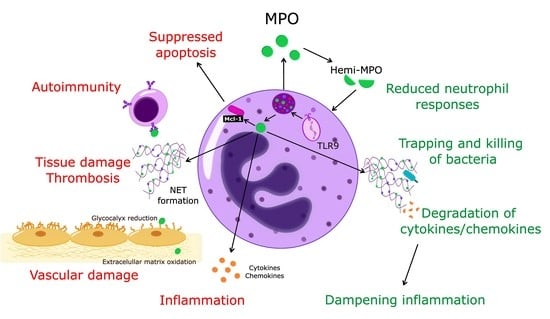
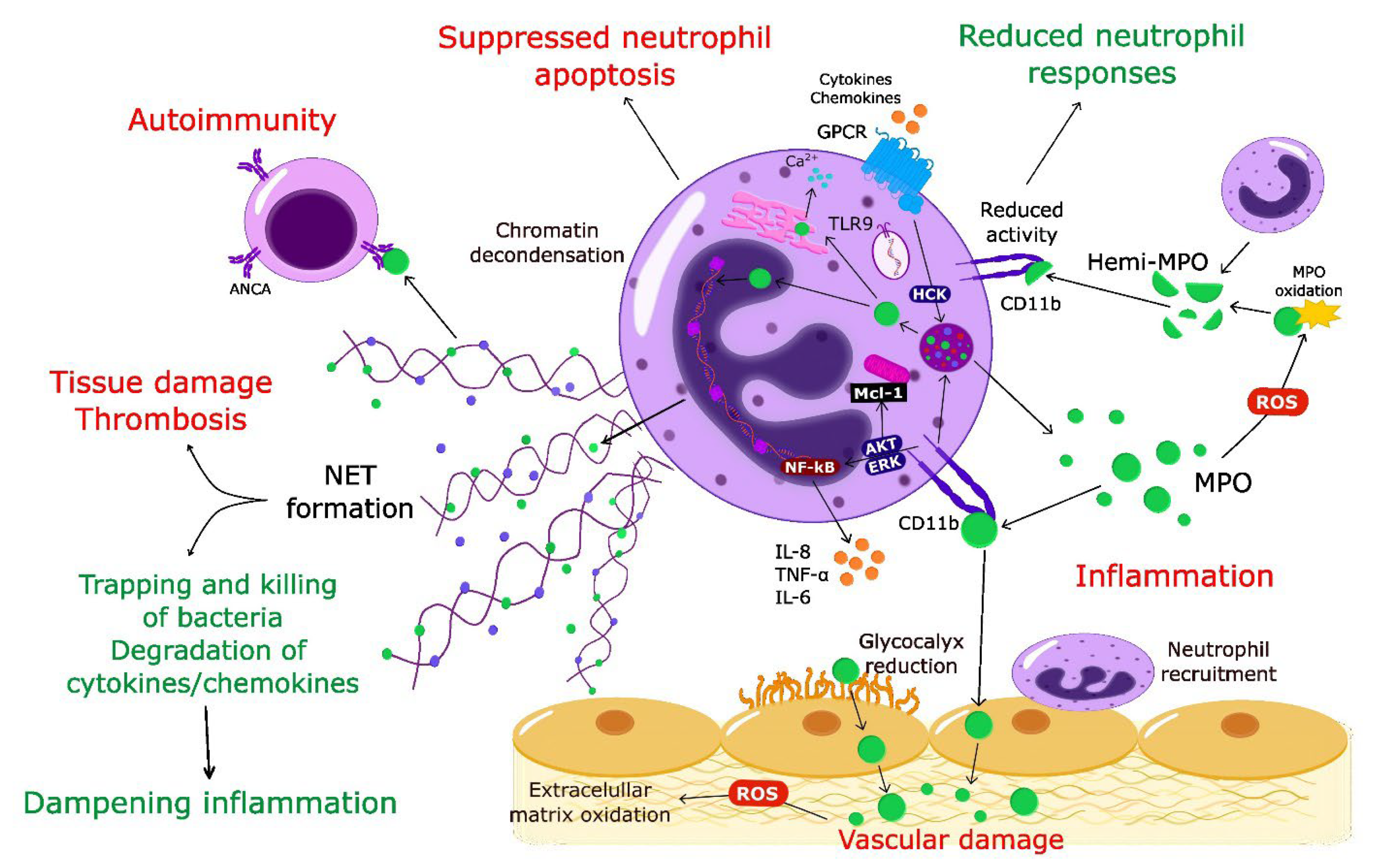
Myeloperoxidase: Regulation of Neutrophil Function and Target for Therapy
Abstract
1. Introduction
2. Myeloperoxidase Expression and Release
3. Enzymatic Properties
4. MPO Regulation of Neutrophil Trafficking
5. MPO, Neutrophil Activation and Phagocytosis
6. MPO Regulation of Neutrophil Lifespan
7. MPO, NET Formation and Autoimmunity
8. Myeloperoxidase as a Therapeutic Target
9. Suppressing MPO Gene and Protein Expression
10. MPO Inhibitors
11. Inhibition of Granule Trafficking, Docking and Degranulation
12. Targeting NET and Silencing the MPO Autoantigens
13. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Kolaczkowska, E.; Kubes, P. Neutrophil Recruitment and Function in Health and Inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Nauseef, W.M.; Borregaard, N. Neutrophils at Work. Nat. Immunol. 2014, 15, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Mayadas, T.N.; Cullere, X.; Lowell, C.A. The Multifaceted Functions of neutrophils. Annu. Rev. Pathol. 2014, 9, 181–218. [Google Scholar] [CrossRef] [PubMed]
- Reeves, E.P.; Lu, H.; Jacobs, H.L.; Messina, C.G.; Bolsover, S.; Gabella, G.; Potma, E.O.; Warley, A.; Roes, J.; Segal, A.W. Killing Activity of Neutrophils is Mediated through Activation of Proteases by K+ flux. Nature 2002, 416, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Liew, P.X.; Kubes, P. The Neutrophil’s Role during Health and Disease. Physiol. Rev. 2019, 99, 1223–1248. [Google Scholar] [CrossRef]
- Malech, H.L.; Gallin, J.I. Current Concepts: Immunology. Neutrophils in Human Diseases. N. Engl. J. Med. 1987, 317, 687–694. [Google Scholar] [CrossRef]
- Gordon, S. Phagocytosis: An Immunobiologic Process. Immunity 2016, 44, 463–475. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Rørvig, S.; Østergaard, O.; Heegaard, N.H.H.; Borregaard, N. Proteome Profiling of Human Neutrophil Granule Subsets, Secretory Vesicles, and Cell Membrane: Correlation with Transcriptome Profiling of Neutrophil Precursors. J. Leukoc. Biol. 2013, 94, 711–721. [Google Scholar] [CrossRef]
- Cassatella, M.; Östberg, N.K.; Tamassia, N.; Soehnlein, O. Biological Roles of Neutrophil-derived Granule Proteins and Cytokines. Trends Immunol. 2019, 40, 648–664. [Google Scholar] [CrossRef]
- Klebanoff, S.J. Myeloperoxidase: Friend and Foe. J. Leukoc. Biol. 2005, 77, 598–625. [Google Scholar] [CrossRef]
- Nathan, C.; Ding, A. Nonresolving inflammation. Cell 2010, 140, 871–882. [Google Scholar] [CrossRef]
- Mantovani, A.; Cassatella, M.A.; Costantini, C.; Jaillon, S. Neutrophils in the Activation and Regulation of Innate and Adaptive Immunity. Nat. Rev. Immunol. 2011, 11, 519–531. [Google Scholar] [CrossRef]
- Baldus, S.; Heeschen, C.; Meinertz, T.; Zeiher, A.M.; Eiserich, J.P.; Münzel, T.; Simoons, M.L.; Hamm, C.W.; CAPTURE Investigators. Myeloperoxidase Serum Levels Predict Risk in Patients with Acute Coronary Syndromes. Circulation 2003, 108, 1440–1445. [Google Scholar] [CrossRef]
- Brennan, M.L.; Penn, M.S.; Van Lente, F.; Nambi, V.; Shishehbor, M.H.; Aviles, R.J.; Goormastic, M.; Pepoy, M.L.; McErlean, E.S.; Topol, E.J.; et al. Prognostic Value of Myeloperoxidase in Patients with Chest Pain. N. Engl. J. Med. 2003, 349, 1595–1604. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Hazen, S.L. Myeloperoxidase and Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1102–1111. [Google Scholar] [CrossRef]
- Kothari, N.; Keshari, R.S.; Bogra, J.; Kohli, M.; Abbas, H.; Malik, A.; Dikshit, M.; Barthwal, M.K. Increased Myeloperoxidase Enzyme Activity in Plasma is an Indicator of Inflammation and Onset of Sepsis. J. Crit. Care 2011, 26, e1–e7. [Google Scholar] [CrossRef]
- Zhu, A.; Ge, D.; Zhang, J.; Teng, Y.; Yuan, C.; Huang, M.; Adcock, I.M.; Barnes, P.J.; Yao, X. Sputum Myeloperoxidase in Chronic Obstructive Pulmonary Disease. Eur. J. Med. Res. 2014, 19, 12. [Google Scholar] [CrossRef]
- Aratani, Y. Myeloperoxidase: Its Role for Host Defense, Inflammation, and Neutrophil Function. Arch. Biochem. Biophys. 2018, 640, 47–52. [Google Scholar] [CrossRef]
- Silvestre-Roig, C.; Hidalgo, A.; Soehnlein, O. Neutrophil Heterogeneity: Implications for Homeostasis and Pathogenesis. Blood 2016, 127, 2173–2181. [Google Scholar] [CrossRef]
- Filep, J.G.; Ariel, A. Inflammation: From Cellular Mechanisms to Immune Cell Education: Neutrophil Heterogeneity and Fate in Inflamed Tissues: Implications for the Resolution of Inflammation. Am. J. Physiol. Cell Physiol. 2020, 319, C510–C532. [Google Scholar] [CrossRef]
- Hansson, M.; Olsson, I.; Nauseef, W.M. Biosynthesis, Processing, and Sorting of Human Myeloperoxidase. Arch. Biochem. Biophys. 2006, 445, 214–224. [Google Scholar] [CrossRef]
- Malle, E.; Furtmüller, P.G.; Sattler, W.; Obinger, C. Myeloperoxidase: A Target for New Drug Development? Br. J. Pharmacol. 2007, 152, 838–854. [Google Scholar] [CrossRef]
- Nauseef, W.M. How human neutrophils kill and degrade microbes: An Integrated View. Immunol. Rev. 2007, 219, 88–102. [Google Scholar] [CrossRef]
- Tobler, A.; Miller, C.W.; Johnson, K.R.; Selsted, M.E.; Rovera, G.; Koeffler, H.P. Regulation of Gene Expression of Myeloperoxidase during Myeloid Differentiation. J. Cell. Physiol. 1988, 136, 215–225. [Google Scholar] [CrossRef]
- Schultz, J.; Kaminker, K. Myeloperoxidase of the Leucocyte of Normal Human Blood. I. Content and Localization. Arch. Biochem. Biophys. 1962, 96, 465–467. [Google Scholar] [CrossRef]
- Borregaard, N.; Cowland, J.B. Granules of the Human Neutrophilic Polymorphonuclear Leukocyte. Blood 1997, 89, 3503–3521. [Google Scholar] [CrossRef] [PubMed]
- Bos, A.; Wever, R.; Roos, D. Characterization and Quantification of the Peroxidase in Human Monocytes. Biochim. Biophys. Acta. BBA—Enzymol. 1978, 525, 37–44. [Google Scholar] [CrossRef]
- Nagra, R.M.; Becher, B.; Tourtellotte, W.W.; Antel, J.P.; Gold, D.; Paladino, T.; Smith, R.A.; Nelson, J.R.; Reynolds, W.F. Immunohistochemical and Genetic Evidence of Myeloperoxidase Involvement in Multiple Sclerosis. J. Neuroimmunol. 1997, 78, 97–107. [Google Scholar] [CrossRef]
- Liu, W.Q.; Zhang, Y.Z.; Wu, Y.; Zhang, J.J.; Li, T.B.; Jiang, T.; Xiong, X.M.; Luo, X.J.; Ma, Q.L.; Peng, J. Myeloperoxidase-Derived Hypochlorous Acid Promotes Ox-LDL-Induced Senescence of Endothelial Cells through a Mechanism Involving β-Catenin Signaling in Hyperlipidemia. Biochem. Biophys. Res. Commun. 2015, 467, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Green, P.S.; Mendez, A.J.; Jacob, J.S.; Crowley, J.R.; Growdon, W.; Hyman, B.T.; Heinecke, J.W. Neuronal Expression of Myeloperoxidase Is Increased in Alzheimer’s Disease. J. Neurochem. 2004, 90, 724–733. [Google Scholar] [CrossRef]
- Maki, R.A.; Tyurin, V.A.; Lyon, R.C.; Hamilton, R.L.; Dekosky, S.T.; Kagan, V.E.; Reynolds, W.F. Aberrant Expression of Myeloperoxidase in Astrocytes Promotes Phospholipid Oxidation and Memory Deficits in a Mouse Model of Alzheimer Disease. J. Biol. Chem. 2009, 284, 3158–3169. [Google Scholar] [CrossRef]
