Palmitic Acid Induced a Long-Lasting Lipotoxic Insult in Human Retinal Pigment Epithelial Cells, which Is Partially Counteracted by TRAIL
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Treatments
2.3. Cell Viability, Proliferation and Apoptosis Evaluation Assays
2.4. Evaluation of Real-Time Effect of Lipotoxicity and TRAIL Treatments
2.5. Assessment of Mitochondrial Morphology
2.6. Analysis of Oxidative Stress
2.7. Evaluation of Surface Expression of TRAIL Receptors
2.8. Cytokines’ Quantification
2.9. Statistical Analysis
3. Results
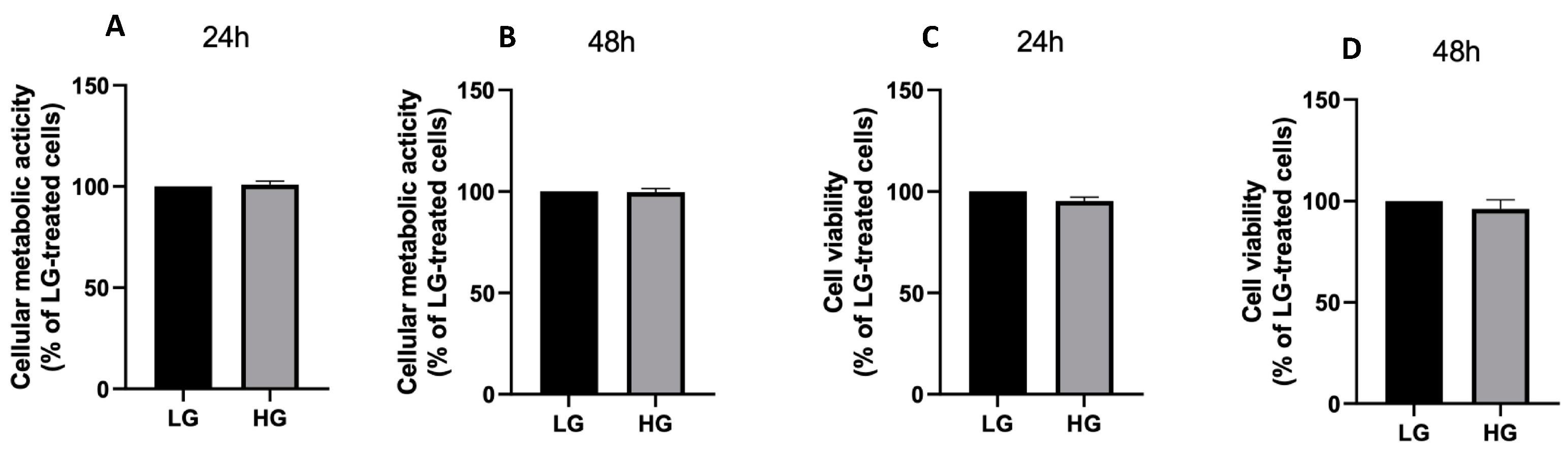
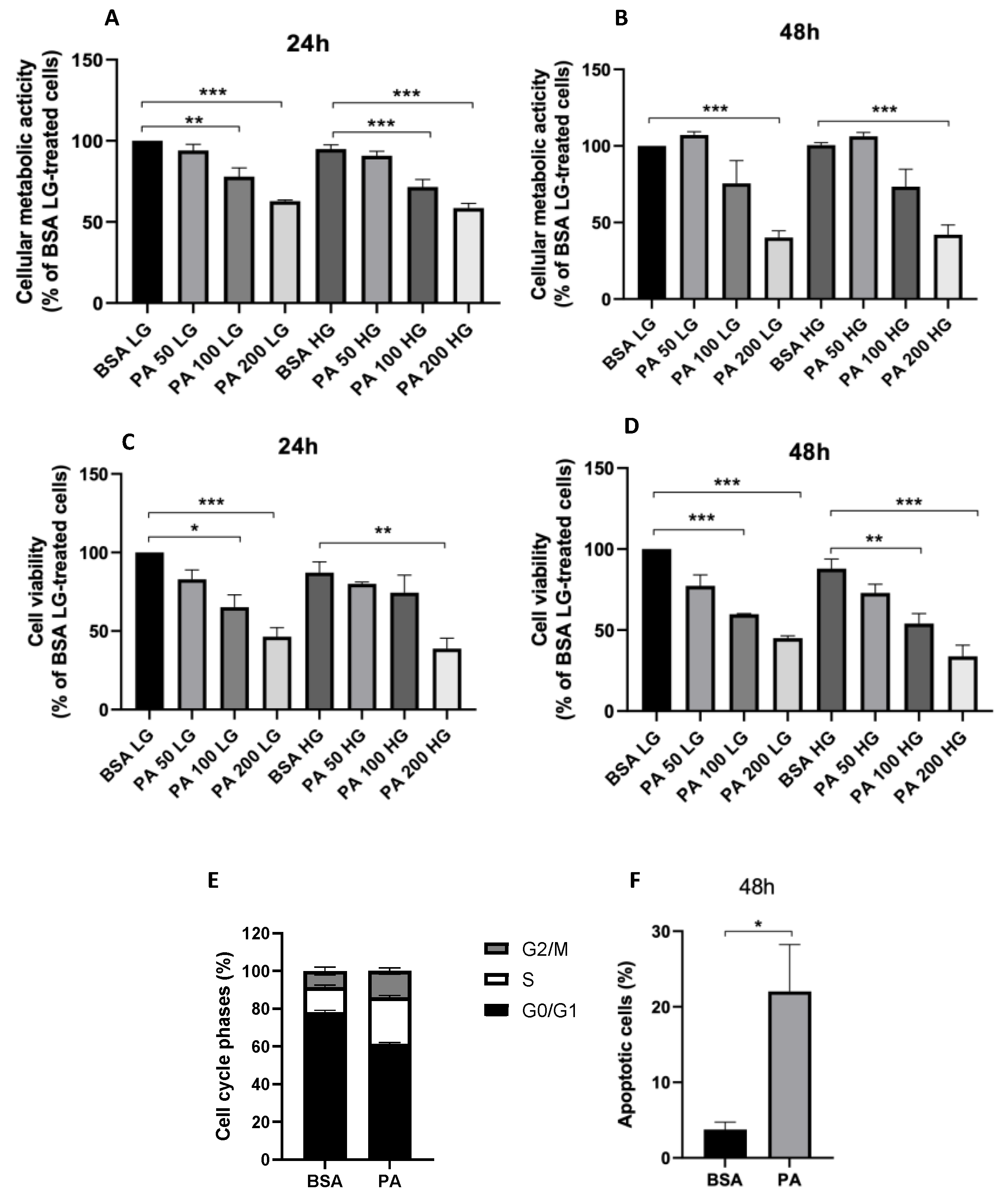
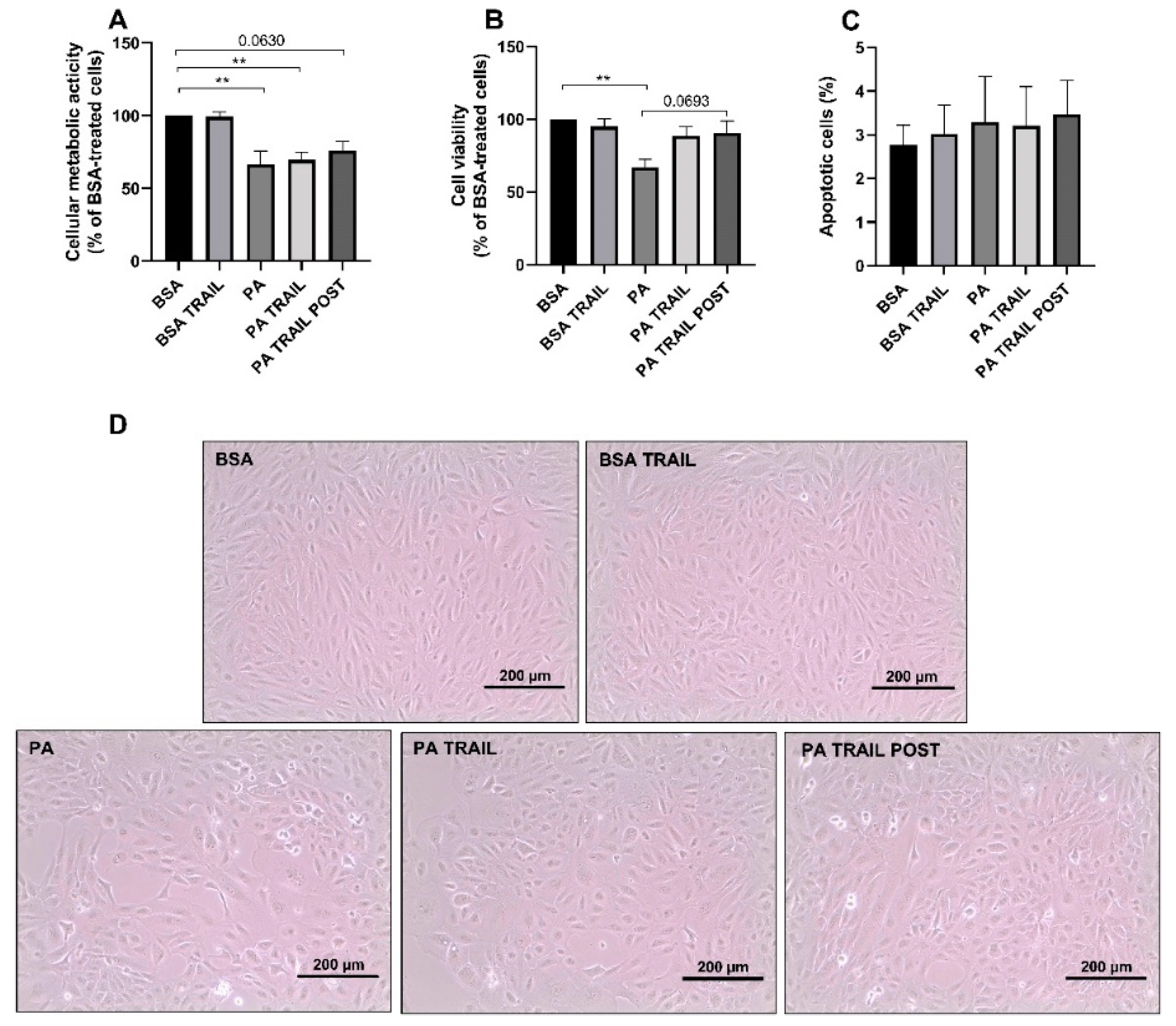
3.1. Effects of High Glucose Concentration and PA on ARPE-19 Cell Viability
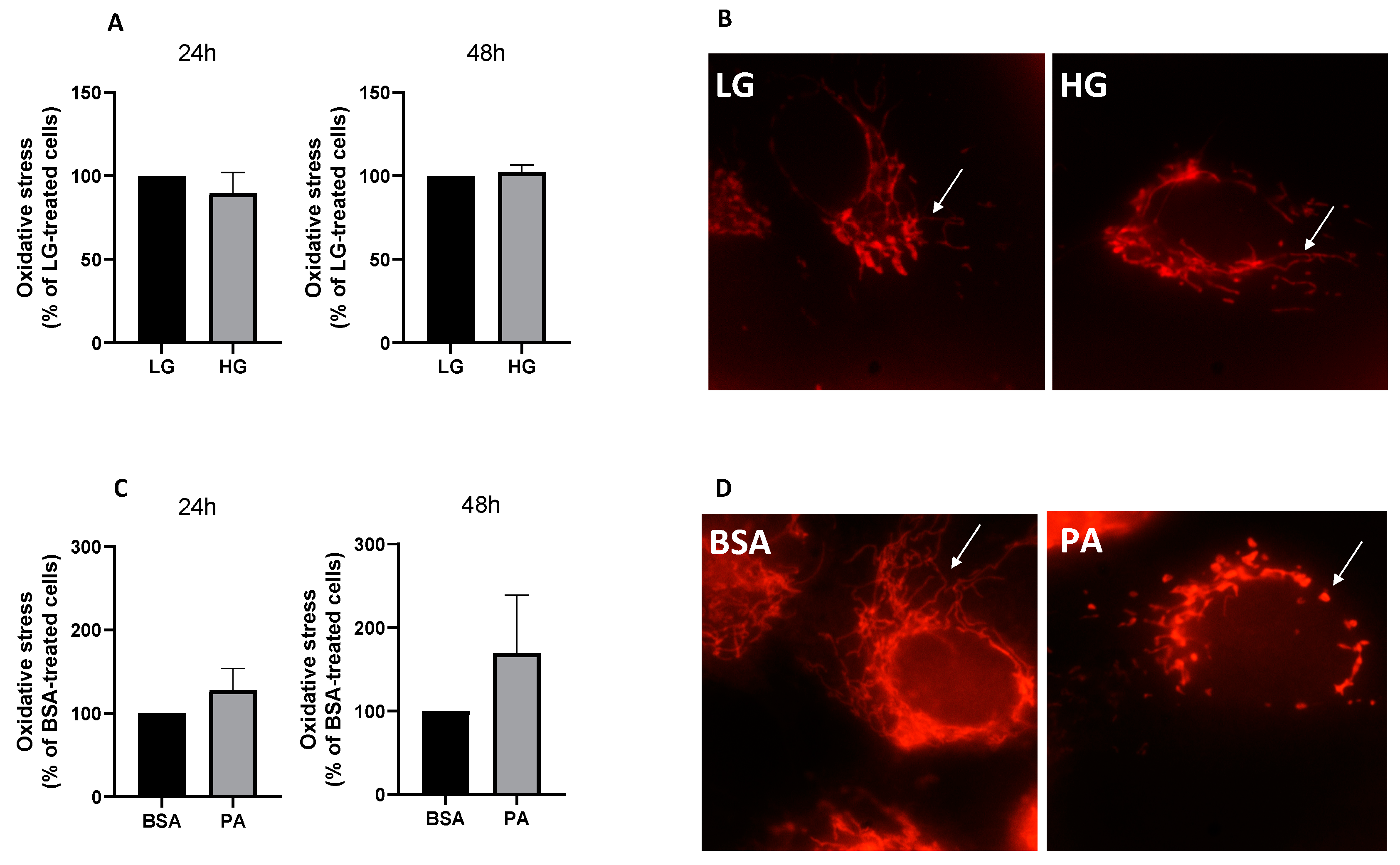
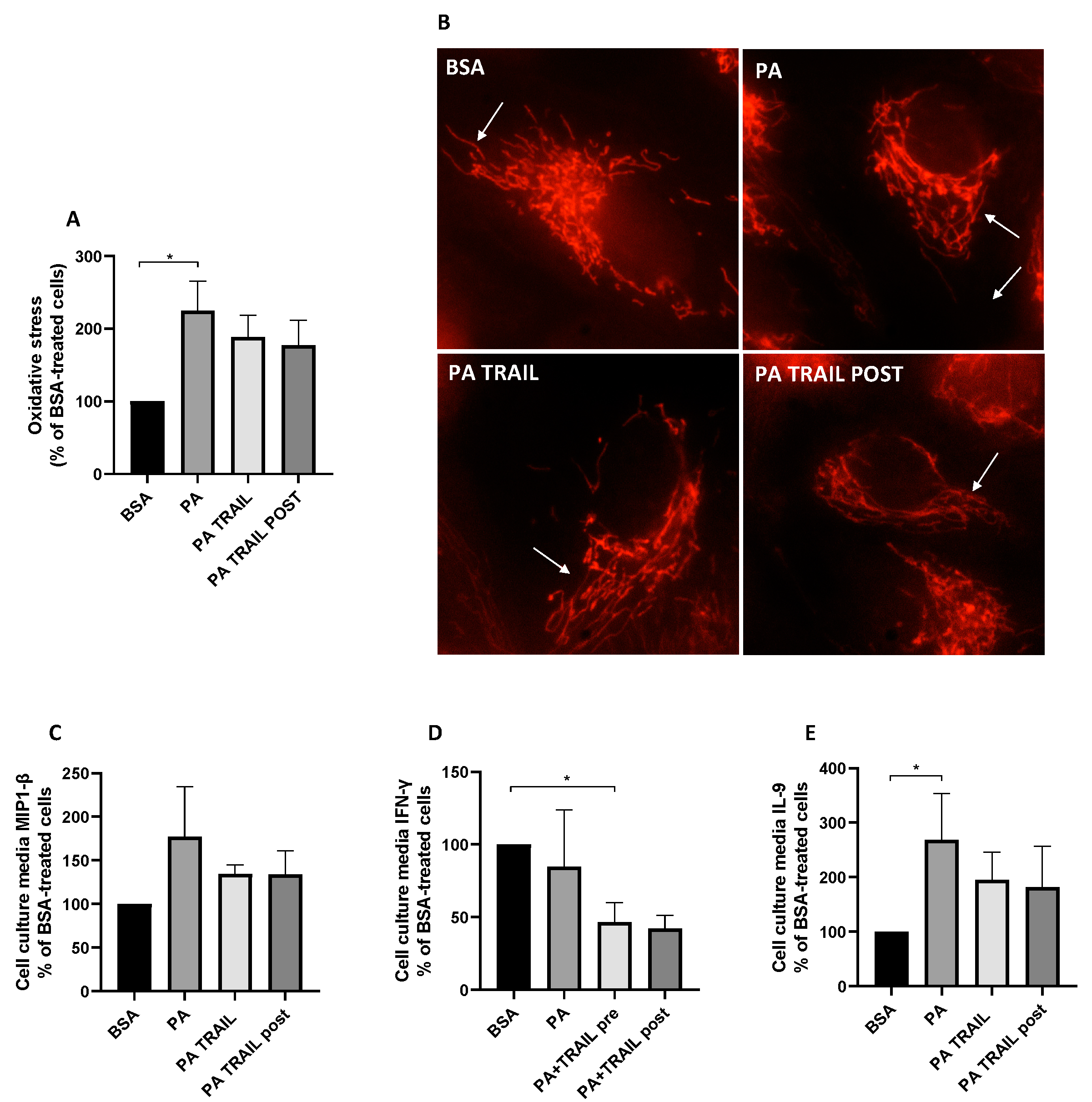
3.2. Effect of PA on Oxidative Stress and Mitochondrial Morphology
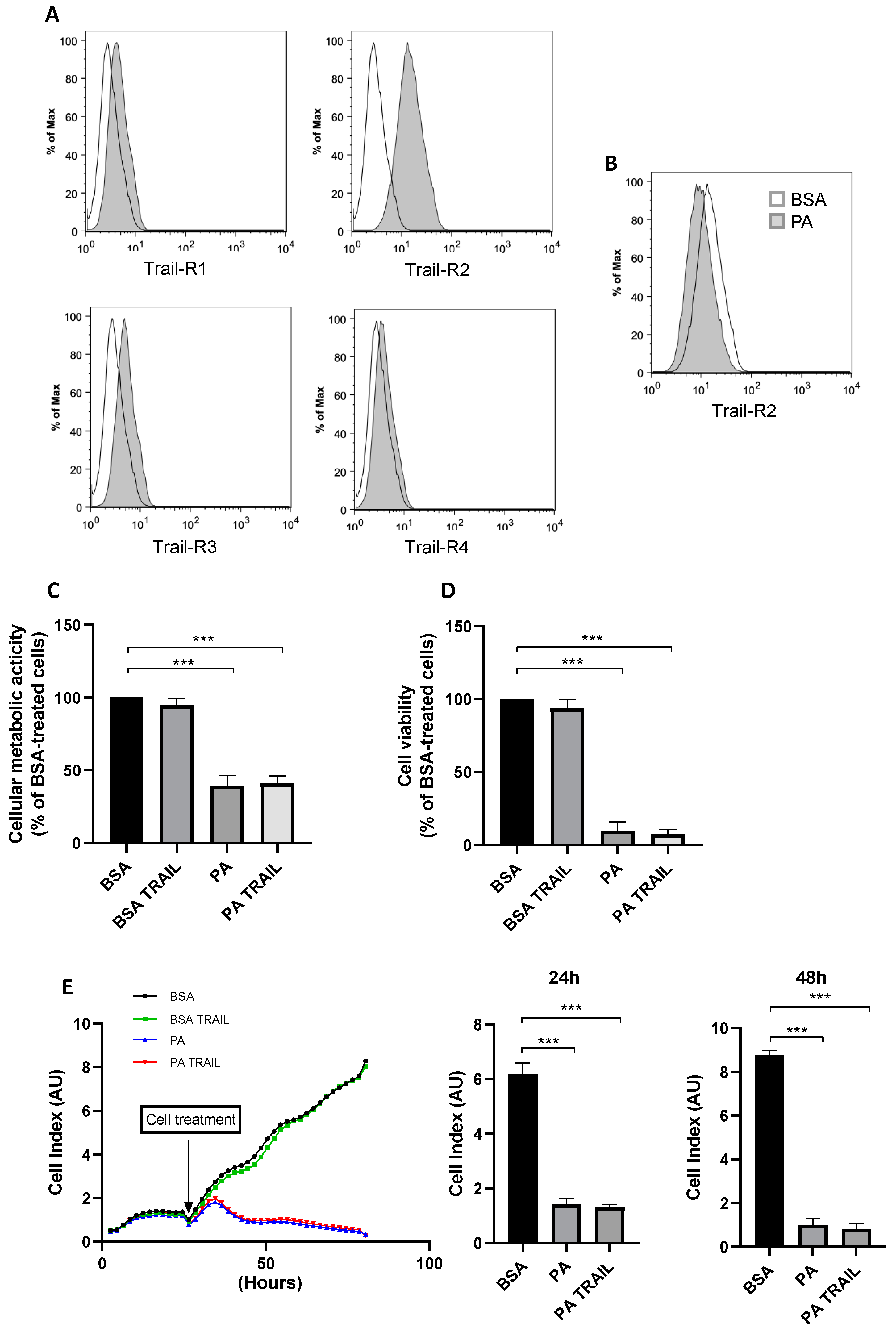
3.3. Characterization of TRAIL Receptors and Effect of Recombinant TRAIL on PA-Induced Cytotoxicity
3.4. Characterization of the Cytotoxic Insult of PA after Its Removal
3.5. Characterization of the Molecular Mechanisms Underlying the Persistence of PA-Induced Cytotoxicity and the Protective Effect of TRAIL
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, T.Y.; Cheung, C.M.; Larsen, M.; Sharma, S.; Simó, R. Diabetic retinopathy. Nat. Rev. Dis. Primers 2016, 17, 16012. [Google Scholar] [CrossRef] [PubMed]
- Hegde, K.; Varma, S. Electron Impact Mass Spectroscopic Studies on Mouse Retinal Fatty Acids: Effect of diabetes. Ophthalmic Res. 2009, 42, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Ontko, C.D.; Capozzi, M.E.; Kim, M.J.; McCollum, G.W.; Penn, J.S. Cytochrome P450-epoxygenated fatty acids inhibit Müller glial inflammation. Sci. Rep. 2021, 11, 9677. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, A.J.; Grant, M.B.; Busik, J.V. Lipids, hyperreflective crystalline deposits and diabetic retinopathy: Potential systemic and retinal-specific effect of lipid-lowering therapies. Diabetologia 2022, 65, 587–603. [Google Scholar] [CrossRef] [PubMed]
