Effect of Different Coffee Brews on Tryptophan Metabolite-Induced Cytotoxicity in HT-29 Human Colon Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. In Vitro Gastrointestinal Digestion
2.3. Cell Culture
2.4. Cell Treatment
2.5. Analysis of Cell Viability
2.6. Assessment of Intracellular ROS Production
2.7. RNA Extraction
2.8. Real-Time PCR Analysis
2.9. Statistical Analysis
3. Results
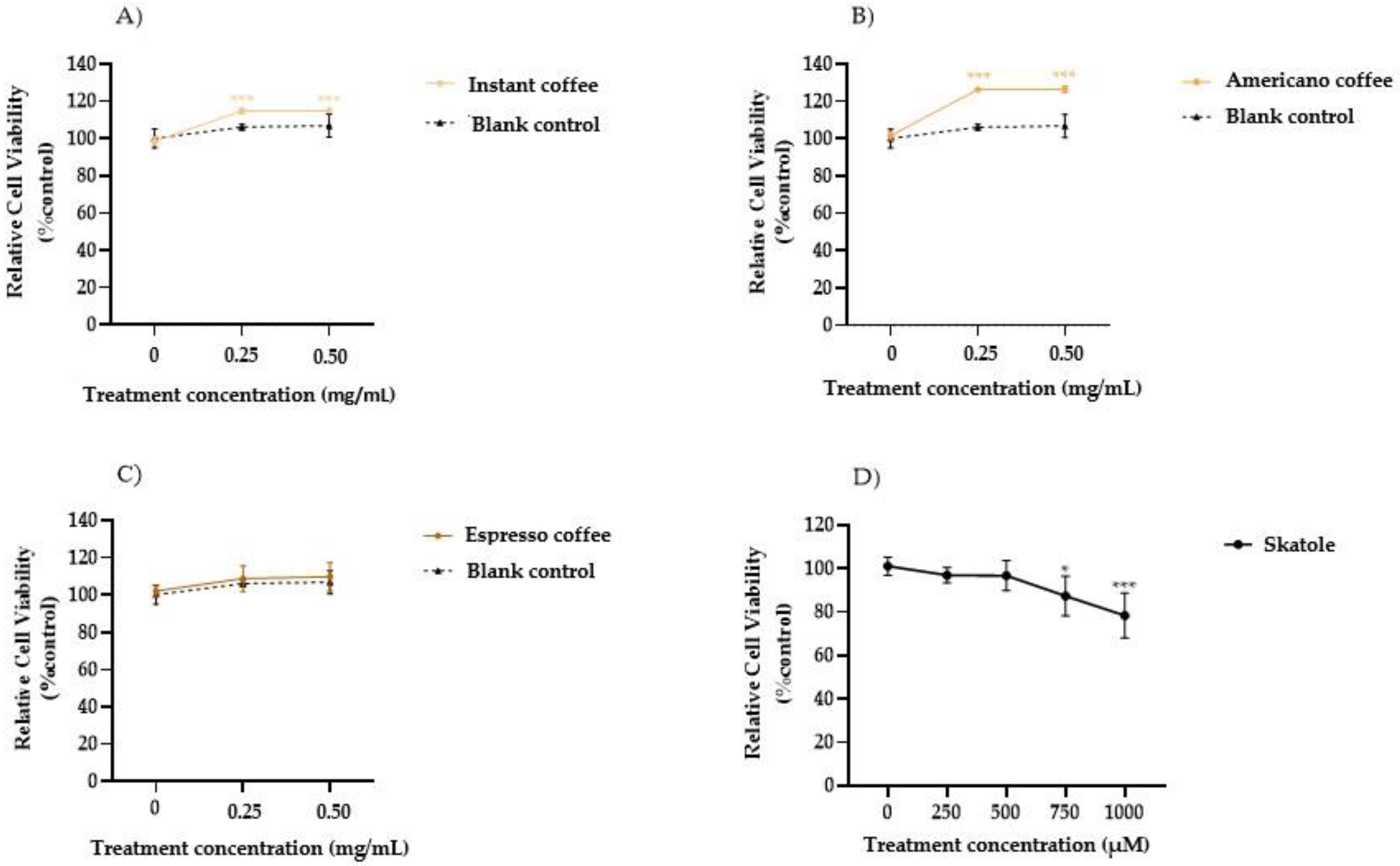
3.1. Cell Viability in HT-29 Cells
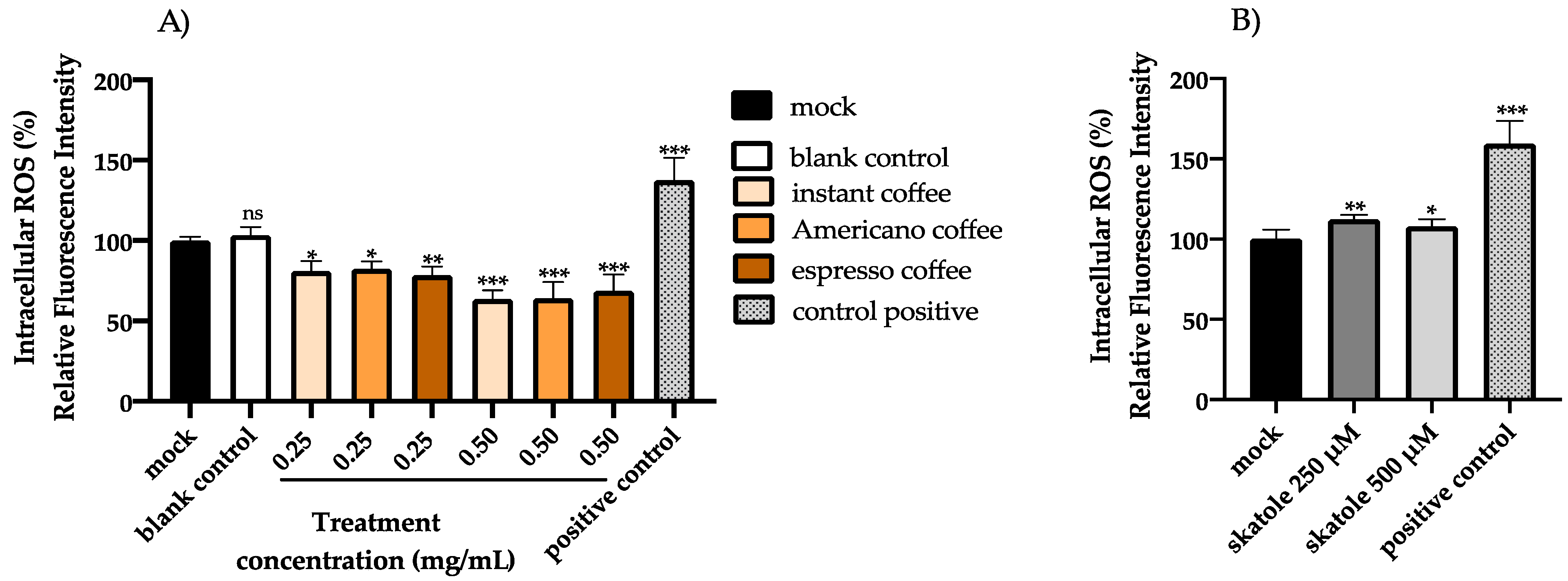
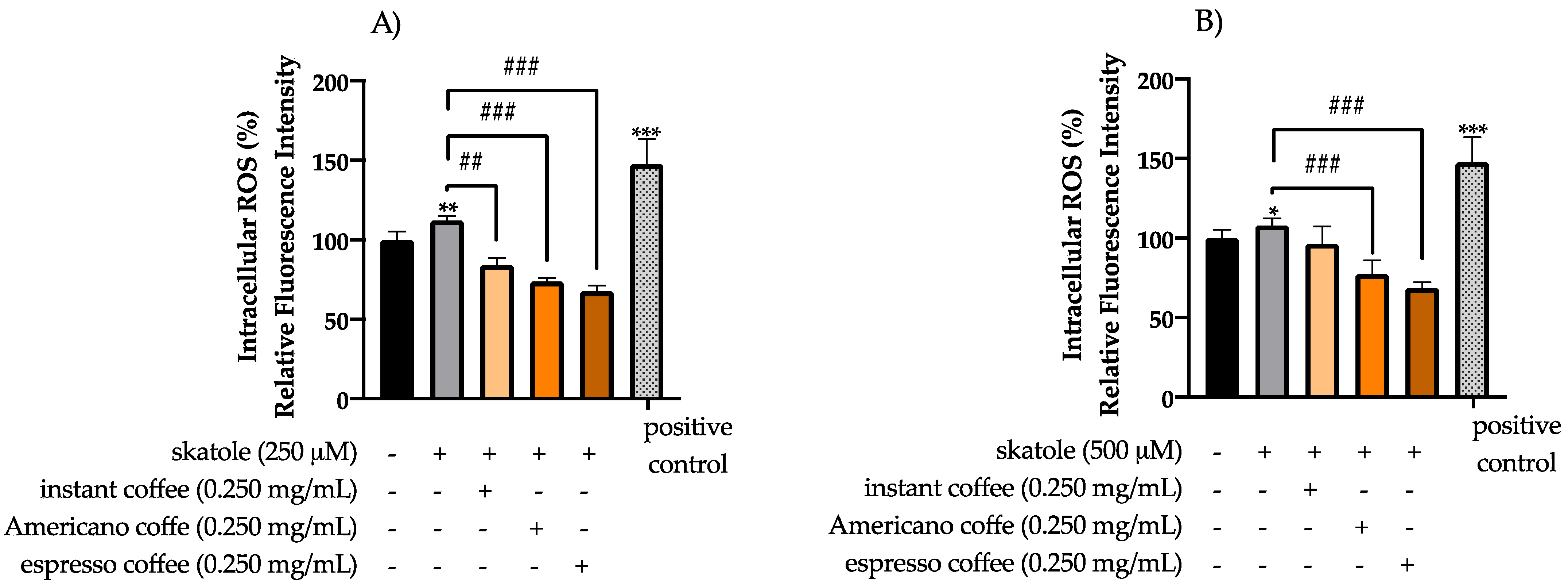
3.2. Intracellular ROS Levels in HT-29 Cells
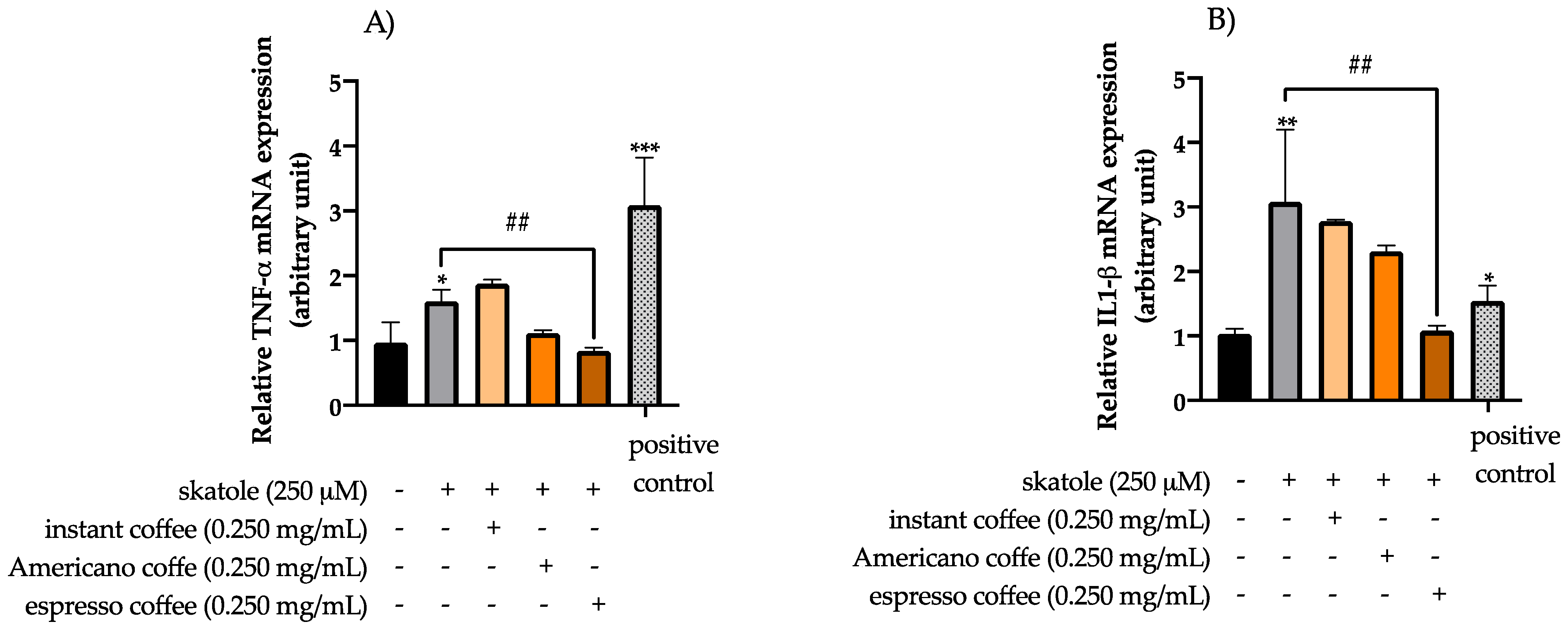
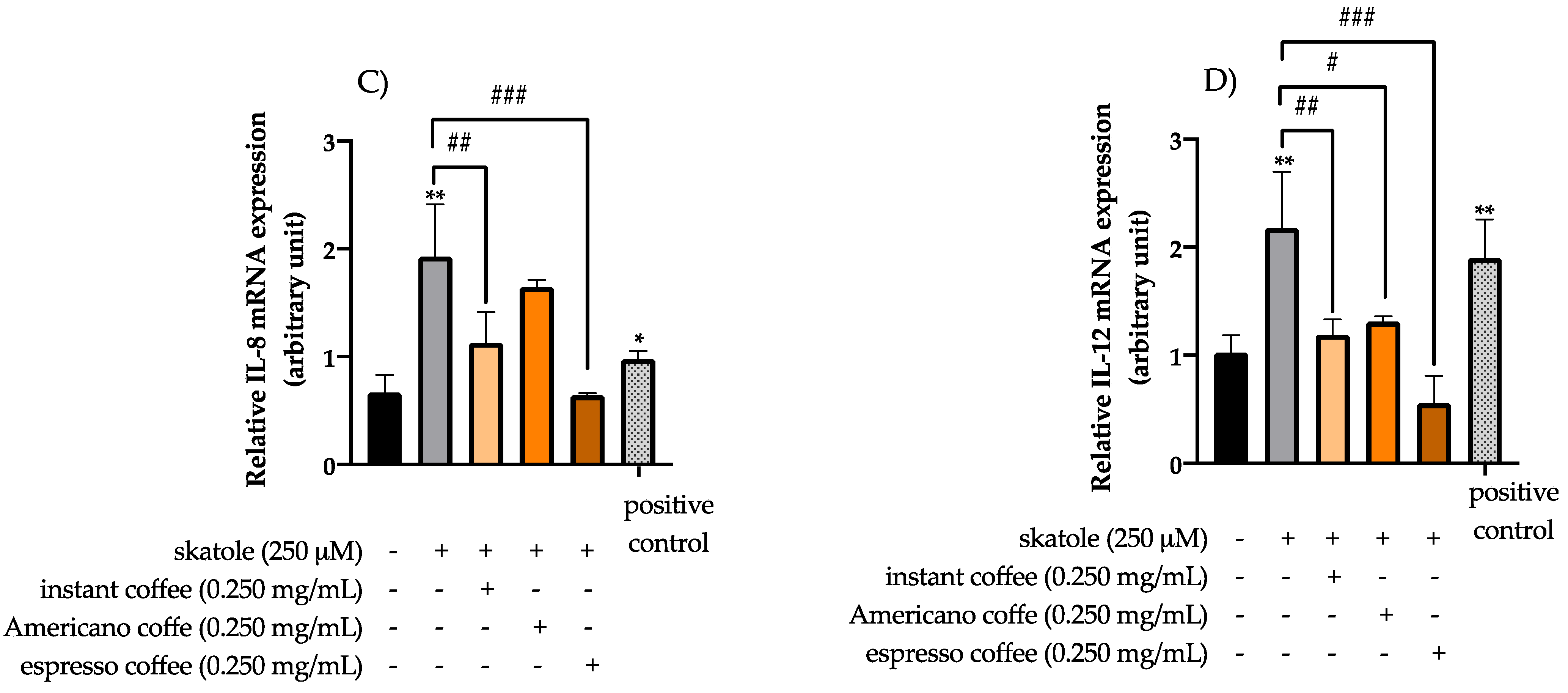
3.3. Anti-Inflammatory Effects of Coffee Extracts on Cytokine mRNA Expression Levels in HT-29 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andersen, L.F.; Jacobs, D.R., Jr.; Carlsen, M.H.; Blomhoff, R. Consumption of coffee is associated with reduced risk of death attributed to inflammatory and cardiovascular diseases in the Iowa Women’s Health Study. Am. J. Clin. Nutr. 2006, 83, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Sarriá, B.; Martínez-López, S.; Mateos, R.; Bravo-Clemente, L. Long-term consumption of a green/roasted coffee blend positively affects glucose metabolism and insulin resistance in humans. Food Res. Int. 2016, 89, 1023–1028. [Google Scholar] [CrossRef]
- Cano-Marquina, A.; Tarín, J.; Cano, A. The impact of coffee on health. Maturitas 2013, 75, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, I.A.; Clifford, M.N.; Lean, M.E.; Ashihara, H.; Crozier, A. Coffee: Biochemistry and potential impact on health. Food Funct. 2014, 5, 1695–1717. [Google Scholar] [CrossRef]
- Perrone, D.; Farah, A.; Donangelo, C.M. Influence of coffee roasting on the incorporation of phenolic compounds into melanoidins and their relationship with antioxidant activity of the brew. J. Agric. Food Chem. 2012, 60, 4265–4275. [Google Scholar] [CrossRef]
- Grosso, G.; Godos, J.; Galvano, F.; Giovannucci, E.L. Coffee, caffeine, and health outcomes: An umbrella review. Annu. Rev. Nutr. 2017, 37, 131–156. [Google Scholar] [CrossRef]
- Martín, M.A.; Goya, L.; Ramos, S. Preventive effects of cocoa and cocoa antioxidants in colon cancer. Diseases 2016, 4, 6. [Google Scholar] [CrossRef]
