Impact of Glyphosate on the Development of Insulin Resistance in Experimental Diabetic Rats: Role of NFκB Signalling Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals and Induction of Diabetes with Glyphosate
2.3. Experimental Time Line
- Group 1.
- Normal control rats treated with vehicle (water only)
- Group 2.
- Normal rats received oral administration of glyphosate dissolved in water (50 mg/kg b. wt./day) for 16 weeks.
- Group 3.
- Normal rats received oral administration of glyphosate dissolved in water (100 mg/kg b. wt./day) for 16 weeks.
- Group 4.
- Normal rats received oral administration of glyphosate dissolved in water (250 mg/kg b. wt./day) for 16 weeks.
2.4. Determination of Fasting Sugar, Serum Insulin and Testosterone
2.5. Determination of Oral Glucose Tolerance (OGT)
2.6. Determination of Insulin Tolerance (IT)
2.7. HOMA-IR and QUICKI
2.8. Measurement of Oxidative Stress Marker
2.9. Measurement of Antioxidant Enzymes
2.10. Determination of Specific Enzymes Involved in Carbohydrate Metabolism
2.11. Total RNA, cDNA Synthesis and Real-Time PCR
2.12. Protein Analysis of Pro-Inflammatory Cytokines and Transcription Factors
2.13. Histopathological Staining
2.14. Immunohistochemical Staining
2.15. Statistical Analysis
3. Results
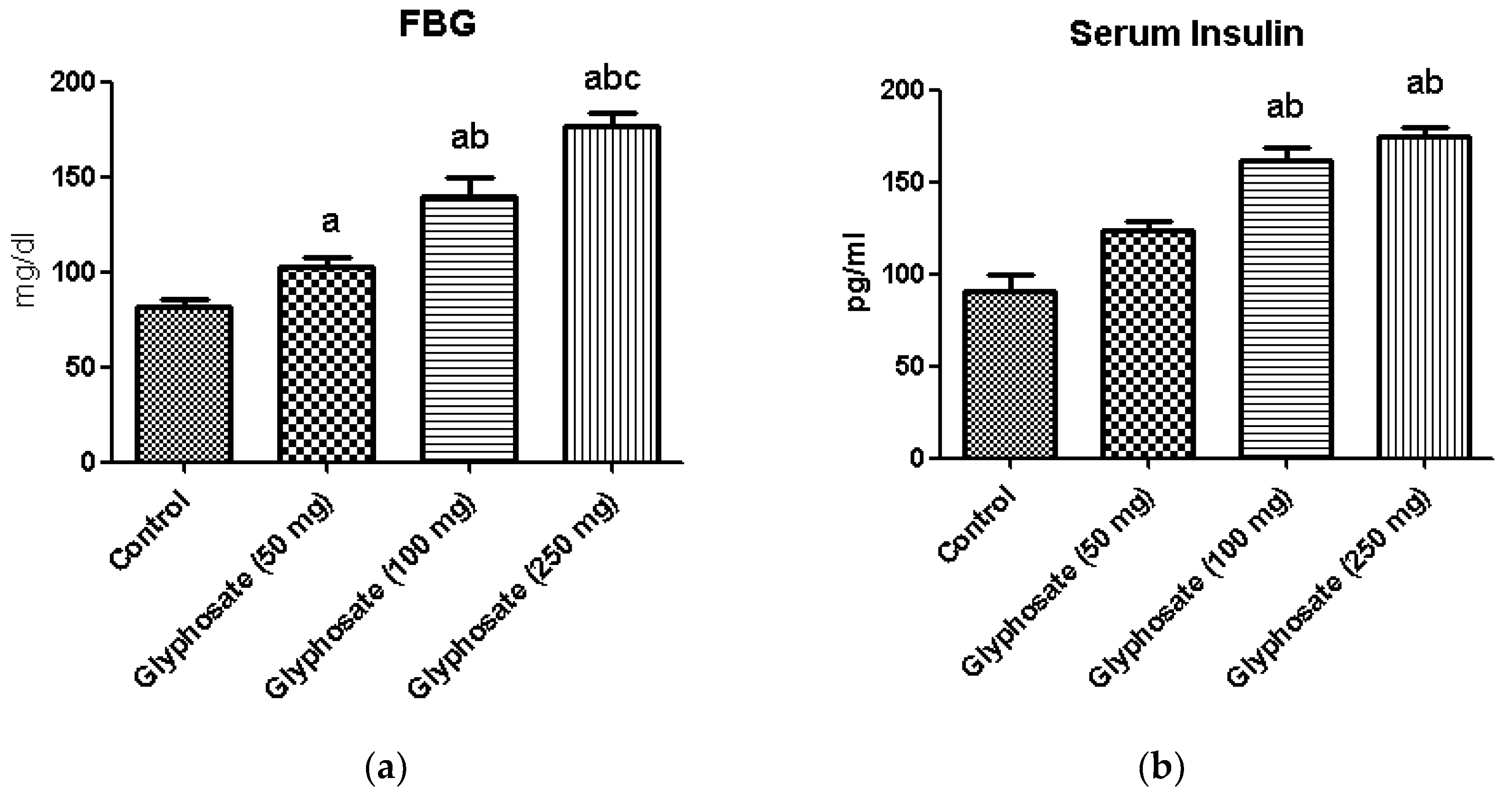
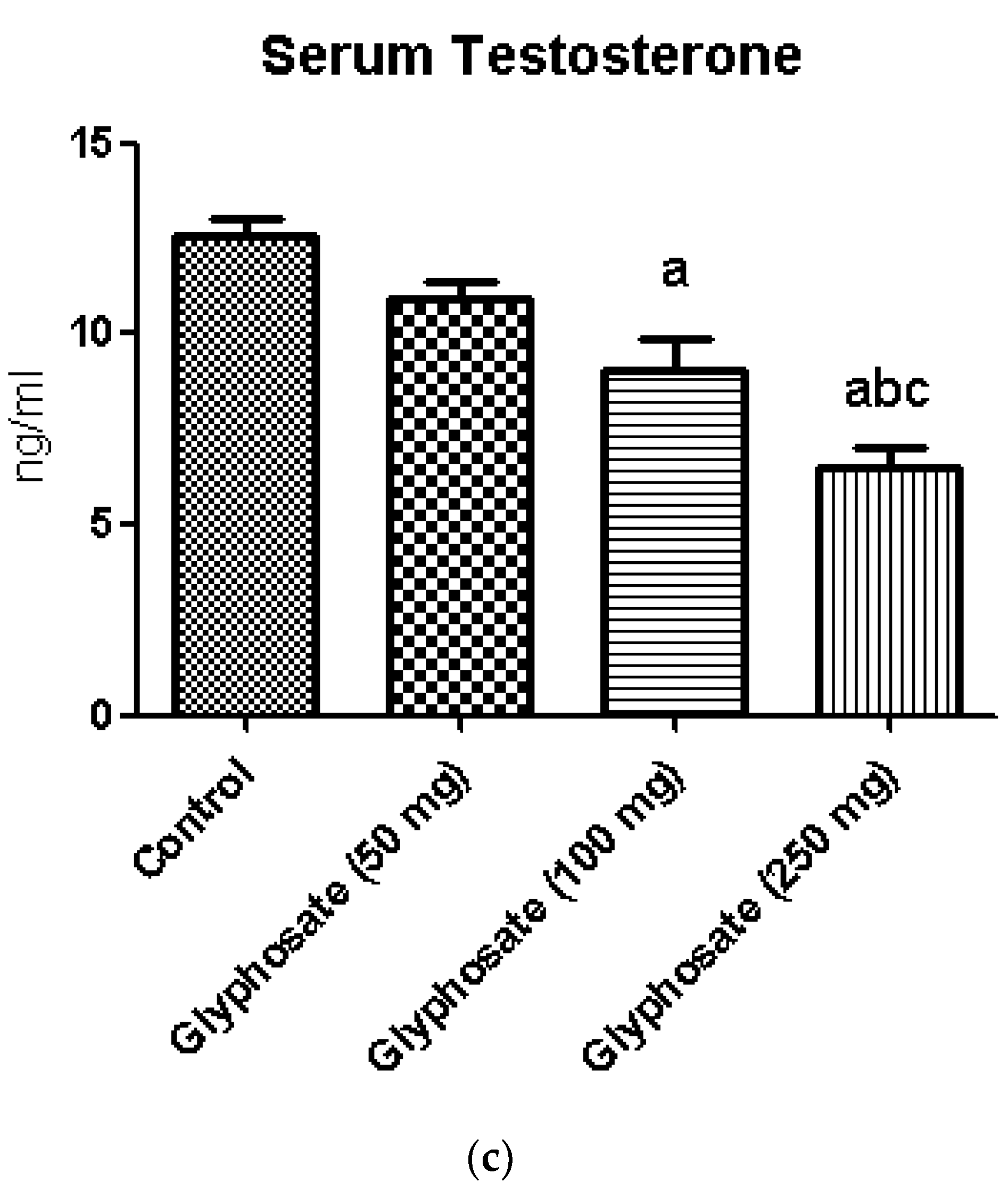
3.1. Impact of Glyphosate on FBG, Insulin and Testosterone
3.2. Impact of Glyphosate on OGT
3.3. Impact of Glyphosate on IT
3.4. Impact of Glyphosate on HOMA-IR and QUICKI
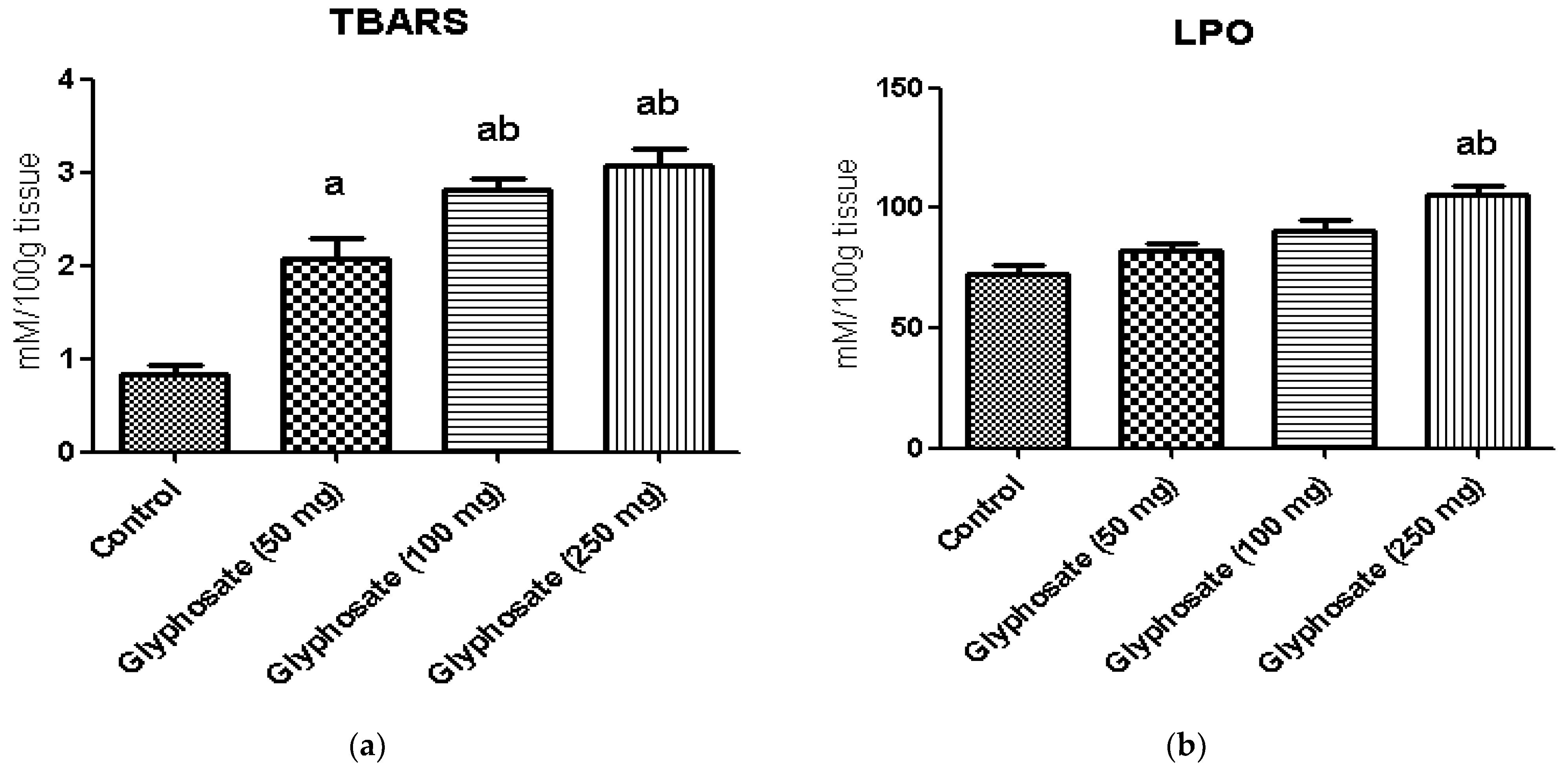
3.5. Impact of Glyphosate on Oxidative Stress Markers
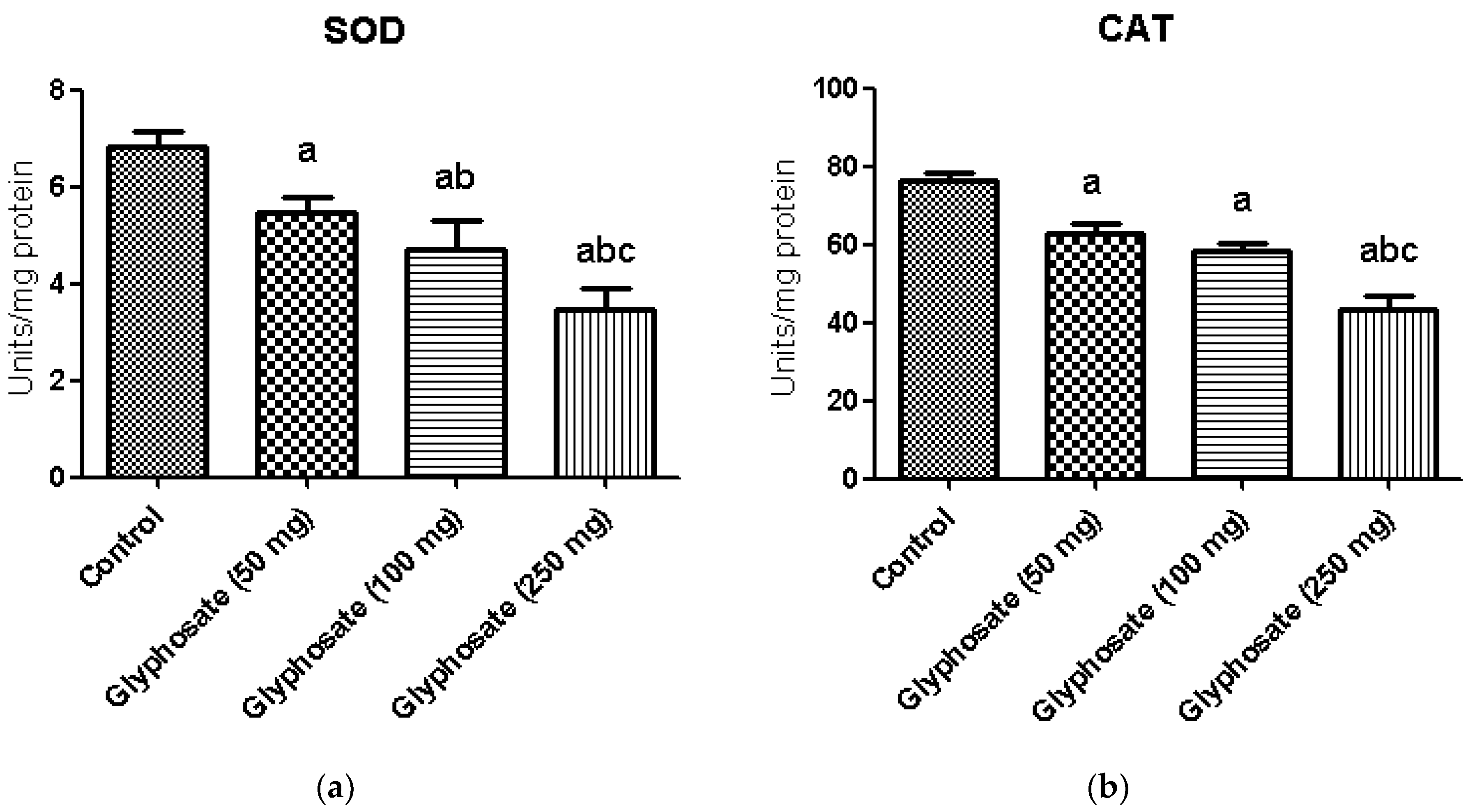
3.6. Impact of Glyphosate on SOD and CAT Enzymes Activity
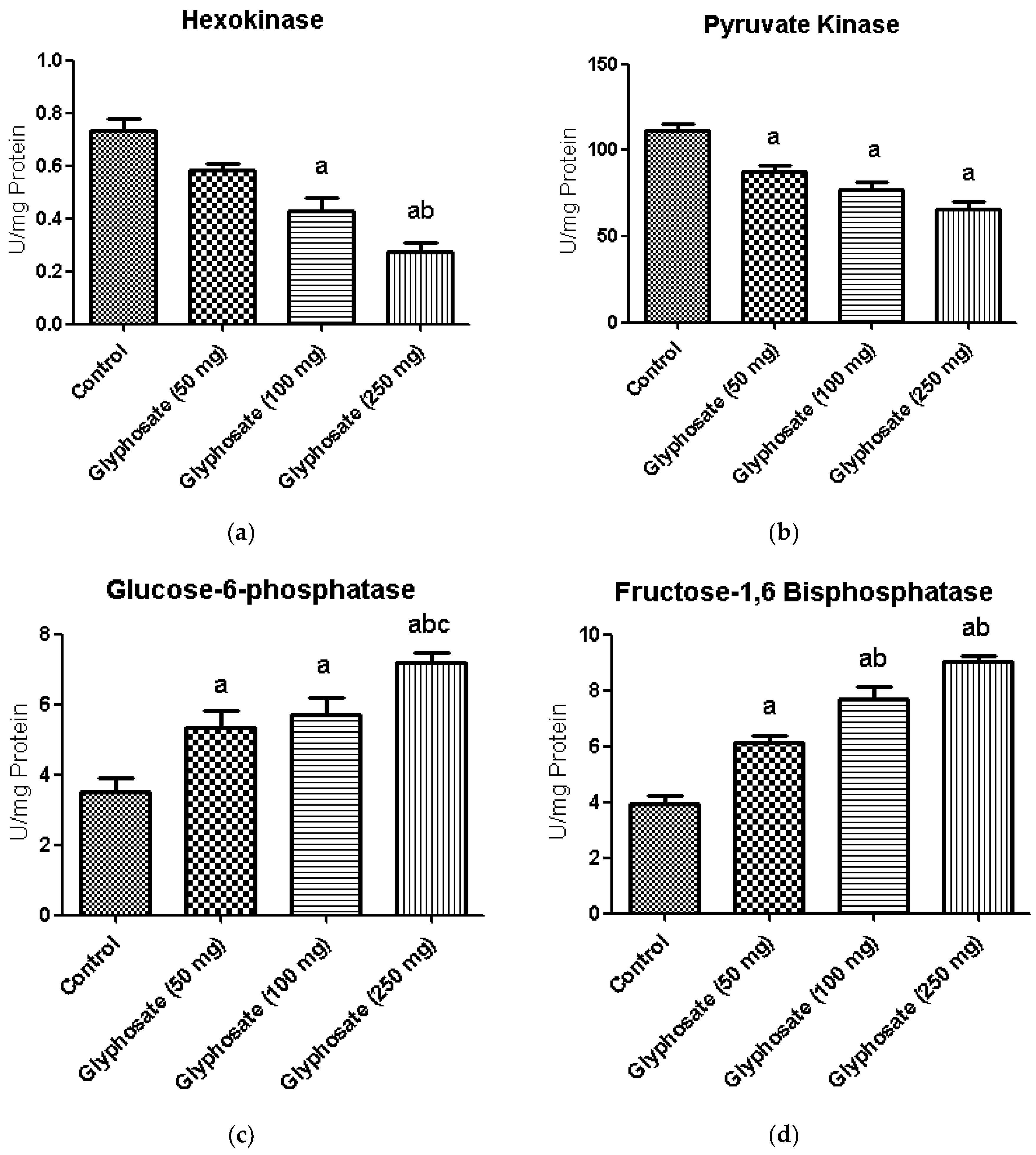
3.7. Impact of Glyphosate on Glycolytic and Gluconeogenic Enzymes
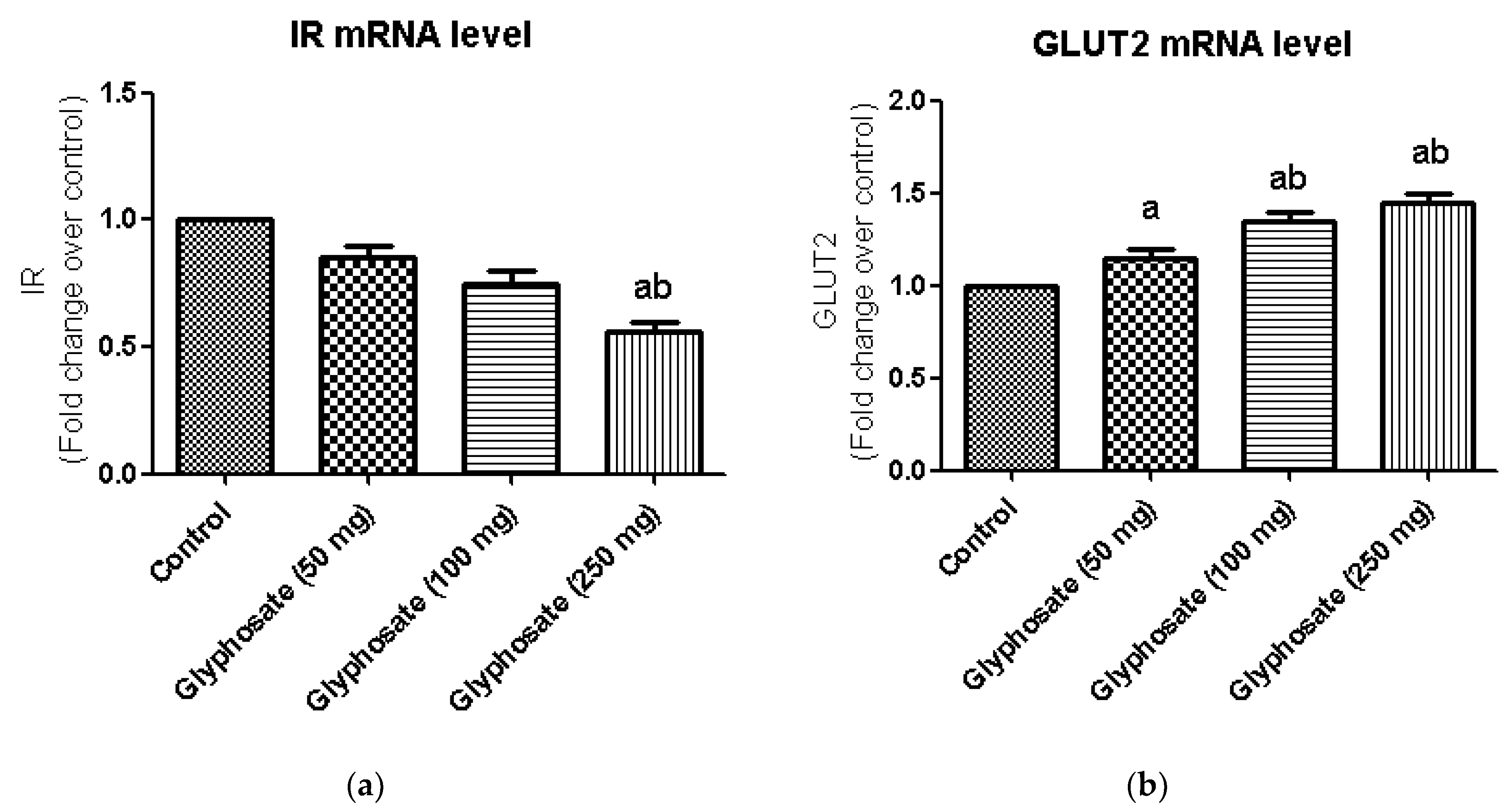
3.8. Impact of Glyphosate on the mRNA Expression of Insulin Signalling Molecules
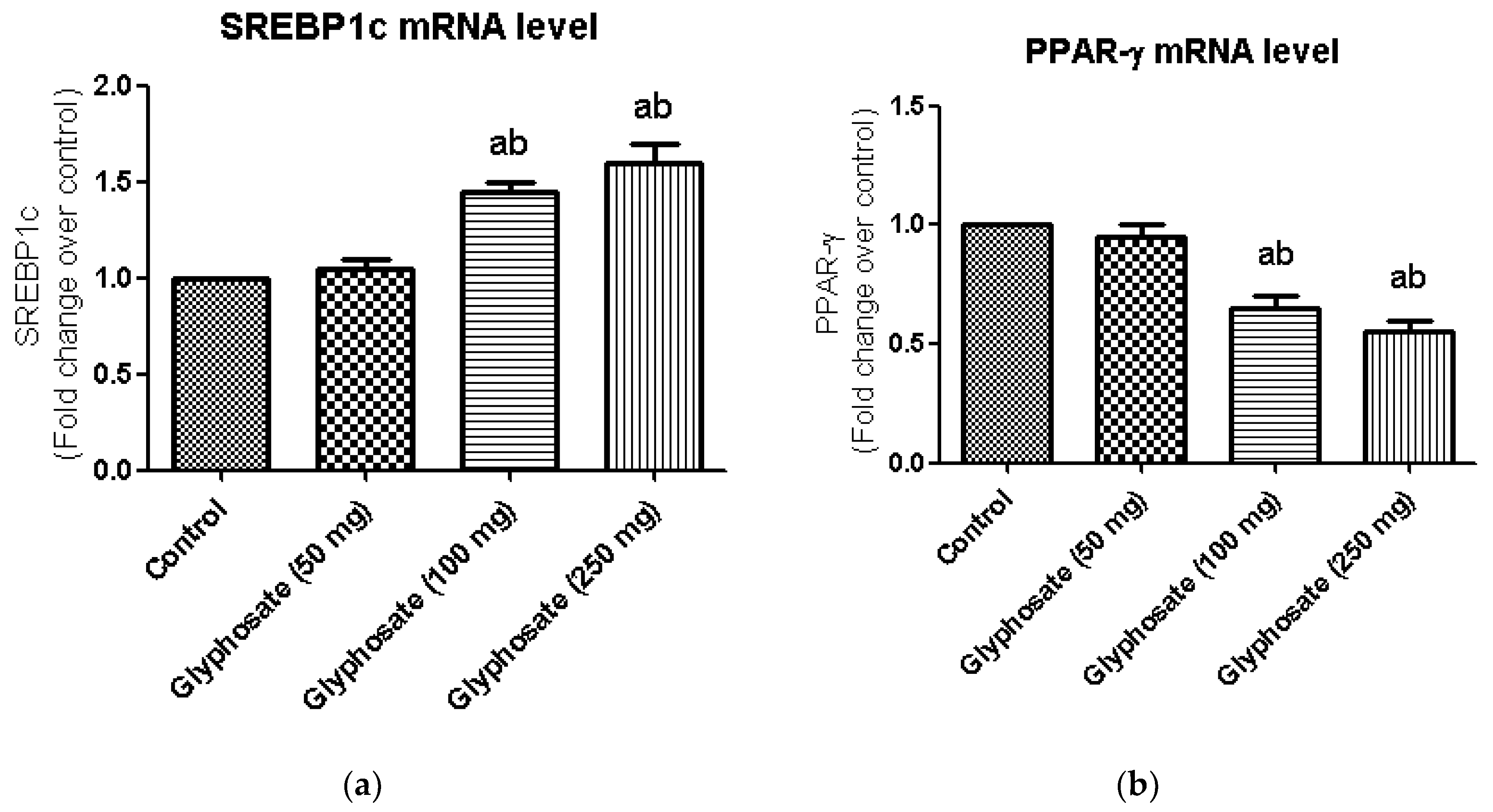
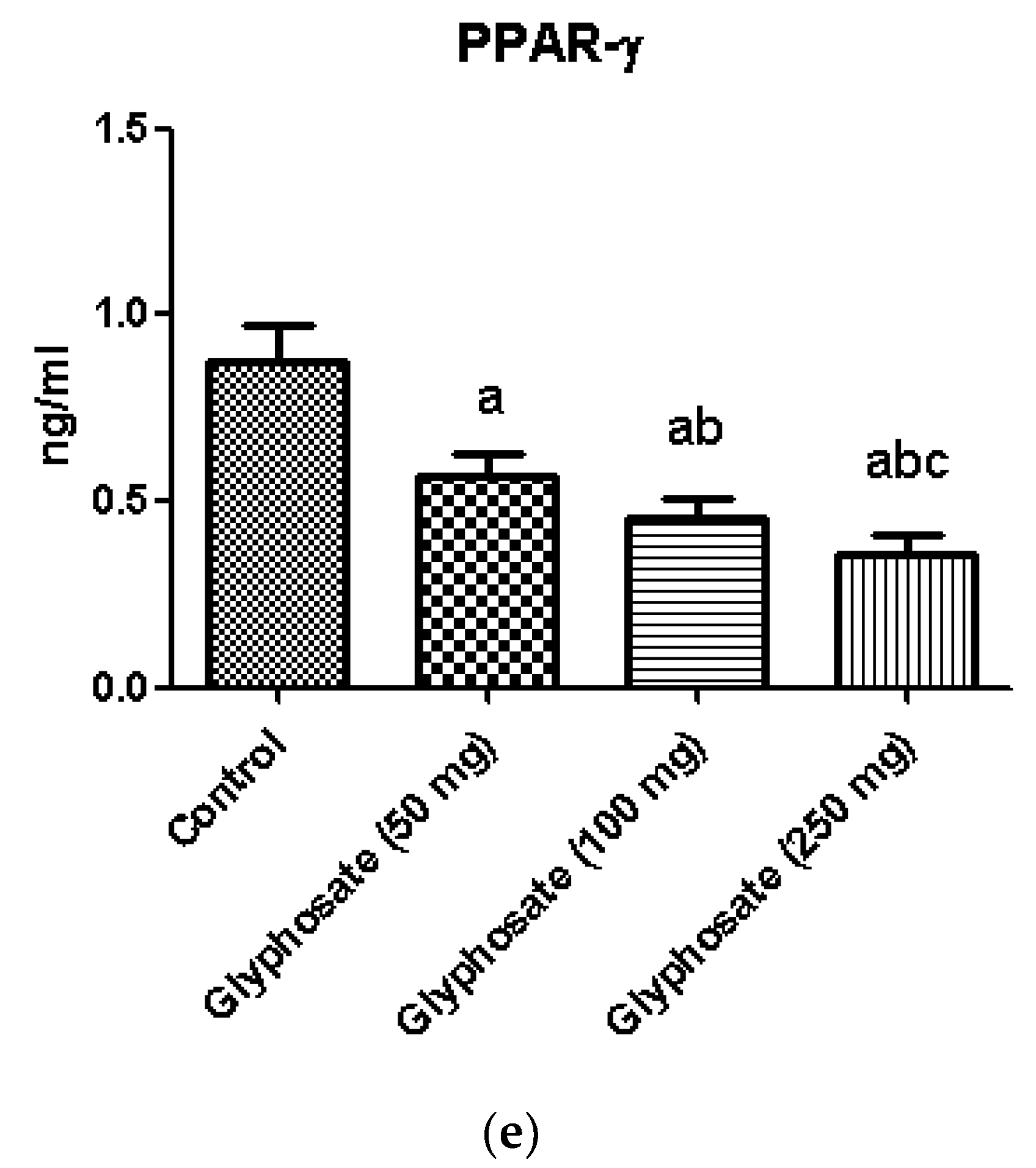
3.9. Impact of Glyphosate on the mRNA Expression of Transcriptional Factors Like PPAR-γ and SREBP1c
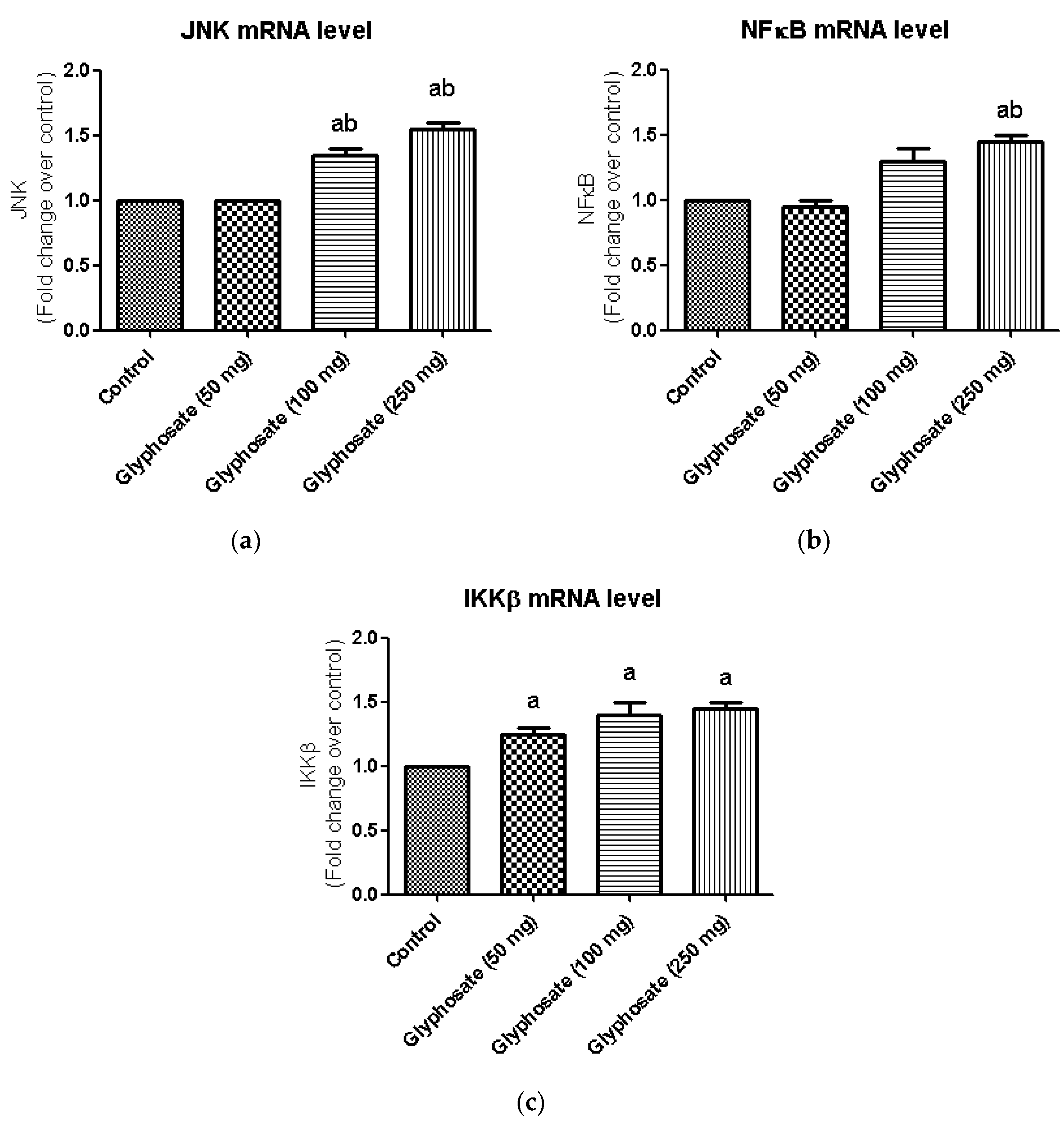
3.10. Impact of Glyphosate on the mRNA Expression of JNK Pathway Related Molecules
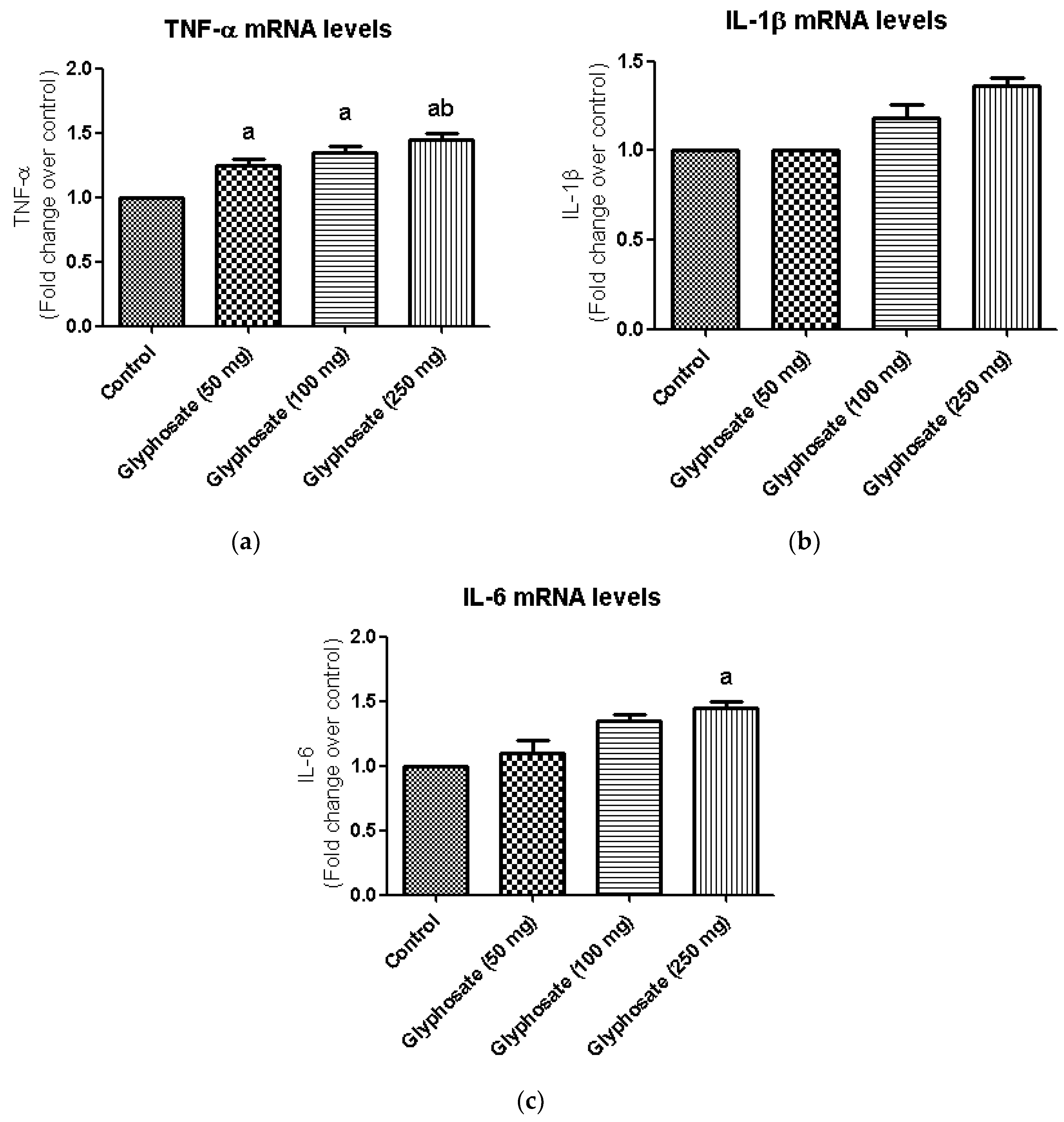
3.11. Impact of Glyphosate on the mRNA Expression of Pro-Inflammatory Cytokines
3.12. Impact of Glyphosate on the Protein Analysis of Pro-Inflammatory Cytokines and Transcription Factors
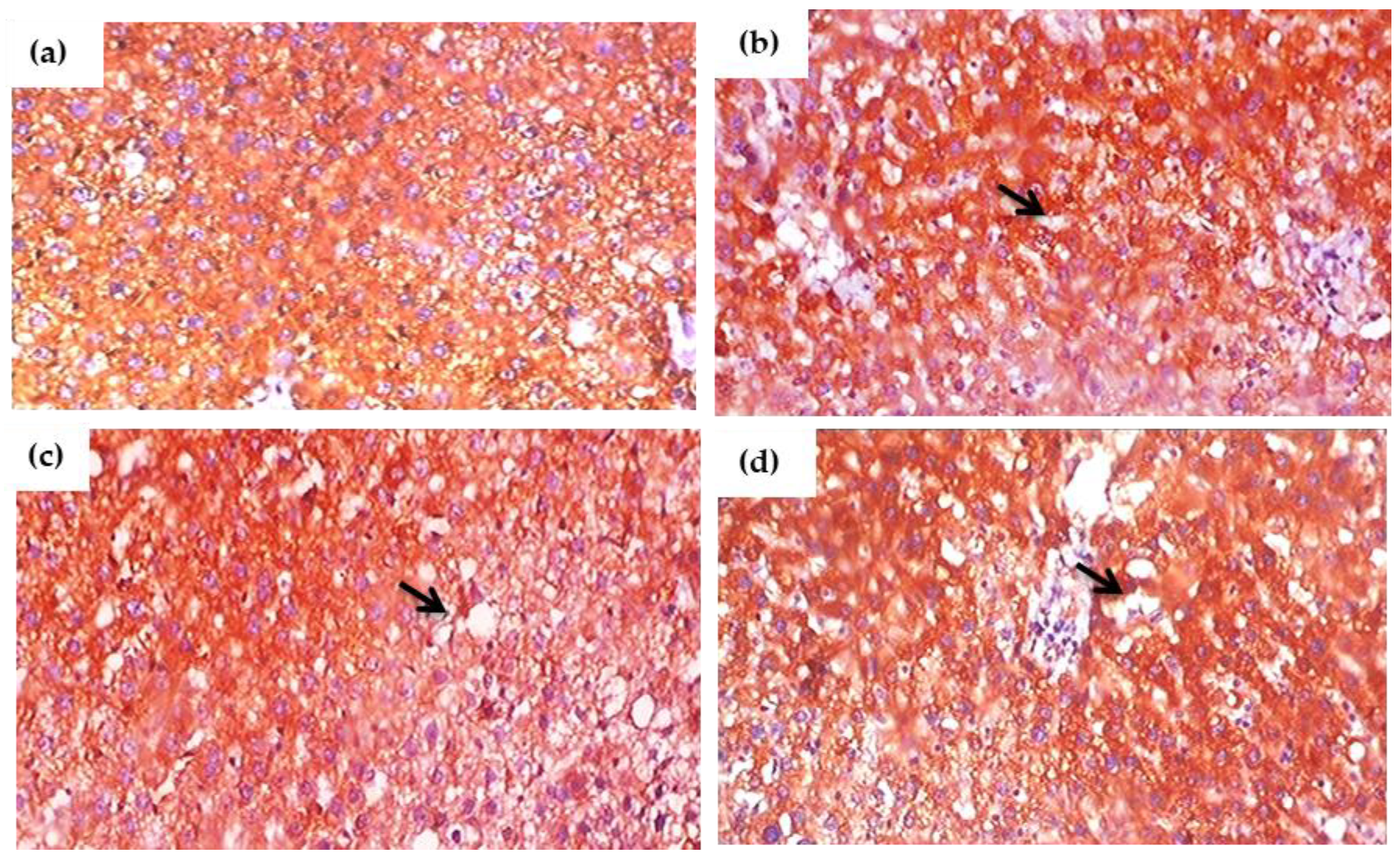
3.13. Impact of Glyphosate on the Expression of Pro-Inflammatory Proteins in Sections of the Liver Using Immunohistochemistry
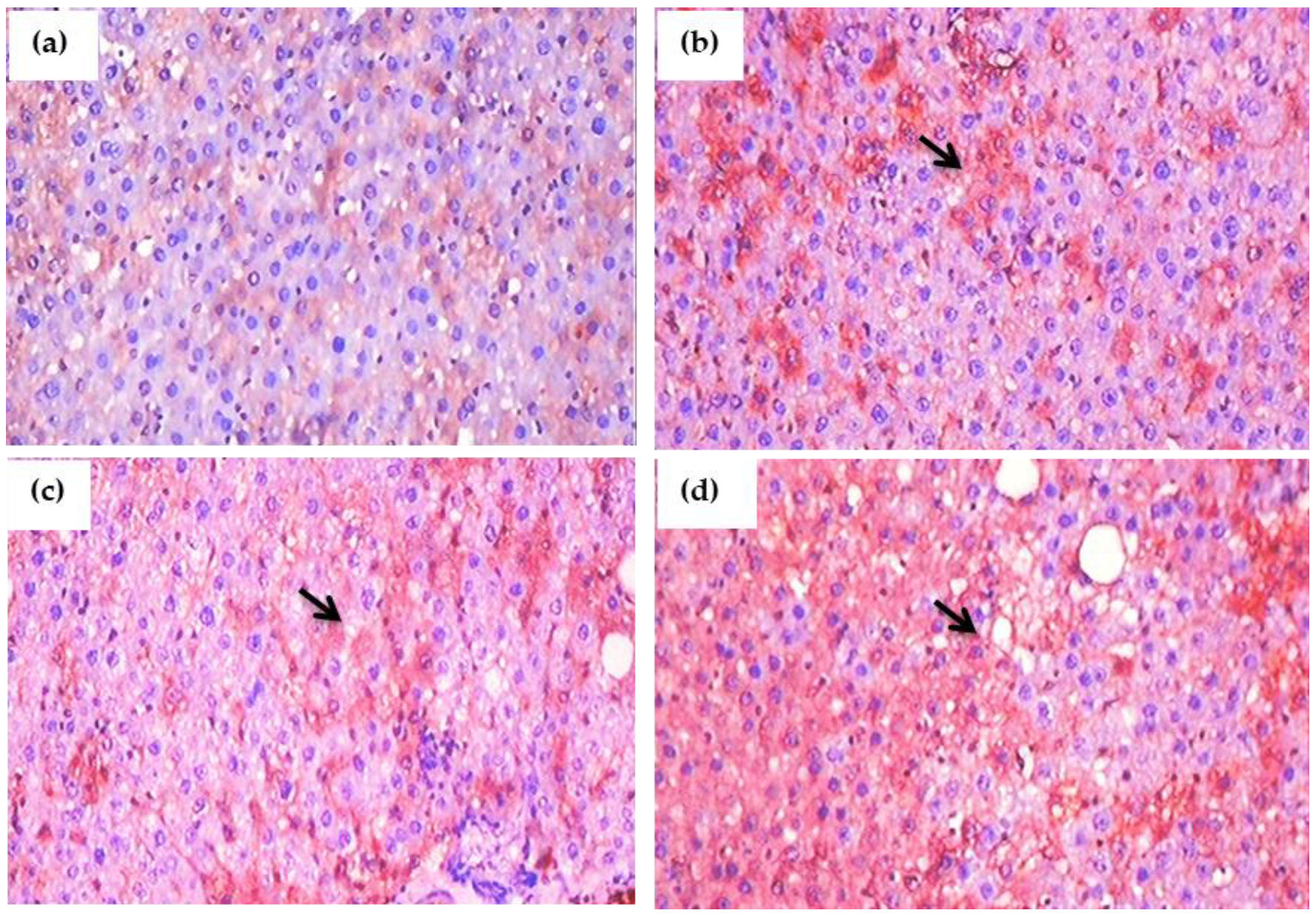
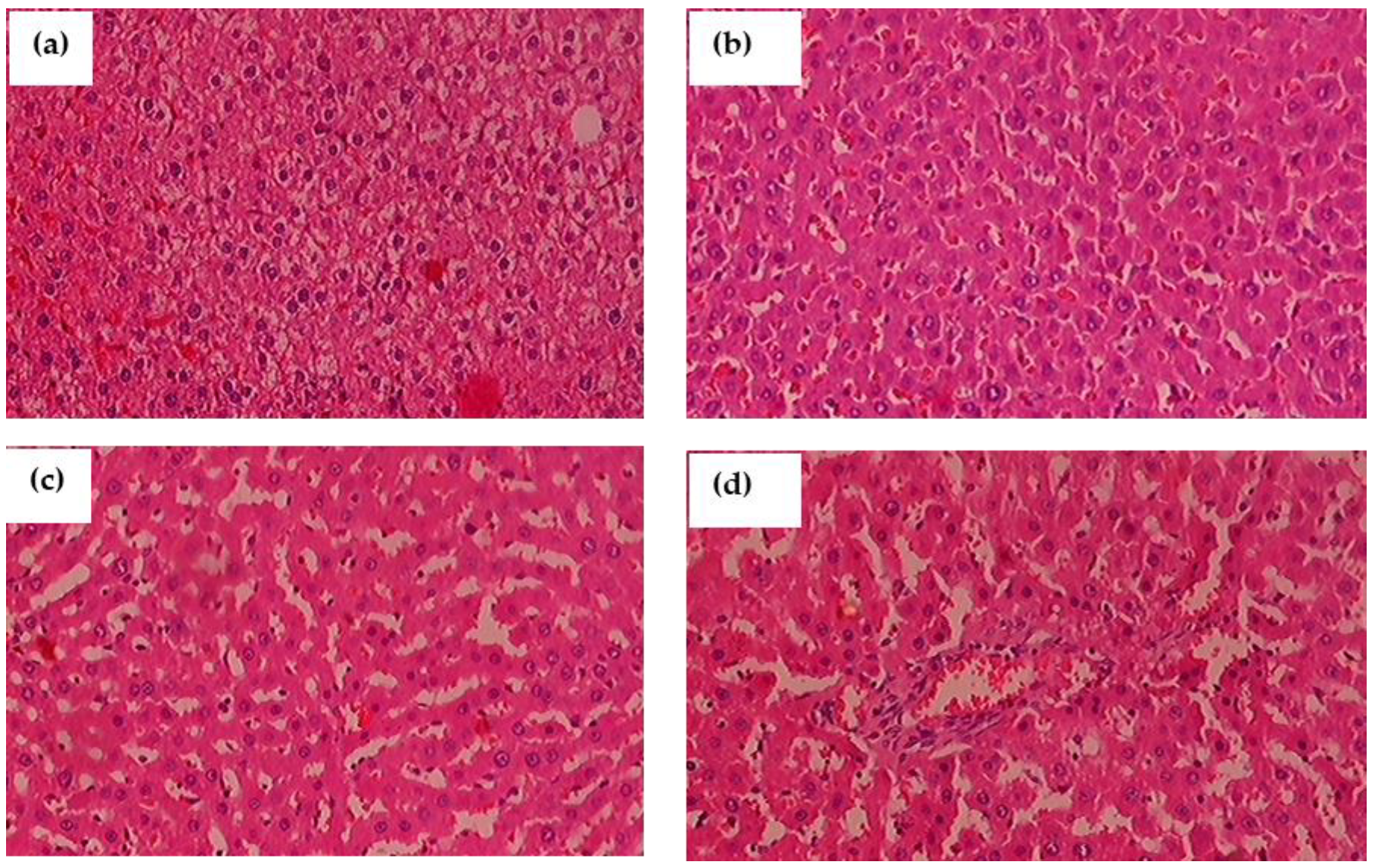
3.14. Histopathological Observation
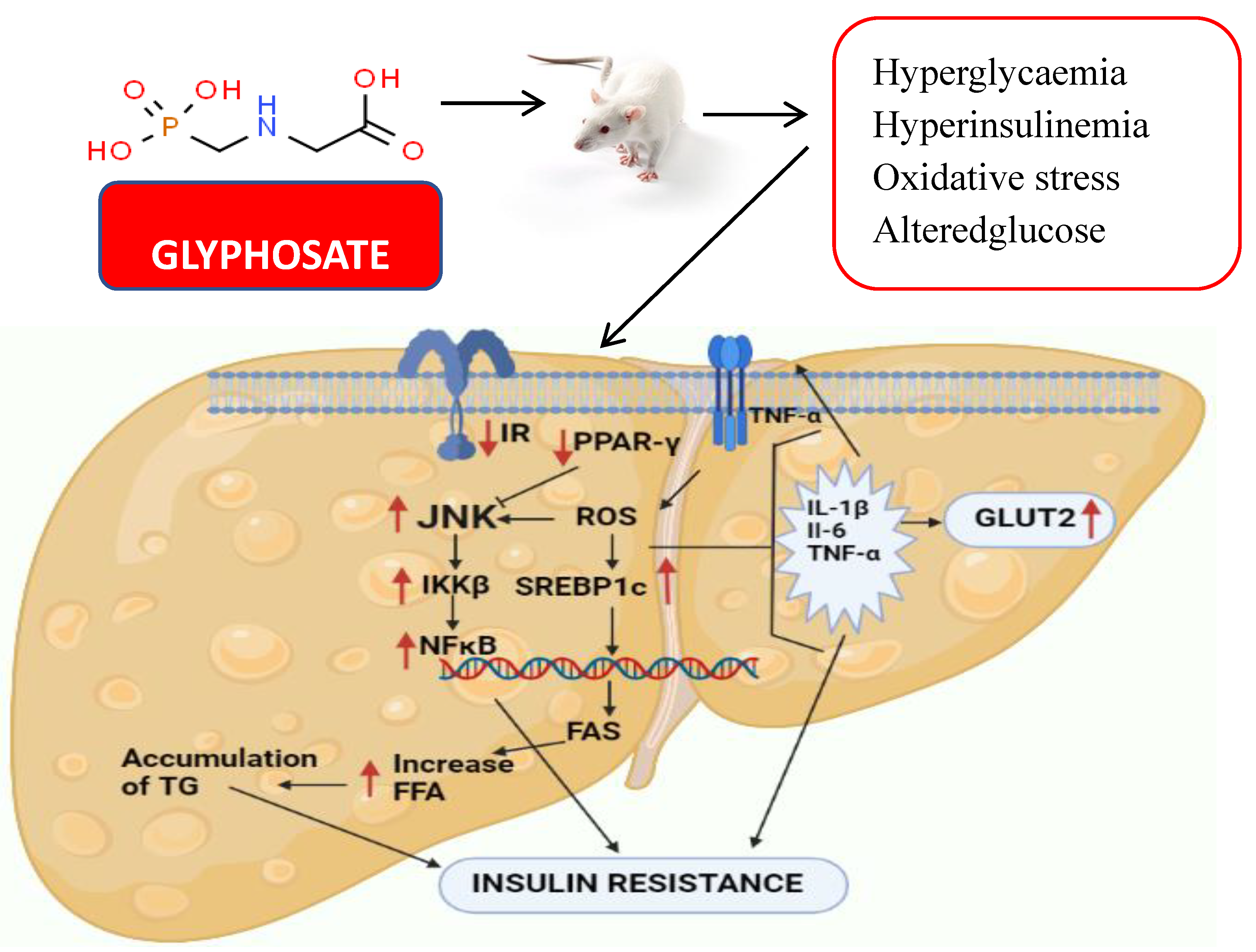
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. IDF Diabetes Atlas Committee (2019), 9th ed. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar]
