K. Comparison effect of dietary astaxanthin and Haematococcus pluvialis on growth performance, antioxidant status and immune response of large yellow croaker Pseudosciaena crocea. Aquaculture 2014, 434, 227–232. [Google Scholar] [CrossRef]
- Hansen, O.J.; Puvanendran, V.; Bangera, R. Broodstock diet with water and astaxanthin improve condition and egg output of broodstock fish and larval survival in Atlantic cod, Gadus morhua L. Aquac. Res. 2016, 47, 819–829. [Google Scholar] [CrossRef]
- Xie, J.; Fang, H.; He, X.; Liao, S.; Liu, Y.; Tian, L.; Niu, J. Study on mechanism of synthetic astaxanthin and Haematococcus pluvialis improving the growth performance and antioxidant capacity under acute hypoxia stress of golden pompano (Trachinotus ovatus) and enhancing antiinflammatory by activating Nrf2-ARE pathway to antagonize the NF-κB pathway. Aquaculture 2020, 518, 734657. [Google Scholar] [CrossRef]
- Pham, M.A.; Byun, H.G.; Kim, K.D.; Lee, S.M. Effects of dietary carotenoid source and level on growth, skin pigmentation, antioxidant activity and chemical composition of juvenile olive flounder Paralichthys olivaceus. Aquaculture 2014, 431, 65–72. [Google Scholar] [CrossRef]
- Wassef, E.A.; Chatzifotis, S.; Sakr, E.M.; Saleh, N.E. Effect of two natural carotenoid sources in diets for gilthead seabream, Sparus aurata, on growth and skincoloration. J. Appl. Aquac. 2010, 22, 216–229. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Leng, X.; Zhang, C.; Han, Z.; Zhang, F. Effects of dietary astaxanthins on pigmentation of flesh and tissue antioxidation of rainbow trout (Oncorhynchus mykiss). Aquacult. Int. 2012, 21, 579–589. [Google Scholar] [CrossRef]
- Rahman, M.; Khosravi, S.; Chang, K.H.; Lee, S.M. Effects of dietary inclusion of astaxanthin on growth, muscle pigmentation and antioxidant capacity of juvenile rainbow trout (Oncorhynchus mykiss). Prev. Nutr. Food Sci. 2016, 21, 281–288. [Google Scholar] [CrossRef]
- Han, B.; Long, W.Q.; He, J.Y.; Liu, Y.J.; Si, Y.Q.; Tian, L.X. Effects of dietary Bacillus licheniformis on growth performance, immunological parameters, intestinal morphology and resistance of juvenile Nile tilapia (Oreochromis niloticus) to challenge infections. Fish Shellfish Immun. 2015, 46, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.J.; Yang, H.J.; Liu, Y.J.; Chen, S.J.; Guo, D.Q.; Yu, Y.Y.; Tian, L.X. Effects of graded levels of threonine on growth performance, biochemical parameters and intestine morphology of juvenile grass carp Ctenopharyngodon idella. Aquaculture 2014, 424–425, 113–119. [Google Scholar] [CrossRef]
- Lin, S.M.; Zhou, X.M.; Zhou, Y.L.; Kuang, W.M.; Chen, Y.J.; Luo, L.; Dai, F.Y. Intestinal morphology, immunity and microbiota response to dietary fibers in largemouth bass, Micropterus salmoide. Fish Shellfish Immun. 2020, 103, 135–142. [Google Scholar] [CrossRef]