- Afrin, S.; Giampieri, F.; Gasparrini, M.; Forbes-Hernández, T.Y.; Cianciosi, D.; Reboredo-Rodriguez, P.; Zhang, J.; Manna, P.P.; Daglia, M.; Atanasov, A.G. Dietary phytochemicals in colorectal cancer prevention and treatment: A focus on the molecular mechanisms involved. Biotechnol. Adv. 2020, 38, 107322. [Google Scholar] [CrossRef]
- Sarnelli, G.; Grosso, M.; Palumbo, I.; Pesce, M.; D’Alessandro, A.; Zaninotto, G.; Annese, V.; Petruzzelli, R.; Izzo, P.; Sepulveres, R. Allele-specific transcriptional activity of the variable number of tandem repeats of the inducible nitric oxide synthase gene is associated with idiopathic achalasia. United Eur. Gastroenterol. J. 2017, 5, 200–207. [Google Scholar] [CrossRef]
- Muruve, D.A.; Pétrilli, V.; Zaiss, A.K.; White, L.R.; Clark, S.A.; Ross, P.J.; Parks, R.J.; Tschopp, J. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature 2008, 452, 103–107. [Google Scholar] [CrossRef]
- Opree, A.; Kress, M. Involvement of the proinflammatory cytokines tumor necrosis factor-α, IL-1β, and IL-6 but not IL-8 in the development of heat hyperalgesia: Effects on heat-evoked calcitonin gene-related peptide release from rat skin. J. Neurosci. 2000, 20, 6289–6293. [Google Scholar] [CrossRef] [PubMed]
- Van der Meeren, A.; Squiban, C.; Gourmelon, P.; Lafont, H.; Gaugler, M.-H. Differential regulation by IL-4 and IL-10 of radiation-induced IL-6 and IL-8 production and ICAM-1 expression by human endothelial cells. Cytokine 1999, 11, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Ullman, T.A.; Itzkowitz, S.H. Intestinal inflammation and cancer. Gastroenterology 2011, 140, 1807–1816.e1. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-J.; Wu, E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 2012, 3, 4–14. [Google Scholar] [CrossRef]
- Illiano, P.; Brambilla, R.; Parolini, C. The mutual interplay of gut microbiota, diet and human disease. FEBS J. 2020, 287, 833–855. [Google Scholar] [CrossRef]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef]
- Hill, M. Intestinal flora and endogenous vitamin synthesis. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ. 1997, 6, S43–S45. [Google Scholar] [CrossRef]
- Mazmanian, S.K.; Liu, C.H.; Tzianabos, A.O.; Kasper, D.L. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 2005, 122, 107–118. [Google Scholar] [CrossRef]
- Blaut, M.; Clavel, T. Metabolic diversity of the intestinal microbiota: Implications for health and disease. J. Nutr. 2007, 137, 751S–755S. [Google Scholar] [CrossRef]