- Chumakov, A.M.; Chumakova, E.A.; Chih, D.; Koeffler, H.P. Molecular Analysis of the Human Myeloperoxidase Promoter Region. Int. J. Oncol. 2000, 16, 4010411. [Google Scholar] [CrossRef]
- Lominadze, G.; Powell, D.W.; Luerman, G.C.; Link, A.J.; Ward, R.A.; McLeish, K.R. Proteomic Analysis of Human Neutrophil Granules. Mol. Cell. Proteom. 2005, 4, 1503–1521. [Google Scholar] [CrossRef]
- Cowland, J.B.; Borregaard, N. The Individual Regulation of Granule Protein mRNA Levels during Neutrophil Maturation Explains the Heterogeneity of Neutrophil Granules. J. Leukoc. Biol. 1999, 66, 989–995. [Google Scholar] [CrossRef]
- Eitzen, G.; Lo, A.N.; Mitchell, T.; Kim, J.D.; Chao, D.V.; Lacy, P. Proteomic Analysis of Secretagogue-stimulated Neutrophils Implicates a Role for Actin and Actin-interacting Proteins in Rac2-mediated Granule Exocytosis. Proteome Sci. 2011, 9, 70. [Google Scholar] [CrossRef]
- Cowland, J.B.; Borregaard, N. Granulopoiesis and Granules of Human Neutrophils. Immunol.Rev. 2016, 273, 11–28. [Google Scholar] [CrossRef]
- Yin, C.; Heit, B. Armed for Destruction: Formation, Function and Trafficking of Neutrophil Granules. Cell Tissue Res. 2018, 371, 455–471. [Google Scholar] [CrossRef]
- Sekheri, M.; El Kebir, D.; Edner, N.; Filep, J.G. 15-Epi-LXA4 and 17-Epi-RvD1 Restore TLR9-Mediated Impaired Neutrophil Phagocytosis and Accelerate Resolution of Lung Inflammation. Proc. Natl. Acad. Sci. USA 2020, 117, 7971–7980. [Google Scholar] [CrossRef]
- Freeman, S.A.; Grinstein, S. Phagocytosis: Receptors, Signal Integration, and the Cytoskeleton. Immunol. Rev. 2014, 262, 193–215. [Google Scholar] [CrossRef]
- Tapper, H.; Karlsson, A.; Mörgelin, M.; Flodgaard, H.; Herwald, H. Secretion of Heparin-Binding Protein from Human Neutrophils Is Determined by Its Localization in Azurophilic Granules and Secretory Vesicles. Blood 2002, 99, 1785–1793. [Google Scholar] [CrossRef]
- Fenna, R.; Zeng, J.; Davey, C. Structure of the Green Heme in Myeloperoxidase. Arch. Biochem. Biophys. 1995, 316, 653–656. [Google Scholar] [CrossRef]
- Fiedler, T.J.; Davey, C.A.; Fenna, R.E. X-Ray Crystal Structure and Characterization of Halide-Binding Sites of Human Myeloperoxidase at 1.8 A Resolution. J. Biol. Chem. 2000, 275, 11964–11971. [Google Scholar] [CrossRef]
- Grishkovskaya, X.I.; Paumann-Page, M.; Tscheliessnig, R.; Stampler, J.; Hofbauer, X.S.; Soudi, M.; Sevcnikar, B.; Oostenbrink, X.C.; Furtmüller, X.P.G.; Djinović-Carugo, X.K.; et al. Structure of Human Promyeloperoxidase (ProMPO) and the Role of the Propeptide in Processing and Maturation. J. Biol. Chem. 2017, 292, 8244–8261. [Google Scholar] [CrossRef]
- Vakhrusheva, T.V.; Grigorieva, D.V.; Gorudko, I.V.; Sokolov, A.V.; Kostevich, V.A.; Lazarev, V.N.; Vasilyev, V.B.; Cherenkevich, S.N.; Panasenko, O.M. Enzymatic and Bactericidal Activity of Myeloperoxidase in Conditions of Halogenative Stress. Biochem. Cell Biol. 2018, 96, 580–591. [Google Scholar] [CrossRef]
- Andrews, P.C.; Parnes, C.; Krinsky, N.I. Comparison of Myeloperoxidase and Hemi-Myeloperoxidase with Respect to Catalysis, Regulation, and Bactericidal Activity. Arch. Biochem. Biophys. 1984, 228, 439–442. [Google Scholar] [CrossRef]
- Galkina, S.I.; Fedorova, N.V.; Serebryakova, M.V.; Arifulin, E.A.; Stadnichuk, V.I.; Gaponova, T.V.; Baratova, L.A.; Sud’ina, G.F. Inhibition of the GTPase Dynamin or Actin Depolymerisation Initiates Outward Plasma Membrane Tubulation/Vesiculation (Cytoneme Formation) in Neutrophils. Biol. Cell 2015, 107, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Galkina, S.I.; Fedorova, N.V.; Serebryakova, M.V.; Romanova, J.M.; Golyshev, S.A.; Stadnichuk, V.I.; Baratova, L.A.; Sud’Ina, G.F.; Klein, T. Proteome Analysis Identified Human Neutrophil Membrane Tubulovesicular Extensions (Cytonemes, Membrane Tethers) as Bactericide Trafficking. Biochim. Biophys. Acta BBA—Gen. Subj. 2012, 1820, 1705–1714. [Google Scholar] [CrossRef] [PubMed]
- Dalli, J.; Montero-Melendez, T.; Norling, L.V.; Yin, X.; Hinds, C.; Haskard, D.; Mayr, M.; Perretti, M. Heterogeneity in Neutrophil Microparticles Reveals Distinct Proteome and Functional Properties. Mol. Cell. Proteom. 2013, 12, 2205–2219. [Google Scholar] [CrossRef]
- Slater, T.W.; Finkielsztein, A.; Mascarenhas, L.A.; Mehl, L.C.; Butin-Israeli, V.; Sumagin, R. Neutrophil Microparticles Deliver Active Myeloperoxidase to Injured Mucosa to Inhibit Epithelial Wound Healing. J. Immunol. 2017, 198, 2886–2897. [Google Scholar] [CrossRef]
- Metzler, K.D.; Fuchs, T.A.; Nauseef, W.M.; Reumaux, D.; Roesler, J.; Schulze, I.; Wahn, V.; Papayannopoulos, V.; Zychlinsky, A. Myeloperoxidase is Required for Neutrophil Extracellular Trap Formation: Implications for Innate Immunity. Blood 2011, 117, 953–959. [Google Scholar] [CrossRef]
- Dinauer, M.C. Disorders of Neutrophil Functions: An Overview. Methods Mol. Biol. 2014, 1124, 501–515. [Google Scholar] [CrossRef]
- Nikpoor, B.; Turecki, G.; Fournier, C.; Théroux, P.; Rouleau, G.A. A Functional Myeloperoxidase Polymorphic Variant is Associated with Coronary Artery Disease in French-Canadians. Am. Heart J. 2001, 142, 336–339. [Google Scholar] [CrossRef]
- Reynolds, W.F.; Stegeman, C.A.; Tervaert, J.W. -463 G/A Myeloperoxidase Promoter Polymorphism is Associated with Clinical Manifestations and the Course of Disease in MPO-ANCA-associated Vasculitis. Clin. Immunol. 2002, 103, 154–160. [Google Scholar] [CrossRef]
- Taioli, E.; Benhamou, S.; Bouchardy, C.; Cascorbi, I.; Cajas-Salazar, N.; Dally, H.; Fong, K.M.; Larsen, J.E.; Le Marchand, L.; London, S.J.; et al. Myeloperoxidase G-463A Polymorphism and Lung Cancer: A HuGE Genetic Susceptibility to Environmental Carcinogens Pooled Analysis. Genet. Med. 2007, 9, 67–73. [Google Scholar] [CrossRef]
- Yang, J.P.; Wang, W.B.; Yang, X.X.; Yang, L.; Ren, L.; Zhou, F.X.; Hu, L.; He, W.; Li, B.Y.; Zhu, Y.; et al. The MPO-463G>A Polymorphism and Lung Cancer Risk: A Meta-analysis Based on 22 Case-control Studies. PLoS ONE 2013, 8, e65778. [Google Scholar] [CrossRef]
- Wheatley-Price, P.; Asomaning, K.; Reid, A.; Zhai, R.; Su, L.; Zhou, W.; Zhu, A.; Ryan, D.P.; Christiani, D.C.; Liu, G. Myeloperoxidase and Superoxide Dismutase Polymorphisms are Associated with an Increased Risk of Developing Pancreatic Adenocarcinoma. Cancer 2008, 112, 1037–1042. [Google Scholar] [CrossRef]
- Pullar, J.M.; Vissers, M.C.; Winterbourn, C.C. Glutathione Oxidation by Hypochlorous Acid in Endothelial Cells Produces Glutathione Sulfonamide as a Major Product but not Glutathione Disulfide. J. Biol. Chem. 2001, 276, 22120–22125. [Google Scholar] [CrossRef]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the Site of Inflammation: The Leukocyte Adhesion Cascade Updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef]
- Johansson, M.W.; Patarroyo, M.; Oberg, F.; Siegbahn, A.; Nilsson, K. Myeloperoxidase Mediates Cell Adhesion via the Alpha M Beta 2 Integrin (Mac-1, CD11b/CD18). J. Cell. Sci. 1997, 110, 1133–1139. [Google Scholar] [CrossRef]
