Post-Translational Modifications Evoked by Reactive Carbonyl Species in Ultraviolet-A-Exposed Skin: Implication in Fibroblast Senescence and Skin Photoaging
Abstract
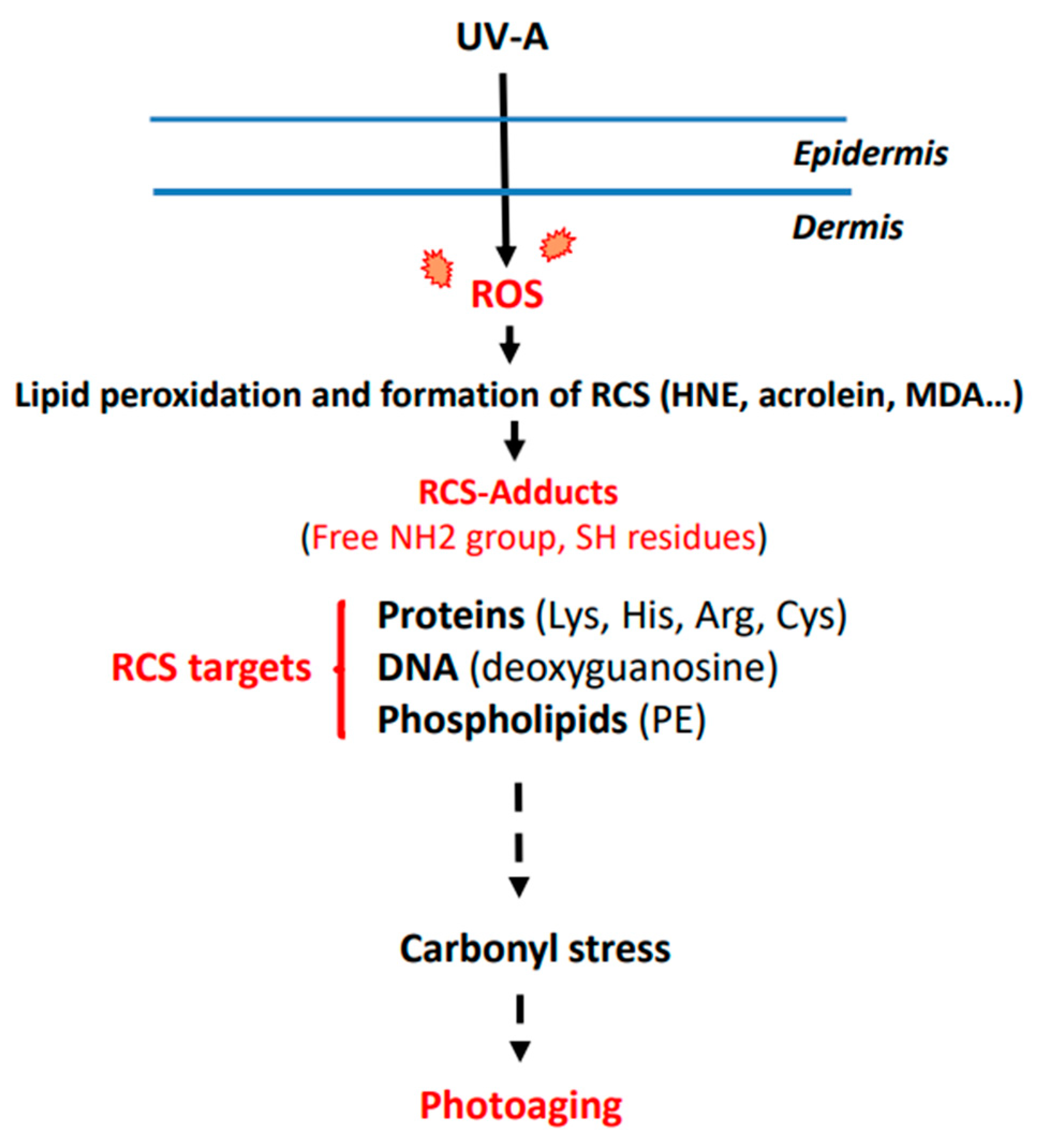
:1. Introduction
2. UV-Induced ROS Production and Photoaging
3. Mechanisms Leading to RCS Generation and Adduct Formation
4. Post-Translational Modifications of Dermis Components by RCS
4.1. Post-Translational Modifications Elicited by RCS in ECM
4.1.1. Collagen
4.1.2. Elastin
4.2. Post-Translational Modifications Evoked by RCS in Dermal Fibroblasts. Implications in Fibroblast Senescence
4.2.1. Fibroblast Senescence in Skin Photoaging
4.2.2. DNA Alterations by Aldehydes in Senescent Fibroblasts
4.2.3. SIRT1 Modifications in Senescent Fibroblasts
4.2.4. Vimentin Modification in Senescent Fibroblasts
5. Prevention of Carbonyl Stress to Limit Skin Photoaging
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnson, F.; Sinclair, D.A.; Guarente, L. Molecular Biology of Aging. Cell 1999, 96, 291–302. [Google Scholar] [CrossRef] [Green Version]
- Höhn, A.; Weber, D.; Jung, T.; Ott, C.; Hugo, M.; Kochlik, B.; Kehm, R.; König, J.; Grune, T.; Castro, J.P. Happily (n) ever after: Aging in the context of oxidative stress, proteostasis loss and cellular senescence. Redox Biol. 2017, 11, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Kligman, L.H. Photoaging. Manifestations, prevention, and treatment. Clin. Geriatr. Med. 1989, 5, 235–251. [Google Scholar] [CrossRef]
- Gilchrest, B.A. Photoaging. J. Investig. Dermatol. 2013, 133, E2–E6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaar, M.; Gilchrest, B.A. Photoageing: Mechanism, prevention and therapy. Br. J. Dermatol. 2007, 157, 874–887. [Google Scholar] [CrossRef] [PubMed]
- Kammeyer, A.; Luiten, R.M. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Kligman, A.M. Early Destructive Effect of Sunlight on Human Skin. JAMA 1969, 210, 2377–2380. [Google Scholar] [CrossRef]
- Lewis, K.G.; Bercovitch, L.; Dill, S.W.; Robinson-Bostom, L. Acquired disorders of elastic tissue: Part I. increased elastic tissue and solar elastotic syndromes. J. Am. Acad. Dermatol. 2004, 51, 1–21. [Google Scholar] [CrossRef]
- El-Domyati, M.; Attia, S.; Saleh, F.; Brown, D.; Birk, D.E.; Gasparro, F.; Ahmad, H.; Uitto, J. Intrinsic aging vs. photoaging: A compar-ative histopathological, immunohistochemical, and ultrastructural study of skin. Exp. Dermatol. 2002, 11, 398–405. [Google Scholar] [CrossRef]
- Sellheyer, K. Pathogenesis of solar elastosis: Synthesis or degradation? J. Cutan. Pathol. 2003, 30, 123–127. [Google Scholar] [CrossRef]
- Weihermann, A.C.; Lorencini, M.; Brohem, C.A.; de Carvalho, C.M. Elastin structure and its involvement in skin photoageing. Int. J. Cosmet. Sci. 2016, 39, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Tigges, J.; Krutmann, J.; Fritsche, E.; Haendeler, J.; Schaal, H.; Fischer, J.W.; Kalfalah, F.; Reinke, H.; Reifenberger, G.; Stühler, K.; et al. The hallmarks of fibroblast ageing. Mech. Ageing Dev. 2014, 138, 26–44. [Google Scholar] [CrossRef] [PubMed]
- Fitsiou, E.; Pulido, T.; Campisi, J.; Alimirah, F.; Demaria, M. Cellular Senescence and the Senescence-Associated Secretory Phenotype as Drivers of Skin Photoaging. J. Investig. Dermatol. 2020, 141, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J. Ultraviolet A radiation-induced biological effects in human skin: Relevance for photoaging and photodermatosis. J. Dermatol. Sci. 2000, 23, S22–S26. [Google Scholar] [CrossRef]
- Wang, S.Q.; Osterwalder, U.; Jung, K. Ex vivo evaluation of radical sun protection factor in popular sunscreens with antioxidants. J. Am. Acad. Dermatol. 2011, 65, 525–530. [Google Scholar] [CrossRef]
- Panich, U.; Sittithumcharee, G.; Rathviboon, N.; Jirawatnotai, S. Ultraviolet Radiation-Induced Skin Aging: The Role of DNA Damage and Oxidative Stress in Epidermal Stem Cell Damage Mediated Skin Aging. Stem Cells Int. 2016, 2016, 7370642. [Google Scholar] [CrossRef] [Green Version]