- Huang, P.; Liu, Y. A reasonable diet promotes balance of intestinal microbiota: Prevention of precolorectal cancer. BioMed Res. Int. 2019, 2019, 3405278. [Google Scholar] [CrossRef] [PubMed]
- Fernstrom, J.D. A perspective on the safety of supplemental tryptophan based on its metabolic fates. J. Nutr. 2016, 146, 2601S–2608S. [Google Scholar] [CrossRef] [PubMed]
- Mosele, J.I.; Macià, A.; Motilva, M.-J. Metabolic and microbial modulation of the large intestine ecosystem by non-absorbed diet phenolic compounds: A review. Molecules 2015, 20, 17429–17468. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, F.; Fiasca, F.; Minelli, M.; Maio, D.; Mattei, A.; Vergallo, I.; Cifone, M.G.; Cinque, B.; Minelli, M. The effects of low-nickel diet combined with oral administration of selected probiotics on patients with systemic nickel allergy syndrome (SNAS) and gut dysbiosis. Nutrients 2020, 12, 1040. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M.; Carlson, J. Microbial metabolites of tryptophan in the intestinal tract with special reference to skatole. Am. J. Clin. Nutr. 1979, 32, 173–178. [Google Scholar] [CrossRef]
- Coras, R.; Murillo-Saich, J.D.; Guma, M. Circulating pro-and anti-inflammatory metabolites and its potential role in rheumatoid arthritis pathogenesis. Cells 2020, 9, 827. [Google Scholar] [CrossRef]
- Ma, Q.; Meng, N.; Li, Y.; Wang, J. Occurrence, impacts, and microbial transformation of 3-methylindole (skatole): A critical review. J. Hazard. Mater. 2021, 416, 126181. [Google Scholar] [CrossRef]
- Leyton, G. Indolic compounds in the urine of schizophrenics. Br. Med. J. 1958, 2, 1136. [Google Scholar] [CrossRef][Green Version]
- Nakao, A. The appearance of a skatole derivative in the urine of schizophrenics. J. Nerv. Ment. Dis. 1960, 130, 417–419. [Google Scholar] [CrossRef]
- Winter, J.; Nyskohus, L.; Young, G.P.; Hu, Y.; Conlon, M.A.; Bird, A.R.; Topping, D.L.; Le Leu, R.K. Inhibition by Resistant Starch of Red Meat–Induced Promutagenic Adducts in Mouse ColonRed Meat Increases Promutagenic Adducts in Mouse Colon. Cancer Prev. Res. 2011, 4, 1920–1928. [Google Scholar] [CrossRef]
- Blachier, F.; Mariotti, F.; Huneau, J.-F.; Tomé, D. Effects of amino acid-derived luminal metabolites on the colonic epithelium and physiopathological consequences. Amino Acids 2007, 33, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-L.; Zhang, A.-H.; Miao, J.-H.; Sun, H.; Yan, G.-L.; Wu, F.-F.; Wang, X.-J. Targeting regulation of tryptophan metabolism for colorectal cancer therapy: A systematic review. RSC Adv. 2019, 9, 3072–3080. [Google Scholar] [CrossRef] [PubMed]