- Yu, L.; Wen, H.; Jiang, M.; Wu, F.; Tian, J.; Lu, X.; Xiao, J.; Liu, W. Effects of ferulic acid on intestinal enzyme activities, morphology, microbiome composition of genetically improved farmed tilapia (Oreochromis niloticus) fed oxidized fish oil. Aquaculture 2020, 528, 735543. [Google Scholar] [CrossRef]
- Zhao, W.; Fang, H.; Liu, Z.; Huang, M.; Su, M.; Zhang, C.; Gao, B.; Niu, J. A newly isolated strain of Haematococcus Pluvialis JNU35 improves the growth, antioxidation, immunity and liver function of golden pompano (Trachinotus Ovatus). Aquac. Nutri. 2021, 27, 342–354. [Google Scholar] [CrossRef]
- Kalinowski, C.T.; Robaina, L.E.; Izquierdo, M.S. Effect of dietary astaxanthin on the growth performance, lipid composition and post-mortem skin colouration of red porgy Pagrus pagrus. Aquacult. Int. 2011, 19, 811–823. [Google Scholar] [CrossRef]
- Christiansen, R.; Torrissen, O.J. Growth and survival of Atlantic salmon, Salmo salar L. fed different dietary levels of astaxanthin. Juveniles. Aquacult. Nutr. 1996, 2, 55–62. [Google Scholar] [CrossRef]
- Yanar, Y.; Bueyuekcapar, H.; Yanar, M.; Goecer, M. Effect of carotenoids from red pepper and marigold flower on pigmentation, sensory properties and fatty acid composition of rainbow trout. Food Chem. 2007, 100, 326–330. [Google Scholar] [CrossRef]
- Hakimi, S.; Kari, N.M.; Ismail, N.; Ismail, M.N.; Ahmad, F. Evaluation of taste active peptides and amino acids from anchovy proteins in fish sauce by insilico approach. Food Sci. Biotechnol. 2022, 31, 767–785. [Google Scholar] [CrossRef]
- Ruiz-Capillas, C.; Moral, A. Free amino acids in muscle of Norway lobster (Nephrops novergicus (L.)) in controlled and modified atmospheres during chilled storage. Food Chem. 2004, 86, 85–91. [Google Scholar] [CrossRef]
- Iaconisi, V.; Secci, G.; Sabatino, G.; Piccolo, G.; Gasco, L.; Papini, A.M.; Parisi, G. Effect of mealworm (Tenebrio molitor L.) larvae meal on amino acid composition of gilthead sea bream (Sparus aurata L.) and rainbow trout (Oncorhynchus mykiss W.) fillets. Aquaculture 2019, 513, 734403. [Google Scholar] [CrossRef]
- Fazio, F. Fish hematology analysis as an important tool of aquaculture: A Review. Aquaculture 2019, 500, 237–242. [Google Scholar] [CrossRef]
- Ghodrati, M.; Islami, H.R.; Shekarabi, S.; Masouleh, A.S.; Mehrgan, M.S. Combined effects of enzymes and probiotics on hemato-biochemical parameters and immunological responses of juvenile Siberian sturgeon (Acipenser Baerii). Fish Shellfish Immunol. 2021, 112, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.C.; Yusoff, F.M.; Shariff, M.; Kamarudin, M.S.; Nagao, N. Dietary supplementation of astaxanthin enhances hemato-biochemistry and innate immunity of Asian seabass, Lates calcarifer (Bloch, 1790). Aquaculture 2019, 512, 734339. [Google Scholar] [CrossRef]
- Sheikhzadeh, N.; Tayefinasrabadi, H.; Oushani, A.K.; Enferadi, M.H. Effects of Haematococcus Pluvialis supplementation on antioxidant system and metabolism in Rainbow Trout (Oncorhynchus Mykiss). Fish Physiol. Biochem. 2012, 38, 413–419. [Google Scholar] [CrossRef]