- Battie, C.; Jitsukawa, S.; Bernerd, F.; Del Bino, S.; Marionnet, C.; Verschoore, M. New insights in photoaging, UVA induced damage and skin types. Exp. Dermatol. 2014, 23, 7–12. [Google Scholar] [CrossRef]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative Stress in Aging Human Skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef] [Green Version]
- Csekes, E.; Račková, L. Skin Aging, Cellular Senescence and Natural Polyphenols. Int. J. Mol. Sci. 2021, 22, 12641. [Google Scholar] [CrossRef]
- Gkogkolou, P.; Böhm, M. Advanced glycation end products: Key players in skin aging? Derm.-Endocrinol. 2012, 4, 259–270. [Google Scholar] [CrossRef]
- Ergin, V.; Hariry, R.E.; Karasu, C. Carbonyl Stress in Aging Process: Role of Vitamins and Phytochemicals as Redox Regulators. Aging Dis. 2013, 4, 276–294. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef]
- Uchida, K. 4-Hydroxy-2-nonenal: A product and mediator of oxidative stress. Prog. Lipid Res. 2003, 42, 318–343. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Giustarini, D.; Colombo, R.; Rossi, R.; Milzani, A. Protein carbonylation in human diseases. Trends Mol. Med. 2003, 9, 169–176. [Google Scholar] [CrossRef]
- Higdon, A.; Diers, A.R.; Oh, J.Y.; Landar, A.; Darley-Usmar, V.M. Cell signalling by reactive lipid species: New concepts and molecular mechanisms. Biochem. J. 2012, 442, 453–464. [Google Scholar] [CrossRef] [Green Version]
- Schaur, R.J.; Siems, W.; Bresgen, N.; Eckl, P.M. 4-Hydroxy-nonenal—A Bioactive Lipid Peroxidation Product. Biomolecules 2015, 5, 2247–2337. [Google Scholar] [CrossRef] [Green Version]
- Eckl, P.M.; Bresgen, N. Genotoxicity of lipid oxidation compounds. Free Radic. Biol. Med. 2017, 111, 244–252. [Google Scholar] [CrossRef]
- Berry, K.; Hallock, K.; Lam, C. Photoaging and Topical Rejuvenation. Facial Plast. Surg. Clin. N. Am. 2022, 30, 291–300. [Google Scholar] [CrossRef]
- Pandel, R.; Poljšak, B.; Godic, A.; Dahmane, R. Skin Photoaging and the Role of Antioxidants in Its Prevention. ISRN Dermatol. 2013, 2013, 930164. [Google Scholar] [CrossRef] [Green Version]
- Haydont, V.; Bernard, B.A.; Fortunel, N.O. Age-related evolutions of the dermis: Clinical signs, fibroblast and extracellular matrix dynamics. Mech. Ageing Dev. 2019, 177, 150–156. [Google Scholar] [CrossRef]
- Davies, J.M.S.; Cillard, J.; Friguet, B.; Cadenas, E.; Cadet, J.; Cayce, R.; Fishmann, A.; Liao, D.; Bulteau, A.-L.; Derbré, F.; et al. The Oxygen Paradox, the French Paradox, and age-related diseases. GeroScience 2017, 39, 499–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debacq-Chainiaux, F.; Leduc, C.; Verbeke, A.; Toussaint, O. UV, stress and aging. Derm.-Endocrinol. 2012, 4, 236–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vernez, D.; Milon, A.; Vuilleumier, L.; Bulliard, J.-L. Anatomical exposure patterns of skin to sunlight: Relative contributions of direct, diffuse and reflected ultraviolet radiation. Br. J. Dermatol. 2012, 167, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Wenk, J.; Brenneisen, P.; Meewes, C.; Wlaschek, M.; Peters, T.; Blaudschun, R.; Ma, W.; Kuhr, L.; Schneider, L.; Scharffet-ter-Kochanek, K. UV-Induced Oxidative Stress and Photoaging. Curr. Probl. Dermatol. 2001, 29, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, B. Photoaging: A review of current concepts of pathogenesis. J. Cutan. Med. Surg. 2011, 15, S374–S377. [Google Scholar] [CrossRef]
- Farris, P.K.; Valacchi, G. Ultraviolet Light Protection: Is It Really Enough? Antioxidants 2022, 11, 1484. [Google Scholar] [CrossRef]
- Bernerd, F.; Passeron, T.; Castiel, I.; Marionnet, C. The Damaging Effects of Long UVA (UVA1) Rays: A Major Challenge to Preserve Skin Health and Integrity. Int. J. Mol. Sci. 2022, 23, 8243. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Photoaging: UV radiation-induced inflammation and immunosuppression accelerate the aging process in the skin. Inflamm. Res. 2022, 71, 817–831. [Google Scholar] [CrossRef]