- Chénard, T.; Prévost, K.; Dubé, J.; Massé, E. Immune system modulations by products of the gut microbiota. Vaccines 2020, 8, 461. [Google Scholar] [CrossRef] [PubMed]
- Mosa, A.; Gerber, A.; Neunzig, J.; Bernhardt, R. Products of gut-microbial tryptophan metabolism inhibit the steroid hormone-synthesizing cytochrome P450 11A1. Endocrine 2016, 53, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Karlin, D.; Mastromarino, A.; Jones, R.; Stroehlein, J.; Lorentz, O. Fecal skatole and indole and breath methane and hydrogen in patients with large bowel polyps or cancer. J. Cancer Res. Clin. Oncol. 1985, 109, 135–141. [Google Scholar] [CrossRef]
- Schmit, S.L.; Rennert, H.S.; Rennert, G.; Gruber, S.B. Coffee consumption and the risk of colorectal cancer. Cancer Epidemiol. Prev. Biomark. 2016, 25, 634–639. [Google Scholar] [CrossRef]
- Mojica, B.E.; Fong, L.E.; Biju, D.; Muharram, A.; Davis, I.M.; Vela, K.O.; Rios, D.; Osorio-Camacena, E.; Kaur, B.; Rojas, S.M. The impact of the roast levels of coffee extracts on their potential anticancer activities. J. Food Sci. 2018, 83, 1125–1130. [Google Scholar] [CrossRef]
- Bakuradze, T.; Lang, R.; Hofmann, T.; Stiebitz, H.; Bytof, G.; Lantz, I.; Baum, M.; Eisenbrand, G.; Janzowski, C. Antioxidant effectiveness of coffee extracts and selected constituents in cell-free systems and human colon cell lines. Mol. Nutr. Food Res. 2010, 54, 1734–1743. [Google Scholar] [CrossRef]
- Fukushima, Y.; Kasuga, M.; Nakao, K.; Shimomura, I.; Matsuzawa, Y. Effects of coffee on inflammatory cytokine gene expression in mice fed high-fat diets. J. Agric. Food Chem. 2009, 57, 11100–11105. [Google Scholar] [CrossRef]
- Yamauchi, R.; Kobayashi, M.; Matsuda, Y.; Ojika, M.; Shigeoka, S.; Yamamoto, Y.; Tou, Y.; Inoue, T.; Katagiri, T.; Murai, A. Coffee and caffeine ameliorate hyperglycemia, fatty liver, and inflammatory adipocytokine expression in spontaneously diabetic KK-Ay mice. J. Agric. Food Chem. 2010, 58, 5597–5603. [Google Scholar] [CrossRef]