- Alnahdi, A.; John, A.; Raza, H. Augmentation of Glucotoxicity, Oxidative Stress, Apoptosis and Mitochondrial Dysfunction in HepG2 Cells by Palmitic Acid. Nutrients 2019, 11, 1979. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, J.; Costa, G.; Renaud, J.; Moitié, A.; Glémet, H.; Sergi, D.; Martinoli, M.-G. The Neuroinflammatory and Neurotoxic Potential of Palmitic Acid Is Mitigated by Oleic Acid in Microglial Cells and Microglial-Neuronal Co-cultures. Mol. Neurobiol. 2021, 58, 3000–3014. [Google Scholar] [CrossRef]
- McLean, F.H.; Campbell, F.M.; Sergi, D.; Grant, C.; Morris, A.C.; Hay, E.A.; MacKenzie, A.; Mayer, C.D.; Langston, R.F.; Williams, L.M. Early and reversible changes to the hippocampal proteome in mice on a high-fat diet. Nutr. Metab. 2019, 16, 57. [Google Scholar] [CrossRef]
- Zeng, Z.-W.; Wen, Q.; Yan, P.-S.; Tang, S.; Zhang, H.-F.; Guo, Y.-Y. Nerve growth factor protects against palmitic acid-induced injury in retinal ganglion cells. Neural Regen. Res. 2016, 11, 1851–1856. [Google Scholar] [CrossRef]
- Yan, P.; Tang, S.; Zhang, H.; Guo, Y.; Zeng, Z.; Wen, Q. Palmitic acid triggers cell apoptosis in RGC-5 retinal ganglion cells through the Akt/FoxO1 signaling pathway. Metab. Brain Dis. 2017, 32, 453–460. [Google Scholar] [CrossRef]
- Capozzi, M.E.; Giblin, M.J.; Penn, J.S. Palmitic Acid Induces Müller Cell Inflammation that is Potentiated by Co-treatment with Glucose. Sci. Rep. 2018, 8, 5459. [Google Scholar] [CrossRef]
- Yu, F.; Ko, M.L.; Ko, G.Y.-P. Decreased MicroRNA-150 Exacerbates Neuronal Apoptosis in the Diabetic Retina. Biomedicines 2021, 9, 1135. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, L.; Zhou, Y.; Zhang, J.; Chen, L. Gambogic acid ameliorates high glucose– and palmitic acid–induced inflammatory response in ARPE-19 cells via activating Nrf2 signaling pathway: Ex vivo. Cell Stress Chaperones 2021, 26, 367–375. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Lin, C.-W.; Chang, Y.-S.; Chen, P.-H.; Li, C.-Y.; Wu, W.-C.; Kao, Y.