Abstract
Phloroglucinol, a phenolic compound, is known to possess a potent antioxidant ability. However, its role in retinal cells susceptible to oxidative stress has not been well elucidated yet. Thus, the objective of this study was to evaluate whether phloroglucinol could protect against oxidative damage in cultured human retinal pigment epithelium ARPE-19 cells. For this purpose, ARPE-19 cells were stimula ted with hydrogen peroxide (H2O2) to mimic oxidative stress. Cell viability, cytotoxicity, apoptosis, reactive oxygen species (ROS) generation, mitochondrial function, DNA damage, and autophagy were then assessed. Our results revealed that phloroglucinol ameliorated cell viability, cytotoxicity, and DNA damage in H2O2-exposued ARPE-19 cells and blocked production of ROS. Phloroglucinol also counteracted H2O2-induced apoptosis by reducing Bax/Bcl-2 ratio, blocking activation of caspase-3, and inhibiting degradation of poly (ADP-ribose) polymerase. H2O2 caused mitochondrial impairment and increased expression levels of mitophagy markers such as PINK1and PARKIN known to be associated with mitochondrial ROS (mtROS) generation and cytosolic release of cytochrome c. However, these changes were significantly attenuated by phloroglucinol. Mito-TEMPO, a selective mitochondrial antioxidant, further enhanced the protective effect of phloroglucinol against dysfunctional mitochondria. Furthermore, H2O2 induced autophagy, but not when ARPE-19 cells were pretreated with phloroglucinol, meaning that autophagy by H2O2 contributed to the pro-survival mechanism and that phloroglucinol protected ARPE-19 cells from apoptosis by blocking autophagy. Taken together, these results suggest that phloroglucinol can inhibit oxidative stress-induced ARPE-19 cell damage and dysfunction by protecting DNA damage, autophagy, and subsequent apoptosis through mitigation of mtROS generation. Thus, phloroglucinol might have therapeutic potential to prevent oxidative stress-mediated damage in RPE cells.
1. Introduction
The retina expends excessive energy for the formation of visual perception. It is very sensitive to oxidative stress. At the same time, the retina serves as a powerful generator of reactive oxygen species (ROS) implicated in several major retinal diseases, including age-related macular degeneration (AMD), a leading cause of vision loss [1,2]. Although the etiology and mechanism of AMD induction remain unclear, oxidative stress-related injury to the retinal pigment epithelium (RPE) is recognized as an early event in in AMD-like pathology [3,4]. Appropriate levels of intracellular ROS including mitochondrial ROS (mtROS) play important physiological roles as modulators of cellular signaling pathways. However, excessive accumulation of ROS by persistent oxidative stress can lead to cellular injury and death and contribute to the initiation of pathological damage to various organs, including eyes [5,6]. In addition, apoptosis and autophagy of RPE cells due to excessive ROS production are accompanied by mitochondrial and DNA damage, ultimately contributing to retina dysfunction [7,8]. Furthermore, since mitochondrial damage in RPE degeneration can induce a cellular defense mechanism known as mitophagy, mitophagy could be a putative therapeutic target in retinal degenerative diseases such as AMD [9,10]. Therefore, the level of ROS must be tightly controlled to protect normal functions of eyes.
Natural resources have long received great attention as sources of drug development. Among them, phenolic compounds derived from natural products having excellent antioxidant activity have attracted attention. Their antioxidant activities mainly involve scavenging of ROS and activation of intracellular antioxidant signaling pathways [5,11,12]. Phloroglucinol, a polyphenol trihydroxybenzene with an aromatic phenyl ring and three hydroxyl groups, is a naturally occurring secondary metabolite present in a variety of organisms including plants, algae, and bacteria [13,14]. This phenolic compound is known to have various pharmacological potentials such as antibacterial, anticonvulsant, anti-allergic, antithrombotic, anti-inflammatory, and cancer chemopreventive activities [15,16]. Recently, the antioxidant potential of phloroglucinol has been validated in several in vitro and in vivo models. For example, Drygalski et al. [17] have reported that phloroglucinol can strengthen antioxidant defense and ameliorate hepatic steatosis and inflammatory response by reducing oxidative/nitrogen damage to cellular macromolecules. In addition, it has been confirmed that phloroglucinol can block oxidative damage caused by hydrogen peroxide (H2O2) treatment and γ-ray irradiation by regulating activities of antioxidant and detoxifying enzymes in the retinal epithelium, hippocampal nerve, renal epithelial cells, and lung fibroblasts [18,19,20]. Moreover, phloroglucinol as an ROS scavenger can modulate synaptic plasticity to attenuate pathological phenomena of neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease [21,22]. Our previous study has shown that phloroglucinol can inhibit DNA damage and apoptosis in H2O2-exposed HaCaT human keratinocytes [23]. Similar results have been confirmed in ultraviolet (UV) B-irradiated keratinocytes and all-trans-retinal-exposed primary rat RPE and mouse photoreceptor cells [19,24]. Recently, Kuo et al. [25] have reported that phloroglucinol can block oxidative cytotoxicity induced by potassium bromate, an AMD inducer, in human RPE ARPE-19 cells by inhibiting ROS production. These results suggest that phloroglucinol could play an antioxidant role in ARPE-19 cells as suggested by Moine et al. [26]. Nevertheless, studies on the protective role of phloroglucinol against cellular damage induced by oxidative stress in RPE cells are lacking. Therefore, the purpose of the current study was to investigate effects of phloroglucinol on oxidative stress-induced mitochondrial and DNA damage and induction of apoptosis and autophagy in RPE cells. For this purpose, a human RPE-derived ARPE-19 cell model treated with H2O2 to mimic oxidative stress was used.
2. Materials and Methods
2.1. Cell Culture and Treatment
ARPE-19 cells (CRL-2302) were purchased from the American Type Culture Collection (Manassas, VA, USA) and routinely cultured in Dulbecco’s Modified Eagle Medium/F-12 supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin (WELGENE Inc., Gyeongsan, Republic of Korea) as described previously [27]. To investigate beneficial effects of phloroglucinol on oxidative damage, cells were cultured in media containing desired concentrations of phloroglucinol and H2O2 (Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 24 h or pretreated with phloroglucinol, N-acetyl-L-cysteine (NAC), Mito-TEMPO, and/or 3-methyladenine (3-MA, Sigma-Aldrich Co., St. Louis, MO, USA) for 1 h prior to treatment with H2O2 for 24 h. To investigate the blocking effect of phloroglucinol on the generation of ROS induced by H2O2, cells were pretreated with phloroglucinol, NAC, and Mito-TEMPO for 1 h and then treated with H2O2 for 1 h. To acquire fluorescence images of ROS generation, γH2AX expression, and autophagic vacuoles, cells cultured on coverslips were stimulated with H2O2 in the presence or absence of phloroglucinol, NAC, and/or Mito-TEMPO. After treatment, cells were washed with phosphate-buffered saline and subjected to fluorescence staining.
2.2. Cell Viability Assay
To investigate viability of ARPE-19 cells cultured under various conditions, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetra-zolium bromide (MTT) assay was performed. In brief, after the necessary experimental treatment, cells were incubated with MTT solution (Thermo Fisher Scientific, Inc.) for 3 h. Formed insoluble formazan products were then dissolved in dimethyl sulfoxide (DMSO, Thermo Fisher Scientific, Inc.) and the absorbance was read at 570 nm using an enzyme-linked immunosorbent assay (ELISA) microplate reader (Molecular Device Co., Sunnyvale, CA, USA) according to a previously described method [28]. Cell viability was expressed as a percentage of untreated control cells.