- Bonciu, E. Cytological effects induced by Agil herbicide to onion. J. Hortic. For. Biotechnol. 2012, 16, 68–72. [Google Scholar]
- Mesi, A.; Kopliku, D.; Golemi, S. The use of higher plants as bio-indicators of environmental pollution—A new approach for toxicity screening in Albania. Mediterr. J. Soc. Sci. 2012, 3, 237–248. [Google Scholar]
- Silveira, M.A.; Ribeiro, D.L.; Dos Santos, T.A.; Vieira, G.M.; Cechinato, C.N.; Kazanovski, M.; Grégio d’Arce, L.P. Mutagenicity of two herbicides widely used on soybean crops by the Allium cepa test. Cytotechnology 2016, 68, 1215–1222. [Google Scholar] [CrossRef]
- Kraehmer, H.; van Almsick, A.; Beffa, R.; Dietrich, H.; Eckes, P.; Hacker, E.; Hain, R.; Strek, H.J.; Stuebler, H.; Willms, L. Herbicides as weed control agents: State of the art: II. Recent achievements. Plant Physiol. 2014, 166, 1132–1148. [Google Scholar] [CrossRef]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical Pesticides and Human Health: The Urgent Need for a New Concept in Agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef]
- Cai, D.; Yuan, M.; Frantz, D.F.; Melendez, P.A.; Hansen, L.; Lee, J.; Shoelson, S.E. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat. Med. 2005, 11, 183–190. [Google Scholar]
- He, Z.; King, G.L. Microvascular complications of diabetes. Endocrinol. Metab. Clin. N. Am. 2004, 33, 215–238. [Google Scholar] [CrossRef]
- Zambrano, S.; De Toma, I.; Piffer, A.; Bianchi, M.E.; Agresti, A. NF-κB oscillations translate into functionally related patterns of gene expression. eLife 2016, 5, e09100. [Google Scholar]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018, 555, 210–215. [Google Scholar] [CrossRef]
- Komives, T.; Schroder, P. On glyphosate. Ecocycles 2016, 2, 1–8. [Google Scholar]
- Thongprakaisang, S.; Thiantanawat, A.; Rangkadilok, N.; Suriyo, T.; Satayavivad, J. Glyphosate induces human breast cancer cells growth via estrogen receptors. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013, 59, 129–136. [Google Scholar] [CrossRef]
- Silva, V.; Montanarella, L.; Jones, A.; Fernández-Ugalde, O.; Mol, H.; Ritsema, C.J.; Geissen, V. Distribution of glyphosate and aminomethylphosphonic acid (AMPA) in agricultural topsoils of the European Union. Sci. Total Environ. 2018, 621, 1352–1359. [Google Scholar] [CrossRef]
- Mertens, M.; Höss, S.; Neumann, G.; Afzal, J.; Reichenbecher, W. Glyphosate, a chelating agent-relevant for ecological risk assessment? Environ. Sci. Pollut. Res. Int. 2018, 25, 5298–5317. [Google Scholar] [CrossRef]
- Williams, G.M.; Berry, C.; Burns, M.; de Camargo, J.L.; Greim, H. Glyphosate rodent carcinogenicity bioassay expert panel review. Crit. Rev. Toxicol. 2016, 46, 44–55. [Google Scholar]
- Kapoor, D.; Malkin, C.J.; Channer, K.S.; Jones, T.H. Androgens, insulin resistance and vascular disease in men. Clin. Endocrinol. 2005, 63, 239–250. [Google Scholar]