-H. Monounsaturated oleic acid modulates autophagy flux and upregulates angiogenic factor production in human retinal pigment epithelial ARPE-19 cells. Life Sci. 2020, 259, 118391. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.; Wong, I.Y.; Wong, T.Y. Ocular Anti-VEGF Therapy for Diabetic Retinopathy: Overview of Clinical Efficacy and Evolving Applications. Diabetes Care 2014, 37, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Blinder, K.J.; Dugel, P.U.; Chen, S.; Jumper, J.M.; Walt, J.G.; Hollander, D.A.; Scott, L.C. Anti-VEGF treatment of diabetic macular edema in clinical practice: Effectiveness and patterns of use (ECHO Study Report 1). Clin. Ophthalmol. 2017, 11, 393–401. [Google Scholar] [CrossRef]
- Bossi, F.; Bernardi, S.; Zauli, G.; Secchiero, P.; Fabris, B. TRAIL Modulates the Immune System and Protects against the Development of Diabetes. J. Immunol. Res. 2015, 2015, 680749. [Google Scholar] [CrossRef]
- Koliaki, C.; Katsilambros, N. Repositioning the Role of Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL) on the TRAIL to the Development of Diabetes Mellitus: An Update of Experimental and Clinical Evidence. Int. J. Mol. Sci. 2022, 23, 3225. [Google Scholar] [CrossRef]
- Cartland, S.P.; Harith, H.; Genner, S.W.; Dang, L.; Cogger, V.C.; Vellozzi, M.; Di Bartolo, B.; Thomas, S.R.; Adams, L.A.; Kavurma, M.M. Non-alcoholic fatty liver disease, vascular inflammation and insulin resistance are exacerbated by TRAIL deletion in mice. Sci. Rep. 2017, 7, 1898. [Google Scholar] [CrossRef]
- Di Bartolo, B.A.; Chan, J.; Bennett, M.R.; Cartland, S.; Bao, S.; Tuch, B.E.; Kavurma, M.M. TNF-related apoptosis-inducing ligand (TRAIL) protects against diabetes and atherosclerosis in Apoe−/− mice. Diabetologia 2011, 54, 3157–3167. [Google Scholar] [CrossRef]
- Zauli, G.; Toffoli, B.; di Iasio, M.G.; Celeghini, C.; Fabris, B.; Secchiero, P. Treatment with Recombinant Tumor Necrosis Factor–Related Apoptosis-Inducing Ligand Alleviates the Severity of Streptozotocin-Induced Diabetes. Diabetes 2010, 59, 1261–1265. [Google Scholar] [CrossRef]