- Gonthier, M.-P.; Verny, M.-A.; Besson, C.; Rémésy, C.; Scalbert, A. Chlorogenic acid bioavailability largely depends on its metabolism by the gut microflora in rats. J. Nutr. 2003, 133, 1853–1859. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.H.; Chung, J.G. The effects of plant phenolics, caffeic acid, chlorogenic acid and ferulic acid on arylamine N-acetyltransferase activities in human gastrointestinal microflora. Anticancer Res. 1999, 19, 133–139. [Google Scholar] [PubMed]
- Jaquet, M.; Rochat, I.; Moulin, J.; Cavin, C.; Bibiloni, R. Impact of coffee consumption on the gut microbiota: A human volunteer study. Int. J. Food Microbiol. 2009, 130, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Gniechwitz, D.; Reichardt, N.; Blaut, M.; Steinhart, H.; Bunzel, M. Dietary fiber from coffee beverage: Degradation by human fecal microbiota. J. Agric. Food Chem. 2007, 55, 6989–6996. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Oishi, K. Influence of coffee (Coffea arabica) and galacto-oligosaccharide consumption on intestinal microbiota and the host responses. FEMS Microbiol. Lett. 2013, 343, 161–168. [Google Scholar] [CrossRef]
- Castaldo, L.; Izzo, L.; Narváez, A.; Rodríguez-Carrasco, Y.; Grosso, M.; Ritieni, A. Colon Bioaccessibility under In Vitro Gastrointestinal Digestion of Different Coffee Brews Chemically Profiled through UHPLC-Q-Orbitrap HRMS. Foods 2021, 10, 179. [Google Scholar] [CrossRef]
- Castaldo, L.; Toriello, M.; Sessa, R.; Izzo, L.; Lombardi, S.; Narváez, A.; Ritieni, A.; Grosso, M. Antioxidant and Anti-Inflammatory Activity of Coffee Brew Evaluated after Simulated Gastrointestinal Digestion. Nutrients 2021, 13, 4368. [Google Scholar] [CrossRef]
- Kurata, K.; Kawahara, H.; Nishimura, K.; Jisaka, M.; Yokota, K.; Shimizu, H. Skatole regulates intestinal epithelial cellular functions through activating aryl hydrocarbon receptors and p38. Biochem. Biophys. Res. Commun. 2019, 510, 649–655. [Google Scholar] [CrossRef]
- Riccio, P.; Sessa, R.; de Nicola, S.; Petruzziello, F.; Trombetti, S.; Menna, G.; Pepe, G.; Maddalena, P.; Izzo, P.; Grosso, M. GATA-1 isoforms differently contribute to the production and compartmentation of reactive oxygen species in the myeloid leukemia cell line K562. J. Cell. Physiol. 2019, 234, 20829–20846. [Google Scholar] [CrossRef]
- Voloboueva, L.A.; Liu, J.; Suh, J.H.; Ames, B.N.; Miller, S.S. (R)-α-lipoic acid protects retinal pigment epithelial cells from oxidative damage. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4302–4310. [Google Scholar] [CrossRef][Green Version]