- Andersson, B.; Mårin, P.; Lissner, L.; Vermeulen, A.; Björntorp, P. Testosterone concentrations in women and men with NIDDM. Diabetes Care 1994, 17, 405–411. [Google Scholar] [CrossRef]
- Muthusamy, T.; Dhevika, S.; Murugesan, P.; Balasubramanian, K. Testosterone deficiency impairs glucose oxidation through defective insulin and its receptor gene expression in target tissues of adult male rats. Life Sci. 2007, 81, 534–542. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Blumberg, B.; Antoniou, M.N.; Benbrook, C.M.; Carroll, L.; Colborn, T.; Everett, L.G.; Hansen, M.; Landrigan, P.J.; Lanphear, B.P.; et al. Is it time to reassess current safety standards for glyphosate-based herbicides? J. Epidemiol. Community Health 2017, 71, 613–618. [Google Scholar] [CrossRef]
- Nardi, J.; Moras, P.B.; Koeppe, C.; Dallegrave, E.; Leal, M.B.; Rossato-Grando, L.G. Prepubertal subchronic exposure to soy milk and glyphosate leads to endocrine disruption. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2017, 100, 247–252. [Google Scholar] [CrossRef]
- Martinez, A.; Al-Ahmad, A.J. Effects of glyphosate and aminomethylphosphonic acid on an isogeneic model of the human blood-brain barrier. Toxicol. Lett. 2019, 304, 39–49. [Google Scholar] [CrossRef]
- Trasande, L.; Aldana, S.I.; Trachtman, H.; Kannan, K.; Morrison, D.; Christakis, D.A.; Whitlock, K.; Messito, M.J.; Gross, R.S.; Karthikraj, R.; et al. Glyphosate exposures and kidney injury biomarkers in infants and young children. Environ. Pollut. 2020, 256, 113334. [Google Scholar] [CrossRef]
- Pandey, A.; Rudraiah, M. Analysis of endocrine disruption effect of Roundup® in adrenal gland of male rats. Toxicol. Rep. 2015, 2, 1075–1085. [Google Scholar] [CrossRef]
- Liu, J.B.; Li, Z.F.; Lu, L.; Wang, Z.Y.; Wang, L. Glyphosate damages blood-testis barrier via NOX1-triggered oxidative stress in rats: Long-term exposure as a potential risk for male reproductive health. Environ. Int. 2022, 159, 107038. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Katz, A.; Nambi, S.S.; Mather, K.; Baron, A.D.; Follmann, D.A.; Sullivan, G.; Quon, M.J. Quantitative insulin sensitivity check index: A simple, accurate method for assessing insulin sensitivity in humans. J. Clin. Endocrinol. Metab. 2000, 85, 2402–2410. [Google Scholar] [CrossRef]
- Fraga, C.G.; Leibovitz, B.E.; Tappel, A.L. Lipid peroxidation measured as thiobarbituric acid-reactive substances in tissue slices: Characterization and comparison with homogenates and microsomes. Free. Radic. Biol. Med. 1988, 4, 155–161. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Hunt, J.V.; Wolff, S.P. Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal. Biochem. 1992, 202, 384–389. [Google Scholar] [CrossRef]
- Kakkar, P.; Das, B.; Viswanathan, P.N. A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys. 1984, 21, 130–132. [Google Scholar]
- Sinha, A.K. Colorimetric assay of catalase. Anal. Biochem. 1972, 47, 389–394. [Google Scholar] [CrossRef]