- Secchiero, P.; Corallini, F.; Ceconi, C.; Parrinello, G.; Volpato, S.; Ferrari, R.; Zauli, G. Potential Prognostic Significance of Decreased Serum Levels of TRAIL after Acute Myocardial Infarction. PLoS ONE 2009, 4, e4442. [Google Scholar] [CrossRef] [PubMed]
- Bisgin, A.; Yalcin, A.; Gorczynski, R. Circulating soluble tumor necrosis factor related apoptosis inducing-ligand (TRAIL) is decreased in type-2 newly diagnosed, non-drug using diabetic patients. Diabetes Res. Clin. Pract. 2012, 96, e84–e86. [Google Scholar] [CrossRef] [PubMed]
- Tornese, G.; Iafusco, D.; Monasta, L.; Agnoletto, C.; Tisato, V.; Ventura, A.; Zauli, G.; Secchiero, P. The levels of circulating TRAIL at the onset of type 1 diabetes are markedly decreased in patients with ketoacidosis and with the highest insulin requirement. Acta Diabetol. 2014, 51, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-W.; Liang, W.; Yao, X.-M.; Zhang, L.; Zhu, L.; Yan, C.; Jin, Y.-L.; Yao, Y.-S. Tumour necrosis factor-related apoptosis-inducing ligand expression in patients with diabetic nephropathy. J. Renin-Angiotensin-Aldosterone Syst. 2018, 19, 1470320318785744. [Google Scholar] [CrossRef] [PubMed]
- Xiang, G.; Zhang, J.; Ling, Y.; Zhao, L. Circulating level of TRAIL concentration is positively associated with endothelial function and increased by diabetic therapy in the newly diagnosed type 2 diabetic patients. Clin. Endocrinol. 2014, 80, 228–234. [Google Scholar] [CrossRef]
- Hubert, K.E.; Davies, M.H.; Stempel, A.J.; Griffith, T.S.; Powers, M.R. TRAIL-Deficient Mice Exhibit Delayed Regression of Retinal Neovascularization. Am. J. Pathol. 2009, 175, 2697–2708. [Google Scholar] [CrossRef]
- Secchiero, P.; Perri, P.; Melloni, E.; Martini, A.; Lamberti, G.; Sebastiani, A.; Zauli, G. Decreased levels of soluble TNF-related apoptosis-inducing ligand (TRAIL) in the conjunctival sac fluid of patients with diabetes affected by proliferative retinopathy. Diabet. Med. 2011, 28, 1277–1278. [Google Scholar] [CrossRef]