2.3. Cytotoxicity Assay
To assess cytotoxicity, lactate dehydrogenase (LDH) release was detected using an LDH Cytotoxicity Assay Kit (Thermo Fisher Scientific, Inc.) according to the manufacturer’s instructions. In brief, culture medium obtained from conditions treated with H2O2 in the presence or absence of phloroglucinol was transferred to a 96-well plate and the amount of released LDH was measured at 490 nm with an ELISA microplate reader.
2.4. Quantitative Assessment of Apoptosis
Annexin V-Fluorescein Isothiocyanate (FITC) Apoptosis Detection Kit was purchased from Abcam Inc. (Cambridge, UK) and used for quantitative evaluation of apoptosis-induced cells upon treatment with phloroglucinol and/or H2O2. After treatment, collected cells were suspended in annexin binding buffer containing annexin V- FITC and propidium Iodide (PI) following the manufacturer’s instructions. The fluorescence of 10,000 events was then acquired using a flow cytometer (Becton Dickinson, San Jose, CA, USA). Annexin V-positive cells were considered as apoptosis-induced cells as described previously [27].
2.5. DNA Fragmentation Assay
To observe fragmented DNA, an apoptosis marker, cell pellet was suspended in a lysis solution as described previously [29]. The supernatant was then incubated with RNase A and proteinase K (Sigma-Aldrich Co.). DNA was precipitated with isopropyl alcohol (Sigma-Aldrich Co.). The extracted DNA was fractionated using 1.0% agarose gel and then stained with ethidium bromide (EtBr, Thermo Fisher Scientific, Inc.) to visualize DNA fragmentation pattern, a characteristic of apoptosis, under UV light.
2.6. Protein Isolation and Western Blotting
Total protein to be used for Western blot analysis was extracted as described previously [30]. Cytoplasmic and mitochondrial proteins were isolated using a Mitochondrial Fractionation Kit (Thermo Fisher Scientific, Inc.) following the manufacturer’s instructions. In brief, proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to Immobilon®-P PVDF membranes (Merck Millipore, Bedford, MA, USA). These membranes were then incubated with specific primary antibodies overnight followed by reaction with horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) at room temperature for 1 h. Immune complexes were visualized with enhanced chemiluminescence reagent (Thermo Fisher Scientific, Inc.) according to the manufacturer’s instruction [31]. Densitometric analysis of the data was performed using the ImageJ® software (v1.48, NIH, Bethesda, MD, USA). Primary antibodies were obtained from Santa Cruz Biotechnology, Inc. and Cell Signaling Technology (Beverly, MA, USA). All antibodies used in this study are listed in Table 1.

Table 1.
List of antibodies used in this study.
2.7. Caspase-3 Activity Assay
Caspase 3 activity was quantified using a Caspase-3 Colorimetric Assay Kit (Abcam, Inc.). In brief, aliquots of cytosolic extracts were mixed with a fluorescent substrate of caspase-3, acetyl-Asp-Glu-Val-Asp-chromophore-p-nitroanilide (Ac-DVAD-pNa), in the buffer provided in the kit according to the manufacturer’s instructions. Enzyme-catalyzed release of pNa was monitored at 405 nm using an ELISA microplate reader. The activity of caspase-3 was presented relative to the control [32].
2.8. Assessment of ROS Generation
Levels of intracellular ROS and mtROS production were detected using fluorescent probes 2′,7′-dichlorofluorescein diacetate (DCF-DA) and MitoSOX (Sigma-Aldrich Co.), respectively. Following exposure to H2O2 with or without phloroglucinol, NAC, and/or Mito-TEMPO, cells were reacted with DCF-DA and MitoSOX to assess levels of intracellular and mitochondrial peroxides, respectively, using flow cytometry. In parallel, fluorescence images of DCF-DA- and MitoSOX-stained cells cultured on coverslips were observed under a fluorescence microscope (Carl Zeiss, Oberkochen, Germany) at Core-Facility Center for Tissue Regeneration, Dong-eui University (Busan, Republic of Korea).
2.9. Comet Assay
The inhibitory effect of phloroglucinol on H2O2-induced DNA damage after appropriate treatment was determined using comet assay (single cell gel electrophoresis). Briefly, collected cells were suspended in 1% low melting point agarose and then spread on comet slides according the manufacturer’s protocol in of a commercially available Comet Assay Kit (Trevigen, Inc., Gaithersburg, MD, USA). After DNA denaturation, electrophoresis was performed and slides were stained with an asymmetric cyanine dye. Resulting images were acquired under a fluorescence microscope.
2.10. γH2AX Immunofluorescence Assay
Immunofluorescence assay was applied to analyze the expression of phosphorylated histone H2AX (p-γH2AX) in cells treated with or without phloroglucinol or NAC before adding H2O2. Following treatment, cells were fixed with formaldehyde, permeabilized with Triton X-100 solutions (Thermo Fisher Scientific, Inc.), and then blocked with bovine serum albumin solution (Sigma-Aldrich Co.). Thereafter, cells were probed with an anti-p-γH2AX antibody (Cell Signaling Technology, Inc.) and then reacted with Alexa Fluor 555-conjugated secondary antibody (Thermo Fisher Scientific, Inc.). For nuclear counterstaining, cells were immersed in a 4’,6-diamidino-2-phenylindol (DAPI) solution (Sigma-Aldrich Co.). Then, p-γH2AX and DAPI fluorescence images were captured using a fluorescence microscope.
2.11. Measurement of 8-Hydroxy-2′-Deoxyguanosine (8-OHdG)
To measure 8-OHdG, a deoxyriboside form of 8-oxoGuanine, an OxiSelect Oxidative DNA Damage ELISA Kit (Cell Biolabs, San Diego, CA, USA) was used. Briefly, DNA was extracted from cells cultured under the same conditions as described above. Subsequently, the DNA of each isolated sample was digested with DNase I (Sigma-Aldrich Co.). The absorbance of the ELISA reaction was then measured at 450 nm following the protocol presented in the kit.
2.12. Mitochondrial Membrane Potential (MMP) Measurement
MMP level was monitored by staining with 5,5′,6,6′-tetrachloro-1,1′3,3′-tetraethyl- imidacarbocyanune iodide (JC-1), a fluorescent carbocyanine probe. For this assay, cells treated with H2O2 in the presence or absence of phloroglucinol or Mito-TEMPO were stained with JC-1 solution (Thermo Fisher Scientific, Inc.). The percentage of JC-1 monomer was analyzed with a flow cytometer to indicate cells that lost MMP.
2.13. Autophagy Detection
Formation of autophagosomes was assessed using a CYTO-ID® Autophagy Detection Kit purchased from Enzo Life Sciences, Inc. (Farmingdale, NY, USA). First, cells were collected for quantitative analysis of autophagy induction. Cyto-ID staining procedure was then performed according to the manufacturer’s instructions. In brief, cells cultured under various conditions were washed with assay buffer included in the kit and fixed with paraformaldehyde. Fluorescently labeled cells were then analyzed by flow cytometry. Next, cells were subjected to DAPI staining after CYTO-ID staining to monitor localizations of autophagosomes and nuclei. To monitor localizations of autophagosomes and nuclei, cells were further subjected to DAPI staining after CYTO-ID staining. The autophagic signal (green) and the nuclear signal (blue) were collected under a fluorescence microscope.
2.14. Statistical Analysis
All statistical analyses were performed using GraphPad Prism (Graphpad Inc., San Diego, CA, USA). Statistical differences were determined by one-way analysis of variance with Tukey’s test. Statistical significance was considered when p-value was less than 0.05. All data are expressed as mean ± standard deviation (SD) (* p < 0.05, ** p < 0.01 and *** p < 0.001 vs. unstimulated control; ## p < 0.01 and ### p < 0.001 vs. H2O2 alone treatment; & p < 0.05 and &&& p < 0.001 vs. phloroglucinol + Mito-TEMPO group).
