Contribution of Non-Coding RNAs to Anticancer Effects of Dietary Polyphenols: Chlorogenic Acid, Curcumin, Epigallocatechin-3-Gallate, Genistein, Quercetin and Resveratrol
Abstract
1. Introduction
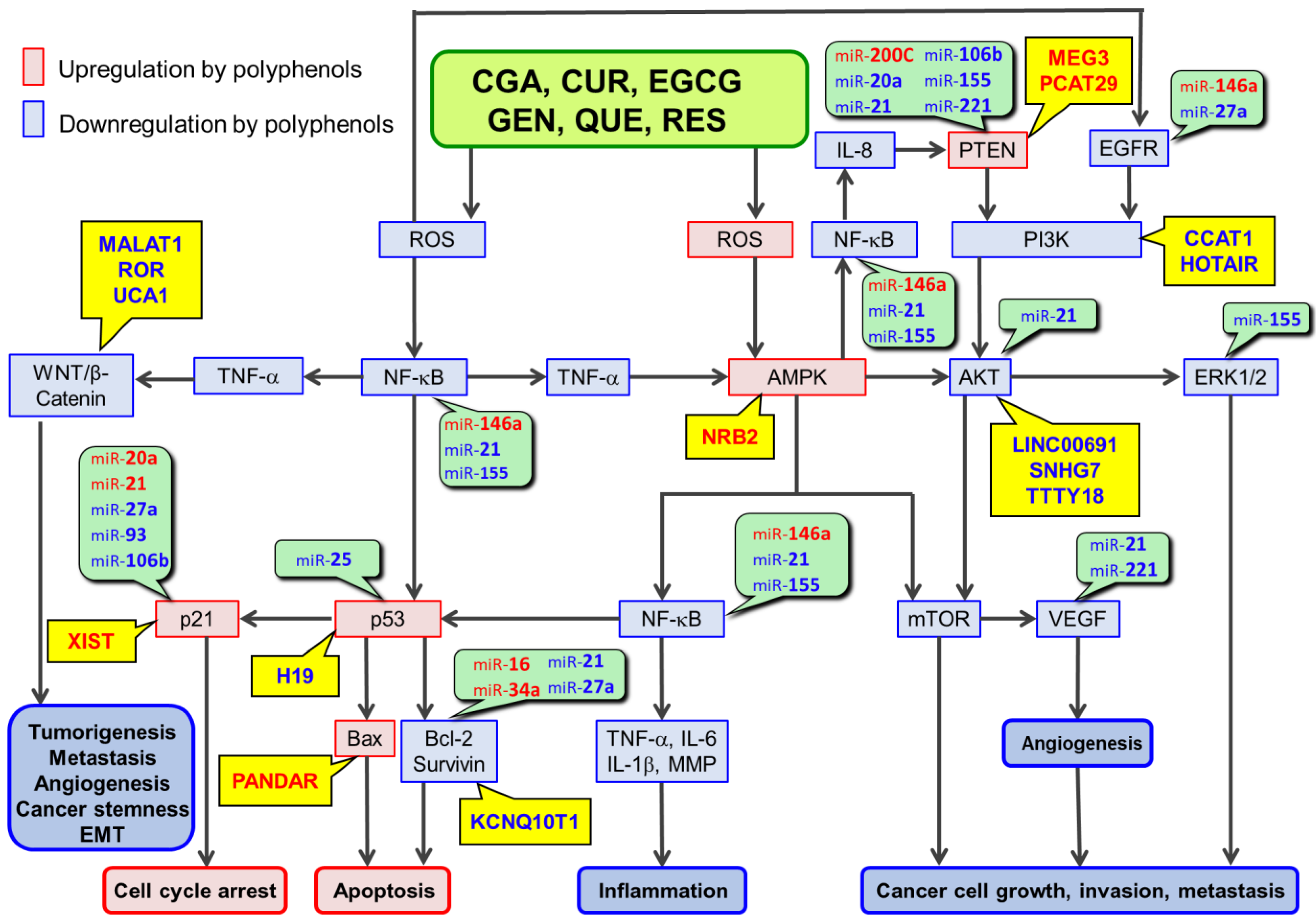
2. Involvements of miRs in Polyphenol-Mediated Anticancer Mechanisms
3. Involvements of lncRs in RSTAPs
3.1. lncR Modulations by CGA
3.2. lncR Modulations by CUR
3.3. lncR Modulations by EGCG
3.4. lncR Modulations by GEN
3.5. lncR Modulations by QUE
3.6. lncR Modulations by RES
3.7. Comparison of the Modulation of lncRs by Six Polyphenols
4. Effects of Dietary Polyphenols on CircRs
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hayakawa, S.; Ohishi, T.; Miyoshi, N.; Oishi, Y.; Nakamura, Y.; Isemura, M. Anti-Cancer Effects of Green Tea Epigallocatchin-3-Gallate and Coffee Chlorogenic Acid. Molecules 2020, 25, 4553. [Google Scholar] [CrossRef] [PubMed]
- Fukutomi, R.; Ohishi, T.; Koyama, Y.; Pervin, M.; Nakamura, Y.; Isemura, M. Beneficial Effects of Epigallocatechin-3-O-Gallate, Chlorogenic Acid, Resveratrol, and Curcumin on Neurodegenerative Diseases. Molecules 2021, 26, 415. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, T.; Fukutomi, R.; Shoji, Y.; Goto, S.; Isemura, M. The Beneficial Effects of Principal Polyphenols from Green Tea, Coffee, Wine, and Curry on Obesity. Molecules 2021, 26, 453. [Google Scholar] [CrossRef] [PubMed]
- Veeraraghavan, V.P.; Mony, U.; Renu, K.; Surapaneni, K.M.; Ammar, R.B.; AlZahrani, A.M.; Ahmed, E.A.; Rajendran, P. Effects of Polyphhenols on NcRNAs in Cancer-An Update. Clin. Exp. Pharmacol. Physiol. 2022, 49, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, H.; Pervin, M.; Goto, S.; Isemura, M.; Nakamura, Y. Beneficial Effects of Plant Polyphenols on Obesity Symbiosis Symbiosis Group. Obes. Control 2017, 4, 1–16. [Google Scholar] [CrossRef]
- Sharma, E.; Attri, D.C.; Sati, P.; Dhyani, P.; Szopa, A.; Sharifi-Rad, J.; Hano, C.; Calina, D.; Cho, W.C. Recent Updates on Anticancer Mechanisms of Polyphenols. Front. Cell Dev. Biol. 2022, 10, 1005910. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, T.; Hayakawa, S.; Miyoshi, N. Involvement of MicroRNA Modifications in Anticancer Effects of Major Polyphenols from Green Tea, Coffee, Wine, and Curry. Crit. Rev. Food Sci. Nutr. 2022, 15, 1–32. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Ohishi, T.; Nakamura, Y.; Fukutomi, R.; Miyoshi, N. Anti-Cancer Effects of Dietary Polyphenols via ROS-Mediated Pathway with Their Modulation of MicroRNAs. Molecules 2022, 27, 3816. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cao, Y.; Sun, J.; Zhang, Y. Curcumin Reduces the Expression of Bcl-2 by Upregulating MiR-15a and MiR-16 in MCF-7 Cells. Med. Oncol. 2010, 27, 1114–1118. [Google Scholar] [CrossRef]
- Tsang, W.P.; Kwok, T.T. Epigallocatechin Gallate Up-Regulation of MiR-16 and Induction of Apoptosis in Human Cancer Cells. J. Nutr. Biochem. 2010, 21, 140–146. [Google Scholar] [CrossRef]
- Sonoki, H.; Sato, T.; Endo, S.; Matsunaga, T.; Yamaguchi, M.; Yamazaki, Y.; Sugatani, J.; Ikari, A. Quercetin Decreases Claudin-2 Expression Mediated by Up-Regulation of MicroRNA MiR-16 in Lung Adenocarcinoma A549 Cells. Nutrients 2015, 7, 4578–4592. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Fang, Z.; Zha, Z.; Sun, Q.; Wang, H.; Sun, M.; Qiao, B. Quercetin Inhibits Cell Viability, Migration and Invasion by Regulating MiR-16/HOXA10 Axis in Oral Cancer. Eur. J. Pharmacol. 2019, 847, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, K.; Kosaka, N.; Yoshioka, Y.; Takahashi, R.-U.; Takeshita, F.; Ochiya, T. Stilbene Derivatives Promote Ago2-Dependent Tumour-Suppressive MicroRNA Activity. Sci. Rep. 2012, 2, 314. [Google Scholar] [CrossRef] [PubMed]
- Azimi, A.; Hagh, M.F.; Talebi, M.; Yousefi, B.; Feizi, A.A.H.p.; Baradaran, B.; Movassaghpour, A.A.; Shamsasenjan, K.; Khanzedeh, T.; Ghaderi, A.H.; et al. Time--and Concentration--Dependent Effects of Resveratrol on MiR 15a and MiR16-1 Expression and Apoptosis in the CCRF-CEM Acute Lymphoblastic Leukemia Cell Line. Asian Pac. J. Cancer Prev. 2015, 16, 6463–6468. [Google Scholar] [CrossRef]
- Sun, M.; Estrov, Z.; Ji, Y.; Coombes, K.R.; Harris, D.H.; Kurzrock, R. Curcumin (Diferuloylmethane) Alters the Expression Profiles of MicroRNAs in Human Pancreatic Cancer Cells. Mol. Cancer Ther. 2008, 7, 464–473. [Google Scholar] [CrossRef]
- Sreenivasan, S.; Thirumalai, K.; Danda, R.; Krishnakumar, S. Effect of Curcumin on MiRNA Expression in Human Y79 Retinoblastoma Cells. Curr. Eye Res. 2012, 37, 421–428. [Google Scholar] [CrossRef]
- Sibbesen, N.A.; Kopp, K.L.; Litvinov, I.V.; Jønson, L.; Willerslev-Olsen, A.; Fredholm, S.; Petersen, D.L.; Nastasi, C.; Krejsgaard, T.; Lindahl, L.M.; et al. Jak3, STAT3, and STAT5 Inhibit Expression of MiR-22, a Novel Tumor Suppressor MicroRNA, in Cutaneous T-Cell Lymphoma. Oncotarget 2015, 6, 20555–20569. [Google Scholar] [CrossRef]
- Li, B.-B.; Huang, G.-L.; Li, H.-H.; Kong, X.; He, Z.-W. Epigallocatechin-3-Gallate Modulates MicroRNA Expression Profiles in Human Nasopharyngeal Carcinoma CNE2 Cells. Chin. Med. J. 2017, 130, 93–99. [Google Scholar] [CrossRef]
- Zhang, C.; Hao, Y.; Sun, Y.; Liu, P. Quercetin Suppresses the Tumorigenesis of Oral Squamous Cell Carcinoma by Regulating MicroRNA-22/WNT1/β-Catenin Axis. J. Pharmacol. Sci. 2019, 140, 128–136. [Google Scholar] [CrossRef]
- Guo, J.; Li, W.; Shi, H.; Xie, X.; Li, L.; Tang, H.; Wu, M.; Kong, Y.; Yang, L.; Gao, J.; et al. Synergistic Effects of Curcumin with Emodin against the Proliferation and Invasion of Breast Cancer Cells through Upregulation of MiR-34a. Mol. Cell. Biochem. 2013, 382, 103–111. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, S.; Liu, C.; Liu, X. Curcumin Promoted MiR-34a Expression and Suppressed Proliferation of Gastric Cancer Cells. Cancer Biother. Radiopharm. 2019, 34, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Toden, S.; Okugawa, Y.; Buhrmann, C.; Nattamai, D.; Anguiano, E.; Baldwin, N.; Shakibaei, M.; Boland, C.R.; Goel, A. Novel Evidence for Curcumin and Boswellic Acid-Induced Chemoprevention through Regulation of MiR-34a and MiR-27a in Colorectal Cancer. Cancer Prev. Res. 2015, 8, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, M.; Khandkar, M.; Banik, N.L.; Ray, S.K. Alterations in Expression of Specific MicroRNAs by Combination of 4-HPR and EGCG Inhibited Growth of Human Malignant Neuroblastoma Cells. Brain Res. 2012, 1454, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, M.; Ai, W.; Banik, N.L.; Ray, S.K. Overexpression of MiR-7-1 Increases Efficacy of Green Tea Polyphenols for Induction of Apoptosis in Human Malignant Neuroblastoma SH-SY5Y and SK-N-DZ Cells. Neurochem. Res. 2013, 38, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Toden, S.; Tran, H.-M.; Tovar-Camargo, O.A.; Okugawa, Y.; Goel, A. Epigallocatechin-3-Gallate Targets Cancer Stem-like Cells and Enhances 5-Fluorouracil Chemosensitivity in Colorectal Cancer. Oncotarget 2016, 7, 16158–16171. [Google Scholar] [CrossRef]
- Mostafa, S.M.; Gamal-Eldeen, A.M.; Maksoud, N.A.E.; Fahmi, A.A. Epigallocatechin Gallate-Capped Gold Nanoparticles Enhanced the Tumor Suppressors Let-7a and MiR-34a in Hepatocellular Carcinoma Cells. An. Acad. Bras. Cienc. 2020, 92, e20200574. [Google Scholar] [CrossRef]
- Hsieh, P.-L.; Liao, Y.-W.; Hsieh, C.-W.; Chen, P.-N.; Yu, C.-C. Soy Isoflavone Genistein Impedes Cancer Stemness and Mesenchymal Transition in Head and Neck Cancer through Activating MiR-34a/RTCB Axis. Nutrients 2020, 12, 1924. [Google Scholar] [CrossRef]
- Xia, J.; Duan, Q.; Ahmad, A.; Bao, B.; Banerjee, S.; Shi, Y.; Ma, J.; Geng, J.; Chen, Z.; Rahman, K.M.W.; et al. Genistein Inhibits Cell Growth and Induces Apoptosis through Up-Regulation of MiR-34a in Pancreatic Cancer Cells. Curr. Drug Targets 2012, 13, 1750–1756. [Google Scholar] [CrossRef]
- Chiyomaru, T.; Yamamura, S.; Fukuhara, S.; Yoshino, H.; Kinoshita, T.; Majid, S.; Saini, S.; Chang, I.; Tanaka, Y.; Enokida, H.; et al. Genistein Inhibits Prostate Cancer Cell Growth by Targeting MiR-34a and Oncogenic HOTAIR. PLoS ONE 2013, 8, e70372. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, K.; Yamamoto, Y.; Ochiya, T. Regulatory Role of Resveratrol, a MicroRNA-Controlling Compound, in HNRNPA1 Expression, Which Is Associated with Poor Prognosis in Breast Cancer. Oncotarget 2018, 9, 24718–24730. [Google Scholar] [CrossRef]