- Wang, T.; Qin, L.; Liu, B.; Liu, Y.; Wilson, B.; Eling, T.; Langenbach, R.; Taniura, S.; Hong, J. Role of reactive oxygen species in LPS-induced production of prostaglandin E2 in microglia. J. Neurochem. 2004, 2, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.; Wen, M. Lipopolysaccharide-mediated reactive oxygen species and signal transduction in the regulation of interleukin-1 gene expression. J. Biol. Chem. 2002, 277, 22131–22139. [Google Scholar] [CrossRef] [PubMed]
- Duary, R.K.; Batish, V.K.; Grover, S. Immunomodulatory activity of two potential probiotic strains in LPS-stimulated HT-29 cells. Genes Nutr. 2014, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Trombetti, S.; Sessa, R.; Catapano, R.; Rinaldi, L.; Lo Bianco, A.; Feliciello, A.; Izzo, P.; Grosso, M. Exploring the Leukemogenic Potential of GATA-1S, the Shorter Isoform of GATA-1: Novel Insights into Mechanisms Hampering Respiratory Chain Complex II Activity and Limiting Oxidative Phosphorylation Efficiency. Antioxidants 2021, 10, 1603. [Google Scholar] [CrossRef]
- Verhoeckx, K.; Cotter, P.; López-Expósito, I.; Kleiveland, C.; Lea, T.; Mackie, A.; Requena, T.; Swiatecka, D.; Wichers, H. The impact of food bioactives on health: In vitro and ex vivo models. In The Impact of Food Bioactives on Health; SpringerOpen: Berlin, Germany, 2015; pp. 113–124. [Google Scholar]
- Castaldo, L.; Narváez, A.; Izzo, L.; Graziani, G.; Ritieni, A. In Vitro Bioaccessibility and Antioxidant Activity of Coffee Silverskin Polyphenolic Extract and Characterization of Bioactive Compounds Using UHPLC-Q-Orbitrap HRMS. Molecules 2020, 25, 2132. [Google Scholar] [CrossRef]
- Bułdak, R.J.; Hejmo, T.; Osowski, M.; Bułdak, Ł.; Kukla, M.; Polaniak, R.; Birkner, E. The Impact of Coffee and Its Selected Bioactive Compounds on the Development and Progression of Colorectal Cancer In Vivo and In Vitro. Molecules 2018, 23, 3309. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Mehta, T.; Esteban-Muñoz, A.; Pastoriza, S.; Paliy, O.; Rufián-Henares, J.Á. Effect of in vitro digestion-fermentation on green and roasted coffee bioactivity: The role of the gut microbiota. Food Chem. 2019, 279, 252–259. [Google Scholar] [CrossRef]
- Vitaglione, P.; Fogliano, V.; Pellegrini, N. Coffee, colon function and colorectal cancer. Food Funct. 2012, 3, 916–922. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D. A standardised static in vitro digestion method suitable for food–an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Nissen, L.; Valerii, M.C.; Spisni, E.; Casciano, F.; Gianotti, A. Multiunit In Vitro Colon Model for the Evaluation of Prebiotic Potential of a Fiber Plus D-Limonene Food Supplement. Foods 2021, 10, 2371. [Google Scholar] [CrossRef]