- Liu, X.J.; Luo, Q.X.; Rakariyatham, K.; Cao, Y.; Goulette, T.; Liu, X.; Xiao, H. Antioxidation and anti-ageing activities of different stereoisomeric astaxanthin in vitro and in vivo. J. Funct. Foods 2016, 25, 50–61. [Google Scholar] [CrossRef]
- Canli, E.G.; Dogan, A.; Canli, M. Serum biomarker levels alter following nanoparticle (Al2O3, CuO, TiO2) exposures in freshwater fish (Oreochromis niloticus). Environ. Toxicol. Pharmacol. 2018, 62, 181–187. [Google Scholar] [CrossRef]
- Tsubakio-Yamamoto, K.; Matsuura, F.; Koseki, M.; Oku, H.; Sandoval, J.C.; Inagaki, M.; Nakatani, K.; Nakaoka, H.; Kawase, R.; Yuasa-Kawase, M.; et al. Adiponectin prevents atherosclerosis by increasing cholesterol efflux from macrophages. Biochem. Biophys. Res. Commun. 2008, 375, 390–394. [Google Scholar] [CrossRef]
- Petri, S.; Korner, S.; Kiaei, M. Nrf2/ARE signaling pathway: Key mediator in oxidative stress and potential therapeutic target in ALS. Neurol. Res. Int. 2012, 2012, 878030. [Google Scholar] [CrossRef]
- Ye, Q.; Huang, B.; Zhang, X.; Zhu, Y.; Chen, X. Astaxanthin protects against mpp+-induced oxidative stress in PC12 cells via the HO-1/NOX2 axis. BMC Neurosci. 2012, 13, 156. [Google Scholar] [CrossRef]
- Banning, A.; Brigelius-Flohé, R. NF-kB, Nrf2, and HO-1 interplay in redox-regulated vcam-1 expression. Antioxid. Redox Sign. 2005, 7, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Saw, C.L.L.; Yang, A.Y.; Guo, Y.; Kong, A.T. Astaxanthin and Omega-3 fatty acids individually and in combination protect against oxidative stress via the Nrf2-ARE pathway. Food Chem. Toxicol. 2013, 62, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.N.; Jena, G.B. Astaxanthin intervention ameliorates cyclophosphamide-induced oxidative stress, DNA damage and early hepatocarcinogenesis in rat: Role of Nrf2, P53, P38 and phase-II enzymes. Mutat. Res. 2010, 696, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Song, X.L.; Wang, L.; Li, X.Q.; Chen, Z.Z.; Liang, G.Y.; Leng, X.J. Dietary astaxanthin improved the body pigmentation and antioxidant function, but not the growth of discus fish (Symphysodon spp.). Aquac. Res. 2017, 48, 1359–1367. [Google Scholar] [CrossRef]
- Liu, F.; Shi, H.Z.; Guo, Q.S.; Yu, Y.B.; Wang, A.M.; Lv, F.; Shen, W.B. Effects of astaxanthin and emodin on the growth, stress resistance and disease resistance of yellow catfish (Pelteobagrus fulvidraco). Fish Shellfish Immunol. 2016, 51, 125–135. [Google Scholar] [CrossRef]
- Ragland, S.A.; Criss, A.K. From bacterial killing to immune modulation: Recent insights into the functions of lysozyme. PLoS Pathog. 2017, 13, e1006512. [Google Scholar] [CrossRef]
- Boshra, H.; Li, J.; Sunyer, J.O. Recent advances on the complement system of teleost fish. Fish Shellfish Immunol. 2006, 20, 239–262. [Google Scholar] [CrossRef]
- Saurabh, S.; Sahoo, P.K. Lysozyme: An important defence molecule of fish innate immune system. Aquac. Res. 2008, 39, 223–239. [Google Scholar] [CrossRef]