- Kumazaki, M.; Noguchi, S.; Yasui, Y.; Iwasaki, J.; Shinohara, H.; Yamada, N.; Akao, Y. Anti-Cancer Effects of Naturally Occurring Compounds through Modulation of Signal Transduction and MiRNA Expression in Human Colon Cancer Cells. J. Nutr. Biochem. 2013, 24, 1849–1858. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Gao, M.; Wang, Z.; Wang, W.; Zhan, L.; Wei, B. Upregulation of MicroRNA-34a Sensitizes Ovarian Cancer Cells to Resveratrol by Targeting Bcl-2. Yonsei Med. J. 2021, 62, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Toden, S.; Okugawa, Y.; Jascur, T.; Wodarz, D.; Komarova, N.L.; Buhrmann, C.; Shakibaei, M.; Boland, C.R.; Goel, A. Curcumin Mediates Chemosensitization to 5-Fluorouracil through MiRNA-Induced Suppression of Epithelial-to-Mesenchymal Transition in Chemoresistant Colorectal Cancer. Carcinogenesis 2015, 36, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.W.; Yan, F.; Zhong, X.; Mazumder, P.B.; Xu-Monette, Z.Y.; Zou, D.; Young, K.H.; Ramos, K.S.; Li, Y. Regulation of P53-Targeting MicroRNAs by Polycyclic Aromatic Hydrocarbons: Implications in the Etiology of Multiple Myeloma. Mol. Carcinog. 2015, 54, 1060–1069. [Google Scholar] [CrossRef]
- Chiyomaru, T.; Fukuhara, S.; Saini, S.; Majid, S.; Deng, G.; Shahryari, V.; Chang, I.; Tanaka, Y.; Enokida, H.; Nakagawa, M.; et al. Long Non-Coding RNA HOTAIR Is Targeted and Regulated by MiR-141 in Human Cancer Cells. J. Biol. Chem. 2014, 289, 12550–12565. [Google Scholar] [CrossRef]
- Tahmasebi Mirgani, M.; Isacchi, B.; Sadeghizadeh, M.; Marra, F.; Bilia, A.R.; Mowla, S.J.; Najafi, F.; Babaei, E. Dendrosomal Curcumin Nanoformulation Downregulates Pluripotency Genes via MiR-145 Activation in U87MG Glioblastoma Cells. Int. J. Nanomed. 2014, 9, 403–417. [Google Scholar] [CrossRef]
- Liu, T.; Chi, H.; Chen, J.; Chen, C.; Huang, Y.; Xi, H.; Xue, J.; Si, Y. Curcumin Suppresses Proliferation and in Vitro Invasion of Human Prostate Cancer Stem Cells by CeRNA Effect of MiR-145 and LncRNA-ROR. Gene 2017, 631, 29–38. [Google Scholar] [CrossRef]
- Wei, D.; Yang, L.; Lv, B.; Chen, L. Genistein Suppresses Retinoblastoma Cell Viability and Growth and Induces Apoptosis by Upregulating MiR-145 and Inhibiting Its Target ABCE1. Mol. Vis. 2017, 23, 385–394. [Google Scholar]
- Zhou, J.; Gong, J.; Ding, C.; Chen, G. Quercetin Induces the Apoptosis of Human Ovarian Carcinoma Cells by Upregulating the Expression of MicroRNA-145. Mol. Med. Rep. 2015, 12, 3127–3131. [Google Scholar] [CrossRef]
- Sachdeva, M.; Liu, Q.; Cao, J.; Lu, Z.; Mo, Y.-Y. Negative Regulation of MiR-145 by C/EBP-β through the Akt Pathway in Cancer Cells. Nucleic Acids Res. 2012, 40, 6683–6692. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Q.; Cai, T.; Chen, Y.-D.; Wang, Z.-F. Induction of MicroRNA-146a Is Involved in Curcumin-Mediated Enhancement of Temozolomide Cytotoxicity against Human Glioblastoma. Mol. Med. Rep. 2015, 12, 5461–5466. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Vandenboom, T.G.; Wang, Z.; Kong, D.; Ali, S.; Philip, P.A.; Sarkar, F.H. MiR-146a Suppresses Invasion of Pancreatic Cancer Cells. Cancer Res. 2010, 70, 1486–1495. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.-F.; He, H.-F.; Chen, Q. Quercetin Inhibits Proliferation and Invasion Acts by Up-Regulating MiR-146a in Human Breast Cancer Cells. Mol. Cell. Biochem. 2015, 402, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Soubani, O.; Ali, A.S.; Logna, F.; Ali, S.; Philip, P.A.; Sarkar, F.H. Re-Expression of MiR-200 by Novel Approaches Regulates the Expression of PTEN and MT1-MMP in Pancreatic Cancer. Carcinogenesis 2012, 33, 1563–1571. [Google Scholar] [CrossRef] [PubMed]
- Karimi Dermani, F.; Saidijam, M.; Amini, R.; Mahdavinezhad, A.; Heydari, K.; Najafi, R. Resveratrol Inhibits Proliferation, Invasion, and Epithelial-Mesenchymal Transition by Increasing MiR-200c Expression in HCT-116 Colorectal Cancer Cells. J. Cell. Biochem. 2017, 118, 1547–1555. [Google Scholar] [CrossRef]
- Huang, S.; Wang, L.-L.; Xue, N.-N.; Li, C.; Guo, H.-H.; Ren, T.-K.; Zhan, Y.; Li, W.-B.; Zhang, J.; Chen, X.-G.; et al. Chlorogenic Acid Effectively Treats Cancers through Induction of Cancer Cell Differentiation. Theranostics 2019, 9, 6745–6763. [Google Scholar] [CrossRef]
- Gandhy, S.U.; Kim, K.; Larsen, L.; Rosengren, R.J.; Safe, S. Curcumin and Synthetic Analogs Induce Reactive Oxygen Species and Decreases Specificity Protein (Sp) Transcription Factors by Targeting MicroRNAs. BMC Cancer 2012, 12, 564. [Google Scholar] [CrossRef]
- Mirzaaghaei, S.; Foroughmand, A.M.; Saki, G.; Shafiei, M. Combination of Epigallocatechin-3-Gallate and Silibinin: A Novel Approach for Targeting Both Tumor and Endothelial Cells. ACS Omega 2019, 4, 8421–8430. [Google Scholar] [CrossRef]
- Dhar, S.; Kumar, A.; Rimando, A.M.; Zhang, X.; Levenson, A.S. Resveratrol and Pterostilbene Epigenetically Restore PTEN Expression by Targeting OncomiRs of the MiR-17 Family in Prostate Cancer. Oncotarget 2015, 6, 27214–27226. [Google Scholar] [CrossRef]
- Dhar, S.; Hicks, C.; Levenson, A.S. Resveratrol and Prostate Cancer: Promising Role for MicroRNAs. Mol. Nutr. Food Res. 2011, 55, 1219–1229. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, F.; Xue, J.; Zhou, X.; Luo, L.; Ma, Q.; Chen, Y.-F.; Zhang, J.; Zhang, S.-L.; Zhao, L. Antischistosomiasis Liver Fibrosis Effects of Chlorogenic Acid through IL-13/MiR-21/Smad7 Signaling Interactions In Vivo and In Vitro. Antimicrob. Agents Chemother. 2017, 61, e01347-16. [Google Scholar] [CrossRef] [PubMed]
- Mudduluru, G.; George-William, J.N.; Muppala, S.; Asangani, I.A.; Kumarswamy, R.; Nelson, L.D.; Allgayer, H. Curcumin Regulates MiR-21 Expression and Inhibits Invasion and Metastasis in Colorectal Cancer. Biosci. Rep. 2011, 31, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, D.; Ponnurangam, S.; Ramamoorthy, P.; Standing, D.; Battafarano, R.J.; Anant, S.; Sharma, P. Curcumin Induces Cell Death in Esophageal Cancer Cells through Modulating Notch Signaling. PLoS ONE 2012, 7, e30590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Bai, W.; Zhang, W. MiR-21 Suppresses the Anticancer Activities of Curcumin by Targeting PTEN Gene in Human Non-Small Cell Lung Cancer A549 Cells. Clin. Transl. Oncol. 2014, 16, 708–713. [Google Scholar] [CrossRef]
- Taverna, S.; Giallombardo, M.; Pucci, M.; Flugy, A.; Manno, M.; Raccosta, S.; Rolfo, C.; De Leo, G.; Alessandro, R. Curcumin Inhibits in Vitro and in Vivo Chronic Myelogenous Leukemia Cells Growth: A Possible Role for Exosomal Disposal of MiR-21. Oncotarget 2015, 6, 21918–21933. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Khan, S.; Maher, D.M.; Ebeling, M.C.; Sundram, V.; Chauhan, N.; Ganju, A.; Balakrishna, S.; Gupta, B.K.; Zafar, N.; et al. Anti-Cancer Activity of Curcumin Loaded Nanoparticles in Prostate Cancer. Biomaterials 2014, 35, 8635–8648. [Google Scholar] [CrossRef]
- Fix, L.N.; Shah, M.; Efferth, T.; Farwell, M.A.; Zhang, B. MicroRNA Expression Profile of MCF-7 Human Breast Cancer Cells and the Effect of Green Tea Polyphenon-60. Cancer Genom. Proteom. 2010, 7, 261–277. [Google Scholar]
- Siddiqui, I.A.; Asim, M.; Hafeez, B.B.; Adhami, V.M.; Tarapore, R.S.; Mukhtar, H. Green Tea Polyphenol EGCG Blunts Androgen Receptor Function in Prostate Cancer. FASEB J. 2011, 25, 1198–1207. [Google Scholar] [CrossRef]
- Zaman, M.S.; Shahryari, V.; Deng, G.; Thamminana, S.; Saini, S.; Majid, S.; Chang, I.; Hirata, H.; Ueno, K.; Yamamura, S.; et al. Up-Regulation of MicroRNA-21 Correlates with Lower Kidney Cancer Survival. PLoS ONE 2012, 7, e31060. [Google Scholar] [CrossRef]
- Tili, E.; Michaille, J.-J.; Alder, H.; Volinia, S.; Delmas, D.; Latruffe, N.; Croce, C.M. Resveratrol Modulates the Levels of MicroRNAs Targeting Genes Encoding Tumor-Suppressors and Effectors of TGFβ Signaling Pathway in SW480 Cells. Biochem. Pharmacol. 2010, 80, 2057–2065. [Google Scholar] [CrossRef]