- Guéraud, F.; Atalay, M.; Bresgen, N.; Cipak, A.; Eckl, P.M.; Huc, L.; Jouanin, I.; Siems, W.; Uchida, K. Chemistry and biochemistry of lipid peroxidation products. Free Radic. Res. 2010, 44, 1098–1124. [Google Scholar] [CrossRef]
- Moldogazieva, N.T.; Mokhosoev, I.M.; Mel’Nikova, T.I.; Porozov, Y.B.; Terentiev, A.A. Oxidative Stress and Advanced Lipoxidation and Glycation End Products (ALEs and AGEs) in Aging and Age-Related Diseases. Oxidative Med. Cell. Longev. 2019, 2019, 3085756. [Google Scholar] [CrossRef]
- Ogura, Y.; Kuwahara, T.; Akiyama, M.; Tajima, S.; Hattori, K.; Okamoto, K.; Okawa, S.; Yamada, Y.; Tagami, H.; Takahashi, M.; et al. Dermal carbonyl modification is related to the yellowish color change of photo-aged Japanese facial skin. J. Dermatol. Sci. 2011, 64, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Larroque-Cardoso, P.; Camaré, C.; Nadal-Wollbold, F.; Grazide, M.H.; Pucelle, M.; Garoby-Salom, S.; Bogdanowicz, P.; Josse, G.; Schmitt, A.-M.; Uchida, K.; et al. Elastin Modification by 4-Hydroxynonenal in Hairless Mice Exposed to UV-A. Role in Photoaging and Actinic Elastosis. J. Investig. Dermatol. 2015, 135, 1873–1881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zucchi, H.; Pageon, H.; Asselineau, D.; Ghibaudo, M.; Sequeira, I.; Girardeau-Hubert, S. Assessing the Role of Carbonyl Adducts, Particularly Malondialdehyde Adducts, in the Development of Dermis Yellowing Occurring during Skin Photoaging. Life 2022, 12, 403. [Google Scholar] [CrossRef]
- Uchida, K.; Kanematsu, M.; Morimitsu, Y.; Osawa, T.; Noguchi, N.; Niki, E. Acrolein Is a Product of Lipid Peroxidation Reaction: Formation of free acrolein and its conjugate with lysine residues in oxidized low-density lipoproteins. J. Biol. Chem. 1998, 273, 16058–16066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domingues, R.M.; Domingues, P.; Melo, T.; Pérez-Sala, D.; Reis, A.; Spickett, C.M. Lipoxidation adducts with peptides and proteins: Deleterious modifications or signaling mechanisms? J. Proteom. 2013, 92, 110–131. [Google Scholar] [CrossRef] [PubMed]
- Barrera, G.; Pizzimenti, S.; Daga, M.; Dianzani, C.; Arcaro, A.; Cetrangolo, G.P.; Giordano, G.; Cucci, M.A.; Graf, M.; Gentile, F. Lipid Peroxidation-Derived Aldehydes, 4-Hydroxynonenal and Malondialdehyde in Aging-Related Disorders. Antioxidants 2018, 7, 102. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Blair, I.A. Characterization of 4-Oxo-2-nonenal as a Novel Product of Lipid Peroxidation. Chem. Res. Toxicol. 2000, 13, 698–702. [Google Scholar] [CrossRef]
- Huang, H.; Kozekov, I.D.; Kozekova, A.; Wang, H.; Lloyd, R.S.; Rizzo, C.J.; Stone, M.P. DNA cross-link induced by trans-4-hydroxynonenal. Environ. Mol. Mutagen. 2010, 51, 625–634. [Google Scholar] [CrossRef] [Green Version]
- Guichardant, M.; Taibi-Tronche, P.; Fay, L.B.; Lagarde, M. Covalent modifications of aminophospholipids by 4-hydroxynonenal. Free Radic. Biol. Med. 1998, 25, 1049–1056. [Google Scholar] [CrossRef]
- Pohl, E.E.; Jovanovic, O. The Role of Phosphatidylethanolamine Adducts in Modification of the Activity of Membrane Proteins under Oxidative Stress. Molecules 2019, 24, 4545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nègre-Salvayre, A.; Garoby-Salom, S.; Swiader, A.; Rouahi, M.; Pucelle, M.; Salvayre, R. Proatherogenic effects of 4-hydroxynonenal. Free Radic. Biol. Med. 2017, 111, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Giera, M.; Lingeman, H.; Niessen, W.M.A. Recent Advancements in the LC- and GC-Based Analysis of Malondialdehyde (MDA): A Brief Overview. Chromatographia 2012, 75, 433–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowdhury, P.K.; Halder, M.; Choudhury, P.K.; Kraus, G.A.; Desai, M.J.; Armstrong, D.W.; Casey, T.A.; Rasmussen, M.A.; Petrich, J.W. Generation of fluorescent adducts of malondialdehyde and amino acids: Toward an understanding of lipofuscin. Photochem. Photobiol. 2004, 79, 21–25. [Google Scholar] [CrossRef]
