Oxidized-LDL Deteriorated the Renal Residual Function and Parenchyma in CKD Rat through Upregulating Epithelial Mesenchymal Transition and Extracellular Matrix-Mediated Tubulointerstitial Fibrosis—Pharmacomodulation of Rosuvastatin
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vitro Study Design
2.1.1. To Elucidate the Impact of Oxidized LDL on Facilitating TGF-β Induced EMT Process in Cell Model
2.1.2. Cell Culture and Cell Grouping
2.1.3. Lipid Droplet Staining
2.1.4. Assessment of Wound Healing Migratory Ability
2.2. In Vivo Study Design
2.2.1. Animal Model of 5/6 Nephrectomy for CKD Induction
2.2.2. Examination of Renal Function Parameters
2.2.3. Collection of 24 h Urine for the Ratio of Urine Protein to Urine Creatinine (RuPr/uCr) Prior to and by Day 42 after CKD Procedure
2.2.4. Histopathological Assessment of Fibrotic Area
2.2.5. Histopathologic Scoring of Kidney Damage by Day 42 after CKD Procedure
2.2.6. Western Blot Assessment of Left Kidney Specimens
2.2.7. Immunofluorescent (IF) Study
2.2.8. Statistical Analysis
3. Results
3.1. Preliminary Results for Pointing out a Corrective Direction of the Study (Figure S1)
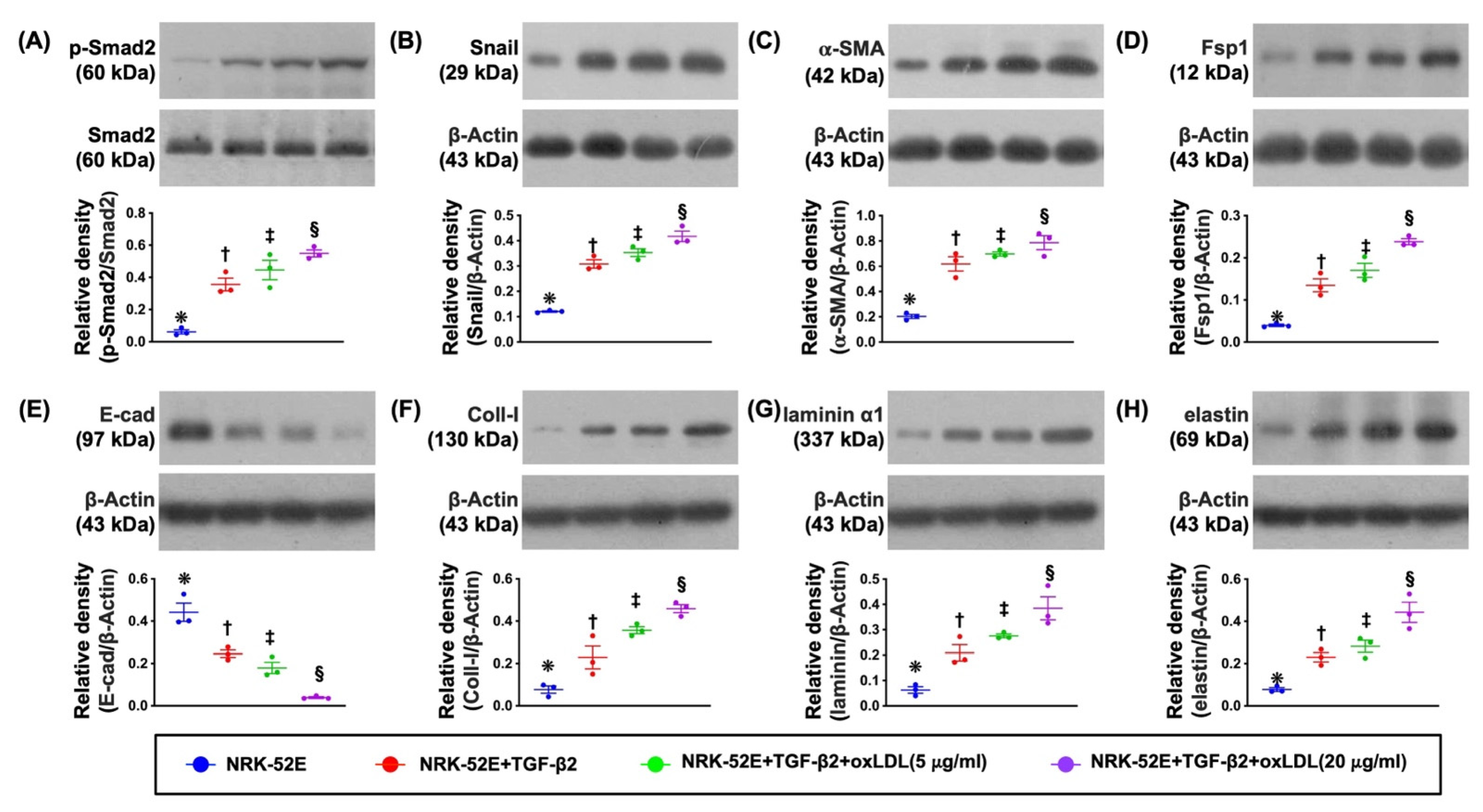
3.2. The Protein Expressions of EMT Biomarkers in NRK-52E Cell Line Undergoing the TGF-β and Oxidized LDL Treatments (Figure 1)
3.3. Cellular Levels of Fibrosis/ECM and Kidney Damaged Biomarkers (Figure 2)
3.4. Morphological Features of NRK-52E Cells Followed by TGF-β and Oxidized LDL Stimulation (Figure S2)
3.5. Lipid Droplets in NRK-52E Cells after Oxidized LDL Treatment and Inflammatory Cells Enhanced Extracellular Matrix (ECM) Production by NRK-52E Cells (Figure S3)
3.6. Impact of Synergic Effect of TGF-β and Oxidized LDL on Wound Healing Process, Migratory Assay, and Cell Viability (Figure 3)
3.7. The Mechanism of Oxidized LDL Boosting TGF-β on the EMT Process in Renal Tubular Cells (Figure 4)