- Sheth, S.; Jajoo, S.; Kaur, T.; Mukherjea, D.; Sheehan, K.; Rybak, L.P.; Ramkumar, V. Resveratrol Reduces Prostate Cancer Growth and Metastasis by Inhibiting the Akt/MicroRNA-21 Pathway. PLoS ONE 2012, 7, e51655. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Liang, H.; Xia, Q.; Li, P.; Kong, H.; Lei, P.; Wang, S.; Tu, Z. Resveratrol Induces Apoptosis of Pancreatic Cancers Cells by Inhibiting MiR-21 Regulation of BCL-2 Expression. Clin. Transl. Oncol. 2013, 15, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jia, Z.; Li, A.; Jenkins, G.; Yang, X.; Hu, J.; Guo, W. Resveratrol Repressed Viability of U251 Cells by MiR-21 Inhibiting of NF-ΚB Pathway. Mol. Cell. Biochem. 2013, 382, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Ding, J.; Wu, Y. Resveratrol Induces Apoptosis of Bladder Cancer Cells via MiR-21 Regulation of the Akt/Bcl-2 Signaling Pathway. Mol. Med. Rep. 2014, 9, 1467–1473. [Google Scholar] [CrossRef]
- Zan, L.; Chen, Q.; Zhang, L.; Li, X. Epigallocatechin Gallate (EGCG) Suppresses Growth and Tumorigenicity in Breast Cancer Cells by Downregulation of MiR-25. Bioengineered 2019, 10, 374–382. [Google Scholar] [CrossRef]
- Noratto, G.D.; Jutooru, I.; Safe, S.; Angel-Morales, G.; Mertens-Talcott, S.U. The Drug Resistance Suppression Induced by Curcuminoids in Colon Cancer SW-480 Cells Is Mediated by Reactive Oxygen Species-Induced Disruption of the MicroRNA-27a-ZBTB10-Sp Axis. Mol. Nutr. Food Res. 2013, 57, 1638–1648. [Google Scholar] [CrossRef]
- Xia, J.; Cheng, L.; Mei, C.; Ma, J.; Shi, Y.; Zeng, F.; Wang, Z.; Wang, Z. Genistein Inhibits Cell Growth and Invasion through Regulation of MiR-27a in Pancreatic Cancer Cells. Curr. Pharm. Des. 2014, 20, 5348–5353. [Google Scholar] [CrossRef]
- Xu, L.; Xiang, J.; Shen, J.; Zou, X.; Zhai, S.; Yin, Y.; Li, P.; Wang, X.; Sun, Q. Oncogenic MicroRNA-27a Is a Target for Genistein in Ovarian Cancer Cells. Anticancer Agents Med. Chem. 2013, 13, 1126–1132. [Google Scholar] [CrossRef]
- Sun, Q.; Cong, R.; Yan, H.; Gu, H.; Zeng, Y.; Liu, N.; Chen, J.; Wang, B. Genistein Inhibits Growth of Human Uveal Melanoma Cells and Affects MicroRNA-27a and Target Gene Expression. Oncol. Rep. 2009, 22, 563–567. [Google Scholar] [CrossRef]
- Singh, B.; Shoulson, R.; Chatterjee, A.; Ronghe, A.; Bhat, N.K.; Dim, D.C.; Bhat, H.K. Resveratrol Inhibits Estrogen-Induced Breast Carcinogenesis through Induction of NRF2-Mediated Protective Pathways. Carcinogenesis 2014, 35, 1872–1880. [Google Scholar] [CrossRef]
- Zeng, J.; Zhang, D.; Wan, X.; Bai, Y.; Yuan, C.; Wang, T.; Yuan, D.; Zhang, C.; Liu, C. Chlorogenic Acid Suppresses MiR-155 and Ameliorates Ulcerative Colitis through the NF-ΚB/NLRP3 Inflammasome Pathway. Mol. Nutr. Food Res. 2020, 64, 1901329–2000452. [Google Scholar] [CrossRef]
- Ma, F.; Liu, F.; Ding, L.; You, M.; Yue, H.; Zhou, Y.; Hou, Y. Anti-Inflammatory Effects of Curcumin Are Associated with down Regulating MicroRNA-155 in LPS-Treated Macrophages and Mice. Pharm. Biol. 2017, 55, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- de la Parra, C.; Castillo-Pichardo, L.; Cruz-Collazo, A.; Cubano, L.; Redis, R.; Calin, G.A.; Dharmawardhane, S. Soy Isoflavone Genistein-Mediated Downregulation of MiR-155 Contributes to the Anticancer Effects of Genistein. Nutr. Cancer 2016, 68, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Boesch-Saadatmandi, C.; Loboda, A.; Wagner, A.E.; Stachurska, A.; Jozkowicz, A.; Dulak, J.; Döring, F.; Wolffram, S.; Rimbach, G. Effect of Quercetin and Its Metabolites Isorhamnetin and Quercetin-3-Glucuronide on Inflammatory Gene Expression: Role of MiR-155. J. Nutr. Biochem. 2011, 22, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Tili, E.; Michaille, J.-J.; Adair, B.; Alder, H.; Limagne, E.; Taccioli, C.; Ferracin, M.; Delmas, D.; Latruffe, N.; Croce, C.M. Resveratrol Decreases the Levels of MiR-155 by Upregulating MiR-663, a MicroRNA Targeting JunB and JunD. Carcinogenesis 2010, 31, 1561–1566. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, D.; Zang, W.; Yin, G.; Dai, J.; Sun, Y.U.; Yang, Z.; Hoffman, R.M.; Guo, X. Synergistic Inhibitory Effect of Traditional Chinese Medicine Astragaloside IV and Curcumin on Tumor Growth and Angiogenesis in an Orthotopic Nude-Mouse Model of Human Hepatocellular Carcinoma. Anticancer Res. 2017, 37, 465–473. [Google Scholar] [CrossRef]
- Allegri, L.; Rosignolo, F.; Mio, C.; Filetti, S.; Baldan, F.; Damante, G. Effects of Nutraceuticals on Anaplastic Thyroid Cancer Cells. J. Cancer Res. Clin. Oncol. 2018, 144, 285–294. [Google Scholar] [CrossRef]
- Chen, Y.; Zaman, M.S.; Deng, G.; Majid, S.; Saini, S.; Liu, J.; Tanaka, Y.; Dahiya, R. MicroRNAs 221/222 and Genistein-Mediated Regulation of ARHI Tumor Suppressor Gene in Prostate Cancer. Cancer Prev. Res. 2011, 4, 76–86. [Google Scholar] [CrossRef]
- Sarkar, S.; Dubaybo, H.; Ali, S.; Goncalves, P.; Kollepara, S.L.; Sethi, S.; Philip, P.A.; Li, Y. Down-Regulation of MiR-221 Inhibits Proliferation of Pancreatic Cancer Cells through up-Regulation of PTEN, P27(Kip1), P57(Kip2), and PUMA. Am. J. Cancer Res. 2013, 3, 465–477. [Google Scholar]
- Ma, J.; Fang, B.; Zeng, F.; Pang, H.; Zhang, J.; Shi, Y.; Wu, X.; Cheng, L.; Ma, C.; Xia, J.; et al. Curcumin Inhibits Cell Growth and Invasion through Up-Regulation of MiR-7 in Pancreatic Cancer Cells. Toxicol. Lett. 2014, 231, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Wang, Y.; Zhang, R.; Yang, G.; Liang, Z.; Wang, Z.; Zhang, G. Curcumin Exerts Its Antitumor Activity through Regulation of MiR-7/Skp2/P21 in Nasopharyngeal Carcinoma Cells. OncoTargets Ther. 2017, 10, 2377–2388. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.-F.; Zhang, X.; Zhang, X.-J.; Shi, X.-Q.; Yu, Z.-J.; Kan, Q.-C. Induction of MicroRNA-9 Mediates Cytotoxicity of Curcumin against SKOV3 Ovarian Cancer Cells. Asian Pac. J. Cancer Prev. 2014, 15, 3363–3368. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Wang, L.; Zhu, L.; Zhang, C.; Zhou, J. Curcumin Inhibits Oral Squamous Cell Carcinoma SCC-9 Cells Proliferation by Regulating MiR-9 Expression. Biochem. Biophys. Res. Commun. 2014, 454, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Yang, J.; Chen, C.; Chen, J.; Ye, L.; Wang, L.; Wu, J.; Xing, C.; Yu, K. Pure Curcumin Decreases the Expression of WT1 by Upregulation of MiR-15a and MiR-16-1 in Leukemic Cells. J. Exp. Clin. Cancer Res. 2012, 31, 27. [Google Scholar] [CrossRef]
- Kang, T.; Sun, W.-L.; Lu, X.-F.; Wang, X.-L.; Jiang, L. MiR-28-5p Mediates the Anti-Proliferative and pro-Apoptotic Effects of Curcumin on Human Diffuse Large B-Cell Lymphoma Cells. J. Int. Med. Res. 2020, 48, 300060520943792. [Google Scholar] [CrossRef]
- Zamani, M.; Sadeghizadeh, M.; Behmanesh, M.; Najafi, F. Dendrosomal Curcumin Increases Expression of the Long Non-Coding RNA Gene MEG3 via up-Regulation of Epi-MiRs in Hepatocellular Cancer. Phytomedicine 2015, 22, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, J.; Liu, L.; Yu, L.; Zhao, N.; Zhou, X.; Lu, X. Curcumin Increases the Sensitivity of Paclitaxel-Resistant NSCLC Cells to Paclitaxel through MicroRNA-30c-Mediated MTA1 Reduction. Tumour Biol. 2017, 39, 1010428317698353. [Google Scholar] [CrossRef]
- Zhang, P.; Bai, H.; Liu, G.; Wang, H.; Chen, F.; Zhang, B.; Zeng, P.; Wu, C.; Peng, C.; Huang, C.; et al. MicroRNA-33b, Upregulated by EF24, a Curcumin Analog, Suppresses the Epithelial-to-Mesenchymal Transition (EMT) and Migratory Potential of Melanoma Cells by Targeting HMGA2. Toxicol. Lett. 2015, 234, 151–161. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, W.; Guo, Y.; Li, Z.; Chen, X.; Wang, Y.; Du, Y.; Zang, W.; Zhao, G. Curcumin Inhibits Cell Growth and Induces Cell Apoptosis through Upregulation of MiR-33b in Gastric Cancer. Tumour Biol. 2016, 37, 13177–13184. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-L.; Chang, J.-M.; Chong, I.-W.; Hung, Y.-L.; Chen, Y.-H.; Huang, W.-T.; Kuo, H.-F.; Hsieh, C.-C.; Liu, P.-L. Curcumin Inhibits LIN-28A through the Activation of MiRNA-98 in the Lung Cancer Cell Line A549. Molecules 2017, 22, 929. [Google Scholar] [CrossRef]
- Li, Y.; Sun, W.; Han, N.; Zou, Y.; Yin, D. Curcumin Inhibits Proliferation, Migration, Invasion and Promotes Apoptosis of Retinoblastoma Cell Lines through Modulation of MiR-99a and JAK/STAT Pathway. BMC Cancer 2018, 18, 1230. [Google Scholar] [CrossRef]
- Bao, B.; Ali, S.; Banerjee, S.; Wang, Z.; Logna, F.; Azmi, A.S.; Kong, D.; Ahmad, A.; Li, Y.; Padhye, S.; et al. Curcumin Analogue CDF Inhibits Pancreatic Tumor Growth by Switching on Suppressor MicroRNAs and Attenuating EZH2 Expression. Cancer Res. 2012, 72, 335–345. [Google Scholar] [CrossRef]