3. Results
3.1. Phloroglucinol Restores Reduced Cell Viability and Increased Cytotoxicity Caused by H2O2
We performed an MTT assay to select the concentration of H2O2 that could induce oxidative damage in ARPE-19 cells. As expected, H2O2 treatment significantly suppressed cell viability in a dose-dependent manner (Figure 1A). At concentration of 0.5 mM, H2O2 reduced the cell viability to be about 60% of the control group (untreated cells). Thus, 0.5 mM was set as the cytotoxicity-inducing concentration of H2O2. In addition, in an experiment to determine the appropriate concentration range of phloroglucinol to evaluate its inhibitory effect on H2O2-induced cytotoxicity, it was found that phloroglucinol had no significant effect on cell survival at concentration up to 20 μg/mL (Figure 1B). Therefore, the highest optimal concentration of phloroglucinol was determined to be 20 μg/mL. Subsequently, we assessed the inhibitory effect of phloroglucinol on H2O2-induced cytotoxicity and found that phloroglucinol significantly restored H2O2-induced reduction in cell viability (Figure 1C). In parallel, pretreatment with NAC, an ROS scavenger used as a positive control, also completely inhibited the downregulation of cell viability in response to H2O2, demonstrating that oxidative stress triggered by H2O2 might mediate H2O2-induced reduction in cell viability. Further analysis of the protective effect of phloroglucinol using the LDH leakage assay showed that phloroglucinol and NAC significantly reduced H2O2-induced LDH release into the cell culture medium (Figure 1D).
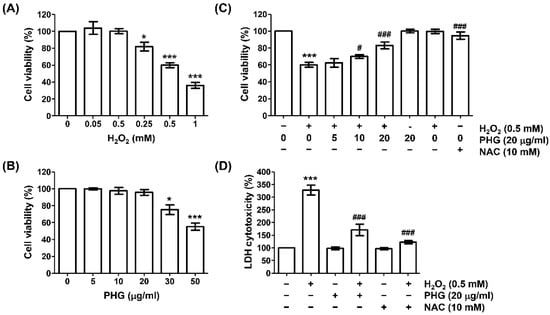
Figure 1.
Phloroglucinol reverses H2O2-induced viability reduction and cytotoxicity of ARPE-19 cells. Cells were treated with different concentrations of phloroglucinol (PHG) or H2O2 alone for 24 h (A,B), or pretreated with or without phloroglucinol and/or N-acetyl-L-cysteine (NAC) for 1 h followed by treatment with phloroglucinol for 24 h (C,D). (A–C) 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetra-zolium bromide (MTT) assay was performed to determine cell viability. (D) Cytotoxicity was measured by lactate dehydrogenase (LDH) assay. * p < 0.05 and *** p < 0.001 vs. unstimulated control; # p < 0.05 and ### p < 0.001 vs. H2O2 alone treatment.
3.2. Phloroglucinol Reveres H2O2-Induced Apoptosis
We next investigated whether the loss of cell survival and induction of cytotoxicity in H2O2-exposued ARPE-19 cells were associated with induction of apoptosis. As demonstrated in Figure 2A,B, flow cytometry results after annexin V/PI staining revealed that much more apoptosis was induced in H2O2-treated cells than in untreated control cells. However, the induction of apoptosis by H2O2 was remarkably attenuated in cells treated with phloroglucinol. Subsequently, DNA fragmentation assay was performed to verify that phloroglucinol prevented H2O2-induced apoptosis. As shown in Figure 2C, H2O2-treated cells exhibited DNA laddering and oligonucleosome-sized DNA fragments. Such patterns were not observed in untreated control cells. However, these patterns were markedly attenuated in cells preincubated with phloroglucinol. H2O2 treatment also suppressed the Bcl-2/Bax ratio known to be correlated with activation of caspase-3 and cleavage of poly(ADP-ribose) polymerase (PARP), a representative substrate protein of activated caspase-3. However, these changes were greatly ameliorated in cells pretreated with phloroglucinol (Figure 2D–F). These results suggest that phloroglucinol can effectively reduce H2O2-induced ARPE-19 cell apoptosis by modulating apoptosis regulators.
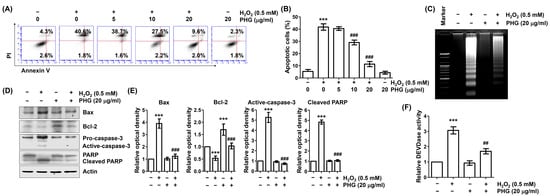
Figure 2.
Phloroglucinol inhibits apoptosis in H2O2-treated ARPE-19 cells. Cells were pretreated with phloroglucinol for 1 h and then treated with or without phloroglucinol for 24 h. (A,B) To quantitatively measure the frequency of apoptosis induction, flow cytometry was performed after double staining with annexin V and propidium iodide (PI). Representative histograms of (A) and quantitative analysis (B) are shown. (C) DNA isolated from cells was stained with ethidium bromide (EtBr) and then observed under UV light. (D) After extracting cell lysate of each treatment group, expression levels of presented proteins were investigated through immunoblotting. (E) Bar diagram showing the relative protein density after normalization with actin based on Western blot analysis. (F) Activity of caspase-3 was measured by DEVDase activity assay using cytoplasmic extracts and presented as relative values compared to control. *** p < 0.001 vs. unstimulated control; ## p < 0.01 and ### p < 0.001 vs. H2O2 alone treatment.
3.3. Phloroglucinol Abrogates H2O2-Induced ROS Generation
To clarify antioxidant properties of phloroglucinol, intracellular ROS generation was determined using DCFH-DA probe. As exhibited in Figure 3A,B, results of flow cytometry indicated that the production of intracellular peroxides in cells treated with H2O2 was greatly increased compared to that in untreated control cells, whereas it was significantly inhibited by pretreatment with phloroglucinol. In addition, ROS hardly occurred in cells treated with phloroglucinol alone. H2O2 treatment was unable to increase ROS levels in cells treated with phloroglucinol, even in the presence of NAC, indicating that phloroglucinol could act as an ROS scavenger. These results were confirmed through fluorescence microscopic observation of cells stained with DCF-DA. It was found pretreatment with phloroglucinol significantly scavenged H2O2-induced DCF fluorescence intensity (Figure 3C).
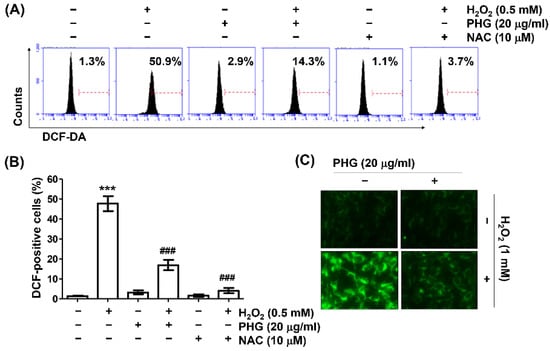
Figure 3.
Phloroglucinol suppresses H2O2-induced reactive oxygen species (ROS) production in ARPE-19 cells. Cells treated with or without phloroglucinol or NAC for 1 h were stimulated with H2O2 for another 1 h. After 2′,7′-dichlorofluorescein diacetate (DCF-DA) staining, ROS generation level was investigated through flow cytometry (A,B) and fluorescence microscopy (C). Representative histograms (A) and quantitative analysis (B) are shown. (C) Representative images of DCF-DA fluorescence are presented. *** p < 0.001 vs. unstimulated control; ### p < 0.001 vs. H2O2 alone treatment.3.4. Phloroglucinol Abolishes H2O2-Induced DNA Damage.