- Lau, D.; Mollnau, H.; Eiserich, J.P.; Freeman, B.A.; Daiber, A.; Gehling, U.M.; Brümmer, J.; Rudolph, V.; Münzel, T.; Heitzer, T.; et al. Myeloperoxidase Mediates Neutrophil Activation by Association with CD11b/CD18 Integrins. Proc. Natl. Acad. Sci. USA 2005, 102, 431–436. [Google Scholar] [CrossRef]
- Gorudko, I.V.; Grigorieva, D.V.; Sokolov, A.V.; Shamova, E.V.; Kostevich, V.A.; Kudryavtsev, I.V.; Syromiatnikova, E.D.; Vasilyev, V.B.; Cherenkevich, S.N.; Panasenko, O.M. Neutrophil Activation in Response to Monomeric Myeloperoxidase. Biochem. Cell. Biol. 2018, 96, 592–601. [Google Scholar] [CrossRef]
- Klinke, A.; Nussbaum, C.; Kubala, L.; Friedrichs, K.; Rudolph, T.K.; Rudolph, V.; Paust, H.J.; Schröder, C.; Benten, D.; Lau, D.; et al. Myeloperoxidase Attracts Neutrophils by Physical Forces. Blood 2011, 117, 1350–1358. [Google Scholar] [CrossRef]
- Manchanda, K.; Kolarova, H.; Kerkenpaß, C.; Mollenhauer, M.; Vitecek, J.; Rudolph, V.; Kubala, L.; Baldus, S.; Adam, M.; Klinke, A. MPO (Myeloperoxidase) Reduces Endothelial Glycocalyx Thickness Dependent on Its Cationic Charge. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1859–1867. [Google Scholar] [CrossRef]
- El Kebir, D.; József, L.; Pan, W.; Filep, J.G. Myeloperoxidase Delays Neutrophil Apoptosis through CD11b/CD18 Integrins and Prolongs Inflammation. Circ. Res. 2008, 103, 352–359. [Google Scholar] [CrossRef]
- Demaret, J.; Venet, F.; Friggeri, A.; Cazalis, M.A.; Plassais, J.; Jallades, L.; Malcus, C.; Poitevin-Later, F.; Textoris, J.; Lepape, A.; et al. Marked Alterations of Neutrophil Functions during Sepsis-induced Immunosuppression. J. Leukoc. Biol. 2015, 98, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Matthijsen, R.A.; Huugen, D.; Hoebers, N.T.; de Vries, B.; Peutz-Kootstra, C.J.; Aratani, Y.; Daha, M.R.; Tervaert, J.W.C.; Buurman, W.A.; Heeringa, P. Myeloperoxidase Is Critically Involved in the Induction of Organ Damage after Renal Ischemia Reperfusion. Am. J. Pathol. 2007, 171, 1743–1752. [Google Scholar] [CrossRef] [PubMed]
- Brovkovych, V.; Gao, X.P.; Ong, E.; Brovkovych, S.; Brennan, M.L.; Su, X.; Hazen, S.L.; Malik, A.B.; Skidgel, R.A. Augmented Inducible Nitric Oxide Synthase Expression and Increased NO Production Reduce Sepsis-Induced Lung Injury and Mortality in Myeloperoxidase-Null Mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 295, L96–L103. [Google Scholar] [CrossRef]
- Kim, H.J.; Wei, Y.; Lee, J.Y.; Wu, Y.; Zheng, Y.; Moskowitz, M.A.; Chen, J.W. Myeloperoxidase Inhibition Increases Neurogenesis after Ischemic Stroke. J. Pharmacol. Exp. Ther. 2016, 359, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Odobasic, D.; Muljadi, R.C.; O’Sullivan, K.M.; Kettle, A.J.; Dickerhof, N.; Summers, S.A.; Kitching, A.R.; Holdsworth, S.R. Suppression of Autoimmunity and Renal Disease in Pristane-Induced Lupus by Myeloperoxidase. Arthritis Rheumatol. 2015, 67, 1868–1880. [Google Scholar] [CrossRef]
- Tseng, A.; Kim, K.; Li, J.; Cho, J. Myeloperoxidase Negatively Regulates Neutrophil-Endothelial Cell Interactions by Impairing αMβ2 Integrin Function in Sterile Inflammation. Front. Med. 2018, 5, 134. [Google Scholar] [CrossRef]
- Reber, L.L.; Gillis, C.M.; Starkl, P.; Jönsson, F.; Sibilano, R.; Marichal, T.; Gaudenzio, N.; Bérard, M.; Rogalla, S.; Contag, C.H.; et al. Neutrophil Myeloperoxidase Diminishes the Toxic Effects and Mortality Induced by Lipopolysaccharide. J. Exp. Med. 2017, 214, 1249–1258. [Google Scholar] [CrossRef]
- Schürmann, N.; Forrer, P.; Casse, O.; Li, J.; Felmy, B.; Burgener, A.V.; Ehrenfeuchter, N.; Hardt, W.D.; Recher, M.; Hess, C.; et al. Myeloperoxidase Targets Oxidative Host Attacks to Salmonella and Prevents Collateral Tissue Damage. Nat. Microbiol. 2017, 2, 16268. [Google Scholar] [CrossRef]
- Zhang, R.; Brennan, M.-L.; Shen, Z.; MacPherson, J.C.; Schmitt, D.; Molenda, C.E.; Hazen, S.L. Myeloperoxidase Functions as a Major Enzymatic Catalyst for Initiation of Lipid Peroxidation at Sites of Inflammation. J. Biol. Chem. 2002, 277, 46116–46122. [Google Scholar] [CrossRef]
- Kubala, L.; Schmelzer, K.R.; Klinke, A.; Kolarova, H.; Baldus, S.; Hammock, B.D.; Eiserich, J.P. Modulation of Arachidonic and Linoleic Acid Metabolites in Myeloperoxidase-deficient Mice During Acute Inflammation. Free Radic. Biol. Med. 2010, 48, 1311–1320. [Google Scholar] [CrossRef]
- Rehring, J.F.; Bui, T.M.; Galán-Enríquez, C.S.; Urbanczyk, J.M.; Ren, X.; Wiesolek, H.L.; Sullivan, D.P.; Sumagin, R. Released Myeloperoxidase Attenuates Neutrophil Migration and Accumulation in Inflamed Tissue. Front. Immunol. 2021, 12, 654259. [Google Scholar] [CrossRef]
- Odobasic, D.; Kitching, A.R.; Semple, T.J.; Holdsworth, S.R. Endogenous Myeloperoxidase Promotes Neutrophil-mediated Renal Injury, but Attenuates T Cell Immunity Inducing Crescentic Glomerulonephritis. J. Am. Soc. Nephrol. 2007, 18, 760–770. [Google Scholar] [CrossRef]
- Rausch, P.G.; Moore, T.G. Granule Enzymes of Polymorphonuclear Neutrophils: A Phylogenetic Comparison. Blood 1975, 46, 913–919. [Google Scholar] [CrossRef]
- Jerke, U.; Rolle, S.; Purfürst, B.; Luft, F.C.; Nauseef, W.M.; Kettritz, R. β2 Integrin-Mediated Cell-Cell Contact Transfers Active Myeloperoxidase from Neutrophils to Endothelial Cells. J. Biol. Chem. 2013, 288, 12910–12919. [Google Scholar] [CrossRef]
- Babior, B.M. NADPH Oxidase. Curr. Opin. Immunol. 2004, 16, 42–47. [Google Scholar] [CrossRef]
- Park, K.J.; Gaynor, R.B.; Tae Kwak, Y. Heat Shock Protein 27 Association with the IκB Kinase Complex Regulates Tumor Necrosis Factor α-Induced NF-κB Activation. J. Biol. Chem. 2003, 278, 35272–35278. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. Integrins: Bidirectional, Allosteric Signaling Machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef]
- Harris, E.S.; McIntyre, T.M.; Prescott, S.M.; Zimmerman, G.A. The Leukocyte Integrins. J. Biol. Chem. 2000, 275, 23409–23412. [Google Scholar] [CrossRef] [PubMed]
- El Kebir, D.; József, L.; Pan, W.; Wang, L.; Petasis, N.A.; Serhan, C.N.; Filep, J.G. 15-Epi-Lipoxin A4 Inhibits Myeloperoxidase Signaling and Enhances Resolution of Acute Lung Injury. Am. J. Respir. Crit. Care Med. 2009, 180, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, A.G.; Caron, E. Integrin-Dependent Phagocytosis–Spreadingfrom Microadhesion to New Concepts. J. Cell. Sci. 2008, 121, 1773–1783. [Google Scholar] [CrossRef]
- Fujimoto, K.; Motowaki, T.; Tamura, N.; Aratani, Y. Myeloperoxidase Deficiency Enhances Zymosan Phagocytosis Associated with Up-Regulation of Surface Expression of CD11b in Mouse Neutrophils. Free Radic. Res. 2016, 50, 1340–1349. [Google Scholar] [CrossRef]
- Müller, J.M.; Rupec, R.A.; Bauerle, P.A. Study of Gene Regulation by NF-κB and AP-1 in Response to Reactive Oxygen Intermediates. Methods 1997, 11, 301–312. [Google Scholar] [CrossRef]