- Wu, G.-Q.; Chai, K.-Q.; Zhu, X.-M.; Jiang, H.; Wang, X.; Xue, Q.; Zheng, A.-H.; Zhou, H.-Y.; Chen, Y.; Chen, X.-C.; et al. Anti-Cancer Effects of Curcumin on Lung Cancer through the Inhibition of EZH2 and NOTCH1. Oncotarget 2016, 7, 26535–26550. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Ruan, T.; Liu, W.; Zhu, X.; Pan, J.; Lu, W.; Yan, C.; Tao, K.; Zhang, W.; Zhang, C. Effect and Mechanism of Curcumin on EZH2-MiR-101 Regulatory Feedback Loop in Multiple Myeloma. Curr. Pharm. Des. 2018, 24, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Pan, Y.; Li, X.; Zhang, X.; Xue, Y.; Wang, T.; Zhao, S.; Hou, Y. Dihydroartemisinin and Curcumin Synergistically Induce Apoptosis in SKOV3 Cells Via Upregulation of MiR-124 Targeting Midkine. Cell. Physiol. Biochem. 2017, 43, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wang, H.; Yang, F.; Chen, H.; He, W.; Wang, J. Curcumin Promotes Osteosarcoma Cell Death by Activating MiR-125a/ERRα Signal Pathway. J. Cell. Biochem. 2017, 118, 74–81. [Google Scholar] [CrossRef]
- Yu, D.; An, F.; He, X.; Cao, X. Curcumin Inhibits the Proliferation and Invasion of Human Osteosarcoma Cell Line MG-63 by Regulating MiR-138. Int. J. Clin. Exp. Pathol. 2015, 8, 14946–14952. [Google Scholar]
- Qiu, B.; Xu, X.; Yi, P.; Hao, Y. Curcumin Reinforces MSC-Derived Exosomes in Attenuating Osteoarthritis via Modulating the MiR-124/NF-KB and MiR-143/ROCK1/TLR9 Signalling Pathways. J. Cell. Mol. Med. 2020, 24, 10855–10865. [Google Scholar] [CrossRef]
- Cao, H.; Yu, H.; Feng, Y.; Chen, L.; Liang, F. Curcumin Inhibits Prostate Cancer by Targeting PGK1 in the FOXD3/MiR-143 Axis. Cancer Chemother. Pharmacol. 2017, 79, 985–994. [Google Scholar] [CrossRef]
- Liu, J.; Li, M.; Wang, Y.; Luo, J. Curcumin Sensitizes Prostate Cancer Cells to Radiation Partly via Epigenetic Activation of MiR-143 and MiR-143 Mediated Autophagy Inhibition. J. Drug Target. 2017, 25, 645–652. [Google Scholar] [CrossRef]
- Kronski, E.; Fiori, M.E.; Barbieri, O.; Astigiano, S.; Mirisola, V.; Killian, P.H.; Bruno, A.; Pagani, A.; Rovera, F.; Pfeffer, U.; et al. MiR181b Is Induced by the Chemopreventive Polyphenol Curcumin and Inhibits Breast Cancer Metastasis via Down-Regulation of the Inflammatory Cytokines CXCL1 and -2. Mol. Oncol. 2014, 8, 581–595. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Zhang, J.; Zhang, J.; Miao, Q.; Yao, L.; Zhang, J. Curcumin Promotes Apoptosis by Activating the P53-MiR-192-5p/215-XIAP Pathway in Non-Small Cell Lung Cancer. Cancer Lett. 2015, 357, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Qiao, F.; Wang, Y.; Xu, Y.; Shang, Y. Curcumin Inhibits Cell Proliferation and Induces Apoptosis of Human Non-Small Cell Lung Cancer Cells through the Upregulation of MiR-192-5p and Suppression of PI3K/Akt Signaling Pathway. Oncol. Rep. 2015, 34, 2782–2789. [Google Scholar] [CrossRef]
- Pan, Y.; Sun, Y.; Liu, Z.; Zhang, C. MiR-192-5p Upregulation Mediates the Suppression of Curcumin in Human NSCLC Cell Proliferation, Migration and Invasion by Targeting C-Myc and Inactivating the Wnt/Β-catenin Signaling Pathway. Mol. Med. Rep. 2020, 22, 1594–1604. [Google Scholar] [CrossRef]
- Wang, N.; Feng, T.; Liu, X.; Liu, Q. Curcumin Inhibits Migration and Invasion of Non-Small Cell Lung Cancer Cells through up-Regulation of MiR-206 and Suppression of PI3K/AKT/MTOR Signaling Pathway. Acta Pharm. 2020, 70, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Li, Y.; Zhao, D. Curcumin Induces Apoptotic Cell Death in Human Pancreatic Cancer Cells via the MiR-340/XIAP Signaling Pathway. Oncol. Lett. 2017, 14, 1811–1816. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, X.; Zhang, Y.; Wang, Z. Curcumin Suppresses the Malignancy of Non-Small Cell Lung Cancer by Modulating the Circ-PRKCA/MiR-384/ITGB1 Pathway. Biomed. Pharmacother. 2021, 138, 111439. [Google Scholar] [CrossRef]
- Li, B.; Shi, C.; Li, B.; Zhao, J.-M.; Wang, L. The Effects of Curcumin on HCT-116 Cells Proliferation and Apoptosis via the MiR-491/PEG10 Pathway. J. Cell. Biochem. 2018, 119, 3091–3098. [Google Scholar] [CrossRef]
- Fan, H.; Shao, M.; Huang, S.; Liu, Y.; Liu, J.; Wang, Z.; Diao, J.; Liu, Y.; Tong, L.I.; Fan, Q. MiR-593 Mediates Curcumin-Induced Radiosensitization of Nasopharyngeal Carcinoma Cells via MDR1. Oncol. Lett. 2016, 11, 3729–3734. [Google Scholar] [CrossRef]
- Zhang, S.; Al-Maghout, T.; Bissinger, R.; Zeng, N.; Pelzl, L.; Salker, M.S.; Cheng, A.; Singh, Y.; Lang, F. Epigallocatechin-3-Gallate (EGCG) up-Regulates MiR-15b Expression Thus Attenuating Store Operated Calcium Entry (SOCE) into Murine CD4+ T Cells and Human Leukaemic T Cell Lymphoblasts. Oncotarget 2017, 8, 89500–89514. [Google Scholar] [CrossRef][Green Version]
- Zhao, Y.; Chen, X.; Jiang, J.; Wan, X.; Wang, Y.; Xu, P. Epigallocatechin Gallate Reverses Gastric Cancer by Regulating the Long Noncoding RNA LINC00511/MiR-29b/KDM2A Axis. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165856. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Xu, C.; Chen, L.; Chen, A.; Wu, X.; Zhou, M.; Haq, I.U.; Mariyam, Z.; Feng, Q. Epigallocatechin-3-Gallate Inhibited Cancer Stem Cell-like Properties by Targeting Hsa-Mir-485-5p/RXRα in Lung Cancer. J. Cell. Biochem. 2018, 119, 8623–8635. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Tsukamoto, S.; Huang, Y.; Makio, A.; Kumazoe, M.; Yamashita, S.; Tachibana, H. Epigallocatechin-3-O-Gallate up-Regulates MicroRNA-Let-7b Expression by Activating 67-KDa Laminin Receptor Signaling in Melanoma Cells. Sci. Rep. 2016, 6, 19225. [Google Scholar] [CrossRef]
- Chiyomaru, T.; Yamamura, S.; Fukuhara, S.; Hidaka, H.; Majid, S.; Saini, S.; Arora, S.; Deng, G.; Shahryari, V.; Chang, I.; et al. Genistein Up-Regulates Tumor Suppressor MicroRNA-574-3p in Prostate Cancer. PLoS ONE 2013, 8, e58929. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.-H.; Zhang, Y.-X.; Tang, L.-H.; Yang, X.-J.; Cui, W.-M.; Han, C.-C.; Ji, W.-Y. MicroRNA-1469, a P53-Responsive MicroRNA Promotes Genistein Induced Apoptosis by Targeting Mcl1 in Human Laryngeal Cancer Cells. Biomed. Pharmacother. 2018, 106, 665–671. [Google Scholar] [CrossRef]
- Asama, H.; Suzuki, R.; Hikichi, T.; Takagi, T.; Masamune, A.; Ohira, H. MicroRNA Let-7d Targets Thrombospondin-1 and Inhibits the Activation of Human Pancreatic Stellate Cells. Pancreatology 2019, 19, 196–203. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Li, J.; Xia, C. Quercetin Antagonizes Esophagus Cancer by Modulating MiR-1-3p/TAGLN2 Pathway-Dependent Growth and Metastasis. Nutr. Cancer 2022, 74, 1872–1881. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Y.; Lu, H.; Wang, H.; Feng, H.; Xu, J.; Zhang, B. Quercetin Radiosensitizes Non-Small Cell Lung Cancer Cells through the Regulation of MiR-16-5p/WEE1 Axis. IUBMB Life 2020, 72, 1012–1022. [Google Scholar] [CrossRef]
- Chai, R.; Xu, C.; Lu, L.; Liu, X.; Ma, Z. Quercetin Inhibits Proliferation of and Induces Apoptosis in Non-Small-Cell Lung Carcinoma via the LncRNA SNHG7/MiR-34a-5p Pathway. Immunopharmacol. Immunotoxicol. 2021, 43, 693–703. [Google Scholar] [CrossRef]
- MacKenzie, T.N.; Mujumdar, N.; Banerjee, S.; Sangwan, V.; Sarver, A.; Vickers, S.; Subramanian, S.; Saluja, A.K. Triptolide Induces the Expression of MiR-142-3p: A Negative Regulator of Heat Shock Protein 70 and Pancreatic Cancer Cell Proliferation. Mol. Cancer Ther. 2013, 12, 1266–1275. [Google Scholar] [CrossRef]
- Hu, S.-A.; Cheng, J.; Zhao, W.-H.; Zhao, H.-Y. Quercetin Induces Apoptosis in Meningioma Cells through the MiR-197/IGFBP5 Cascade. Environ. Toxicol. Pharmacol. 2020, 80, 103439. [Google Scholar] [CrossRef] [PubMed]
- Nwaeburu, C.C.; Abukiwan, A.; Zhao, Z.; Herr, I. Quercetin-Induced MiR-200b-3p Regulates the Mode of Self-Renewing Divisions in Pancreatic Cancer. Mol. Cancer 2017, 16, 23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, Q.; Chen, J.; Chen, Z. Quercetin Enhances Cisplatin Sensitivity of Human Osteosarcoma Cells by Modulating MicroRNA-217-KRAS Axis. Mol. Cells 2015, 38, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lim, W.; Bazer, F.W.; Whang, K.-Y.; Song, G. Quercetin Inhibits Proliferation of Endometriosis Regulating Cyclin D1 and Its Target MicroRNAs in Vitro and in Vivo. J. Nutr. Biochem. 2019, 63, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xia, J.-S.; Wu, J.-H.; Chen, Y.-G.; Qiu, C.-J. Quercetin Suppresses Cell Survival and Invasion in Oral Squamous Cell Carcinoma via the MiR-1254/CD36 Cascade in Vitro. Hum. Exp. Toxicol. 2021, 40, 1413–1421. [Google Scholar] [CrossRef]