- Brandstrup, N.; Kirk, J.E.; Bruni, C. The hexokinase and phosphoglucoisomerase activities of aortic and pulmonary artery tissue in individuals of various ages. J. Gerontol. 1957, 12, 166–171. [Google Scholar] [CrossRef]
- Valentine, W.N.; Tanaka, K.R. Pyruvate kinase: Clinical aspects. Methods Enzym. 1966, 9, 468–473. [Google Scholar]
- Koide, H.; Oda, T. Pathological occurrence of glucose-6-phosphatase in serum in liver diseases. Clin. Chim. Acta 1959, 4, 554–561. [Google Scholar]
- Gancedo, J.M.; Gancedo, C. Fructose-1,6-diphosphatase, phosphofructokinase and glucose-6-phosphate dehydrogenase from fermenting and non fermenting yeasts. Arch. Mikrobiol. 1971, 76, 132–138. [Google Scholar] [CrossRef]
- Fourney, R.M.; Day, M.J. Randall RJRS. Northern blotting: Efficient RNA staining and transfer. Focus 1988, 10, 5–7. [Google Scholar]
- Jayashree, S.; Indumathi, D.; Akilavalli, N.; Sathish, S.; Selvaraj, J.; Balasubramanian, K. Effect of Bisphenol-A on insulin signal transduction and glucose oxidation in liver of adult male albino rat. Environ. Toxicol. Pharmacol. 2013, 35, 300–310. [Google Scholar] [CrossRef]
- Bizeau, M.E.; MacLean, P.S.; Johnson, G.C.; Wei, Y. Skeletal Muscle Sterol Regulatory Element Binding Protein-1c Decreases with Food Deprivation and Increases with Feeding in Rats. J. Nutr. 2003, 133, 1787–1792. [Google Scholar]
- Mahmoud, A.M.; Abdel-Rahman, M.M.; Bastawy, N.A.; Eissa, H.M. Modulatory effect of berberine on adipose tissue PPAR, adipocytokines and oxidative stress in high fat diet/streptozotocin-induced diabetic rats. J. Appl. Pharm. Sci. 2017, 7, 1–10. [Google Scholar]
- Al-Rasheed, N.M.; Fadda, L.M.; Al-Rasheed, N.M.; Ali, H.M.; Yacoub, H.I. Down-Regulation of NFκB, Bax, TGF-α, Smad-2mRNA expression in the Livers of Carbon Tetrachloride Treated Rats using Different Natural Antioxidants. Braz. Arch. Biol. Technol. 2016, 59, e16150553. [Google Scholar]
- Zhou, H.; Li, Y.J.; Wang, M.; Zhang, L.H.; Guo, B.Y.; Zhao, Z.S.; Meng, F.L.; Deng, Y.G.; Wang, R.Y. Involvement of RhoA/ROCK in myocardial fibrosis in a rat model of type 2 diabetes. Acta Pharmacol. Sin. 2011, 32, 999–1008. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, Q.; Pu, L.J.; Xu, X.W.; Zhang, R.Y.; Zhang, J.S.; Hu, J.; Yang, Z.K.; Lü, A.K.; Ding, F.H.; et al. Elevation of tumor necrosis factor-alpha, interleukin-1beta and interleukin-6 levels in aortic intima of Chinese Guizhou minipigs with streptozotocin-induced diabetes. Chin. Med. J. 2007, 120, 479–484. [Google Scholar]
- Qiu, L.L.; Wang, C.; Yao, S.; Li, N.; Hu, Y.; Yu, Y.; Xia, R.; Zhu, J.; Ji, M.; Zhang, Z.; et al. Fenvalerate induces oxidative hepatic lesions through an overload of intracellular calcium triggered by the ERK/IKK/NF-κB pathway. FASEB J. 2019, 33, 2782–2795. [Google Scholar] [CrossRef]
- Dange, R.B.; Agarwal, D.; Teruyama, R.; Francis, J. Toll-like receptor 4 inhibition within the paraventricular nucleus attenuates blood pressure and inflammatory response in a genetic model of hypertension. J. Neuroinflamm. 2015, 12, 31. [Google Scholar] [CrossRef]