- József, L.; Zouki, C.; Petasis, N.A.; Serhan, C.N.; Filep, J.G. Lipoxin A4 and Aspirin-triggered 15-epi-lipoxin A4 Inhibit Peroxynitrite Formation, NF-κB and AP-1 Activation, and IL-8 Gene Expression in Human Leukocytes. Proc. Natl. Acad. Sci. USA 2002, 99, 13266–13271. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Hampton, M.B.; Livesey, J.H.; Kettle, A.J. Modeling the Reactions of Superoxide and Myeloperoxidase in the Neutrophil Phagosome: Implications for Microbial Killing. J. Biol. Chem. 2006, 281, 39860–39869. [Google Scholar] [CrossRef]
- Lane, A.E.; Tan, J.T.M.; Hawkins, C.L.; Heather, A.K.; Davies, M.J. The Myeloperoxidase-Derived Oxidant HOSCN Inhibits Protein Tyrosine Phosphatases and Modulates Cell Signalling via the Mitogen-Activated Protein Kinase (MAPK) Pathway in Macrophages. Biochem. J. 2010, 430, 161–169. [Google Scholar] [CrossRef]
- Guo, C.; Davies, M.J.; Hawkins, C.L. Role of Thiocyanate in the Modulation of Myeloperoxidase-Derived Oxidant Induced Damage to Macrophages. Redox. Biol. 2020, 36, 101666. [Google Scholar] [CrossRef]
- Haegens, A.; Heeringa, P.; van Suylen, R.J.; Steele, C.; Aratani, Y.; O’Donoghue, R.J.J.; Mutsaers, S.E.; Mossman, B.T.; Wouters, E.F.M.; Vernooy, J.H.J. Myeloperoxidase Deficiency Attenuates Lipopolysaccharide-Induced Acute Lung Inflammation and Subsequent Cytokine and Chemokine Production. J. Immunol. 2009, 182, 7990–7996. [Google Scholar] [CrossRef]
- Endo, D.; Saito, T.; Umeki, Y.; Suzuki, K.; Aratani, Y. Myeloperoxidase Negatively Regulates the Expression of Proinflammatory Cytokines and Chemokines by Zymosan-Induced Mouse Neutrophils. Inflamm. Res. 2015, 65, 151–159. [Google Scholar] [CrossRef]
- Savill, J.; Dransfield, I.; Gregory, C.; Haslett, C. A Blast from the Past: Clearance of Apoptotic Cells Regulates Immune Responses. Nat. Rev. Immunol. 2002, 2, 965–975. [Google Scholar] [CrossRef]
- Rubel, C.; Gómez, S.; Fernández, G.C.; Isturiz, M.A.; Caamaño, J.; Palermo, M.S. Fibrinogen-CD11b/CD18 Interaction Activates the NF-κB Pathway and Delays Apoptosis in Human Neutrophils. Eur. J. Immunol. 2003, 33, 1429–1438. [Google Scholar] [CrossRef]
- Yan, M.; Mao, J.; Wei, Y.; Zhong, J.; Yang, S.; Xu, R. Study on Intracellular Trafficking of Mac-1 by Direct Visualization. Sci. China C Life Sci. 2004, 47, 521–529. [Google Scholar] [CrossRef]
- Whitlock, B.B.; Gardai, S.; Fadok, V.; Bratton, D.; Henson, P.M. Differential Roles for αMβ2 Integrin Clustering or Activation in the Control of Apoptosis via Regulation of Akt and ERK Survival Mechanisms. J. Cell Biol. 2000, 151, 1305–1320. [Google Scholar] [CrossRef]
- Dzhagalov, I.; St. John, A.; He, Y.W. The Antiapoptotic Protein Mcl-1 Is Essential for the Survival of Neutrophils but Not Macrophages. Blood 2007, 109, 1620–1626. [Google Scholar] [CrossRef]
- Pluskota, E.; Soloviev, D.A.; Szpak, D.; Weber, C.; Plow, E.F. Neutrophil Apoptosis: Selective Regulation by Different Ligands of Integrin αMβ2. J. Immunol. 2008, 181, 3609–3619. [Google Scholar] [CrossRef]
- Rossi, A.G.; Sawatzky, D.A.; Walker, A.; Ward, C.; Sheldrake, T.A.; Riley, N.A.; Caldicott, A.; Martinez-Losa, M.; Walker, T.R.; Duffin, R.; et al. Cyclin-Dependent Kinase Inhibitors Enhance the Resolution of Inflammation by Promoting Inflammatory Cell Apoptosis. Nat. Med. 2006, 12, 1056–1064, Erratum in Nat. Med. 2006, 12, 1434. [Google Scholar] [CrossRef]
- Wagner, B.A.; Buettner, G.R.; Oberley, L.W.; Darby, C.J.; Burns, C.P. Myeloperoxidase Is Involved in H2O2-Induced Apoptosis of HL-60 Human Leukemia Cells. J. Biol. Chem. 2000, 275, 22461–22469. [Google Scholar] [CrossRef] [PubMed]
- Kanayama, A.; Miyamoto, Y. Apoptosis Triggered by Phagocytosis-Related Oxidative Stress through FLIPS down-Regulation and JNK Activation. J. Leukoc. Biol. 2007, 82, 1344–1352. [Google Scholar] [CrossRef] [PubMed]
- Bardoel, B.W.; Kenny, E.F.; Sollberger, G.; Zychlinsky, A. The Balancing Act of Neutrophils. Cell Host Microbe 2014, 15, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Urban, C.F.; Reichard, U.; Brinkmann, V.; Zychlinsky, A. Neutrophil Extracellular Traps Capture and Kill Candida albicans Yeast and Hyphal Forms. Cell Microbiol. 2006, 8, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Erreni, M.; Manfredi, A.A.; Garlanda, C.; Mantovani, A.; Rovere-Querini, P. The Long Pentraxin PTX3: A Prototypical Sensor of Tissue Injury and a Regulator of Homeostasis. Immunol. Rev. 2017, 280, 112–125. [Google Scholar] [CrossRef]
- Yuen, J.; Pluthero, F.G.; Douda, D.N.; Riedl, M.; Cherry, A.; Ulanova, M.; Kahr, W.H.; Palaniyar, N.; Licht, C. NETosing Neutrophils Activate Complement both on their own NETs and Bacteria via Alternative and Non-alternative Pathways. Front. Immunol. 2016, 7, 137. [Google Scholar] [CrossRef]
- Papayannopoulos, V.; Metzler, K.D.; Hakkim, A.; Zychlinsky, A. Neutrophil Elastase and Myeloperoxidase Regulate the Formation of Neutrophil Extracellular Traps. J. Cell Biol. 2010, 191, 677–691. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schulze, I.; Wahn, V.; Weinrauch, Y.; Brinkmann, V.; Zychlinsky, A. Novel Cell Death Program Leads to Neutrophil Extracellular Traps. J. Cell. Biol. 2007, 176, 231–241. [Google Scholar] [CrossRef]
- Tessarz, P.; Kouzarides, T. Histone Core Modifications Regulating Nucleosome Structure and Dynamics. Nat. Rev. Mol. Cell. Biol. 2014, 15, 703–708. [Google Scholar] [CrossRef]
- Pilsczek, F.H.; Salina, D.; Poon, K.K.H.; Fahey, C.; Yipp, B.G.; Sibley, C.D.; Robbins, S.M.; Green, F.H.Y.; Surette, M.G.; Sugai, M.; et al. A Novel Mechanism of Rapid Nuclear Neutrophil Extracellular Trap Formation in Response to Staphylococcus aureus. J. Immunol. 2010, 185, 7413–7425. [Google Scholar] [CrossRef]
- Byrd, A.S.; O’Brien, X.M.; Johnson, C.M.; Lavigne, L.M.; Reichner, J.S. An Extracellular Matrix-Based Mechanism of Rapid Neutrophil Extracellular Trap Formation in Response to Candida albicans. J. Immunol. 2013, 190, 4136–4148. [Google Scholar] [CrossRef]
- Cristinziano, L.; Modestino, L.; Loffredo, S.; Varricchi, G.; Braile, M.; Ferrara, A.L.; de Paulis, A.; Antonelli, A.; Marone, G.; Galdiero, M.R. Anaplastic Thyroid Cancer Cells Induce the Release of Mitochondrial Extracellular DNA Traps by Viable Neutrophils. J. Immunol. 2020, 204, 1362–1372. [Google Scholar] [CrossRef]
- Yousefi, S.; Mihalache, C.; Kozlowski, E.; Schmid, I.; Simon, H.U. Viable Neutrophils Release Mitochondrial DNA to Form Neutrophil Extracellular Traps. Cell Death Differ. 2009, 16, 1438–1444. [Google Scholar] [CrossRef]
- Cristinziano, L.; Modestino, L.; Antonelli, A.; Marone, G.; Simon, H.U.; Varricchi, G.; Galdiero, M.R. Neutrophil Extracellular Traps in Cancer. Semin. Cancer Biol. 2022, 79, 91–104. [Google Scholar] [CrossRef]
- Yousefi, S.; Stojkov, D.; Germic, N.; Simon, D.; Wang, X.; Benarafa, C.; Simon, H.U. Untangling “NETosis” from NETs. Eur. J. Immunol. 2019, 49, 221–227. [Google Scholar] [CrossRef]
- Manfredi, A.A.; Ramirez, G.A.; Rovere-Querini, P.; Maugeri, N. The Neutrophil’s Choice: Phagocytose vs Make Neutrophil Extracellular Traps. Front. Immunol. 2018, 9, 288. [Google Scholar] [CrossRef]