- Shaalan, Y.M.; Handoussa, H.; Youness, R.A.; Assal, R.A.; El-Khatib, A.H.; Linscheid, M.W.; El Tayebi, H.M.; Abdelaziz, A.I. Destabilizing the Interplay between MiR-1275 and IGF2BPs by Tamarix Articulata and Quercetin in Hepatocellular Carcinoma. Nat. Prod. Res. 2018, 32, 2217–2220. [Google Scholar] [CrossRef] [PubMed]
- Appari, M.; Babu, K.R.; Kaczorowski, A.; Gross, W.; Herr, I. Sulforaphane, Quercetin and Catechins Complement Each Other in Elimination of Advanced Pancreatic Cancer by MiR-Let-7 Induction and K-Ras Inhibition. Int. J. Oncol. 2014, 45, 1391–1400. [Google Scholar] [CrossRef] [PubMed]
- Nwaeburu, C.C.; Bauer, N.; Zhao, Z.; Abukiwan, A.; Gladkich, J.; Benner, A.; Herr, I. Up-Regulation of MicroRNA Let-7c by Quercetin Inhibits Pancreatic Cancer Progression by Activation of Numbl. Oncotarget 2016, 7, 58367–58380. [Google Scholar] [CrossRef]
- El-Kott, A.F.; Shati, A.A.; Ali Al-Kahtani, M.; Alharbi, S.A. The Apoptotic Effect of Resveratrol in Ovarian Cancer Cells Is Associated with Downregulation of Galectin-3 and Stimulating MiR-424-3p Transcription. J. Food Biochem. 2019, 43, e13072. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, S.; Ying, Y.; Zhou, R.; Mao, P. MiR-196b/MiR-1290 Participate in the Antitumor Effect of Resveratrol via Regulation of IGFBP3 Expression in Acute Lymphoblastic Leukemia. Oncol. Rep. 2017, 37, 1075–1083. [Google Scholar] [CrossRef]
- Li, X.; Xie, W.; Xie, C.; Huang, C.; Zhu, J.; Liang, Z.; Deng, F.; Zhu, M.; Zhu, W.; Wu, R.; et al. Curcumin Modulates MiR-19/PTEN/AKT/P53 Axis to Suppress Bisphenol A-Induced MCF-7 Breast Cancer Cell Proliferation. Phytother. Res. 2014, 28, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Chan, J.Y.-W.; Wong, T.-S. Curcumin Exerts Inhibitory Effects on Undifferentiated Nasopharyngeal Carcinoma by Inhibiting the Expression of MiR-125a-5p. Clin. Sci. 2014, 127, 571–579. [Google Scholar] [CrossRef]
- Dou, H.; Shen, R.; Tao, J.; Huang, L.; Shi, H.; Chen, H.; Wang, Y.; Wang, T. Curcumin Suppresses the Colon Cancer Proliferation by Inhibiting Wnt/β-Catenin Pathways via MiR-130a. Front. Pharmacol. 2017, 8, 877. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Tan, S.-L.; Lu, Q.; Xu, R.; Cao, J.; Wu, S.-Q.; Wang, Y.-H.; Zhao, X.-K.; Zhong, Z.-H. Curcumin Suppresses MicroRNA-7641-Mediated Regulation of P16 Expression in Bladder Cancer. Am. J. Chin. Med. 2018, 46, 1357–1368. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Wu, X.; Wang, X.; Huang, W.; Feng, Q. NEAT1 Upregulates EGCG-Induced CTR1 to Enhance Cisplatin Sensitivity in Lung Cancer Cells. Oncotarget 2016, 7, 43337–43351. [Google Scholar] [CrossRef]
- Zaman, M.S.; Thamminana, S.; Shahryari, V.; Chiyomaru, T.; Deng, G.; Saini, S.; Majid, S.; Fukuhara, S.; Chang, I.; Arora, S.; et al. Inhibition of PTEN Gene Expression by Oncogenic MiR-23b-3p in Renal Cancer. PLoS ONE 2012, 7, e50203. [Google Scholar] [CrossRef]
- Chiyomaru, T.; Yamamura, S.; Zaman, M.S.; Majid, S.; Deng, G.; Shahryari, V.; Saini, S.; Hirata, H.; Ueno, K.; Chang, I.; et al. Genistein Suppresses Prostate Cancer Growth through Inhibition of Oncogenic MicroRNA-151. PLoS ONE 2012, 7, e43812. [Google Scholar] [CrossRef]
- Ma, J.; Cheng, L.; Liu, H.; Zhang, J.; Shi, Y.; Zeng, F.; Miele, L.; Sarkar, F.H.; Xia, J.; Wang, Z. Genistein Down-Regulates MiR-223 Expression in Pancreatic Cancer Cells. Curr. Drug Targets 2013, 14, 1150–1156. [Google Scholar] [CrossRef]
- Ma, J.; Zeng, F.; Ma, C.; Pang, H.; Fang, B.; Lian, C.; Yin, B.; Zhang, X.; Wang, Z.; Xia, J. Synergistic Reversal Effect of Epithelial-to-Mesenchymal Transition by MiR-223 Inhibitor and Genistein in Gemcitabine-Resistant Pancreatic Cancer Cells. Am. J. Cancer Res. 2016, 6, 1384–1395. [Google Scholar]
- Yu, Y.; Xing, Y.; Zhang, Q.; Zhang, Q.; Huang, S.; Li, X.; Gao, C. Soy Isoflavone Genistein Inhibits Hsa_circ_0031250/MiR-873-5p/FOXM1 Axis to Suppress Non-Small-Cell Lung Cancer Progression. IUBMB Life 2021, 73, 92–107. [Google Scholar] [CrossRef]
- Hirata, H.; Hinoda, Y.; Shahryari, V.; Deng, G.; Tanaka, Y.; Tabatabai, Z.L.; Dahiya, R. Genistein Downregulates Onco-MiR-1260b and Upregulates SFRP1 and Smad4 via Demethylation and Histone Modification in Prostate Cancer Cells. Br. J. Cancer 2014, 110, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Hirata, H.; Ueno, K.; Nakajima, K.; Tabatabai, Z.L.; Hinoda, Y.; Ishii, N.; Dahiya, R. Genistein Downregulates Onco-MiR-1260b and Inhibits Wnt-Signalling in Renal Cancer Cells. Br. J. Cancer 2013, 108, 2070–2078. [Google Scholar] [CrossRef] [PubMed]
- C Yilmaz, U.; Bagca, B.G.; Karaca, E.; Durmaz, A.; Durmaz, B.; Aykut, A.; Kayalar, H.; Avci, C.B.; Susluer, S.Y.; Pariltay, E.; et al. Propolis Extract Regulates MicroRNA Expression in Glioblastoma and Brain Cancer Stem Cells. Anticancer Agents Med. Chem. 2022, 22, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Hottiger, M.O. Crosstalk between Wnt/β-Catenin and NF-ΚB Signaling Pathway during Inflammation. Front. Immunol. 2016, 7, 378. [Google Scholar] [CrossRef]
- Vallée, A.; Lecarpentier, Y. Crosstalk Between Peroxisome Proliferator-Activated Receptor Gamma and the Canonical WNT/β-Catenin Pathway in Chronic Inflammation and Oxidative Stress During Carcinogenesis. Front. Immunol. 2018, 9, 745. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Hong, H.S.; Liu, Z.X.; Kim, R.H.; Kang, M.K.; Park, N.-H.; Shin, K.-H. TNFα Enhances Cancer Stem Cell-like Phenotype via Notch-Hes1 Activation in Oral Squamous Cell Carcinoma Cells. Biochem. Biophys. Res. Commun. 2012, 424, 58–64. [Google Scholar] [CrossRef]
- Zhang, L.; Jiao, M.; Wu, K.; Li, L.; Zhu, G.; Wang, X.; He, D.; Wu, D. TNF-α Induced Epithelial Mesenchymal Transition Increases Stemness Properties in Renal Cell Carcinoma Cells. Int. J. Clin. Exp. Med. 2014, 7, 4951–4958. [Google Scholar]
- Chen, Y.; Wen, H.; Zhou, C.; Su, Q.; Lin, Y.; Xie, Y.; Huang, Y.; Qiu, Q.; Lin, J.; Huang, X.; et al. TNF-α Derived from M2 Tumor-Associated Macrophages Promotes Epithelial-Mesenchymal Transition and Cancer Stemness through the Wnt/β-Catenin Pathway in SMMC-7721 Hepatocellular Carcinoma Cells. Exp. Cell Res. 2019, 378, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, H.; Makabel, B.; Cui, Q.; Li, J.; Su, C.; Ashby, C.R.; Chen, Z.; Zhang, J. The Targeting of Non-coding RNAs by Curcumin: Facts and Hopes for Cancer Therapy (Review). Oncol. Rep. 2019, 42, 20–34. [Google Scholar] [CrossRef]
- Gong, Z.; Shen, G.; Huang, C.; Zhang, J.; Ji, J. Downregulation of LncRNA NEAT1 Inhibits the Proliferation of Human Cutaneous Squamous Cell Carcinoma in Vivo and in Vitro. Ann. Transl. Med. 2022, 10, 79. [Google Scholar] [CrossRef]
- Dong, P.; Xiong, Y.; Yue, J.; J B Hanley, S.; Kobayashi, N.; Todo, Y.; Watari, H. Exploring LncRNA-Mediated Regulatory Networks in Endometrial Cancer Cells and the Tumor Microenvironment: Advances and Challenges. Cancers 2019, 11, 234. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Yan, F.; Li, Q.; Liang, Y.; Yu, J.; Li, Z.; He, F.; Li, R.; Li, M. Chlorogenic Acid Promotes Autophagy and Alleviates Salmonella Typhimurium Infection Through the LncRNAGAS5/MiR-23a/PTEN Axis and the P38 MAPK Pathway. Front. Cell Dev. Biol. 2020, 8, 552020. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Li, J.; Zhu, G.; Wang, Y.; Zheng, G.; Kan, Q. Chlorogenic Acid Relieved Oxidative Stress Injury in Retinal Ganglion Cells through IncRNA-TUG1/Nrf2. Cell Cycle 2019, 18, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Long, F.; Lin, H.; Wang, T. Dietary Phytochemicals Targeting Nrf2 for Chemoprevention in Breast Cancer. Food Funct. 2022, 13, 4273–4285. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.-J.; Cheng, X.-D.; Zhang, J.; Zhang, W.-D. Dual Roles and Therapeutic Potential of Keap1-Nrf2 Pathway in Pancreatic Cancer: A Systematic Review. Cell Commun. Signal. 2019, 17, 121. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Fan, H.; Liu, Y.; Yin, Z.; Cai, H.; Liu, J.; Wang, Z.; Shao, M.; Sun, X.; Diao, J.; et al. Curcumin Enhances the Radiosensitivity in Nasopharyngeal Carcinoma Cells Involving the Reversal of Differentially Expressed Long Non-Coding RNAs. Int. J. Oncol. 2014, 44, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Esmatabadi, M.J.D.; Motamedrad, M.; Sadeghizadeh, M. Down-Regulation of LncRNA, GAS5 Decreases Chemotherapeutic Effect of Dendrosomal Curcumin (DNC) in Breast Cancer Cells. Phytomedicine 2018, 42, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Guo, Y.; Song, Y.; Shang, C. Long Noncoding RNA GAS5 Inhibits Malignant Proliferation and Chemotherapy Resistance to Doxorubicin in Bladder Transitional Cell Carcinoma. Cancer Chemother. Pharmacol. 