3.8. The Time Courses of Circulating Levels of BUN and Creatinine and the Ratio of Urine Protein to Urine Creatinine (Figure 5)
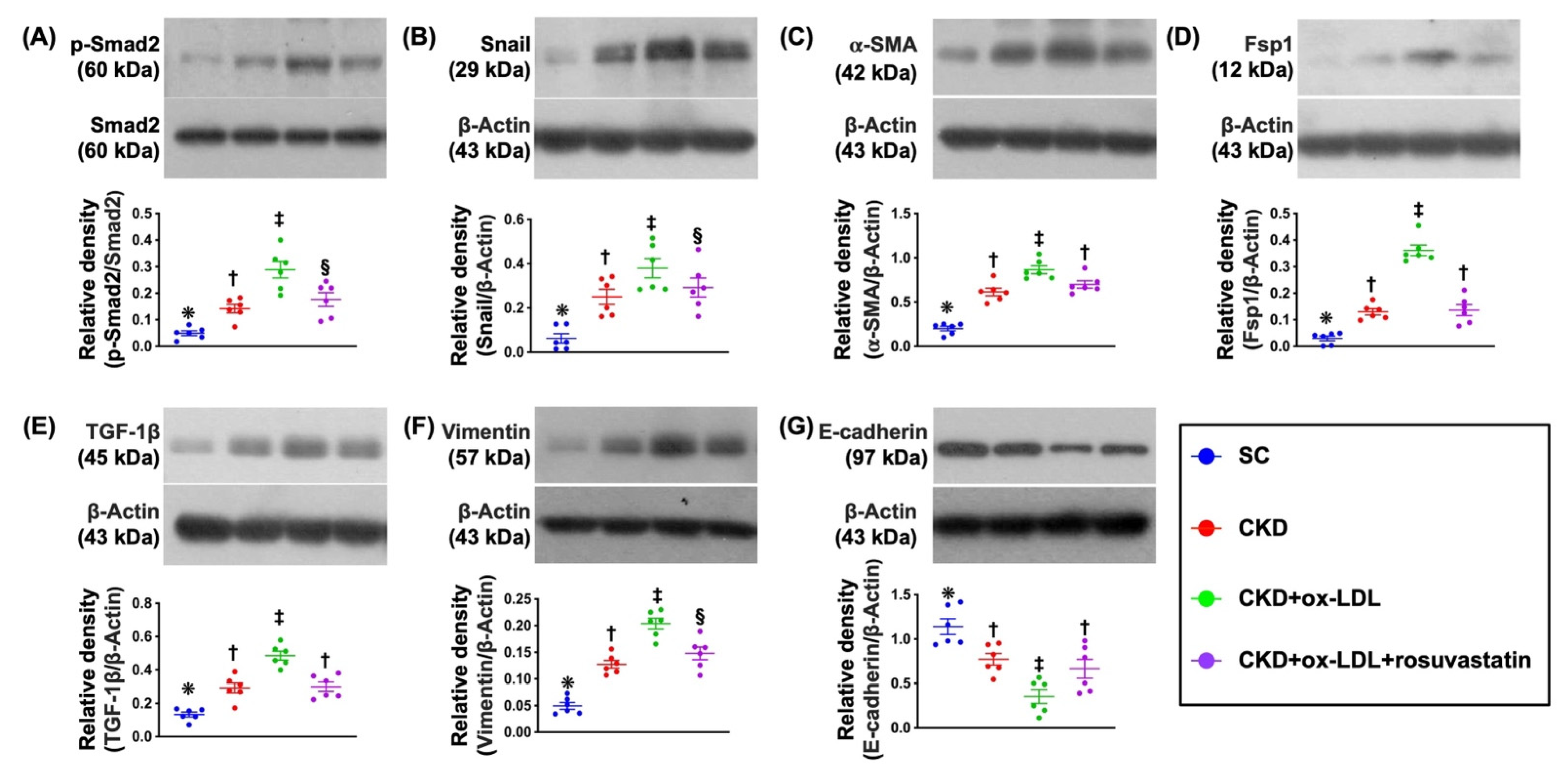
3.9. Protein Expressions of EMT Biomarkers in Kidney Parenchyma by Day 42 after CKD Induction (Figure 6)
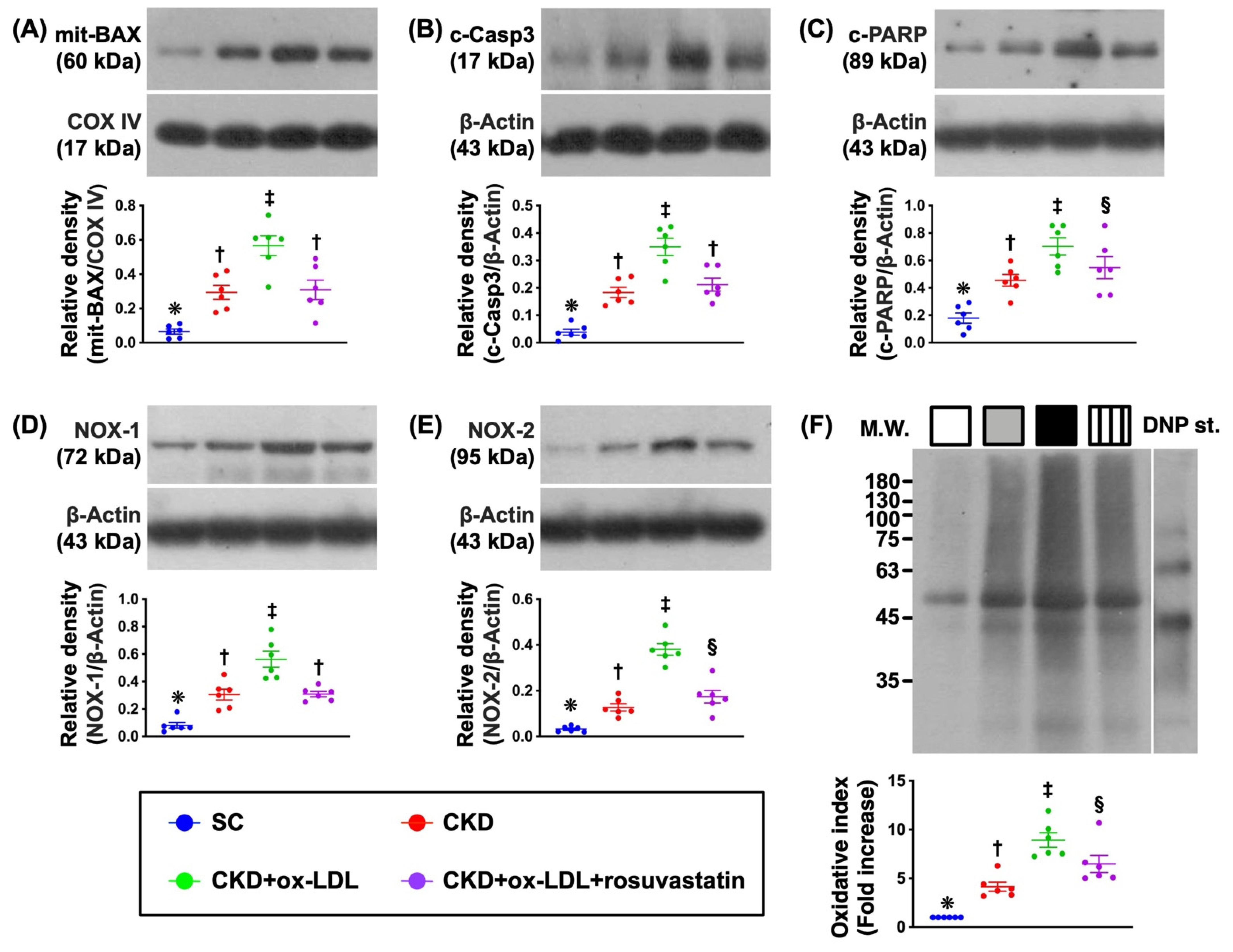
3.10. Protein Expressions of Apoptotic and Oxidative Stress Biomarkers in Kidney Parenchyma by Day 42 after CKD Induction (Figure 7)
3.11. Protein Expression of ECM in Kidney Parenchyma by Day 42 after CKD Induction (Figure 8)
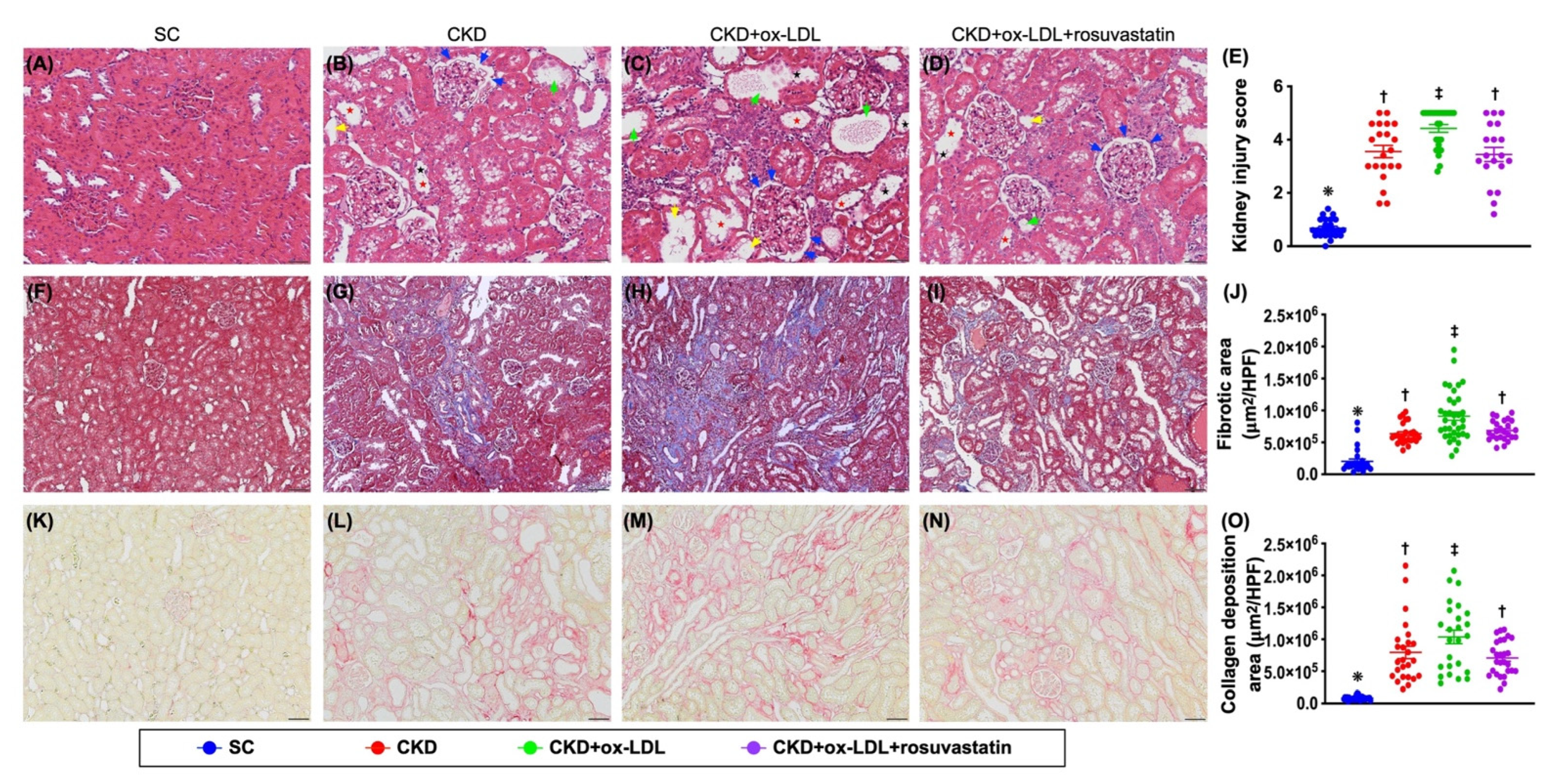
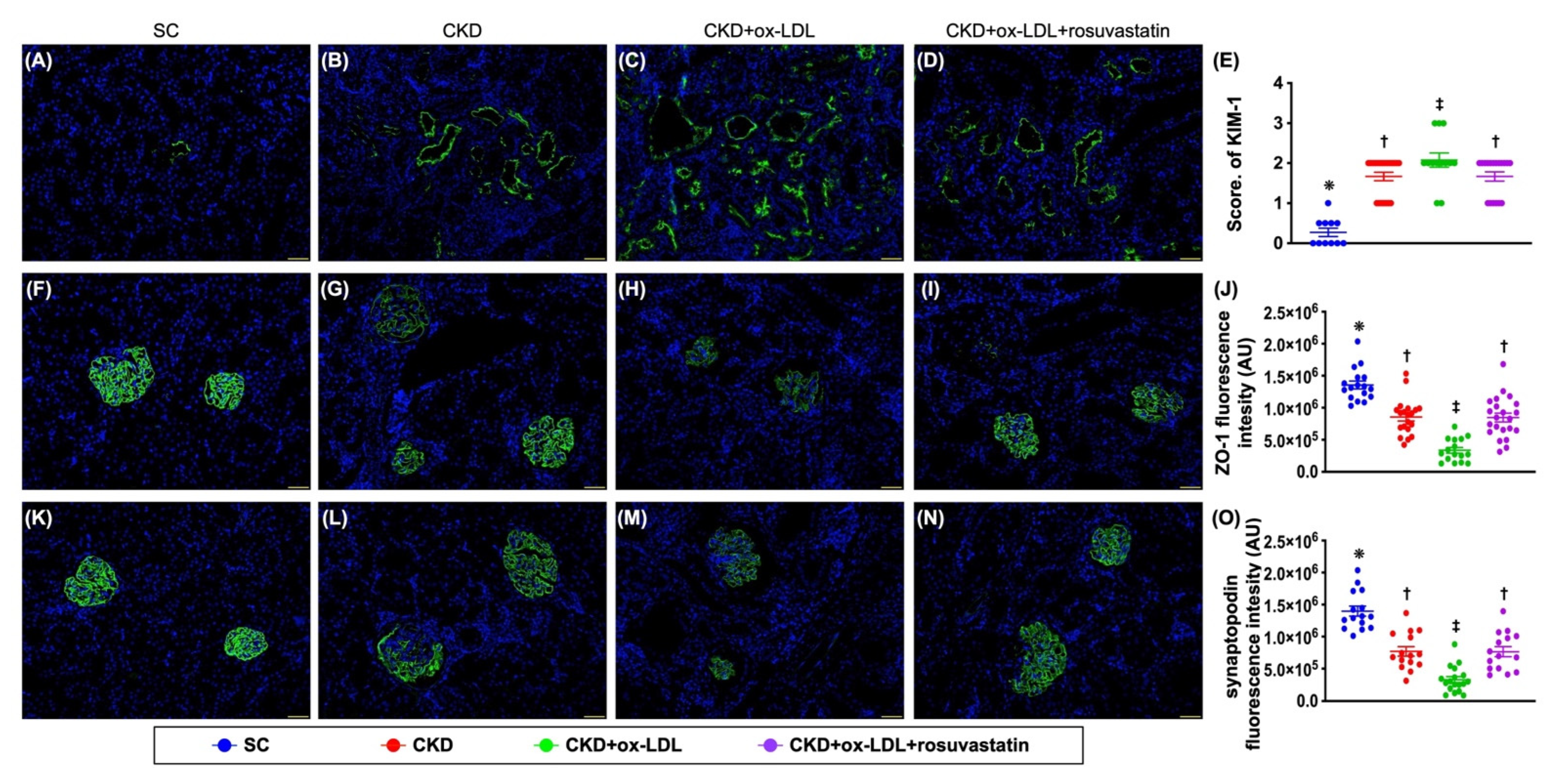
3.12. The Histopathological Analyses of Kidney Injury Score, Fibrosis, Kidney Injury Molecule, and Podocyte Components in Kidney Parenchyma by Day 42 after CKD Induction (Figure 9 and Figure 10)
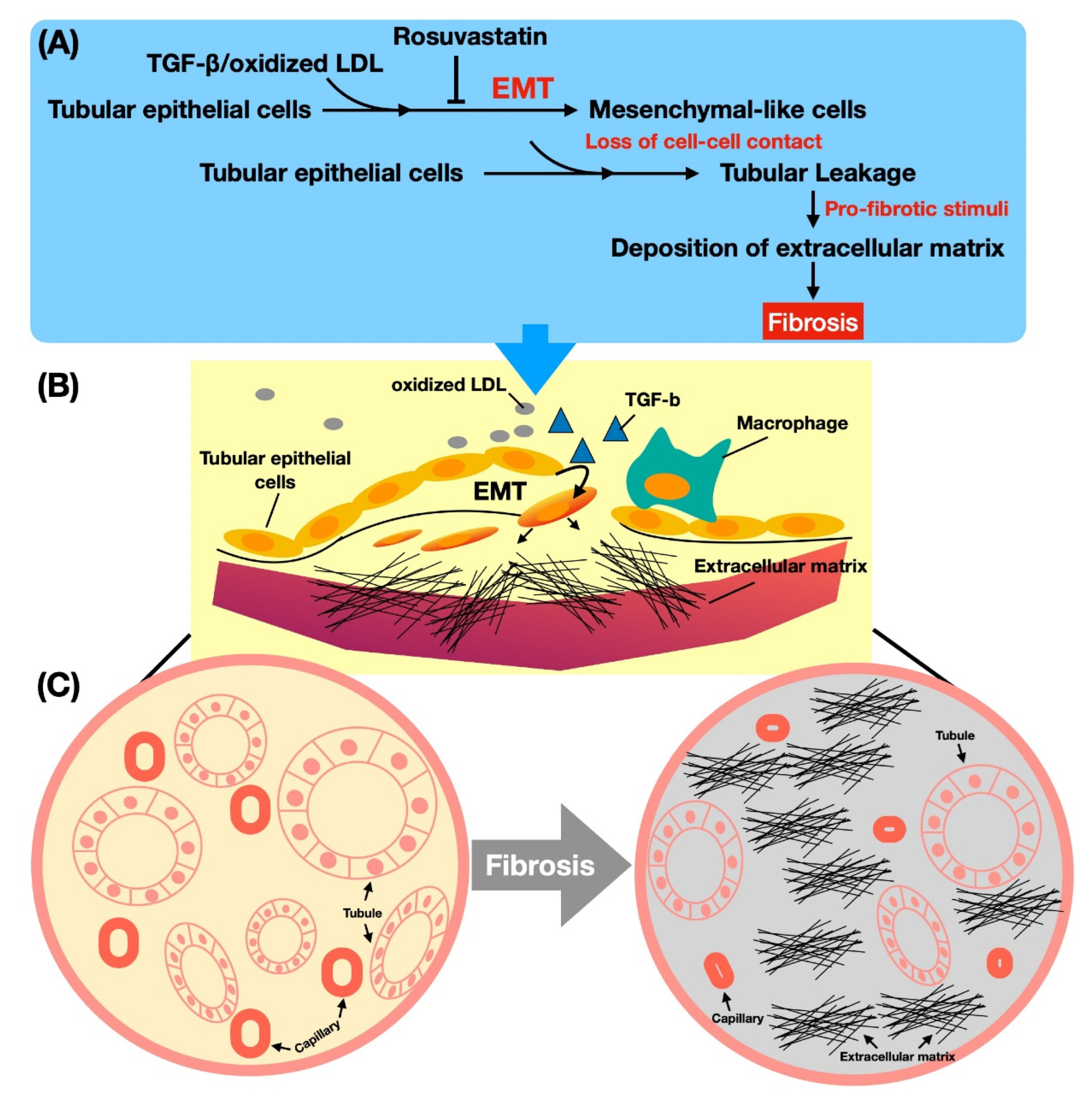
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nath, K.A. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am. J. Kidney Dis. 1992, 20, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ning, X.; Li, R.; Yang, Z.; Yang, X.; Sun, S.; Qian, Q. Signalling pathways involved in hypoxia-induced renal fibrosis. J. Cell Mol. Med. 2017, 21, 1248–1259. [Google Scholar] [CrossRef] [PubMed]
- Schoenberg, N.C.; Argula, R.G.; Klings, E.S.; Wilson, K.C.; Farber, H.W. Prevalence and Mortality of Pulmonary Hypertension in ESRD: A Systematic Review and Meta-analysis. Lung 2020, 198, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, A.; Pires, M.J.; Oliveira, P.A. Pathophysiological Mechanisms of Renal Fibrosis: A Review of Animal Models and Therapeutic Strategies. Vivo 2017, 31, 1–22. [Google Scholar] [CrossRef]
- Humphreys, B.D. Mechanisms of Renal Fibrosis. Annu. Rev. Physiol. 2018, 80, 309–326. [Google Scholar] [CrossRef]
- Ma, T.T.; Meng, X.M. TGF-β/Smad and Renal Fibrosis. Adv. Exp. Med. Biol. 2019, 1165, 347–364. [Google Scholar]
