Nrf2 Regulates Oxidative Stress and Its Role in Cerebral Ischemic Stroke
Abstract
1. Introduction
1.1. Cerebral Ischemic Stroke
1.2. Nrf2
1.3. Oxidative Stress
1.4. The Role of Nrf2 in Cerebral Ischemic Stroke
2. The Structure and Function of Nrf2
3. Oxidative Stress and Cerebral Ischemic Stroke
4. Activation of Nrf2 Signaling Pathway in Cerebral Ischemic Stroke
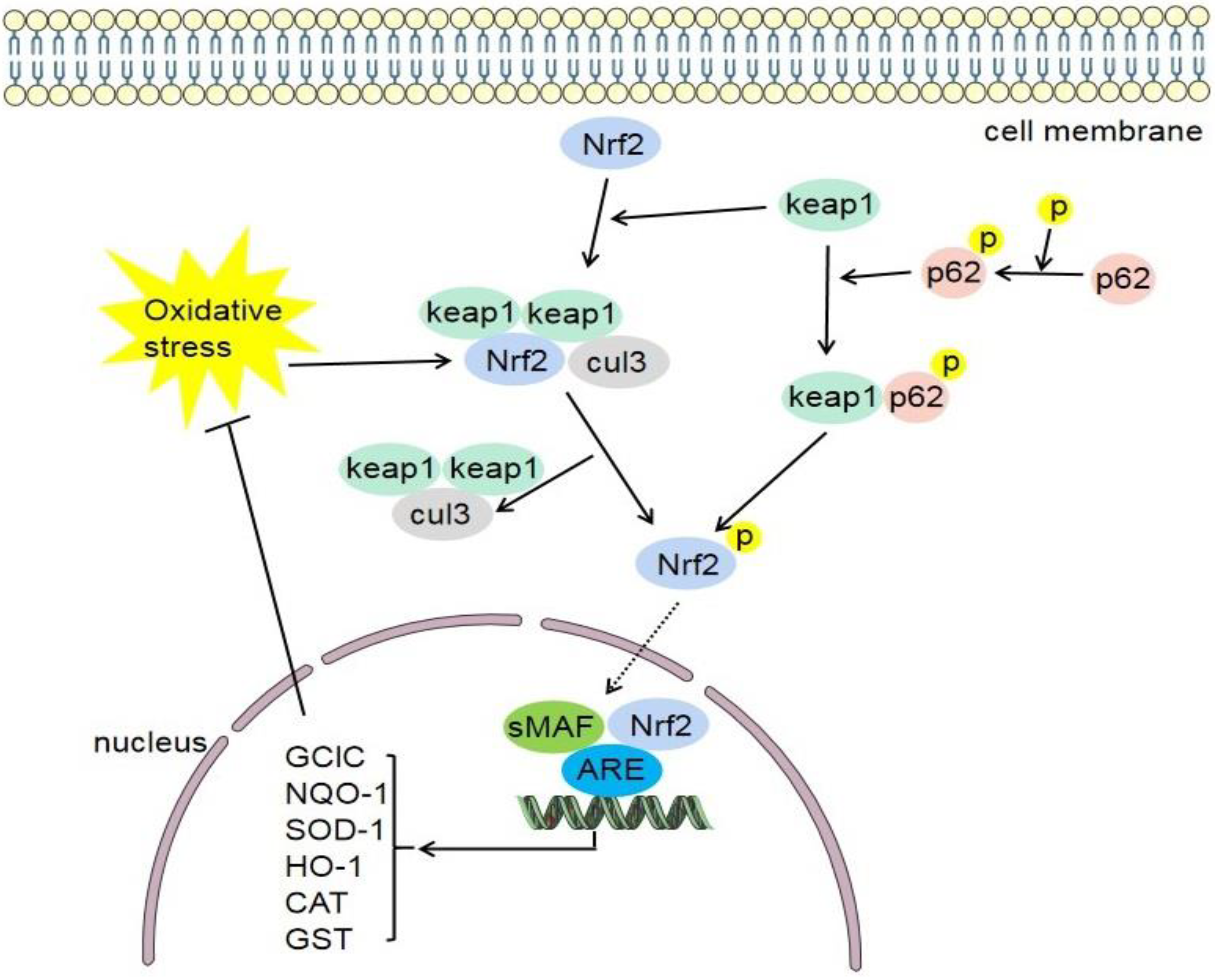
4.1. Keap1/Nrf2/ARE Signaling Pathway
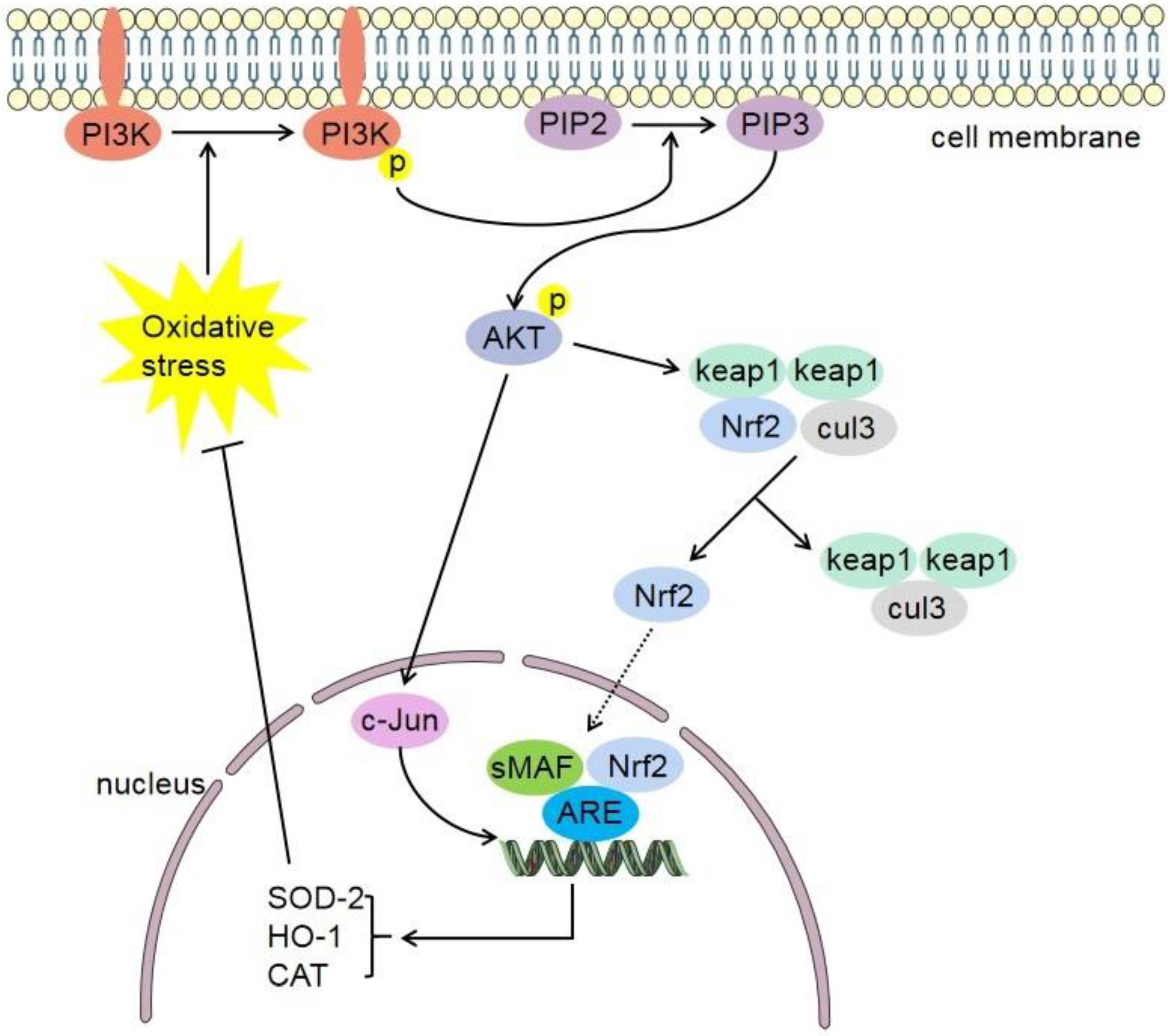
4.2. Phosphatidylinositol-4,5-Bisphosphate 3-Kinase (PI3K)/Protein Kinase B (Akt)-Nrf2 Signaling Pathway
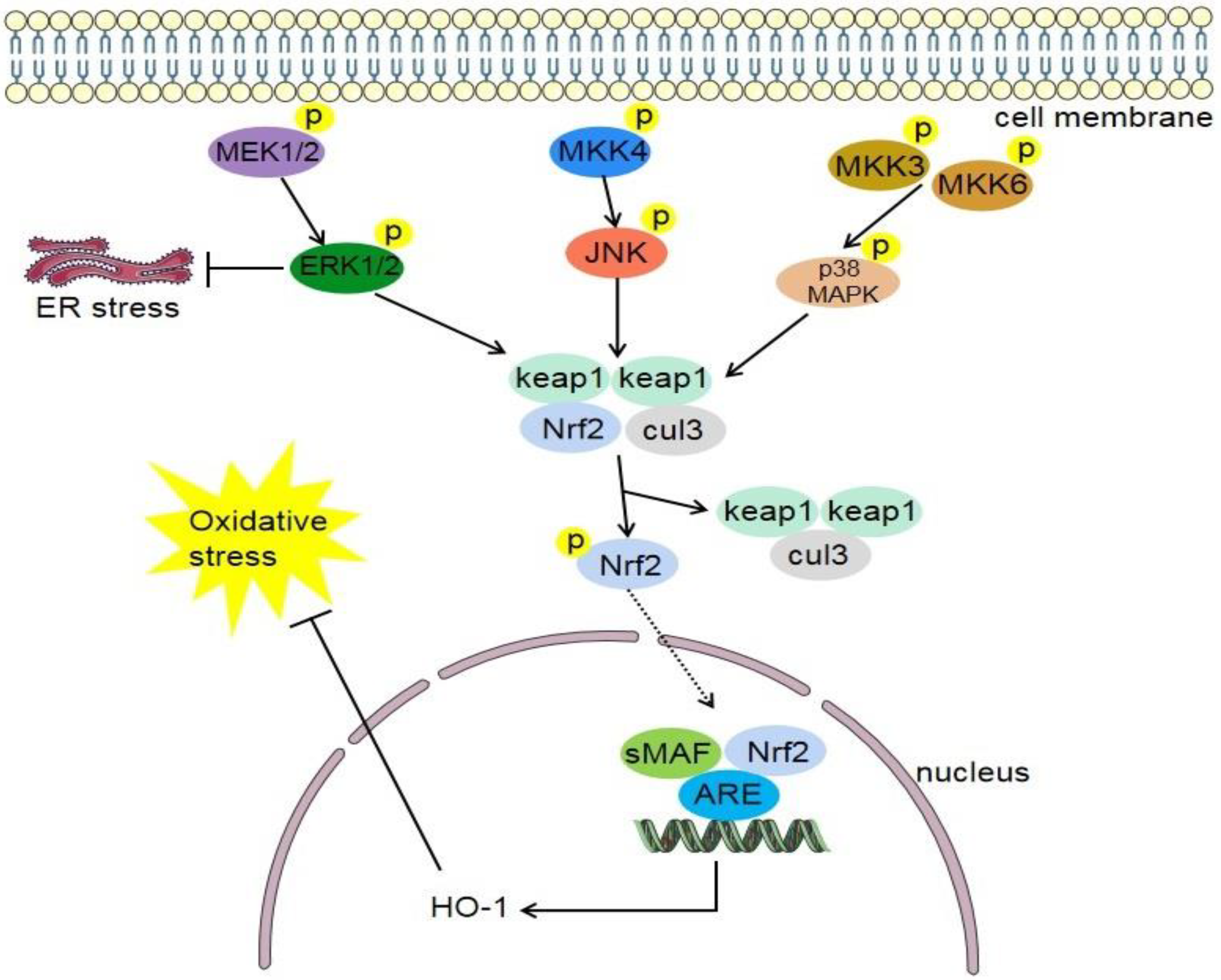
4.3. MAPK/Nrf2 Signaling Pathway
4.4. Nrf2/Nuclear Transcription Factor-Kappa B (NF-κB) Signaling Pathway
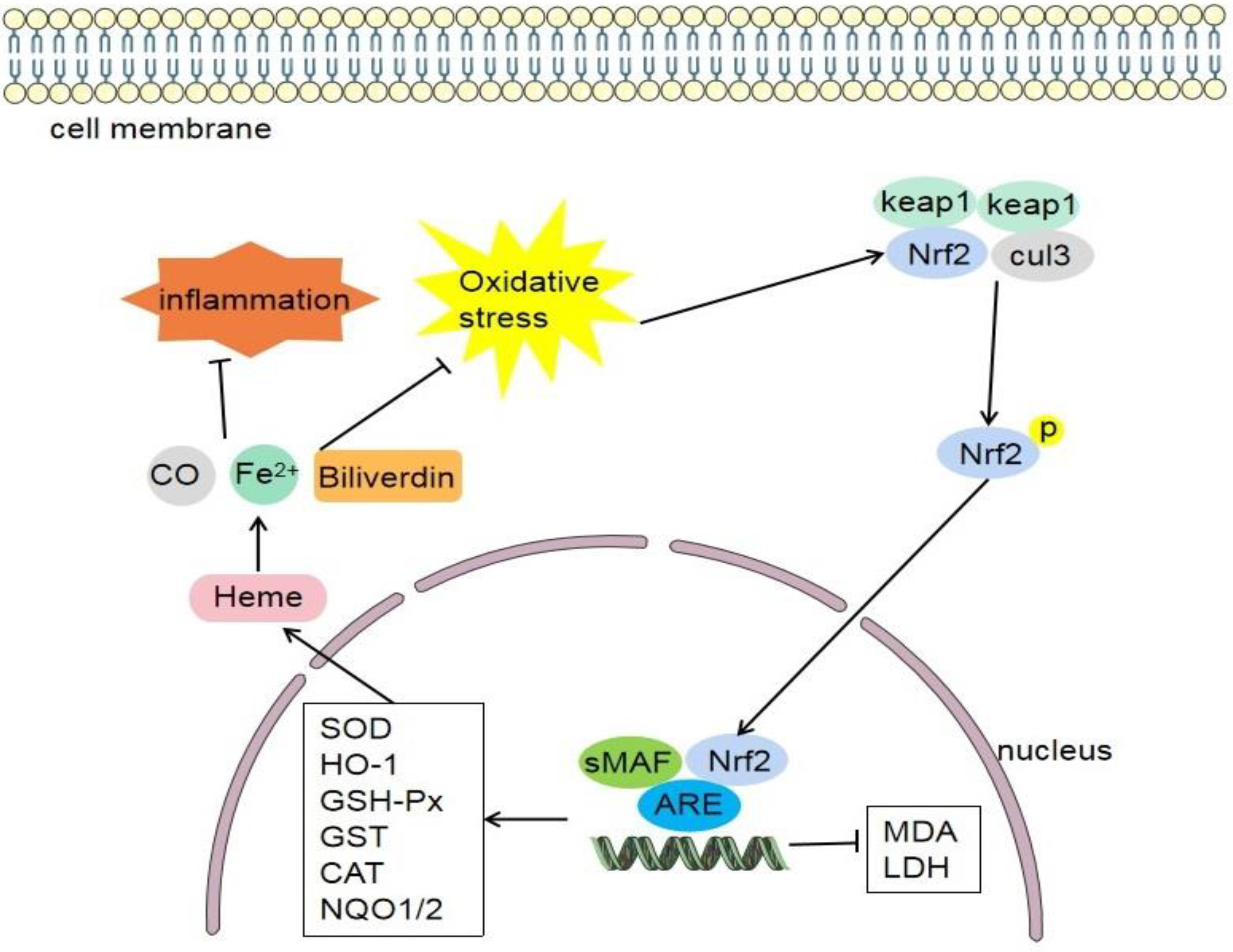
4.5. Nrf2/HO-1 Signaling Pathway
5. The Role and Mechanism of Nrf2 in Cerebral Ischemic Stroke
5.1. Nrf2 Regulates Oxidative Stress and Antioxidant Effect
5.2. Nrf2 Regulates Inflammation and Anti-Inflammatory Effects
5.3. Nrf2 and Regulation of Mitochondrial Function
5.4. Nrf2 and Protection of Blood–Brain Barrier Function
5.5. Nrf2 Regulates Ferroptosis
6. Drugs or Compounds Affect Cerebral Ischemic Stroke by Regulating Nrf2 Signaling Pathways
7. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Wu, S.; Wu, B.; Liu, M.; Chen, Z.; Wang, W.; Anderson, C.; Sandercock, P.; Wang, Y.; Huang, Y.; Cui, L.; et al. Stroke in China: Advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 2019, 18, 394–405. [Google Scholar] [CrossRef]
- Hankey, G. Stroke. Lancet 2017, 389, 641–654. [Google Scholar] [CrossRef]
- Hitomi, E.; Simpkins, A.; Luby, M.; Latour, L.; Leigh, R.; Leigh, R. Blood-ocular barrier disruption in patients with acute stroke. Neurology 2018, 90, e915–e923. [Google Scholar] [CrossRef]
- Lassen, N.; Fieschi, C.; Lenzi, G. Ischemic penumbra and neuronal death: Comments on the therapeutic window in acute stroke with particular reference to thrombolytic therapy. Cerebrovasc. Dis. 1991, 1, 32–35. [Google Scholar] [CrossRef]
- Amer, A.; Oorschot, D. Xenon Combined With Hypothermia in Perinatal Hypoxic-Ischemic Encephalopathy: A Noble Gas, a Noble Mission. Pediatr. Neurol. 2018, 84, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, C.; Chio, I.; Tuveson, D. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef] [PubMed]
- Hagan, S.; Khurana, N.; Chandra, S.; Abdel-Mageed, A.; Mondal, D.; Hellstrom, W.; Sikka, S. Differential expression of novel biomarkers (TLR-2, TLR-4, COX-2, and Nrf-2) of inflammation and oxidative stress in semen of leukocytospermia patients. Andrology 2015, 3, 848–855. [Google Scholar] [CrossRef]
- Thanas, C.; Ziros, P.; Chartoumpekis, D.; Renaud, C.; Sykiotis, G. The Keap1/Nrf2 Signaling Pathway in the Thyroid-2020 Update. Antioxidants 2020, 9, 1082. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Kensler, T.; Motohashi, H. The Keap1-Nrf2 System: A Thiol-Based Sensor-Effector Apparatus for Maintaining Redox Homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef]
- Khodakarami, A.; Adibfar, S.; Karpisheh, V.; Abolhasani, S.; Jalali, P.; Mohammadi, H.; Navashenaq, J.G.; Mohammad Hojjat-Farsangi, M.; Jadidi-Niaragh, F. The molecular biology and therapeutic potential of Nrf2 in leukemia. Cancer Cell Int. 2022, 22, 241. [Google Scholar]
- Saeed, S.; Shad, K.; Saleem, T.; Javed, F.; Khan, M. Some new prospects in the understanding of the molecular basis of the pathogenesis of stroke. Exp. Brain Res. 2007, 182, 1–10. [Google Scholar] [CrossRef]
- Mazur, A.; Fangman, M.; Ashouri, R.; Arcenas, A.; Dore, S. Nrf2 as a therapeutic target in ischemic stroke. Expert Opin. Ther. Targets 2021, 25, 163–166. [Google Scholar] [CrossRef]
- Farina, M.; Vieira, L.; Buttari, B.; Profumo, E.; Saso, L. The Nrf2 Pathway in Ischemic Stroke: A Review. Molecules 2021, 26, 5001. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Vollmer, M.; Fernandez, V.; Dweik, Y.; Kim, H.; Dore, S. Korean Red Ginseng Pretreatment Protects Against Long-Term Sensorimotor Deficits After Ischemic Stroke Likely Through Nrf2. Front. Cell. Neurosci. 2018, 12, 74. [Google Scholar] [CrossRef]
- Lee, C.; Yu, H. Role of mitochondria, ROS, and DNA damage in arsenic induced carcinogenesis. Front. Biosci. 2016, 8, 312–320. [Google Scholar]
- De Bont, R.; Van Larebeke, N. Endogenous DNA damage in humans: A review of quantitative data. Mutagenesis 2004, 19, 169–185. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Oxidative stress and neurodegeneration: Where are we now? J. Neurochem. 2006, 97, 1634–1658. [Google Scholar] [CrossRef] [PubMed]
- Bano, T.; Kumar, N.; Dudhe, R. Free radical scavenging properties of pyrimidine derivatives. Org. Med. Chem. Lett. 2012, 2, 34. [Google Scholar] [CrossRef]
- Caputo, F.; Vegliante, R.; Ghibelli, L. Redox modulation of the DNA damage response. Biochem. Pharmacol. 2012, 84, 1292–1306. [Google Scholar] [PubMed]
- Yao, Y.; Miao, W.; Liu, Z.; Han, W.; Shi, K.; Shen, Y.; Li, H.; Liu, Q.; Fu, Y.; Huang, D.; et al. Dimethyl Fumarate and Monomethyl Fumarate Promote Post-Ischemic Recovery in Mice. Transl. Stroke Res. 2016, 7, 535–547. [Google Scholar] [CrossRef]
- Park, H.; Lee, H.; Lee, J.; Yim, N.; Gu, M.; Ma, J. Protective Effects of Spatholobi Caulis Extract on Neuronal Damage and Focal Ischemic Stroke/Reperfusion Injury. Mol. Neurobiol. 2018, 55, 4650–4666. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, R.; Tao, Z.; Yan, F.; Gao, L.; Liu, X.; Wang, N.; Min, L.; Jia, Y.; Zhao, Y.; et al. Activation of T-LAK-cell-originated protein kinase-mediated antioxidation protects against focal cerebral ischemia-reperfusion injury. FEBS J. 2014, 281, 4411–4420. [Google Scholar] [CrossRef]
- Singh, L.; Singh, A.; Bhatti, R. Mechanistic interplay of various mediators involved in mediating the neuroprotective effect of daphnetin. Pharmacol. Rep. 2021, 73, 1220–1229. [Google Scholar] [CrossRef]
- Lee, C.; Yu, L.; Wang, J. Nitroxide antioxidant as a potential strategy to attenuate the oxidative/nitrosative stress induced by hydrogen peroxide plus nitric oxide in cultured neurons. Nitric Oxide 2016, 54, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Kao, T.; Chen, W.; Ou, Y.; Li, J.; Liao, S.; Raung, S.; Chen, C. Tetramethylpyrazine inhibits neutrophil activation following permanent cerebral ischemia in rats. Biochem. Biophys. Res. Commun. 2015, 463, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Michalickova, D.; Hrncir, T.; Canova, N.; Slanar, O. Targeting Keap1/Nrf2/ARE signaling pathway in multiple sclerosis. Eur. J. Pharmacol. 2020, 873, 172973. [Google Scholar] [PubMed]
- Park, Y.; Park, H.; Kim, E.; Lee, H.; Hwang, J.; Jeon, Y.; Lim, Y. The antioxidant effect of preischemic dexmedetomidine in a rat model: Increased expression of Nrf2/HO-1 via the PKC pathway. Braz. J. Anesthesiol. 2021. [Google Scholar] [CrossRef]
- Yu, H.; Wu, Z.; Wang, X.; Gao, C.; Liu, R.; Kang, F.; Dai, M. Protective effects of combined treatment with mild hypothermia and edaravone against cerebral ischemia/reperfusion injury via oxidative stress and Nrf2 pathway regulation. Int. J. Oncol. 2020, 57, 500–508. [Google Scholar] [CrossRef]
- Shi, H.; Jing, X.; Wei, X.; Perez, R.; Ren, M.; Zhang, X.; Lou, H. S-allyl cysteine activates the Nrf2-dependent antioxidant response and protects neurons against ischemic injury in vitro and in vivo. J. Neurochem. 2015, 133, 298–308. [Google Scholar] [CrossRef]
- Ashabi, G.; Khalaj, L.; Khodagholi, F.; Goudarzvand, M.; Sarkaki, A. Pre-treatment with metformin activates Nrf2 antioxidant pathways and inhibits inflammatory responses through induction of AMPK after transient global cerebral ischemia. Metab. Brain Dis. 2015, 30, 747–754. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, M.; Wang, M.; Li, Y.; Wen, A. Posttreatment with 11-Keto-beta-Boswellic Acid Ameliorates Cerebral Ischemia-Reperfusion Injury: Nrf2/HO-1 Pathway as a Potential Mechanism. Mol. Neurobiol. 2015, 52, 1430–1439. [Google Scholar] [CrossRef]