2017, 79, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Novak Kujundzić, R.; Grbesa, I.; Ivkić, M.; Katdare, M.; Gall-Troselj, K. Curcumin Downregulates H19 Gene Transcription in Tumor Cells. J. Cell. Biochem. 2008, 104, 1781–1792. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Xiang, T.; Wu, Q.-F.; Wang, W.-X. Curcumin Suppresses the Proliferation of Gastric Cancer Cells by Downregulating H19. Oncol. Lett. 2016, 12, 5156–5162. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Sun, H.; Zheng, B.; Xie, M.; Xu, C.; Zhang, G.; Huang, X.; Zhuang, J. Curcumin Attenuates LncRNA H19-induced Epithelial-mesenchymal Transition In Tamoxifen-resistant Breast Cancer Cells. Mol. Med. Rep. 2021, 23, 13. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.-H.; You, H.-Y.; Feng, Y.-J.; Zhang, Z.-T. LncRNA KCNQ1OT1 Is a Key Factor in the Reversal Effect of Curcumin on Cisplatin Resistance in the Colorectal Cancer Cells. Mol. Cell. Biochem. 2021, 476, 2575–2585. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gao, Y.; Li, L.; Xie, S. Curcumin Inhibits Papillary Thyroid Cancer Cell Proliferation by Regulating LncRNA LINC00691. Anal. Cell. Pathol. 2022, 2022, 5946670. [Google Scholar] [CrossRef]
- Garitano-Trojaola, A.; José-Enériz, E.S.; Ezponda, T.; Unfried, J.P.; Carrasco-León, A.; Razquin, N.; Barriocanal, M.; Vilas-Zornoza, A.; Sangro, B.; Segura, V.; et al. Deregulation of Linc-PINT in Acute Lymphoblastic Leukemia Is Implicated in Abnormal Proliferation of Leukemic Cells. Oncotarget 2018, 9, 12842–12852. [Google Scholar] [CrossRef] [PubMed]
- Alghanimi, Y.K.; Ghasemian, A. Inhibitory Traits of Dendrosome Curcumin (DNC) on Breast Cancer Compared to Curcumin Single Compound. J. Gastrointest. Cancer 2020, 51, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Gao, R.; Chen, S.; Wei, M.; Wang, J.; Zhang, B.; Wu, S.; Xu, Y.; Wu, P.; Chen, X.; et al. Downregulation of MEG3 and Upregulation of EZH2 Cooperatively Promote Neuroblastoma Progression. J. Cell. Mol. Med. 2022, 26, 2377–2391. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Shao, T.; Zheng, W.; Ding, J. Curcumin Suppresses Tumor Growth of Gemcitabine-Resistant Non-Small Cell Lung Cancer by Regulating LncRNA-MEG3 and PTEN Signaling. Clin. Transl. Oncol. 2021, 23, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Xie, Y.; Zhou, Z.; Wu, Z.; Dai, X.; Xu, B. Curcumin Regulates the Progression of Colorectal Cancer via LncRNA NBR2/AMPK Pathway. Technol. Cancer Res. Treat. 2019, 18, 1533033819870781. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Yang, P.; Wang, H.; He, Z.-Y. Silence of Long Noncoding RNA PANDAR Switches Low-Dose Curcumin-Induced Senescence to Apoptosis in Colorectal Cancer Cells. OncoTargets Ther. 2017, 10, 483–491. [Google Scholar] [CrossRef]
- Ming, L.; Wang, P.; Bank, A.; Yu, J.; Zhang, L. PUMA Dissociates Bax and Bcl-X(L) to Induce Apoptosis in Colon Cancer Cells. J. Biol. Chem. 2006, 281, 16034–16042. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Toden, S.; Ravindranathan, P.; Han, H.; Goel, A. Curcumin Sensitizes Pancreatic Cancer Cells to Gemcitabine by Attenuating PRC2 Subunit EZH2, and the LncRNA PVT1 Expression. Carcinogenesis 2017, 38, 1036–1046. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Shi, C.-J.; Li, Y.; Zhang, F.-W.; Pan, F.-F.; Fu, W.-M.; Zhang, J.-F. LincROR Mediates the Suppressive Effects of Curcumin on Hepatocellular Carcinoma Through Inactivating Wnt/β-Catenin Signaling. Front. Pharmacol. 2020, 11, 847. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Lu, H.; Sun, Y.; Liu, L.; Wang, H. Prognostic Value of Octamer Binding Transcription Factor 4 for Patients with Solid Tumors: A Meta-Analysis. Medicine 2020, 99, e22804. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-H.; Chen, J.; Zhang, B.-R.; Lu, S.-J.; Wang, F.; Peng, L.; Dai, J.-H.; Sun, Y.-Z. Curcumin Inhibits Proliferation and Enhances Apoptosis in A549 Cells by Downregulating LncRNA UCA1. Pharmazie 2018, 73, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, M.; Zhou, X. Long Non-Coding RNA UCA1 Promotes Retinoblastoma Progression by Modulating the MiR-124/c-Myc Axis. Am. J. Transl. Res. 2022, 14, 1592–1605. [Google Scholar] [PubMed]
- Sun, K.; Jia, Z.; Duan, R.; Yan, Z.; Jin, Z.; Yan, L.; Li, Q.; Yang, J. Long Non-Coding RNA XIST Regulates MiR-106b-5p/P21 Axis to Suppress Tumor Progression in Renal Cell Carcinoma. Biochem. Biophys. Res. Commun. 2019, 510, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Sabry, D.; Abdelaleem, O.O.; El Amin Ali, A.M.; Mohammed, R.A.; Abdel-Hameed, N.D.; Hassouna, A.; Khalifa, W.A. Anti-Proliferative and Anti-Apoptotic Potential Effects of Epigallocatechin-3-Gallate and/or Metformin on Hepatocellular Carcinoma Cells: In Vitro Study. Mol. Biol. Rep. 2019, 46, 2039–2047. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Jiang, P.; Zeb, F.; Wu, X.; Xu, C.; Chen, L.; Feng, Q. EGCG Regulates CTR1 Expression through Its Pro-Oxidative Property in Non-Small-Cell Lung Cancer Cells. J. Cell. Physiol. 2020, 235, 7970–7981. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Chen, A.; Wu, X.; Zhou, M.; Ul Haq, I.; Mariyam, Z.; Feng, Q. NEAT1 Acts as an Inducer of Cancer Stem Cell-like Phenotypes in NSCLC by Inhibiting EGCG-Upregulated CTR1. J. Cell. Physiol. 2018, 233, 4852–4863. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, D.; Zhu, K. SOX2OT Variant 7 Contributes to the Synergistic Interaction between EGCG and Doxorubicin to Kill Osteosarcoma via Autophagy and Stemness Inhibition. J. Exp. Clin. Cancer Res. 2018, 37, 37. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.-L.; Wang, G.; Yu, J.; Zhang, L.-H.; Huang, Y.-F.; Wang, D.; Zhou, H.-H. Epigallocatechin-3-gallate Modulates Long Non-coding RNA and MRNA Expression Profiles in Lung Cancer Cells. Mol. Med. Rep. 2019, 19, 1509–1520. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lin, C.; Yong, W.; Ye, Y.; Huang, Z. Calycosin and Genistein Induce Apoptosis by Inactivation of HOTAIR/p-Akt Signaling Pathway in Human Breast Cancer MCF-7 Cells. Cell. Physiol. Biochem. 2015, 35, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Imai-Sumida, M.; Dasgupta, P.; Kulkarni, P.; Shiina, M.; Hashimoto, Y.; Shahryari, V.; Majid, S.; Tanaka, Y.; Dahiya, R.; Yamamura, S. Genistein Represses HOTAIR/Chromatin Remodeling Pathways to Suppress Kidney Cancer. Cell. Physiol. Biochem. 2020, 54, 53–70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-H.; Hu, P.; Xie, Y.-Q.; Kang, Y.-J.; Li, M. Long Noncoding RNA HOTAIR Promotes Endometrial Carcinoma Cell Proliferation by Binding to PTEN via the Activating Phosphatidylinositol 3-Kinase/Akt Signaling Pathway. Mol. Cell. Biol. 2019, 39, e00251-19. [Google Scholar] [CrossRef] [PubMed]
- Sadeghalvad, M.; Mansouri, K.; Mohammadi-Motlagh, H.-R.; Noorbakhsh, F.; Mostafaie, A.; Alipour, S.; Rezaei, N. Long Non-Coding RNA HOTAIR Induces the PI3K/AKT/MTOR Signaling Pathway in Breast Cancer Cells. Rev. Assoc. Med. Bras. 2022, 68, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, Y.; Gu, J.; Liang, P.; Shen, M.; Xi, J.; Qin, J. Anti-Invasive Effect and Pharmacological Mechanism of Genistein against Colorectal Cancer. Biofactors 2020, 46, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kou, J.; Wu, T.; Zheng, P.; Chao, X. Screening of Therapeutic Candidate Genes of Quercetin for Cervical Cancer and Analysis of Their Regulatory Network. OncoTargets Ther. 2021, 14, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Chen, D.; Yang, F.; Xing, N. Quercetin Inhibits Epithelial-to-Mesenchymal Transition (EMT) Process and Promotes Apoptosis in Prostate Cancer via Downregulating LncRNA MALAT1. Cancer Manag. Res. 2020, 12, 1741–1750. [Google Scholar] [CrossRef] [PubMed]
- Esteghlal, S.; Mokhtari, M.J.; Beyzaei, Z. Quercetin Can Inhibit Angiogenesis via the Down Regulation of MALAT1 and MIAT LncRNAs in Human Umbilical Vein Endothelial Cells. Int. J. Prev. Med. 2021, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Sheng, B.; Zhao, L.; Zang, X.; Zhen, J.; Liu, Y.; Bian, W.; Chen, W. Quercetin Inhibits Caerulein-Induced Acute Pancreatitis through Regulating MiR-216b by Targeting MAP2K6 and NEAT1. Inflammopharmacology 2021, 29, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ye, D.; Liu, L.; Li, X.; Liu, J.; Su, S.; Lu, W.; Yu, Z. Long Noncoding RNA SNHG7 Accelerates Proliferation, Migration and Invasion of Non-Small Cell Lung Cancer Cells by Suppressing MiR-181a-5p Through AKT/MTOR Signaling Pathway. Cancer Manag. Res. 2020, 12, 8303–8312. [Google Scholar] [CrossRef]