- Abu El-Asrar, A.M.; Ahmad, A.; Alam, K.; Bittoun, E.; Siddiquei, M.M.; Mohammad, G.; Mousa, A.; De Hertogh, G.; Opdenakker, G. Unbalanced Vitreous Levels of Osteoprotegerin, RANKL, RANK, and TRAIL in Proliferative Diabetic Retinopathy. Ocul. Immunol. Inflamm. 2018, 26, 1248–1260. [Google Scholar] [CrossRef]
- Secchiero, P.; Melloni, E.; Heikinheimo, M.; Mannisto, S.; Di Pietro, R.; Iacone, A.; Zauli, G. TRAIL regulates normal erythroid maturation through an ERK-dependent pathway. Blood 2004, 103, 517–522. [Google Scholar] [CrossRef]
- Sergi, D.; Morris, A.C.; Kahn, D.E.; McLean, F.H.; Hay, E.A.; Kubitz, P.; MacKenzie, A.; Martinoli, M.G.; Drew, J.E.; Williams, L.M. Palmitic acid triggers inflammatory responses in N42 cultured hypothalamic cells partially via ceramide synthesis but not via TLR4. Nutr. Neurosci. 2018, 23, 321–334. [Google Scholar] [CrossRef]
- Zauli, G.; Voltan, R.; Bosco, R.; Melloni, E.; Marmiroli, S.; Rigolin, G.M.; Cuneo, A.; Secchiero, P. Dasatinib Plus Nutlin-3 Shows Synergistic Antileukemic Activity in Both p53wild-type and p53mutated B Chronic Lymphocytic Leukemias by Inhibiting the Akt Pathway. Clin. Cancer Res. 2011, 17, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Melloni, E.; Secchiero, P.; Celeghini, C.; Campioni, D.; Grill, V.; Guidotti, L.; Zauli, G. Functional expression of TRAIL and TRAIL-R2 during human megakaryocytic development. J. Cell. Physiol. 2005, 204, 975–982. [Google Scholar] [CrossRef]
- Eid, S.; Sas, K.M.; Abcouwer, S.F.; Feldman, E.L.; Gardner, T.W.; Pennathur, S.; Fort, P.E. New insights into the mechanisms of diabetic complications: Role of lipids and lipid metabolism. Diabetologia 2019, 62, 1539–1549. [Google Scholar] [CrossRef] [PubMed]
- Sobczak, A.I.S.; Blindauer, C.A.; Stewart, A.J. Changes in Plasma Free Fatty Acids Associated with Type-2 Diabetes. Nutrients 2019, 11, 2022. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Hood, D.A. Oxidative stress-induced mitochondrial fragmentation and movement in skeletal muscle myoblasts. Am. J. Physiol.-Cell Physiol. 2014, 306, C1176–C1183. [Google Scholar] [CrossRef] [PubMed]