- Sandberg, M.; Patil, J.; D’Angelo, B.; Weber, S.; Mallard, C. Nrf2-regulation in brain health and disease: Implication of cerebral inflammation. Neuropharmacology 2014, 79, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wei, R.; Zhang, L.; Tan, Y.; Qian, C. Sirtuin 6 protects the brain from cerebral ischemia/reperfusion injury through Nrf2 activation. Neuroscience 2017, 366, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, H.; Ji, X.; Zhu, L.; Sun, Q.; Cong, Z.; Zhou, Y.; Liu, H.; Zhou, M. Differential Nrf2 expression between glioma stem cells and non-stem-like cells in glioblastoma. Oncol. Lett. 2014, 7, 693–698. [Google Scholar] [CrossRef]
- Kryszczuk, M.; Kowalczuk, O. Significance of NRF2 in physiological and pathological conditions an comprehensive review. Arch. Biochem. Biophys. 2022, 730, 109417. [Google Scholar] [CrossRef]
- Hayes, J.; Dinkova-Kostova, A. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014, 39, 199–218. [Google Scholar] [CrossRef]
- Horie, Y.; Suzuki, T.; Inoue, J.; Iso, T.; Wells, G.; Moore, T.; Mizushima, T.; Dinkova-Kostova, A.; Kasai, T.; Kamei, T.; et al. Molecular basis for the disruption of Keap1-Nrf2 interaction via Hinge & Latch mechanism. Commun. Biol. 2021, 4, 576. [Google Scholar] [PubMed]
- Wang, T.; Liang, X.; Abeysekera, I.; Iqbal, U.; Duan, Q.; Naha, G.; Lin, L.; Yao, X. Activation of the Nrf2-Keap 1 Pathway in Short-Term Iodide Excess in Thyroid in Rats. Oxid. Med. Cell. Longev. 2017, 2017, 4383652. [Google Scholar] [CrossRef]
- Krajka-Kuzniak, V.; Paluszczak, J.; Baer-Dubowska, W. The Nrf2-ARE signaling pathway: An update on its regulation and possible role in cancer prevention and treatment. Pharmacol. Rep. 2017, 69, 393–402. [Google Scholar] [CrossRef]
- Wang, H.; Liu, K.; Geng, M.; Gao, P.; Wu, X.; Hai, Y.; Li, Y.; Li, Y.; Luo, L.; Hayes, J.; et al. RXRalpha inhibits the Nrf2-ARE signaling pathway through a direct interaction with the Neh7 domain of Nrf2. Cancer Res. 2013, 73, 3097–3108. [Google Scholar] [CrossRef] [PubMed]
- Takagi, T.; Kitashoji, A.; Iwawaki, T.; Tsuruma, K.; Shimazawa, M.; Yoshimura, S.; Iwama, T.; Hara, H. Temporal activation of Nrf2 in the penumbra and Nrf2 activator-mediated neuroprotection in ischemia-reperfusion injury. Free Radic. Biol. Med. 2014, 72, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Christopher, E.; Loan, J.; Samarasekera, N.; McDade, K.; Rose, J.; Barrington, J.; Hughes, J.; Smith, C.; Al-Shahi, S.R. Nrf2 activation in the human brain after stroke due to supratentorial intracerebral haemorrhage: A case-control study. BMJ Neurol. Open 2022, 4, e000238. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A.; Rojo, A.; Wells, G.; Hayes, J.; Cousin, S.; Rumsey, W.; Attucks, O.; Franklin, S.; Levonen, A.; Kensler, T.; et al. Therapeutic targeting of the Nrf2 and Keap1 partnership in chronic diseases. Nat. Rev. Drug Discov. 2019, 18, 295–317. [Google Scholar] [PubMed]
- Rinaldi, T.M.; Bocanegra, V.; Manucha, W.; Gil, L.A.; Valles, P. The Nrf2-Keap1 cellular defense pathway and heat shock protein 70 (Hsp70) response. Role in protection against oxidative stress in early neonatal unilateral ureteral obstruction (UUO). Cell Stress Chaperones 2011, 16, 57–68. [Google Scholar] [CrossRef]
- Li, H.; Wu, S.; Shi, N.; Lian, S.; Lin, W. Nrf2/HO-1 pathway activation by manganese is associated with reactive oxygen species and ubiquitin-proteasome pathway, not MAPKs signaling. J. Appl. Toxicol. 2011, 31, 690–697. [Google Scholar] [CrossRef]
- Jansen, T.; Hortmann, M.; Oelze, M.; Opitz, B.; Steven, S.; Schell, R.; Knorr, M.; Karbach, S.; Schuhmacher, S.; Wenzel, P.; et al. Conversion of biliverdin to bilirubin by biliverdin reductase contributes to endothelial cell protection by heme oxygenase-1-evidence for direct and indirect antioxidant actions of bilirubin. J. Mol. Cell. Cardiol. 2010, 49, 186–195. [Google Scholar] [CrossRef]
- Fuse, Y.; Kobayashi, M. Conservation of the Keap1-Nrf2 System: An Evolutionary Journey through Stressful Space and Time. Molecules 2017, 22, 436. [Google Scholar] [CrossRef]
- Pardo, M.; Qiu, X.; Zimmermann, R.; Rudich, Y. Particulate Matter Toxicity Is Nrf2 and Mitochondria Dependent: The Roles of Metals and Polycyclic Aromatic Hydrocarbons. Chem. Res. Toxicol. 2020, 33, 1110–1120. [Google Scholar] [CrossRef]
- Singh, S.; Nagalakshmi, D.; Sharma, K.; Ravichandiran, V. Natural antioxidants for neuroinflammatory disorders and possible involvement of Nrf2 pathway: A review. Heliyon 2021, 7, e06216. [Google Scholar] [CrossRef]
- Tu, W.; Wang, H.; Li, S.; Liu, Q.; Sha, H. The Anti-Inflammatory and Anti-Oxidant Mechanisms of the Keap1/Nrf2/ARE Signaling Pathway in Chronic Diseases. Aging Dis. 2019, 10, 637–651. [Google Scholar] [CrossRef]
- Cederbaum, A. Nrf2 and antioxidant defense against CYP2E1 toxicity. Subcell Biochem. 2013, 67, 105–130. [Google Scholar] [PubMed]
- Novo, E.; Parola, M. Redox mechanisms in hepatic chronic wound healing and fibrogenesis. Fibrogenesis Tissue Repair. 2008, 1, 5. [Google Scholar]
- Niizuma, K.; Yoshioka, H.; Chen, H.; Kim, G.; Jung, J.; Katsu, M.; Okami, N.; Chan, P. Mitochondrial and apoptotic neuronal death signaling pathways in cerebral ischemia. Biochim. Biophys. Acta 2010, 1802, 92–99. [Google Scholar] [CrossRef]
- Iyer, M.; Cinar, R.; Katz, A.; Gao, M.; Erdelyi, K.; Jourdan, T.; Coffey, N.; Pacher, P.; Kunos, G. Design, Synthesis, and Biological Evaluation of Novel, Non-Brain-Penetrant, Hybrid Cannabinoid CB1R Inverse Agonist/Inducible Nitric Oxide Synthase (iNOS) Inhibitors for the Treatment of Liver Fibrosis. J. Med. Chem. 2017, 60, 1126–1141. [Google Scholar] [CrossRef] [PubMed]
- Cerkezkayabekir, A.; Sanal, F.; Bakar, E.; Ulucam, E.; Inan, M. Naringin protects viscera from ischemia/reperfusion injury by regulating the nitric oxide level in a rat model. Biotech. Histochem. 2017, 92, 252–263. [Google Scholar] [CrossRef]
- Yao, X.; Carlson, D.; Sun, Y.; Ma, L.; Wolf, S.; Minei, J.; Zang, Q. Mitochondrial ROS Induces Cardiac Inflammation via a Pathway through mtDNA Damage in a Pneumonia-Related Sepsis Model. PLoS ONE 2015, 10, e0139416. [Google Scholar] [CrossRef] [PubMed]
- Enzmann, G.; Kargaran, S.; Engelhardt, B. Ischemia-reperfusion injury in stroke: Impact of the brain barriers and brain immune privilege on neutrophil function. Ther. Adv. Neurol. Disord. 2018, 11, 1756286418794184. [Google Scholar] [CrossRef]
- Su, X.; Wang, L.; Ma, S.; Cao, Y.; Yang, N.; Lin, L.; Fisher, M.; Yang, J.; Liu, C. Mechanisms of Acupuncture in the Regulation of Oxidative Stress in Treating Ischemic Stroke. Oxid. Med. Cell. Longev. 2020, 2020, 7875396. [Google Scholar] [CrossRef]
- Malko, P.; Jiang, L. TRPM2 channel-mediated cell death: An important mechanism linking oxidative stress-inducing pathological factors to associated pathological conditions. Redox Biol. 2020, 37, 101755. [Google Scholar] [CrossRef]
- Radak, D.; Resanovic, I.; Isenovic, E. Link between oxidative stress and acute brain ischemia. Angiology 2014, 65, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yang, J.; Lin, T.; Yang, D. Emerging Roles of Sestrins in Neurodegenerative Diseases: Counteracting Oxidative Stress and Beyond. J. Clin. Med. 2019, 8, 1001. [Google Scholar] [CrossRef]
- Guo, J.; Ma, L.; Shi, H.; Zhu, J.; Wu, J.; Ding, Z.; An, Y.; Zou, Y.; Ge, J. Alginate Oligosaccharide Prevents Acute Doxorubicin Cardiotoxicity by Suppressing Oxidative Stress and Endoplasmic Reticulum-Mediated Apoptosis. Mar. Drugs 2016, 14, 231. [Google Scholar] [CrossRef]
- Zuo, M.; Wang, A.; Song, G.; Yang, Z. miR-652 protects rats from cerebral ischemia/reperfusion oxidative stress injury by directly targeting NOX2. Biomed. Pharmacother. 2020, 124, 109860. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Xiang, Y.; Zuo, M.; Mao, L.; Hu, G.; Song, G.; Sheikh, M.; Tan, L. lncRNA PINK1-AS Aggravates Cerebral Ischemia/Reperfusion Oxidative Stress Injury through Regulating ATF2 by Sponging miR-203. Oxid. Med. Cell. Longev. 2022, 2022, 1296816. [Google Scholar] [CrossRef]