- Maugeri, N.; Rovere-Querini, P.; Evangelista, V.; Covino, C.; Capobianco, A.; Bertilaccio, M.T.S.; Piccoli, A.; Totani, L.; Cianflone, D.; Maseri, A.; et al. Neutrophils Phagocytose Activated Platelets in Vivo: A Phosphatidylserine, P-Selectin, and β2 Integrin-Dependent Cell Clearance Program. Blood 2009, 113, 5254–5265. [Google Scholar] [CrossRef]
- Papayannopoulos, V. Neutrophil Extracellular Traps in Immunity and Disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef]
- Mutua, V.; Gershwin, L.J. A Review of Neutrophil Extracellular Traps (NETs) in Disease: Potential Anti-NETs Therapeutics. Clin. Rev. Allergy Immunol. 2021, 61, 194–211. [Google Scholar] [CrossRef]
- Sørensen, O.E.; Borregaard, N. Neutrophil Extracellular Traps—the Dark Side of Neutrophils. J. Clin. Investig. 2016, 126, 1612–1620. [Google Scholar] [CrossRef]
- Jorch, S.K.; Kubes, P. An Emerging Role for Neutrophil Extracellular Traps in Noninfectious Disease. Nat. Med. 2017, 23, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Ribon, M.; Seninet, S.; Mussard, J.; Sebbag, M.; Clavel, C.; Serre, G.; Boissier, M.C.; Semerano, L.; Decker, P. Neutrophil Extracellular Traps Exert both Pro- and Anti-inflammatory Actions in Rheumatoid Arthritis that are Modulated by C1q and LL-37. J. Autoimmun. 2019, 98, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Hahn, J.; Schauer, C.; Czegley, C.; Kling, L.; Petru, L.; Schmid, B.; Weidner, D.; Reinwald, C.; Biermann, M.H.C.; Blunder, S.; et al. Aggregated Neutrophil Extracellular Traps Resolve Inflammation by Proteolysis of Cytokines and Chemokines and Protection from Antiproteases. FASEB J. 2019, 33, 1401–1414. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cao, X.; Liu, Y.; Zhao, Y.; Herrmann, M. Neutrophil Extracellular Traps Formation and Aggregation Orchestrate Induction and Resolution of Sterile Crystal-Mediated Inflammation. Front. Immunol. 2018, 9, 1559. [Google Scholar] [CrossRef] [PubMed]
- Jennette, J.C.; Xiao, H.; Falk, R.J. Pathogenesis of Vascular Inflammation by Anti-Neutrophil Cytoplasmic Antibodies. J. Am. Soc. Nephrol. 2006, 17, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kaplan, M.J. The Role of Neutrophils and NETosis in Autoimmune and Renal Diseases. Nat. Rev. Nephrol. 2016, 12, 402–413. [Google Scholar] [CrossRef]
- Austin, K.; Janagan, S.; Wells, M.; Crawshaw, H.; McAdoo, S.; Robson, J.C. ANCA Associated Vasculitis Subtypes: Recent Insights and Future Perspectives. J. Inflamm. Res. 2022, 15, 2567–2582. [Google Scholar] [CrossRef]
- Hakkim, A.; Fürnrohr, B.G.; Amann, K.; Laube, B.; Abed, U.A.; Brinkmann, V.; Herrmann, M.; Voll, R.E.; Zychlinsky, A. Impairment of Neutrophil Extracellular Trap Degradation Is Associated with Lupus Nephritis. Proc. Natl. Acad. Sci. USA 2010, 107, 9813–9818. [Google Scholar] [CrossRef]
- Csernok, E. Anti-Neutrophil Cytoplasmic Antibodies and Pathogenesis of Small Vessel Vasculitides. Autoimmun. Rev. 2003, 2, 158–164. [Google Scholar] [CrossRef]
- Nakazawa, D.; Shida, H.; Tomaru, U.; Yoshida, M.; Nishio, S.; Atsumi, T.; Ishizu, A. Enhanced Formation and Disordered Regulation of NETs in Myeloperoxidase-ANCA-Associated Microscopic Polyangiitis. J. Am. Soc. Nephrol. 2014, 25, 990–997. [Google Scholar] [CrossRef]
- Xiao, H.; Heeringa, P.; Hu, P.; Liu, Z.; Zhao, M.; Aratani, Y.; Maeda, N.; Falk, R.J.; Jennette, J.C. Antineutrophil Cytoplasmic Autoantibodies Specific for Myeloperoxidase Cause Glomerulonephritis and Vasculitis in Mice. J. Clin. Investig. 2002, 110, 955–963. [Google Scholar] [CrossRef]
- Békési, G.; Heinle, H.; Kakucs, R.; Pázmány, T.; Szombath, D.; Dinya, M.; Tulassay, Z.; Fehér, J.; Rácz, K.; Székács, B.; et al. Effect of Inhibitors of Myeloperoxidase on the Development of Aortic Atherosclerosis in an Animal Model. Exp. Gerontol. 2005, 40, 199–208. [Google Scholar] [CrossRef]
- Ugolini, A.; Tyurin, V.A.; Tyurina, Y.Y.; Tcyganov, E.N.; Donthireddy, L.; Kagan, V.E.; Gabrilovich, D.I.; Veglia, F. Polymorphonuclear Myeloid-Derived Suppressor Cells Limit Antigen Cross-Presentation by Dendritic Cells in Cancer. JCI Insight 2020, 5, e138581. [Google Scholar] [CrossRef]
- Maiocchi, S.; Rees, M.; Morris, J.; Thomas, S. Endothelial-Targeted Nitroxides Inhibit MPO-Mediated Endothelial Dysfunction. Free Radic. Biol. Med. 2017, 112, 207. [Google Scholar] [CrossRef]
- Piek, A.; Koonen, D.P.Y.; Schouten, E.M.; Lindtstedt, E.L.; Michaëlsson, E.; de Boer, R.A.; Silljé, H.H.W. Pharmacological Myeloperoxidase (MPO) Inhibition in an Obese/Hypertensive Mouse Model Attenuates Obesity and Liver Damage, but Not Cardiac Remodeling. Sci. Rep. 2019, 9, 18765. [Google Scholar] [CrossRef]
- Cheng, D.; Talib, J.; Stanley, C.P.; Rashid, I.; Michaëlsson, E.; Lindstedt, E.L.; Croft, K.D.; Kettle, A.J.; Maghzal, G.J.; Stocker, R. Inhibition of MPO (Myeloperoxidase) Attenuates Endothelial Dysfunction in Mouse Models of Vascular Inflammation and Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1448–1457. [Google Scholar] [CrossRef]
- Tidén, A.K.; Sjögren, T.; Svensson, M.; Bernlind, A.; Senthilmohan, R.; Auchère, F.; Norman, H.; Markgren, P.O.; Gustavsson, S.; Schmidt, S.; et al. 2-Thioxanthines are Mechanism-Based Inactivators of Myeloperoxidase that Block Oxidative Stress during Inflammation. J. Biol. Chem. 2011, 286, 37578–37589. [Google Scholar] [CrossRef]
- Churg, A.; Marshall, C.V.; Sin, D.D.; Bolton, S.; Zhou, S.; Thain, K.; Cadogan, E.B.; Maltby, J.; Soars, M.G.; Mallinder, P.R.; et al. Late Intervention with a Myeloperoxidase Inhibitor Stops Progression of Experimental Chronic Obstructive Pulmonary Disease. Am. J. Resp. Crit. Care Med. 2012, 185, 34–43. [Google Scholar] [CrossRef]
- Antonelou, M.; Michaëlsson, E.; Evans, R.D.R.; Wang, C.J.; Henderson, S.R.; Walker, L.S.K.; Unwin, R.J.; Salama, A.D.; RAVE-ITN Investigators. Therapeutic Myeloperoxidase Inhibition Attenuates Neutrophil Activation, ANCA-Mediated Endothelial Damage, and Crescentic GN. J. Am. Soc. Nephrol. 2020, 31, 350–364. [Google Scholar] [CrossRef]
- Jucaite, A.; Svenningsson, P.; Rinne, J.O.; Cselényi, Z.; Varnäs, K.; Johnström, P.; Amini, N.; Kirjavainen, A.; Helin, S.; Minkwitz, M.; et al. Effect of the Myeloperoxidase Inhibitor AZD3241 on Microglia: A PET Study in Parkinson’s Disease. Brain 2015, 138, 2687–2700. [Google Scholar] [CrossRef]
- Kaindlstorfer, C.; Sommer, P.; Georgievska, B.; Mather, R.J.; Kugler, A.R.; Poewe, W.; Wenning, G.K.; Stefanova, N. Failure of Neuroprotection Despite Microglial Suppression by Delayed-Start Myeloperoxidase Inhibition in a Model of Advanced Multiple System Atrophy: Clinical Implications. Neurotox. Res. 2015, 28, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Stefanova, N.; Georgievska, B.; Eriksson, H.; Poewe, W.; Wenning, G.K. Myeloperoxidase Inhibition Ameliorates Multiple System Atrophy-Like Degeneration in a Transgenic Mouse Model. Neurotox. Res. 2012, 21, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, G.; Chami, B.; Liu, Y.; Schroder, A.L.; San Gabriel, P.T.; Gao, A.; Fong, G.; Wang, X.S.; Witting, P.K. The Synthetic Myeloperoxidase Inhibitor AZD3241 Ameliorates Dextran Sodium Sulfate Stimulated Experimental Colitis. Front. Pharmacol. 2020, 11, 556020. [Google Scholar] [CrossRef]