- Tuomela, K.; Ambrose, A.R.; Davis, D.M. Escaping Death: How Cancer Cells and Infected Cells Resist Cell-Mediated Cytotoxicity. Front. Immunol. 2022, 13, 867098. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z.-G. Crosstalk of reactive oxygen species and NF-kappaκB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef]
- Sergi, D.; Luscombe-Marsh, N.; Heilbronn, L.K.; Birch-Machin, M.; Naumovski, N.; Lionetti, L.; Proud, C.G.; Abeywardena, M.Y.; O’Callaghan, N. The Inhibition of Metabolic Inflammation by EPA Is Associated with Enhanced Mitochondrial Fusion and Insulin Signaling in Human Primary Myotubes. J. Nutr. 2021, 151, 810–819. [Google Scholar] [CrossRef]
- Sergi, D.; Luscombe-Marsh, N.; Naumovski, N.; Abeywardena, M.; O’Callaghan, N. Palmitic Acid, but Not Lauric Acid, Induces Metabolic Inflammation, Mitochondrial Fragmentation, and a Drop in Mitochondrial Membrane Potential in Human Primary Myotubes. Front. Nutr. 2021, 8, 663838. [Google Scholar] [CrossRef]
- Monnier, L.; Lapinski, H.; Colette, C. Contributions of Fasting and Postprandial Plasma Glucose Increments to the Overall Diurnal Hyperglycemia of Type 2 Diabetic Patients: Variations with increasing levels of HbA(1c). Diabetes Care 2003, 26, 881–885. [Google Scholar] [CrossRef]
- Brownlee, M. The Pathobiology of Diabetic Complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Perdiz, D.; Oziol, L.; Poüs, C. Early mitochondrial fragmentation is a potential in vitro biomarker of environmental stress. Chemosphere 2019, 223, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Jheng, H.-F.; Tsai, P.-J.; Guo, S.-M.; Kuo, L.-H.; Chang, C.-S.; Su, I.-J.; Chang, C.-R.; Tsai, Y.-S. Mitochondrial Fission Contributes to Mitochondrial Dysfunction and Insulin Resistance in Skeletal Muscle. Mol. Cell. Biol. 2012, 32, 309–319. [Google Scholar] [CrossRef]
- Fucho, R.; Casals, N.; Serra, D.; Herrero, L. Ceramides and mitochondrial fatty acid oxidation in obesity. FASEB J. 2017, 31, 1263–1272. [Google Scholar] [CrossRef]
- Calderon, G.D.; Juarez, O.H.; Hernandez, G.E.; Punzo, S.M.; De La Cruz, Z.D. Oxidative stress and diabetic retinopathy: Development and treatment. Eye 2017, 31, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Willems, P.H.; Rossignol, R.; Dieteren, C.E.; Murphy, M.P.; Koopman, W.J. Redox Homeostasis and Mitochondrial Dynamics. Cell Metab. 2015, 22, 207–218. [Google Scholar] [CrossRef]