- Raval, A.; Dave, K.; DeFazio, R.; Perez-Pinzon, M. epsilonPKC phosphorylates the mitochondrial K(+) (ATP) channel during induction of ischemic preconditioning in the rat hippocampus. Brain Res. 2007, 1184, 345–353. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cadenas, E.; Davies, K. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic. Biol. Med. 2000, 29, 222–230. [Google Scholar] [CrossRef]
- Masiero, S.; Celia, A.; Rosati, G.; Armani, M. Robotic-assisted rehabilitation of the upper limb after acute stroke. Arch. Phys. Med. Rehabil. 2007, 88, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Orrenius, S.; Gogvadze, V.; Zhivotovsky, B. Mitochondrial oxidative stress: Implications for cell death. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 143–183. [Google Scholar] [CrossRef]
- Sanderson, T.; Reynolds, C.; Kumar, R.; Przyklenk, K.; Huttemann, M. Molecular mechanisms of ischemia-reperfusion injury in brain: Pivotal role of the mitochondrial membrane potential in reactive oxygen species generation. Mol. Neurobiol. 2013, 47, 9–23. [Google Scholar]
- Halestrap, A. Calcium, mitochondria and reperfusion injury: A pore way to die. Biochem. Soc. Trans. 2006, 34, 232–237. [Google Scholar] [CrossRef]
- Cai, Z.; Zeng, W.; Tao, K.; Lu, F.; Gao, G.; Yang, Q. Myricitrin alleviates MPP(+)-induced mitochondrial dysfunction in a DJ-1-dependent manner in SN4741 cells. Biochem. Biophys. Res. Commun. 2015, 458, 227–233. [Google Scholar] [CrossRef]
- Qu, Y.; Zhang, H.; Zhang, X.; Jiang, H. Arachidonic acid attenuates brain damage in a rat model of ischemia/reperfusion by inhibiting inflammatory response and oxidative stress. Hum. Exp. Toxicol. 2018, 37, 135–141. [Google Scholar] [CrossRef]
- Janyou, A.; Wicha, P.; Jittiwat, J.; Suksamrarn, A.; Tocharus, C.; Tocharus, J. Dihydrocapsaicin Attenuates Blood Brain Barrier and Cerebral Damage in Focal Cerebral Ischemia/Reperfusion via Oxidative Stress and Inflammatory. Sci. Rep. 2017, 7, 10556. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Zhang, Z.; Zhao, Y. Acteoside Attenuates Oxidative Stress and Neuronal Apoptosis in Rats with Focal Cerebral Ischemia-Reperfusion Injury. Biol. Pharm. Bull. 2018, 41, 1645–1651. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, M.; Ju, J.; Wang, Y.; Liu, Z.; Zhao, X.; Yan, Y.; Yan, S.; Luo, X.; Fang, Y. Schizandrin A protects against cerebral ischemia-reperfusion injury by suppressing inflammation and oxidative stress and regulating the AMPK/Nrf2 pathway regulation. Am. J. Transl. Res. 2019, 11, 199–209. [Google Scholar]
- Li, Y.; Meng, F. Effects of icariside II on brain tissue oxidative stress and Nrf2/HO-1 expression in rats with cerebral ischemia-reperfusion injury1. Acta Cir. Bras. 2019, 34, e201900208. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Hu, L.; Chen, Y. Rutaecarpine may improve neuronal injury, inhibits apoptosis, inflammation and oxidative stress by regulating the expression of ERK1/2 and Nrf2/HO-1 pathway in rats with cerebral ischemia-reperfusion injury. Drug Des. Dev. Ther. 2019, 13, 2923–2931. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Lu, H.; Qin, J.; Qu, S.; Wang, W.; Guo, Y.; Liao, W.; Song, M.; Chen, J.; Wang, Y. Biochanin A Provides Neuroprotection Against Cerebral Ischemia/Reperfusion Injury by Nrf2-Mediated Inhibition of Oxidative Stress and Inflammation Signaling Pathway in Rats. Med. Sci. Monit. 2019, 25, 8975–8983. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, X.; Wu, H.; Yang, Z.; Fei, L.; Zhu, J. Theaflavin attenuates cerebral ischemia/reperfusion injury by abolishing miRNA1283pmediated Nrf2 inhibition and reducing oxidative stress. Mol. Med. Rep. 2019, 20, 4893–4904. [Google Scholar]
- Song, Y.; Wang, L.; Bei, Y.; Qin, D.; Ai, L.; Ma, Q.; Lin, P. Carvacryl acetate, a semisynthetic monoterpenic ester obtained from essential oils, provides neuroprotection against cerebral ischemia reperfusion-induced oxidative stress injury via the Nrf2 signalling pathway. Food Funct. 2020, 11, 1754–1763. [Google Scholar] [CrossRef]
- Shalavadi, M.; Chandrashekhar, V.; Muchchandi, I. Neuroprotective effect of Convolvulus pluricaulis Choisy in oxidative stress model of cerebral ischemia reperfusion injury and assessment of MAP2 in rats. J. Ethnopharmacol. 2020, 249, 112393. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Piao, X.; Wu, Y.; Liang, S.; Han, F.; Liang, Q.; Shao, S.; Zhao, D. Cepharanthine attenuates cerebral ischemia/reperfusion injury by reducing NLRP3 inflammasome-induced inflammation and oxidative stress via inhibiting 12/15-LOX signaling. Biomed. Pharmacother. 2020, 127, 110151. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xu, J. Sanggenon C Ameliorates Cerebral Ischemia-Reperfusion Injury by Inhibiting Inflammation and Oxidative Stress through Regulating RhoA-ROCK Signaling. Inflammation 2020, 43, 1476–1487. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Yin, G.; Hu, Y.; Shi, S.; Jiang, J.; Song, X.; Zhang, Z.; Wei, Z.; Tang, C.; Lyu, H. Coicis semen protects against focal cerebral ischemia-reperfusion injury by inhibiting oxidative stress and promoting angiogenesis via the TGFbeta/ALK1/Smad1/5 signaling pathway. Aging 2020, 13, 877–893. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, W.; Liu, J.; Cui, Y.; Cui, J. Piceatannol protects against cerebral ischemia/reperfusioninduced apoptosis and oxidative stress via the Sirt1/FoxO1 signaling pathway. Mol. Med. Rep. 2020, 22, 5399–5411. [Google Scholar] [CrossRef]
- Kang, Y.; Sun, Y.; Li, T.; Ren, Z. Garcinol protects against cerebral ischemia-reperfusion injury in vivo and in vitro by inhibiting inflammation and oxidative stress. Mol. Cell. Probes 2020, 54, 101672. [Google Scholar] [CrossRef]
- Zhang, P.; Cui, J. Neuroprotective Effect of Fisetin Against the Cerebral Ischemia-Reperfusion Damage via Suppression of Oxidative Stress and Inflammatory Parameters. Inflammation 2021, 44, 1490–1506. [Google Scholar] [CrossRef]
- Yang, Y.; He, B.; Zhang, X.; Yang, R.; Xia, X.; Chen, L.; Li, R.; Shen, Z.; Chen, P. Geraniin Protects against Cerebral Ischemia/Reperfusion Injury by Suppressing Oxidative Stress and Neuronal Apoptosis via Regulation of the Nrf2/HO-1 Pathway. Oxid. Med. Cell. Longev. 2022, 2022, 2152746. [Google Scholar] [CrossRef]
- Chu, X.; Zhang, L.; Zhou, Y.; Fang, Q. Cucurbitacin B alleviates cerebral ischemia/reperfusion injury by inhibiting NLRP3 inflammasome-mediated inflammation and reducing oxidative stress. Biosci. Biotechnol. Biochem. 2022, 86, 846–854. [Google Scholar] [CrossRef]
- Yao, H.; Zhao, J.; Song, X. Protective effects of fraxin on cerebral ischemia-reperfusion injury by mediating neuroinflammation and oxidative stress through PPAR-gamma/NF-kappaB pathway. Brain Res. Bull. 2022, 187, 49–62. [Google Scholar] [CrossRef]
- Deng, M.; Sun, J.; Peng, L.; Huang, Y.; Jiang, W.; Wu, S.; Zhou, L.; Chung, S.; Cheng, X. Scutellarin acts on the AR-NOX axis to remediate oxidative stress injury in a mouse model of cerebral ischemia/reperfusion injury. Phytomedicine 2022, 103, 154214. [Google Scholar] [CrossRef] [PubMed]
- Uruno, A.; Motohashi, H. The Keap1-Nrf2 system as an in vivo sensor for electrophiles. Nitric Oxide 2011, 25, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Baird, L.; Dinkova-Kostova, A. The cytoprotective role of the Keap1-Nrf2 pathway. Arch. Toxicol. 2011, 85, 241–272. [Google Scholar] [PubMed]
- Zhang, M.; An, C.; Gao, Y.; Leak, R.; Chen, J.; Zhang, F. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog. Neurobiol. 2013, 100, 30–47. [Google Scholar] [PubMed]
- Zborowski, V.; Heck, S.; Vencato, M.; Pinton, S.; Marques, L.; Nogueira, C. Keap1/Nrf2/HO-1 signaling pathway contributes to p-chlorodiphenyl diselenide antidepressant-like action in diabetic mice. Psychopharmacology 2020, 237, 363–374. [Google Scholar] [CrossRef]
- Velichkova, M.; Hasson, T. Keap1 regulates the oxidation-sensitive shuttling of Nrf2 into and out of the nucleus via a Crm1-dependent nuclear export mechanism. Mol. Cell. Biol. 2005, 25, 4501–4513. [Google Scholar] [CrossRef]