3.4. Phloroglucinol Abolishes H2O2-Induced DNA Damage
Next, we evaluated whether phloroglucinol could prevent H2O2-induced DNA damage in ARPE-19 cells. The blocking effect of phloroglucinol on DNA damage induced by H2O2-treatment was first investigated using a comet assay. As expected, an increase in comet tail moment was clearly observed in H2O2-treated cells, indicating that DNA damage was induced by H2O2 treatment (Figure 4A). To validate this finding, the expression of γH2AX was analyzed. Immunofluorescence results demonstrated that the fluorescence intensity of γH2AX in the nuclei of H2O2-treated cells was clearly increased compared to that in the nuclei of untreated cells. Further testing to quantify oxidative damage to nucleic acids also showed that levels of 8-OHdG were significantly elevated upon exposure to H2O2 (Figure 4C). However, increases of DNA migration, γH2AX expression, and 8-OHdG/8-oxoGuanine ratio caused by H2O2 treatment were markedly weakened in the presence of NAC as well as phloroglucinol, suggesting that phloroglucinol could attenuated oxidative DNA damage caused by H2O2.
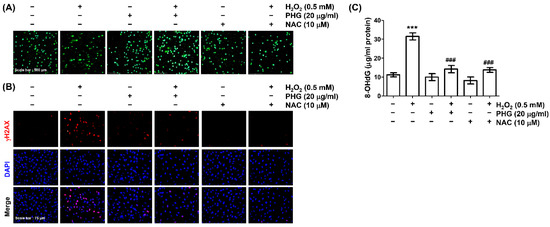
Figure 4.
Phloroglucinol alleviates H2O2-induced DNA damage in ARPE-19 cells. Cells were treated with or without phloroglucinol or NAC for 1 h prior to treatment with H2O2 for 24 h. (A) Representative immunofluorescence images of comet assay are indicated. (B) Representative images of γH2AX immunofluorescence (red) observed with a fluorescence microscope are shown. The location of the nucleus was indicated by counterstaining with DAPI (blue). (C) After treatment, contents of 8-hydroxy-2′-deoxyguanosine (8-OHdG) were measured using an ELISA kit. *** p < 0.001 vs. unstimulated control; ### p < 0.001 vs. H2O2 alone treatment.
3.5. Phloroglucinol Reduces H2O2-Induced mtROS Production
To determine whether mitochondria are major sources of H2O2-induced ROS and whether phloroglucinol can inhibit them, we used MitoSOX-red, a mitochondrial superoxide-specific dye. As shown in Figure 5A, strong red fluorescence intensity was evident in H2O2-treated ARPE-19 cells, but not in untreated control cells or cells treated with phloroglucinol alone. However, H2O2-induced fluorescence intensity was suppressed in the presence of phloroglucinol. It was further eliminated in cells pretreated with phloroglucinol and Mito-TEMPO, a mitochondria-targeted antioxidant. These results were consistent with flow cytometry results, which directly measured the frequency of MitoSOX-red-positive cells (Figure 5B,C). Moreover, phloroglucinol suppressed H2O2-induced accumulation of PINK1 and PARKIN known to be key mitochondrial autophagy proteins (Figure 5D). These findings suggest that phloroglucinol can contribute to mitophagy inhibition through its role as a scavenger of H2O2-induced mtROS in ARPE-19 cells.
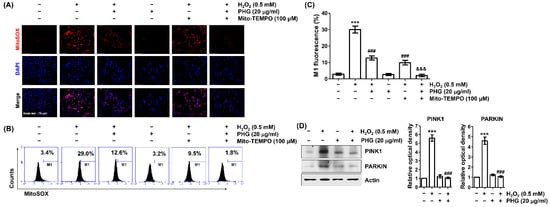
Figure 5.
Phloroglucinol eliminates H2O2-mediated mtROS generation in ARPE-19 cells. MitoSOX staining was performed to determine the abundance of mtROS in cells treated with H2O2 for 24 h after pretreatment for 1 h with or without phloroglucinol and/or Mito-TEMPO. (A) Representative images of cells stained for mitochondrial peroxide (MitoSOX, red) and nuclei (4’,6-diamidino-2-phenylindol, DAPI, blue) are shown. (B,C) For quantitative evaluation of mtROS production, flow cytometric analysis was performed. Representative histograms (B) and average values (C) are shown. (D) Isolated total cell lysates were immunoblotted with antibodies corresponding to indicated mitophagy-marker proteins. (*** p < 0.001 vs. unstimulated control; ### p < 0.001 vs. H2O2 alone treatment; &&& p < 0.001 vs. phloroglucinol + Mito-TEMPO group.
3.6. Phloroglucinol Protects H2O2-Induced Mitochondrial Impairment
To determine whether phloroglucinol could protect against H2O2-induced mitochondrial damage, we estimated MMP following JC-1 staining. Flow cytometry analysis results (Figure 6A,B) showed that the frequency of JC-1 monomer was significantly increased whereas the frequency of JC-1 aggregates was decreased in H2O2-treated cells, indicating that H2O2 induced the collapse of MMP. However, these changes were significantly attenuated by phloroglucinol pretreatment. Moreover, when phloroglucinol and Mito-TEMPO were used for pretreatment together, the loss of MMP induced by H2O2 was almost entirely reduced to the control level. In addition, after H2O2 treatment, the expression level of cytochrome c was increased in the cytoplasm but decreased in the mitochondria. Phloroglucinol pretreatment was able to restore these changes (Figure 6C,D). These results illustrate that blockade of H2O2-induced mtROS generation by phloroglucinol can preserve mitochondrial function.
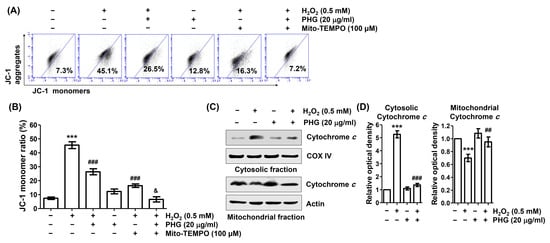
Figure 6.
Phloroglucinol protects H2O2-induced mitochondrial impairment and cytosolic release of cytochrome c in ARPE-19 cells. Cells were preincubated with or without phloroglucinol and/or Mito-TEMPO for 1 h, followed by treatment with H2O2 for another 24 h. (A,B) After with 5,5′,6,6′-tetrachloro-1,1′3,3′-tetraethyl-imidacarbocyanune iodide (JC-1) staining, representative histograms (A) and average values of JC-1 monomer ratios (B) are presented. (C) After isolation of mitochondrial and cytoplasmic fractions, the expression of cytochrome c in each fraction was investigated by immunoblotting. (D) Bar diagram showing the relative protein density after normalization with actin based on Western blot analysis. *** p < 0.001 vs. unstimulated control; ## p < 0.01 and ### p < 0.001 vs. H2O2 alone treatment; & p < 0.05 vs. phloroglucinol + Mito-TEMPO group.
3.7. Phloroglucinol Abrogates H2O2-Induced Autophagy
Finally, we evaluated the effect of phloroglucinol on H2O2-induced autophagy in ARPE-19 cells. As shown in Figure 7A,B, flow cytometry analysis using a Cyto-ID tracer dye capable of monitoring autophagic vacuoles showed that H2O2 dramatically induced autophagy. However, pretreated with phloroglucinol or 3-MA, a selective autophagic inhibitor, dramatically reduced H2O2-induced autophagy in cells. Consistent with these results, the formation of Cyto-ID puncta was enhanced in response to H2O2, whereas it was reduced almost completely after phloroglucinol pretreatment (Figure 7C), indicating that H2O2-induced autophagy could be reversed by phloroglucinol. We next verified H2O2-induced autophagy by detecting autophagy biomarkers such as microtubule-associated protein-1 light chain-3 (LC3), Beclin-1, and p62 by immunoblotting. As indicated in Figure 7D,E, H2O2 enhanced the conversion of LC3-I to LC3-II and induced Beclin-1 expression, but downregulated the expression of p62. However, these changes caused by H2O2 were all abrogated by phloroglucinol, supporting flow cytometry results that H2O2-mediated autophagy could be protected by phloroglucinol.