- Ishii, T.; Yamada, T.; Mori, T.; Kumazawa, S.; Uchida, K.; Nakayama, T. Characterization of acrolein-induced protein cross-links. Free Radic. Res. 2007, 41, 1253–1260. [Google Scholar] [CrossRef]
- Abordo, E.A.; Minhas, H.S.; Thornalley, P.J. Accumulation of α-oxoaldehydes during oxidative stress: A role in cytotoxicity. Biochem. Pharmacol. 1999, 58, 641–648. [Google Scholar] [CrossRef]
- Vistoli, G.; De Maddis, D.; Cipak, A.; Zarkovic, N.; Carini, M.; Aldini, G. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): An overview of their mechanisms of formation. Free Radic. Res. 2013, 47, 3–27. [Google Scholar] [CrossRef] [Green Version]
- Papaccio, F.; D′arino, A.; Caputo, S.; Bellei, B. Focus on the Contribution of Oxidative Stress in Skin Aging. Antioxidants 2022, 11, 1121. [Google Scholar] [CrossRef]
- Sander, C.S.; Chang, H.; Salzmann, S.; Müller, C.S.L.; Ekanayake-Mudiyanselage, S.; Elsner, P.; Thiele, J.J. Photoaging is Associated with Protein Oxidation in Human Skin In Vivo. J. Investig. Dermatol. 2002, 118, 618–625. [Google Scholar] [CrossRef] [Green Version]
- Mizutani, T.; Sumida, H.; Sagawa, Y.; Okano, Y.; Masaki, H. Carbonylated proteins exposed to UVA and to blue light generate reactive oxygen species through a type I photosensitizing reaction. J. Dermatol. Sci. 2016, 84, 314–321. [Google Scholar] [CrossRef]
- Tanaka, N.; Tajima, S.; Ishibashi, A.; Uchida, K.; Shigematsu, T. Immunohistochemical detection of lipid peroxidation products, protein-bound acrolein and 4-hydroxynonenal protein adducts, in actinic elastosis of photodamaged skin. Arch. Dermatol. Res. 2001, 293, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Lamore, S.D.; Azimian, S.; Horn, D.; Anglin, B.L.; Uchida, K.; Cabello, C.M.; Wondrak, G.T. The malondialdehyde-derived fluorophore DHP-lysine is a potent sensitizer of UVA-induced photooxidative stress in human skin cells. J. Photochem. Photobiol. B Biol. 2010, 101, 251–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jørgensen, P.; Milkovic, L.; Zarkovic, N.; Waeg, G.; Rattan, S.I.S. Lipid peroxidation-derived 4-hydroxynonenal-modified proteins accumulate in human facial skin fibroblasts during ageing In Vitro. Biogerontology 2014, 15, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Larroque-Cardoso, P.; Mucher, E.; Grazide, M.-H.; Josse, G.; Schmitt, A.-M.; Nadal-Wolbold, F.; Zarkovic, K.; Salvayre, R.; Nègre-Salvayre, A. 4-Hydroxynonenal impairs transforming growth factor-β1-induced elastin synthesis via epidermal growth factor receptor activation in human and murine fibroblasts. Free Radic. Biol. Med. 2014, 71, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Le Boulch, M.; Ahmed, E.K.; Rogowska-Wrzesinska, A.; Baraibar, M.A.; Friguet, B. Proteome oxidative carbonylation during oxidative stress-induced premature senescence of WI-38 human fibroblasts. Mech. Ageing Dev. 2018, 170, 59–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petkovic, I.; Bresgen, N.; Gilardoni, E.; Regazzoni, L.; Uchida, K.; Aldini, G.; Siems, W.; Eckl, P. In Vitro Aging of Human Skin Fibroblasts: Age-Dependent Changes in 4-Hydroxynonenal Metabolism. Antioxidants 2020, 9, 150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swiader, A.; Camaré, C.; Guerby, P.; Salvayre, R.; Negre-Salvayre, A. 4-Hydroxynonenal Contributes to Fibroblast Senescence in Skin Photoaging Evoked by UV-A Radiation. Antioxidants 2021, 10, 365. [Google Scholar] [CrossRef]
- Baraibar, M.A.; Friguet, B. Oxidative proteome modifications target specific cellular pathways during oxidative stress, cellular senescence and aging. Exp. Gerontol. 2013, 48, 620–625. [Google Scholar] [CrossRef]