- Peinnequin, A.; Mouret, C.; Birot, O.; Alonso, A.; Mathieu, J.; Clarençon, D.; Agay, D.; Chancerelle, Y.; Multon, E. Rat proinflammatory cytokine and cytokine related mRNA quantification by real-time polymerase chain reaction using SYBR green. BMC Immunol. 2004, 3, 3. [Google Scholar]
- Gabe, M. Techniques Histologiques; Massie e Cie: Paris, France, 1968; p. 1113. [Google Scholar]
- Gill, J.P.K.; Sethi, N.; Mohan, A.; Datta, S.; Girdhar, M. Glyphosate toxicity for animals. Environ. Chem. Lett. 2018, 16, 401–426. [Google Scholar] [CrossRef]
- Mesnage, R.; Renney, G.; Séralini, G.E.; Ward, M.; Antoniou, M.N. Multiomics reveal non-alcoholic fatty liver disease in rats following chronic exposure to an ultra-low dose of Roundup herbicide. Sci. Rep. 2017, 7, 39328. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J. Clin. Investig. 2016, 126, 12–22. [Google Scholar]
- Dallegrave, E.; Mantese, F.D.; Oliveira, R.T.; Andrade, A.J.; Dalsenter, P.R.; Langeloh, A. Pre-and postnatal toxicity of the commercial glyphosate formulation in Wistar rats. Arch. Toxicol. 2007, 81, 665–673. [Google Scholar]
- Romano, R.M.; Romano, M.A.; Bernardi, M.M.; Furtado, P.V.; Oliveira, C.A.D. Prepubertal exposure to commercial formulation of the herbicide glyphosate alters testosterone levels and testicular morphology. Arch. Toxicol. 2010, 84, 309–317. [Google Scholar]
- Mechanick, J.I. Metabolic mechanisms of stress hyperglycemia. JPEN J. Parenter. Enteral. Nutr. 2006, 30, 157–163. [Google Scholar]
- Tizhe, E.; Ibrahim, N.; Fatihu, M.; Ambali, S.; Igbokwe, I.; Tizhe, U. Pancreatic function and histoarchitecture in Wistar rats following chronic exposure to Bushfire®: The mitigating role of zinc. J. Int. Med. Res. 2018, 46, 3296–3305. [Google Scholar] [CrossRef]
- Rajesh, P.; Sathish, S.; Srinivasan, C.; Selvaraj, J.; Balasubramanian, K. Phthalate is associated with insulin resistance in adipose tissue of male rat: Role of antioxidant vitamins. J. Cell. Biochem. 2013, 114, 558–569. [Google Scholar] [CrossRef]
- Ponnulakshmi, R.; Shyamaladevi, B.; Vijayalakshmi, P.; Selvaraj, J. In silico and in vivo analysis to identify the antidiabetic activity of beta sitosterol in adipose tissue of high fat diet and sucrose induced type-2 diabetic experimental rats. Toxicol. Mech. Methods 2019, 29, 276–290. [Google Scholar] [CrossRef]
- Bagchi, D.; Bagchi, M.; Hassoun, E.A.; Stohs, S.J. In vitro and in vivo generation of reactive oxygen species, DNA damage and lactate dehydrogenase leakage by selected pesticides. Toxicology 1995, 104, 129–140. [Google Scholar] [CrossRef]
- Palmeira, C.M.; Moreno, A.J.; Madeira, V.M. Thiols metabolism is altered by the herbicides paraquat, dinoseb and 2,4-D: A study in isolated hepatocytes. Toxicol. Lett. 1995, 81, 115–123. [Google Scholar] [CrossRef]