- Zhu, X.M.; Li, M.Y.; Liu, X.Y.; Xia, C.G.; Niu, X.T.; Wang, G.Q.; Zhang, D.M. Effects of dietary astaxanthin on growth, blood biochemistry, antioxidant, immune and inflammatory response in lipopolysaccharide-challenged Channa argus. Aqua. Res. 2020, 51, 1980–1991. [Google Scholar] [CrossRef]

| Ingredients | Control | LP | CP | EP | PR | HP |
|---|---|---|---|---|---|---|
| Fish meal | 43 | 43 | 43 | 43 | 43 | 43 |
| Soybean meal | 5 | 5 | 5 | 5 | 5 | 5 |
| Soy protein isolate | 10 | 10 | 10 | 10 | 10 | 10 |
| Corn starch | 10.7 | 10.6 | 10.6 | 10.6 | 9.7 | 9.7 |
| Tenebrio molitor meal | 8 | 8 | 8 | 8 | 8 | 8 |
| Cassava starch | 5 | 5 | 5 | 5 | 5 | 5 |
| Fish oil | 12 | 12 | 12 | 12 | 12 | 12 |
| Soybean lecithin | 2 | 2 | 2 | 2 | 2 | 2 |
| Ca (H2PO4)2 | 1 | 1 | 1 | 1 | 1 | 1 |
| Vitamin premix a | 1 | 1 | 1 | 1 | 1 | 1 |
| Mineral premix b | 1 | 1 | 1 | 1 | 1 | 1 |
| Choline | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Vitamin C | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| DL-Met | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Lys-HCL (99%) | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Lucantin Pink CWD (10%) c | 0 | 0.1 | 0 | 0 | 0 | 0 |
| Carophyll Pink (10%) d | 0 | 0 | 0.1 | 0 | 0 | 0 |
| Essention Pink (10%) e | 0 | 0 | 0 | 0.1 | 0 | 0 |
| Phaffia rhodozyma (1%) f | 0 | 0 | 0 | 0 | 1 | 0 |
| Haematococcus Pluvialis (1%) g | 0 | 0 | 0 | 0 | 0 | 1 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 |
| Nutrient levels h | ||||||
| Crude protein | 46.47 | 46.75 | 46.76 | 46.02 | 46.45 | 46.47 |
| Crude lipid | 15.46 | 16.12 | 15.66 | 15.68 | 15.49 | 15.37 |
| Moisture | 9.33 | 9.58 | 9.61 | 9.77 | 9.16 | 9.39 |
| Gene | Primer Sequence (5′ to 3′) | Genbank No. |
|---|---|---|
| Nrf2-F | GCAGAGGTCTGCCCACCTGAAT | HQ916348.1 |
| Nrf2-R | GCCACAAGGCAGGGTGACACTT | |
| HO-1-F | CGCCTACACCCGTTACCTAG | AF099079.2 |
| HO-1-R | CTCTCCGCTGCTTAACCCAA | |
| Lyz-F Lyz-R | GAAACAGCCTGCCCAACT GTCCAACACCACACGCTT | AF452171.1 |
| C3-F | GGCCAGTCCCTGGTGGTTA | XM_036955530.1 |
| C3-R | GGTGGACTGTGTGGATCCGTA | |
| β-actin-F | TACAACGAGCTGAGGGTGGC | AJ438158.1 |
| β-actin-R | GGCAGGGGTGTTGAAGGTCT |
| Lightness (L*) | Redness (a*) | Yellowness (b*) | |
|---|---|---|---|
| Control | 39.51 ± 1.11 b | 7.21 ± 0.74 a | 7.98 ± 0.39 a |
| LP | 31.76 ± 0.99 a | 12.79 ± 0.58 c | 9.74 ± 0.62 bc |
| CP | 33.59 ± 0.75 a | 12.17 ± 0.56 c | 10.43 ± 0.56 c |
| EP | 33.57 ± 0.78 a | 12.37 ± 0.61 c | 10.18 ± 0.42 c |
| PR | 34.43 ± 1.17 a | 9.22 ± 0.59 b | 7.74 ± 0.35 a |
| HP | 34.02 ± 0.85 a | 9.78 ± 0.72 b | 8.40 ± 0.45 ab |
| Control | LP | CP | EP | PR | HP | |
|---|---|---|---|---|---|---|
| Whole body | ||||||
| Astaxanthin content | 2.76 ± 0.28 a | 6.32 ± 0.42 c | 5.91 ± 0.49 c | 5.79 ± 0.55 c | 4.01 ± 0.54 b | 4.42 ± 0.66 b |
| Dorsal muscle | ||||||
| Astaxanthin content | 1.59 ± 0.08 a | 5.65 ± 0.44 c | 5.22 ± 0.46 c | 4.93 ± 0.22 c | 2.93 ± 0.24 b | 3.23 ± 0.43 b |
| Item | Control | LP | CP | EP | PR | HP |
|---|---|---|---|---|---|---|
| Astaxanthin content in feeds before extrusion (mg/kg) | ||||||
| 5.02 | 94.35 | 104.02 | 96.13 | 96.32 | 97.03 | |