- Radrezza, S.; Carini, M.; Baron, G.; Aldini, G.; Negre-Salvayre, A.; D’Amato, A. Study of Carnosine’s effect on nude mice skin to prevent UV-A damage. Free Radic. Biol. Med. 2021, 173, 97–103. [Google Scholar] [CrossRef]
- Boldyrev, A.A.; Aldini, G.; Derave, W. Physiology and Pathophysiology of Carnosine. Physiol. Rev. 2013, 93, 1803–1845. [Google Scholar] [CrossRef]
- Slatter, D.A.; Paul, R.G.; Murray, M.; Bailey, A.J. Reactions of Lipid-derived Malondialdehyde with Collagen. J. Biol. Chem. 1999, 274, 19661–19669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.Q.; Bernstein, E.F.; Tamai, K.; Shepley, K.J.; Resnik, K.S.; Zhang, H.; Tuan, R.; Mauviel, A.; Uitto, J. Enhanced Elastin and Fibrillin Gene Expression in Chronically Photodamaged Skin. J. Investig. Dermatol. 1994, 103, 182–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernstein, E.F.; Uitto, J. The effect of photodamage on dermal extracellular matrix. Clin. Dermatol. 1996, 14, 143–151. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Tanaka, H.; Okada, T.; Konishi, H.; Takahashi, M.; Ito, M.; Asai, J. Effect of Reactive Oxygen Species on the Elastin mRNA Expression in Cultured Human Dermal Fibroblasts. Free Radic. Biol. Med. 1997, 23, 162–165. [Google Scholar] [CrossRef]
- Sproul, E.P.; Argraves, W.S. A cytokine axis regulates elastin formation and degradation. Matrix Biol. 2013, 32, 86–94. [Google Scholar] [CrossRef]
- Muto, J.; Kuroda, K.; Wachi, H.; Hirose, S.; Tajima, S. Accumulation of Elafin in Actinic Elastosis of Sun-Damaged Skin: Elafin Binds to Elastin and Prevents Elastolytic Degradation. J. Investig. Dermatol. 2007, 127, 1358–1366. [Google Scholar] [CrossRef] [Green Version]
- Schalkwijk, J. Cross-Linking of Elafin/SKALP to Elastic Fibers in Photodamaged Skin: Too Much of a Good Thing? J. Investig. Dermatol. 2007, 127, 1286–1287. [Google Scholar] [CrossRef] [Green Version]
- Yoshinaga, E.; Kawada, A.; Ono, K.; Fujimoto, E.; Wachi, H.; Harumiya, S.; Nagai, R.; Tajima, S. Nε-(Carboxymethyl)lysine Modification of Elastin Alters Its Biological Properties: Implications for the Accumulation of Abnormal Elastic Fibers in Actinic Elastosis. J. Investig. Dermatol. 2012, 132, 315–323. [Google Scholar] [CrossRef] [Green Version]
- Zarkovic, K.; Larroque-Cardoso, P.; Pucelle, M.; Salvayre, R.; Waeg, G.; Nègre-Salvayre, A.; Zarkovic, N. Elastin aging and lipid oxidation products in human aorta. Redox Biol. 2015, 4, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Dhital, B.; Durlik, P.; Rathod, P.; Gul-E-Noor, F.; Wang, Z.; Sun, C.; Chang, E.J.; Itin, B.; Boutis, G.S. Ultraviolet radiation reduces desmosine cross-links in elastin. Biochem. Biophys. Rep. 2017, 10, 172–177. [Google Scholar] [CrossRef]
- Baurain, R.; Larochelle, J.-F.; Lamy, F. Photolysis of Desmosine and Isodesmosine by Ultraviolet Light. Eur. J. Biochem. 1976, 67, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Thulabandu, V.; Chen, D.; Atit, R.P. Dermal fibroblast in cutaneous development and healing. Wiley Interdiscip. Rev. Dev. Biol. 2018, 7, e307. [Google Scholar] [CrossRef]
- Wlaschek, M.; Tantcheva-Poór, I.; Naderi, L.; Ma, W.; Schneider, L.A.; Razi-Wolf, Z.; Schüller, J.; Scharffetter-Kochanek, K. Solar UV irradiation and dermal photoaging. J. Photochem. Photobiol. B Biol. 2001, 63, 41–51. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozano-Torres, B.; Estepa-Fernández, A.; Rovira, M.; Orzáez, M.; Serrano, M.; Martínez-Máñez, R.; Sancenón, F. The chemistry of senescence. Nat. Rev. Chem. 2019, 3, 426–441. [Google Scholar] [CrossRef]
- Rodier, F.; Campisi, J. Four faces of cellular senescence. J. Cell Biol. 2011, 192, 547–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O.; et al. A biomarker that identifies senescent human cells in culture and in aging skin In Vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mah, L.-J.; El-Osta, A.; Karagiannis, T.C. Gamma H2AX as a molecular marker of aging and disease. Epigenetics 2010, 5, 129–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kueper, T.; Grune, T.; Prahl, S.; Lenz, H.; Welge, V.; Biernoth, T.; Vogt, Y.; Muhr, G.-M.; Gaemlich, A.; Jung, T.; et al. Vimentin Is the Specific Target in Skin Glycation. Structural prerequisites, functional consequences, and role in skin aging. J. Biol. Chem. 2007, 282, 23427–23436. [Google Scholar] [CrossRef] [Green Version]