- Qi, R.; Yang, C. Renal tubular epithelial cells: The neglected mediator of tubulointerstitial fibrosis after injury. Cell Death Dis. 2018, 9, 1126. [Google Scholar] [CrossRef]
- Leung, G.; Kirpalani, A.; Szeto, S.G.; Deeb, M.; Foltz, W.; Simmons, C.A.; Yuen, D.A. Could MRI Be Used To Image Kidney Fibrosis? A Review of Recent Advances and Remaining Barriers. Clin. J. Am. Soc. Nephrol. 2017, 12, 1019–1028. [Google Scholar] [CrossRef]
- Pushpakumar, S.; Kundu, S.; Pryor, T.; Givvimani, S.; Lederer, E.; Tyagi, S.C.; Sen, U. Angiotensin-II induced hypertension and renovascular remodelling in tissue inhibitor of metalloproteinase 2 knockout mice. J. Hypertens 2013, 31, 2270–2281. [Google Scholar] [CrossRef]
- Pushpakumar, S.B.; Kundu, S.; Metreveli, N.; Tyagi, S.C.; Sen, U. Matrix Metalloproteinase Inhibition Mitigates Renovascular Remodeling in Salt-Sensitive Hypertension. Physiol. Rep. 2013, 1, e00063. [Google Scholar] [CrossRef]
- Klymkowsky, M.W.; Savagner, P. Epithelial-mesenchymal transition: A cancer researcher’s conceptual friend and foe. Am. J. Pathol. 2009, 174, 1588–1593. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lamouille, S.; Derynck, R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009, 19, 156–172. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Zhuang, S. New Insights Into the Role and Mechanism of Partial Epithelial-Mesenchymal Transition in Kidney Fibrosis. Front. Physiol. 2020, 11, 569322. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.Y. Tubular epithelial-myofibroblast transdifferentiation mechanisms in proximal tubule cells. Curr. Opin. Nephrol. Hypertens 2003, 12, 25–29. [Google Scholar] [CrossRef]
- Liu, Y. Epithelial to mesenchymal transition in renal fibrogenesis: Pathologic significance, molecular mechanism, and therapeutic intervention. J. Am. Soc. Nephrol. 2004, 15, 1–12. [Google Scholar] [CrossRef]