- Cuevas, S.; Yang, Y.; Konkalmatt, P.; Asico, L.; Feranil, J.; Jones, J.; Villar, V.; Armando, I.; Jose, P. Role of nuclear factor erythroid 2-related factor 2 in the oxidative stress-dependent hypertension associated with the depletion of DJ-1. Hypertension 2015, 65, 1251–1257. [Google Scholar] [CrossRef]
- Waz, S.; Heeba, G.; Hassanin, S.; Abdel-Latif, R. Nephroprotective effect of exogenous hydrogen sulfide donor against cyclophosphamide-induced toxicity is mediated by Nrf2/HO-1/NF-kappaB signaling pathway. Life Sci. 2021, 264, 118630. [Google Scholar] [CrossRef]
- Liu, Y.; Kern, J.; Walker, J.; Johnson, J.; Schultz, P.; Luesch, H. A genomic screen for activators of the antioxidant response element. Proc. Natl. Acad. Sci. USA 2007, 104, 5205–5210. [Google Scholar] [CrossRef]
- Kwon, J.; Han, E.; Bui, C.; Shin, W.; Lee, J.; Lee, S.; Choi, Y.; Lee, A.; Lee, K.; Park, C.; et al. Assurance of mitochondrial integrity and mammalian longevity by the p62-Keap1-Nrf2-Nqo1 cascade. EMBO Rep. 2012, 13, 150–156. [Google Scholar] [CrossRef]
- Bae, S.; Sung, S.; Oh, S.; Lim, J.; Lee, S.; Park, Y.; Lee, H.; Kang, D.; Rhee, S. Sestrins activate Nrf2 by promoting p62-dependent autophagic degradation of Keap1 and prevent oxidative liver damage. Cell Metab. 2013, 17, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; He, L.; Wang, T.; Hua, W.; Qin, H.; Wang, J.; Wang, L.; Gu, W.; Li, T.; Li, N.; et al. Activation of p62-Keap1-Nrf2 Pathway Protects 6-Hydroxydopamine-Induced Ferroptosis in Dopaminergic Cells. Mol. Neurobiol. 2020, 57, 4628–4641. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Chen, N.; Li, N.; Xu, F.; Wang, W.; Lei, Y.; Shi, J.; Gong, Q. Neuroprotective Effects of Trilobatin, a Novel Naturally Occurring Sirt3 Agonist from Lithocarpus polystachyus Rehd.; Mitigate Cerebral Ischemia/Reperfusion Injury: Involvement of TLR4/NF-kappaB and Nrf2/Keap-1 Signaling. Antioxid. Redox Signal. 2020, 33, 117–143. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Hu, S.; Zhang, C.; Zhou, Q.; Wang, H.; Yang, Y.; Liu, C.; Ding, H. Ginsenoside Rd attenuates cerebral ischemia/reperfusion injury by exerting an anti-pyroptotic effect via the miR-139-5p/FoxO1/Keap1/Nrf2 axis. Int. Immunopharmacol. 2022, 105, 108582. [Google Scholar] [CrossRef]
- Fan, S.; Liu, X.; Wang, Y.; Ren, X.; Liu, Y.; Dong, Y.; Fan, Q.; Wei, J.; Ma, J.; Yu, A.; et al. Thymus quinquecostatus Celak. ameliorates cerebral ischemia-reperfusion injury via dual antioxidant actions: Activating Keap1/Nrf2/HO-1 signaling pathway and directly scavenging ROS. Phytomedicine 2021, 91, 153673. [Google Scholar] [CrossRef]
- Xie, Y.; Shi, X.; Sheng, K.; Han, G.; Li, W.; Zhao, Q.; Jiang, B.; Feng, J.; Li, J.; Gu, Y. PI3K/Akt signaling transduction pathway, erythropoiesis and glycolysis in hypoxia (Review). Mol. Med. Rep. 2019, 19, 783–791. [Google Scholar] [CrossRef]
- Huang, L.; Cao, J.; Cao, L.; Gao, L.; Yang, Y.; Xu, L. Puerarin induces cell apoptosis in human chondrosarcoma cell line SW1353 via inhibition of the PI3K/Akt signaling pathway. Oncol. Lett. 2017, 14, 5585–5590. [Google Scholar] [CrossRef][Green Version]
- Yin, X.; Wang, X.; Fan, Z.; Peng, C.; Ren, Z.; Huang, L.; Liu, Z.; Zhao, K. Hyperbaric Oxygen Preconditioning Attenuates Myocardium Ischemia-Reperfusion Injury Through Upregulation of Heme Oxygenase 1 Expression: PI3K/Akt/Nrf2 Pathway Involved. J. Cardiovasc. Pharmacol. Ther. 2015, 20, 428–438. [Google Scholar] [CrossRef]
- Liu, Q.; Jin, Z.; Xu, Z.; Yang, H.; Li, L.; Li, G.; Li, F.; Gu, S.; Zong, S.; Zhou, J.; et al. Antioxidant effects of ginkgolides and bilobalide against cerebral ischemia injury by activating the Akt/Nrf2 pathway in vitro and in vivo. Cell Stress Chaperones 2019, 24, 441–452. [Google Scholar] [CrossRef]
- Xu, Y.; Han, F.; Tan, J. Edaravone protects the retina against ischemia/reperfusioninduced oxidative injury through the PI3K/Akt/Nrf2 pathway. Mol. Med. Rep. 2017, 16, 9210–9216. [Google Scholar] [CrossRef]
- Wang, J.; Wang, A.; He, H.; She, X.; He, Y.; Li, S.; Liu, L.; Luo, T.; Huang, N.; Luo, H.; et al. Trametenolic acid B protects against cerebral ischemia and reperfusion injury through modulation of microRNA-10a and PI3K/Akt/mTOR signaling pathways. Biomed. Pharmacother. 2019, 112, 108692. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Wu, Y.; Liu, S.; Luo, C.; Lu, Y.; Liu, M.; Wang, L.; Zhang, Y.; Liu, X. Rehmannioside A improves cognitive impairment and alleviates ferroptosis via activating PI3K/AKT/Nrf2 and SLC7A11/GPX4 signaling pathway after ischemia. J. Ethnopharmacol. 2022, 289, 115021. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Song, J.; Yan, R.; Li, L.; Xiao, Z.; Zhou, W.; Wang, Z.; Xiao, W.; Du, G. Diterpene ginkgolides protect against cerebral ischemia/reperfusion damage in rats by activating Nrf2 and CREB through PI3K/Akt signaling. Acta Pharmacol. Sin. 2018, 39, 1259–1272. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Hou, W.; Wu, W.; Zhao, Y.; Dong, X.; Bai, X.; Peng, L.; Song, L. 6’-O-Galloylpaeoniflorin Attenuates Cerebral Ischemia Reperfusion-Induced Neuroinflammation and Oxidative Stress via PI3K/Akt/Nrf2 Activation. Oxid. Med. Cell. Longev. 2018, 2018, 8678267. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.; Xue, L.; Cheng, J.; Bai, Y. Preconditioning of H2S inhalation protects against cerebral ischemia/reperfusion injury by induction of HSP70 through PI3K/Akt/Nrf2 pathway. Brain Res. Bull. 2016, 121, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Choi, E. Compromised MAPK signaling in human diseases: An update. Arch. Toxicol. 2015, 89, 867–882. [Google Scholar] [CrossRef]
- Zhang, H.; Yuan, B.; Huang, H.; Qu, S.; Yang, S.; Zeng, Z. Gastrodin induced HO-1 and Nrf2 up-regulation to alleviate H2O2-induced oxidative stress in mouse liver sinusoidal endothelial cells through p38 MAPK phosphorylation. Braz. J. Med. Biol. Res. 2018, 51, e7439. [Google Scholar] [CrossRef]
- Wang, Z.; Ka, S.; Lee, Y.; Park, B.; Bae, E. Butein induction of HO-1 by p38 MAPK/Nrf2 pathway in adipocytes attenuates high-fat diet induced adipose hypertrophy in mice. Eur. J. Pharmacol. 2017, 799, 201–210. [Google Scholar] [CrossRef]
- Mahli, A.; Saugspier, M.; Koch, A.; Sommer, J.; Dietrich, P.; Lee, S.; Thasler, R.; Schulze-Luehrmann, J.; Luehrmann, A.; Thasler, W.; et al. ERK activation and autophagy impairment are central mediators of irinotecan-induced steatohepatitis. Gut 2018, 67, 746–756. [Google Scholar]
- Sun, G.; Chen, Z.; Jasmer, K.; Chuang, D.; Gu, Z.; Hannink, M.; Simonyi, A. Quercetin Attenuates Inflammatory Responses in BV-2 Microglial Cells: Role of MAPKs on the Nrf2 Pathway and Induction of Heme Oxygenase-1. PLoS ONE 2015, 10, e0141509. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, U.; Kini, S.; Garg, V.; Agrawal, S.; Tomar, P.; Pathak, P.; Chaudhary, A.; Gupta, P.; Malik, A. JNK pathway signaling: A novel and smarter therapeutic targets for various biological diseases. Future Med. Chem. 2015, 7, 2065–2086. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Barrett, T.; Brehm, M.; Davis, R. Inflammation Mediated by JNK in Myeloid Cells Promotes the Development of Hepatitis and Hepatocellular Carcinoma. Cell Rep. 2016, 15, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhu, P.; Fujino, M.; Nishio, Y.; Chen, J.; Ito, H.; Takahashi, K.; Nakajima, M.; Tanaka, T.; Zhao, L. 5-Aminolevulinic acid with sodium ferrous citrate induces autophagy and protects cardiomyocytes from hypoxia-induced cellular injury through MAPK-Nrf-2-HO-1 signaling cascade. Biochem. Biophys. Res. Commun. 2016, 28, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Chi, P.; Lin, C.; Chen, Y.; Hsiao, L.; Yang, C. CO Induces Nrf2-Dependent Heme Oxygenase-1 Transcription by Cooperating with Sp1 and c-Jun in Rat Brain Astrocytes. Mol. Neurobiol. 2015, 52, 277–292. [Google Scholar] [CrossRef] [PubMed]
- Serizawa, F.; Patterson, E.; Potter, R.; Fraser, D.; Cepinskas, G. Pretreatment of human cerebrovascular endothelial cells with CO-releasing molecule-3 interferes with JNK/AP-1 signaling and suppresses LPS-induced proadhesive phenotype. Microcirculation 2015, 22, 28–36. [Google Scholar] [CrossRef]