- Frescas, D.; Roux, C.M.; Aygun-Sunar, S.; Gleiberman, A.S.; Krasnov, P.; Kurnasov, O.V.; Strom, E.; Virtuoso, L.P.; Wrobel, M.; Osterman, A.L.; et al. Senescent cells expose and secrete an oxidized form of membrane-bound vimentin as revealed by a natural polyreactive antibody. Proc. Natl. Acad. Sci. USA 2017, 114, E1668–E1677. [Google Scholar] [CrossRef]
- Treiber, N.; Maity, P.; Singh, K.; Kohn, M.; Keist, A.F.; Ferchiu, F.; Sante, L.; Frese, S.; Bloch, W.; Kreppel, F.; et al. Accelerated aging phenotype in mice with conditional deficiency for mitochondrial superoxide dismutase in the connective tissue. Aging Cell 2011, 10, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Meyer, P.; Maity, P.; Burkovski, A.; Schwab, J.; Müssel, C.; Singh, K.; Ferreira, F.F.; Krug, L.; Maier, H.J.; Wlaschek, M.; et al. A model of the onset of the senescence associated secretory phenotype after DNA damage induced senescence. PLoS Comput. Biol. 2017, 13, e1005741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wlaschek, M.; Maity, P.; Makrantonaki, E.; Scharffetter-Kochanek, K. Connective Tissue and Fibroblast Senescence in Skin Aging. J. Investig. Dermatol. 2021, 141, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Bulteau, A.; Moreau, M.; Nizard, C.; Friguet, B. Proteasome and Photoaging: The Effects of UV Irradiation. Ann. N. Y. Acad. Sci. 2007, 1100, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Chondrogianni, N.; Gonos, E.S. Proteasome Function Determines Cellular Homeostasis and the Rate of Aging. Adv. Exp. Med. Biol. 2010, 694, 38–46. [Google Scholar] [CrossRef]
- Debacq-Chainiaux, F.; Borlon, C.; Pascal, T.; Royer, V.; Eliaers, F.; Ninane, N.; Carrard, G.; Friguet, B.; de Longueville, F.; Boffe, S.; et al. Repeated exposure of human skin fibroblasts to UVB at subcytotoxic level triggers premature senescence through the TGF-β1 signaling pathway. J. Cell Sci. 2005, 118, 743–758. [Google Scholar] [CrossRef] [Green Version]
- Bertrand-Vallery, V.; Boilan, E.; Ninane, N.; Demazy, C.; Friguet, B.; Toussaint, O.; Poumay, Y.; Debacq-Chainiaux, F. Repeated exposures to UVB induce differentiation rather than senescence of human keratinocytes lacking p16INK-4A. Biogerontology 2010, 11, 167–181. [Google Scholar] [CrossRef]
- Kurtz, A.J.; Lloyd, R.S. 1,N2-Deoxyguanosine Adducts of Acrolein, Crotonaldehyde, and trans-4-Hydroxynonenal Cross-link to Peptides via Schiff Base Linkage. J. Biol. Chem. 2003, 278, 5970–5976. [Google Scholar] [CrossRef] [Green Version]
- Alzolibani, A.A.; Al Robaee, A.A.; Al-Shobaili, H.A.; Rasheed, Z. 4-Hydroxy-2-nonenal modified histone-H2A: A possible antigenic stimulus for systemic lupus erythematosus autoantibodies. Cell. Immunol. 2013, 284, 154–162. [Google Scholar] [CrossRef]
- Doyle, K.; Fitzpatrick, F. Redox Signaling, Alkylation (Carbonylation) of Conserved Cysteines Inactivates Class I Histone Deacetylases 1, 2, and 3 and Antagonizes Their Transcriptional Repressor Function. J. Biol. Chem. 2010, 285, 17417–17424. [Google Scholar] [CrossRef]