- Milić, M.; Žunec, S.; Micek, V.; Kašuba, V.; Mikolić, A.; Lovaković, B.T.; Semren, T.Ž.; Pavičić, I.; Čermak, A.; Pizent, A.; et al. Oxidative stress, cholinesterase activity, and DNA damage in the liver, whole blood, and plasma of Wistar rats following a 28-day exposure to glyphosate. Arh. Hig. Rada Toksikol. 2018, 69, 154–168. [Google Scholar] [CrossRef]
- Rikans, L.E.; Yamano, T. Mechanisms of cadmium mediated acute hepatotoxicity. J. Biochem. Mol. Toxicol. 2000, 14, 110–117. [Google Scholar]
- Chance, B.; Greenstein, D.S.; Roughton, R.J.W. The mechanism of catalase action 1-steady state analysis. Arch. Biochem. Biophys. 1952, 37, 301–339. [Google Scholar]
- Jurczuk, M.; Brzoska, M.M.; Moniuszko-Jakoniuk, J.; Galazyn-Sidorczuk, M.; Kulikowska-Karpinska, E. Antioxidant enzymes activity and lipid peroxidation in liver and kidney of rats exposed to cadmium and ethanol. Food Chem. Toxicol. 2004, 42, 429–438. [Google Scholar]
- Djeffal, A.; Messarah, M.; Boumendjel, A.; Kadeche, L.; Feki, A.E. Protective effects of vitamin C and selenium supplementation on methomyl-induced tissue oxidative stress in adult rats. Toxicol. Ind. Health 2015, 31, 31–43. [Google Scholar]
- Baquer, N.Z.; Gupta, D.; Raju, J. Regulation of metabolic pathways in liver and kidney during experimental diabetes: Effects of antidiabetic compounds. Indian J. Clin. Biochem. 1998, 13, 63–80. [Google Scholar] [CrossRef]
- Vats, V.; Yadav, S.P.; Grover, J.K. Effect of T. foenumgraecum on glycogen content of tissues and the key enzymes of carbohydrate metabolism. J. Ethnopharmacol. 2003, 85, 237–242. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Biswas, T.K.; Rokeya, B.; Ali, L.; Mosihuzzaman, M.; Nahar, N.; Khan, A.K.; Mukherjee, B. Advanced studies on the hypoglycemic effect of Caesalpinia bonducella F. in type 1 and 2 diabetes in Long Evans rats. J. Ethnopharmacol. 2003, 84, 41–46. [Google Scholar] [CrossRef]
- Mugabo, Y.; Lim, G.E. Scaffold proteins: From coordinating signaling pathways to metabolic regulation. Endocrinology 2018, 159, 3615–3630. [Google Scholar]
- Uren Webster, T.M.; Santos, E.M. Global transcriptomic profiling demonstrates induction of oxidative stress and of compensatory cellular stress responses in brown trout exposed to glyphosate and Roundup. BMC Genom. 2015, 16, 32. [Google Scholar] [CrossRef]
- da Silva Rosa, S.C.; Nayak, N.; Caymo, A.M.; Gordon, J.W. Mechanisms of muscle insulin resistance and the cross-talk with liver and adipose tissue. Physiol. Rep. 2020, 8, e14607. [Google Scholar]
- Hamdaoui, L.; Naifar, M.; Mzid, M.; Ben Salem, M.; Chtourou, A.; Ayedi, F.; Sahnoun, Z.; Rebai, T. Nephrotoxicity of Kalach 360 SL: Biochemical and histopathological findings. Toxicol. Mech. Methods 2016, 26, 685–691. [Google Scholar]
- Yazdinezhad, A.; Abbasian, M.; Hojjat Hosseini, S.; Naserzadeh, P.; Agh-Atabay, A.H.; Hosseini, M.J. Protective effects of Ziziphora tenuior extract against chlorpyrifos induced liver and lung toxicity in rat: Mechanistic approaches in subchronic study. Environ. Toxicol. 2017, 32, 2191–2202. [Google Scholar] [CrossRef]