- Chen, L.; Guo, Y.; Qu, S.; Li, K.; Yang, T.; Yang, Y.; Zheng, Z.; Liu, H.; Wang, X.; Deng, S.; et al. The Protective Effects of Shengmai Formula Against Myocardial Injury Induced by Ultrafine Particulate Matter Exposure and Myocardial Ischemia are Mediated by the PI3K/AKT/p38 MAPK/Nrf2 Pathway. Front. Pharmacol. 2021, 12, 619311. [Google Scholar] [CrossRef]
- Jie, P.; Hong, Z.; Tian, Y.; Li, Y.; Lin, L.; Zhou, L.; Du, Y.; Chen, L.; Chen, L. Activation of transient receptor potential vanilloid 4 induces apoptosis in hippocampus through downregulating PI3K/Akt and upregulating p38 MAPK signaling pathways. Cell Death Dis. 2015, 6, e1775. [Google Scholar] [CrossRef]
- Lu, H.; Wang, B.; Cui, N.; Zhang, Y. Artesunate suppresses oxidative and inflammatory processes by activating Nrf2 and ROS dependent p38 MAPK and protects against cerebral ischemiareperfusion injury. Mol. Med. Rep. 2018, 17, 6639–6646. [Google Scholar]
- Zhang, Z.; Xu, C.; Hao, J.; Zhang, M.; Wang, Z.; Yin, T.; Lin, K.; Liu, W.; Jiang, Q.; Li, Z.; et al. Beneficial consequences of Lupeol on middle cerebral artery-induced cerebral ischemia in the rat involves Nrf2 and P38 MAPK modulation. Metab. Brain Dis. 2020, 35, 841–848. [Google Scholar] [CrossRef]
- Ma, H.; Wang, L.; Lv, W.; Lv, Z. Effects of miR-7 on Hcy-induced rat cerebral arterial vascular smooth muscle cell proliferation, migration and inflammatory factor expression by targeting MMP-14 to regulate TLR4/NF-κB signaling pathway. Cell Mol. Biol. 2020, 66, 12–17. [Google Scholar] [CrossRef]
- Capece, D.; Veraella, D.; Flati, I.; Arboretto, P.; Cornice, J.; Franzoso, G. NF-κB: Blending metabolism, immunity, and inflammation. Trends Immunol. 2022, 43, 757–775. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhong, Z.; Karin, M. NF-κB: A Double-Edged Sword Controlling Inflammation. Biomedicines 2022, 10, 1250. [Google Scholar] [CrossRef] [PubMed]
- Roberti, A.; Chaffey, L.; Greaves, D. NF-κB Signaling and Inflammation-Drug Repurposing to Treat Inflammatory Disorders? Biology 2022, 11, 372. [Google Scholar] [CrossRef] [PubMed]
- Medunjanin, S.; Schleithoff, L.; Fiegehenn, C.; Weinert, S.; Zuschratter, W.; Braun-Dullaeus, R. GSK-3β controls NF-kappaB activity via IKKγ/NEMO. Sci. Rep. 2016, 6, 38553. [Google Scholar] [CrossRef] [PubMed]
- Howell, J.; Bidwell, G. Targeting the NF-κB pathway for therapy of ischemic stroke. Ther. Deliv. 2020, 11, 113–123. [Google Scholar] [CrossRef]
- Wu, X.; Wang, B.; Li, J.; Yang, Z.; Zhou, Y.; Ma, X.; Kou, Z.; Jiang, L.; Song, J. Inhibition of PRMT5 attenuates cerebral ischemia/reperfusion-Induced inflammation and pyroptosis through suppression of NF-kappaB/NLRP3 axis. Neurosci. Lett. 2022, 776, 136576. [Google Scholar] [CrossRef]
- Zhang, L.; Sui, R.; Zhang, L. Fingolimod protects against cerebral ischemia reperfusion injury in rats by reducing inflammatory cytokines and inhibiting the activation of p38 MAPK and NF-kappaB signaling pathways. Neurosci. Lett. 2022, 771, 136413. [Google Scholar] [CrossRef]
- Li, Q.; Tian, Z.; Wang, M.; Kou, J.; Wang, C.; Rong, X.; Li, J.; Xie, X.; Pang, X. Luteoloside attenuates neuroinflammation in focal cerebral ischemia in rats via regulation of the PPARgamma/Nrf2/NF-kappaB signaling pathway. Int. Immunopharmacol. 2019, 66, 309–316. [Google Scholar] [CrossRef]
- He, J.; Zhou, D.; Yan, B. Eriocitrin alleviates oxidative stress and inflammatory response in cerebral ischemia reperfusion rats by regulating phosphorylation levels of Nrf2/NQO-1/HO-1/NF-kappaB p65 proteins. Ann. Transl. Med. 2020, 8, 757. [Google Scholar] [CrossRef]
- Gao, Y.; Hu, M.; Niu, X.; Li, M.; Xu, L.; Xiao, Y.; Zhang, J.; Wang, H.; Li, L.; Chu, B.; et al. Dl-3-n-Butylphthalide Improves Neuroinflammation in Mice with Repeated Cerebral Ischemia-Reperfusion Injury through the Nrf2-Mediated Antioxidant Response and TLR4/MyD88/NF-kappaB Signaling Pathway. Oxid. Med. Cell. Longev. 2022, 2022, 8652741. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.; Cui, L.; Wang, L.; Liu, H.; Ji, H.; Du, Y. Ursolic acid promotes the neuroprotection by activating Nrf2 pathway after cerebral ischemia in mice. Brain Res. 2013, 1497, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, V.; Wagener, F.; Immenschuh, S. The macrophage heme-heme oxygenase-1 system and its role in inflammation. Biochem. Pharmacol. 2018, 153, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [PubMed]
- Paladino, S.; Conte, A.; Caggiano, R.; Pierantoni, G.; Faraonio, R. Nrf2 Pathway in Age-Related Neurological Disorders: Insights into MicroRNAs. Cell. Physiol. Biochem. 2018, 47, 1951–1976. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Weian, C.; Susu, H.; Hanmin, W. Protective effects of mangiferin on cerebral ischemia-reperfusion injury and its mechanisms. Eur. J. Pharmacol. 2016, 771, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.; Cao, G.; Li, R.; Liu, J.; Dong, Z.; Xu, L. beta-Caryophyllene Attenuates Focal Cerebral Ischemia-Reperfusion Injury by Nrf2/HO-1 Pathway in Rats. Neurochem. Res. 2016, 41, 1291–1304. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Chen, M.; Zheng, H.; Li, C.; Yang, F.; Niu, Q. Pelargonidin ameliorates MCAO-induced cerebral ischemia/reperfusion injury in rats by the action on the Nrf2/HO-1 pathway. Transl. Neurosci. 2021, 12, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Maharjan, S.; Wang, Q.; Sun, Y.; Han, X.; Wang, S.; Mao, Z.; Xin, Y.; Zhang, B. alpha-Lipoic Acid Promotes Neurological Recovery After Ischemic Stroke by Activating the Nrf2/HO-1 Pathway to Attenuate Oxidative Damage. Cell. Physiol. Biochem. 2017, 43, 1273–1287. [Google Scholar] [CrossRef]
- Lu, J.; Huang, Q.; Zhang, D.; Lan, T.; Zhang, Y.; Tang, X.; Xu, P.; Zhao, D.; Cong, D.; Zhao, D.; et al. The Protective Effect of DiDang Tang Against AlCl3-Induced Oxidative Stress and Apoptosis in PC12 Cells Through the Activation of SIRT1-Mediated Akt/Nrf2/HO-1 Pathway. Front. Pharmacol. 2020, 11, 466. [Google Scholar] [CrossRef]
- Wang, B.; Cao, W.; Biswal, S.; Dore, S. Carbon monoxide-activated Nrf2 pathway leads to protection against permanent focal cerebral ischemia. Stroke 2011, 42, 2605–2610. [Google Scholar] [CrossRef]
- Hu, Q.; Zuo, T.; Deng, L.; Chen, S.; Yu, W.; Liu, S.; Liu, J.; Wang, X.; Fan, X.; Dong, Z. beta-Caryophyllene suppresses ferroptosis induced by cerebral ischemia reperfusion via activation of the Nrf2/HO-1 signaling pathway in MCAO/R rats. Phytomedicine 2022, 102, 154112. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Alfieri, A.; Siow, R.; Mann, G.; Fraser, P. Temporal and spatial distribution of Nrf2 in rat brain following stroke: Quantification of nuclear to cytoplasmic Nrf2 content using a novel immunohistochemical technique. J. Physiol. 2013, 591, 3525–3538. [Google Scholar] [CrossRef] [PubMed]
- Shih, A.; Li, P.; Murphy, T. A small-molecule-inducible Nrf2-mediated antioxidant response provides effective prophylaxis against cerebral ischemia in vivo. J. Neurosci. 2005, 25, 10321–10335. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Han, Z.; Ji, X.; Luo, Y. Epigenetic Regulation of Oxidative Stress in Ischemic Stroke. Aging Dis. 2016, 7, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.; Kostov, R.; Canning, P. Keap1, the cysteine-based mammalian intracellular sensor for electrophiles and oxidants. Arch. Biochem. Biophys. 2017, 617, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Xu, M.; Wang, Y.; Xie, F.; Zhang, G.; Qin, X. Nrf2-a Promising Therapeutic Target for Defensing Against Oxidative Stress in Stroke. Mol. Neurobiol. 2017, 54, 6006–6017. [Google Scholar] [PubMed]
- Liu, H.; Wu, X.; Luo, J.; Zhao, L.; Li, X.; Guo, H.; Bai, H.; Cui, W.; Guo, W.; Feng, D.; et al. Adiponectin peptide alleviates oxidative stress and NLRP3 inflammasome activation after cerebral ischemia-reperfusion injury by regulating AMPK/GSK-3beta. Exp. Neurol. 2020, 329, 113302. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Liang, J. Activation of the Nrf2 defense pathway contributes to neuroprotective effects of phloretin on oxidative stress injury after cerebral ischemia/reperfusion in rats. J. Neurol. Sci. 2015, 351, 88–92. [Google Scholar] [CrossRef]