| Astaxanthin content in feeds after extrusion (mg/kg) | ||||||
| 4.93 | 92.34 | 100.02 | 91.12 | 93.31 | 94.21 | |
| Astaxanthin retention in feeds after extrusion (%) | ||||||
| 98.21 | 97.87 | 96.15 | 94.79 | 96.88 | 97.09 | |
| Astaxanthin content in feeds after 1-month (mg/kg) | ||||||
| 4.62 | 86.32 | 90.02 | 85.12 | 84.28 | 86.57 | |
| Astaxanthin retention in feed after 1-month (%) | ||||||
| 93.71 | 93.48 | 90.00 | 93.41 | 90.32 | 91.89 | |
| Astaxanthin content in feeds after 2-month (mg/kg) | ||||||
| 4.14 | 76.00 | 76.52 | 72.96 | 67.06 | 72.36 | |
| Astaxanthin retention in feed after 2-month (%) | ||||||
| 89.66 | 88.04 | 85.00 | 85.71 | 79.57 | 83.59 | |
| Control | LP | CP | EP | PR | HP | |
|---|---|---|---|---|---|---|
| IBW (g) | 251.35 ± 1.27 | 249.92 ± 0.63 | 251.22 ± 1.12 | 251.43 ± 0.98 | 250.46 ± 0.87 | 251.86 ± 0.58 |
| FBW (g) | 618.01 ± 5.20 a | 649.80 ± 6.18 b | 652.32 ± 4.44 b | 670.39 ± 3.86 c | 651.98 ± 6.21 b | 672.71 ± 7.10 c |
| WGR (%) | 145.92 ± 3.05 a | 160.04 ± 2.44 b | 159.67 ± 1.61 b | 166.66 ± 2.53 c | 160.33 ± 2.75 b | 165.22 ± 5.89 bc |
| SGR (%/d) | 1.00 ± 0.01 a | 1.06 ± 0.01 b | 1.06 ± 0.01 b | 1.09 ± 0.01 b | 1.06 ± 0.01 b | 1.08 ± 0.01 b |
| SR (%) | 100 ± 0 | 100 ± 0 | 97.5 ± 1.60 | 100 ± 0 | 98.34 ± 0.96 | 99.17 ± 0.83 |
| FCR | 1.66 ± 0.06 b | 1.42 ± 0.04 a | 1.52 ± 0.08 ab | 1.40 ± 0.04 a | 1.56 ± 0.06 ab | 1.52 ± 0.07 ab |
| CF (g/cm3) | 1.21 ± 0.02 | 1.22 ± 0.02 | 1.16 ± 0.03 | 1.18 ± 0.04 | 1.17 ± 0.03 | 1.17 ± 0.02 |
| Item | Control | LP | CP | EP | PR | HP |
|---|---|---|---|---|---|---|
| Whole-body, % dry weight | ||||||
| Moisture | 69.24 ± 0.45 | 68.74 ± 0.71 | 69.99 ± 1.02 | 70.64 ± 0.42 | 69.31 ± 0.34 | 69.93 ± 0.40 |
| Crude protein | 55.17 ± 0.65 a | 59.71 ± 0.38 b | 59.03 ± 1.03 b | 59.07 ± 1.13 b | 56.64 ± 0.17 ab | 58.71 ± 0.99 b |
| Crude lipid | 33.48 ± 0.48 c | 29.39 ± 0.15 b | 28.12 ± 0.21 a | 28.19 ± 0.20 a | 30.41 ± 0.63 b | 26.98 ± 0.22 a |
| Dorsal muscle, % dry weight | ||||||
| Moisture | 78.30 ± 0.36 | 76.84 ± 0.35 | 78.62 ± 0.30 | 76.62 ± 0.77 | 75.96 ± 0.63 | 75.96 ± 0.63 |
| Crude protein | 75.97 ± 0.68 a | 80.44 ± 0.31 c | 81.54 ± 0.44 c | 79.97 ± 0.68 bc | 77.96 ± 0.34 b | 77.96 ± 0.34 b |
| Crude lipid | 9.49 ± 0.58 | 8.74 ± 0.60 | 8.98 ± 0.63 | 8.58 ± 0.37 | 8.61 ± 0.89 | 8.61 ± 0.89 |
| Control | LP | CP | EP | PR | HP | |
|---|---|---|---|---|---|---|
| Methionine (Met) * | 2.32 ± 0.03 a | 2.31 ± 0.06 a | 2.42 ± 0.04 ab | 2.36 ± 0.02 ab | 2.48 ± 0.05 b | 2.44 ± 0.06 ab |
| Lysine (Lys) * | 6.50 ± 0.07 a | 6.52 ± 0.12 a | 6.87 ± 0.13 ab | 6.57 ± 0.09 ab | 6.96 ± 0.12 b | 6.83 ± 0.19 ab |
| Threonine (Thr) * | 3.16 ± 0.04 a | 3.16 ± 0.06 a | 3.31 ± 0.04 ab | 3.29 ± 0.05 ab | 3.47 ± 0.07 b | 3.45 ± 0.07 b |
| Phenylalanine (Phe) * | 3.07 ± 0.03 ab | 3.08 ± 0.07 ab | 3.32 ± 0.02 c | 3.00 ± 0.04 a | 3.20 ± 0.06 bc | 3.14 ± 0.06 ab |
| Isoleucine (Ile) * | 3.25 ± 0.03 a | 3.26 ± 0.06 a | 3.40 ± 0.04 ab | 3.30 ± 0.03 a | 3.48 ± 0.05 b | 3.43 ± 0.09 ab |