- Zheng, W.; Warner, R.; Ruggeri, R.; Su, C.; Cortes, C.; Skoura, A.; Ward, J.; Ahn, K.; Kalgutkar, A.; Sun, D.; et al. PF-1355, a Mechanism-Based Myeloperoxidase Inhibitor, Prevents Immune Complex Vasculitis and Anti–Glomerular Basement Membrane Glomerulonephritis. J. Pharmacol. Exp. Ther. 2015, 353, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.Q.; Varma, M.V.; Wolford, A.; Ryder, T.; Di, L.; Feng, B.; Terra, S.G.; Sagawa, K.; Kalgutkar, A.S. Pharmacokinetics and Disposition of the Thiouracil Derivative PF-06282999, an Orally Bioavailable, Irreversible Inactivator of Myeloperoxidase Enzyme, across Animals and Humans. Drug. Metab. Dispos. 2016, 44, 209–219. [Google Scholar] [CrossRef]
- MPO Inhibitor A_Zeneca for HFpEF—Clinical Trials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03611153 (accessed on 20 August 2022).
- Lam, C.S.P.; Voors, A.A.; Shah, S.J.; Erlinge, D.; Saraste, A.; Pirazzi, C.; Grove, E.L.; Barasa, A.; Schou, M.; Aziz, A.; et al. Myeloperoxidase Inhibitor AZD4831 Target Engagement and Safety in a Phase 2a Study in Patients with Heart Failure with Preserved Ejection Fraction (SATELLITE). Eur. J. Heart Fail. 2021, 23, 2–322. [Google Scholar]
- Zhou, T.; Zhou, S.-H.; Qi, S.-S.; Shen, X.-G.; Zeng, G.-F.; Zhou, H.-N. The Effect of Atorvastatin on Serum Myeloperoxidase and CRP Levels in Patients with Acute Coronary Syndrome. Clin. Chim. Acta 2006, 368, 168–172. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Rodríguez-Ayala, E.; Massy, Z.A.; Qureshi, A.R.; Barany, P.; Fellström, B.; Heimburger, O.; Lindholm, B.; Alvestrand, A. Statin Treatment and Diabetes Affect Myeloperoxidase Activity in Maintenance Hemodialysis Patients. Clin. J. Am. Soc. Nephrol. 2006, 1, 281–287. [Google Scholar] [CrossRef]
- Retinoids in ANCA Small Vessel Vasculitis: Silencing Autoantigens—Clinical Trials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01275274. (accessed on 20 August 2022).
- Johnson, J.L.; Ramadass, M.; He, J.; Brown, S.J.; Zhang, J.; Abgaryan, L.; Biris, N.; Gavathiotis, E.; Rosen, H.; Catz, S.D. Identification of Neutrophil Exocytosis Inhibitors (Nexinhibs), Small Molecule Inhibitors of Neutrophil Exocytosis and Inflammation: DRUGGABILITY OF THE SMALL GTPase Rab27a. J. Biol. Chem. 2016, 291, 25965–25982. [Google Scholar] [CrossRef]
- Fan, Z.; Liu, W.; Cronin, C.G.; Wang, C.; Ruan, J.; Johnson, J.L.; Catz, S.; Sun, H.; Groisman, A.; Chen, Y.; et al. Nexinhib20 Prevents Myocardial Ischemia-Reperfusion Injury by Inhibiting Neutrophil Adhesion and β2 Integrin Activation. FASEB J. 2022, 36. [Google Scholar] [CrossRef]
- Arnett, E.; Vadia, S.; Nackerman, C.C.; Oghumu, S.; Satoskar, A.R.; McLeish, K.R.; Uriarte, S.M.; Seveau, S. The Pore-Forming Toxin Listeriolysin O is Degraded by Neutrophil Metalloproteinase-8 and Fails to Mediate Listeria Monocytogenes Intracellular Survival in Neutrophils. J. Immunol. 2014, 192, 234–244. [Google Scholar] [CrossRef]
- Uriarte, S.M.; Rane, M.J.; Luerman, G.C.; Barati, M.T.; Ward, R.A.; Nauseef, W.M.; McLeish, K.R. Granule Exocytosis Contributes to Priming and Activation of the Human Neutrophil Respiratory Burst. J. Immunol. 2011, 187, 391–400. [Google Scholar] [CrossRef]
- Uriarte, S.M.; Rane, M.J.; Merchant, M.L.; Jin, S.; Lentsch, A.B.; Ward, R.A.; McLeish, K.R. Inhibition of Neutrophil Exocytosis Ameliorates Acute Lung Injury in Rats. Shock 2013, 39, 286–292. [Google Scholar] [CrossRef]
- Bai, J.; Tang, L.; Lomas-Neira, J.; Chen, Y.; McLeish, K.R.; Uriarte, S.M.; Chung, C.-S.; Ayala, A. TATSNAP-23 Treatment Inhibits the Priming of Neutrophil Functions Contributing to Shock and/or Sepsis-induced Extra-pulmonary Acute Lung Injury. Innate. Immun. 2015, 21, 42–54. [Google Scholar] [CrossRef]
- Patel, S.; Kumar, S.; Jyoti, A.; Srinag, B.S.; Keshari, R.S.; Saluja, R.; Verma, A.; Mitra, K.; Barthwal, M.K.; Krishnamurthy, H.; et al. Nitric Oxide Donors Release Extracellular Traps from Human Neutrophils by Augmenting Free Radical Generation. Nitric. Oxide 2010, 22, 226–234. [Google Scholar] [CrossRef]
- Munafo, D.B.; Johnson, J.L.; Brzezinska, A.A.; Ellis, B.A.; Wood, M.R.; Catz, S.D. DNase I Inhibits a Late Phase of Reactive Oxygen Species Production in Neutrophils. J. Innate. Immun. 2009, 1, 527–542. [Google Scholar] [CrossRef]
- Willis, V.C.; Gizinski, A.M.; Banda, N.K.; Causey, C.P.; Knuckley, B.; Cordova, K.N.; Luo, Y.; Levitt, B.; Glogowska, M.; Chandra, P.; et al. N-α-Benzoyl-N5-(2-Chloro-1-Iminoethyl)-L-Ornithine Amide, a Protein Arginine Deiminase Inhibitor, Reduces the Severity of Murine Collagen-Induced Arthritis. J. Immunol. 2011, 186, 4396–4404. [Google Scholar] [CrossRef]
- Knight, J.S.; Luo, W.; O’Dell, A.A.; Yalavarthi, S.; Zhao, W.; Subramanian, V.; Guo, C.; Grenn, R.C.; Thompson, P.R.; Eitzman, D.T.; et al. Peptidylarginine Deiminase Inhibition Reduces Vascular Damage and Modulates Innate Immune Responses in Murine Models of Atherosclerosis. Circ. Res. 2014, 114, 947–956. [Google Scholar] [CrossRef]
- Kusunoki, Y.; Nakazawa, D.; Shida, H.; Hattanda, F.; Miyoshi, A.; Masuda, S.; Nishio, S.; Tomaru, U.; Atsumi, T.; Ishizu, A. Peptidylarginine Deiminase Inhibitor Suppresses Neutrophil Extracellular Trap Formation and MPO-ANCA Production. Front. Immunol. 2016, 7, 227. [Google Scholar] [CrossRef]
- Chiang, N.; Sakuma, M.; Rodriguez, A.R.; Spur, B.W.; Irimia, D.; Serhan, C.N. Resolvin T-series Reduce Neutrophil Extracellular Traps. Blood 2022, 139, 1222–1233. [Google Scholar] [CrossRef]
- Kutter, D.; Devaquet, P.; Vanderstocken, G.; Paulus, J.M.; Marchal, V.; Gothot, A. Consequences of Total and Subtotal Myeloperoxidase Deficiency: Risk or Benefit? Acta Haematol. 2000, 104, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.P.; Reynolds, W.F. Statins Downregulate Myeloperoxidase Gene Expression in Macrophages. Biochem. Biophys. Res. Commun. 2005, 331, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Galijasevic, S. The Development of Myeloperoxidase Inhibitors. Bioorg. Med. Chem. Lett. 2019, 29, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Maiocchi, S.L.; Morris, J.C.; Rees, M.D.; Thomas, S.R. Regulation of the Nitric Oxide Oxidase Activity of Myeloperoxidase by Pharmacological Agents. Biochem. Pharmacol. 2017, 135, 90–115. [Google Scholar] [CrossRef] [PubMed]
- Vago, J.P.; Tavares, L.P.; Sugimoto, M.A.; Lima, G.L.N.; Galvão, I.; de Caux, T.R.; Lima, K.M.; Ribeiro, A.L.C.; Carneiro, F.S.; Nunes, F.F.C.; et al. Proresolving Actions of Synthetic and Natural Protease Inhibitors Are Mediated by Annexin A1. J. Immunol. 2016, 196, 1922–1932. [Google Scholar] [CrossRef]
- AZD3241 PET MSA Trial, Phase 2, Randomized,12 Week Safety and Tolerability Trial with PET in MSA Patients—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02388295 (accessed on 8 May 2022).