- Annunziata, G.; Maisto, M.; Schisano, C.; Ciampaglia, R.; Daliu, P.; Narciso, V.; Tenore, G.C.; Novellino, E. Colon bioaccessibility and antioxidant activity of white, green and black tea polyphenols extract after in vitro simulated gastrointestinal digestion. Nutrients 2018, 10, 1711. [Google Scholar] [CrossRef] [PubMed]
- Eker, M.E.; Karakaya, S. Influence of the addition of chia seeds and germinated seeds and sprouts on the nutritional and beneficial properties of yogurt. Int. J. Gastron. Food Sci. 2020, 22, 100276. [Google Scholar] [CrossRef]
- Colombo, R.; Ferron, L.; Frosi, I.; Papetti, A. Advances in static in vitro digestion models after COST action Infogest consensus protocol. Food Funct. 2021, 12, 7619–7636. [Google Scholar] [CrossRef]
- Castaldo, L.; Izzo, L.; De Pascale, S.; Narváez, A.; Rodriguez-Carrasco, Y.; Ritieni, A. Chemical Composition, In Vitro Bioaccessibility and Antioxidant Activity of Polyphenolic Compounds from Nutraceutical Fennel Waste Extract. Molecules 2021, 26, 1968. [Google Scholar] [CrossRef]
- Izzo, L.; Rodríguez-Carrasco, Y.; Pacifico, S.; Castaldo, L.; Narváez, A.; Ritieni, A. Colon Bioaccessibility under In Vitro Gastrointestinal Digestion of a Red Cabbage Extract Chemically Profiled through UHPLC-Q-Orbitrap HRMS. Antioxidants 2020, 9, 955. [Google Scholar] [CrossRef]
- Castaldo, L.; Lombardi, S.; Gaspari, A.; Rubino, M.; Izzo, L.; Narváez, A.; Ritieni, A.; Grosso, M. In Vitro Bioaccessibility and Antioxidant Activity of Polyphenolic Compounds from Spent Coffee Grounds-Enriched Cookies. Foods 2021, 10, 1837. [Google Scholar] [CrossRef] [PubMed]
- Van Nuenen, M.H.; Venema, K.; Van Der Woude, J.C.; Kuipers, E.J. The metabolic activity of fecal microbiota from healthy individuals and patients with inflammatory bowel disease. Dig. Dis. Sci. 2004, 49, 485–491. [Google Scholar] [CrossRef]
- Kanazawa, K.; Konishi, F.; Mitsuoka, T.; Terada, A.; Itoh, K.; Narushima, S.; Kumemura, M.; Kimura, H. Factors influencing the development of sigmoid colon cancer: Bacteriologic and biochemical studies. Cancer Interdiscip. Int. J. Am. Cancer Soc. 1996, 77, 1701–1706. [Google Scholar]