| Leucine (Leu) * | 5.73 ± 0.05 ab | 5.77 ± 0.12 ab | 6.05 ± 0.07 b | 5.57 ± 0.07 a | 5.94±0.12b | 5.86 ± 0.15 ab |
| Valine (Val) * | 3.89 ± 0.04 a | 3.89 ± 0.06 a | 4.12 ± 0.06 b | 3.83 ± 0.03 a | 4.01 ± 0.06 ab | 3.99 ± 0.10 ab |
| Histidine (His) * | 2.88 ± 0.05 b | 2.92 ± 0.02 b | 3.09 ± 0.06 c | 1.91 ± 0.05 a | 2.01 ± 0.02 a | 1.98 ± 0.03 a |
| Arginine (Arg) * | 4.24 ± 0.07 ab | 4.14 ± 0.06 a | 4.40 ± 0.08 ab | 4.14 ± 0.07 a | 4.44 ± 0.09 b | 4.40 ± 0.12 ab |
| Glycine (Gly) ** | 3.69 ± 0.10 bc | 3.39 ± 0.06 a | 3.78 ± 0.09 c | 3.49 ± 0.03 ab | 3.63 ± 0.05 bc | 3.71 ± 0.09 bc |
| Serine (Ser) ** | 2.76 ± 0.04 a | 2.74 ± 0.05 a | 2.91 ± 0.03 ab | 2.82 ± 0.03 a | 3.01 ± 0.08 b | 3.01 ± 0.06 b |
| Proline (Pro) ** | 2.35 ± 0.12 ab | 2.23 ± 0.02 a | 2.47 ± 0.06 b | 2.29 ± 0.02 ab | 2.35 ± 0.05 ab | 2.36 ± 0.01 ab |
| Alanine (Ala) ** | 4.35 ± 0.05 ab | 4.32 ± 0.08 ab | 4.57 ± 0.07 b | 4.27 ± 0.06 a | 4.49 ± 0.07 ab | 4.42 ± 0.11 ab |
| Aspartic acid (Asp) ** | 7.48 ± 0.09 ab | 7.47±0.14 ab | 7.85 ± 0.06 b | 7.28 ± 0.08 a | 7.71 ± 0.13 ab | 7.59 ± 0.06 ab |
| Glutamic acid (Glu) ** | 10.00 ± 0.14 a | 10.02 ± 0.19 ab | 10.66 ± 0.18 b | 10.12 ± 0.14 a | 10.62 ± 0.20 b | 10.54 ± 0.29 ab |
| Essential amino acids | 35.04 ± 0.40 ab | 35.04 ± 0.62 ab | 36.99 ± 0.45 b | 33.96 ± 0.45 a | 36.01 ± 0.63 ab | 35.52 ± 0.87 ab |
| Non-essential amino acids | 30.77 ± 0.47 | 30.17 ± 0.52 | 32.23 ± 0.47 | 30.28 ± 0.30 | 31.81 ± 0.58 | 31.63 ± 0.75 |
| Total amino acids | 65.81 ± 0.85 | 65.21 ± 1.14 | 69.22 ± 0.92 | 64.24 ± 0.74 | 67.81 ± 1.21 | 67.15 ± 1.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Guo, Y.-C.; Huai, M.-Y.; Li, L.; Man, C.; Pelletier, W.; Wei, H.-L.; Yao, R.; Niu, J. Comparison of the Retention Rates of Synthetic and Natural Astaxanthin in Feeds and Their Effects on Pigmentation, Growth, and Health in Rainbow Trout (Oncorhynchus mykiss). Antioxidants 2022, 11, 2473. https://doi.org/10.3390/antiox11122473
Zhao W, Guo Y-C, Huai M-Y, Li L, Man C, Pelletier W, Wei H-L, Yao R, Niu J. Comparison of the Retention Rates of Synthetic and Natural Astaxanthin in Feeds and Their Effects on Pigmentation, Growth, and Health in Rainbow Trout (Oncorhynchus mykiss). Antioxidants. 2022; 11(12):2473. https://doi.org/10.3390/antiox11122473
Chicago/Turabian StyleZhao, Wei, Yu-Cai Guo, Ming-Yan Huai, Lily Li, Chi Man, Wolf Pelletier, Han-Lin Wei, Rong Yao, and Jin Niu. 2022. "Comparison of the Retention Rates of Synthetic and Natural Astaxanthin in Feeds and Their Effects on Pigmentation, Growth, and Health in Rainbow Trout (Oncorhynchus mykiss)" Antioxidants 11, no. 12: 2473. https://doi.org/10.3390/antiox11122473
APA StyleZhao, W., Guo, Y.-C., Huai, M.-Y., Li, L., Man, C., Pelletier, W., Wei, H.-L., Yao, R., & Niu, J. (2022). Comparison of the Retention Rates of Synthetic and Natural Astaxanthin in Feeds and Their Effects on Pigmentation, Growth, and Health in Rainbow Trout (Oncorhynchus mykiss). Antioxidants, 11(12), 2473. https://doi.org/10.3390/antiox11122473