- Namba, T.; Nolte, C.T.; Jackrel, J.; Grob, D. Poisoning due to organophosphate insecticides: Acute and chronic manifestations. Am. J. Med. 1971, 50, 475–492. [Google Scholar]
- Ren, X.; Dai, P.; Perveen, A.; Tang, Q.; Zhao, L.; Jia, X.; Li, Y.; Li, C. Effects of chronic glyphosate exposure to pregnant mice on hepatic lipid metabolism in offspring. Environ. Pollut. 2019, 254(Pt. A), 112906. [Google Scholar] [CrossRef]
- Blaschke, F.; Takata, Y.; Caglayan, E.; Law, R.E.; Hsueh, W.A. Obesity, peroxisome proliferator-activated receptor, and atherosclerosis in type 2 diabetes. Arter. Thromb Vasc. Biol 2006, 26, 28–40. [Google Scholar]
- Leonardini, A.; Laviola, L.; Perrini, S.; Natalicchio, A.; Giorgino, F. Cross-Talk between PPARgamma and Insulin Signaling and Modulation of Insulin Sensitivity. PPAR Res. 2009, 2009, 818945. [Google Scholar] [CrossRef]
- Martini, C.N.; Gabrielli, M.; Brandani, J.N.; Vila Mdel, C. Glyphosate Inhibits PPAR Gamma Induction and Differentiation of Preadipocytes and is able to Induce Oxidative Stress. J. Biochem. Mol. Toxicol. 2016, 30, 404–413. [Google Scholar]
- Jayaraman, S.; Devarajan, N.; Rajagopal, P.; Babu, S.; Ganesan, S.K.; Veeraraghavan, V.P.; Palanisamy, C.P.; Cui, B.; Periyasamy, V.; Chandrasekar, K. β-Sitosterol Circumvents Obesity Induced Inflammation and Insulin Resistance by down-Regulating IKKβ/NF-κB and JNK Signaling Pathway in Adipocytes of Type 2 Diabetic Rats. Molecules 2021, 26, 2101. [Google Scholar] [CrossRef]
- Prasad, M.; Jayaraman, S.; Rajagopal, P.; Veeraraghavan, V.P.; Kumar, P.K.; Piramanayagam, S.; Pari, L. Diosgenin inhibits ER stress-induced inflammation in aorta via iRhom2/TACE mediated signaling in experimental diabetic rats: An in vivo and in silico approach. Chem.-Biol. Interact. 2022, 358, 109885. [Google Scholar] [CrossRef]
- Pandey, A.; Dhabade, P.; Kumarasamy, A. Inflammatory Effects of Subacute Exposure of Roundup in Rat Liver and Adipose Tissue. Dose-Response A Publ. Int. Hormesis Soc. 2019, 17, 1559325819843380. [Google Scholar] [CrossRef]

| S. No. | Gene Details | Primer Details | Reference |
| 1. | GLUT2 | Forward: 5′-CTC GGG CCT TAC GTG TTC TTC CTT-3′ Reverse: 5′-TGG TTC CCT TCT GGT CTG TTC CTG-3′ | [37] |
| 2. | IR | Forward: 5′-GCC ATC CCG AAA GCG AAG ATC-3′ Reverse: 5′-TCT GGG GAG TCC TGA TTG CAT-3′ | [37] |
| 3. | SREBP1c | Forward: 5′-GGA GCC ATG GAT TGC ACA TT-3′ Reverse: 5′-GCT TCC AGA GAG GAG CCC AG-3′ | [38] |
| 4. | PPAR-γ | Forward: 5′-CCT GAA GCT CCA AGA ATA CC-3′ Reverse: 5′-GAT GCT TTA TCC CCA CAG AC-3′ | [39] |
| 5. | NFκB | Forward: 5′-CAT GAA GAG AAG ACA CTG ACC ATG GAA A-3′ Reverse: 5′-TGG ATA GAG GCT AAG TGT AGA CAC G-3′ | [40] |
| 6. | JNK | Forward: 5′-TCA GAA TCC GAA CGA GAC AAA AT-3′ Reverse: 5′-AAG CCA GAG TCC TTC ACA GAC AA-3′ | [41] |
| 7. | IL-6 | Forward: 5′-GTG AGA AGT ATG AGA AGT GTG A-3′ Reverse: 5′-GCA GGA TGA GAA TGA TCT TTG-3′ | [42] |
| 8. | IKKβ | Forward: 5′-TGG CAT GGA AAC GGA TAA CTG A-3′ Reverse: 5′-CTG GAA CTC TGT GCC TGT GGA A-3′ | [43] |