- Rezaie, F.; Mokhtari, M.J.; Kalani, M. Quercetin Arrests in G2 Phase, Upregulates INXS LncRNA and Downregulates UCA1 LncRNA in MCF-7 Cells. Int. J. Mol. Cell. Med. 2021, 10, 208–216. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, E.; Dai, J.; Liu, B.; Han, Z.; Wu, J.; Zhang, S.; Peng, B.; Zhang, Y.; Jiang, Y. A Novel Long Noncoding RNA AK001796 Acts as an Oncogene and Is Involved in Cell Growth Inhibition by Resveratrol in Lung Cancer. Toxicol. Appl. Pharmacol. 2015, 285, 79–88. [Google Scholar] [CrossRef]
- Kay, M.K.; Zhang, J.; Choudhury, M. Screening for Alternative Splicing of LncRNA Dleu2 in the Mouse Liver Cell Line AML-12. Biomed. Rep. 2021, 14, 50. [Google Scholar] [CrossRef]
- Lerner, M.; Harada, M.; Lovén, J.; Castro, J.; Davis, Z.; Oscier, D.; Henriksson, M.; Sangfelt, O.; Grandér, D.; Corcoran, M.M. DLEU2, Frequently Deleted in Malignancy, Functions as a Critical Host Gene of the Cell Cycle Inhibitory MicroRNAs MiR-15a and MiR-16-1. Exp. Cell Res. 2009, 315, 2941–2952. [Google Scholar] [CrossRef]
- Li, T.; Zhang, X.; Cheng, L.; Li, C.; Wu, Z.; Luo, Y.; Zhou, K.; Li, Y.; Zhao, Q.; Huang, Y. Modulation of LncRNA H19 Enhances Resveratrol-Inhibited Cancer Cell Proliferation and Migration by Regulating Endoplasmic Reticulum Stress. J. Cell. Mol. Med. 2022, 26, 2205–2217. [Google Scholar] [CrossRef]
- Ji, Q.; Liu, X.; Fu, X.; Zhang, L.; Sui, H.; Zhou, L.; Sun, J.; Cai, J.; Qin, J.; Ren, J.; et al. Resveratrol Inhibits Invasion and Metastasis of Colorectal Cancer Cells via MALAT1 Mediated Wnt/β-Catenin Signal Pathway. PLoS ONE 2013, 8, e78700. [Google Scholar] [CrossRef]
- Geng, W.; Guo, X.; Zhang, L.; Ma, Y.; Wang, L.; Liu, Z.; Ji, H.; Xiong, Y. Resveratrol Inhibits Proliferation, Migration and Invasion of Multiple Myeloma Cells via NEAT1-Mediated Wnt/β-Catenin Signaling Pathway. Biomed. Pharmacother. 2018, 107, 484–494. [Google Scholar] [CrossRef]
- Al Aameri, R.F.H.; Sheth, S.; Alanisi, E.M.A.; Borse, V.; Mukherjea, D.; Rybak, L.P.; Ramkumar, V. Tonic Suppression of PCAT29 by the IL-6 Signaling Pathway in Prostate Cancer: Reversal by Resveratrol. PLoS ONE 2017, 12, e0177198. [Google Scholar] [CrossRef]
- Lu, B.; Lv, H.; Yang, Z.; Shu, J.; Zhang, H. LncRNA PCAT29 Up-Regulates the Expression of PTEN by Down-Regulating MiR-494 in Non-Small-Cell Lung Cancer to Suppress Tumor Progression. Crit. Rev. Eukaryot. Gene Expr. 2021, 31, 9–15. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, S.; Yu, W.; Jiang, J.; Zhuo, F.; Qiu, G.; Xu, S.; Jiang, X. Altered Expression of Long Non-Coding RNAs during Genotoxic Stress-Induced Cell Death in Human Glioma Cells. J. Neurooncol. 2015, 122, 283–292. [Google Scholar] [CrossRef]
- Ruan, Z.; Ma, H.; Li, J.; Liu, H.; Jia, H.; Li, F. The Long Non-Coding RNA NEAT1 Contributes to Extracellular Matrix Degradation in Degenerative Human Nucleus Pulposus Cells. Exp. Biol. Med. 2018, 243, 595–600. [Google Scholar] [CrossRef]
- Luan, L.; Hu, Q.; Wang, Y.; Lu, L.; Ling, J. Knockdown of LncRNA NEAT1 Expression Inhibits Cell Migration, Invasion and EMT by Regulating the MiR-24-3p/LRG1 Axis in Retinoblastoma Cells. Exp. Ther. Med. 2021, 21, 367. [Google Scholar] [CrossRef] [PubMed]
- Du, X.-T.; Wang, X.-Y.; Zheng, Y.-H.; Liu, D.-P. Propofol Suppresses the Growth and Invasion of Cervical Carcinoma Cells by Inhibiting MIR155HG. Aging 2021, 13, 24464–24475. [Google Scholar] [CrossRef] [PubMed]
- Cesmeli, S.; Goker Bagca, B.; Caglar, H.O.; Ozates, N.P.; Gunduz, C.; Biray Avci, C. Combination of Resveratrol and BIBR1532 Inhibits Proliferation of Colon Cancer Cells by Repressing Expression of LncRNAs. Med. Oncol. 2021, 39, 12. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Alduais, Y.; Zhang, K.; Zhu, X.; Chen, B. CCAT1/FABP5 Promotes Tumour Progression through Mediating Fatty Acid Metabolism and Stabilizing PI3K/AKT/MTOR Signalling in Lung Adenocarcinoma. J. Cell. Mol. Med. 2021, 25, 9199–9213. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Shao, M.; Yang, J.; Fang, M.; Liu, S.; Lou, D.; Gao, R.; Liu, Y.; Li, A.; Lv, Y.; et al. Curcumin Enhances Radiosensitization of Nasopharyngeal Carcinoma via Mediating Regulation of Tumor Stem-like Cells by a CircRNA Network. J. Cancer 2020, 11, 2360–2370. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Guo, H.; Zong, Y.; Zhao, X. Curcumin Restrains Hepatocellular Carcinoma Progression Depending on the Regulation of the Circ_0078710/MiR-378b/PRIM2 Axis. J. Recept. Signal Transduct. Res. 2022, 42, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, B.; Xu, P.; Yang, B. Integrated Whole Transcriptome Profiling and Bioinformatics Analysis for Revealing Regulatory Pathways Associated With Quercetin-Induced Apoptosis in HCT-116 Cells. Front. Pharmacol. 2019, 10, 798. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhu, D.; Liu, S.; Shao, M.; Liu, Y.; Li, A.; Lv, Y.; Huang, M.; Lou, D.; Fan, Q. Curcumin Enhances Radiosensitization of Nasopharyngeal Carcinoma by Regulating CircRNA Network. Mol. Carcinog. 2020, 59, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Tao, Y.; Yuan, Y.; Qu, W.; Wang, W. Curcumin Suppresses Renal Carcinoma Tumorigenesis by Regulating Circ-FNDC3B/MiR-138-5p/IGF2 Axis. Anticancer Drugs 2021, 32, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lee, I.-M.; Zhang, S.M.; Blumberg, J.B.; Buring, J.E.; Sesso, H.D. Dietary Intake of Selected Flavonols, Flavones, and Flavonoid-Rich Foods and Risk of Cancer in Middle-Aged and Older Women. Am. J. Clin. Nutr. 2009, 89, 905–912. [Google Scholar] [CrossRef] [PubMed]
- D’Arcy, M.S. A Review of Biologically Active Flavonoids as Inducers of Autophagy and Apoptosis in Neoplastic Cells and as Cytoprotective Agents in Non-Neoplastic Cells. Cell Biol. Int. 2022, 46, 1179–1195. [Google Scholar] [CrossRef] [PubMed]
- Drețcanu, G.; Știrbu, I.; Leoplold, N.; Cruceriu, D.; Danciu, C.; Stănilă, A.; Fărcaș, A.; Borda, I.M.; Iuhas, C.; Diaconeasa, Z. Chemical Structure, Sources and Role of Bioactive Flavonoids in Cancer Prevention: A Review. Plants 2022, 11, 1117. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.; Osipo, C. Targeting Breast Cancer Stem Cells Using Naturally Occurring Phytoestrogens. Int. J. Mol. Sci. 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, Y.; Kumazoe, M.; Tachibana, H. 67-KDa Laminin Receptor-Mediated Cellular Sensing System of Green Tea Polyphenol EGCG and Functional Food Pairing. Molecules 2022, 27, 5130. [Google Scholar] [CrossRef] [PubMed]
| miRs | miR-16 | miR-22 | miR-34a | miR-141 | miR-145 | miR-146a | miR-200c |
|---|---|---|---|---|---|---|---|
| Polyphenols | CUR Yang et al. [9] EGCG Tsang et al. [10] QUE Sonoki et al. [11]; Zhao et al. [12] RES Hagiwara et al. [13]; Azimi et al. [14] | CUR Sun et al. [15]; Sreenivasan et al. [16]; Sibbesen et al. [17] EGCG Li et al. [18] QUE Zhang et al. [19] | CUR Guo et al. [20]; Sun et al. [21]; Toden et al. [22]; Sun et al. [15] EGCG Chakrabarti et al. [23]; Li et al. [18]; Chakrabarti et al. [24]; Toden et al. [25]; Mostafa et al. [26] GEN Hsieh et al. [27]; Xia et al. [28]; Chiyomaru et al. [29] RES Hagiwara et al. [13]; Otsuka et al. [30]; Kumazaki et al. [31]; Yao et al. [32] | CUR Toden et al. [33] EGCG Gordon et al. [34] GEN Chiyomaru et al. [35] RES Hagiwara et al. [13] | CUR Mirgani et al. [36]; Liu et al. [37] EGCG Toden et al. [25] GEN Wei et al. [38] QUE Zhou et al. [39] RES Sachdeva et al. [40] | CUR Wu et al. [41] GEN Li et al. [42] QUE Tao et al. [43] | CUR Toden et al. [33]; Soubani et al. [44] EGCG Toden et al. [25] RES Hagiwara et al. [13]; Dermani et al. [45] |