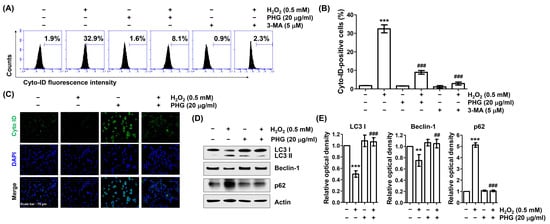
Figure 7.
Phloroglucinol attenuates ARPE-19 cells against H2O2-induced autophagy. (A,B) Cells were incubated with phloroglucinol or 3-MA for 1 h and then treated with H2O2 for 24 h, stained with Cyto-ID, and subjected to flow cytometry. Representative histograms (A) and mean values of Cyto-ID-positive cells (B) are presented. (C) H2O2-teated Cells in the presence or absence of phloroglucinol were stained with Cyto-ID. Representative images are shown. (D) Isolated total proteins were immunoblotted with indicated antibodies corresponding to autophagy-marker proteins. (E) Bar diagram showing the relative protein density after normalization with actin based on Western blot analysis. ** p < 0.01 and *** p < 0.001 vs. unstimulated control; ## p < 0.01 and ### p < 0.001 vs. H2O2 alone treatment.
4. Discussion
In the current study, we induced oxidative stress using H2O2 to examine whether phloroglucinol could protect human RPE ARPE-19 cells from oxidative injury. We found that H2O2 induced apoptosis, accompanied by mitochondrial dysfunction, DNA damage, and autophagy through an increase in ROS generation. However, phloroglucinol was able to block H2O2-induced cellular damage and scavenge ROS.
Induction of cytotoxicity including DNA damage and cell death by oxidative stimulation is mostly accompanied by mitochondrial dysfunction associated with ROS generation [33,34]. In healthy retinal cells, ROS levels remain low as a result of normal cellular metabolism. However, accumulation of ROS caused by oxidative stress can act as an initiator in the pathogenesis of degenerative diseases of the retina [4,35]. In this study, inhibition of cell survival, induction of cytotoxicity, and generation of ROS by H2O2 in ARPE-19 cells were significantly suppressed by pretreatment with phloroglucinol or NAC, a free-radical scavenger used as a positive control. These results showed the possibility that phloroglucinol could block ROS generation caused by oxidative stress. Many previous studies have shown that DNA damage and apoptosis can be induced in RPE cells exposed to oxidative stimuli [35,36]. This finding was also confirmed in H2O2-treated ARPE-19 cells. We first performed a comet assay, a widely used method to detect DNA strand breaks in eukaryotic cells [37], to evaluate whether pretreatment of phloroglucinol could inhibit H2O2-induced DNA damage. We found that phloroglucinol effectively inhibited the comet tail moment (DNA migration) observed in cells treated with H2O2. In addition, the expression of p-γH2AX, a biomarker of DNA double-strand break [38], and the amount of 8-OHdG, an indicator of oxidative stress-mediated DNA damage [39], were increased by H2O2 treatment. However, these changes were all canceled by treatment with phloroglucinol. The blocking effect of phloroglucinol on these three indicators was similarly observed in cells pretreated with NAC. Our results well support results shown in H2O2-treated human keratinocytes and UVB-irradiated mouse skin [18,23,24]. These results suggest that the ROS scavenging ability of phloroglucinol might contribute to the reduction in H2O2-induced DNA damage in RPE cells.
Apoptosis is usually divided into extrinsic and intrinsic pathways. Overload of ROS by oxidative stress can depolarize the mitochondrial membrane, which contributes to the activation of mitochondria-mediated intrinsic apoptosis pathway [40,41], resulting in the collapse of MMP indicative of dysfunctional mitochondria and leading to cytosolic release of cytochrome c. Released cytochrome c can activate the caspase cascade required for the intrinsic apoptosis pathway, causing degradation of caspase-dependent proteins such as PARP, thereby terminating apoptosis [40,42,43]. As reported in previous studies [23,44,45], the reduction in MMP and cytoplasmic release of cytochrome c are major events during mitochondria-mediated apoptosis. These events were increased in H2O2-treated ARPE-19 cells in the present study. However, there changes were markedly blocked by phloroglucinol. Furthermore, expression of Bcl-2 family proteins, activation of caspase-3, and cleavage of PARP by H2O2 were maintained at control levels after phloroglucinol pretreatment, in good agreement with our previous study using human keratinocytes [23]. Accumulated prior studies have shown that the intrinsic pathway is critically controlled by Bcl-2 family members. Among them, anti-apoptotic proteins including Bcl-2 are essential to maintain stability of the mitochondrial membrane barrier, whereas anti-apoptotic proteins such as Bax are key executors of mitochondrial poration, thereby enhancing mitochondrial membrane permeability and releasing mitochondrial cytochrome c [8,40]. These findings well support our finding that phloroglucinol can prevent apoptosis by suppressing the intrinsic apoptotic pathway. Taken together, our findings indicate that the antioxidant activity of phloroglucinol is responsible for H2O2-induced blockade of apoptosis in ARPE-19 cells.
Although the primary targets of intracellular ROS are mitochondria, mitochondria are also major sources of ROS. Increased ROS in turn can inhibit mitochondrial efficiency, which can lead to more ROS production in mitochondria by a self-destructive vicious cycle [46,47]. Therefore, we evaluated whether ROS generated by H2O2 was derived from mitochondria by applying MitoSOX-red, a mitochondrial superoxide indicator, and Mito-TEMPO, a specific antioxidant for mtROS based on previous studies showing that the generation of ROS induced by H2O2 in ARPE-19 cells occurs in mitochondria [48,49]. MMP lost by H2O2 was also significantly abolished by treatment with phloroglucinol or Mito-TEMPO alone. However, in cells pretreated with both phloroglucinol and Mito-TEMPO, MMP was almost completely restored to untreated control levels. Moreover, H2O2-induced cytosolic release of cytochrome c and expression of mitophagy markers such as PINK1 and PARKIN were not observed in cells pretreated with phloroglucinol, which might be due to blockade of mtROS production by phloroglucinol. During mitophagy, a type of autophagy unique to mitochondria, PINK1 recruits PARKIN for autophagosome formation, which in turn initiates the removal of damaged mitochondria via autophagy and proteasome mechanisms [50,51]. In AMD-like pathology associated with RPE injury, accumulation of mitochondrial damage and reduction in biogenesis are closely related to the induction of mitophagy, a phenomenon that appears prominently as aging progresses [4,10]. In particular, an aged retina is characterized by increased ROS accumulation, impaired autophagy, and mitochondrial damage associated with the pathogenesis of AMD. Rohrer et al. [52] have demonstrated that RPE cells isolated from eyes of elderly donors are more sensitive to oxidative stress and that a further decrease in mitochondrial metabolism might be associated with increased mitophagy. In addition, Kim et al. [9] have recently shown that mitochondrial dysfunction in H2O2-injured rat retina and RPE cells is responsible for the induction of mitophagy. As in other cells, oxidative stress-induced mitophagy in RPE cells occurs through the PINK1-PARKIN signaling pathway, a process that clears damaged mitochondria through autophagy [53,54]. Therefore, our results suggest that suppression of mtROS production and preservation of mitochondrial function by phloroglucinol in ARPE-19 cells exposed to H2O2 are mediated by blockade of mtROS production.