- Zhao, K.; Harshaw, R.; Chai, X.; Marmorstein, R. Structural basis for nicotinamide cleavage and ADP-ribose transfer by NAD (+) -dependent Sir2 histone/protein deacetylases. Proc. Natl. Acad. Sci. USA 2004, 101, 8563–8568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grabowska, W.; Sikora, E.; Bielak-Zmijewska, A. Sirtuins, a promising target in slowing down the ageing process. Biogerontology 2017, 18, 447–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merksamer, P.I.; Liu, Y.; He, W.; Hirschey, M.D.; Chen, D.; Verdin, E. The sirtuins, oxidative stress and aging: An emerging link. Aging 2013, 5, 144–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; Mack, N.J.; Ahmad, N. The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid. Redox Signal. 2018, 28, 643–661. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.W.; Choi, Y.J.; Park, M.H.; Jang, E.J.; Kim, D.H.; Park, B.H.; Yu, B.P.; Chung, H.Y. Molecular Insights into SIRT1 Protection Against UVB-Induced Skin Fibroblast Senescence by Suppression of Oxidative Stress and p53 Acetylation. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 959–968. [Google Scholar] [CrossRef]
- Hwang, J.-W.; Yao, H.; Caito, S.; Sundar, I.K.; Rahman, I. Redox regulation of SIRT1 in inflammation and cellular senescence. Free. Radic. Biol. Med. 2013, 61, 95–110. [Google Scholar] [CrossRef] [Green Version]
- Yamaba, H.; Haba, M.; Kunita, M.; Sakaida, T.; Tanaka, H.; Yashiro, Y.; Nakata, S. Morphological change of skin fibroblasts induced by UV Irradiation is involved in photoaging. Exp. Dermatol. 2016, 25, 45–51. [Google Scholar] [CrossRef]
- Gadoni, E.; Olivero, A.; Miglietta, A.; Bocca, C.; Gabriel, L. Cytoskeletal modifications induced by 4-hydroxynonenal. Cytotechnology 1993, 11, S62–S64. [Google Scholar] [CrossRef]
- Sliogeryte, K.; Gavara, N. Vimentin Plays a Crucial Role in Fibroblast Ageing by Regulating Biophysical Properties and Cell Migration. Cells 2019, 8, 1164. [Google Scholar] [CrossRef] [Green Version]
- Cheng, F.; Shen, Y.; Mohanasundaram, P.; Lindström, M.; Ivaska, J.; Ny, T.; Eriksson, J.E. Vimentin coordinates fibroblast proliferation and keratinocyte differentiation in wound healing via TGF-β–Slug signaling. Proc. Natl. Acad. Sci. USA 2016, 113, E4320–E4327. [Google Scholar] [CrossRef]
- Mónico, A.; Duarte, S.; Pajares, M.; Pérez-Sala, D. Vimentin disruption by lipoxidation and electrophiles: Role of the cysteine residue and filament dynamics. Redox Biol. 2019, 23, 101098. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sala, D.; Oeste, C.L.; Martínez, A.E.; Carrasco, M.J.; Garzón, B.; Cañada, F.J. Vimentin filament organization and stress sensing depend on its single cysteine residue and zinc binding. Nat. Commun. 2015, 6, 7287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viedma-Poyatos, A.; Pajares, M.; Pérez-Sala, D. Type III intermediate filaments as targets and effectors of electrophiles and oxidants. Redox Biol. 2020, 36, 101582. [Google Scholar] [CrossRef] [PubMed]
- Amaro-Ortiz, A.; Yan, B.; D’Orazio, J.A. Ultraviolet Radiation, Aging and the Skin: Prevention of Damage by Topical cAMP Manipulation. Molecules 2014, 19, 6202–6219. [Google Scholar] [CrossRef] [PubMed]
- Gruber, F.; Ornelas, C.M.; Karner, S.; Narzt, M.-S.; Nagelreiter, I.M.; Gschwandtner, M.; Bochkov, V.; Tschachler, E. Nrf2 deficiency causes lipid oxidation, inflammation, and matrix-protease expression in DHA-supplemented and UVA-irradiated skin fibroblasts. Free Radic. Biol. Med. 2015, 88, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Singhal, S.S.; Singh, S.P.; Singhal, P.; Horne, D.; Singhal, J.; Awasthi, S. Antioxidant role of glutathione S-transferases: 4-Hydroxynonenal, a key molecule in stress-mediated signaling. Toxicol. Appl. Pharmacol. 2015, 289, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Shireman, L.M.; Kripps, K.A.; Balogh, L.M.; Conner, K.P.; Whittington, D.; Atkins, W.M. Glutathione transferase A4-4 resists adduction by 4-hydroxynonenal. Arch. Biochem. Biophys. 2010, 504, 182–189. [Google Scholar] [CrossRef] [Green Version]
- Castro, J.P.; Jung, T.; Grune, T.; Siems, W. 4-Hydroxynonenal (HNE) modified proteins in metabolic diseases. Free Radic. Biol. Med. 2017, 111, 309–315. [Google Scholar] [CrossRef] [Green Version]
- Aldini, G.; Altomare, A.; Baron, G.; Vistoli, G.; Carini, M.; Borsani, L.; Sergio, F. N-Acetylcysteine as an antioxidant and disulphide breaking agent: The reasons why. Free. Radic. Res. 2018, 52, 751–762. [Google Scholar] [CrossRef] [Green Version]