- Yako, T.; Nakamura, M.; Nakamura, S.; Hara, H.; Shimazawa, M. Pharmacological inhibition of mitochondrial fission attenuates oxidative stress-induced damage of retinal pigmented epithelial cells. J. Pharmacol. Sci. 2021, 146, 149–159. [Google Scholar] [CrossRef]
- Fulda, S. The dark side of TRAIL signaling. Cell Death Differ. 2013, 20, 845–846. [Google Scholar] [CrossRef]
- Secchiero, P.; Zerbinati, C.; Rimondi, E.; Corallini, F.; Milani, D.; Grill, V.; Forti, G.; Capitani, S.; Zauli, G. TRAIL promotes the survival, migration and proliferation of vascular smooth muscle cells. Cell. Mol. Life Sci. 2004, 61, 1965–1974. [Google Scholar] [CrossRef]
- Forde, H.; Harper, E.; Rochfort, K.D.; Wallace, R.G.; Davenport, C.; Smith, D.; Cummins, P.M. TRAIL inhibits oxidative stress in human aortic endothelial cells exposed to pro-inflammatory stimuli. Physiol. Rep. 2020, 8, e14612. [Google Scholar] [CrossRef]
- Batumalaie, K.; Amin, M.A.; Murugan, D.D.; Sattar, M.Z.A.; Abdullah, N.A. Withaferin A protects against palmitic acid-induced endothelial insulin resistance and dysfunction through suppression of oxidative stress and inflammation. Sci. Rep. 2016, 6, 27236. [Google Scholar] [CrossRef] [PubMed]
- Mangali, S.; Bhat, A.; Udumula, M.P.; Dhar, I.; Sriram, D.; Dhar, A. Inhibition of protein kinase R protects against palmitic acid–induced inflammation, oxidative stress, and apoptosis through the JNK/NF-kB/NLRP3 pathway in cultured H9C2 cardiomyocytes. J. Cell. Biochem. 2019, 120, 3651–3663. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Prather, E.R.; Stetskiv, M.; Garrison, D.E.; Meade, J.R.; Peace, T.I.; Zhou, T. Inflammaging and oxidative stress in human diseases: From molecular mechanisms to novel treatments. Int. J. Mol. Sci. 2019, 20, 4472. [Google Scholar] [CrossRef]