Recently, the importance of autophagy in AMD pathology has been steadily rising. It has been shown that mtROS-mediated autophagy induced by oxidative stress may contribute to retinal damage [55,56]. Autophagy is a critical catabolic process for adapting to metabolic stress and maintaining homeostasis by removing damaged intracellular organelles (including mitochondria) and proteins through formation of autophagosomes. This process is involved in the promotion and inhibition of apoptosis depending on stimulators that induce autophagy, the type of cell, and the environment surrounding the cell [50,51]. One of the features of retinal aging is the accumulation of autophagy proteins associated with mitochondrial damage [57,58]. In this respect, pharmacological manipulation of autophagic activity could be a therapeutic target for retinal damage-related disorders. Although autophagy in RPE cells exposed to oxidative stress, particularly H2O2, is known to contribute to apoptosis induction [49], RPE cells might also be protected from oxidative stress and apoptosis through promotion of autophagy [59]. In this study, H2O2-induced autophagy in ARPE-19 cells was blocked by 3-MA, an autophagosome blocker, suggesting that H2O2-mediated autophagy might contribute to apoptosis induction. Phloroglucinol also conferred a protection against H2O2-induced autophagy, similar to 3-MA. In addition, as is commonly observed during autophagy, in H2O2-treated cells, the conversion of LC3-I to LC3-II was increased and p62 was down-regulated while Beclin-1 was up-regulated. However, H2O2 stimulation in the presence of phloroglucinol failed to induce an increase in LC3-II/LC3-I value or Beclin-1 expression, which could serve as markers of autophagy because they were involved in the formation of autophagosomes [7,60]. On the other hand, p62, an indicator of autophagic flux due to degradation in autolysosomes [61,62], was maintained at the control level. Therefore, phloroglucinol might protect ARPE-19 cells from H2O2-induced cellular damage, a pro-apoptotic mechanism, by counteracting the process of autophagy.
5. Conclusions
Taken together, our results suggest that phloroglucinol can protect RPE cells from H2O2-induced oxidative damage, thereby lessening DNA and mitochondrial damage, reducing apoptosis, and improving cell survival. This protective effect of phloroglucinol can at least be achieved by regulating mitochondrial-mediated autophagy through blockade of mtROS production (Figure 8). Although our results support the beneficial potential of phloroglucinol for the prevention and treatment of oxidative damage-mediated retinal diseases, many limitations need to be addressed. First, further studies on the role of other signaling pathways including intracellular antioxidant signaling and PI3K/AKT/mTOR signaling are needed to clearly understand the blocking mechanism of mtROS by phloroglucinol. Additionally, since only in vitro experiments challenged with H2O2 were employed, animal experiments should be performed in the future to verify our findings in vivo along with additional studies with other oxidative stress inducers.
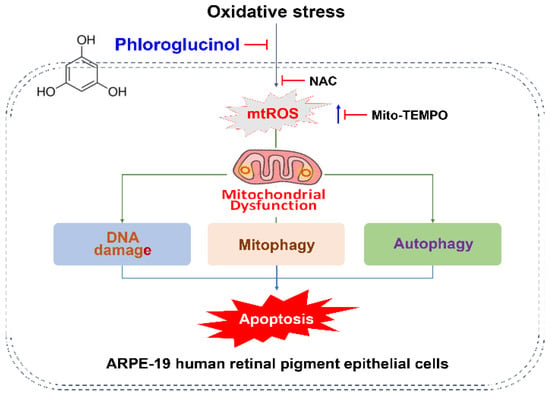
Figure 8.
Schematic showing the protective effect of phloroglucinol on oxidative injury in human RPE ARPE-19 cells. As a scavenger of mtROS, phloroglucinol protects against oxidative stress-induced apoptosis by blocking mitochondrial and DNA damage and autophagy.
Author Contributions
Conceptualization, C.P., H.-J.C., H.L. and Y.H.C.; methodology, M.Y.K., E.B., S.-K.M. and S.J.Y.; software, S.-K.M., S.J.Y. and W.-J.K.; validation, J.S.N., G.-Y.K. and S.C.; formal analysis, C.P., H.-J.C., S.J.Y., W.-J.K. and J.S.N.; investigation, C.P., H.-J.C., M.Y.K. and E.B.; resources, J.S.N., G.-Y.K., S.C. and Y.H.C.; data curation, C.P., H.-J.C., G.-Y.K., S.C., H.L. and Y.H.C.; writing—original draft preparation, C.P., H.-J.C., H.L. and Y.H.C.; writing—review and editing, C.P., H.-J.C., H.L. and Y.H.C.; visualization, C.P., H.-J.C., S.-K.M. and S.J.Y.; supervision, Y.H.C.; project administration, S.-K.M. and S.J.Y.; funding acquisition, H.L. and Y.H.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Basic Science Research Program through the National Research Foundation of Korea (NRF) grant funded by the Korea government (2021R1A2C200954911 and 2022R1I1A1A01070768) and Korea Institute of Marine Science & Technology Promotion (KIMST) funded by the Ministry of Oceans and Fisheries, Korea (20220488).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are contained within this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nebbioso, M.; Franzone, F.; Lambiase, A.; Bonfiglio, V.; Limoli, P.G.; Artico, M.; Taurone, S.; Vingolo, E.M.; Greco, A.; Polimeni, A. Oxidative stress implication in retinal diseases—A review. Antioxidants 2022, 11, 1790. [Google Scholar] [CrossRef]
- Ozawa, Y. Oxidative stress in the light-exposed retina and its implication in age-related macular degeneration. Redox. Biol. 2020, 37, 101779. [Google Scholar] [CrossRef] [PubMed]
- Nashine, S. Potential therapeutic candidates for age-related macular degeneration (AMD). Cells 2021, 10, 2483. [Google Scholar] [CrossRef] [PubMed]
- Kaarniranta, K.; Uusitalo, H.; Blasiak, J.; Felszeghy, S.; Kannan, R.; Kauppinen, A.; Salminen, A.; Sinha, D.; Ferrington, D. Mechanisms of mitochondrial dysfunction and their impact on age-related macular degeneration. Prog. Retin. Eye Res. 2020, 79, 100858. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Bhardwaj, K.; Nepovimova, E.; Kuča, K.; Dhanjal, D.S.; Bhardwaj, S.; Bhatia, S.K.; Verma, R.; Kumar, D. Antioxidant functionalized nanoparticles: A combat against oxidative stress. Nanomaterials 2020, 10, 1334. [Google Scholar] [CrossRef]
- Chan, T.C.; Wilkinson Berka, J.L.; Deliyanti, D.; Hunter, D.; Fung, A.; Liew, G.; White, A. The role of reactive oxygen species in the pathogenesis and treatment of retinal diseases. Exp. Eye Res. 2020, 201, 108255. [Google Scholar] [CrossRef] [PubMed]
- Kaarniranta, K.; Blasiak, J.; Liton, P.; Boulton, M.; Klionsky, D.J.; Sinha, D. Autophagy in age-related macular degeneration. Autophagy 2022, 1, 1–13. [Google Scholar] [CrossRef]
- Brook, N.; Brook, E.; Dharmarajan, A.; Chan, A.; Dass, C.R. The role of pigment epithelium-derived factor in protecting against cellular stress. Free Radic. Res. 2019, 53, 1166–1180. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, S.; Park, H.J.; Kim, S.H.; Lew, H.; Kim, G.J. PEDF-mediated mitophagy triggers the visual cycle by enhancing mitochondrial functions in a H2O2-injured rat model. Cells 2021, 10, 1117. [Google Scholar] [CrossRef]