- Avantaggiato, A.; Palmieri, A.; Bertuzzi, G.; Carinci, F. Fibroblasts Behavior after N-Acetylcysteine and Amino Acids Exposure: Extracellular Matrix Gene Expression. Rejuvenation Res. 2014, 17, 285–290. [Google Scholar] [CrossRef]
- Lan, C.-C.E.; Hung, Y.-T.; Fang, A.-H.; Ching-Shuang, W. Effects of irradiance on UVA-induced skin aging. J. Dermatol. Sci. 2019, 94, 220–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wondrak, G.T.; Cervantes-Laurean, D.; Roberts, M.J.; Qasem, J.G.; Kim, M.; Jacobson, E.L.; Jacobson, M.K. Identification of α-dicarbonyl scavengers for cellular protection against carbonyl stress. Biochem. Pharmacol. 2002, 63, 361–373. [Google Scholar] [CrossRef]
- Baye, E.; Ukropcova, B.; Ukropec, J.; Hipkiss, A.; Aldini, G.; de Courten, B. Physiological and therapeutic effects of carnosine on cardiometabolic risk and disease. Amino Acids 2016, 48, 1131–1149. [Google Scholar] [CrossRef]
- Song, B.C.; Joo, N.-S.; Aldini, G.; Yeum, K.-J. Biological functions of histidine-dipeptides and metabolic syndrome. Nutr. Res. Pract. 2014, 8, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caruso, G.; Privitera, A.; Antunes, B.M.; Lazzarino, G.; Lunte, S.M.; Aldini, G.; Caraci, F. The Therapeutic Potential of Carnosine as an Antidote against Drug-Induced Cardiotoxicity and Neurotoxicity: Focus on Nrf2 Pathway. Molecules 2022, 27, 4452. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.D.; Sale, C.; Garner, A.C.; Hipkiss, A.R. Anti-cancer actions of carnosine and the restoration of normal cellular homeostasis. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 119117. [Google Scholar] [CrossRef] [PubMed]
- Hipkiss, A.R.; Baye, E.; de Courten, B. Carnosine and the processes of ageing. Maturitas 2016, 93, 28–33. [Google Scholar] [CrossRef]
- Babizhayev, M.A.; Vishnyakova, K.S.; Yegorov, Y.E. Oxidative Damage Impact on Aging and Age-Related Diseases: Drug Targeting of Telomere Attrition and Dynamic Telomerase Activity Flirting with Imidazole-Containing Dipeptides. Recent Pat. Drug Deliv. Formul. 2014, 8, 163–192. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Effect of Antioxidants on the Fibroblast Replicative Lifespan In Vitro. Oxidative Med. Cell. Longev. 2020, 2020, 6423783. [Google Scholar] [CrossRef]
- Regazzoni, L.; Fumagalli, L.; Artasensi, A.; Gervasoni, S.; Gilardoni, E.; Mazzolari, A.; Aldini, G.; Vistoli, G. Cyclo (His-Pro) Exerts Protective Carbonyl Quenching Effects through Its Open Histidine Containing Dipeptides. Nutrients 2022, 14, 1775. [Google Scholar] [CrossRef]
- Zhong, H.; Yin, H. Role of lipid peroxidation derived 4-hydroxynonenal (4-HNE) in cancer: Focusing on mitochondria. Redox Biol. 2015, 4, 193–199. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negre-Salvayre, A.; Salvayre, R. Post-Translational Modifications Evoked by Reactive Carbonyl Species in Ultraviolet-A-Exposed Skin: Implication in Fibroblast Senescence and Skin Photoaging. Antioxidants 2022, 11, 2281. https://doi.org/10.3390/antiox11112281
Negre-Salvayre A, Salvayre R. Post-Translational Modifications Evoked by Reactive Carbonyl Species in Ultraviolet-A-Exposed Skin: Implication in Fibroblast Senescence and Skin Photoaging. Antioxidants. 2022; 11(11):2281. https://doi.org/10.3390/antiox11112281
Chicago/Turabian StyleNegre-Salvayre, Anne, and Robert Salvayre. 2022. "Post-Translational Modifications Evoked by Reactive Carbonyl Species in Ultraviolet-A-Exposed Skin: Implication in Fibroblast Senescence and Skin Photoaging" Antioxidants 11, no. 11: 2281. https://doi.org/10.3390/antiox11112281
APA StyleNegre-Salvayre, A., & Salvayre, R. (2022). Post-Translational Modifications Evoked by Reactive Carbonyl Species in Ultraviolet-A-Exposed Skin: Implication in Fibroblast Senescence and Skin Photoaging. Antioxidants, 11(11), 2281. https://doi.org/10.3390/antiox11112281