- Liu, Y. New insights into epithelial-mesenchymal transition in kidney fibrosis. J. Am. Soc. Nephrol. 2010, 21, 212–222. [Google Scholar] [CrossRef]
- Ng, Y.Y.; Huang, T.P.; Yang, W.C.; Chen, Z.P.; Yang, A.H.; Mu, W.; Nikolic-Paterson, D.J.; Atkins, R.C.; Lan, H.Y. Tubular epithelial-myofibroblast transdifferentiation in progressive tubulointerstitial fibrosis in 5/6 nephrectomized rats. Kidney Int. 1998, 54, 864–876. [Google Scholar] [CrossRef]
- Parthasarathy, S.; Raghavamenon, A.; Garelnabi, M.O.; Santanam, N. Oxidized low-density lipoprotein. Methods Mol. Biol. 2010, 610, 403–417. [Google Scholar]
- Tekin, I.O.; Orem, A.; Shiri-Sverdlov, R. Oxidized LDL in inflammation: From bench to bedside. Mediat. Inflamm. 2013, 2013, 762759. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Wu, H.; Li, L.; Yang, C.; Song, S.; Peng, P.; Shao, M.; Zhang, M.; Zhao, J.; et al. Lectin-like oxidized low-density lipoprotein receptor-1 facilitates metastasis of gastric cancer through driving epithelial-mesenchymal transition and PI3K/Akt/GSK3β activation. Sci. Rep. 2017, 7, 45275. [Google Scholar] [CrossRef]
- Jiang, L.; Jiang, S.; Zhou, W.; Huang, J.; Lin, Y.; Long, H.; Luo, Q. Oxidized low density lipoprotein receptor 1 promotes lung metastases of osteosarcomas through regulating the epithelial-mesenchymal transition. J. Transl. Med. 2019, 17, 369. [Google Scholar] [CrossRef] [PubMed]
- Revilla, G.; Corcoy, R.; Moral, A.; Escolà-Gil, J.C.; Mato, E. Cross-Talk between Inflammatory Mediators and the Epithelial Mesenchymal Transition Process in the Development of Thyroid Carcinoma. Int. J. Mol. Sci. 2019, 20, 2466. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Shen, Q. IRF2BP2 prevents ox-LDL-induced inflammation and EMT in endothelial cells via regulation of KLF2. Exp. Ther. Med. 2021, 21, 481. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chen, Y.; He, Z.; Wang, C.; Wang, X.; Pan, G.; Peng, J.Y.; Chen, Q.; Wang, X. Hsa_circ_0001879 promotes the progression of atherosclerosis by regulating the proliferation and migration of oxidation of low density lipoprotein (ox-LDL)-induced vascular endothelial cells via the miR-6873-5p-HDAC9 axis. Bioengineered 2021, 12, 10420–10429. [Google Scholar] [CrossRef]
- Lamo-Espinosa, J.M.; Mora, G.; Blanco, J.F.; Granero-Moltó, F.; Núñez-Córdoba, J.M.; López-Elío, S.; Andreu, E.; Sánchez-Guijo, F.; Aquerreta, J.D.; Bondía, J.M.; et al. Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: Long-term follow up of a multicenter randomized controlled clinical trial (phase I/II). J. Transl. Med. 2018, 16, 213. [Google Scholar] [CrossRef]
- Dai, Y.; Palade, P.; Wang, X.; Mercanti, F.; Ding, Z.; Dai, D.; Mehta, J.L. High fat diet causes renal fibrosis in LDLr-null mice through MAPK-NF-κB pathway mediated by Ox-LDL. J. Cardiovasc. Pharmacol. 2014, 63, 158–166. [Google Scholar] [CrossRef]
- Deng, S.; Jin, T.; Zhang, L.; Bu, H.; Zhang, P. Mechanism of tacrolimus-induced chronic renal fibrosis following transplantation is regulated by ox-LDL and its receptor, LOX-1. Mol. Med. Rep. 2016, 14, 4124–4134. [Google Scholar] [CrossRef]
- Villa, M.; Cerda-Opazo, P.; Jimenez-Gallegos, D.; Garrido-Moreno, V.; Chiong, M.; Quest, A.F.; Toledo, J.; Garcia, L. Pro-fibrotic effect of oxidized LDL in cardiac myofibroblasts. Biochem. Biophys Res. Commun. 2020, 524, 696–701. [Google Scholar] [CrossRef]
- Roumeliotis, S.; Roumeliotis, A.; Stamou, A.; Panagoutsos, S.; Manolopoulos, V.G.; Tsetsos, F.; Georgitsi, M.; Liakopoulos, V. Association of rs11780592 Polymorphism in the Human Soluble Epoxide Hydrolase Gene (EPHX2) with Oxidized LDL and Mortality in Patients with Diabetic Chronic Kidney Disease. Oxid. Med. Cell Longev. 2021, 2021, 8817502. [Google Scholar] [CrossRef]