- Sridevi Gurubaran, I.; Viiri, J.; Koskela, A.; Hyttinen, J.M.T.; Paterno, J.J.; Kis, G.; Antal, M.; Urtti, A.; Kauppinen, A.; Felszeghy, S.; et al. Mitophagy in the retinal pigment epithelium of dry age-related macular degeneration investigated in the NFE2L2/PGC-1α−/− mouse model. Int. J. Mol. Sci. 2020, 21, 1976. [Google Scholar] [CrossRef]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health benefits of polyphenols: A concise review. J. Food Biochem. 2022, 13, e14264. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Luo, M.; Wei, S. The bioprotective effects of polyphenols on metabolic syndrome against oxidative stress: Evidences and perspectives. Oxid. Med. Cell. Longev. 2019, 2019, 6713194. [Google Scholar] [CrossRef] [PubMed]
- Phang, Y.L.; Liu, S.; Zheng, C.; Xu, H. Recent advances in the synthesis of natural products containing the phloroglucinol motif. Nat. Prod. Rep. 2022, 39, 1766–1802. [Google Scholar] [CrossRef]
- Monteiro, P.; Lomartire, S.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Call the eckols: Present and future potential cancer therapies. Mar. Drugs 2022, 20, 387. [Google Scholar] [CrossRef]
- Khan, F.; Tabassum, N.; Bamunuarachchi, N.I.; Kim, Y.M. Phloroglucinol and its derivatives: Antimicrobial properties toward microbial pathogens. J. Agric. Food Chem. 2022, 70, 4817–4838. [Google Scholar] [CrossRef] [PubMed]
- Clara, B.; Paul, V.; Denis, P.; Stéphanie, M.; Hélène, V.R.; Rémy, B. Efficacy of phloroglucinol for the treatment of pain of gynaecologic or obstetrical origin: A systematic review of literature of randomised controlled trials. Eur. J. Clin. Pharmacol. 2020, 76, 1–6. [Google Scholar] [CrossRef]
- Drygalski, K.; Fereniec, E.; Zalewska, A.; Krętowski, A.; Żendzian-Piotrowska, M.; Maciejczyk, M. Phloroglucinol prevents albumin glycation as well as diminishes ROS production, glycooxidative damage, nitrosative stress and inflammation in hepatocytes treated with high glucose. Biomed. Pharmacother. 2021, 142, 111958. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.A.; Zhang, R.; Chae, S.; Lee, S.J.; Kim, J.; Kim, J.; Jeong, J.; Lee, J.; Shin, T.; Lee, N.H.; et al. Phloroglucinol (1,3,5-trihydroxybenzene) protects against ionizing radiation-induced cell damage through inhibition of oxidative stress in vitro and in vivo. Chem. Biol. Interact. 2010, 185, 215–226. [Google Scholar] [CrossRef]
- Cia, D.; Cubizolle, A.; Crauste, C.; Jacquemot, N.; Guillou, L.; Vigor, C.; Angebault, C.; Hamel, C.P.; Vercauteren, J.; Brabet, P. Phloroglucinol protects retinal pigment epithelium and photoreceptor against all-trans-retinal-induced toxicity and inhibits A2E formation. J. Cell. Mol. Med. 2016, 20, 1651–1663. [Google Scholar] [CrossRef] [PubMed]
- So, M.J.; Cho, E.J. Phloroglucinol attenuates free radical-induced oxidative stress. Prev. Nutr. Food Sci. 2014, 19, 129–135. [Google Scholar] [CrossRef]
- Yang, E.J.; Kim, H.; Kim, H.S.; Chang, M.J. Phloroglucinol attenuates oligomeric amyloid beta peptide1-42-induced astrocytic activation by reducing oxidative stress. J. Pharmacol. Sci. 2021, 145, 308–312. [Google Scholar] [CrossRef]
- Ryu, J.; Zhang, R.; Hong, B.H.; Yang, E.J.; Kang, K.A.; Choi, M.; Kim, K.C.; Noh, S.J.; Kim, H.S.; Lee, N.H.; et al. Phloroglucinol attenuates motor functional deficits in an animal model of Parkinson’s disease by enhancing Nrf2 activity. PLoS ONE 2013, 8, e71178. [Google Scholar] [CrossRef]
- Park, C.; Cha, H.J.; Hong, S.H.; Kim, G.Y.; Kim, S.; Kim, H.S.; Kim, B.W.; Jeon, Y.J.; Choi, Y.H. Protective effect of phloroglucinol on oxidative stress-induced DNA damage and apoptosis through activation of the Nrf2/HO-1 signaling pathway in HaCaT human keratinocytes. Mar. Drugs 2019, 17, 225. [Google Scholar] [CrossRef] [PubMed]
- Piao, M.J.; Ahn, M.J.; Kang, K.A.; Kim, K.C.; Zheng, J.; Yao, C.W.; Cha, J.W.; Hyun, C.L.; Kang, H.K.; Lee, N.H.; et al. Phloroglucinol inhibits ultraviolet B radiation-induced oxidative stress in the mouse skin. Int. J. Radiat. Biol. 2014, 90, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.C.; Li, Y.; Cheng, K.C.; Hsu, C.C.; Cheng, J.T.; Lau, H.H. Potassium bromate-induced cell model of age-related macular degeneration in vitro. Mol. Med. Rep. 2021, 23, 216. [Google Scholar] [CrossRef] [PubMed]
- Moine, E.; Brabet, P.; Guillou, L.; Durand, T.; Vercauteren, J.; Crauste, C. New lipophenol antioxidants reduce oxidative damage in retina pigment epithelial cells. Antioxidants 2018, 7, 197. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Noh, J.S.; Jung, Y.; Leem, S.H.; Hyun, J.W.; Chang, Y.C.; Kwon, T.K.; Kim, G.Y.; Lee, H.; Choi, Y.H. Fisetin attenuated oxidative stress-induced cellular damage in ARPE-19 human retinal pigment epithelial cells through Nrf2-mediated activation of heme oxygenase-1. Front. Pharmacol. 2022, 13, 927898. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.J.; Lim, D.S.; Kim, S.O.; Park, C.; Leem, S.H.; Lee, H.; Kim, G.Y.; Jeong, S.J.; Choi, Y.H. Protection of oxidative stress-induced DNA damage and apoptosis by rosmarinic acid in murine myoblast C2C12 cells. Biotechnol. Bioprocess Eng. 2022, 27, 171–182. [Google Scholar] [CrossRef]
- Choi, Y.H. Trans-cinnamaldehyde protects C2C12 myoblasts from DNA damage, mitochondrial dysfunction and apoptosis caused by oxidative stress through inhibiting ROS production. Genes Genom. 2021, 43, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Park, J.P.; Yun, J.W. Carboxylesterase3 (Ces3) interacts with bone morphogenetic protein 11 and promotes differentiation of osteoblasts via Smad1/5/9 pathway. Biotechnol. Bioprocess Eng. 2022, 27, 1–16. [Google Scholar] [CrossRef]
- Kim, C.; Kong, G.; Lee, H.; Tran, Q.; Vo, T.T.; Kwon, S.H.; Park, J.; Kim, S.H.; Park, J. Scavenger receptor class F member 2 (SCARF2) as a novel therapeutic target in glioblastoma. Toxicol. Res. 2022, 38, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Sim, K.H.; Shu, M.S.; Kim, S.; Kim, J.Y.; Choi, B.H.; Lee, Y.J. Cilostazol induces apoptosis and inhibits proliferation of hepatocellular carcinoma cells by activating AMPK. Biotechnol. Bioprocess Eng. 2021, 26, 776–785. [Google Scholar] [CrossRef]
- Tjahjono, E.; Kirienko, D.R.; Kirienko, N.V. The emergent role of mitochondrial surveillance in cellular health. Aging Cell 2022, 11, e13710. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, P.; Sulejczak, D.; Kleczkowska, P.; Bukowska-Ośko, I.; Kucia, M.; Popiel, M.; Wietrak, E.; Kramkowski, K.; Wrzosek, K.; Kaczyńska, K. Mitochondrial oxidative stress—A causative factor and therapeutic target in many diseases. Int. J. Mol. Sci. 2021, 22, 13384. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Zhang, Z.; Wang, S. Role of mitochondria in retinal pigment epithelial aging and degeneration. Front. Aging 2022, 3, 926627. [Google Scholar] [CrossRef] [PubMed]