- Mei, Z.; Du, L.; Liu, X.; Chen, X.; Tian, H.; Deng, Y.; Zhang, W. Diosmetin alleviated cerebral ischemia/reperfusion injury in vivo and in vitro by inhibiting oxidative stress via the SIRT1/Nrf2 signaling pathway. Food Funct. 2022, 13, 198–212. [Google Scholar] [CrossRef]
- Tobin, K.; Bonds, J.; Minshall, R.; Pelligrino, D.; Testai, F.; Lazarov, O. Neurogenesis and inflammation after ischemic stroke: What is known and where we go from here. J. Cereb. Blood Flow Metab. 2014, 34, 1573–1584. [Google Scholar] [CrossRef]
- Koyama, R.; Shichita, T. Glial roles in sterile inflammation after ischemic stroke. Neurosci. Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Przykaza, Ł. Understanding the Connection Between Common Stroke Comorbidities, Their Associated Inflammation, and the Course of the Cerebral Ischemia/Reperfusion Cascade. Front. Immunol. 2021, 12, 782569. [Google Scholar] [CrossRef] [PubMed]
- Maestrini, I.; Tagzirt, M.; Gautier, S.; Dupont, A.; Mendyk, A.; Susen, S.; Tailleux, A.; Vallez, E.; Staels, B.; Cordonnier, C.; et al. Analysis of the association of MPO and MMP-9 with stroke severity and outcome: Cohort study. Neurology 2020, 95, e97–e108. [Google Scholar] [CrossRef]
- Kang, L.; Yu, H.; Yang, X.; Zhu, Y.; Bai, X.; Wang, R.; Cao, Y.; Xu, H.; Luo, H.; Lu, L.; et al. Neutrophil extracellular traps released by neutrophils impair revascularization and vascular remodeling after stroke. Nat. Commun. 2020, 11, 2488. [Google Scholar] [CrossRef] [PubMed]
- Sakai, S.; Shichita, T. Inflammation and neural repair after ischemic brain injury. Neurochem. Int. 2019, 130, 104316. [Google Scholar] [CrossRef]
- Geetha, T.; Zheng, C.; McGregor, W.; Douglas White, B.; Diaz-Meco, M.T.; Moscat, J.; Babu, J. TRAF6 and p62 inhibit amyloid β-induced neuronal death through p75 neurotrophin receptor. Neurochem. Int. 2012, 61, 1289–1293. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Shoorei, H.; Poornajaf, Y.; Hussen, B.; Hajiesmaeili, Y.; Abak, A.; Taheri, M.; Eghbali, A. NLRP3: Role in ischemia/reperfusion injuries. Front. Immunol. 2022, 13, 926895. [Google Scholar] [CrossRef]
- Bai, R.; Lang, Y.; Shao, J.; Deng, Y.; Refuhati, R.; Cui, L. The Role of NLRP3 Inflammasome in Cerebrovascular Diseases Pathology and Possible Therapeutic Targets. ASN Neuro 2021, 13, 17590914211018100. [Google Scholar] [CrossRef]
- Wang, K.; Yao, Y.; Zhu, X.; Zhang, K.; Zhou, F.; Zhu, L. Amyloid beta induces NLRP3 inflammasome activation in retinal pigment epithelial cells via NADPH oxidase- and mitochondria-dependent ROS production. J. Biochem. Mol. Toxicol. 2017, 31, e21887. [Google Scholar] [CrossRef]
- Ito, M.; Shichita, T.; Okada, M.; Komine, R.; Noguchi, Y.; Yoshimura, A.; Morita, R. Bruton’s tyrosine kinase is essential for NLRP3 inflammasome activation and contributes to ischaemic brain injury. Nat. Commun. 2015, 6, 7360. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, Y.; Chen, N.; Yu, H.; Liu, S.; Qian, M.; Zhang, Z. PHLDA1 Blockade Alleviates Cerebral Ischemia/Reperfusion Injury by Affecting Microglial M1/M2 Polarization and NLRP3 Inflammasome Activation. Neuroscience 2022, 487, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wang, Y.; He, Q.; Li, L.; Xie, H.; Zhao, Y.; Zhao, J. Nrf2 inhibits NLRP3 inflammasome activation through regulating Trx1/TXNIP complex in cerebral ischemia reperfusion injury. Behav. Brain Res. 2018, 336, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhang, S.; Li, C.; Tang, J.; Che, F.; Lu, Y. Roles of the Nrf2/HO-1 pathway in the anti-oxidative stress response to ischemia-reperfusion brain injury in rats. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1532–1540. [Google Scholar] [PubMed]
- Dotson, A.; Chen, Y.; Zhu, W.; Libal, N.; Alkayed, N.; Offner, H. Partial MHC Constructs Treat Thromboembolic Ischemic Stroke Characterized by Early Immune Expansion. Transl. Stroke Res. 2016, 7, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.; Behrouz, R. Impact of Infection on Stroke Morbidity and Outcomes. Curr. Neurol. Neurosci. Rep. 2016, 16, 83. [Google Scholar] [CrossRef] [PubMed]
- Narne, P.; Pandey, V.; Phanithi, P. Interplay between mitochondrial metabolism and oxidative stress in ischemic stroke: An epigenetic connection. Mol. Cell. Neurosci. 2017, 82, 176–194. [Google Scholar] [CrossRef] [PubMed]
- Anne, S.R.; Leak, R.; Gao, Y.; Chen, J. The dynamics of the mitochondrial organelle as a potential therapeutic target. J. Cereb. Blood Flow Metab. 2013, 33, 22–32. [Google Scholar]
- Xavier, J.; Rodrigues, C.; Sola, S. Mitochondria: Major Regulators of Neural Development. Neuroscientist 2016, 22, 346–358. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, S.; Tang, Y.; Mou, C.; Hu, X.; Shao, F.; Yan, W.; Wu, Q. Mitochondrial Fusion and Fission in Neuronal Death Induced by Cerebral Ischemia-Reperfusion and Its Clinical Application: A Mini-Review. Med. Sci. Monit. 2020, 26, e928651. [Google Scholar] [CrossRef]
- Sekerdag, E.; Solaroglu, I.; Gursoy-Ozdemir, Y. Cell Death Mechanisms in Stroke and Novel Molecular and Cellular Treatment Options. Curr. Neuropharmacol. 2018, 16, 1396–1415. [Google Scholar] [CrossRef]
- Anrather, J.; Iadecola, C. Inflammation and Stroke: An Overview. Neurotherapeutics 2016, 13, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Barcena, C.; Mayoral, P.; Quiros, P. Mitohormesis, an Antiaging Paradigm. Int. Rev. Cell Mol. Biol. 2018, 340, 35–77. [Google Scholar] [PubMed]
- Tsushima, M.; Liu, J.; Hirao, W.; Yamazaki, H.; Tomita, H.; Itoh, K. Emerging evidence for crosstalk between Nrf2 and mitochondria in physiological homeostasis and in heart disease. Arch. Pharm. Res. 2020, 43, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Kasai, S.; Yamazaki, H.; Tanji, K.; Engler, M.; Matsumiya, T.; Itoh, K. Role of the ISR-ATF4 pathway and its cross talk with Nrf2 in mitochondrial quality control. J. Clin. Biochem. Nutr. 2019, 64, 1–12. [Google Scholar] [CrossRef]
- Murata, H.; Takamatsu, H.; Liu, S.; Kataoka, K.; Huh, N.; Sakaguchi, M. Nrf2 Regulates PINK1 Expression under Oxidative Stress Conditions. PLoS ONE 2015, 10, e0142438. [Google Scholar] [CrossRef]
- Imhoff, B.; Hansen, J. Extracellular redox status regulates Nrf2 activation through mitochondrial reactive oxygen species. Biochem. J. 2009, 424, 491–500. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W.; Lv, C.; Wang, Y.; Ma, B.; Zhang, H.; Fan, Z.; Li, M.; Li, X. A novel biscoumarin compound ameliorates cerebral ischemia reperfusion-induced mitochondrial oxidative injury via Nrf2/Keap1/ARE signaling. Neuropharmacology 2020, 167, 107918. [Google Scholar] [CrossRef]
- Bahar, E.; Kim, J.; Yoon, H. Quercetin Attenuates Manganese-Induced Neuroinflammation by Alleviating Oxidative Stress through Regulation of Apoptosis, iNOS/NF-kappaB and HO-1/Nrf2 Pathways. Int. J. Mol. Sci. 2017, 18, 1989. [Google Scholar] [CrossRef]
- Armulik, A.; Genove, G.; Mae, M.; Nisancioglu, M.; Wallgard, E.; Niaudet, C.; He, L.; Norlin, J.; Lindblom, P.; Strittmatter, K.; et al. Pericytes regulate the blood-brain barrier. Nature 2010, 468, 557–561. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, L.; Pu, H.; Mao, L.; Hu, X.; Jiang, X.; Xu, N.; Stetler, R.; Zhang, F.; Liu, X.; et al. Rapid endothelial cytoskeletal reorganization enables early blood-brain barrier disruption and long-term ischaemic reperfusion brain injury. Nat. Commun. 2016, 7, 10523. [Google Scholar] [CrossRef]
- Chen, H.; He, Y.; Chen, S.; Qi, S.; Shen, J. Therapeutic targets of oxidative/nitrosative stress and neuroinflammation in ischemic stroke: Applications for natural product efficacy with omics and systemic biology. Pharmacol. Res. 2020, 158, 104877. [Google Scholar] [PubMed]