- Moradi, H.; Pahl, M.V.; Elahimehr, R.; Vaziri, N.D. Impaired antioxidant activity of high-density lipoprotein in chronic kidney disease. Transl. Res. 2009, 153, 77–85. [Google Scholar] [CrossRef]
- Yip, H.K.; Lin, K.C.; Sung, P.H.; Chiang, J.Y.; Yin, T.C.; Wu, R.W.; Chen, K.H. Umbilical cord-derived MSC and hyperbaric oxygen therapy effectively protected the brain in rat after acute intracerebral haemorrhage. J. Cell Mol. Med. 2021, 25, 5640–5654. [Google Scholar] [CrossRef] [PubMed]
- Sheu, J.J.; Sung, P.H.; Wallace, C.G.; Yang, C.C.; Chen, K.H.; Shao, P.L.; Chu, Y.C.; Huang, C.R.; Chen, Y.L.; Ko, S.F.; et al. Intravenous administration of iPS-MSC(SPIONs) mobilized into CKD parenchyma and effectively preserved residual renal function in CKD rat. J. Cell Mol. Med. 2020, 24, 3593–3610. [Google Scholar] [CrossRef]
- Huang, T.H.; Chen, Y.T.; Sung, P.H.; Chiang, H.J.; Chen, Y.L.; Chai, H.T.; Chung, S.Y.; Tsai, T.H.; Yang, C.C.; Chen, C.H.; et al. Peripheral blood-derived endothelial progenitor cell therapy prevented deterioration of chronic kidney disease in rats. Am. J. Transl. Res. 2015, 7, 804–824. [Google Scholar]
- Kim, Y.J.; Oh, S.H.; Ahn, J.S.; Yook, J.M.; Kim, C.D.; Park, S.H.; Cho, J.H.; Kim, Y.L. The Crucial Role of Xanthine Oxidase in CKD Progression Associated with Hypercholesterolemia. Int. J. Mol. Sci. 2020, 21, 7444. [Google Scholar] [CrossRef] [PubMed]
- Roumeliotis, S.; Georgianos, P.I.; Roumeliotis, A.; Eleftheriadis, T.; Stamou, A.; Manolopoulos, V.G.; Panagoutsos, S.; Liakopoulos, V. Oxidized LDL Modifies the Association between Proteinuria and Deterioration of Kidney Function in Proteinuric Diabetic Kidney Disease. Life 2021, 11, 504. [Google Scholar] [CrossRef] [PubMed]
- Roumeliotis, S.; Roumeliotis, A.; Georgianos, P.I.; Stamou, A.; Manolopoulos, V.G.; Panagoutsos, S.; Liakopoulos, V. Oxidized LDL Is Associated with eGFR Decline in Proteinuric Diabetic Kidney Disease: A Cohort Study. Oxid. Med. Cell Longev. 2021, 2021, 2968869. [Google Scholar] [CrossRef] [PubMed]