- Ruggeri, R.B.; Buckbinder, L.; Bagley, S.W.; Carpino, P.A.; Conn, E.L.; Dowling, M.S.; Fernando, D.P.; Jiao, W.; Kung, D.W.; Orr, S.T.M.; et al. Discovery of 2-(6-(5-Chloro-2-Methoxyphenyl)-4-Oxo-2-Thioxo-3,4-Dihydropyrimidin-1(2H)-Yl) Acetamide (PF-06282999): A Highly Selective Mechanism-Based Myeloperoxidase Inhibitor for the Treatment of Cardiovascular Diseases. J. Med. Chem. 2015, 58, 8513–8528. [Google Scholar] [CrossRef]
- Doxycycline and Airway Inflammation in Chronic Obstructive Pulmonary Disease (COPD)—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT00857038 (accessed on 8 May 2022).
- Catz, S.D.; Mcleish, K.R. Therapeutic Targeting of Neutrophil Exocytosis. J. Leukoc. Biol. 2020, 107, 393–408. [Google Scholar] [CrossRef]
- Wang, H.; Ishizaki, R.; Xu, J.; Kasai, K.; Kobayashi, E.; Gomi, H.; Izumi, T. The Rab27a Effector Exophilin 7 Promotes Fusion of Secretory Granules that have not been Docked to the Plasma Membrane. Mol. Biol. Cell 2013, 24, 319–330. [Google Scholar] [CrossRef]
- Johnson, J.L.; Hong, H.; Monfregola, J.; Kiosses, W.B.; Catz, S.D. Munc13-4 Restricts Motility of Rab27a-expressing Vesicles to Facilitate Lipopolysaccharide-induced Priming of Exocytosis in Neutrophils. J. Biol. Chem. 2011, 286, 5647–5656. [Google Scholar] [CrossRef]
- Serhan, C.N.; Levy, B.D. Resolvins in Inflammation: Emergence of the Pro-resolving Superfamily of Mediators. J. Clin. Investig. 2018, 128, 2657–2669. [Google Scholar] [CrossRef]
- Serhan, C.N. Pro-resolving Lipid Mediators are Leads for Resolution Physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef]
- Serhan, C.N.; Gupta, S.K.; Perretti, M.; Godson, C.; Brennan, E.; Li, Y.; Soehnlein, O.; Shimizu, T.; Werz, O.; Chiurchiù, V.; et al. The Atlas of Inflammation Resolution (AIR). Mol. Aspects Med. 2020, 74, 100894. [Google Scholar] [CrossRef]
- Levy, B.D.; De Sanctis, G.T.; Devchand, P.R.; Kim, E.; Ackerman, K.; Schmidt, B.A.; Szczeklik, W.; Drazen, J.M.; Serhan, C.N. Multi-pronged Inhibition of Airway Hyper-responsiveness and Inflammation by Lipoxin A4. Nat. Med. 2002, 8, 1018–1023. [Google Scholar] [CrossRef]
- Godson, C.; Mitchell, S.; Harvey, K.; Petasis, N.A.; Hogg, N.; Brady, H.R. Cutting Edge: Lipoxins Rapidly Stimulate Nonphlogistic Phagocytosis of Apoptotic Neutrophils by Monocyte-derived Macrophages. J. Immunol. 2000, 164, 1663–1667. [Google Scholar] [CrossRef]
- Schwab, J.M.; Chiang, N.; Arita, M.; Serhan, C.N. Resolvin E1 and Protectin D1 Activate Inflammation-resolution Programmes. Nature 2007, 447, 869–874. [Google Scholar] [CrossRef]
- Karp, C.L.; Flick, L.M.; Park, K.W.; Softic, S.; Greer, T.M.; Keledjian, R.; Yang, R.; Uddin, J.; Guggino, W.B.; Atabani, S.F.; et al. Defective Lipoxin-mediated Anti-inflammatory Activity in the Cystic Fibrosis Airway. Nat. Immunol. 2004, 5, 388–392. [Google Scholar] [CrossRef]
- Chiang, N.; Fredman, G.; Bäckhed, F.; Oh, S.F.; Vickery, T.; Schmidt, B.A.; Serhan, C.N. Infection Regulates Pro-Resolving Mediators that Lower Antibiotic Requirements. Nature 2012, 484, 524–528. [Google Scholar] [CrossRef]
- Knight, J.S.; Kaplan, M.J. Lupus Neutrophils: “NET” Gain in Understanding Lupus Pathogenesis. Curr. Opin. Rheumatol. 2012, 24, 441–450. [Google Scholar] [CrossRef]
- Li, P.; Li, M.; Lindberg, M.R.; Kennett, M.J.; Xiong, N.; Wang, Y. PAD4 is Essential for Antibacterial Innate Immunity Mediated by Neutrophil Extracellular Traps. J. Exp. Med. 2010, 207, 1853–1862. [Google Scholar] [CrossRef]
- Martinod, K.; Fuchs, T.A.; Zitomersky, N.L.; Wong, S.L.; Demers, M.; Gallant, M.; Wang, Y.; Wagner, D.D. PAD4-Deficiency does not Affect Bacteremia in Polymicrobial Sepsis and Ameliorates Endotoxemic Shock. Blood 2015, 125, 1948–1956. [Google Scholar] [CrossRef]
- Murata, N.; Mogi, C.; Tobo, M.; Nakakura, T.; Sato, K.; Tomura, H.; Okajima, F. Inhibition of Superoxide Anion Production by Extracellular Acidification in Neutrophils. Cell Immunol. 2009, 259, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Liu, P.; Zhu, H.; Gong, H.; Yao, J.; Sun, Y.; Geng, G.; Wang, T.; Feng, S.; Han, M.; et al. Extracellular Acidification Acts as a Key Modulator of Neutrophil Apoptosis and Functions. PLoS ONE 2015, 10, e0137221. [Google Scholar] [CrossRef] [PubMed]
- Behnen, M.; Möller, S.; Brozek, A.; Klinger, M.; Laskay, T. Extracellular Acidification Inhibits the ROS-Dependent Formation of Neutrophil Extracellular Traps. Front. Immunol. 2017, 8, 184. [Google Scholar] [CrossRef] [PubMed]
- Cherpokova, D.; Jouvene, C.C.; Libreros, S.; De Roo, E.P.; Chu, L.; de la Rosa, X.; Norris, P.C.; Wagner, D.D.; Serhan, C.N. Resolvin D4 Attenuates the Severity of Pathological Thrombosis in Mice. Blood 2019, 134, 1458–1468. [Google Scholar] [CrossRef] [PubMed]
- Yipp, B.G.; Kubes, P. NETosis: How Vital Is It? Blood 2013, 122, 2784–2794. [Google Scholar] [CrossRef]
- Cedervall, J.; Zhang, Y.; Huang, H.; Zhang, L.; Femel, J.; Dimberg, A.; Olsson, A.K. Neutrophil Extracellular Traps Accumulate in Peripheral Blood Vessels and Compromise Organ Function in Tumor-Bearing Animals. Cancer Res. 2015, 75, 2653–2662. [Google Scholar] [CrossRef]
- Macanovic, M.; Sinicropi, D.; Shak, S.; Baughman, S.; Thiru, S.; Lachmann, P.J. The Treatment of Systemic Lupus Erythematosus (SLE) in NZB/W F1Hybrid Mice: Studies with Recombinant Murine DNase and with Dexamethasone. Clin. Exp. Immunol. 1996, 106, 243–252. [Google Scholar] [CrossRef]
- Li, H.; Zhou, X.; Tan, H.; Hu, Y.; Zhang, L.; Liu, S.; Dai, M.; Li, Y.; Li, Q.; Mao, Z.; et al. Neutrophil Extracellular Traps Contribute to the Pathogenesis of Acid Aspiration-induced ALI/ARDS. Oncotarget 2018, 9, 1772–1784. [Google Scholar] [CrossRef]
- Sayah, D.M.; Mallavia, B.; Liu, F.; Ortiz-Muñoz, G.; Caudrillier, A.; Der Hovanessian, A.; Ross, D.J.; Lynch, J.P., 3rd; Saggar, R.; Ardehali, A.; et al. Neutrophil Extracellular Traps are Pathogenic in Primary Graft Dysfunction after Lung Transplantation. Am. J. Respir. Crit. Care Med. 2015, 191, 455–463. [Google Scholar] [CrossRef]
- Pottecher, J. Inhaled Dornase Alpha to Reduce Respiratory Failure After Severe Trauma. Available online: https://clinicaltrials.gov/ct2/show/NCT03368092 (accessed on 10 December 2020).