- Moskowitz, M.; Lo, E.; Iadecola, C. The science of stroke: Mechanisms in search of treatments. Neuron 2010, 67, 181–198. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Kim, Y.; Jeong, S.; Lee, K.; Kim, H.; Park, E. Extracellular signal-regulated kinase1/2-dependent changes in tight junctions after ischemic preconditioning contributes to tolerance induction after ischemic stroke. Brain Struct. Funct. 2015, 220, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Sun, Y.; Mao, L.; Zhang, M.; Li, Q.; Zhang, L.; Shi, Y.; Leak, R.; Chen, J.; Zhang, F. Brain ischemic preconditioning protects against ischemic injury and preserves the blood-brain barrier via oxidative signaling and Nrf2 activation. Redox Biol. 2018, 17, 323–337. [Google Scholar] [CrossRef]
- Haorah, J.; Ramirez, S.; Schall, K.; Smith, D.; Pandya, R.; Persidsky, Y. Oxidative stress activates protein tyrosine kinase and matrix metalloproteinases leading to blood-brain barrier dysfunction. J. Neurochem. 2007, 101, 566–576. [Google Scholar] [CrossRef]
- Gu, Y.; Dee, C.; Shen, J. Interaction of free radicals, matrix metalloproteinases and caveolin-1 impacts blood-brain barrier permeability. Front. Biosci. 2011, 3, 1216–1231. [Google Scholar] [CrossRef]
- Kunze, R.; Urrutia, A.; Hoffmann, A.; Liu, H.; Helluy, X.; Pham, M.; Reischl, S.; Korff, T.; Marti, H. Dimethyl fumarate attenuates cerebral edema formation by protecting the blood-brain barrier integrity. Exp. Neurol. 2015, 266, 99–111. [Google Scholar] [CrossRef]
- Ibbotson, K.; Yell, J.; Ronaldson, P. Nrf2 signaling increases expression of ATP-binding cassette subfamily C mRNA transcripts at the blood-brain barrier following hypoxia-reoxygenation stress. Fluids Barriers CNS 2017, 14, 6. [Google Scholar] [CrossRef]
- Alfieri, A.; Srivastava, S.; Siow, R.; Cash, D.; Modo, M.; Duchen, M.; Fraser, P.; Williams, S.; Mann, G. Sulforaphane preconditioning of the Nrf2/HO-1 defense pathway protects the cerebral vasculature against blood-brain barrier disruption and neurological deficits in stroke. Free Radic. Biol. Med. 2013, 65, 1012–1022. [Google Scholar] [CrossRef]
- Mao, L.; Yang, T.; Li, X.; Lei, X.; Sun, Y.; Zhao, Y.; Zhang, W.; Gao, Y.; Sun, B.; Zhang, F. Protective effects of sulforaphane in experimental vascular cognitive impairment: Contribution of the Nrf2 pathway. J. Cereb. Blood Flow Metab. 2019, 39, 352–366. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, Y.; Liu, B.; Li, C.; Wu, J.; Li, Y. Nomilin protects against cerebral ischemia-reperfusion induced neurological deficits and blood-brain barrier disruption via the Nrf2 pathway. Food Funct. 2019, 10, 5323–5332. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, P.; Huang, F.; Jin, J.; Wu, H.; Zhang, B.; Wang, Z.; Shi, H.; Wu, X. Astragaloside IV protects blood-brain barrier integrity from LPS-induced disruption via activating Nrf2 antioxidant signaling pathway in mice. Toxicol. Appl. Pharmacol. 2018, 340, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.; Lemberg, K.; Lamprecht, M.; Skouta, R.; Zaitsev, E.; Gleason, C.; Patel, D.; Bauer, A.; Cantley, A.; Yang, W.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Ding, W.; Ji, X.; Ao, X.; Liu, Y.; Yu, W.; Wang, J. Molecular mechanisms of ferroptosis and its role in cancer therapy. J. Cell. Mol. Med. 2019, 23, 4900–4912. [Google Scholar]
- Yu, Y.; Yan, Y.; Niu, F.; Wang, Y.; Chen, X.; Su, G.; Liu, Y.; Zhao, X.; Qian, L.; Liu, P.; et al. Ferroptosis: A cell death connecting oxidative stress, inflammation and cardiovascular diseases. Cell Death Discov. 2021, 7, 193. [Google Scholar]
- Li, J.; Cao, F.; Yin, H.; Huang, Z.; Lin, Z.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, present and future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef]
- DeGregorio-Rocasolano, N.; Marti-Sistac, O.; Gasull, T. Deciphering the Iron Side of Stroke: Neurodegeneration at the Crossroads Between Iron Dyshomeostasis, Excitotoxicity, and Ferroptosis. Front. Neurosci. 2019, 13, 85. [Google Scholar]
- Tuo, Q.; Lei, P.; Jackman, K.; Li, X.; Xiong, H.; Li, X.; Liuyang, Z.; Roisman, L.; Zhang, S.; Ayton, S.; et al. Tau-mediated iron export prevents ferroptotic damage after ischemic stroke. Mol. Psychiatry 2017, 22, 1520–1530. [Google Scholar] [CrossRef]
- Chen, G.; Li, L.; Tao, H. Bioinformatics Identification of Ferroptosis-Related Biomarkers and Therapeutic Compounds in Ischemic Stroke. Front. Neurol. 2021, 12, 745240. [Google Scholar] [CrossRef]
- Lan, B.; Ge, J.; Cheng, S.; Zheng, X.; Liao, J.; He, C.; Rao, Z.; Wang, G. Extract of Naotaifang, a compound Chinese herbal medicine, protects neuron ferroptosis induced by acute cerebral ischemia in rats. J. Integr. Med. 2020, 18, 344–350. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Kerins, M.; Ooi, A. The Roles of Nrf2 in Modulating Cellular Iron Homeostasis. Antioxid. Redox Signal. 2018, 29, 1756–1773. [Google Scholar] [CrossRef]
- Sarutipaiboon, I.; Settasatian, N.; Komanasin, N.; Kukongwiriyapan, U.; Sawanyawisuth, K.; Intharaphet, P.; Senthong, V.; Settasatian, C. Association of Genetic Variations in Nrf2, NQO1, HMOX1, and MT with Severity of Coronary Artery Disease and Related Risk Factors. Cardiovasc. Toxicol. 2020, 20, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Mohsenpour, H.; Pesce, M.; Patruno, A.; Bahrami, A.; Pour, P.; Farzaei, M. A Review of Plant Extracts and Plant-Derived Natural Compounds in the Prevention/Treatment of Neonatal Hypoxic-Ischemic Brain Injury. Int. J. Mol. Sci. 2021, 22, 833. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Wang, J.; Bi, F.; Bai, Z. Diosmetin alleviates cerebral ischemia-reperfusion injury through Keap1-mediated Nrf2/ARE signaling pathway activation and NLRP3 inflammasome inhibition. Environ. Toxicol. 2022, 37, 1529–1542. [Google Scholar] [CrossRef]
- Xu, L.; Gao, Y.; Hu, M.; Dong, Y.; Xu, J.; Zhang, J.; Lv, P. Edaravone dexborneol protects cerebral ischemia reperfusion injury through activating Nrf2/HO-1 signaling pathway in mice. Fundam. Clin. Pharmacol. 2022, 36, 790–800. [Google Scholar] [CrossRef]
- Li, K.; Jiang, J.; Shi, Z.; Zhan, L.; Peng, L.; Sun, W.; Tang, Y.; Zuo, X.; Xu, E. Neuroprotective Effects of Rhodiola Sacra on Transient Global Cerebral Ischemia Through Activating AMPK/Nrf2 Pathway in Rats. Antioxid. Redox Signal. 2022, 36, 567–591. [Google Scholar] [CrossRef]
- Xu, H.; Shen, J.; Xiao, J.; Chen, F.; Wang, M. Neuroprotective effect of cajaninstilbene acid against cerebral ischemia and reperfusion damages by activating AMPK/Nrf2 pathway. J. Adv. Res. 2021, 34, 199–210. [Google Scholar] [CrossRef]
- Xue, Z.; Zhao, K.; Sun, Z.; Wu, C.; Yu, B.; Kong, D.; Xu, B. Isorhapontigenin ameliorates cerebral ischemia/reperfusion injury via modulating Kinase Cepsilon/Nrf2/HO-1 signaling pathway. Brain Behav. 2021, 11, e02143. [Google Scholar] [CrossRef]
- Tang, C.; Hong, J.; Hu, C.; Huang, C.; Gao, J.; Huang, J.; Wang, D.; Geng, Q.; Dong, Y. Palmatine Protects against Cerebral Ischemia/Reperfusion Injury by Activation of the AMPK/Nrf2 Pathway. Oxid. Med. Cell. Longev. 2021, 2021, 6660193. [Google Scholar] [CrossRef]
- Liu, D.; Wang, H.; Zhang, Y.; Zhang, Z. Protective Effects of Chlorogenic Acid on Cerebral Ischemia/Reperfusion Injury Rats by Regulating Oxidative Stress-Related Nrf2 Pathway. Drug Des. Devel. Ther. 2020, 14, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Liu, M.; Ding, Y.; Yao, M.; Zhu, Y.; Zhao, J.; Cheng, L.; Bai, J.; Wang, F.; Cao, J.; et al. A phenolic amide (LyA) isolated from the fruits of Lycium barbarum protects against cerebral ischemia-reperfusion injury via PKCepsilon/Nrf2/HO-1 pathway. Aging 2019, 11, 12361–12374. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wei, W.; Lan, X.; Liu, N.; Li, Y.; Ma, H.; Sun, T.; Peng, X.; Zhuang, C.; Yu, J. Neuroprotective Effect of Swertiamain on Cerebral Ischemia/Reperfusion Injury by Inducing the Nrf2 Protective Pathway. ACS Chem. Neurosci. 2019, 10, 2276–2286. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Cui, H.; Jia, A.; Jia, S.; Yuan, K. The Protective Effect of the Total Flavonoids of Abelmoschus esculentus L. Flowers on Transient Cerebral Ischemia-Reperfusion Injury Is due to Activation of the Nrf2-ARE Pathway. Oxid. Med. Cell. Longev. 2018, 2018, 8987173. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Cao, J.; Han, J.; Li, J.; Li, Z.; Shi, N.; He, J. Liraglutide Activates the Nrf2/HO-1 Antioxidant Pathway and Protects Brain Nerve Cells against Cerebral Ischemia in Diabetic Rats. Comput. Intell. Neurosci. 2018, 2018, 3094504. [Google Scholar] [CrossRef]