- Mahendra, C.K.; Tan, L.T.H.; Pusparajah, P.; Htar, T.T.; Chuah, L.H.; Lee, V.S.; Low, L.E.; Tang, S.Y.; Chan, K.G.; Goh, B.H. Detrimental effects of UVB on retinal pigment epithelial cells and its role in age-related macular degeneration. Oxid. Med. Cell. Longev. 2020, 2020, 1904178. [Google Scholar] [CrossRef] [PubMed]
- Møller, P.; Stopper, H.; Collins, A.R. Measurement of DNA damage with the comet assay in high-prevalence diseases: Current status and future directions. Mutagenesis 2020, 35, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Rahmanian, N.; Shokrzadeh, M.; Eskandani, M. Recent advances in γH2AX biomarker-based genotoxicity assays: A marker of DNA damage and repair. DNA Repair 2021, 108, 103243. [Google Scholar] [CrossRef] [PubMed]
- Goh, X.X.; Tang, P.Y.; Tee, S.F. 8-Hydroxy-2′-deoxyguanosine and reactive oxygen species as biomarkers of oxidative stress in mental illnesses: A meta-analysis. Psychiatry Investig. 2021, 18, 603–618. [Google Scholar] [CrossRef]
- Tiwari, S.; Dewry, R.K.; Srivastava, R.; Nath, S.; Mohanty, T.K. Targeted antioxidant delivery modulates mitochondrial functions, ameliorates oxidative stress and preserve sperm quality during cryopreservation. Theriogenology 2022, 179, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Urbani, A.; Prosdocimi, E.; Carrer, A.; Checchetto, V.; Szabò, I. Mitochondrial ion channels of the inner membrane and their regulation in cell death signaling. Front. Cell. Dev. Biol. 2021, 8, 620081. [Google Scholar] [CrossRef]
- Sarwar, M.S.; Xia, Y.X.; Liang, Z.M.; Tsang, S.W.; Zhang, H.J. Mechanistic pathways and molecular targets of plant-derived anticancer ent-Kaurane diterpenes. Biomolecules 2020, 10, 144. [Google Scholar] [CrossRef] [PubMed]
- Kiraz, Y.; Adan, A.; Kartal Yandim, M.; Baran, Y. Major apoptotic mechanisms and genes involved in apoptosis. Tumor Biol. 2016, 37, 8471–8486. [Google Scholar] [CrossRef] [PubMed]
- Clementi, M.E.; Pizzoferrato, M.; Bianchetti, G.; Brancato, A.; Sampaolese, B.; Maulucci, G.; Tringali, G. Cytoprotective effect of idebenone through modulation of the intrinsic mitochondrial pathway of apoptosis in human retinal pigment epithelial cells exposed to oxidative stress induced by hydrogen peroxide. Biomedicines 2022, 10, 503. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Huang, T.Y.; Chen, H.Y.; Huang, T.C.; Lin, L.C.; Chang, Y.J.; Hsia, S.M. Protective effect of melatonin against oxidative stress-induced apoptosis and enhanced autophagy in human retinal pigment epithelium cells. Oxid. Med. Cell. Longev. 2018, 2018, 9015765. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wong, H.S. Are mitochondria the main contributor of reactive oxygen species in cells? J. Exp. Biol. 2021, 224, jeb221606. [Google Scholar] [CrossRef] [PubMed]
- Brillo, V.; Chieregato, L.; Leanza, L.; Muccioli, S.; Costa, R. Mitochondrial dynamics, ROS, and cell signaling: A blended overview. Life 2021, 11, 332. [Google Scholar] [CrossRef]
- He, Y.; Chen, Z.; Zhang, R.; Quan, Z.; Xu, Y.; He, B.; Ren, Y. Mitochondrial-targeted antioxidant peptide SS31 prevents RPE cell death under oxidative stress. Biomed. Res. Int. 2022, 2022, 6180349. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Mitter, S.K.; Qi, X.; Beli, E.; Rao, H.V.; Ding, J.; Ip, C.S.; Gu, H.; Akin, D.; Dunn, W.A., Jr.; et al. Oxidative stress-mediated NFκB phosphorylation upregulates p62/SQSTM1 and promotes retinal pigmented epithelial cell survival through increased autophagy. PLoS ONE 2017, 12, e0171940. [Google Scholar] [CrossRef] [PubMed]
- Jîtcă, G.; Ősz, B.E.; Tero-Vescan, A.; Miklos, A.P.; Rusz, C.M.; Bătrînu, M.G.; Vari, C.E. Positive aspects of oxidative stress at different levels of the human body: A review. Antioxidants 2022, 11, 572. [Google Scholar] [CrossRef]
- Terman, A.; Gustafsson, B.; Brunk, U.T. Autophagy, organelles and ageing. J. Pathol. 2007, 211, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Rohrer, B.; Bandyopadhyay, M.; Beeson, C. Reduced metabolic capacity in aged primary retinal pigment epithelium (RPE) is correlated with increased susceptibility to oxidative stress. Adv. Exp. Med. Biol. 2016, 854, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yao, T.; Chen, J.; Zhang, Z.; Yang, W.; Gao, X.; Dan, Y.; He, Y. Effect of Sirt3 on retinal pigment epithelial cells in high glucose through Foxo3a/ PINK1-Parkin pathway mediated mitophagy. Exp. Eye Res. 2022, 218, 109015. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xi, X.; Mei, Y.; Zhao, X.; Zhou, L.; Ma, M.; Liu, S.; Zha, X.; Yang, Y. High-glucose induces retinal pigment epithelium mitochondrial pathways of apoptosis and inhibits mitophagy by regulating ROS/PINK1/Parkin signal pathway. Biomed. Pharmacother. 2019, 111, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Dammak, A.; Huete-Toral, F.; Carpena-Torres, C.; Martin-Gil, A.; Pastrana, C.; Carracedo, G. From oxidative stress to inflammation in the posterior ocular diseases: Diagnosis and treatment. Pharmaceutics 2021, 13, 1376. [Google Scholar] [CrossRef] [PubMed]
- Nita, M.; Grzybowski, A. Interplay between reactive oxygen species and autophagy in the course of age-related macular degeneration. EXCLI J. 2020, 19, 1353–1371. [Google Scholar] [CrossRef]
- Blasiak, J.; Pawlowska, E.; Sobczuk, A.; Szczepanska, J.; Kaarniranta, K. The aging stress response and its implication for AMD pathogenesis. Int. J. Mol. Sci. 2020, 21, 8840. [Google Scholar] [CrossRef] [PubMed]
- Mitter, S.K.; Rao, H.V.; Qi, X.; Cai, J.; Sugrue, A.; Dunn, W.A., Jr.; Grant, M.B.; Boulton, M.E. Autophagy in the retina: A potential role in age-related macular degeneration. Adv. Exp. Med. Biol. 2012, 723, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Li, D.; Cui, Y.; Xie, T.; Cai, J.; Yao, Y. Decorin protects retinal pigment epithelium cells from oxidative stress and apoptosis via AMPK-mTOR-regulated autophagy. Oxid. Med. Cell, Longev. 2022, 2022, 3955748. [Google Scholar] [CrossRef] [PubMed]
- Buzun, K.; Gornowicz, A.; Lesyk, R.; Bielawski, K.; Bielawska, A. Autophagy modulators in cancer therapy. Int. J. Mol. Sci. 2021, 22, 5804. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Huang, T.; Tong, Y.; Fan, Z.; Yang, Z.; Yang, D.; Mao, X.; Yang, M. p62 works as a hub modulation in the ageing process. Ageing Res. Rev. 2022, 73, 101538. [Google Scholar] [CrossRef]
- Yoshii, S.R.; Mizushima, N. Monitoring and measuring autophagy. Int. J. Mol. Sci. 2017, 18, 1865. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).