- Kessenbrock, K.; Krumbholz, M.; Schönermarck, U.; Back, W.; Gross, W.L.; Werb, Z.; Gröne, H.J.; Brinkmann, V.; Jenne, D.E. Netting Neutrophils in Autoimmune Small-vessel Vasculitis. Nat. Med. 2009, 15, 623–625. [Google Scholar] [CrossRef]
- Sangaletti, S.; Tripodo, C.; Chiodoni, C.; Guarnotta, C.; Cappetti, B.; Casalini, P.; Piconese, S.; Parenza, M.; Guiducci, C.; Vitali, C.; et al. Neutrophil Extracellular Traps Mediate Transfer of Cytoplasmic Neutrophil Antigens to Myeloid Dendritic Cells toward ANCA Induction and Associated Autoimmunity. Blood 2012, 120, 3007–3018. [Google Scholar] [CrossRef]
- Jennette, J.C.; Falk, R.J. Pathogenesis of Antineutrophil Cytoplasmic Autoantibody-mediated Disease. Nat. Rev. Rheumatol. 2014, 10, 463–473. [Google Scholar] [CrossRef]
- Knight, J.S.; Zhao, W.; Luo, W.; Subramanian, V.; O’Dell, A.A.; Yalavarthi, S.; Hodgin, J.B.; Eitzman, D.T.; Thompson, P.R.; Kaplan, M.J. Peptidylarginine Deiminase Inhibition is Immunomodulatory and Vasculoprotective in Murine Lupus. J. Clin. Investig. 2013, 123, 2981–2993. [Google Scholar] [CrossRef]
- Chumanevich, A.A.; Causey, C.P.; Knuckley, B.A.; Jones, J.E.; Poudyal, D.; Chumanevich, A.P.; Davis, T.; Matesic, L.E.; Thompson, P.R.; Hofseth, L.J. Suppression of Colitis in Mice by Cl-amidine: A Novel Peptidylarginine Deiminase Inhibitor. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G929–G938. [Google Scholar] [CrossRef]
- Deniset, J.F.; Kubes, P. Neutrophil Heterogeneity: Bone Fide Subsets or Polarization States? J. Leukoc. Biol. 2018, 103, 829–838. [Google Scholar] [CrossRef]
- Ng, L.G.; Ostuni, R.; Hidalgo, A. Heterogeneity of Neutrophils. Nat. Rev. Immunol. 2019, 19, 255–265. [Google Scholar] [CrossRef]
- Grieshaber-Bouyer, R.; Nigrovic, P.A. Neutrophil Heterogeneity as Therapeutic Opportunity in Immune-Mediated Disease. Front. Immunol. 2019, 10, 346. [Google Scholar] [CrossRef]
| Molecule | Species | Disease/Model | Key Mechanisms | Effects on Disease | References |
|---|---|---|---|---|---|
| Blocking Enzymatic Activities | |||||
| 4-Aminobenzoic acid hydrazide (4-ABAH) | Rabbit | Atherosclerosis | Irreversible inhibition of HOCl production | ↓ Peroxidase activity | [132] |
| Mice | Lung carcinoma | ↓ Tumor progression | [133] | ||
| Mice | Ischemic stroke | ↑ Cell proliferation and neurogenesis | [69] | ||
| Polyamine-Conjugated Piperidine Nitroxides | Bovine | Aortic endothelial cells | ↓ H2O2↓ HOCl, •NO2 scavenging | ↓ Endothelial HOCl ↓ Protein nitration, ↓ NO oxidation | [134] |
| AZM198 (2-thioxanthin) | Mice | Obesity and hypertension | Irreversibly inhibition by covalent attachment to the heme group | ↓ Body weight ↓ Fat accumulation, ↓ Inflammation ↓ Non-alcoholic steatohepatitis | [135] |
| Mice | Vascular inflammation | ↑eNOS/NO | [136] | ||
| Mice | Peritonitis | ↓ Tissue damage | [137] | ||
| Guinea pig | Chronic obstructive pulmonary disease | ↓ Tissue damage and remodeling | [138] | ||
| Mice | Nephritis | ↓ MPO deposition ↓ Glomerular damage | [139] | ||
| AZD3241 (Pyrrolo (3, 2-d) pyrimidin-4-one derivative) | Human | Parkinson’s disease | Selective and irreversible MPO inhibitor | ↓ Neuro-inflammation | [140] |
| Mice | Multiple system atrophy | ↓ Microglial activation and motor impairment | [141,142] | ||
| Mice | Colitis | ↓ Weight loss ↑ Clinical score | [143] | ||
| PF-1355 (Thiouracil derivative) | Mice | Small vessel vasculitis | Irreversible MPO inhibitor | ↓ HOCl ↓ Vascular edema, ↓ Neutrophil recruitment | [144] |
| PF-06282999 (Thiouracil derivative) | Human | Irreversible MPO inhibitor | [145] | ||
| AZD4831 | Human | Heart failure with preserved ejection fraction | Selective extracellular MPO inhibitor | ↓ Morbidity and mortality | [146,147] |
| Suppression of MPO gene expression | |||||
| Atorvastatin | Human | Acute coronary syndrome | ? | ↓ Serum MPO | [148] |
| Human | Diabetes with renal failure | ? | ↓ Serum MPO | [149] | |
| All-trans retinoic acid (tretinoin) | Human | ANCA vasculitis | ? | ↓ Disease relapse ? | [150] |
| Inhibition of granule trafficking, docking and degranulation | |||||
| Nexinhib 20 | Mice | Endotoxemia | Selective inhibition of release of azurophilic granules | ↓ Neutrophil infiltration | [151] |
| Mice | Myocardial ischemia-reperfusion injury | ↓ Neutrophil recruitment and exocytosis ↓ Infarct size | [152] | ||
| TAT-STX-4 | Human | Neutrophils (L. monocytogenes infection) | Inhibition of degranulation | ↓ Degranulation of all granule subsets | [153] |
| TAT-SNAP-23 | Human | Neutrophils (S. aureus infection) | Inhibition of azurophilic granule release | ↓ Exocytosis ↓ Respiratory burst | [154] |
| Rats Mice | Acute lung injury | ↓ Neutrophil recruitment ↓ Exocytosis | [155,156] | ||
| 15-epi-lipoxin A4 and 17-epi-resolvin D1 | Human | Activation of ALX/FPR2 | ↓ MPO release ↑ Phagocytosis and bacterial killing | [39] | |
| Mice | Acute lung injury | ↑ Bacterial clearance ↑ Resolution | [39] | ||
| Targeting NETs and MPO auto-antigens | |||||
| 4-Aminobenzoic acid hydrazide (4-ABAH) | Human | Neutrophils | ↓ NET formation | [157] | |
| Deoxyribonuclease I (DNase I) | Human Mice | Bacterial infection | NET degradation | ↓ Reactive oxygen species | [158] |
| N-a-benzoyl-N5-(2-chloro-1-iminoethyl)-L-ornithine am-ide (Cl-amidine) | Mice | Rheumatoid arthritis | Peptidyl arginine deiminase (1-4) inhibitor | ↓ Disease severity | [159] |
| Mice | Atherosclerosis | ↓ Lesion area ↓ Neutrophil and macrophages recruitment | [160] | ||
| Mice | ANCA vasculitis | ↓ MPO-ANCA production | [161] | ||
| 13-series resolvins (RvTs) | Human | Neutrophils | ↓ MPO release ↓ NET formation ↑ NET degradation | [162] | |
| Mice | Bacterial infection (Skin air pouch) | ↓ Neutrophil accumulation ↑ Bacterial clearance | [162] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizo-Téllez, S.A.; Sekheri, M.; Filep, J.G. Myeloperoxidase: Regulation of Neutrophil Function and Target for Therapy. Antioxidants 2022, 11, 2302. https://doi.org/10.3390/antiox11112302
Rizo-Téllez SA, Sekheri M, Filep JG. Myeloperoxidase: Regulation of Neutrophil Function and Target for Therapy. Antioxidants. 2022; 11(11):2302. https://doi.org/10.3390/antiox11112302
Chicago/Turabian StyleRizo-Téllez, Salma A., Meriem Sekheri, and János G. Filep. 2022. "Myeloperoxidase: Regulation of Neutrophil Function and Target for Therapy" Antioxidants 11, no. 11: 2302. https://doi.org/10.3390/antiox11112302
APA StyleRizo-Téllez, S. A., Sekheri, M., & Filep, J. G. (2022). Myeloperoxidase: Regulation of Neutrophil Function and Target for Therapy. Antioxidants, 11(11), 2302. https://doi.org/10.3390/antiox11112302