| Targets * | Bcl-2↓ CUR: Yang et al. [9]; EGCG: Tsang et al. [10] HOXA10↓ QUE: Zhao et al. [12] | SP1↓, ESR1↓ CUR: Sun et al. [15] Erbb3↓ CUR: Sreenivasan et al. [16] NCoA1↓, HDAC6↓, MYCBP↓, PTEN↓, CUR: Sibbesen et al. [17] Wnt1/β-catenin↓ QUE: Zhang et al. [19] | Bcl-2↓, Bmi-1↓ CUR: Guo et al. [20] Bcl-2↓, CDK4↓, Cyclin D1↓ CUR: Sun et al. [21] Cyclin D↓, c-Myc↓, CDK6↓, Bcl-2↓ CUR: Toden et al. [22] miR-92↓, miR-93↓, miR-106b↓, miR-7-1↑, miR-34a↑, miR-99a↑ EGCG: Chakrabarti et al. [23] EMT↓, RTCB↓, ROS↑ GEN: Hsieh et al. [27] HOTAIR↓ GEN: Chiyomaru et al. [29] Notch-1↓ GEN: Xia et al. [28] Sirt1↓ via E2F3 RES: Kumazaki et al. [31] HNRNPA1↓ RES: Otsuka et al. [30] Bcl-2↓ RES: Yao et al. [32] | EMT↓ CUR: Toden et al. [33] HOTAIR↓ GEN: Chiyomaru et al. [35] Cancer stemness↓ RES: Hagiwara et al. [13] | Oct4↓, SOX-2↓, Oct4B1↓, CUR: Mirgani et al. [36] Oct4↓, CD44↓, CD133↓, Cyclin D1↓, Cdk4↓ CUR: Liu et al. [37] c-Myc↓ EGCG: Toden et al. [25] ABCE1↓ GEN: Wei et al. [38] Caspase-3↑ QUE: Zhou et al. [39] | NF-κB↓ CUR: Wu et al. [41] EGFR↓ MTA-2↓, IRAK-1↓, NF-κB↓ GEN Li et al. [42] Caspase 3↑, Bax↑, EGFR↓ QUE: Tao et al. [43] | EMT↓ CUR: Toden et al. [33] PTEN↑, MT1-MMP↓ CUR: Soubani et al. [44] Cancer stemness↓ EGCG: Toden et al. [25] Cancer stemness↓ RES: Hagiwara et al. [13] EMT↓ via vimentin, ZEB1, E-cadherin RES: Dermani et al. [45] |
| miRs | miR-20a | miR-21 | miR-25 | miR-27a | miR-93 | miR-106b | miR-155 | miR-221 |
|---|---|---|---|---|---|---|---|---|
| Polyphenols | CGA Huang et al. [46] CUR Gandhy et al. [47] EGCG Mirzaaghaei et al. [48] RES Dhar et al. [49]; Dhar et al. [50] | CGA Wang et al. [51] CUR Mudduluru et al. [52]; Subramaniam et al. [53]; Zhang et al. [54]; Taverna et al. [55]; Yallapu et al. [56] EGCG Fix et al. [57] **; Siddiqui et al. [58] GEN Zaman et al. [59] RES Tili et al. [60]; Sheth et al. [61]; Liu et al. [62]; Li et al. [63]; Zhou et al. [64] | CUR Sun et al. [15] EGCG Fix et al. [57] **; Gordon et al. [34]; Zan et al. [65] RES Tili et al. [60] | CUR Toden et al. [22]; Noratto et al. [66] EGCG Fix et al. [57] ** GEN Xia et al. [67]; Xu et al. [68]; Sun et al. [69] | CGA Huang et al. [46] EGCG Chakrabarti et al. [23]; Chakrabarti et al. [24] RES Singh et al. [70] | CGA Huang et al. [46] EGCG Chakrabarti et al. [23] RES Dhar et al. [50]; Dhar et al. [49] | CGA Zeng et al. [71] CUR Ma et al. [72] GEN de la Parra et al. [73] QUE Boesch-Saadatmandi et al. [74] RES Tili et al. [75] | CUR Zhang et al. [76]; Allegri et al. [77] EGCG Allegri et al. [77] GEN Chen et al. [78] |
| Targets * | p21↑ CGA: Huang et al. [46] PTEN↑ RES: Dhar et al. [49] | Smad7↑ CGA: Wang et al. [51] PDCD4↑ CUR: Mudduluru et al. [52] PTEN↑ CUR: Zhang et al. [54] PTEN↑ CUR: Taverna et al. [55] p21↑, p38 MAPK↑, Cyclin E2↓ GEN: Zaman et al. [59] PDCD4↑ RES: Sheth et al. [61] Bcl-2↓ RES: Liu et al. [62] NF-κB↓ RES: Liu et al. [63] AKT↓, Bcl-2↓ RES: Zhou et al. [64] | p53↑ EGCG: Gordon et al. [34] PARP1↑, Caspases 3↑, Caspases 9↑ EGCG: Zan et al. [65] | Cyclin E1↓, c-Myc↓ via FBXW7 CUR: Toden et al. [22] ZBTB10-Sp↑ CUR: Noratto et al. [66] Sp1↓, Sp3↓ Sp4↓, EGFR↓, hepatocyte growth factor receptor↓, survivin↓, Bcl-2↓, Cyclin D1↓, NFκB↓, ZBTB4↑ CUR: Grandhy et al. [47] Spry2↑ GEN: Xu et al. [68] | p21↑ CGA: Huang et al. [46] Caspase 8↑, tBid↑, Calpain↑, Caspase 3↑ EGCG: Chakrabarti et al. [23] | p21↑ CGA: Huang et al. [46] PTEN↑ RES: Dhar et al. [50]; Dhar et al. [49] | Inflammation↓ via NF-κB/NLRP3 CGA: Zeng et al. [71] SOCS1↓, IL-6↓, CUR: Ma et al. [72] PTEN↑, FOXO3a↑ GEN: de la Parra et al. [73] AP-1↓ via miR-663 RES: Tili et al. [75] | PTEN↑, p27↑, p57↑, PUMA↑ CUR: Sarkar et al. [79] FGF2↓, MMP2↓, VEGF↓, HGF↓, CUR: Zhang et al. [76] miR-21↓, miR-146b↓, miR-221↓, miR-222↓ CUR: Allegri et al. [77] miR-221↓, EGCG: Allegri et al. [77] ARHI↑ GEN:Chen et al. [78] |
| CUR | EGCG | GEN | QUE | RES | |
|---|---|---|---|---|---|
| miR-7 SET8↓, Bcl-2↓, p53↑ [80]; Skp2↓, p57↑, p21↑ [81] miR-9 AKT↓, FOXO1↓ [82]; GSK-3β↑, β-catenin↑, Cyclin D1↓ [83] miR-15a Bcl-2↓ [9]; WT1↓ [84] miR-16-1 WT1↓ [84] miR-28-5p BECN1↓ [85] miR-29a DNMT1↓, 3A↓, 3B↓ [86] miR-30c-5p MTA1↓ [87] miR-33b HMGA2↓ [88]; XIAP↓ [89] miR-98 LIN28A↓, MMP2↓, MMP9↓ [90] miR-99a JAK1↓, STAT1↓, STAT3↓ [91] miR-101 EZH2↓, EpCAM↓ [92]; Notch1↓ [93]; EZH2↓ [94] miR-124 Midkine↓ [95] | miR-125a ERRα↓ [96] miR-138 Smad4↓, NF-kB↓, Cyclin D3↓ [97] miR-143 NF-kB↓ [98]; PGK1↓ [99]; Autophagy via ATG2B↓ [100] miR-181b CXCL1↓ [101] miR-185 DNMT1↓, 3A↓, 3B↓ [86] miR-192-5p XIAP↓ [102]; PI3K↓, AKT↓ [103]; Wnt/β-catenin↓ [104] miR-196b ** BCR-ABL↓ [55] miR-206 mTOR↓, AKT↓ [105] miR-215 XIAP↓ [102] miR-340 XIAP↓ [106] miR-384 circ-PRKCA↓ [107] miR-491 PEG10↓ [108] miR-593 MDR1↓ [109] | miR-15b STIM2↓, Orai1↓ [110] miR-29b KDM2A↓ [111] miR-485-5p RXRα↓ [112] let-7b HMGA2↓ [113] | miR-574-3p RAC1↓, EGFR↓, EP300↓ [114] miR-1469 Mcl1↓ [115] let-7d THBS1↓ [116] | miR-1-3p TAGLN2↓ [117] miR-16-5p WEE1↓ [118] miR-22 Wnt1↓ [19] miR-34a-5p SNHG7↓ [119] miR-142-3p HSP70 ↓ [120] miR-197 IGFBP5↓ [121] miR-200b-3p Notch1↓ [122] miR-217 KRAS↓ [123] miR-503-5p Cyclin D1↓ [124] miR-1254 CD36↓ [125] miR-1275 IGF2BP1↓, IGF2BP3↓ [126] let7-a KRAS↓ [127] let-7c Numbl/Notch1↑ [128] | miR-424-3p Galectin-3↓ [129] |
| CGA | CUR | EGCG | GEN | QUE | RES |
|---|---|---|---|---|---|
| miR-17 p21↑, G0/G1 arrest↑ [46] | miR-19a,b PTEN↑ [131] miR-125a-5p TP53↑ [132] miR-130a Nkd2↑ [133] miR-7641 p16↑ [134] | miR-98-5p CTR1↑ [135] | miR-23b-3p PTEN↑ [136] miR-151a-5p CASZ1↑, IL1RAPL1↑, SOX17↑, N4BP1↑, ARHGDIA↑ [137] miR-155 PTEN↑ [73] miR-221 miR-222 ARHI↑ [78] miR-223 Fbw7↑ [138] miR-223 E-cadherin↑ [139] miR-873-5p FOXM1↓ [140] miR-1260b sFRP1↑, Smad4↑, Dkk2↑ [141,142] | miR-30d-5p Notch↓ Wnt↓ [143] | miR-196b ** miR-1290 IGFBP3↑ [130] |
| lncR | Upregulation | Downregulation | Effects of Polyphenols on Proposed Targets of lncRs (↑, Upregulation; ↓, Downregulation) |
|---|---|---|---|
| AF085935 | EGCG [177] | Not specified | |
| AK001796 | RES [193] | Cell-cycle arrest↑ [193] | |
| AK294004 | CUR [156] | Cyclin D1↓ [156] | |
| CCAT1 | RES [205] | PI3K/AKT/mTOR↓ [206] | |
| DLEU2 | RES [194] | Cyclins E1 and D1↓ [195] | |
| GAS5 | CGA [152] CUR [157] | miR-23a↓ by sponging [152] | |
| H19 | RES (at 50 μM) [196] | CUR [159,160,161] RES (at 200 μM) [162] | Cell cycle arrest at S-phase↑ [196], p53↑ [160] EMT↓, invasion and migration via upregulating Snail [161] |
| HOTAIR | GEN [29,35,182,183] RES, [205] | PI3K/AKT/mTOR signaling pathway↓ [184,185] | |
| KCNQ1OT1 | CUR [162] | Bcl-2↓ via miR-497 [162] | |
| LINC00511 | EGCG [111] | miR-29b↑ [111] | |
| LINC00691 | CUR [163] | AKT↓ [163] | |
| LINC-PINT | CUR [164] | Cell cycle arrest at G2/M↑ [164] | |
| MALAT1 | RES [196] | QUE [187,188,189] RES [197] | Wnt/β-catenin signaling↓ [197] |
| MEG3 | CUR [165,167] | PTEN↑ [167] | |
| NEAT1 | EGCG [135,178] | EGCG [179] QUE [190] RES [198] | ERK1/2↓ [178] miR-98-5p↑ [135] by sponging Cancer cell stemness↓ [179] |
| NRB2 | CUR [168] | AMPK/mTOR pathway↑ [168] | |
| PANDAR | CUR [169] | Bax↑ via upregulation of PUMA [169,170] | |
| PCAT29 | RES [199] | PTEN↑ via downregulation of miR-494 [200] | |
| ROR | CUR [37,172] | Wnt/β-catenin↓ [172] Oct4↓ via sponging miR-145 [37] | |
| SNHG7 | QUE [119] | AKT/mTOR↓ [191] | |
| SOX2OT variant 7 | EGCG [180] | Cancer cell stemness↓ in combination with doxorubicin [180] | |
| TTTY18 | GEN [186] | AKT↓ [186] | |
| TUG1 | CGA [153] | Not specified | |
| UCA1 | CUR [174] QUE [192] | Wnt/mTOR↓ [174] c-Myc↓ by sponging miR-124 [175] | |
| XIST | CUR [176] | p21↑ [176] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayakawa, S.; Ohishi, T.; Oishi, Y.; Isemura, M.; Miyoshi, N. Contribution of Non-Coding RNAs to Anticancer Effects of Dietary Polyphenols: Chlorogenic Acid, Curcumin, Epigallocatechin-3-Gallate, Genistein, Quercetin and Resveratrol. Antioxidants 2022, 11, 2352. https://doi.org/10.3390/antiox11122352
Hayakawa S, Ohishi T, Oishi Y, Isemura M, Miyoshi N. Contribution of Non-Coding RNAs to Anticancer Effects of Dietary Polyphenols: Chlorogenic Acid, Curcumin, Epigallocatechin-3-Gallate, Genistein, Quercetin and Resveratrol. Antioxidants. 2022; 11(12):2352. https://doi.org/10.3390/antiox11122352
Chicago/Turabian StyleHayakawa, Sumio, Tomokazu Ohishi, Yumiko Oishi, Mamoru Isemura, and Noriyuki Miyoshi. 2022. "Contribution of Non-Coding RNAs to Anticancer Effects of Dietary Polyphenols: Chlorogenic Acid, Curcumin, Epigallocatechin-3-Gallate, Genistein, Quercetin and Resveratrol" Antioxidants 11, no. 12: 2352. https://doi.org/10.3390/antiox11122352
APA StyleHayakawa, S., Ohishi, T., Oishi, Y., Isemura, M., & Miyoshi, N. (2022). Contribution of Non-Coding RNAs to Anticancer Effects of Dietary Polyphenols: Chlorogenic Acid, Curcumin, Epigallocatechin-3-Gallate, Genistein, Quercetin and Resveratrol. Antioxidants, 11(12), 2352. https://doi.org/10.3390/antiox11122352