- Renshaw, S.; Parmar, J.S.; Singleton, V.; Rowe, S.J.; Dockrell, D.; Dower, S.K.; Bingle, C.; Chilvers, E.; Whyte, M.K.B. Acceleration of Human Neutrophil Apoptosis by TRAIL. J. Immunol. 2003, 170, 1027–1033. [Google Scholar] [CrossRef]
- Bernardi, S.; Milani, D.; Fabris, B.; Secchiero, P.; Zauli, G. TRAIL as biomarker and potential therapeutic tool for cardiovascular diseases. Curr. Drug Targets 2012, 13, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Al-Kharashi, A.S. Role of oxidative stress, inflammation, hypoxia and angiogenesis in the development of diabetic retinopathy. Saudi J. Ophthalmol. 2018, 32, 318–323. [Google Scholar] [CrossRef]
- Kang, Q.; Yang, C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef] [PubMed]
- Forrester, J.V.; Kuffova, L.; Delibegovic, M. The Role of Inflammation in Diabetic Retinopathy. Front. Immunol. 2020, 11, 583687. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sergi, D.; Zauli, E.; Casciano, F.; Secchiero, P.; Zauli, G.; Fields, M.; Melloni, E. Palmitic Acid Induced a Long-Lasting Lipotoxic Insult in Human Retinal Pigment Epithelial Cells, which Is Partially Counteracted by TRAIL. Antioxidants 2022, 11, 2340. https://doi.org/10.3390/antiox11122340
Sergi D, Zauli E, Casciano F, Secchiero P, Zauli G, Fields M, Melloni E. Palmitic Acid Induced a Long-Lasting Lipotoxic Insult in Human Retinal Pigment Epithelial Cells, which Is Partially Counteracted by TRAIL. Antioxidants. 2022; 11(12):2340. https://doi.org/10.3390/antiox11122340
Chicago/Turabian StyleSergi, Domenico, Enrico Zauli, Fabio Casciano, Paola Secchiero, Giorgio Zauli, Matteo Fields, and Elisabetta Melloni. 2022. "Palmitic Acid Induced a Long-Lasting Lipotoxic Insult in Human Retinal Pigment Epithelial Cells, which Is Partially Counteracted by TRAIL" Antioxidants 11, no. 12: 2340. https://doi.org/10.3390/antiox11122340
APA StyleSergi, D., Zauli, E., Casciano, F., Secchiero, P., Zauli, G., Fields, M., & Melloni, E. (2022). Palmitic Acid Induced a Long-Lasting Lipotoxic Insult in Human Retinal Pigment Epithelial Cells, which Is Partially Counteracted by TRAIL. Antioxidants, 11(12), 2340. https://doi.org/10.3390/antiox11122340