| Medicine | Model (s) | Related Signaling Pathway | Related Mechanism | Effect | Reference |
|---|---|---|---|---|---|
| Dihydrocapsaicin (DHC) | MCAO/R | Activate SOD and GPx, downregulate ROS, NOX2, NOX4, NF-ĸB, NO, and MMP-9 | Attenuate oxidative stress and inflammation | Protective | [73] |
| Acteoside (ACT) | MCAO/R | Decrease ROS and MDA, increase SOD and CAT | Attenuate oxidative stress and neuronal apoptosis | Protective | [74] |
| Schizandrin A (Sch A) | MCAO/R and OGD/R | Downregulate iNOS, COX-2, IL-1β, IL-6, and TNF-α, increase SOD, CAT, HO-1 and NQO-1 | Suppress inflammation and oxidative stress | Protective | [75] |
| Icariside II | MCAO/R | Decrease ROS and MDA, increase SOD, GSH-Px, catalase, Nrf2, and HO-1 | Attenuate oxidative stress | Protective | [76] |
| Rutaecarpine (Rut) | MCAO/R | Alleviate IL-6, IL-1β, LDH, MDA, and ROS, increase IL-4, IL-10, SOD, HO-1, and NQO1 | Alleviate inflammatory response and oxidative stress | Protective | [77] |
| Biochanin A | MCAO/R | Activate SOD, GSH-Px, and HO-1, suppress MDA, NF-kB | Antioxidative and anti-inflammatory actions | Protective | [78] |
| Theaflavin | MCAO/R and OGD/R | Decrease ROS and MDA, increase SOD and GSH-Px | Restore the impaired antioxidant defense system | Protective | [79] |
| Carvacryl acetate (CA) | MCAO/R | Decrease ROS and MDA, increase SOD | Antioxidant stress | Protective | [80] |
| Convolvulus pluricaulis Choisy | BCCA | Increase SOD, catalase, glutathione, and total thiol | Protect against oxidative damage | Protective | [81] |
| Cepharanthine (CEP) | MCAO/R and OGD/R | Decrease ROS and MDA, NLRP3, ASC, and cleaved caspase-1, increase SOD | Inhibit microglia activation, inflammation, and reduce oxidative stress | Protective | [82] |
| Sanggenon C (SC) | MCAO/R and OGD/R | Decrease TNF-α, IL-1β, IL-6, ROS, and MDA, increase SOD | Inhibit inflammation and oxidative stress | Protective | [83] |
| Coicis Semen | MCAO/R and OGD/R | Decrease ROS and MDA, increase SOD, GSH-Px, ZO-1 and occludin, CD31, and VEGF | Inhibit oxidative stress and promote angiogenesis | Protective | [84] |
| Piceatannol (Pic) | MCAO/R | Decrease ROS, increase SOD, CAT, and GSH-Px | Suppress oxidative stress | Protective | [85] |
| Garcinol | MCAO/R and OGD/R | Inhibit IL-1β, IL-6, TNF-α, NF-κB, MDA, and nitric oxide (NO), increase SOD | Attenuate inflammation and oxidative stress | Protective | [86] |
| Fisetin | MCAO/R and OGD/R | Decrease IL-1, TNF-α, iNOS, IL-1β, COX-2, IL-6, and PGE2 | Inhibit inflammation and oxidative stress | Protective | [87] |
| Geraniin | MCAO/R and OGD/R | Decrease LDH, NO, nNOS and MDA, increase SOD | Decrease oxidative stress and neuronal apoptosis | Protective | [88] |
| Cucurbitacin B (CuB) | MCAO/R and OGD/R | Decrease LDH, ROS, and NLRP3 | Inhibit oxidative stress and inflammation | Protective | [89] |
| Fraxin | MCAO/R and OGD/R | Decrease ROS, NF-κB, IKK-β, p38 MAPK, ERK1/2, and Keap1 | Inhibit oxidative stress, inflammatory response, and cell apoptosis | Protective | [90] |
| Scutellarin | tMCAO | Decrease ROS, 4-HNE, 8-OHDG, NT-3, PARP1, NOX1, NOX2, and NOX4 | Suppress oxidative stress | Protective | [91] |
| Medicine | Model (s) | Related Signaling Pathway | Related Mechanism | Effect | Reference |
|---|---|---|---|---|---|
| β-Caryophyllene (BCP) | MCAO/R and OGD/R | Activate Nrf2/HO-1 pathway | Protect against ferroptosis | Protective | [151] |
| Diosmetin | MCAO/R and OGD/R | Activate Keap1/Nrf2/ARE pathway | Attenuate oxidative stress and inflammation | Protective | [215] |
| Dl-3-n-butylphthalide (NBP) | Repeated CIRI | Nrf2-modulated TLR4/MyD88/NF-κB pathway | Antioxidant, antineuroinflammatory | Protective | [140] |
| Edaravone dexborneol | Repeated CIRI | Activate Nrf2/HO-1 pathway | Antioxidant, antineuroinflammatory | Protective | [216] |
| Rhodiola sacra | tGCI | Activate AMPK/Nrf2 pathway | prevent oxidant stress | Protective | [217] |
| Geraniin | MCAO/R and OGD/R | Activate Nrf2/HO-1 pathway | Suppress oxidative stress and neuronal apoptosis. | Protective | [88] |
| Cajaninstilbene acid (CSA) | MCAO/R and OGD/R | Activate AMPK/Nrf2 pathway | Reduce oxidative stress and mitochondrial disfunction | Protective | [218] |
| Thymus quinquecostatus Celak | tMCAO | Activate Keap1/Nrf2/HO-1 pathway | Antioxidant stress | Protective | [105] |
| Isorhapontigenin (ISO) | MCAO/R and OGD/R | Activate PKCε/Nrf2/HO-1 pathway | Protect against oxidative damage | Protective | [219] |
| Palmatine (PAL) | tMCAO | Activate AMPK/Nrf2 pathway | Reduce oxidative stress and inflammatory response | Protective | [220] |
| Pelargonidin | MCAO/R | Activate Nrf2/HO-1 pathway | Reduce oxidative stress and inflammatory response | Protective | [147] |
| Eriocitrin | MCAO/R | Activate Nrf2/HO-1/NQO1/NF-κB pathway | Attenuate oxidative injury and inflammatory response | Protective | [139] |
| Lupeol | MCAO/R | Involve Nrf2 and P38 MAPK modulation | Suppress oxidative stress and inflammatory response | Protective | [129] |
| A biscoumarin compound COM 3 | MCAO/R and OGD/R | Modulate Nrf2/Keap1/ARE pathway | Antioxidant stress | Protective | [187] |
| Chlorogenic acid (CGA) | CI/R model | Activate Nrf2/HO-1 pathway | Regulate oxidative stress | Protective | [221] |
| Lyciumamide A (LyA) | MCAO/R and OGD/R | Activate PKCε/Nrf2/HO-1 pathway | Ameliorate oxidative damage and neuronal apoptosis | Protective | [222] |
| Nomilin | MCAO/R and OGD/R | Activate Nrf2/NQO1 pathway | Mitigate oxidative stress | Protective | [201] |
| Rutaecarpine (Rut) | MCAO/R | Activate Nrf2/HO-1 pathway | Inhibit apoptosis, inflammation, and oxidative stress | Protective | [77] |
| Swertiamarin (Swe) | MCAO/R and OGD/R | Activate Nrf2/HO-1 pathway | Suppress oxidative stress | Protective | [223] |
| Ginkgolides and bilobalide | MCAO/R and OGD/R | Mediate Akt/Nrf2 pathway | Inhibit oxidative stress | Protective | [109] |
| Icariside II | MCAO/R | Activate Nrf2/HO-1 pathway | Inhibit oxidative stress | Protective | [76] |
| Schizandrin A (Sch A) | MCAO/R and OGD/R | Regulate AMPK/Nrf2 pathway | Suppress inflammation and oxidative stress | Protective | [75] |
| Luteoloside | MCAO/R | Regulate PPARγ/Nrf2/NF-κB pathway | Inhibit neuroinflammation | Protective | [138] |
| Total Flavonoids from A. esculentus | TCI-RI model | Activate Nrf2-ARE pathway | Inhibit oxidative stress | Protective | [224] |
| Diterpene ginkgolides | MCAO/R and OGD/R | Activate PI3K/Akt/Nrf2 pathway | Inhibit oxidative stress | Protective | [113] |
| Artesunate | CI/RI model | Activate MAPK/Nrf2 pathway | Suppress oxidative stress and inflammatory process | Protective | [128] |
| 6’-O-galloylpaeoniflorin (GPF) | MCAO/R and OGD/R | Activate PI3K/Akt/Nrf2 pathway | Prevent oxidative stress, inflammation, and apoptosis | Protective | [114] |
| Liraglutide | MCAO/R | Activate Nrf2/HO-1 pathway | Antioxidant stress | Protective | [225] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Zhang, X.; Xiong, X.; Zhu, H.; Chen, R.; Zhang, S.; Chen, G.; Jian, Z. Nrf2 Regulates Oxidative Stress and Its Role in Cerebral Ischemic Stroke. Antioxidants 2022, 11, 2377. https://doi.org/10.3390/antiox11122377
Wang L, Zhang X, Xiong X, Zhu H, Chen R, Zhang S, Chen G, Jian Z. Nrf2 Regulates Oxidative Stress and Its Role in Cerebral Ischemic Stroke. Antioxidants. 2022; 11(12):2377. https://doi.org/10.3390/antiox11122377
Chicago/Turabian StyleWang, Lei, Xu Zhang, Xiaoxing Xiong, Hua Zhu, Ran Chen, Shudi Zhang, Gang Chen, and Zhihong Jian. 2022. "Nrf2 Regulates Oxidative Stress and Its Role in Cerebral Ischemic Stroke" Antioxidants 11, no. 12: 2377. https://doi.org/10.3390/antiox11122377
APA StyleWang, L., Zhang, X., Xiong, X., Zhu, H., Chen, R., Zhang, S., Chen, G., & Jian, Z. (2022). Nrf2 Regulates Oxidative Stress and Its Role in Cerebral Ischemic Stroke. Antioxidants, 11(12), 2377. https://doi.org/10.3390/antiox11122377