| 9. | TNF-α | Forward: 5′-GTC GTA GCA AAC CAC CAA GC-3′ Reverse: 5′-TGT GGG TGA GGA GCA CAT AG-3′ | [44] |
| 10. | IL-1β | Forward: 5′- GCA ATG GTC GGG ACA TAG TT-3′ Reverse: 5′-AGA CCT GAC TTG GCA GAG A-3′ | [44] |
| 11. | β-actin | Forward: 5′-AAG TCC CTC ACC CTC CCA AAA G-3′ Reverse: 5′-AAG CAA TGC TGT CAC CTT CCC-3′ | [35] |
| Groups | 0 h | 60 min | 120 min | 180 min |
|---|---|---|---|---|
| Control | 69 ± 1.2 | 72 ±1.4 | 75 ±3.2 | 76 ± 4.2 |
| Glyphosate (50 mg) | 110 ± 1.5 a | 115 ± 2.2 a | 120 ± 3.5 a | 121 ± 3.4 a |
| Glyphosate (100 mg) | 132 ± 2.5 ab | 138 ± 5.9 ab | 149 ± 6.2 ab | 150 ± 6.2 ab |
| Glyphosate (250 mg) | 138 ± 0.5 ab | 140 ± 6.7 ab | 152 ± 7.8 ab | 159 ± 7.7 ab |
| Group | 0 h | 15 min | 30 min | 45 min | 60 min |
|---|---|---|---|---|---|
| Control | 68 ± 2.9 | 70 ±4.2 | 62 ±1.2 | 69 ±3.2 | 70 ±2.9 |
| Glyphosate (50 mg) | 109 ± 7.29 a | 110 ± 6.29 a | 109 ±4.2 a | 110 ±4.9 a | 101 ±5.2 a |
| Glyphosate (100 mg) | 129 ± 1.9 a | 117 ± 1.5 a | 116 ±5.2 ab | 110 ±6.7 a | 112 ± 6.5 a |
| Glyphosate (250 mg) | 127 ± 6.2 b | 122 ±5.9 ab | 129 ±6.2 ab | 127 ±7.9 ab | 112 ±6.4 a |
| Groups | HOMA-IR | QUICKI |
|---|---|---|
| Control | 2.76 ± 0.11 | 0.92 ± 0.05 |
| Glyphosate (50 mg) | 9 ± 0.52 a | 0.8 ± 0.03 a |
| Glyphosate (100 mg) | 10± 0.42 a | 0.62 ± 0.02 ab |
| Glyphosate (250 mg) | 12± 0.25 ab | 0.6 ± 0.03 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasad, M.; Gatasheh, M.K.; Alshuniaber, M.A.; Krishnamoorthy, R.; Rajagopal, P.; Krishnamoorthy, K.; Periyasamy, V.; Veeraraghavan, V.P.; Jayaraman, S. Impact of Glyphosate on the Development of Insulin Resistance in Experimental Diabetic Rats: Role of NFκB Signalling Pathways. Antioxidants 2022, 11, 2436. https://doi.org/10.3390/antiox11122436
Prasad M, Gatasheh MK, Alshuniaber MA, Krishnamoorthy R, Rajagopal P, Krishnamoorthy K, Periyasamy V, Veeraraghavan VP, Jayaraman S. Impact of Glyphosate on the Development of Insulin Resistance in Experimental Diabetic Rats: Role of NFκB Signalling Pathways. Antioxidants. 2022; 11(12):2436. https://doi.org/10.3390/antiox11122436
Chicago/Turabian StylePrasad, Monisha, Mansour K. Gatasheh, Mohammad A. Alshuniaber, Rajapandiyan Krishnamoorthy, Ponnulakhmi Rajagopal, Kalaiselvi Krishnamoorthy, Vijayalakshmi Periyasamy, Vishnu Priya Veeraraghavan, and Selvaraj Jayaraman. 2022. "Impact of Glyphosate on the Development of Insulin Resistance in Experimental Diabetic Rats: Role of NFκB Signalling Pathways" Antioxidants 11, no. 12: 2436. https://doi.org/10.3390/antiox11122436
APA StylePrasad, M., Gatasheh, M. K., Alshuniaber, M. A., Krishnamoorthy, R., Rajagopal, P., Krishnamoorthy, K., Periyasamy, V., Veeraraghavan, V. P., & Jayaraman, S. (2022). Impact of Glyphosate on the Development of Insulin Resistance in Experimental Diabetic Rats: Role of NFκB Signalling Pathways. Antioxidants, 11(12), 2436. https://doi.org/10.3390/antiox11122436