- Rodríguez-Carrasco, Y.; Castaldo, L.; Gaspari, A.; Graziani, G.; Ritieni, A. Development of an UHPLC-Q-Orbitrap HRMS method for simultaneous determination of mycotoxins and isoflavones in soy-based burgers. LWT-Food Sci. Technol. 2019, 99, 34–42. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients 2016, 8, 16. [Google Scholar] [CrossRef]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-S.; Chen, P.-W.; Wang, J.-Y.; Kuo, T.-C. Assessment of Cellular Mutagenicity of Americano Coffees from Popular Coffee Chains. J. Food Prot. 2017, 80, 1489–1495. [Google Scholar]
| Transcript | Primer | Sequence 5′-3′ | Amplicon Size (bp) |
|---|---|---|---|
| TNF-α | For | AGCCCATGTTGTAGCAAACC | 134 |
| Rev | TGAGGTACAGGCCCTCTGAT | ||
| IL-1β | For | CATGGGATAACGAGGCTTATG | 149 |
| Rev | CCACTTGTTGCTCCATATCC | ||
| IL-8 | For | TGGCTCTCTTGGCAGCCTTC | 238 |
| Rev | TGCACCCAGTTTTCCTTGGG | ||
| IL-12 | For | TTCACCACTCCCAAAACCTGC | 225 |
| Rev | GAGGCCAGGCAACTCCCATTA | ||
| β-actin | For | CGACAGGATGCAGAAGGAGA | 160 |
| Rev | CGTCATACTCCTGCTTGCTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castaldo, L.; Toriello, M.; Izzo, L.; Sessa, R.; Lombardi, S.; Trombetti, S.; Rodríguez-Carrasco, Y.; Ritieni, A.; Grosso, M. Effect of Different Coffee Brews on Tryptophan Metabolite-Induced Cytotoxicity in HT-29 Human Colon Cancer Cells. Antioxidants 2022, 11, 2458. https://doi.org/10.3390/antiox11122458
Castaldo L, Toriello M, Izzo L, Sessa R, Lombardi S, Trombetti S, Rodríguez-Carrasco Y, Ritieni A, Grosso M. Effect of Different Coffee Brews on Tryptophan Metabolite-Induced Cytotoxicity in HT-29 Human Colon Cancer Cells. Antioxidants. 2022; 11(12):2458. https://doi.org/10.3390/antiox11122458
Chicago/Turabian StyleCastaldo, Luigi, Marianna Toriello, Luana Izzo, Raffaele Sessa, Sonia Lombardi, Silvia Trombetti, Yelko Rodríguez-Carrasco, Alberto Ritieni, and Michela Grosso. 2022. "Effect of Different Coffee Brews on Tryptophan Metabolite-Induced Cytotoxicity in HT-29 Human Colon Cancer Cells" Antioxidants 11, no. 12: 2458. https://doi.org/10.3390/antiox11122458
APA StyleCastaldo, L., Toriello, M., Izzo, L., Sessa, R., Lombardi, S., Trombetti, S., Rodríguez-Carrasco, Y., Ritieni, A., & Grosso, M. (2022). Effect of Different Coffee Brews on Tryptophan Metabolite-Induced Cytotoxicity in HT-29 Human Colon Cancer Cells. Antioxidants, 11(12), 2458. https://doi.org/10.3390/antiox11122458