- Reeve, J.L.V.; Davis, M.; Twomey, P.J. Observations from a teaching hospital in Ireland: Changing from MDRD to CKD-EPI eGFR in routine practice. J. Clin. Pathol. 2021, 74, 608–611. [Google Scholar] [CrossRef]
- Liu, Y. Cellular and molecular mechanisms of renal fibrosis. Nat. Rev. Nephrol. 2011, 7, 684–696. [Google Scholar] [CrossRef]
- Gewin, L.S. Renal fibrosis: Primacy of the proximal tubule. Matrix Biol. 2018, 68–69, 248–262. [Google Scholar] [CrossRef]
- Tanaka, T. A mechanistic link between renal ischemia and fibrosis. Med. Mol. Morphol. 2017, 50, 1–8. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Yeh, J.N.; Chiang, J.Y.; Sung, P.H.; Chen, Y.L.; Liu, F.; Yip, H.K. Intrarenal arterial administration of human umbilical cord-derived mesenchymal stem cells effectively preserved the residual renal function of diabetic kidney disease in rat. Stem. Cell Res. Ther. 2022, 13, 186. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Chaykovska, L.; Chu, C.; Chen, X.; Hasan, A.A.; Krämer, B.K.; Tepel, M.; Hocher, B. Head-to-Head Comparison of Oxidative Stress Biomarkers for All-Cause Mortality in Hemodialysis Patients. Antioxidants 2022, 11, 1975. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.H.; Sun, C.K.; Wallance, C.G.; Lin, Y.C.; Chang, L.T.; Chen, Y.L.; Tsai, T.H.; Kao, Y.H.; Shao, P.L.; Hsieh, C.Y.; et al. Continuing exposure to low-dose nonylphenol aggravates adenine-induced chronic renal dysfunction and role of rosuvastatin therapy. J. Transl. Med. 2012, 10, 147. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.H.; Lin, K.C.; Leu, S.; Sun, C.K.; Chang, L.T.; Chai, H.T.; Chung, S.Y.; Chang, H.W.; Ko, S.F.; Chen, Y.T.; et al. Chronic exposure to environmental contaminant nonylphenol exacerbates adenine-induced chronic renal insufficiency: Role of signaling pathways and therapeutic impact of rosuvastatin. Eur. J. Pharm. Sci. 2012, 46, 455–467. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, P.-H.; Cheng, B.-C.; Hsu, T.-W.; Chiang, J.Y.; Chiang, H.-J.; Chen, Y.-L.; Yang, C.-C.; Yip, H.-K. Oxidized-LDL Deteriorated the Renal Residual Function and Parenchyma in CKD Rat through Upregulating Epithelial Mesenchymal Transition and Extracellular Matrix-Mediated Tubulointerstitial Fibrosis—Pharmacomodulation of Rosuvastatin. Antioxidants 2022, 11, 2465. https://doi.org/10.3390/antiox11122465
Sung P-H, Cheng B-C, Hsu T-W, Chiang JY, Chiang H-J, Chen Y-L, Yang C-C, Yip H-K. Oxidized-LDL Deteriorated the Renal Residual Function and Parenchyma in CKD Rat through Upregulating Epithelial Mesenchymal Transition and Extracellular Matrix-Mediated Tubulointerstitial Fibrosis—Pharmacomodulation of Rosuvastatin. Antioxidants. 2022; 11(12):2465. https://doi.org/10.3390/antiox11122465
Chicago/Turabian StyleSung, Pei-Hsun, Ben-Chung Cheng, Tsuen-Wei Hsu, John Y Chiang, Hsin-Ju Chiang, Yi-Ling Chen, Chih-Chao Yang, and Hon-Kan Yip. 2022. "Oxidized-LDL Deteriorated the Renal Residual Function and Parenchyma in CKD Rat through Upregulating Epithelial Mesenchymal Transition and Extracellular Matrix-Mediated Tubulointerstitial Fibrosis—Pharmacomodulation of Rosuvastatin" Antioxidants 11, no. 12: 2465. https://doi.org/10.3390/antiox11122465
APA StyleSung, P.-H., Cheng, B.-C., Hsu, T.-W., Chiang, J. Y., Chiang, H.-J., Chen, Y.-L., Yang, C.-C., & Yip, H.-K. (2022). Oxidized-LDL Deteriorated the Renal Residual Function and Parenchyma in CKD Rat through Upregulating Epithelial Mesenchymal Transition and Extracellular Matrix-Mediated Tubulointerstitial Fibrosis—Pharmacomodulation of Rosuvastatin. Antioxidants, 11(12), 2465. https://doi.org/10.3390/antiox11122465





